Abstract
Janus tyrosine kinase 3 (Jak3) is essential for signaling by interleukin-2 (IL-2) family cytokines and proper immune function. Dysfunctional regulation of Jak3 may result in certain disease states. However, the molecular mechanisms governing Jak3 activation are not fully understood. In this study, we used a functional-proteomics approach to identify two novel tyrosine phosphorylation sites within Jak3, Y904 and Y939, which are conserved among Jak family proteins. By using phosphospecific antibodies, both residues were observed to be rapidly induced by stimulation of cells with IL-2 or other γc cytokines. Mechanistic studies indicated that Y904 and Y939 regulate Jak3 activities. A phenylalanine substitution at either site greatly reduced Jak3 kinase activity in vitro and its ability to phosphorylate signal transducer and activator of transcription 5 (Stat5) in vivo, suggesting that phosphorylation of these previously unrecognized residues positively regulates Jak3 activity. Y904 and Y939 were required for optimal ATP usage by Jak3, while phosphorylation of Y939 preferentially promoted Stat5 activity in intact cells. Together, these findings demonstrate positive functional roles for two novel Jak3 phosphoregulatory sites which may be similarly important for other Jak family members. Identification of these sites also provides new therapeutic opportunities to modulate Jak3 function.
The Janus kinase (Jak) family of cytoplasmic tyrosine kinases associates with a variety of cell surface receptors to perform essential roles for transducing intracellular signals (9, 15). There are four Jak family members in vertebrates: Jak1, Jak2, Jak3, and Tyk2. While Jak1, Jak2, and Tyk2 are ubiquitously expressed, Jak3 is predominantly expressed in hematopoietic cells (20, 30, 41). Jak3 specifically associates with the cytokine receptor γ common (γc) chain and can be activated by interleukin-2 (IL-2) family cytokines such as IL-2, IL-4, IL-7, and IL-9 (40, 45). Inhibitory mutations in Jak3 or its binding partner, γc, can result in severe combined immunodeficiency (SCID) syndrome in humans and mice, which is clinically manifested by limited numbers of T, natural killer, and functional B cells (34, 35). Hyperactivation of Jak3 has also been associated with diseases such as asthma (31) and cancers of the immune system (44). The restricted expression and function of Jak3 has made it a promising target for controlling these diseases (6, 33, 39).
The activation of Jak proteins contributes to multiple cellular processes, including cell growth, proliferation, and differentiation (1). Following receptor engagement by cytokines, the activation of Jak proteins is believed to occur by auto- or transphosphorylation of key tyrosine residues located within their activation loops (12). Stimulation of hematopoietic cells with IL-2 family growth factors results in the phosphorylation and enzymatic activation of γc-associated Jak3 and another Jak family member, Jak1, which may bind to a cytokine-specific receptor subunit cooperatively with γc (19). Activated Jak1 and/or Jak3 then phosphorylate tyrosine residues on the associated receptors to produce docking sites for SH2- or PTB-containing proteins such as signal transducer and activator of transcription 5 (Stat5) (14, 24, 25), leading to their phosphorylation and subsequent activation. These proteins then regulate many downstream events, including gene transcription.
Phosphorylation plays a critical role in regulating Jak3 kinase activity. It has been reported that two adjacent tyrosines located in the Jak3 kinase activation loop are phosphorylated to positively (Y980) or negatively (Y981) regulate its catalytic activity (47). Phosphorylation of Jak proteins can also provide binding sites for other signaling molecules. For example, phosphorylation of Jak3 on Y785 has been reported to create a binding site for the adaptor protein SH2B-β, although the functional significance of this interaction is unknown (23). Negative regulatory mechanisms of Jak3 activity include dephosphorylation by CD45 and T-cell protein tyrosine phosphatase (17, 38). Suppressor of cytokine signaling family proteins form a classical negative feedback loop to attenuate cytokine signaling that can also act through the Jak/Stat pathway (2).
To determine whether other phosphosites exist, we mutated the three known residues, Y980, Y981, and Y785, and found no significant change in total tyrosine phosphorylation. Using mass spectrometry, we identified two additional phosphotyrosines in Jak3 at Y904 and Y939. Phosphospecific antibodies confirmed that phosphorylation of Jak3 on these sites occurred in response to IL-2 and other IL-2 family cytokines in multiple cell types, including primary human T cells. Phenylalanine substitution of these residues inhibited Jak3 tyrosine phosphorylation and catalytic activity. Evidence is provided to suggest that Y904 is required for ATP binding while Y939 may be required for substrate association. More importantly, these sites are conserved in other Jak family members, suggesting a universal regulatory role for these tyrosine residues.
MATERIALS AND METHODS
Plasmids and recombinant proteins.
The human Stat5a clone, the human γ common chain (γc) clone, pcDNA3.1(+), and pcDNA3.1/GS were purchased from Invitrogen. The pRL-TK vector was obtained from Promega. The β-casein-luciferase reporter plasmid was generated by cloning a triple repeat of the Stat5 consensus site corresponding to the β-casein gene promoter (5′-AGATTTCTAGGAATTCAATCC-3′) into the pGL3-Promoter vector (Promega) by using SacI and XhoI restriction sites (42). The human Jak3 cDNA was kindly provided by John J. O'Shea (National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH).
The Jak3 cDNA was subcloned into pcDNA3.1(+) by using EcoRI and XhoI. Mutant forms of Jak3 were prepared with the QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. The primers used for Jak3 mutant forms were as follows: for K855A, 5′-CTGGTGGCCGTGGCACAGCTGCAGCACAG-3′; for Y980, 5′-CGCTTGACAAAGACTTCTACGTGGTCCGCGAG-3′; for Y981F, 5′-GACAAAGACTACTTCGTGGTCCGCGAGCCA-3′; for Y785F, 5′-ATCTCTTCAGACTTTGAGCTCCTCTCAG-3′; for Y904F, 5′-GCTGGTCATGGAGTTTCTGCCCAGCGGC-3′; for Y939F, 5′-CAAGGGCATGGAGTTCCTGGGCTCCCGC-3′. The cytoplasmic region of human γc cDNA (nucleotides 863 to 1107) was subcloned into pGEXKG (Amersham Biosciences) by using EcoRI and XhoI. All subclones and mutations were verified by DNA sequencing.
For production of glutathione S-transferase (GST)-γc, Escherichia coli BL21/DE3 was transformed with pGEXKG/γc and grown at 37°C to an optical density at 600 nm of 0.8. Expression was induced with isopropyl-β-d-thiogalactopyranoside (IPTG; 400 μM, 4 h). Bacteria were pelleted and lysed in GST lysis buffer (20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1 mM DTT, 1 mM EDTA, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, 1 mM phenylmethylsulfonyl fluoride). Insoluble proteins were pelleted by centrifugation (35,000 × g, 30 min, 4°C). GST-γc was purified by incubating the soluble lysate for 1 h at 4°C with 2 ml of a 50% slurry of preswelled glutathione-agarose (Sigma). Bound GST-γc was precipitated by centrifugation, and the slurry was washed three times with lysis buffer. GST-γc was eluted from the beads by incubation with 5 mM free glutathione and then dialyzed overnight in phosphate-buffered saline plus 10% glycerol and stored in aliquots at −80°C.
Antibodies.
The Jak3 antibody used for immunoprecipitation and immunoblotting was raised against a peptide derived from the unique COOH terminus of human Jak3 (residues 1104 to 1124) as described previously (27). The anti-pY(904)Jak3 and anti-pY(939)Jak3 polyclonal antibodies were produced by immunizing rabbits with the peptides CLVME(pY)LPSG and CKGME(pY)LGSR conjugated to keyhole limpet hemocyanin, respectively (Sigma-Genosys). The antiphosphotyrosine antibody 4G10 (anti-pY), the anti-pY Stat5 monoclonal antibody, and the anti-Stat5 monoclonal antibody were purchased from Upstate Biotechnology. The Stat5a polyclonal antibody was raised against a peptide derived from the C terminus of human Stat5a (residues 775 to 794) as described previously (46). The anti-GST monoclonal antibody was purchased from Santa Cruz Biotechnology.
Cell culture and treatment.
Human YT and HEK293 cell lines were maintained in RPMI 1640 (Cellgro) medium containing 10% fetal bovine serum (Atlanta Biologicals), 2 mM l-glutamine, and penicillin-streptomycin (50 IU/ml and 50 mg/ml, respectively) as previously reported (43). The IL-2-dependent T-cell line Kit225 (kindly provided by J. Johnston, Queens University, United Kingdom) was maintained in the above medium containing 1 nM recombinant human IL-2 (rhIL-2) and made quiescent before cytokine stimulation by overnight incubation in IL-2-free medium. Freshly explanted human T lymphocytes were purified and maintained in the above medium in the presence of phytohemagglutinin (PHA; 1 μg/ml; Sigma) for 72 h as previously described (21). T lymphocytes were subsequently made quiescent by washing and incubation for 24 h in RPMI 1640 medium containing 1% fetal bovine serum prior to exposure to cytokines. For cytokine treatments, YT cells, quiescent Kit225 cells, or human T lymphocytes were treated with 100 nM rhIL-2 at 37°C for the times indicated. Quiescent Kit225 cells were also treated with 100 nM rhIL-9 under the same conditions. The rhIL-2 was obtained from Hoffman-La Roche, and the rhIL-9 was purchased from PeproTech. Transient transfections of HEK293 cells were performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Cells were harvested 30 h later for protein analysis.
Solubilization of membrane proteins, immunoprecipitation, and Western blot analysis.
Cell pellets were solubilized in Triton lysis buffer (10 mM Tris-HCl [pH 7.6], 5 mM EDTA [pH 8.0], 50 mM NaCl, 30 mM Na4P2O7, 50 mM NaF, 1 mM Na3VO4, 1% Triton X-100) containing 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, 2 μg/ml leupeptin, and 1 μg/ml pepstatin A and clarified by centrifugation (16,000 × g, 10 min, 4°C). For immunoprecipitation reactions, supernatants were rotated with 2 μl of Jak3 antibody for 2 h at 4°C. The Jak3 immune complexes were captured by incubation for 30 min at 4°C with protein A-Sepharose beads (Rockland Immunochemicals). The beads were then washed three times with cold lysis buffer and eluted by boiling in 1× sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris-HCl [pH 6.8], 100 mM dithiothreitol, 2% SDS, 0.02% bromophenol blue, 10% glycerol [pH 6.8]). Samples were resolved by 7.5% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene difluoride (PVDF) membrane (Amersham Biosciences). Western blot analysis was performed with the indicated primary antibodies either overnight [anti-pY(904)Jak3 and anti-pY(939)Jak3] or for 1 h (other primary antibodies) at room temperature. Western blot assays were developed with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG; heavy plus light chains) or goat anti-rabbit IgG (heavy plus light chains; KPL) and visualized by using enhanced chemiluminescence and X-ray film. Blots were also incubated with either IRDye800-conjugated anti-rabbit IgG (Rockland Immunochemicals) or Alexa Fluor 680 goat anti-mouse IgG (Molecular Probes) and visualized with the Odyssey infrared imaging system (LI-COR Biosciences). Enhanced-chemiluminescence blots were quantified with Scion image software. Infrared-imaged blots were quantified with LI-COR Odyssey 2.0 software. When reblotting, PVDF membranes were cleaned in stripping buffer (100 mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl [pH 6.7]) at 55°C for 30 min, blocked, and then probed with a second primary antibody.
In vitro kinase assays and mass spectrometry analysis.
HEK293 cells were transfected with the appropriate expression vectors for Jak3 and lysed in Triton lysis buffer. Jak3 proteins were immunoprecipitated with anti-Jak3 antibody and captured by protein A-Sepharose as described above. The beads were washed three times with cold lysis buffer and once with 100 mM NaCl-10 mM HEPES-KOH (pH 7.5) and then resuspended in a kinase buffer (20 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 5 mM MnCl2, 100 μM ATP) in the absence or presence of 2 μg of GST-γc. Reaction mixtures were incubated at 30°C for the indicated times, and reactions were terminated by adding SDS sample buffer. When determining the affinity of immunoprecipitated Jak3 proteins for ATP, assays were performed at 30°C for 20 min in kinase buffer with increasing concentrations of ATP. This condition was previously determined to be within the linear range for GST-γc phosphorylation by immunoprecipitated Jak3 (see Fig. 4). Samples were resolved by 7.5% SDS-PAGE (see Fig. 2B) or by 12% SDS-PAGE (see Fig. 4 and 7), and tyrosine phosphorylation levels of Jak3 or GST-γc were assessed by Western blotting with anti-pY antibodies and quantified by infrared imaging.
FIG. 4.
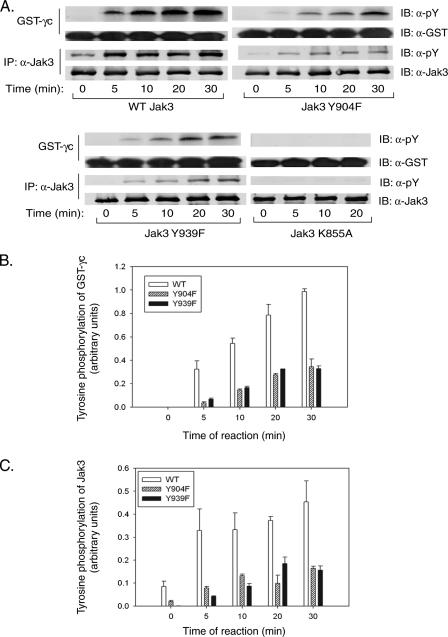
Jak3 Y904F and Y939F variants have decreased autophosphorylation and kinase activity toward the exogenous substrate GST-γc. (A) HEK293 cells were transfected with plasmids for WT Jak3 or the Y939F, Y939F, or K855A variant. Jak3 was immunoprecipitated (IP) from cell lysates and tested for kinase activity in the presence of the Jak3 substrate GST-γc. The pY levels of Jak3 and GST-γc were assessed by quantitative anti-pY antibody Western blotting (immunoblotting [IB]) normalized to Jak3 and GST-γc. (B) Phosphorylation of GST-γc was quantified, and data from two representative independent experiments were plotted. (C) Jak3 autophosphorylation was quantified, and data from two representative independent experiments were plotted.
FIG. 2.
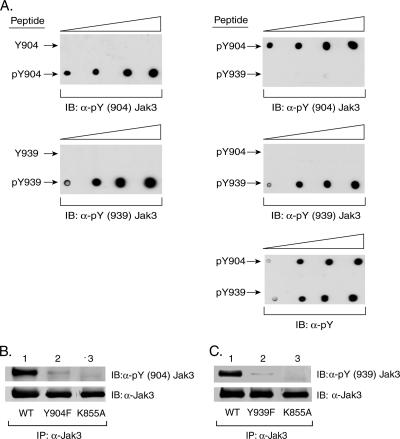
Characterization of anti-pY(904)Jak3 and anti-pY(939)Jak3 antibodies. (A) Phospho-specific Jak3 antibodies to pY904 and pY939 are selective targets. Increasing amounts of Y904 and pY904 peptides (left panel, top), as well as Y939 and pY939 peptides (left panel, bottom), were spotted onto PVDF membranes and tested for recognition by rabbit anti-pY(904)Jak3 and anti-pY(939)Jak3 antibodies by Western blotting (immunoblotting [IB]). Additionally, increasing amounts of pY904 and pY993 peptides were spotted onto PVDF membranes and tested for recognition by rabbit anti-pY(904)Jak3 (right panel, top), anti-pY(939)Jak3 (right panel, middle), and anti-pY (right panel, bottom) antibodies by Western blotting. (B) Anti-pY(904)Jak3 antibody recognizes activated WT Jak3. HEK293 cells were transfected with plasmids for WT Jak3 or Y904F or kinase-inactive K855A mutant Jak3. Jak3 proteins were immunoprecipitated (IP) with anti-Jak3 antibody, followed by an in vitro kinase assay in the presence of 100 μM ATP for 30 min as described in Materials and Methods. Phosphorylation of Y904 was detected by blotting with anti-pY(904)Jak3 (upper panel). The Jak3 expression level was monitored by reblotting the membrane with anti-Jak3 antibody (lower panel). Shown are representative data from three independent experiments. (C) Anti-pY(939)Jak3 antibody preferentially recognizes activated WT Jak3. HEK293 cells were transfected with plasmids for WT or Y939F or K855A mutant Jak3. Jak3 proteins were immunoprecipitated, and tyrosine phosphorylation was monitored following an in vitro kinase assay as described for panel B. The phosphorylation of Jak3 on Y939 was detected by blotting with anti-pY(939)Jak3 antibody (upper panel). The blot was reprobed with anti-Jak3 antibody (lower panel). Shown are representative data from three independent experiments.
FIG. 7.
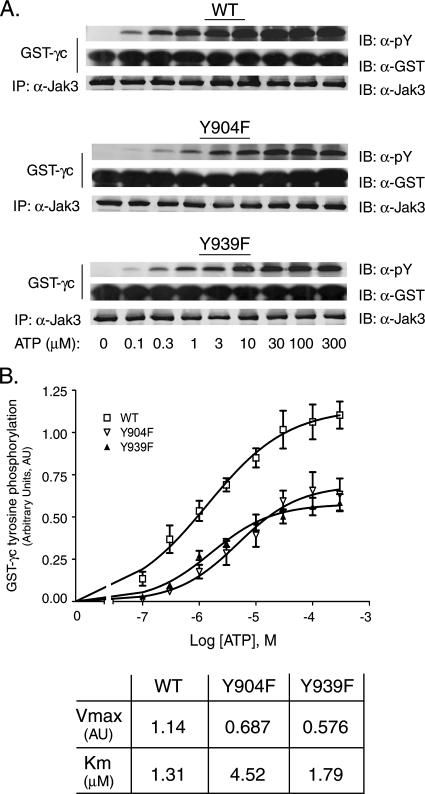
The Jak3 Y904F mutant form shows impaired ATP binding activity. (A) HEK293 cells were transfected with plasmids for WT Jak3 or the Y904F or Y939F mutant form. Jak3 immunoprecipitation (IP) kinase assays were performed with increasing concentrations of ATP in the presence of 2 μg GST-γc for 20 min as indicated. pY levels of GST-γc were assessed by quantitative Western blotting (immunoblotting [IB]) with anti-pY and anti-GST antibodies. Jak3 expression levels were determined by Western blotting with anti-Jak3 antibody. (B) Quantification of the KmATP and Vmax for WT Jak3 and the Y904F and Y939F variants were performed with GraphPad Prism 4 software and Michaelis-Menten kinetics. Each datum point represents at least three independent experiments. Error bars represent the standard error of the mean.
For phosphorylation site mapping, immunopurified Jak3 from the in vitro autokinase assay described above was resolved by 7.5% SDS-PAGE and the resulting Coomassie blue-stained Jak3 protein was excised and stored at −80°C before analysis. Liquid chromatography-tandem mass spectrometry analysis was performed by the Taplin Biological Mass Spectrometry Facility (Harvard University) or the Biomolecule Analysis Core Facility (University of Texas at El Paso) by following their standard procedures.
Luciferase assays.
Subconfluent HEK293 cells in six-well dishes were transfected with a triple repeat of β-casein-luciferase reporter plasmid (0.2 μg), pRL-TK vector (0.1 μg), Jak3 plasmids (1 μg), and Stat5a plasmid (1 μg). Cells were harvested 30 h after transfection, and firefly and Renilla luciferase activities were measured with a dual-luciferase assay reporter system (Promega) according to the manufacturer's instructions. Equivalent amounts of cell lysates were also examined for Jak3 and Stat5a expression by Western blotting.
Peptide pull-down assays.
The peptides CKGMEY(939)LGSR and CKGMEpY(939)LGSR were dissolved in coupling buffer (0.1 M NaHCO3 [pH 8.3], 0.5 M NaCl) and conjugated to activated CNBr-Sepharose beads overnight at 4°C. After washing with coupling buffer to remove unbound peptides, the remaining active groups were blocked with 0.1 M Tris-HCl (pH 8.0). Peptide-conjugated beads were then incubated with cell lysates from IL-2-treated or untreated YT cells for 1 h at 4°C. Complexes were washed five times with cold Triton lysis buffer containing 500 mM NaCl and once with regular Triton lysis buffer. Bound proteins were then eluted with 1× SDS sample buffer, resolved by 7.5% SDS-PAGE, and analyzed by Western blotting with anti-pY Stat5, anti-Stat5, and anti-pY antibodies.
RESULTS
Jak3 is phosphorylated on previously unrecognized tyrosine residues.
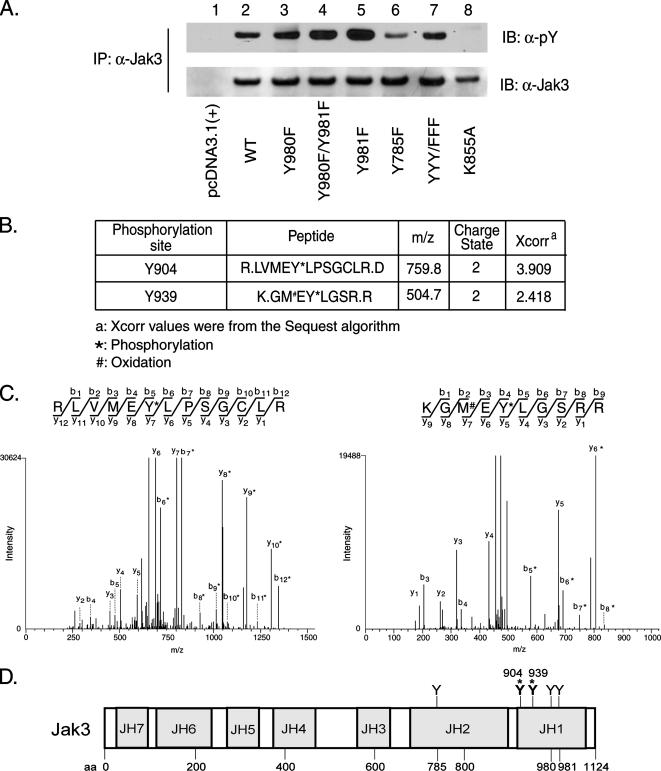
Three tyrosine residues in Jak3, Y980, Y981, and Y785, were previously identified as phosphoregulatory sites (23, 47). Mutational analysis was used to reexamine the regulatory role of these tyrosines and to determine whether Jak3 has additional unrecognized regulatory tyrosine phosphorylation sites. Jak3 Y980, Y981, and Y785 were mutated to phenylalanine individually or in combination and transfected into HEK293 cells. The transfected Jak3 proteins were then immunoprecipitated and immunoblotted with anti-pY or anti-Jak3 antibodies (Fig. 1A). We observed that the Y980F mutant form of Jak3 and wild-type (WT) Jak3 had comparable levels of tyrosine phosphorylation (lanes 2 and 3). Similar to earlier findings, we observed that the Y981F mutant form exhibited elevated tyrosine phosphorylation (lane 5), as did the Y980F/Y981F mutant form (lane 4), suggesting that Y981 functions as a negative regulator in controlling Jak3 activity. We also observed that the tyrosine phosphorylation of the Y785F mutant form (lane 6) was decreased by 50% compared to WT Jak3, indicating that this site may account for a substantial portion of the overall tyrosine phosphorylation of transfected Jak3. Strikingly, the Jak3 Y980F/Y981F/Y785F triple-mutant form was also readily tyrosine phosphorylated (lane 7), strongly suggesting that novel Jak3 tyrosine phosphorylation sites are present within this enzyme. Since the Jak3 kinase-inactive K855A mutant form was not tyrosine phosphorylated (lane 8), the phosphorylation of WT Jak3 and Y980F/Y981F/Y785F mutant Jak3 is presumably mediated through autophosphorylation at distinct Jak3 sites within this model system.
FIG. 1.
Identification of novel phosphorylated tyrosine residues in human Jak3. (A) Jak3 is phosphorylated on novel tyrosine sites in HEK293 cells. HEK293 cells were transfected with empty vector or plasmids for WT Jak3 or Jak3 mutant forms Y980F, Y980F/Y981F, Y981F, Y785F, Y980F/Y981F/Y785F (YYY/FFF), and K855A, as indicated. Cells were harvested at 30 h posttransfection and lysed, and Jak3 proteins were immunoprecipitated (IP) from soluble lysates with anti-Jak3 antibody. The immunoprecipitates were then Western blotted (immunoblotted [IB]) with pY or anti-Jak3 antibodies as indicated. Shown are representative data from three independent experiments. (B) Summary of peptides detected as phosphorylated at Y904 and Y939. (C) Tandem mass spectra of monophosphorylated peptides showing site localization to either Y904 (left panel) or Y939 (right panel), as indicated by asterisks. Methionine oxidation is denoted (#). (D) Domain architecture of human Jak3 with known and newly identified (asterisks) tyrosine phosphorylation sites. Numbers indicate amino acid residues of human Jak3.
To identify these novel tyrosine phosphorylation sites, WT Jak3 was immunoprecipitated from transfected HEK293 cells and subjected to an in vitro autokinase assay for 30 min. The sample was separated by 7.5% SDS-PAGE and visualized by Coomassie blue staining. The corresponding 116-kDa band was excised and subjected to trypsin digestion, and the resulting peptides were analyzed by liquid chromatography-tandem mass spectrometry. Two novel Jak3 phosphorylated peptides were identified with the Sequest search algorithm. R.LVMEYLPSGCLR.D contained phosphorylated Y904 (underlined), and K.GMEYLGSR.R harbored phosphorylated Y939 (underlined) as a second site (Fig. 1B). Tandem mass spectra for each peptide are shown in Fig. 1C. The positions of these novel and previously identified Jak3 phosphorylation sites are shown in Fig. 1D.
Generation of anti-pY(904)Jak3 and anti-pY(939)Jak3 phospho-specific antibodies.
To verify that Jak3 is phosphorylated at tyrosine 904 and tyrosine 939 and to confirm the regulatory roles of these phosphorylation sites, two phospho-specific antibodies [anti-pY(904)Jak3 and anti-pY(939)Jak3] were generated. Dot blot analysis was performed with the immunizing phosphopeptides and the corresponding nonphosphorylated peptides (see Materials and Methods for sequences) to determine if the Jak3 phospho-specific antibodies cross-react with regions distal to the phosphorylated tyrosines. Increasing amounts of Y904 and pY904 peptides (Fig. 2A, left panel, top) or Y939 and pY939 peptides (Fig. 2A, left panel, bottom) were spotted onto PVDF membranes and immunoblotted with the anti-pY(904)Jak3 or anti-pY(939)Jak3 antibody. The anti-pY(904)Jak3 and anti-pY(939)Jak3 antibodies recognized the corresponding phosphorylated peptides but not the nonphosphorylated counterparts, indicating that these two phospho-specific antibodies do not cross-react significantly with the nonphosphorylated peptide. Because of the amino acid similarities between these two peptides (60%), their specificity was tested against the corresponding immunizing phosphopeptides. Increasing amounts of peptides containing pY904 or pY939 were spotted onto PVDF membranes and immunoblotted with the anti-pY(904)Jak3 (Fig. 2A, right panel, top), anti-pY(939)Jak3 (Fig. 2A, right panel, middle), and anti-pY (Fig. 2A, right panel, bottom) antibodies. The anti-pY(904)Jak3 antibody recognized the pY904 but not the pY939 peptide, thus validating its specificity. Similarly, the anti-pY(939)Jak3 antibody recognized the pY939 but not the pY904 peptide. Immunoblotting with the anti-pY antibody indicated that the same amount of each peptide was spotted onto the membrane.
To further characterize these phospho-specific antibodies, HEK293 cells were transfected with cDNA encoding WT Jak3 or the Y904F, Y939F, or kinase-inactive K855A mutant form of Jak3. The Jak3 proteins were then immunoprecipitated and subjected to an in vitro autophosphorylation reaction. The Jak3 proteins present in the kinase assays were then resolved by SDS-PAGE, transferred to PVDF membrane, and examined for phosphorylation of Y904 and Y939 by Western blotting. As shown in Fig. 2B (lanes 1 and 2), the anti-pY(904)Jak3 antibody recognized autophosphorylated WT Jak3 but not the Y904F mutant form. Similarly, anti-pY(939)Jak3 preferentially recognized autophosphorylated WT Jak3 but not the Y939F mutant form (Fig. 2C, lanes 1 and 2). Reprobing these blots with anti-Jak3 antibody confirmed similar Jak3 protein expression (Fig. 2B and C, lower panels). Under the same experimental conditions, neither phospho-specific antibody recognized the kinase-inactive K855A mutant form (Fig. 2B and C, lanes 3). These data suggest that intact kinase activity is required for the phosphorylation of Jak3 at Y904 and Y939.
Phosphorylation of Jak3 at Y904 and Y939 occurs in vivo.
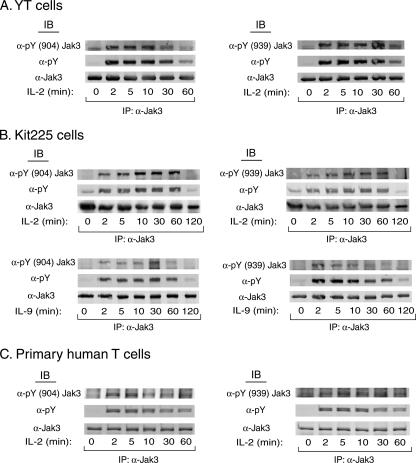
γc-containing cytokines such as IL-2, IL-4, IL-7, and IL-9 are critical for the development and function of the immune system (16, 18). Binding of these cytokines to their receptors results in the rapid phosphorylation of Jak3 and transmission of their downstream signals to control cellular function (19). To determine whether Jak3 is phosphorylated at Y904 and Y939 in response to physiological stimuli, YT cells were stimulated with IL-2 and endogenous Jak3 was then immunoprecipitated and examined for phosphorylation (Fig. 3A). We observed that both tyrosine 904 and tyrosine 939 in Jak3 were rapidly and transiently phosphorylated following IL-2 stimulation. Phosphorylation of these two tyrosines reached maximal levels after 2 min and returned to the baseline by 60 min (Fig. 3A). In contrast, the human T-cell leukemia line Kit225 showed that maximum IL-2-induced phosphorylation of Jak3 at Y904 and Y939 occurred at 5 to 10 min and did not return to basal levels until 120 min post IL-2 stimulation (Fig. 3B). To determine whether Jak3 is phosphorylated at Y904 and Y939 in response to other γc-containing cytokines, Kit225 cells were stimulated with IL-9 as described in the legend to Fig. 3B. Jak3 protein was immunoprecipitated, and Western blotting was performed with these two phospho-specific antibodies. We observed that IL-9 was able to induce Jak3 phosphorylation at Y904 and Y939, although the strength and kinetics of phosphorylation on these sites differed from the treatment with IL-2. Nonetheless, these results suggest that γc cytokines, in general, can result in Jak3 phosphorylation at these sites.
FIG. 3.
Phosphorylation of Jak3 Y904 and Y939 in YT, Kit225, and primary human T cells is mediated by γc-containing cytokines. (A) YT cells were stimulated with IL-2 for the indicated times. Endogenous Jak3 was then immunoprecipitated (IP) with anti-Jak3 antibody and Western blotted (immunoblotted [IB]) with anti-pY(904)Jak3 (left panels) or anti-pY(939)Jak3 (right panels) antibody. Blots were then reprobed with anti-pY and anti-Jak3 antibodies. Shown are representative data from three independent experiments. (B) Kit225 cells were made quiescent in IL-2-free medium overnight and then stimulated with IL-2 or IL-9 for the indicated times. Phosphorylation of Jak3 at Y904 and Y939 was monitored as described for panel A. Shown are representative data from two independent experiments. (C) Purified human T lymphocytes were activated with PHA for 72 h, subsequently made quiescent for 24 h, and then stimulated with IL-2 for the indicated times. Phosphorylation of Jak3 on Y904 and Y939 was determined by Western blotting with the respective phospho-specific antibodies. Representative data from three independent experiments are shown.
To test whether Y904 and Y939 are phosphorylated in nontumorigenic primary lymphocytes, PHA-activated primary human T cells were made quiescent and then stimulated with IL-2. IL-2-stimulated Jak3 phosphorylation of Y904 and Y939, although less robust than that in tumor cell lines (panels A and B), was detectable within 2 min before returning to basal levels at 60 min (Fig. 3C). Moreover, IL-2-induced phosphorylation of Y904 and Y939 had kinetics similar to those of total tyrosine phosphorylation of Jak3, as detected by anti-pY blotting, except that phosphorylation did not return to basal levels after 60 min. Thus, the phosphorylation of Y904 and Y939 occurred in multiple cell types, including primary human T cells, with activation profiles indicating a universal mechanism of Jak3 activation.
Y904 and Y939 are required for optimal Jak3 autophosphorylation and kinase activity in vitro.
Phosphorylation represents an important posttranslational modification that regulates the catalytic activities of protein kinases. To determine whether phosphorylation at tyrosine 904 or 939 affects the kinase activity of Jak3, HEK293 cells were transfected with plasmids encoding WT Jak3 or the Y904F, Y939F, or K855A mutant form, and these Jak3 proteins were subsequently immunoprecipitated and tested for autophosphorylation, as well as kinase activity toward the exogenous substrate GST-γc. The tyrosine phosphorylation of Jak3 and GST-γc was measured by quantitative Western blotting with anti-pY antibody that was normalized to the total amounts of Jak3 or GST-γc. Jak3 and GST-γc levels were detected with anti-Jak3 and anti-GST antibodies, respectively.
In these assays, WT Jak3 exhibited robust, time-dependent autophosphorylation, as well as kinase activity on the GST-γc substrate (Fig. 4A). This activity originated from immunoprecipitated Jak3, since no phosphorylation of GST-γc was observed in immunoprecipitates of the Jak3 kinase-inactive K855A mutant form. Importantly, in contrast to WT Jak3, both the Y904F and Y939F mutant forms showed nearly 60% less autophosphorylation (Fig. 4C) and kinase activity toward GST-γc (Fig. 4B). These results suggest that both Y904 and Y939 are vital for Jak3 autophosphorylation and the kinase activity toward exogenous substrates. Hence, these decreased kinase activities suggest that phosphorylation of Jak3 at Y904 or Y939 positively regulates this enzyme.
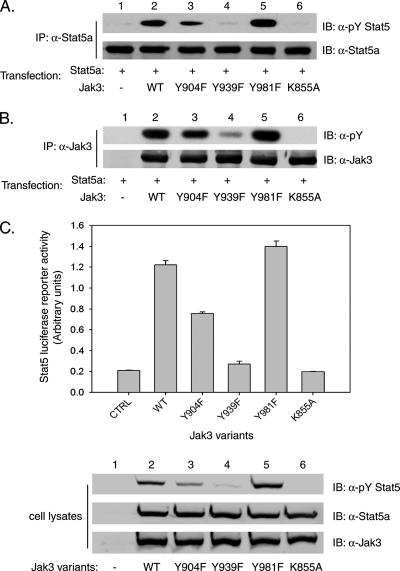
Jak3 Y904 and Y939 are required for optimal Stat5 tyrosine phosphorylation and transcriptional activity.
Stat5a and Stat5b are two of seven Stat family members that share high homology due to a gene duplication event (11). Human Stat5a is tyrosine phosphorylated at Y694, while human Stat5b is tyrosine phosphorylated at Y699, by Jak proteins and other tyrosine kinases. Phosphorylation of cytokine receptor-based tyrosines recruits Stat5, allowing their subsequent tyrosine phosphorylation, which is required for their dimerization, nuclear translocation, and gene regulation activity (25). To determine whether Y904 and Y939 of Jak3 are required for optimal Stat5 activation in vivo, plasmids encoding WT Jak3 or Y904F, Y939F, Y981F, or K855A mutant Jak3 were cotransfected into HEK293 cells with an expression plasmid for Stat5a. Stat5a was immunoprecipitated and tested for tyrosine phosphorylation by Western blotting with an anti-pY Stat5 antibody that recognized pY694 in Stat5a (Fig. 5A). For these assays, coexpression of WT Jak3 with Stat5a was found to result in tyrosine phosphorylation of Stat5a (lane 2) mediated by Jak3, since Stat5a coexpressed with the kinase-inactive K855A mutant form of Jak3 was not tyrosine phosphorylated (lane 6). As expected, expression of the hyperactive Y981F mutant form of Jak3 increased Stat5a tyrosine phosphorylation dramatically (lane 5). In contrast, tyrosine phosphorylation of Stat5a was reduced nearly 50% for the Y904F mutant form compared to WT Jak3 (lane 3) and was almost completely abolished for the Y939F mutant form (lane 4). Reprobing this blot with anti-Stat5a antibody confirmed that similar amounts of Stat5a protein were tested and measured (Fig. 5A, lower panel). As an additional control, Jak3 was also immunoprecipitated and tested for its levels of total tyrosine phosphorylation by blotting with anti-pY antibody (Fig. 5B). In contrast to WT Jak3 (lane 2), the Jak3 Y981F mutant form (lane 5) showed more pronounced tyrosine phosphorylation, while the tyrosine phosphorylation of Y904F mutant Jak3 (lane 3) was modestly decreased and tyrosine phosphorylation of Y939F mutant Jak3 (lane 4) was significantly reduced. Similar levels of Jak3 protein were expressed for each variant, as determined by Western blotting with anti-Jak3 antibodies (Fig. 5B, lower panel). These data further support the model in which Y904 and Y939 are required for optimal Jak3 activation and subsequent tyrosine phosphorylation of endogenous substrates such as Stat5.
FIG. 5.
Jak3 Y939 and Y904 are required for optimal Stat5 activity in vivo. (A) HEK293 cells were cotransfected with plasmids encoding the Stat5a and Jak3 proteins as indicated. At 30 h posttransfection, cells were harvested and cell lysates were immunoprecipitated (IP) with anti-Stat5a antibody. Stat5a activation was then assessed by Western blotting (immunoblotting [IB]) with anti-pY Stat5 antibody. Total Stat5a levels were determined by reprobing the membrane with anti-Stat5a antibody, as shown in the panel below. (B) Cell lysates were also immunoprecipitated with antibodies to Jak3 and Western blotted for tyrosine phosphorylation. Total Jak3 levels were monitored by reprobing with anti-Jak3 antibodies as shown in the panel below. Shown are representative data from four independent experiments. (C) In six-well dishes, subconfluent cells were transfected in triplicate with the Stat5-activated β-casein-luciferase reporter plasmid, the TK-Renilla luciferase vector, Stat5a, and WT Jak3 or Jak3 mutant forms, as indicated. Control (CTRL) wells were transfected with identical amounts of luciferase and empty vectors. Cells were lysed, and the luciferase activities were determined at 30 h posttransfection. Representative data from three independent experiments are shown. Cell lysates were also immunoblotted with anti-pY Stat5, anti-Stat5a, and anti-Jak3 antibodies to verify equivalent expression of Jak3 and Stat5a (lower panels).
Tyrosine-phosphorylated Stat proteins dissociate from the receptor complex to form dimers, translocate to the nucleus, and cooperate with other factors to stimulate gene transcription (24). To analyze the functional role of Jak3 Y904 and Y939 in Stat5-dependent transcriptional activity, HEK293 cells were transfected with Stat5a and the appropriate Jak3 expression plasmids, along with a β-casein-firefly luciferase reporter construct harboring three Stat5 response elements in tandem. Cells were also transfected with a second Renilla luciferase gene under the control of a constitutive thymidine kinase promoter to control for transfection efficiency. In these experiments, WT Jak3 promoted a sixfold induction of Stat5 activated β-casein-luciferase reporter activity compared to the Jak3 kinase-inactive K855A mutant form. In contrast to WT Jak3, Y904F mutant Jak3 showed a 40% decrease in this Stat5 reporter activity, while a Y939F Jak3 variant was almost completely inactive (Fig. 5C). These data indicate that Jak3 Y904 and Y939 are required for activation of Stat5 transcriptional activity. As a control, cell lysates were immunoblotted with anti-pYStat5, anti-Stat5a, and anti-Jak3 antibodies to confirm activation and expression (Fig. 5C, lower panel). These results demonstrate that the reduced levels of Stat5a tyrosine phosphorylation, when cotransfected with the Y904F and Y939F Jak3 variants, resulted in a direct reduction in its transcriptional activity (lanes 2 to 4). Interestingly, the Jak3 Y981F mutant form did not induce significantly higher luciferase gene expression compared to WT Jak3, although the Jak3 Y981F variant mediated substantially stronger tyrosine phosphorylation of Stat5a (lane 5).
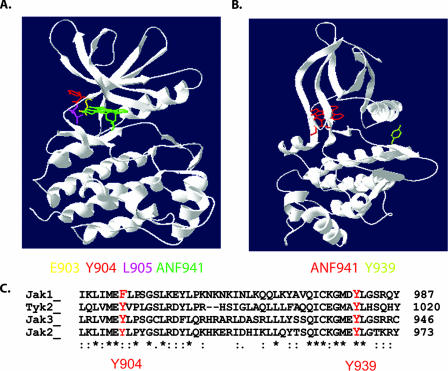
Y904 and Y939 are localized to distinct regions of the Jak3 kinase domain and conserved among other Jak family members.
The Jak3 kinase domain has been crystallized in a complex with the staurosporine analogue ANF941 (4). Based upon this structure, Y904 and Y939 were localized in Jak3 to predict their potential roles in Jak3 catalytic activity. Both tyrosine residues are at least partially exposed to the solvent. Y904 localizes to the N lobe of the kinase domain. Interestingly, the two residues flanking tyrosine 904, glutamate 903 and leucine 905, make direct contact with ANF941 (Fig. 6A). Since ANF941 is also an ATP analogue, it seemed plausible to expect that tyrosine 904 may be important for regulating ATP binding during catalytic reactions. Conversely, Y939 is localized to α-helix E in the C lobe of the kinase domain (Fig. 6B). Typically, this kinase region promotes substrate access to the catalytic cleft, suggesting that phosphorylation of Y939 may regulate the interaction between Jak3 and Stat5 or other substrates. In addition, phosphorylation of Y939 creates a potential Stat5 SH2 binding site (pY[VLTFIC]XX) that may mediate high-affinity interaction with Stat5 (32).
FIG. 6.
Y904 and Y939 are located within distinct regions of the Jak3 catalytic domain and are conserved among Jak family members. (A) Y904 localizes to the ATP binding pocket of the N lobe of the kinase domain. (B) Y939 is harbored within α-helix E of the C lobe of the kinase domain. Models were made with the DeepView Swiss-pdbviewer 3.7 program, based on the crystal structure of the Jak3 kinase domain solved in complex with the staurosporine analogue ANF941 (4) (accession number 1YVJ). Residues E903, Y904, L905, and Y939 and ANF941 are indicated in color beneath the Jak3 structure. (C) The amino acid sequences surrounding the novel phosphotyrosine sites in human Jak3 were aligned with human Jak1, Jak2, and Tyk2.
Alignment of the amino acid sequences of the four human Jak proteins revealed that Y904 is conserved in Jak2, Tyk2, and Jak3 but is replaced by a phenylalanine in Jak1 (Fig. 6C). Since this is a highly phosphorylated residue in Jak3, it is tempting to speculate that this substitution may explain the lower level of cytokine-stimulated tyrosine phosphorylation of Jak1 compared to Jak3 which has been reported (22, 26). On the other hand, Y939 is conserved among the four Jak proteins (Fig. 6C) and also some other receptor tyrosine kinases, including the epidermal growth factor receptor family members EGFR, HER2, and ErbB4 (not shown). Taken together, this model suggests that phosphorylation of this conserved tyrosine may regulate Jak family kinases through a common mechanism.
Phenylalanine substitution of Y904 in Jak3 impairs its ATP binding affinity.
To determine whether Y904 in Jak3 is important for its interaction with ATP, the ATP binding affinity of WT Jak3 or the Y904F or Y939F mutant form of Jak3 was examined. HEK293 cells were transfected with WT Jak3 or the Y904F or Y939F mutant form, and the Jak3 proteins were immunoprecipitated and tested for kinase activity toward GST-γc in the presence of gradually increasing concentrations of ATP. Tyrosine phosphorylation of GST-γc was assessed by quantitative Western blotting with anti-pY antibodies (Fig. 7A). Blots were also probed with anti-GST and anti-Jak3 antibodies to normalize protein levels. Data quantified from several experiments are shown in Fig. 7B. Consistent with our finding that Y904 and Y939 are required for optimal in vitro catalytic activity of Jak3 (Fig. 4), the Vmax for the Y904F or Y939F mutant form was decreased by 50% compared to that for WT Jak3. In addition, the KmATP for Jak3 was increased from 1.31 μM for the WT to 4.52 μM for the Y904F variant, while WT Jak3 and the Y939F mutant form showed similar ATP binding affinities (the KmATP for Y939F mutant Jak3 was 1.79 μM). These data suggest that Y904 or Y939 can affect the ability of Jak3 to consume ATP.
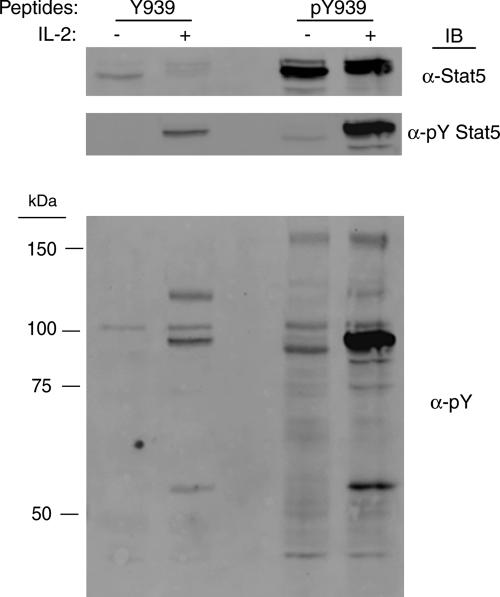
Stat5 binds to Y939 phosphorylated peptide.
The previous data (Fig. 5) support the notion that phosphorylation of Y939 in Jak3 is essential for Stat5 tyrosine phosphorylation and transcriptional activity. However, in vitro data indicated that the kinase activity of the Jak3 Y939F mutant form toward GST-γc was only reduced approximately 60% compared to that of WT Jak3 (Fig. 4). In addition to ATP binding, this suggests that Y939 may be important for optimal interaction between Jak3 and Stat5. To determine the possible direct regulatory mechanism of Jak3 Y939 in Stat5 activation, peptides containing phosphorylated or nonphosphorylated Y939 were coupled to CNBr-activated Sepharose and used in a pull-down assay with YT-cell lysates. Captured Stat5 and pY Stat5 were examined by Western blotting. Indeed, endogenous Stat5 was found to bind the phosphorylated Y939 peptide but not its nonphosphorylated counterpart (Fig. 8, upper panel). Similarly, the phosphorylated Y904 peptide also captured Stat5 (data not shown). In either instance, this occurred whether or not the cells had been stimulated with IL-2. Interestingly, reprobing the membrane with anti-pY antibodies indicated that additional tyrosine-phosphorylated proteins other than Stat5 coprecipitated with the phosphorylated Y939 peptide in an IL-2-dependent manner (Fig. 8, lower panel). These data correlate with previous reports that peptides derived from the corresponding phosphorylation site in Jak2, Y966, bound to a number of signaling proteins containing SH2 domains, such as Stat5, SHC, phosphatidylinositol 3-kinase, and a novel protein tentatively named p70 (5). Taken as a whole, these data indicate that, in addition to regulating the intrinsic catalytic activity of Jak3, phosphorylation of Y939 and Y904 may provide a docking site for interaction with Jak3 substrates such as Stat5.
FIG. 8.
Association of Stat5 with phosphorylated Y939 peptides. Peptides containing nonphosphorylated or phosphorylated Y939 were coupled to CNBr-activated beads and used to probe for interacting proteins from YT-cell lysates. YT cells (3 × 107/sample) were left untreated or stimulated with 100 nM IL-2 for 10 min. Soluble cell lysates were incubated with the indicated peptides for 1 h at 4°C. After washing, bound proteins were eluted from the beads with SDS sample buffer. Binding of Stat5, pY Stat5, and other tyrosine-phosphorylated proteins was assessed by Western blotting (immunoblotting [IB]) with anti-Stat5, anti-pY Stat5, and anti-pY antibodies. Molecular mass markers are indicated on the left. One representative set of data from three independent experiments is shown.
DISCUSSION
Jak3 is a key immunoregulatory enzyme responsible for promoting normal and abnormal immune responses. Thus, understanding its mechanism of regulation is fundamentally important. For this work, two novel tyrosine phosphorylation sites in Jak3 were identified by mass spectrometry and further characterized with site-specific phospho-specific antibodies. Y904 and Y939 were determined to be rapidly and transiently phosphorylated in response to IL-2 in YT, Kit225, and primary human T cells. IL-9 similarly activated the phosphorylation of Jak3 at Y904 and Y939 in Kit225 cells. The tyrosine phosphorylation kinetics of Y904 or Y939 peaked at 2 min and returned to baseline by 60 min in YT and human T lymphocytes. In contrast, Kit225 cells showed protracted phosphorylation kinetics, with pY904 and pY939 only returning to basal levels after 120 min of IL-2 stimulation. Additionally, Y904 and Y939 were readily autophosphorylated and required for Jak3 catalytic activity to phosphorylate a defined substrate. This is likely due to the requirements for Y904 and Y939 in ATP and substrate binding.
For protein kinases, phosphorylation of key residues within the activation loop, which is localized between subdomains VII and VIII of the kinase domain, induces a conformational change which facilitates the access of substrates to the active site. Hence, phosphorylation of these residues often increases the intrinsic catalytic activities of kinases (29), as is true for Y1162 in the insulin receptor kinase (13). For Jak family proteins, two adjacent tyrosine residues within the activation loop have been implicated in the control of their catalytic activities. In Jak3, Y980 is a positive regulatory site while Y981 negatively controls its activity (47). Similarly, mutation of positionally conserved Y1007 to phenylalanine in Jak2 blocked its activation while phenylalanine substitution of Y1008 had no effect (7).
Jak proteins have seven regions of sequence similarity named Janus homology (JH) domains. The tyrosine kinase domain is localized to the C terminus in JH1. Jak proteins are unique in having a catalytically inactive pseudokinase domain (JH2), which has been shown to regulate their kinase activity (36, 37). The N-terminal JH4 to JH7 domains of Jak proteins are involved in receptor association. This region has a band 4.1, ezrin, radixin, and moesin (FERM) domain (10). Several mutations in the FERM domain of Jak3 were found in SCID patients (9). Except for tyrosine phosphorylation within the activation loop of the kinase domain, few studies have expanded the functional roles of other putative phosphorylation sites in Jak proteins. Among the four Jak kinases, autophosphorylation of Jak2 may be the best characterized. Indeed, Y221 in the FERM domain and Y570 in the JH2 domain are sites of autophosphorylation in Jak2 that differently regulate its catalytic activity (3). Phosphorylation of Jak2 on Y119 in the FERM domain was found to down-regulate erythropoietin signaling by promoting the dissociation of Jak2 from the receptor complex (8). Y813 in the JH2 domain of Jak2 represents another site of phosphorylation which is required for binding to the adaptor protein SH2-Bβ, which further enhances the activity of this enzyme. Positionally conserved corresponding tyrosine 785 in Jak3 is phosphorylated in response to IL-2 and is also important for binding to SH2-Bβ (23). Phosphorylation of Jak2 on S523 was recently demonstrated to function as a regulatory feedback mechanism to dampen the activity of this enzyme (28). While little is known about serine phosphorylation in other Jak proteins, we also observed that Jak3 was serine phosphorylated upon IL-2 stimulation (data not shown). Additional work seeks to identify this serine residue(s).
Extending the current model, our data indicate that phosphorylation of both Y904 and Y939 positively regulates Jak3 activity. This is based on our observations that mutation of either site to phenylalanine impairs the autocatalytic activity of Jak3, as well as its ability to phosphorylate substrates such as GST-γc in vitro (Fig. 4). Phenylalanine mutation of either Y904 or Y939 impaired its ability to stimulate Stat5 tyrosine phosphorylation and transcriptional activity (Fig. 5). Our data provide insight into the molecular mechanisms by which phosphorylation of Y904 and Y939 positively regulates Jak3 activity. Based upon the crystal structure of the Jak3 kinase domain, Y904 is likely confined to the ATP binding pocket between two amino acids shown to make contact with the ATP analogue ANF941(Fig. 6). Indeed, phenylalanine substitution of Y904 increased the Km of Jak3 for ATP from 1.31 to 4.52 μM and reduced the Vmax of Jak3 toward GST-γc by approximately 50% (Fig. 7). Although Y939 lies outside the proposed ATP binding domain, this residue also changed the Km of Jak3 for ATP from 1.31 to 1.79 μM and reduced the Vmax of Jak3 toward GST-γc by approximately 50%. Nonetheless, these data indicate that Y904 and Y939 are important for Jak3 ATP binding affinity and that phosphorylation of these sites promotes Jak3 catalytic activity.
Interestingly, Y939 is localized to the α-helix E of the C lobe, suggesting that phosphorylation of this site may influence substrate interaction. Moreover, phosphorylation of this residue is predicted to create a Stat5 SH2 binding site (pY[VLTFIC]XX). To test this notion, phenylalanine substitution of Y939 in Jak3 was found to result in reduced catalytic activity in vitro (Fig. 4) and completely blocked its ability to activate Stat5a in vivo (Fig. 5). Moreover, a 10-mer peptide harboring pY939, but not its nonphosphorylated counterpart, was able to capture endogenous Stat5 from YT-cell lysates (Fig. 8). It is also noteworthy that this peptide readily coassociated with other, unknown, tyrosine-phosphorylated proteins, indicating that phosphorylated Y939 may regulate other signaling molecules. These data lead us to propose that Y939 serves two roles in the Jak3 activation mechanism. When phosphorylated, it may enhance access to the catalytic cleft for substrates lacking an SH2 domain (e.g., GST-γc) and may also provide a docking site for SH2 domain-containing proteins such as Stat5. Results in Fig. 5 indicate that Y904 is less effective than Y939 in mediating Stat5 activation. These data may explain how Stat5 can be activated by Jak2 or Jak3 independently of a receptor involvement (23, 47). Based on our findings, we hypothesize that, in the absence of cytokine receptors, phosphorylation of Jak3 at Y939, and possibly Y904, is required for Stat5 association and subsequent activation. This may be especially important in tumor models where constitutively active Jak3 may activate substrates independently of a receptor.
In summary, we have identified Y904 and Y939 in Jak3 as two novel sites of cytokine-mediated phosphorylation. Phosphoantibodies that specifically recognized these residues confirmed that Jak3 Y904 and Y939 are rapidly and transiently induced in YT, Kit225, and primary human T cells. Y904 and Y939 positively regulate the enzymatic activity of Jak3 and its substrate Stat5. Lastly, we provide evidence that phosphorylation of Y904 and Y939 of Jak3 regulates Stat5 and ATP binding activity. These data provide new insight into the mechanisms that regulate Jak3 activation and its downstream signaling.
Acknowledgments
This work was supported by grants from the Lizanell and Colbert Coldwell Foundation; JP Morgan Chase Bank, N.A., Trustee; the National Institutes of Health (AI053566); and the National Center for Research Resources (5G12RR008124), a component of the National Institutes of Health, to R. A. Kirken.
Footnotes
Published ahead of print on 4 February 2008.
REFERENCES
- 1.Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who don't know JAK-STAT. Science 2961653-1655. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, W. S., and D. J. Hilton. 2004. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu. Rev. Immunol. 22503-529. [DOI] [PubMed] [Google Scholar]
- 3.Argetsinger, L. S., J. L. Kouadio, H. Steen, A. Stensballe, O. N. Jensen, and C. Carter-Su. 2004. Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity. Mol. Cell. Biol. 244955-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boggon, T. J., Y. Li, P. W. Manley, and M. J. Eck. 2005. Crystal structure of the Jak3 kinase domain in complex with a staurosporine analog. Blood 106996-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpino, N., R. Kobayashi, H. Zang, Y. Takahashi, S. T. Jou, J. Feng, H. Nakajima, and J. N. Ihle. 2002. Identification, cDNA cloning, and targeted deletion of p70, a novel, ubiquitously expressed SH3 domain-containing protein. Mol. Cell. Biol. 227491-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Changelian, P. S., M. E. Flanagan, D. J. Ball, C. R. Kent, K. S. Magnuson, W. H. Martin, B. J. Rizzuti, P. S. Sawyer, B. D. Perry, W. H. Brissette, S. P. McCurdy, E. M. Kudlacz, M. J. Conklyn, E. A. Elliott, E. R. Koslov, M. B. Fisher, T. J. Strelevitz, K. Yoon, D. A. Whipple, J. Sun, M. J. Munchhof, J. L. Doty, J. M. Casavant, T. A. Blumenkopf, M. Hines, M. F. Brown, B. M. Lillie, C. Subramanyam, C. Shang-Poa, A. J. Milici, G. E. Beckius, J. D. Moyer, C. Su, T. G. Woodworth, A. S. Gaweco, C. R. Beals, B. H. Littman, D. A. Fisher, J. F. Smith, P. Zagouras, H. A. Magna, M. J. Saltarelli, K. S. Johnson, L. F. Nelms, S. G. Des Etages, L. S. Hayes, T. T. Kawabata, D. Finco-Kent, D. L. Baker, M. Larson, M. S. Si, R. Paniagua, J. Higgins, B. Holm, B. Reitz, Y. J. Zhou, R. E. Morris, J. J. O'Shea, and D. C. Borie. 2003. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science 302875-878. [DOI] [PubMed] [Google Scholar]
- 7.Feng, J., B. A. Witthuhn, T. Matsuda, F. Kohlhuber, I. M. Kerr, and J. N. Ihle. 1997. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 172497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funakoshi-Tago, M., S. Pelletier, T. Matsuda, E. Parganas, and J. N. Ihle. 2006. Receptor specific downregulation of cytokine signaling by autophosphorylation in the FERM domain of Jak2. EMBO J. 254763-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadina, M., D. Hilton, J. A. Johnston, A. Morinobu, A. Lighvani, Y. J. Zhou, R. Visconti, and J. J. O'Shea. 2001. Signaling by type I and II cytokine receptors: ten years after. Curr. Opin. Immunol. 13363-373. [DOI] [PubMed] [Google Scholar]
- 10.Girault, J. A., G. Labesse, J. P. Mornon, and I. Callebaut. 1999. The N-termini of FAK and JAKs contain divergent band 4.1 domains. Trends Biochem. Sci. 2454-57. [DOI] [PubMed] [Google Scholar]
- 11.Grimley, P. M., F. Dong, and H. Rui. 1999. Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev. 10131-157. [DOI] [PubMed] [Google Scholar]
- 12.Hou, S. X., Z. Zheng, X. Chen, and N. Perrimon. 2002. The Jak/STAT pathway in model organisms: emerging roles in cell movement. Dev. Cell 3765-778. [DOI] [PubMed] [Google Scholar]
- 13.Hubbard, S. R., L. Wei, L. Ellis, and W. A. Hendrickson. 1994. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature 372746-754. [DOI] [PubMed] [Google Scholar]
- 14.Ihle, J. N. 2001. The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 13211-217. [DOI] [PubMed] [Google Scholar]
- 15.Ihle, J. N., W. Thierfelder, S. Teglund, D. Stravopodis, D. Wang, J. Feng, and E. Parganas. 1998. Signaling by the cytokine receptor superfamily. Ann. N. Y. Acad. Sci. 8651-9. [DOI] [PubMed] [Google Scholar]
- 16.Ihle, J. N., B. A. Witthuhn, F. W. Quelle, K. Yamamoto, and O. Silvennoinen. 1995. Signaling through the hematopoietic cytokine receptors. Annu. Rev. Immunol. 13369-398. [DOI] [PubMed] [Google Scholar]
- 17.Irie-Sasaki, J., T. Sasaki, W. Matsumoto, A. Opavsky, M. Cheng, G. Welstead, E. Griffiths, C. Krawczyk, C. D. Richardson, K. Aitken, N. Iscove, G. Koretzky, P. Johnson, P. Liu, D. M. Rothstein, and J. M. Penninger. 2001. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature 409349-354. [DOI] [PubMed] [Google Scholar]
- 18.Johnston, J. A., C. M. Bacon, M. C. Riedy, and J. J. O'Shea. 1996. Signaling by IL-2 and related cytokines: JAKs, STATs, and relationship to immunodeficiency. J. Leukoc. Biol. 60441-452. [DOI] [PubMed] [Google Scholar]
- 19.Johnston, J. A., M. Kawamura, R. A. Kirken, Y. Q. Chen, T. B. Blake, K. Shibuya, J. R. Ortaldo, D. W. McVicar, and J. J. O'Shea. 1994. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature 370151-153. [DOI] [PubMed] [Google Scholar]
- 20.Kawamura, M., D. W. McVicar, J. A. Johnston, T. B. Blake, Y.-Q. Chen, B. K. Lal, A. R. Lloyd, D. J. Kelvin, J. E. Staples, J. R. Ortaldo, and J. J. O'Shea. 1994. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc. Natl. Acad. Sci. USA 916374-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirken, R. A., R. A. Erwin, D. Taub, W. J. Murphy, F. Behbod, L. Wang, F. Pericle, and W. L. Farrar. 1999. Tyrphostin AG-490 inhibits cytokine-mediated JAK3/STAT5a/b signal transduction and cellular proliferation of antigen-activated human T cells. J. Leukoc Biol. 65891-899. [DOI] [PubMed] [Google Scholar]
- 22.Kirken, R. A., H. Rui, M. G. Malabarba, O. M. Howard, M. Kawamura, J. J. O'Shea, and W. L. Farrar. 1995. Activation of JAK3, but not JAK1, is critical for IL-2-induced proliferation and STAT5 recruitment by a COOH-terminal region of the IL-2 receptor beta-chain. Cytokine 7689-700. [DOI] [PubMed] [Google Scholar]
- 23.Kurzer, J. H., L. S. Argetsinger, Y.-J. Zhou, J. L. Kouadio, J. J. O'Shea, and C. Carter-Su. 2004. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-Bβ. Mol. Cell. Biol. 244557-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy, D. E., and J. E. Darnell, Jr. 2002. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell. Biol. 3651-662. [DOI] [PubMed] [Google Scholar]
- 25.Lin, J. X., and W. J. Leonard. 2000. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene 192566-2576. [DOI] [PubMed] [Google Scholar]
- 26.Malabarba, M. G., R. A. Kirken, H. Rui, K. Koettnitz, M. Kawamura, J. J. O'Shea, F. S. Kalthoff, and W. L. Farrar. 1995. Activation of JAK3, but not JAK1, is critical to interleukin-4 (IL4) stimulated proliferation and requires a membrane-proximal region of IL4 receptor α. J. Biol. Chem. 2709630-9637. [DOI] [PubMed] [Google Scholar]
- 27.Malabarba, M. G., H. Rui, H. H. Deutsch, J. Chung, F. S. Kalthoff, W. L. Farrar, and R. A. Kirken. 1996. Interleukin-13 is a potent activator of JAK3 and STAT6 in cells expressing interleukin-2 receptor-γ and interleukin-4 receptor-α. Biochem. J. 319(Pt. 3)865-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazurkiewicz-Munoz, A. M., L. S. Argetsinger, J. L. Kouadio, A. Stensballe, O. N. Jensen, J. M. Cline, and C. Carter-Su. 2006. Phosphorylation of JAK2 at serine 523: a negative regulator of JAK2 that is stimulated by growth hormone and epidermal growth factor. Mol. Cell. Biol. 264052-4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan, D. O., and H. L. De Bondt. 1994. Protein kinase regulation: insights from crystal structure analysis. Curr. Opin. Cell Biol. 6239-246. [DOI] [PubMed] [Google Scholar]
- 30.Musso, T., J. A. Johnston, D. Linnekin, L. Varesio, T. K. Rowe, J. J. O'Shea, and D. W. McVicar. 1995. Regulation of JAK3 expression in human monocytes: phosphorylation in response to interleukins 2, 4, and 7. J. Exp. Med. 1811425-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pernis, A. B., and P. B. Rothman. 2002. JAK-STAT signaling in asthma. J. Clin. Investig. 1091279-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puntervoll, P., R. Linding, C. Gemund, S. Chabanis-Davidson, M. Mattingsdal, S. Cameron, D. M. Martin, G. Ausiello, B. Brannetti, A. Costantini, F. Ferre, V. Maselli, A. Via, G. Cesareni, F. Diella, G. Superti-Furga, L. Wyrwicz, C. Ramu, C. McGuigan, R. Gudavalli, I. Letunic, P. Bork, L. Rychlewski, B. Kuster, M. Helmer-Citterich, W. N. Hunter, R. Aasland, and T. J. Gibson. 2003. ELM server: a new resource for investigating short functional sites in modular eukaryotic proteins. Nucleic Acids Res. 313625-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross, J. A., Z. S. Nagy, H. Cheng, S. M. Stepkowski, and R. A. Kirken. 2007. Regulation of T cell homeostasis by JAKs and STATs. Arch. Immunol. Ther. Exp. 55231-245. [DOI] [PubMed] [Google Scholar]
- 34.Russell, S. M., J. A. Johnston, M. Noguchi, M. Kawamura, C. M. Bacon, M. Friedmann, M. Berg, D. W. McVicar, B. A. Witthuhn, O. Silvennoinen, et al. 1994. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science 2661042-1045. [DOI] [PubMed] [Google Scholar]
- 35.Russell, S. M., N. Tayebi, H. Nakajima, M. C. Riedy, J. L. Roberts, M. J. Aman, T. S. Migone, M. Noguchi, M. L. Markert, R. H. Buckley, J. J. O'Shea, and W. J. Leonard. 1995. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270797-800. [DOI] [PubMed] [Google Scholar]
- 36.Saharinen, P., and O. Silvennoinen. 2002. The pseudokinase domain is required for suppression of basal activity of Jak2 and Jak3 tyrosine kinases and for cytokine-inducible activation of signal transduction. J. Biol. Chem. 27747954-47963. [DOI] [PubMed] [Google Scholar]
- 37.Saharinen, P., M. Vihinen, and O. Silvennoinen. 2003. Autoinhibition of Jak2 tyrosine kinase is dependent on specific regions in its pseudokinase domain. Mol. Biol. Cell 141448-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simoncic, P. D., A. Lee-Loy, D. L. Barber, M. L. Tremblay, and C. J. McGlade. 2002. The T cell protein tyrosine phosphatase is a negative regulator of Janus family kinases 1 and 3. Curr. Biol. 12446-453. [DOI] [PubMed] [Google Scholar]
- 39.Stepkowski, S. M., J. Kao, M. E. Wang, N. Tejpal, H. Podder, L. Furian, J. Dimmock, A. Jha, U. Das, B. D. Kahan, and R. A. Kirken. 2005. The Mannich base NC1153 promotes long-term allograft survival and spares the recipient from multiple toxicities. J. Immunol. 1754236-4246. [DOI] [PubMed] [Google Scholar]
- 40.Sugamura, K., H. Asao, M. Kondo, N. Tanaka, N. Ishii, K. Ohbo, M. Nakamura, and T. Takeshita. 1996. The interleukin-2 receptor γ chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu. Rev. Immunol. 14179-205. [DOI] [PubMed] [Google Scholar]
- 41.Tortolani, P. J., B. K. Lal, A. Riva, J. A. Johnston, Y. Q. Chen, G. H. Reaman, M. Beckwith, D. Longo, J. R. Ortaldo, K. Bhatia, I. McGrath, J. Kehrl, J. Tuscano, D. W. McVicar, and J. J. O'Shea. 1995. Regulation of JAK3 expression and activation in human B cells and B cell malignancies. J. Immunol. 1555220-5226. [PubMed] [Google Scholar]
- 42.Wakao, H., F. Gouilleux, and B. Groner. 1994. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 132182-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, Y., M. G. Malabarba, Z. S. Nagy, and R. A. Kirken. 2004. Interleukin 4 regulates phosphorylation of serine 756 in the transactivation domain of Stat6. Roles for multiple phosphorylation sites and Stat6 function. J. Biol. Chem. 27925196-25203. [DOI] [PubMed] [Google Scholar]
- 44.Ward, A. C., I. Touw, and A. Yoshimura. 2000. The Jak-Stat pathway in normal and perturbed hematopoiesis. Blood 9519-29. [PubMed] [Google Scholar]
- 45.Witthuhn, B. A., O. Silvennoinen, O. Miura, K. S. Lai, C. Cwik, E. T. Liu, and J. N. Ihle. 1994. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature 370153-157. [DOI] [PubMed] [Google Scholar]
- 46.Yamashita, H., J. Xu, R. A. Erwin, W. L. Farrar, R. A. Kirken, and H. Rui. 1998. Differential control of the phosphorylation state of proline-juxtaposed serine residues Ser725 of Stat5a and Ser730 of Stat5b in prolactin-sensitive cells. J. Biol. Chem. 27330218-30224. [DOI] [PubMed] [Google Scholar]
- 47.Zhou, Y. J., E. P. Hanson, Y. Q. Chen, K. Magnuson, M. Chen, P. G. Swann, R. L. Wange, P. S. Changelian, and J. J. O'Shea. 1997. Distinct tyrosine phosphorylation sites in JAK3 kinase domain positively and negatively regulate its enzymatic activity. Proc. Natl. Acad. Sci. USA 9413850-13855. [DOI] [PMC free article] [PubMed] [Google Scholar]