Abstract
The nuclear receptor Rev-erbα is a potent transcriptional repressor that regulates circadian rhythm and metabolism. Here we demonstrate a dissociation between Rev-erbα mRNA and protein levels that profoundly influences adipocyte differentiation. During adipogenesis, Rev-erbα gene expression initially declines and subsequently increases. Remarkably, Rev-erbα protein levels are nearly the opposite, increasing early in adipogenesis and then markedly decreasing in adipocytes. The Rev-erbα protein is necessary for the early mitotic events that are required for adipogenesis. The subsequent reduction in Rev-erbα protein, due to increased degradation via the 26S proteasome, is also required for adipocyte differentiation because Rev-erbα represses the expression of PPARγ2, the master transcriptional regulator of adipogenesis. Thus, opposite to what might be predicted from Rev-erbα gene expression, Rev-erbα protein levels must rise and then fall for adipocyte differentiation to occur.
The conversion of fibroblastic preadipocytes to mature adipocytes involves the temporal and hierarchical coordination of intracellular signaling molecules and transcription factors (11, 34). Differentiation of the widely used 3T3-L1 preadipocyte cell line requires extracellular signals, including cell contact, glucocorticoids, and serum-derived factors, as well as intracellular accumulation of cyclic AMP (39). Together, these initiators lead confluent preadipocytes to undergo two rounds of cell division that are required for adipogenesis (41). They also induce early transcription factors, notably, CCAAT enhancer-binding proteins (C/EBP) β and δ, to instigate a cascade of transcriptional events that ultimately results in withdrawal from the cell cycle in the process of differentiation into mature postmitotic adipocytes (8, 47, 49). C/EBPβ, in particular, induces nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) (17, 35, 36, 47), the master transcriptional regulator of adipogenesis whose high-level expression in adipocytes is both necessary and sufficient for adipocyte-specific gene expression and the adipocyte phenotypes (6, 42, 43). PPARγ and C/EBPα induce one another to maintain the differentiated adipocyte phenotype (9, 33). Other transcription factors, such as ADD1/SREBP and KLF5, also play important roles in adipogenesis (23, 30).
Rev-erbα is an orphan nuclear receptor that has also been implicated in adipocyte differentiation (5, 12, 24). It is highly expressed in adipose tissue, and its gene expression is specifically induced in differentiating adipocytes (5, 14). Rev-erbα mRNA is transcribed from the opposite strand of the thyroid hormone receptor (TR) α gene and is antisense to the TRα2 splice product which encodes a non-thyroid hormone-binding TR variant (26, 29). The Rev-erbα protein lacks the classical nuclear receptor C-terminal activation domain but binds to nuclear receptor corepressor (N-CoR) and hence functions as a potent transcriptional repressor (18). Recently, Rev-erbα has been shown to function as a core negative component of the circadian clock, driving antiphasic expression of the positive feedback loop involving Bmal1 and Clock (3, 31). Rev-erbα protein stability is subject to regulated, ubiquitin-targeted proteasomal degradation that is required for synchronization of cellular clocks (51). Rev-erbα expression is circadian in adipose tissue (52), and Rev-erbα directly modulates the rhythmic expression of plasminogen activator inhibitor 1, which is an adipokine (46). However, the function of the Rev-erbα protein in adipocyte differentiation remains obscure.
Here we report that, surprisingly, levels of Rev-erbα mRNA and protein are dissociated during adipogenesis, with the protein increasing early and decreasing late in the process. Rev-erbα is required for adipogenesis, where it is critical for the early mitotic events. However, constitutive expression of Rev-erbα inhibits the adipogenic program by repressing the expression of the gene for PPARγ2. Proteasomal degradation is responsible for the decrease in endogenous Rev-erbα protein levels that is normally permissive for adipogenesis. Thus, the dynamic expression of Rev-erbα is an important determinant of adipocyte differentiation.
MATERIALS AND METHODS
Plasmids and reagents.
The murine PPARγ2-luciferase reporter construct was generated by PCR amplifying 700 bp of the proximal PPARγ2 promoter, ending at the ATG start codon in exon 1, from mouse genomic DNA with the following primers: forward, 5′-TTCCTTTTTATAGAATTTGGATAGCA-3′; reverse, 5′-CCCAGAGTTTCACCCATAACA-3′. The PCR product was digested with KpnI and SacI and subcloned into a short-half-life pGL4.15 luc2P/Hygro vector (Promega, Madison, WI). The expression vectors encoding human Rev-erbα have been described previously (51). Components of the adipocyte differentiation cocktail, including dexamethasone, insulin, and 3-isobutyl-1-methylxanthine (IBMX), as well as the protein synthesis inhibitor cycloheximide (CHX), were purchased from Sigma (St. Louis, MO).
Mammalian cell culture and transfection.
3T3-L1 cells were obtained from the American Type Culture Collection (Manassas, VA). 293T-derived BOSC viral packaging cells were a gift from M. Birnbaum (University of Pennsylvania). All cells were maintained in high-glucose, antibiotic-free Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum. Cells were grown at 37°C in 5% CO2. Stable Tet-Off 3T3-L1 cell lines expressing ectopic wild-type (WT) or 55/59SD mutant Rev-erbα were constructed by transient transfection with pTet-Off and pTRE-Rev-erbα vectors (51) and subsequent selection in G418 and hygromycin. For the repression assay, cells were transfected in 12-well plates in triplicate with FuGENE (Roche) according to the manufacturer's instructions. Luciferase activities were assayed 48 h later with a Reporter Assay kit (Promega, Madison, WI).
Retroviral infection.
Ecotropic BOSC packaging cells were a gift from M. Birnbaum (University of Pennsylvania) and were grown in six-well plates and transfected with FuGENE with the pMSCV backbone vector or the pMSCV-mPPARγ2 expression vector (15). At 48 h posttransfection, filtered viral supernatants were used to infect target 3T3-L1 preadipocytes. Infection was performed twice over 48 h, and target cells were harvested for protein analysis or plated for differentiation.
Adipocyte differentiation.
For in vitro adipogenic induction, 3T3-L1 preadipocytes were grown to confluence in growth medium consisting of DMEM supplemented with 10% differentiation grade fetal bovine serum (U.S. Biotechnologies) at 37°C. Differentiation was induced on day 0 (cells at 2 days postconfluence) by addition of 10 μg/ml insulin, 0.5 mM IBMX, and 0.25 μM dexamethasone. After 48 h, the medium was replaced with DMEM containing 10% fetal bovine serum and 10 μg/ml insulin only. Medium was changed every 48 h until cells had differentiated into mature adipocytes, at 6 to 7 days. Differentiation outcome was assessed by morphological examination by phase-contrast microscopy and Oil Red O staining for lipid accumulation and by protein and RNA analysis for the adipocyte marker gene aP2.
Oil Red O staining.
Dishes were washed twice with phosphate-buffered saline and fixed by 100% methanol for 1 min at room temperature. After fixation, cells were stained with Oil Red O solution (PolyScientific Reagents) for 1 h at room temperature. Cells were rinsed with deionized water and differentiated by 85% propylene glycol for 1 min. Cells were rinsed again in deionized water and counterstained with hematoxylin for 30 s, followed by a final rinse in water, and then imaged.
Quantitative reverse transcription (RT)-PCR.
Total mRNA was prepared with the RNeasy kit (Qiagen, Valencia, CA). RT was performed with 3 μg total RNA and the ImpromII RT kit (Promega, Madison, WI) according to the manufacturer's instructions. The cDNA was subjected to quantitative RT-PCR with an ABI Prism 7900 HT detection system (Applied Biosystems, Foster City, CA). All primers and probes were purchased from Applied Biosystems. Target gene expression was normalized to the housekeeping gene 36B4. The average cycle threshold value from each triplicate was used to calculate the relative induction of the gene, with the control group normalized to 1.
RNA silencing.
Vectors expressing small hairpin interfering RNAs (shRNAs) under the control of the human U6 promoter were previously described (20, 51). The target sequences were as follows: β-galactosidase (β-gal), 5′-GTGCACCTGGTAAATCTTAT-3′; Rev-erbα, 5′-GCCGGAGCATCCAACAGAATA-3′. Plasmids were electroporated into target cells with an Amaxa Nucleofection apparatus according to the manufacturer's instructions. Cells were allowed to recover for 24 to 48 h before use for protein and RNA analysis.
Immunoprecipitation (IP) and Western blotting.
Cells were lysed in whole-cell lysis buffer (150 mM NaCl, 10 mM Tris [pH 7.6], 0.1% sodium dodecyl sulfate, 5 mM EDTA) with protease inhibitor, homogenized by vortexing, and centrifuged for 10 min at 4°C at 14,000 × g. The protein concentration of the supernatant was determined by a NanoDrop spectrophotometer, and 500 μg of whole-cell extract per sample was incubated with anti-Flag M2 agarose beads (Sigma) overnight at 4°C in Flag IP buffer (150 mM NaCl, 50 mM Tris [pH 8.0], 0.1% NP-40, 2 mM EDTA). Beads were subsequently pelleted, washed four times in IP buffer, and eluted by boiling in 2× sodium dodecyl sulfate buffer at 95°C for 10 min. Western blots were probed with the following antibodies: rabbit anti-Rev-erbα (Cell Signaling), mouse anti-PPARγ E8 antibody and anti-β-actin antibody (Santa Cruz), anti-glyceraldehyde-3-phosphate dehydrogenase-horseradish peroxidase (Abcam, Cambridge, MA), and rabbit anti-aP2 (gift from D. Bernlohr, University of Minnesota, Minneapolis). Quantitation of Western blot bands was performed with Photoshop CS2 software by selecting each band area and integrating the mean intensity and pixel value and then dividing the product by that of the standard band, which was either β-actin or glyceraldehyde-3-phosphate dehydrogenase. Relative intensity was then normalized to the control treatment or the initial time point, which was assigned a value of 1.
Bromodeoxyuridine (BrdU) assay.
BrdU incorporation was assessed by a BrdU Cell Proliferation Assay kit (Calbiochem). 3T3-L1 preadipocytes first received shRNA against β-gal or endogenous Rev-erbα and were plated in 96-well plates. At 48 h later, cells were treated with differentiation medium and labeled with BrdU for 12 h, after which cells were fixed and stained with anti-BrdU antibody and visualized in a colorimetric immunoassay. Spectrum absorbance was measured on a Bio-Tek Synergy HT plate reader.
RESULTS
Rev-erbα mRNA and protein levels are uncoupled during adipogenesis.
We confirmed that Rev-erbα mRNA decreases in the first 24 h and then is markedly induced during adipocyte differentiation (5, 14) (Fig. 1A). Surprisingly, we found that Rev-erbα protein levels increase during the initial 24 h but then decrease (Fig. 1B), the opposite of the mRNA expression pattern. Given that Rev-erbα is regulated posttranscriptionally and represses its own gene expression (1, 51), we hypothesized that the discrepancy between Rev-erbα mRNA levels in the mature adipocyte is due to enhanced proteasomal degradation of the protein. Indeed, an acute 4-h treatment with the proteasome inhibitor MG132 at 20 μM stabilized Rev-erbα protein in differentiating adipocytes, an effect that was much more pronounced in cells after day 4 than in day 0 preadipocytes (Fig. 1C). Thus, Rev-erbα protein appears to be regulated by increasing proteasomal degradation during late adipogenesis.
FIG. 1.
Uncoupling of Rev-erbα mRNA and protein expressions during adipogenesis. (A) Quantitative RT-PCR showing initial repression and subsequent induction of Rev-erbα mRNA during normal 3T3-L1 adipocyte differentiation. (B) Western blot assay showing the initial decline and the subsequent decrease in Rev-erbα protein during adipogenesis. β-Actin served as a loading control, and aP2 was a positive control for differentiation. (C) Rev-erbα protein levels in adipocytes are increased by a 4-h treatment with the proteasome inhibitor MG132 at 20 μM. Ethanol (EtOH) was the solvent and served as a vehicle control.
Endogenous Rev-erbα is required for adipocyte differentiation.
To determine the role of Rev-erbα expression in adipocyte differentiation, we used an shRNA against murine Rev-erbα to inhibit the expression of Rev-erbα in 3T3-L1 preadipocytes prior to subjecting the cells to differentiation induction (Fig. 2A). Compared to control cells treated with an irrelevant shRNA, knockdown of Rev-erbα dramatically reduced the differentiation capacity of the cells, as assessed by morphological examination and Oil Red O staining (Fig. 2B).
FIG. 2.
Knockdown of endogenous Rev-erbα blocks adipocyte differentiation. (A) Quantitative PCR and Western blot assay showing knockdown of Rev-erbα in 3T3-L1 preadipocytes with a control β-gal- or Rev-erbα-specific shRNA. *, P < 0.05 (n = 3). (B) Phase-contrast microscopy and Oil Red O staining of day 7 3T3-L1 cells treated with control β-gal shRNA (upper panels) or Rev-erbα shRNA (lower panels). (C) Preadipocytes lacking Rev-erbα do not undergo the cell division normally required for adipogenesis. BrdU incorporation at day 2 in cells treated with no shRNA, β-gal control shRNA, or specific Rev-erbα shRNA is shown. Black bars indicate growth medium (GM), which was the negative control; gray bars indicate treatment with differentiation medium (DM). *, P < 0.05 (n = 3) compared with other shRNA treatments in differentiation medium. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Since mitotic clonal expansion is required for adipocyte differentiation (41), we examined whether Rev-erbα knockdown had altered the proliferative capacity of the cells. Indeed, cells depleted of Rev-erbα had a significantly decreased mitotic rate, as determined by BrdU uptake upon exposure to differentiation medium (Fig. 2C). Thus, the early increase in Rev-erbα protein is required for the mitotic events that are an obligatory step in adipocyte differentiation.
Proteasomal degradation of Rev-erbα is required for adipogenesis.
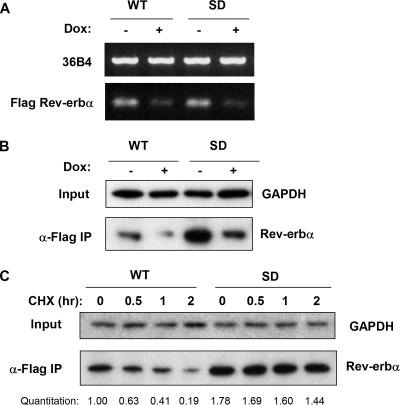
Since Rev-erbα gene expression increases during adipocyte differentiation, we and others have suggested that Rev-erbα would enhance adipogenesis (5, 12), an idea that is consistent with our observation that Rev-erbα knockdown prevents adipogenesis. However, having noted that Rev-erbα protein levels decrease as adipogenesis progresses, due to proteasomal degradation, we hypothesized that the loss of Rev-erbα protein is also critical for late stages of adipocyte differentiation. To test this, we used a Tet-off system to conditionally express Flag-tagged WT Rev-erbα or the S55D/S59D Rev-erbα mutant (SD) that is resistant to proteasomal degradation (51). In the absence of doxycycline, these 3T3-L1-derived cell lines stably expressed the ectopic Rev-erbα mRNA and protein, while the addition of 2 μg/ml doxycycline led to a marked decrease in the transgenes (Fig. 3A and B). Consistent with our expectations, steady-state levels of the SD Rev-erbα protein were higher than WT Rev-erbα levels despite similar mRNA expression levels, reflecting the increased stability of the SD protein. Analysis of protein levels following CHX treatment confirmed that the half-life of the SD Rev-erbα protein was markedly longer than that of WT Rev-erbα in 3T3-L1 cells (Fig. 3C).
FIG. 3.
3T3-L1 cells conditionally expressing WT and degradation-resistant Rev-erbα. (A) Expression of Flag-tagged WT or degradation-resistant S55D/S59D (SD) Rev-erbα mRNA in Tet-off 3T3-L1 cells. Transgene expression is sensitive to inhibition by 2 μg/ml doxycycline (Dox). (B) Flag IP, followed by Western blotting, showing that the Tet-off WT and SD Rev-erbα proteins are also sensitive to doxycycline inhibition. (C) Western blot assay of WT and SD Rev-erbα proteins in Tet-off stable 3T3-L1 preadipocytes at various times after treatment with 20 μM CHX. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
We next tested the functional outcome of Rev-erbα protein stabilization in adipogenesis. In the presence of doxycycline, both the WT and SD cells displayed normal adipogenesis (Fig. 4A, upper panels), suggesting that the genetic manipulation of the cells had not nonspecifically altered their function as preadipocytes. In the absence of doxycycline, cells that expressed ectopic WT Rev-erbα also differentiated normally (Fig. 4A, lower left panel). In contrast, expression of degradation-resistant SD Rev-erbα markedly impaired adipocyte differentiation (Fig. 4A, lower right panel). Differentiation status was also monitored by induction of the adipogenic marker aP2, which was greatly diminished in SD Rev-erbα-expressing cells, at both the mRNA (Fig. 4B) and protein (Fig. 4C) levels. Note that the ectopic WT and SD Rev-erbα proteins followed different patterns of expression during adipogenesis. In the absence of doxycycline, the WT protein was initially expressed but became destabilized after day 4 (Fig. 4D), much like the endogenous protein (cf. Fig. 1A). By contrast, the SD protein was constitutively expressed at a higher level throughout adipogenesis (Fig. 4D), likely explaining its more marked effect on adipogenesis.
FIG. 4.
Ectopic expression of degradation-resistant Rev-erbα blocks adipocyte differentiation. (A) Preadipocytes expressing WT or SD Rev-erbα expression vectors were differentiated for 9 days with or without 2 μg/ml doxycycline (Dox) and stained with Oil Red O. (B) aP2 mRNA levels in cells expressing ectopic WT and SD Rev-erbα. (C) aP2 protein levels in cells expressing ectopic WT and SD Rev-erbα. β-Actin served as a loading control. (D) Expression of the ectopic SD and WT Rev-erbα proteins during adipogenesis. β-Actin served as a loading control.
Stable expression of Rev-erbα protein prevents induction of PPARγ.
To determine the mechanism by which constitutive Rev-erbα protein expression inhibited adipogenesis, we examined the expression of PPARγ2, the “master” transcriptional regulator of adipogenesis. As expected, PPARγ2 expression robustly increased during adipogenesis of control preadipocytes, as well as in cells expressing ectopic WT Rev-erbα (Fig. 5A). By contrast, the induction of PPARγ2 was dramatically blunted in cells expressing degradation-resistant SD Rev-erbα. To test whether the failure to induce PPARγ2 was responsible for the inability of these cells to differentiate, we used retroviral vectors to force the expression of PPARγ2 (Fig. 5B). Indeed, ectopic expression of PPARγ2 rescued the adipogenic phenotype (Fig. 5C), indicating that the differentiation block resulted from repression of PPARγ2 expression by the SD Rev-erbα protein.
FIG. 5.
Rev-erbα expression represses PPARγ2. (A) Ectopic expression of degradation-resistant (SD), but not WT, Rev-erbα blocks PPARγ2 induction. (B) Retroviral expression of ectopic PPARγ2 in 3T3-L1 preadipocytes. (C) Ectopic expression of PPARγ2 rescues adipogenesis in 3T3-L1 cells ectopically expressing degradation-resistant SD Rev-erbα, as assessed by Oil Red O staining on day 7. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Rev-erbα represses PPARγ2 gene expression.
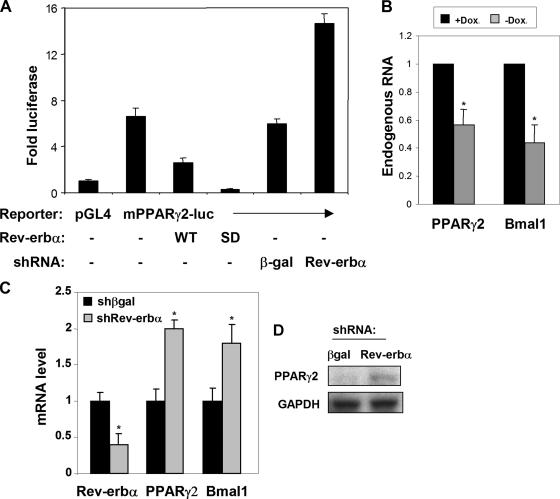
Because Rev-erbα is a potent transcriptional repressor, we hypothesized that it might directly repress PPARγ expression. Indeed, overexpression of WT Rev-erbα repressed the luciferase activity of a murine PPARγ2 reporter in 3T3-L1 cells (Fig. 6A). Expression of SD Rev-erbα led to even greater repression of the mPPARγ2 promoter, indicating that the SD Rev-erbα mutant was not functionally defective and was, in fact, a more potent repressor, likely due to its expression at higher levels. Conversely, knockdown of endogenous Rev-erbα led to increased mPPARγ2 promoter activity, indicating that, at its endogenous level, Rev-erbα suppresses PPARγ2 gene expression (Fig. 6A). Ectopic expression of SD Rev-erbα by removal of doxycycline significantly repressed endogenous PPARγ2 and Bmal1 expression in mature adipocytes (Fig. 6B). Consistent with this, Rev-erbα knockdown increased native PPARγ2 mRNA, as well as the expression of Bmal1, a known Rev-erbα target gene (Fig. 6C). A similar result was obtained with a second, nonoverlapping shRNA targeting Rev-erbα (not shown), but not an off-target effect because it was not seen with a control shRNA directed at β-gal (Fig. 6C). Knockdown of Rev-erbα also induced endogenous PPARγ2 protein in preadipocytes (Fig. 6D). Thus, Rev-erbα appears to be a regulator of PPARγ2 in 3T3-L1 cells and constitutive expression of Rev-erbα protein prevents adipogenesis by inhibiting PPARγ2 induction.
FIG. 6.
Rev-erbα directly represses PPARγ2 promoter activity and expression. (A) Expression of PPARγ2-luciferase reporter transfected into 3T3-L1 preadipocytes along with 1 μg of either a WT or SD Rev-erbα expression plasmid or shRNA knockdown of endogenous Rev-erbα. Data shown are the averages of three independent experiments. Error bars represent standard deviations. (B) Ectopic expression of SD Rev-erbα in mature 3T3-L1 adipocytes reduces PPARγ2 and Bmal1 gene expression. *, P < 0.05 (n = 3). (C) shRNA knockdown of endogenous Rev-erbα increases the expression of the native PPARγ2 mRNA in preadipocytes, as well as a known Rev-erbα target gene, that for Bmal1. *, P < 0.05 (n = 3). (D) shRNA knockdown of endogenous Rev-erbα increases the expression of endogenous PPARγ2 protein in preadipocytes. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Dox, doxycycline.
DISCUSSION
Since expression of the gene for Rev-erbα is induced during adipogenesis, it has been suggested that Rev-erbα may be proadipogenic. Here we show that Rev-erbα actually has a bipartite function, reflected by the dissociation between its mRNA and protein expressions. Rev-erbα is indeed required for adipocyte differentiation; this requirement is early, during the period of greatest Rev-erbα protein expression, and due to a permissive role for Rev-erbα during the cell proliferation stage that is crucial for adipogenesis of 3T3-L1 cells. Remarkably, this period is when Rev-erbα mRNA levels are lowest. Later in adipogenesis, when Rev-erbα gene expression is highest, Rev-erbα protein levels are actually low and forced expression of Rev-erbα prevents adipogenesis by repressing expression of the master adipogenic transcription factor PPARγ.
The lack of correlation between Rev-erbα mRNA and protein levels is interesting, and similar observations have been reported for a number of other genes (21). Of note, while this work was in progress, the Nuclear Receptor Signaling Alliance confirmed our findings for Rev-erbα protein, as well as mRNA, during adipogenesis (48). The patterns of Rev-erbα protein and mRNA expression are nearly antiphasic, most likely because Rev-erbα potently represses its own gene expression (1, 51) but is independently and posttranslationally regulated by proteasomal degradation. Increased proteasomal degradation of Rev-erbα in late adipogenesis reduces the steady-state protein level, which depresses Rev-erbα gene expression. Consistent with this, we have observed that ectopic expression of degradation-resistant SD Rev-erbα markedly suppresses the endogenous Rev-erbα mRNA level in 3T3-L1 cells (data not shown).
The coupling of Rev-erbα protein stabilization to early clonal expansion in adipogenesis suggests a potential linkage between circadian cycle and cell cycle regulators. Consistent with this, other core circadian cycle proteins, such as PER and TIM, have been shown to interact with components of the cell cycle machinery (16, 44). Furthermore, the expression of cell cycle genes such as those for Wee1, cyclins, and c-Myc is under circadian regulation (13, 28). Given the role of Rev-erbα as a major feedback circadian cycle regulator, Rev-erbα may participate in the circadian control of cell cycle gene expression. It should be noted that while mitotic clonal expansion is a requirement for adipocyte differentiation of 3T3-L1 cells, this may be less critical in other models of adipogenesis (7, 10).
Rev-erbα protein loss in late adipogenesis is due to reduced stability that is mediated by proteasomal degradation, which we have demonstrated by assessing the effect of the 26S proteasomal inhibitor MG132 on successive days in adipocyte differentiation. Rev-erbα protein is stabilized by GSK3β-dependent phosphorylation at S55 and S59 (51), and hence, the reduced stability of Rev-erbα is potentially explained by the decrease in GSK3β activity that has been shown to occur during 3T3-L1 adipocyte differentiation (4, 27). However, we and others have not consistently observed altered phosphorylation of GSK3β, which regulates its activity, during adipogenesis (40; data not shown). Thus, it is possible that other mechanisms play a role in destabilizing Rev-erbα protein in adipogenesis.
Mutation of serines 55 and 59 to aspartate, which mimics phosphorylation, results in a protein (SD) that resists degradation and disrupts differentiation in adipocytes. Rev-erbα protein stabilization leads to suppression of PPARγ2 gene transcription, which is normally induced in adipogenesis by a hierarchical regulatory cascade that is initiated by C/EBPβ (36, 47) and perpetuated by C/EBPα, as well as positive feedback from PPARγ2 itself (11, 33, 37). Our data demonstrate that SD Rev-erbα is capable of dominantly repressing PPARγ2 induction during adipogenesis, and hence, the reduction in Rev-erbα protein level seen in normal adipogenesis may play a permissive role in differentiation. Repression of PPARγ2 promoter activity could be a direct effect of Rev-erbα, although mutation of two putative Rev-erbα-responsive elements in the PPAR2 promoter did not abrogate the effect of Rev-erbα (data not shown). Thus, the effect of Rev-erbα could be due to cryptic Rev-erbα-responsive sequences or could be indirect, for example, by repression of the transcriptional activator Bmal1, which is encoded by a well-established Rev-erbα target gene (50), which has been shown to promote adipogenesis (38).
The finding that Rev-erbα protein decreases in adipogenesis is surprising given the increase in its mRNA, which raises the question of whether induction of Rev-erbα mRNA during adipocyte differentiation has a function. Rev-erbα has an important function in the circadian clock which may be important for mature adipocytes, whose circadian clock oscillations are robust and coordinated with the expression of many adipokines and metabolic enzymes (2, 52). Thus, it is possible that the relatively high level of Rev-erbα gene expression in mature adipocytes reflects a circadian function. It is also intriguing that Rev-erbα mRNA expression modulates the alternative splicing of the gene for TRα, which governs the ratio of TRα1 and TRα2, two factors that facilitate or inhibit thyroid hormone action, respectively (19, 25). An accumulation of Rev-erbα mRNA in mature adipocytes would therefore favor TRα1 mRNA production and increase the cellular response to thyroid hormone, which is important for maintaining metabolic homeostasis (22, 32, 45).
In summary, we have uncovered a mechanism by which the rise and fall of Rev-erbα protein may benefit adipocyte differentiation. The striking dissociation between Rev-erbα mRNA and protein during adipogenesis indicates that Rev-erbα may be regulated differently at the transcriptional and posttranslational levels. Indeed, stabilization of the Rev-erbα protein, mediated by phosphorylation at serines 55 and 59, has a dominant effect in suppressing the adipogenic gene expression program. It will be interesting to explore, in future studies, the regulatory pathways that lead to altered Rev-erbα protein expression in adipogenesis.
Acknowledgments
We thank Morris Birnbaum (University of Pennsylvania, Philadelphia) for the gift of BOSC retroviral packaging cells and David Bernlohr (University of Minnesota, Minneapolis) for anti-aP2 antiserum. We also thank Lei Yin for helpful discussions and other members of the Lazar laboratory for support.
This work was supported by NIH grant DK45586 (to M.A.L.).
Footnotes
Published ahead of print on 28 January 2008.
REFERENCES
- 1.Adelmant, G., A. Begue, D. Stehelin, and V. Laudet. 1996. A functional Rev-erbα responsive element located in the human Rev-erbα promoter mediates a repressing activity. Proc. Natl. Acad. Sci. USA 933553-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando, H., H. Yanagihara, Y. Hayashi, Y. Obi, S. Tsuruoka, T. Takamura, S. Kaneko, and A. Fujimura. 2005. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology 1465631-5636. [DOI] [PubMed] [Google Scholar]
- 3.Balsalobre, A., F. Damiola, and U. Schibler. 1998. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93929-937. [DOI] [PubMed] [Google Scholar]
- 4.Brady, M. J., F. J. Bourbonais, and A. R. Saltiel. 1998. The activation of glycogen synthase by insulin switches from kinase inhibition to phosphatase activation during adipogenesis in 3T3-L1 cells. J. Biol. Chem. 27314063-14066. [DOI] [PubMed] [Google Scholar]
- 5.Chawla, A., and M. A. Lazar. 1993. Induction of Rev-ErbAα, an orphan receptor encoded on the opposite strand of the α-thyroid hormone receptor gene, during adipocyte differentiation. J. Biol. Chem. 26816265-16269. [PubMed] [Google Scholar]
- 6.Chawla, A., E. J. Schwarz, D. D. Dimaculangan, and M. A. Lazar. 1994. Peroxisome proliferator-activated receptor (PPAR) γ: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 135798-800. [DOI] [PubMed] [Google Scholar]
- 7.Cho, Y. C., W. Zheng, M. Yamamoto, X. Liu, P. R. Hanlon, and C. R. Jefcoate. 2005. Differentiation of pluripotent C3H10T1/2 cells rapidly elevates CYP1B1 through a novel process that overcomes a loss of Ah receptor. Arch. Biochem. Biophys. 439139-153. [DOI] [PubMed] [Google Scholar]
- 8.Darlington, G. J., S. E. Ross, and O. A. MacDougald. 1998. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 27330057-30060. [DOI] [PubMed] [Google Scholar]
- 9.El-Jack, A. K., J. K. Hamm, P. F. Pilch, and S. R. Farmer. 1999. Reconstitution of insulin-sensitive glucose transport in fibroblasts requires expression of both PPARγ and C/EBPα. J. Biol. Chem. 2747946-7951. [DOI] [PubMed] [Google Scholar]
- 10.Entenmann, G., and H. Hauner. 1996. Relationship between replication and differentiation in cultured human adipocyte precursor cells. Am. J. Physiol. 270C1011-C1016. [DOI] [PubMed] [Google Scholar]
- 11.Farmer, S. R. 2006. Transcriptional control of adipocyte formation. Cell. Metab. 4263-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontaine, C., G. Dubois, Y. Duguay, T. Helledie, N. Vu-Dac, P. Gervois, F. Soncin, S. Mandrup, J. C. Fruchart, J. Fruchart-Najib, and B. Staels. 2003. The orphan nuclear receptor Rev-Erbα is a peroxisome proliferator-activated receptor (PPAR) γ target gene and promotes PPARγ-induced adipocyte differentiation. J. Biol. Chem. 27837672-37680. [DOI] [PubMed] [Google Scholar]
- 13.Fu, L., M. S. Patel, A. Bradley, E. F. Wagner, and G. Karsenty. 2005. The molecular clock mediates leptin-regulated bone formation. Cell 122803-815. [DOI] [PubMed] [Google Scholar]
- 14.Fu, M., T. Sun, A. L. Bookout, M. Downes, R. T. Yu, R. M. Evans, and D. J. Mangelsdorf. 2005. Adipogenic expression patterns of nuclear receptors. www.nursa.org/10.1621/datasets.
- 15.Ge, K., M. Guermah, C. X. Yuan, M. Ito, A. E. Wallberg, B. M. Spiegelman, and R. G. Roeder. 2002. Transcription coactivator TRAP220 is required for PPARγ 2-stimulated adipogenesis. Nature 417563-567. [DOI] [PubMed] [Google Scholar]
- 16.Gery, S., N. Komatsu, L. Baldjyan, A. Yu, D. Koo, and H. P. Koeffler. 2006. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol. Cell 22375-382. [DOI] [PubMed] [Google Scholar]
- 17.Hamm, J. K., B. H. Park, and S. R. Farmer. 2001. A role for C/EBPβ in regulating peroxisome proliferator-activated receptor γ activity during adipogenesis in 3T3-L1 preadipocytes. J. Biol. Chem. 27618464-18471. [DOI] [PubMed] [Google Scholar]
- 18.Harding, H. P., and M. A. Lazar. 1995. The monomer-binding orphan receptor Rev-Erb represses transcription as a dimer on a novel direct repeat. Mol. Cell. Biol. 154791-4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hastings, M. L., H. A. Ingle, M. A. Lazar, and S. H. Munroe. 2000. Post-transcriptional regulation of thyroid hormone receptor expression by cis-acting sequences and a naturally occurring antisense RNA. J. Biol. Chem. 27511507-11513. [DOI] [PubMed] [Google Scholar]
- 20.Ishizuka, T., and M. A. Lazar. 2003. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol. Cell. Biol. 235122-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izzotti, A., M. Bagnasco, C. Cartiglia, M. Longobardi, and S. De Flora. 2004. Proteomic analysis as related to transcriptome data in the lung of chromium(VI)-treated rats. Int. J. Oncol. 241513-1522. [PubMed] [Google Scholar]
- 22.Jiang, W., T. Miyamoto, T. Kakizawa, T. Sakuma, S. Nishio, T. Takeda, S. Suzuki, and K. Hashizume. 2004. Expression of thyroid hormone receptor α in 3T3-L1 adipocytes; triiodothyronine increases the expression of lipogenic enzyme and triglyceride accumulation. J. Endocrinol. 182295-302. [DOI] [PubMed] [Google Scholar]
- 23.Kim, J. B., and B. M. Spiegelman. 1996. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 101096-1107. [DOI] [PubMed] [Google Scholar]
- 24.Laitinen, S., C. Fontaine, J. C. Fruchart, and B. Staels. 2005. The role of the orphan nuclear receptor Rev-Erbα in adipocyte differentiation and function. Biochimie 8721-25. [DOI] [PubMed] [Google Scholar]
- 25.Lazar, M. A., R. A. Hodin, G. Cardona, and W. W. Chin. 1990. Gene expression from the c-erbAα/Rev-ErbAα genomic locus. Potential regulation of alternative splicing by opposite strand transcription. J. Biol. Chem. 26512859-12863. [PubMed] [Google Scholar]
- 26.Lazar, M. A., R. A. Hodin, D. S. Darling, and W. W. Chin. 1989. A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbAα transcriptional unit. Mol. Cell. Biol. 91128-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacAulay, K., E. Hajduch, A. S. Blair, M. P. Coghlan, S. A. Smith, and H. S. Hundal. 2003. Use of lithium and SB-415286 to explore the role of glycogen synthase kinase-3 in the regulation of glucose transport and glycogen synthase. Eur. J. Biochem. 2703829-3838. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo, T., S. Yamaguchi, S. Mitsui, A. Emi, F. Shimoda, and H. Okamura. 2003. Control mechanism of the circadian clock for timing of cell division in vivo. Science 302255-259. [DOI] [PubMed] [Google Scholar]
- 29.Miyajima, N., R. Horiuchi, Y. Shibuya, S. Fukushige, K. Matsubara, K. Toyoshima, and T. Yamamoto. 1989. Two erbA homologs encoding proteins with different T3 binding capacities are transcribed from opposite DNA strands of the same genetic locus. Cell 5731-39. [DOI] [PubMed] [Google Scholar]
- 30.Oishi, Y., I. Manabe, K. Tobe, K. Tsushima, T. Shindo, K. Fujiu, G. Nishimura, K. Maemura, T. Yamauchi, N. Kubota, R. Suzuki, T. Kitamura, S. Akira, T. Kadowaki, and R. Nagai. 2005. Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 127-39. [DOI] [PubMed] [Google Scholar]
- 31.Preitner, N., F. Damiola, L. Lopez-Molina, J. Zakany, D. Duboule, U. Albrecht, and U. Schibler. 2002. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110251-260. [DOI] [PubMed] [Google Scholar]
- 32.Romero, R., B. Casanova, N. Pulido, A. I. Suarez, E. Rodriguez, and A. Rovira. 2000. Stimulation of glucose transport by thyroid hormone in 3T3-L1 adipocytes: increased abundance of GLUT1 and GLUT4 glucose transporter proteins. J. Endocrinol. 164187-195. [DOI] [PubMed] [Google Scholar]
- 33.Rosen, E. D., C. H. Hsu, X. Wang, S. Sakai, M. W. Freeman, F. J. Gonzalez, and B. M. Spiegelman. 2002. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev. 1622-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen, E. D., and O. A. MacDougald. 2006. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7885-896. [DOI] [PubMed] [Google Scholar]
- 35.Rosen, E. D., P. Sarraf, A. E. Troy, G. Bradwin, K. Moore, D. S. Milstone, B. M. Spiegelman, and R. M. Mortensen. 1999. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4611-617. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz, E. J., M. J. Reginato, D. Shao, S. L. Krakow, and M. A. Lazar. 1997. Retinoic acid blocks adipogenesis by inhibiting C/EBPβ-mediated transcription. Mol. Cell. Biol. 171552-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shao, D., and M. A. Lazar. 1997. Peroxisome proliferator activated receptor γ, CCAAT/enhancer-binding protein α, and cell cycle status regulate the commitment to adipocyte differentiation. J. Biol. Chem. 27221473-21478. [DOI] [PubMed] [Google Scholar]
- 38.Shimba, S., N. Ishii, Y. Ohta, T. Ohno, Y. Watabe, M. Hayashi, T. Wada, T. Aoyagi, and M. Tezuka. 2005. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci. USA 10212071-12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiegelman, B. M., and H. Green. 1981. Cyclic AMP-mediated control of lipogenic enzyme synthesis during adipose differentiation of 3T3 cells. Cell 24503-510. [DOI] [PubMed] [Google Scholar]
- 40.Summers, S. A., A. W. Kao, A. D. Kohn, G. S. Backus, R. A. Roth, J. E. Pessin, and M. J. Birnbaum. 1999. The role of glycogen synthase kinase 3β in insulin-stimulated glucose metabolism. J. Biol. Chem. 27417934-17940. [DOI] [PubMed] [Google Scholar]
- 41.Tang, Q. Q., T. C. Otto, and M. D. Lane. 2003. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 10044-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tontonoz, P., E. Hu, R. A. Graves, A. I. Budavari, and B. M. Spiegelman. 1994. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 81224-1234. [DOI] [PubMed] [Google Scholar]
- 43.Tontonoz, P., E. Hu, and B. M. Spiegelman. 1994. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 791147-1156. [DOI] [PubMed] [Google Scholar]
- 44.Unsal-Kacmaz, K., T. E. Mullen, W. K. Kaufmann, and A. Sancar. 2005. Coupling of human circadian and cell cycles by the timeless protein. Mol. Cell. Biol. 253109-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viguerie, N., L. Millet, S. Avizou, H. Vidal, D. Larrouy, and D. Langin. 2002. Regulation of human adipocyte gene expression by thyroid hormone. J. Clin. Endocrinol. Metab. 87630-634. [DOI] [PubMed] [Google Scholar]
- 46.Wang, J., L. Yin, and M. A. Lazar. 2006. The orphan nuclear receptor Rev-erbα regulates circadian expression of plasminogen activator inhibitor type 1. J. Biol. Chem. 28133842-33848. [DOI] [PubMed] [Google Scholar]
- 47.Wu, Z., Y. Xie, N. L. Bucher, and S. R. Farmer. 1995. Conditional ectopic expression of C/EBPβ in NIH-3T3 cells induces PPARγ and stimulates adipogenesis. Genes Dev. 92350-2363. [DOI] [PubMed] [Google Scholar]
- 48.Xie, X., S. Y. Tsai, and M.-J. Tsai. 2007. Western analysis of nuclear receptors during adipogenesis of 3T3L1 cells. www.nursa.org/10.1621/datasets.
- 49.Yeh, W. C., Z. Cao, M. Classon, and S. L. McKnight. 1995. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 9168-181. [DOI] [PubMed] [Google Scholar]
- 50.Yin, L., and M. A. Lazar. 2005. The orphan nuclear receptor Rev-erbα recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol. Endocrinol. 191452-1459. [DOI] [PubMed] [Google Scholar]
- 51.Yin, L., J. Wang, P. S. Klein, and M. A. Lazar. 2006. Nuclear receptor Rev-erbα is a critical lithium-sensitive component of the circadian clock. Science 3111002-1005. [DOI] [PubMed] [Google Scholar]
- 52.Zvonic, S., A. A. Ptitsyn, S. A. Conrad, L. K. Scott, Z. E. Floyd, G. Kilroy, X. Wu, B. C. Goh, R. L. Mynatt, and J. M. Gimble. 2006. Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55962-970. [DOI] [PubMed] [Google Scholar]