Abstract
It has previously been shown that E3 ubiquitin ligase Casitas B-lineage lymphoma-b (Cbl-b) negatively regulates T-cell activation, but the molecular mechanism(s) underlying this inhibition is not completely defined. In this study, we report that the loss of Cbl-b selectively results in aberrant activation of NF-κB upon T-cell antigen receptor (TCR) ligation, which is mediated by phosphatidylinositol 3-kinase (PI3-K)/Akt and protein kinase C-θ (PKC-θ). TCR-induced hyperactivation of Akt in the absence of Cbl-b may potentiate the formation of caspase recruitment domain-containing membrane-associated guanylate kinase protein 1 (CARMA1)-B-cell lymphoma/leukemia 10 (Bcl10)-mucosa-associated lymphatic tissue 1(MALT1) (CBM) complex, which appears to be independent of PKC-θ. Cbl-b associates with PKC-θ upon TCR stimulation and regulates TCR-induced PKC-θ activation via Vav-1, which couples PKC-θ to PI3-K and allows it to be phosphorylated. PKC-θ then couples IκB kinases (IKKs) to the CBM complex, resulting in the activation of the IKK complex. Therefore, our data provide the first evidence to demonstrate that the down-regulation of TCR-induced NF-κB activation by Cbl-b is mediated coordinately by both Akt-dependent and PKC-θ-dependent signaling pathways in primary T cells.
The Casitas B-lineage lymphoma (Cbl) family of proteins consists of an N-terminal (variant) Src homology 2 domain, a RING finger domain, and a C-terminal proline-rich region with potential tyrosine phosphorylation sites. It is known that Cbl functions as an E3 ubiquitin (Ub) ligase; its RING finger domain recruits an Ub-conjugating enzyme (E2), and its Src homology 2 domain recognizes target proteins for Ub conjugation (19, 42). It has been demonstrated that Cbl-b negatively regulates T-cell activation. T cells from Cbl-b−/− mice show enhanced proliferation and interleukin-2 production in response to T-cell antigen receptor (TCR) stimulation (2, 4). The reduction of the cell activation threshold (caused by the loss of Cbl-b) has been correlated with an increased susceptibility to the development of autoimmunity (2, 4). Therefore, Cbl-b is believed to be a key regulator of susceptibility to autoimmunity.
Interestingly, the loss of Cbl-b relieves a T cell from requiring CD28 costimulation (2, 4), suggesting that Cbl-b may be involved in CD28-dependent T-cell activation. In support of this idea, the elimination of Cbl-b may favor CD28-mediated Vav-1 activation and cytoskeletal reorganization (2, 4, 25), possibly through a phosphatidylinositol 3-kinase (PI3-K)-dependent mechanism (7). Although Cbl-b inhibits TCR-induced Vav-1 activation, it does not regulate the activation of mitogen-activated protein kinases (MAPKs), such as extracellular signal-regulated kinase (ERK), c-Jun-NH2-terminal kinase (JNK), and Ca2+ influx (2, 25), which have been shown to be downstream of either PI3-K or Vav-1 (38-40). Therefore, the specific signaling pathway(s) emanating from the TCR and leading to hyper-T-cell activity in the absence of Cbl-b has not been fully characterized.
NF-κB proteins are present in the cytoplasm in association with inhibitors of NF-κB (IκBs). Engagement of the TCR leads to the activation of an IκB kinase (IKK) complex that phosphorylates IκBα. This phosphorylation is then recognized by the β-TrCP-containing Skp1/Cell/F-box Ub ligase complex, leading to its ubiquitination and degradation by the proteasome and thereby releasing NF-κB dimers from the cytoplasmic NF-κB-IκB complex, which allows them to translocate to the nucleus (43). T-cell activation is dependent upon not only TCR signaling but also costimulatory signaling. Although TCR signaling itself can activate NF-κB modestly, CD28 costimulatory signaling is required for the efficient activation of NF-κB.
Protein kinase C-θ (PKC-θ), a Ca2+-independent PKC isoform, plays a central role in TCR and CD28 costimulatory signaling pathways (28, 43). Two proteins have been suggested to couple PKC-θ to NF-κB activation in T cells. One of those proteins is B-cell lymphoma/leukemia 10 (Bcl10), which is a caspase recruitment domain (CARD)-containing adaptor protein. The other protein is the CARD-containing membrane-associated guanylate kinase protein 1 (CARMA1), which is the only lymphocyte-specific member in the family of membrane-associated guanylate kinase scaffolding proteins that interacts with Bcl10 by way of CARD-CARD interactions. Bcl10 also associates with mucosa-associated lymphatic tissue 1 (MALT1), which depends upon amino acids 106 to 120 of Bcl10 and the immunoglobulin (Ig)-like domains of MALT1 (28, 43). The signaling complex consisting of CARMA1, Bcl10, and MALT1 (CBM complex) functions as an intermediate, linking the TCR to the IKK complex, and this process is facilitated by CD28 costimulation (28, 43). PKC-θ associates with and phosphorylates CARMA1 in Jurkat T cells (31, 52), suggesting that PKC-θ may favor the formation of the CBM complex. Mice deficient in CBM show severe defects in antigen receptor-induced NF-κB activation (6, 12, 21), placing CBM in the same pathway, leading to the activation of NF-κB. It has been shown, however, that the physical association of PKC-θ with a lipid raft-associated IKK complex plays a role in the activation of the NF-κB cascade by both TCR and CD28 (23). Therefore, the relationship between PKC-θ, CARMA1, Bcl10, MALT1, and IKKs in activating NF-κB remains to be further determined, especially for primary T cells.
To define the potential signaling pathway(s) regulated by Cbl-b in T cells, we compared the activation states of MAPKs, activator protein 1 (AP-1), NF-κB, and nuclear factor of activated T cells (NF-AT) induced by either TCR or TCR/CD28 stimulation. We demonstrate that the loss of Cbl-b does not affect the activation of MAPKs, AP-1, and NF-AT but selectively results in the hyperactivation of NF-κB upon TCR ligation, obviating the need for optimal NF-κB activation from CD28 costimulation. TCR-induced hyperactivation of NF-κB in Cbl-b−/− T cells is mediated by Akt, which is required for the formation of the CBM complex, and by PKC-θ, which couples IKKs to the CBM complex in primary T cells.
MATERIALS AND METHODS
Mice.
Wild-type (WT) BALB/c and C57BL/6 mice were purchased from the Jackson Laboratory (Frederick, MD). Vav-1−/−, Cbl-b−/−, and PKC-θ−/− mice have previously been described (2, 25, 46). CARMA1−/− mice (6) were obtained from Daniel Littman (New York University School of Medicine, New York, NY). Mice were used for experiments at 6 to 10 weeks of age.
Reagents.
Purified anti-mouse CD3 (clone no. 145-2C11) and anti-mouse CD28 (clone no. 37.51) monoclonal antibodies (MAbs) were purchased from BD Pharmingen (San Diego, CA). Protein G-agarose and Abs against Cbl-b (clone no. G-1), NF-ATc (clone no. 7A6), PKC-θ (clone no. C-18), PI3-K p85 (clone no. B-9), Vav-1 (clone no. c-14), Bcl-10 (clone no. H-197), MALT1 (clone no. H-300), IKKβ (clone no. S-13), and IKKγ (clone no. FL-419) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-Abs against Akt (Thr308), IκBα (clone no. 14D4), PKC-θ (Thr538), glycogen synthase kinase 3β (GSK-3β) (Ser9), PDK1 (Ser241), ERK (Thr202/Tyr204), JNK (Thr183/Tyr185), and p38 MAPK (Thr180/Tyr182) (clone no. 12FB) were purchased from Cell Signaling Technology (Beverly, MA). The glutathione S-transferase (GST)-IκBα fusion protein was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). T-cell enrichment columns were obtained from R&D Systems (Minneapolis, MN). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and rabbit anti-mouse IgG were purchased from Kierkegaard & Perry Laboratories (Gaithersburg, MD). Myelin basic protein (MBP; clone no. m1891), rabbit anti-hamster IgG, rabbit anti-mouse IgG, and antiactin (clone no. AC40) Abs, biotin-cholera toxin B subunit (CTx B), and streptavidin-HRP were purchased from Sigma (St. Louis, MO). The pan-PKC inhibitor, bisindolylmaleimide I (BIM I), and the Akt inhibitor, Akti 1/2, were obtained from Calbiochem (San Diego, CA). Anti-CARMA1 (CARD11) (clone no. AL220) Ab was purchased from Alexis Biotechnology (San Diego, CA).
T-cell isolation and activation.
Splenic T cells from naive wild-type (WT) and Cbl-b−/− mice were isolated (purity of ≥95% as determined by fluorescence-activated cell sorter analysis of CD3+ cell surface expression) on T-cell enrichment columns. For in vitro activation, T cells were incubated with anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) MAbs for 30 min on ice, followed by cross-linking with rabbit anti-hamster IgG (10 μg/ml). The cells were lysed in either 0.5 or 1% NP-40 lysis buffer (10 mM Tris [pH 7.5], 150 mM NaCl, 2 mM EGTA, 50 mM β-glycerophosphate, 2 mM Na3VO4, 10 mM NaF, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/ml leupeptin, and 10 μg/ml aprotinin).
Lipid raft isolation.
Purification of the lipid raft fraction was performed as described previously (41). Cells were lysed by a brief sonication in ice-cold lysis buffer containing 25 mM MES (morpholineethanesulfonic acid) (pH 6.5), 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100, supplemented with a mixture of protease and phosphatase inhibitors. Lysates were mixed with an equal volume of 80% sucrose made in lysis buffer and overlaid with 2 ml of 30% sucrose and 1 ml of 5% sucrose. Lipid rafts were collected from the 5%-30% interface following an overnight ultracentrifugation at 200,000 × g. The relative purity of these fractions was monitored by the presence of CTx B (insoluble fractions) and its absence in the Triton X-100 soluble membrane fractions.
Coimmunoprecipitation and Western blotting.
Protein concentrations in the cell lysates were determined using a bicinchoninic acid assay kit (Pierce, Rockford, IL). Cell lysates were precleared, postnuclear cell lysates were normalized for protein concentration levels, and proteins were immunoprecipitated (overnight at 4°C) with specific polyclonal Abs or control isotype-matched preimmune immunoglobulin coupled to protein G-agarose. The immunoprecipitates were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (HybondC Super; Amersham). Blots were blocked for 1 h at room temperature in phosphate-buffered saline (PBS) containing 2% bovine serum albumin (BSA) and 0.05% Tween 20. Membranes were incubated overnight with specific Abs, washed three times in PBS containing 0.05% Tween 20, and detected using HRP-conjugated goat anti-rabbit IgG or rabbit anti-mouse IgG. After three washes in PBS containing 0.05% Tween 20, signals were revealed by an enhanced chemiluminescence detection system (Amersham) and visualized by autoradiography. To determine the nuclear entry rate of NF-ATc between WT and Cbl-b−/− T cells upon either TCR or TCR/CD28 stimulation, cytoplasmic and nuclear NF-ATc bands were quantitated using the Molecular Imager system and Molecular Analyst imaging software (Bio-Rad Labs, Hercules, CA).
Nuclear extract preparation and electrophoretic mobility shift assay (EMSA) of AP-1 and NF-κB.
Nuclear extracts were harvested according to protocols described previously (55). In brief, 6 × 106-purified T cells, either untreated or stimulated for 30 min with various treatments, were washed twice with PBS and resuspended in 500 μl buffer A (10 mM HEPES [pH 7.8], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, and 1 mM PMSF) and then incubated on ice for 5 min. After centrifugation, cytoplasmic proteins were removed and the pelleted nuclei were resuspended in 50 μl buffer C (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and 1 mM PMSF). After a 30-min agitation at 4°C, the samples were centrifuged and the supernatants, containing nuclear proteins, were transferred to a fresh vial. Equal amounts of nuclear extracts (5 μg protein), as determined using the Bio-Rad protein assay, were incubated with a [32P]dATP-labeled, double-stranded, AP-1-specific oligonucleotide probe (5′-CGCTTGATGACTCAGCCGGAA-3′) and a NF-κB-specific oligonucleotide probe (5′-CAACGGCAGGGGAATTCCCCTCTCCTT-3′) (15). The reaction was performed in a total of 20 μl binding buffer [5 mM HEPES (pH 7.8), 50 mM KCl, 0.5 mM DTT, 1 μg poly(dI-dC), and 10% glycerol] for 30 min at room temperature. After incubation, samples were fractionated on a 4% nondenaturing polyacrylamide gel and visualized by autoradiography.
In vitro kinase assay.
After stimulation, cells were lysed in ice-cold lysis buffer containing 1% NP-40, 10 mM Tris, pH 7.5, 150 mM NaCl, 2 mM EGTA, 50 mM β-glycerophosphate, 2 mM Na3VO4, 10 mM NaF, 1 mM DTT, 1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. Lysates were clarified by centrifugation at 12,000 rpm for 10 min at 4°C, and their protein content was determined by the Bradford assay using BSA as a standard. Lysates were incubated for 1 h at 4°C with anti-PKC-θ Ab and further reacted with protein G-agarose for an additional 2 h at 4°C. Immunoprecipitates were washed three times with lysis buffer and twice with kinase buffer (20 mM HEPES [pH 7.5], 20 mM MgCl2, 20 mM MnCl2, 2 mM DTT, 25 mM β-glycerophosphate, and 100 nM Na3VO4). Immunoprecipitates were resuspended in 18 μl kinase buffer containing 5 μg MBP in the presence of 20 μM cold ATP and 20 μCi-[γ-32P]ATP (Amersham Life Science, Arlington Heights, IL) and incubated for 30 min at 30°C. Equal loading of precipitated proteins was confirmed by probing an aliquot of the same lysates with antiactin Ab. For in vitro IKK assay, the IKKβ immunoprecipitates were incubated with GST-IκBα fusion protein in the presence of kinase buffer. IκBα phosphorylation was detected using an anti-phospho-IκBα Ab.
Measurement of Ca2+ mobilization.
For the detection of cytosolic Ca2+, 5 × 106 freshly isolated splenic T cells were loaded with 2 μg/ml of INDO-1 (Molecular Probes; Eugene, OR) for 45 min at 37°C in the presence of probenecid (4 mM) in cell loading medium (Hanks' balanced salt solution supplemented with 1 mM CaCl2, 1 mM MgCl2 and 0.5% BSA). After loading, cells were incubated with anti-CD3 for 30 min at 4°C and cross-linked using rabbit anti-hamster IgG. Cytosolic Ca2+ flux was recorded by a BD LSR2 flow cytometer. The final data were analyzed by FlowJo flow cytometric data analysis software.
RESULTS
Cbl-b selectively inhibits TCR-induced activation of NF-κB but not that of MAPKs, AP-1, and NF-AT.
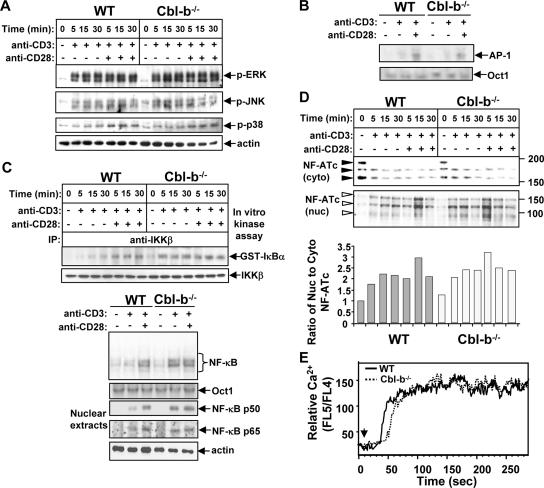
Although Cbl-b has been shown to negatively regulate Vav-1-mediated actin polymerization (25), the precise biochemical mechanisms underlying the effects of Cbl-b deficiency on downstream signaling pathways have not previously been examined. To further define the target signaling pathway(s) regulated by Cbl-b, both WT and Cbl-b−/− T cells were stimulated with anti-CD3 Ab in the presence or absence of anti-CD28 Ab and the phosphorylation state of MAPKs was determined using specific phospho-Abs against ERK, JNK, and p38 MAPK. The loss of Cbl-b did not have any effect on the activation of ERK, JNK, and p38 MAPK (Fig. 1A). Consistent with this observation, AP-1 activity, as revealed by EMSA, was comparable between WT and Cbl-b−/− T cells upon either TCR or TCR/CD28 stimulation (Fig. 1B). To investigate whether the NF-κB signaling pathway is affected by the loss of Cbl-b, both WT and Cbl-b−/− T cells were stimulated as above and IKKβ kinase activity was determined by in vitro kinase assay using GST-IκBα as a substrate. Although optimal IKKβ activation required CD28 costimulation in WT T cells, IKKβ was optimally activated in Cbl-b−/− T cells in response to CD3 stimulation alone (Fig. 1C, upper panel). To confirm this observation, NF-κB EMSA and assays for the nuclear expression of p50 and p65 were performed. Analyses of EMSA and the nuclear translocation of p50 and p65 showed that although CD28 costimulation was required for the activation of NF-κB in WT T cells, the loss of Cbl-b obviated the need for optimal NF-κB activation by CD28 costimulation (Fig. 1C, lower panel). In support of our results, double-positive thymocytes from c-Cbl/Cbl-b double-knockout mice were shown to have constitutively high NF-κB activity (15).
FIG. 1.
Cbl-b negatively regulates the activation of NF-κB but not that of MAPKs, AP-1, and NF-AT in T cells. (A) WT and Cbl-b−/− T cells were stimulated with anti-CD3 Ab or anti-CD3 and anti-CD28 Abs for 5, 15, and 30 min and lysed. The cell lysates were analyzed with Abs against phospho-ERK, phospho-JNK, and phospho-p38 MAPK. The membranes were reprobed with antiactin Ab. (B) For detection of AP-1 activity, both WT and Cbl-b−/− T cells were stimulated with anti-CD3 Ab or anti-CD3 and anti-CD28 Abs for 30 min and nuclear extracts were prepared. AP-1 activity was detected by EMSA. (C) WT and Cbl-b−/− T cells were stimulated as in A. The cell lysates were immunoprecipitated with anti-IKKβ, and incubated with GST-IκBα fusion protein. The phosphorylation of IκBα was detected using anti-phospho-IκBα Ab (upper panel). WT and Cbl-b−/− T cells were stimulated with anti-CD3 Ab or anti-CD3 and anti-CD28 Abs for 30 min, and nuclear extracts were subjected to EMSA analysis with either the NF-κB or the Oct-1 probe. Alternatively, the nuclear extracts were blotted with anti-p50 and -p65 Abs (lower panel). (D) WT and Cbl-b−/− T cells were stimulated with anti-CD3 Ab or anti-CD3 and anti-CD28 Abs for 5, 15, and 30 min, and cytosolic and nuclear fractions were prepared and analyzed with anti-NF-ATc Ab. NF-ATc translocates rapidly from the cytoplasm (cyto) to the nucleus (nuc). NF-ATc in its phosphorylated state is present as several bands migrating with apparent sizes of 160 to 190 kDa (filled arrows). Nuclear NF-ATc is dephosphorylated and migrates more rapidly as apparent bands at 80 to 150 kDa (open arrows). The cytoplasmic and nuclear NF-ATc bands were quantitated by densitometry, and the ratio of nuclear to cytoplasmic NF-ATc was calculated. The WT nuclear to cytoplasmic NF-ATc ratio was defined as 1. (E) WT and Cbl-b−/− T cells were loaded with INDO-1, incubated with anti-CD3, and cross-linked with rabbit anti-hamster IgG. Ca2+ flux was monitored by flow cytometry. Arrowheads indicate time of addition of cross-linking Ab. The data shown are representative of three independent experiments. −, absence of; +, presence of.
The NF-AT family of transcription factors has been implicated in the activation of the interleukin-2 gene. Whereas NF-AT proteins are phosphorylated and reside in the cytoplasm in resting cells, these proteins are dephosphorylated and translocate rapidly to the nucleus upon stimulation of cells with calcium-mobilizing agents (32, 34). To investigate whether the loss of Cbl-b also affects the activation of NF-AT, both WT and Cbl-b−/− T cells were stimulated with anti-CD3 or anti-CD3 plus anti-CD28 Ab. The cytosolic and nuclear fractions were prepared and blotted with anti-NF-ATc Ab. The translocation of NF-ATc from the cytoplasm to the nucleus was not significantly changed between WT and Cbl-b−/− T cells (Fig. 1D, upper panel). To further verify whether the loss of Cbl-b affects TCR-induced activation of NF-AT, which is dependent upon Ca2+ signaling, we performed Ca2+ influx assay. Consistent with previous publications (4, 25), Ca2+ influx in response to CD3 stimulation was comparable between WT and Cbl-b−/− T cells (Fig. 1E). These data support the notion that TCR-induced NF-AT pathway is not affected in the absence of Cbl-b. Therefore, our data suggest that Cbl-b selectively regulates TCR-induced NF-κB activation. Note that flow cytometric analysis revealed that the surface expression of TCRβ, CD3, CD25, CD69, CD44, and CD62L was comparable between WT and Cbl-b−/− T cells (data not shown), suggesting that Cbl-b−/− T cells are not activated in vivo. Therefore, the hyperactivation of NF-κB induced by TCR stimulation in Cbl-b−/− T cells does not result from an activated/memory phenotype.
Loss of Cbl-b releases the need for activation of PI3-K/Akt and PKC-θ from CD28 costimulation.
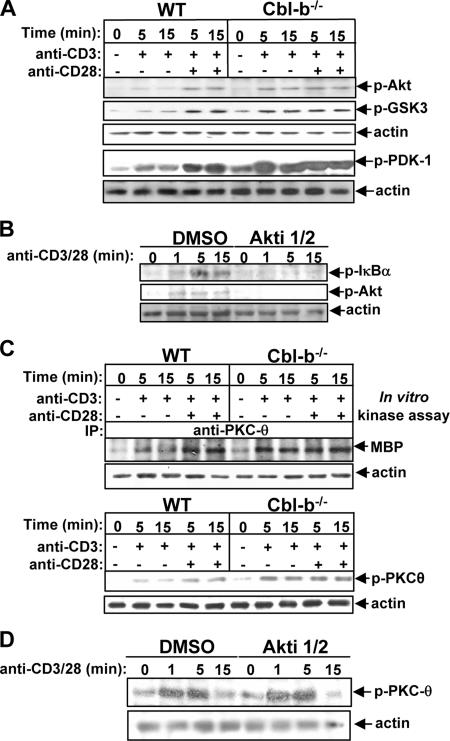
Having shown that the hyperactivation of NF-κB was induced by TCR stimulation in Cbl-b−/− T cells, we examined the activation status of two key mediators in T cells lacking Cbl-b, PI3-K and PKC-θ, which have been shown to be involved in TCR-induced NF-κB activation (8, 37). PI3-K phosphorylates PI lipids at the D3 position of the inositol ring to form active lipid second messengers. It also recruits both Akt and PDK-1 to the plasma membrane through the binding of these active lipid products to the pleckstrin homology (PH) domains of Akt and PDK-1, allowing for direct phosphorylation of Akt by PDK-1 at Thr308 (24). Therefore, the phosphorylation state of Akt can be used as a surrogate indicator of PI3-K activation (22, 35). To examine whether Cbl-b downregulates PI3-K/Akt activity in T cells, both WT and Cbl-b−/− T cells were stimulated with anti-CD3 Ab with or without anti-CD28 Ab and lysed. The cell lysates were analyzed using anti-phospho-Akt (Thr308) Ab. CD28 costimulation synergistically enhanced PI3-K/Akt activity induced by TCR stimulation in WT T cells, whereas optimal PI3-K/Akt phosphorylation did not require CD28 costimulation in Cbl-b−/− T cells (Fig. 2A). To determine the functional activity of phosphorylated Akt in Cbl-b−/− T cells, we reprobed the membrane with an Ab specific to phospho-GSK-3, a well-characterized substrate of Akt that exists in an activated nonphosphorylated form in resting cells (5). As shown in Fig. 2A, activated Akt in Cbl-b−/− T cells was able to target GSK-3 and cross-linking CD3 alone was sufficient to activate this pathway. In keeping with this finding, PDK-1 activity was optimally induced without CD28 costimulation in Cbl-b−/− T cells (Fig. 2A). To ascertain the role of Akt in TCR-induced NF-κB activation, BALB/c T cells were pretreated with Akti 1/2, a specific Akt inhibitor, and then stimulated with anti-CD3 and anti-CD28 Abs. The cell lysates were analyzed with anti-phospho-IκBα. The inhibition of Akt activity significantly suppressed TCR/CD28-induced activation of NF-κB (Fig. 2B).
FIG. 2.
Cbl-b down-regulates TCR-induced Akt and PKC-θ activation in primary mouse T cells. (A) WT and Cbl-b−/− T cells were stimulated as in Fig. 1 and lysed. The cell lysates were analyzed with anti-phospho-Akt (Thr308) Ab, anti-phospho-GSK Ab, and anti-phospho-PDK-1 Ab, and reprobed with antiactin Ab. (B) BALB/c T cells were pretreated with Akt inhibitor for 15 min, stimulated with anti-CD3 and anti-CD28 Abs for 1, 5, and 15 min, and then lysed. The cell lysates were analyzed with anti-phospho-IκBα and anti-phospho-Akt Abs. The membrane was reprobed with antiactin Ab. (C) The cell lysates from A were immunoprecipitated with anti-PKC-θ, and the kinase activity associated with PKC-θ immunoprecipitates was determined by in vitro kinase assay using MBP as a substrate, or alternatively, analyzed by anti-phospho-PKC-θ Ab and reprobed with antiactin Ab. (D) Inhibition of Akt does not affect TCR/CD28-induced PKC-θ activation. BALB/c T cells were treated with 10 μM Akti 1/2 for 30 min, stimulated with anti-CD3 and anti-CD28 Abs for 1, 5, and 15 min, and lysed. The phosphorylation of PKC-θ was detected by anti-phospho-PKC-θ Ab, and the membrane was stripped and reprobed with antiactin Ab. Data are from one of four independent experiments. −, absence of; +, presence of.
PKC-θ is a member of the “novel” subclass of PKC family members and is critical for TCR/CD28-induced activation of NF-κB (28, 43). The activation of PKC-θ is associated with both auto- or heterophosphorylation events that regulate its enzymatic activity (29, 36). PDK-1 is believed to be responsible for the phosphorylation of PKC-θ at Thr538 in the activation loop (26). Although the phosphorylation of PKC-θ at Thr538 may not correlate with its catalytic activity, the mutation of this residue results in the abrogation of PKC-θ activity, suggesting that this residue is required for PKC-θ activation (3, 29). To examine the kinase activation state of PKC-θ, we used both an in vitro kinase assay and analysis of the phosphorylation status of PKC-θ (Thr538). As expected, CD28 costimulation promoted TCR-induced activation of PKC-θ in WT T cells. The loss of Cbl-b obviated the requirement for PKC-θ activation from CD28 stimulation (Fig. 2C). Note that the inhibition of Akt does not affect PKC-θ phosphorylation (Fig. 2D), suggesting that Akt and PKC-θ reside in parallel downstream pathways of PI3-K. Therefore, the data presented above indicate that Cbl-b negatively regulates the activation of both Akt and PKC-θ, two key molecules involved in TCR- and CD28-signaling pathways, leading to NF-κB activation. Although PKC-θ is activated by diacylglycerol, a product of the cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) by phospholipase Cγ1 (PLC-γ1) (1, 3, 16), the data generated from our laboratory and others indicate that the loss of Cbl-b does not affect PLC-γ1 activation and Ca2+ influx (Fig. 1E) (2, 4, 18, 25), suggesting that Cbl-b has a minimal effect on PLC-γ1-mediated activation of PKC-θ in T cells.
Suppression of TCR-induced PKC-θ activation by Cbl-b requires PI3-K and Vav-1.
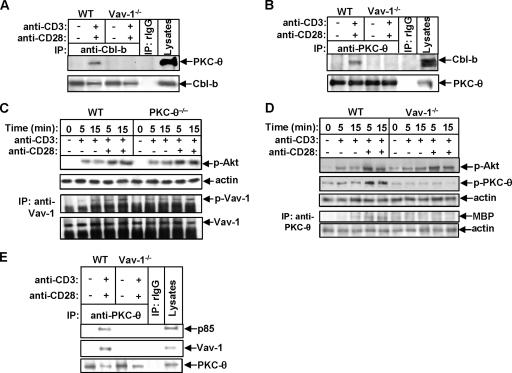
It has previously been shown that Cbl-b targets PKC-θ for monoubiquitination in anergic T cells (14), suggesting that Cbl-b plays a crucial role in the induction of T-cell anergy. Vav-1 appears to be required for the recruitment of PKC-θ to the plasma membrane (50). Whether Cbl-b directly associates with PKC-θ or whether this association, if it occurs, is mediated by Vav-1 is currently unknown. To test this hypothesis, WT and Vav-1−/− T cells were stimulated with anti-CD3 and anti-CD28 Abs and lysed. The cell lysates were immunoprecipitated with anti-Cbl-b Ab and blotted with anti-PKC-θ Ab. In WT T cells, Cbl-b associated with PKC-θ upon TCR/CD28 stimulation, while in the absence of Vav-1, Cbl-b failed to bind to PKC-θ (Fig. 3A). Our findings indicate that Cbl-b does not directly bind to PKC-θ. To confirm this suggestion, a reciprocal experiment was performed. Both WT and Vav-1−/− T cells were stimulated as above and immunoprecipitated with anti-PKC-θ Ab and blotted with anti-Cbl-b Ab. Consistent with the result shown in Fig. 3A, PKC-θ inducibly bound to Cbl-b in WT but not Vav-1−/− T cells (Fig. 3B). These data suggest that Cbl-b regulates PKC-θ activation in T cells in a Vav-1-dependent manner. We did not observe any ubiquitination of PKC-θ upon TCR/CD28 stimulation (data not shown), suggesting that PKC-θ ubiquitination is unique to anergic T cells.
FIG. 3.
Inhibition of TCR-induced PKC-θ activation by Cbl-b requires PI3-K and Vav-1. (A) WT and Vav-1−/− T cells were stimulated with anti-CD3 and anti-CD28 Abs for 15 min and lysed in 0.5% NP-40 lysis buffer; the cell lysates were immunoprecipitated with anti-Cbl-b Ab and analyzed with anti-PKC-θ and anti-Cbl-b Abs, respectively. (B) WT and Vav-1−/− T cells were stimulated and lysed as described for panel A; the cell lysates were immunoprecipitated with anti-PKC-θ Ab and analyzed with anti-Cbl-b and anti-PKC-θ Abs, respectively. (C) WT and PKC-θ−/− T cells were stimulated with anti-CD3 Ab in the presence or absence of anti-CD28 Ab for 5 and 15 min and lysed. The cell lysates were blotted with anti-phospho-Akt (Thr308) Ab, or alternatively, the cell lysates were immunoprecipitated (IP) with anti-Vav-1 Ab and analyzed with antiphosphotyrosine MAb (PY20). The membrane was stripped and reprobed with anti-Vav-1 Ab. (D) T cells from WT and Vav-1−/− mice were stimulated and lysed as described for panel C. The cell lysates were analyzed with anti-phospho-Akt (Thr308) and anti-phospho-PKC-θ Abs and reprobed with antiactin Ab. Alternatively, the cell lysates were immunoprecipitated with anti-PKC-θ Ab, and the kinase activity associated with PKC-θ immunoprecipitates was detected by in vitro kinase assay using MBP as a substrate. (E) WT and Vav-1−/− T cells were stimulated as described for panel A and lysed. The cell lysates were immunoprecipitated with anti-PKC-θ and analyzed with anti-p85, anti-Vav-1, and anti-PKC-θ Abs. Data represent one of three independent experiments. −, absence of; +, presence of.
PKC-θ is also an important downstream effector molecule of Vav-1 in T cells (50, 51). It was reported that PI3-K may regulate the exchange activity of Vav-1 in vitro through its product phosphatidylinositol 3,4,5-trisphosphate (PIP3), which binds to the PH domain of Vav-1 and recruits Vav-1 to the plasma membrane (10, 30). However, it has also been reported that PI3-K may act downstream of Rac-1, a downstream substrate of Vav-1 (9). Therefore, the relationship among Vav-1, PI3-K, and PKC-θ in primary T cells is not fully characterized. To further define the relationship among these three molecules in T-cell activation, both WT and PKC-θ−/− T cells were stimulated with anti-CD3 Ab, with or without anti-CD28 Ab, and lysed. The cell lysates were analyzed with anti-phospho-Akt Ab or immunoprecipitated with anti-Vav-1 Ab and blotted with anti-phospho-Tyr MAb. Both Akt and Vav-1 activation in PKC-θ−/− T cells was normal compared with that in WT T cells (Fig. 3C), supporting the idea that PI3-K and Vav-1 may lie upstream of PKC-θ in primary T cells. To ascertain the relationship between PI3-K and Vav-1, T cells from WT and Vav-1−/− mice were stimulated as described above, and the cell lysates were analyzed with anti-phospho-Akt and anti-phospho-PKC-θ Abs, respectively. The phosphorylation of Akt was comparable between WT and Vav-1−/− T cells, but the activation of PKC-θ was defective in T cells lacking Vav-1 in response to either TCR or TCR/CD28 stimulation (Fig. 3D). These data suggest that Vav-1 is downstream of, or parallel with, PI3-K/Akt, but upstream of PKC-θ in primary T cells. Since it has been demonstrated that PDK-1 is required for the phosphorylation of PKC-θ at Thr538, which is essential for its activity (3), the failure to induce PKC-θ phosphorylation in the absence of Vav-1 suggests that Vav-1 may bring PKC-θ in the proximity of PI3-K and allow it to be phosphorylated by PDK-1. To test this hypothesis, both WT and Vav-1−/− T cells were stimulated with anti-CD3 Ab plus anti-CD28 Ab and lysed. The cell lysates were immunoprecipitated with anti-PKC-θ Ab and blotted with anti-p85 and anti-Vav-1 Abs. PKC-θ was inducibly associated with PI3-K in the presence of Vav-1. In contrast, the loss of Vav-1 resulted in the uncoupling of PI3-K from PKC-θ (Fig. 3E). Taken together, these data indicate that Cbl-b regulates TCR/CD28-mediated PKC-θ activation via Vav-1 which functions as an adaptor protein to bring PKC-θ in the proximity of PI3-K-dependent kinase(s).
Cbl-b controls formation of a signaling complex consisting of PKC-θ, CBM, and IKKs in primary mouse T cells.
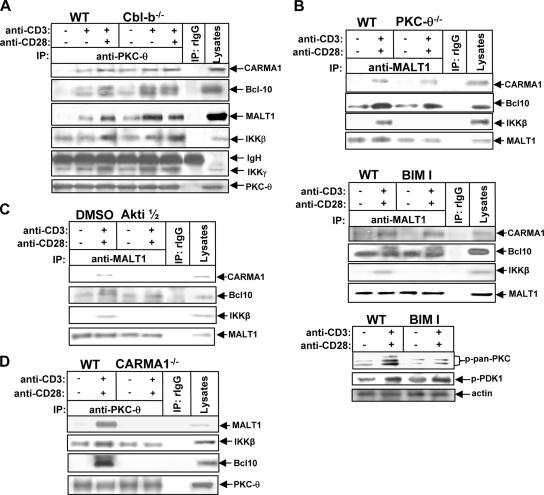
Although how signaling pathways induced by TCR/CD28 costimulation lead to the activation of NF-κB is not fully understood, PKC-θ is essential for the TCR/CD28 costimulation-induced NF-κB activation through a CBM-dependent pathway (28, 47). One could expect the loss of Cbl-b to result in increased recruitment of PKC-θ with CBM and IKKs. To test whether this increased recruitment is the case, both WT and Cbl-b−/− T cells were stimulated with anti-CD3 Ab with or without anti-CD28 Ab and lysed in 0.5% NP-40 lysis buffer. The cell lysates were immunoprecipitated with anti-PKC-θ Ab and blotted with Abs against CARMA1, Bcl10, MALT1, IKKβ, and IKKγ. As shown in Fig. 4A, a small amount of CARMA1, Bcl10, MALT1, IKKβ, and IKKγ associated with PKC-θ following TCR stimulation in WT T cells, and CD28 costimulation potentiated this TCR-induced formation of the signaling complex. The loss of Cbl-b obviated the requirement from CD28 engagement for the formation of the PKC-θ-associated signaling complex, which was present even in unstimulated Cbl-b−/− T cells (Fig. 4A). Although it has been reported that TCR/CD28-induced PKC-θ activation is mediated by PDK-1 (26), we could not detect any physical association between PDK-1 and PKC-θ in either WT or Cbl-b−/− T cells following TCR or TCR/CD28 stimulation, suggesting that the association of PDK-1 with PKC-θ might be very weak or transient. Interestingly, PKC-θ was found to be constitutively associated with IKKs in the absence of the CBM complex in resting WT T cells (Fig. 4A), suggesting that PKC-θ may couple IKKs to the CBM complex.
FIG. 4.
Cbl-b controls the assembly of a signaling complex consisting of PKC-θ, CARMA-1, Bcl-10, MALT-1, IKKβ, and IKKγ. (A) WT and Cbl-b−/− T cells (108/ml) were stimulated with anti-CD3 Ab in the presence or absence of anti-CD28 Ab for 15 min and lysed in 0.5% NP-40 lysis buffer. The cell lysates were immunoprecipitated (IP) with anti-PKC-θ Ab and analyzed with anti-CARMA1, anti-Bcl10, anti-MALT1, anti-IKKβ, anti-IKKγ, and PKC-θ Abs. (B) WT and PKC-θ−/− T cells or BALB/c T cells pretreated with bisindolylmaleimide I (0.5 μM) for 15 min were stimulated with anti-CD3 and anti-CD28 Abs for 15 min, immunoprecipitated with anti-MALT1 Ab, and analyzed with anti-CARMA1, anti-MALT1, anti-Bcl10, and anti-IKKβ Abs, respectively. The specificity of BIM I was determined by analyzing the lysates of T cells treated with BIM I with anti-pan-phospho-PKC and anti-phospho-PDK-1 Abs, respectively. (C) BALB/c T cells were pretreated with a specific Akt inhibitor, Akti 1/2, for 15 min, stimulated with anti-CD3 and anti-CD28 Abs for 15 min, and lysed. The formation of the CBM complex was determined as in B. (D) WT and CARMA1−/− T cells were stimulated with anti-CD3 and anti-CD28 Abs as above, immunoprecipitated with anti-PKC-θ Ab, and analyzed with anti-Bcl10, anti-MALT1, and anti-IKKβ Abs, respectively. Data represent one of three independent experiments. −, absence of; +, presence of.
To test this notion, both WT and PKC-θ−/− T cells were stimulated with anti-CD3 and anti-CD28 Abs and the formation of CBM-IKK complex was determined. The absence of PKC-θ did not affect the formation of the CBM complex, but this complex could no longer associate with IKKs (Fig. 4B, upper panel). These data indicate that PKC-θ is indeed responsible for coupling IKKs to the CBM complex. To rule out the possibility that another isoform(s) of PKC may compensate for the deficiency in PKC-θ, BALB/c T cells were treated with bisindolylmaleimide I, a pan-PKC inhibitor, and then stimulated with anti-CD3 and anti-CD28 Abs. In the presence of the pan-PKC inhibitor, the formation of CBM was still intact, but this complex no longer bound to IKKβ (Fig. 4B, lower panel), suggesting that there is PKC-independent formation of the CBM complex. To assess whether Akt could potentiate the formation of the CBM complex, BALB/c T cells were pretreated with Akti 1/2 and stimulated with anti-CD3 and anti-CD28 Abs. The inhibition of Akt activity suppressed TCR-induced formation of the CBM complex and uncoupled IKKβ from the Bcl10-MALT1 complex (Fig. 4C). This observation suggests that the recruitment of CARMA1 is required for the coupling of the PKC-θ-IKK complex to the Bcl10-MALT1 complex. If this is true, one could expect that CARMA1 deficiency might result in the uncoupling of PKC-θ-IKKs from the Bcl10-MALT1 complex. To test this hypothesis, WT and CARMA1−/− T cells were stimulated with anti-CD3 and anti-CD28 Abs. The cell lysates were immunoprecipitated with anti-PKC-θ Ab and blotted with anti-CARMA1, anti-Bcl10, anti-MALT1, and anti-IKKβ Abs. In the absence of CARMA1, the PKC-θ-IKK complex no longer bound to the Bcl10-MALT1 complex (Fig. 4D). Consistent with our observation, CARMA1 was reported to connect the PKC-θ-IKK complex to the BCL10-MALT1 complex in Jurkat T cells (44). These data suggest that Akt is required for the formation of the CBM complex, whereas CARMA1 links PKC-θ-IKKs to the Bcl10-MALT1 complex to form a large functional signaling complex that leads to the activation of NF-κB.
Cbl-b regulates the aggregation of PKC-θ, CARMA1, Bcl10, and IKKβ in lipid rafts.
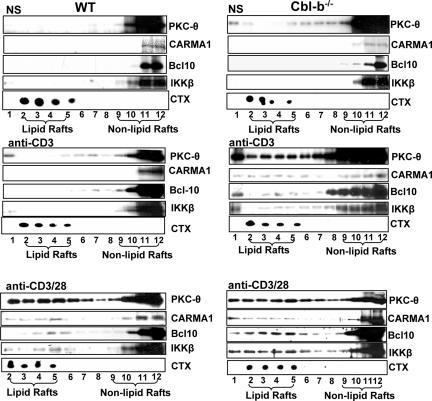
Plasma membranes of many cell types contain domains enriched in specific lipids and cholesterol, called lipid rafts. In T cells, key TCR signaling molecules associate with rafts, and the disruption of the raft association of certain of these molecules abrogates TCR signaling. The TCR itself associates with lipid rafts, and TCR cross-linking causes the aggregation of raft-associated proteins (13). T-cell activation induced by CD3 stimulation is associated with the translocation of PKC-θ from the cytosol to the plasma membrane, which can be further enhanced by CD28 costimulation (1, 48-51). To substantiate whether Cbl-b controls the aggregation of PKC-θ, CARMA1, Bcl10, and IKKβ in the lipid rafts, both WT and Cbl-b−/− T cells were stimulated with anti-CD3 or anti-CD3 Ab plus anti-CD28 Ab, and lipid rafts were isolated using sucrose gradient centrifugation (41). In resting WT T cells, PKC-θ, CARMA1, Bcl10, and IKKβ were localized to the nonlipid raft fraction, whereas CD28 costimulation favored TCR-induced translocation of these molecules into lipid rafts. In contrast, small amounts of PKC-θ, CARMA1, Bcl10, MALT1, IKKβ, and IKKγ were present in lipid rafts in unstimulated Cbl-b−/− T cells and the loss of Cbl-b obviated the need of CD28 to propel these molecules into lipid rafts (Fig. 5).
FIG. 5.
Aggregation of PKC-θ, CARMA1, Bcl-10, and IKKβ in lipid rafts is negatively modulated by Cbl-b. T cells from WT and Cbl-b−/− mice were stimulated with anti-CD3 Ab or anti-CD3 plus anti-CD28 Abs or were left unstimulated. The lipid rafts were isolated by sucrose-gradient ultracentrifugation, and the lipid raft and nonlipid raft fractions were blotted with Abs against PKC-θ, CARMA-1, Bcl-10, and IKKβ. The purity of lipid rafts was determined by CTx analysis. The data represent one of four independent experiments. −, absence of; +, presence of.
DISCUSSION
We have demonstrated that Cbl-b is one of the key regulators that influences the threshold for T-cell activation regulated by CD28 and CTLA-4 (27, 54). This notion is supported by the recent findings that Cbl-b plays a crucial role in the induction of T-cell anergy (14, 18). In this report, we have further characterized the signaling pathway regulated by Cbl-b in T cells. We demonstrate that (i) Cbl-b selectively downregulates TCR-induced NF-κB activation (Fig. 1); (ii) the inhibition of NF-κB-activation by Cbl-b is mediated by Akt, which is required for CBM formation, and by PKC-θ, which is responsible for the coupling of IKKs to the CBM complex (Fig. 2 to 4); (iii) Cbl-b-mediated inhibition of PKC-θ is regulated accordingly by PI3-K and Vav-1, the latter of which functions as an adaptor protein to bring PKC-θ into proximity of PI3-K, resulting in its activation (Fig. 3); and (iv) Cbl-b negatively regulates the assembly of a signaling complex consisting of PKC-θ, CARMA1, Bcl10, and IKKs and their aggregation into lipid rafts (Fig. 4 and 5).
Although it was reported that Cbl-b selectively regulates Vav-1 activation in T cells, presumably through a PI3-K-dependent, proteolysis-independent mechanism (7), the signaling pathway downstream of Cbl-b is still poorly defined. In this study, we found that NF-κB is hyperactivated by TCR stimulation in T cells lacking Cbl-b, and the loss of Cbl-b obviates the requirement for CD28 engagement for optimal NF-κB activation, which closely correlates with PI3-K/Akt and PKC-θ activity (Fig. 1 and 2). Therefore, to our knowledge, these data provide the first piece of evidence for the regulation of T-cell activation by Cbl-b via an NF-κB-dependent mechanism. It has previously been shown that Akt plays a role in NF-κB induction in T cells (20, 33). Our data reveal that the loss of Cbl-b results in the optimal activation of Akt in the absence of CD28 costimulation (Fig. 2A), and the inhibition of Akt attenuates TCR-induced activation of NF-κB, possibly by inhibiting the recruitment of CARMA1 to the Bcl10-MALT1 complex (Fig. 4C). Consistent with our findings, CARMA1 was reported to be required for Akt-mediated NF-κB activation in T cells (17).
PI3-K may regulate Vav-1 via the interaction between PIP3, a PI3-K substrate, and the PH domain of Vav-1 (10, 30). We showed that the Cbl-b-PKC-θ association is mediated by Vav-1 (Fig. 3A and B). Furthermore, Vav-1 has been shown to be required for the membrane targeting of PKC-θ (50). It was also suggested that PI3-K may act downstream of Rac-1, a downstream substrate of Vav-1 (9). Therefore, the relationship between PI3-K, Vav-1, and PKC-θ is still not fully defined. The inability of PKC-θ to bind to p85 as well as the defective PKC-θ activation in the absence of Vav-1 (Fig. 3C to E), suggests that Vav-1 functions as an adaptor protein to recruit PKC-θ to the cell membrane in proximity to p85, subsequently allowing it to be phosphorylated by PI3-K-dependent kinase(s). Although during T-cell activation, membrane-associated Vav-1-SLP-76 leads to PLC-γ activation and production of diacylglycerol, which is important for PKC-θ activation (1, 3, 16), the loss of Cbl-b does not elicit elevated activation of PLC-γ1 or increased Ca2+ influx in response to TCR stimulation (Fig. 1E) (2, 4, 25), arguing that Cbl-b may have a specific effect on Vav-1-mediated signaling events, independent of PLC-γ activation. Therefore, Cbl-b negatively regulates TCR-induced NF-κB activation via a PI3-K-Vav-1-PKC-θ-dependent pathway.
Recently, it has been shown that in transformed cells, PKC-θ can associate with and phosphorylate the CARMA1 linker region, which binds to and blocks the accessibility of the CARD motif (31, 45, 52). It has previously been suggested that TCR-triggered, PKC-dependent linker phosphorylation is required to release this inhibition, thereby allowing for Bcl10 recruitment and signal propagation to the IKK complex (31, 45, 52). Recent studies also indicate that CARMA1 links PKC-θ to downstream signaling components that lead to the activation of NF-κB. These studies suggest that PKC-θ is essential for TCR/CD28-induced NF-κB activation, possibly via a CBM-dependent pathway. It has been reported that CARMA1 recruits IKKs to lipid rafts at the immunological synapse, whereas the translocation of PKC-θ to lipid rafts at the immunological synapse may be independent of CARMA1 (11). On the other hand, other studies indicate that CARMA1 controls the recruitment of PKC-θ, Bcl10, MALT1, and IKKβ to the immunological synapse (52). Therefore, the precise role of PKC-θ and CARMA1 in mediating NF-κB activation is still not completely elucidated, especially for primary T cells. Surprisingly, we found that the formation of the CBM complex is largely independent of PKC (Fig. 4B), which contradicts two recent reports (31, 45). We speculated that this discrepancy is due to different cell types used, namely primary mouse T cells versus Jurkat T cells. Nevertheless, our data suggest that there is a PKC-θ-independent pathway that induces formation of the CBM complex. Indeed, either Akt or CaM kinase II may associate with CARMA1 or induce CARMA1 phosphorylation (17, 33), which may represent this alternative pathway. In support of this notion, the inhibition of Akt blocks CBM complex formation and the subsequent coupling of PKC-θ-IKKs to the Bcl10-MALT1 complex (Fig. 4C). We should note that in the absence of PKC-θ, the CBM complex fails to associate with IKKs upon TCR ligation (Fig. 4B), indicating that PKC-θ is responsible for coupling IKKs to the CBM complex and leads to NF-κB activation. An analysis of CARMA1−/− T cells suggests that the loss of CARMA1 results in the uncoupling of PKC-θ-IKKs from the Bcl10-MALT1 complex (Fig. 4D). Our findings are consistent with the observations made by Shambharkar et al., in which CARMA1 was shown to link the PKC-θ-IKK complex to the Bcl10-MALT1 complex and to be essential for the regulation of IKKγ ubiquitination upon TCR stimulation (44). Note that we did not observe any constitutive association of IKKs with the MALT1-Bcl10 complex, which is different from the results of a recent report in which Jurkat T cells were used (53). Therefore, our data provide an additional, but not mutually exclusive, mechanism by which both PKC-θ and CARMA1 mediate TCR/CD28-induced NF-κB activation.
In summary, we demonstrate that in primary T cells, Cbl-b is a key negative regulator of NF-κB activation in a manner mediated by both PI3-K/Akt and PKC-θ. Akt may potentiate the formation of the CBM complex, whereas PKC-θ couples IKKs to the CBM complex, resulting in the activation of IKKβ (Fig. 6). Our findings indicate that Cbl-b regulates TCR-induced NF-κB activation via Akt-dependent and PKC-θ-dependent pathways in primary T cells.
FIG. 6.
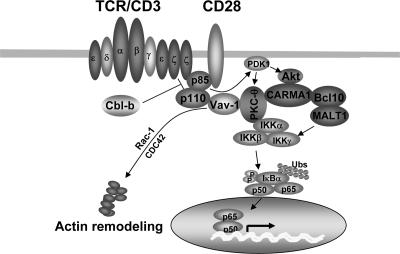
Model of Cbl-b action on T-cell activation. TCR/CD28 ligation induces the activation of several pathways, including that of PI3-K, which is targeted by Cbl-b for ubiquitination. PI3-K may activate Vav-1 through the interaction of its product PIP3 and the PH domain of Vav-1, resulting in the recruitment of Vav-1 to the plasma membrane. Vav-1 then functions as an adaptor protein to bring PKC-θ into the proximity of PI3-K and allow it to be phosphorylated by PDK-1. The formation of the CBM complex may be dependent on Akt, a downstream target of PI3-K, but independent of PKC-θ, which couples IKKs to the CBM complex, whereas CARMA1 links PKC-θ-IKKs to the Bcl10-MALT1 complex. In the absence of Cbl-b, both Akt and PKC-θ are hyperactivated upon TCR stimulation. Consequently, in Cbl-b−/− T cells, hyperactivated Akt induced by TCR stimulation initiates the enhanced formation of the CBM complex, whereas hyperactivated PKC-θ couples additional IKKs to the CBM complex, resulting in a large functional signaling complex and leading to an elevated activation of NF-κB.
Acknowledgments
We thank Daniel Littman for providing CARMA1−/− mice. We also thank Ryan Duggan for helping with Ca2+ flux studies and Marcus Clark for critical reading of the manuscript and helpful discussions.
The project described here was supported by grants K02 AR049047 and R01AR049775 to J.Z. from the National Institutes of Health and grants-in-aid (0355509Z and 0650181Z) to J.Z. from the American Heart Association. The University of Chicago Cancer Research Center Flow Cytometry Facility is supported in part by P30-CA14599.
Footnotes
Published ahead of print on 28 January 2008.
REFERENCES
- 1.Altman, A., N. Isakov, and G. Baier. 2000. Protein kinase Ctheta: a new essential superstar on the T-cell stage. Immunol. Today 21567-573. [DOI] [PubMed] [Google Scholar]
- 2.Bachmaier, K., C. Krawczyk, I. Kozieradzki, Y.-Y. Kong, T. Sasaki, A. J. Oliveira-dos-Santos, S. Mariathasan, D. Bouchard, A. Wakeham, A. Itie, J. Le, P. S. Ohashi, I. Sarosi, H. Nishina, S. Lipkowitz, and J. M. Penninger. 2000. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 403211-216. [DOI] [PubMed] [Google Scholar]
- 3.Baier, G. 2003. The PKC gene module: molecular biosystematics to resolve its T cell functions. Immunol. Rev. 19264-79. [DOI] [PubMed] [Google Scholar]
- 4.Chiang, Y. J., H. K. Kole, K. Brown, M. Naramura, S. Fukuhara, R.-J. Hu, I. K. Jang, J. S. Gutkind, E. Shevach, and H. Gu. 2000. Cbl-b regulates the CD28 dependence of T-cell activation. Nature 403216-220. [DOI] [PubMed] [Google Scholar]
- 5.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378785-789. [DOI] [PubMed] [Google Scholar]
- 6.Egawa, T., B. Albrecht, B. Favier, M. J. Sunshine, K. Mirchandani, W. O'Brien, M. Thome, and D. R. Littman. 2003. Requirement for CARMA1 in antigen receptor-induced NF-kappa B activation and lymphocyte proliferation. Curr. Biol. 131252-1258. [DOI] [PubMed] [Google Scholar]
- 7.Fang, D., and Y.-C. Liu. 2001. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat. Immunol. 2870-875. [DOI] [PubMed] [Google Scholar]
- 8.Gao, M., and M. Karin. 2005. Regulating the regulators: control of protein ubiquitination and ubiquitin-like modifications by extracellular stimuli. Mol. Cell 19581-593. [DOI] [PubMed] [Google Scholar]
- 9.Genot, E. M., C. Arrieumerlou, G. Ku, B. M. Burgering, A. Weiss, and I. M. Kramer. 2000. The T-cell receptor regulates Akt (protein kinase B) via a pathway involving Rac1 and phosphatidylinositide 3-kinase. Mol. Cell. Biol. 205469-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han, J., K. Luby-Phelps, B. Das, X. Shu, Y. Xia, R. D. Mosteller, U. M. Krishna, J. R. Falck, M. A. White, and D. Broek. 1998. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279558-560. [DOI] [PubMed] [Google Scholar]
- 11.Hara, H., C. Bakal, T. Wada, D. Bouchard, R. Rottapel, T. Saito, and J. M. Penninger. 2004. The molecular adapter carma1 controls entry of IκB kinase into the central immune synapse. J. Exp. Med. 2001167-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara, H., T. Wada, C. Bakal, I. Kozieradzki, S. Suzuki, N. Suzuki, M. Nghiem, E. K. Griffiths, C. Krawczyk, B. Bauer, F. D'Acquisto, S. Ghosh, W. C. Yeh, G. Baier, R. Rottapel, and J. M. Penninger. 2003. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity 18763-775. [DOI] [PubMed] [Google Scholar]
- 13.Harder, T. 2004. Lipid raft domains and protein networks in T-cell receptor signal transduction. Curr. Opin. Immunol. 16353-359. [DOI] [PubMed] [Google Scholar]
- 14.Heissmeyer, V., F. Macian, S. H. Im, R. Varma, S. Feske, K. Venuprasad, H. Gu, Y. C. Liu, M. L. Dustin, and A. Rao. 2004. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 5255-265. [DOI] [PubMed] [Google Scholar]
- 15.Huang, F., Y. Kitaura, I. Jang, M. Naramura, H. H. Kole, L. Liu, H. Qin, M. S. Schlissel, and H. Gu. 2006. Establishment of the major compatibility complex-dependent development of CD4+ and CD8+ T cells by the Cbl family proteins. Immunity 25571-581. [DOI] [PubMed] [Google Scholar]
- 16.Isakov, N., and A. Altman. 2002. Protein kinase C(theta) in T cell activation. Annu. Rev. Immunol. 20761-794. [DOI] [PubMed] [Google Scholar]
- 17.Ishiguro, K., T. Green, J. Rapley, H. Wachtel, C. Giallourakis, A. Landry, Z. Cao, N. Lu, A. Takafumi, H. Goto, M. J. Daly, and R. J. Xavier. 2006. Ca2+/calmodulin-dependent protein kinase II is a modulator of CARMA1-mediated NF-κB activation. Mol. Cell. Biol. 265497-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon, M. S., A. Atfield, K. Venuprasad, C. Krawczyk, R. Sarao, C. Elly, C. Yang, S. Arya, K. Bachmaier, L. Su, D. Bouchard, R. Jones, M. Gronski, P. Ohashi, T. Wada, D. Bloom, C. G. Fathman, Y. C. Liu, and J. M. Penninger. 2004. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity 21167-177. [DOI] [PubMed] [Google Scholar]
- 19.Joazeiro, C. A. P., S. S. Wing, H.-K. Huang, J. D. Leverson, T. Hunter, and Y.-C. Liu. 1999. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286309-312. [DOI] [PubMed] [Google Scholar]
- 20.Jones, R. G., M. Parsons, M. Bonnard, V. S. Chan, W. C. Yeh, J. R. Woodgett, and P. S. Ohashi. 2000. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J. Exp. Med. 1911721-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jun, J. E., L. E. Wilson, C. G. Vinuesa, S. Lesage, M. Blery, L. A. Miosge, M. C. Cook, E. M. Kucharska, H. Hara, J. M. Penninger, H. Domashenz, N. A. Hong, R. J. Glynne, K. A. Nelms, and C. C. Goodnow. 2003. Identifying the MAGUK protein Carma-1 as a central regulator of humoral immune responses and atopy by genome-wide mouse mutagenesis. Immunity 18751-762. [DOI] [PubMed] [Google Scholar]
- 22.Kane, L. P., P. G. Andres, K. C. Howland, A. K. Abbas, and A. Weiss. 2001. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat. Immunol. 237-44. [DOI] [PubMed] [Google Scholar]
- 23.Khoshnan, A., D. Bae, C. A. Tindell, and A. E. Nel. 2000. The physical association of protein kinase C theta with a lipid raft-associated inhibitor of kappa B factor kinase (IKK) complex plays a role in the activation of the NF-kappa B cascade by TCR and CD28. J. Immunol. 1656933-6940. [DOI] [PubMed] [Google Scholar]
- 24.Koyasu, S. 2003. The role of PI3K in immune cells. Nat. Immunol. 4313-319. [DOI] [PubMed] [Google Scholar]
- 25.Krawczyk, C., K. Bachmaier, T. Sasaki, R. G. Jones, S. B. Snapper, D. Bouchard, I. Kozieradzki, P. S. Ohashi, F. W. Alt, and J. M. Penninger. 2000. Cbl-b is a negative regulator of receptor clustering and raft aggregation in T cells. Immunity 13463-473. [DOI] [PubMed] [Google Scholar]
- 26.Lee, K. Y., F. D'Acquisto, M. S. Hayden, J. H. Shim, and S. Ghosh. 2005. PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation. Science 308114-118. [DOI] [PubMed] [Google Scholar]
- 27.Li, D., I. Gal, C. Vermes, M. L. Alegre, A. S. Chong, L. Chen, Q. Shao, V. Adarichev, X. Xu, T. Koreny, K. Mikecz, A. Finnegan, T. T. Glant, and J. Zhang. 2004. Cutting edge: Cbl-b: one of the key molecules tuning CD28- and CTLA-4-mediated T cell costimulation. J. Immunol. 1737135-7139. [DOI] [PubMed] [Google Scholar]
- 28.Lin, X., and D. Wang. 2004. The roles of CARMA1, Bcl10, and MALT1 in antigen receptor signaling. Semin. Immunol. 16429-435. [DOI] [PubMed] [Google Scholar]
- 29.Liu, Y., C. Graham, A. Li, R. J. Fisher, and S. Shaw. 2002. Phosphorylation of the protein kinase C-theta activation loop and hydrophobic motif regulates its kinase activity, but only activation loop phosphorylation is critical to in vivo nuclear-factor-kappaB induction. Biochem. J. 361255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma, A. D., A. Metjian, S. Bagrodia, S. Taylor, and C. S. Abrams. 1998. Cytoskeletal reorganization by G protein-coupled receptors is dependent on phosphoinositide 3-kinase gamma, a Rac guanosine exchange factor, and Rac. Mol. Cell. Biol. 184744-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto, R., D. Wang, M. Blonska, H. Li, M. Kobayashi, B. Pappu, Y. Chen, D. Wang, and X. Lin. 2005. Phosphorylation of CARMA1 plays a critical role in T cell receptor-mediated NF-kappaB activation. Immunity 23575-585. [DOI] [PubMed] [Google Scholar]
- 32.Michel, F., G. Mangino, G. Attal-Bonnefoy, L. Tuosto, A. Alcover, A. Roumier, D. Olive, and O. Acuto. 2000. CD28 utilizes Vav-1 to enhance TCR-proximal signaling and NF-AT activation. J. Immunol. 1653820-3829. [DOI] [PubMed] [Google Scholar]
- 33.Narayan, P., B. Holt, R. Tosti, and L. P. Kane. 2006. CARMA1 is required for Akt-mediated NF-κB activation in T cells. Mol. Cell. Biol. 262327-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamura, H., and A. Rao. 2001. Transcriptional regulation in lymphocytes. Curr. Opin. Cell Biol. 13239-243. [DOI] [PubMed] [Google Scholar]
- 35.Okkenhaug, K., L. Wu, K. M. Garza, J. La Rose, W. Khoo, B. Odermatt, T. W. Mak, P. S. Ohashi, and R. Rottapel. 2001. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat. Immunol. 2325-332. [DOI] [PubMed] [Google Scholar]
- 36.Parekh, D. B., W. Ziegler, and P. J. Parker. 2000. Multiple pathways control protein kinase C phosphorylation. EMBO J. 19496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitcher, L. A., and N. S. van Oers. 2003. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 24554-560. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds, L. F., C. de Bettignies, T. Norton, A. Beeser, J. Chernoff, and V. L. Tybulewicz. 2004. Vav1 transduces T cell receptor signals to the activation of the Ras/ERK pathway via LAT, Sos, and RasGRP1. J. Biol. Chem. 27918239-18246. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds, L. F., L. A. Smyth, T. Norton, N. Freshney, J. Downward, D. Kioussis, and V. L. Tybulewicz. 2002. Vav1 transduces T cell receptor signals to the activation of phospholipase C-gamma1 via phosphoinositide 3-kinase-dependent and -independent pathways. J. Exp. Med. 1951103-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salojin, K. V., J. Zhang, and T. L. Delovitch. 1999. TCR and CD28 are coupled via ZAP-70 to the activation of the Vav/Rac-1-PAK-1/p38 MAPK signaling pathway. J. Immunol. 163844-853. [PubMed] [Google Scholar]
- 41.Salojin, K. V., J. Zhang, C. Meagher, and T. L. Delovitch. 2000. ZAP-70 is essential for the T cell antigen receptor-induced plasma membrane targeting of SOS and Vav in T cells. J. Biol. Chem. 2755966-5975. [DOI] [PubMed] [Google Scholar]
- 42.Sawasdikosol, S., J. C. Pratt, W. Meng, M. J. Eck, and S. J. Burakoff. 2000. Adapting to multiple personalities: Cbl is also a RING finger ubiquitin ligase. Biochim. Biophys. Acta 1471M1-M12. [DOI] [PubMed] [Google Scholar]
- 43.Schulze-Luehrmann, J., and S. Ghosh. 2006. Antigen-receptor signaling to nuclear factor kappaB. Immunity 25701-715. [DOI] [PubMed] [Google Scholar]
- 44.Shambharkar, P. B., M. Blonska, B. P. Pappu, H. Li, Y. You, H. Sakurai, B. G. Darnay, H. Hara, J. Penninger, and X. Lin. 2007. Phosphorylation and ubiquitination of the IkappaB kinase complex by two distinct signaling pathways. EMBO J. 261794-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sommer, K., B. Guo, J. L. Pomerantz, A. D. Bandaranayake, M. E. Moreno-Garcia, Y. L. Ovechkina, and D. J. Rawlings. 2005. Phosphorylation of the CARMA1 linker controls NF-kappaB activation. Immunity 23561-574. [DOI] [PubMed] [Google Scholar]
- 46.Sun, Z., C. W. Arendt, W. Ellmeier, E. M. Schaeffer, M. J. Sunshine, L. Gandhi, J. Annes, D. Petrzilka, A. Kupfer, P. L. Schwartzberg, and D. R. Littman. 2000. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 404402-407. [DOI] [PubMed] [Google Scholar]
- 47.Thome, M. 2004. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat. Rev. Immunol. 4348-359. [DOI] [PubMed] [Google Scholar]
- 48.Tuosto, L., and O. Acuto. 1998. CD28 affects the earliest signaling events generated by TCR engagement. Eur. J. Immunol. 282131-2142. [DOI] [PubMed] [Google Scholar]
- 49.van der Merwe, P. A. 2002. Formation and function of the immunological synapse. Curr. Opin. Immunol. 14293-298. [DOI] [PubMed] [Google Scholar]
- 50.Villalba, M., K. Bi, J. Hu, Y. Altman, P. Bushway, E. Reits, J. Neefjes, G. Baier, R. T. Abraham, and A. Altman. 2002. Translocation of PKCθ in T cells is mediated by a nonconventional, PI3-K- and Vav-dependent pathway, but does not absolutely require phospholipase C. J. Cell Biol. 157253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villalba, M., N. Coudronniere, M. Deckert, E. Teixeiro, P. Mas, and A. Altman. 2000. A novel functional interaction between Vav and PKCtheta is required for TCR-induced T cell activation. Immunity 12151-160. [DOI] [PubMed] [Google Scholar]
- 52.Wang, D., R. Matsumoto, Y. You, T. Che, X. Y. Lin, S. L. Gaffen, and X. Lin. 2004. CD3/CD28 costimulation-induced NF-κB activation is mediated by recruitment of protein kinase C-θ, Bcl10, and IκB kinase β to the immunological synapse through CARMA1. Mol. Cell. Biol. 24164-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wegener, E., A. Oeckinghaus, N. Papadopoulou, L. Lavitas, M. Schmidt-Supprian, U. Ferch, T. W. Mak, J. Ruland, V. Heissmeyer, and D. Krappmann. 2006. Essential role for IkappaB kinase beta in remodeling Carma1-Bcl10-Malt1 complexes upon T cell activation. Mol. Cell 2313-23. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, J., T. Bardos, D.-D. Li, I. Gal, C. Vermes, J.-Y. Xu, K. Mikecz, A. Finnegan, S. Lipkowitz, and T. T. Glant. 2002. Cutting edge: regulation of T cell activation threshold by CD28 costimulation by targeting Cbl-b for ubiquitination. J. Immunol. 1692236-2240. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, J., K. V. Salojin, and T. L. Delovitch. 2000. CD28 costimulation restores T-cell responsiveness in nonobese diabetic mice by overcoming deficiencies in Rac-1/p38 MAPK signaling and IL-2 and IL-4 gene transcription. Int. Immunol. 13377-384. [DOI] [PubMed] [Google Scholar]