Abstract
RNA editing in Trypanosoma brucei is posttranscriptional uridylate removal/addition, generally at vast numbers of pre-mRNA sites, but to date, only single editing cycles have been examined in vitro. We here demonstrate achieving sequential cycles of U deletion in vitro, with editing products confirmed by sequence analysis. Notably, the subsequent editing cycle is much more efficient and occurs far more rapidly than single editing cycles; plus, it has different recognition requirements. This indicates that the editing complex acts in a concerted manner and does not dissociate from the RNA substrate between these cycles. Furthermore, the multicycle substrate exhibits editing that is unexpected from a strictly 3′-to-5′ progression, reminiscent of the unexpected editing that has been shown to occur frequently in T. brucei mRNAs edited in vivo. This unexpected editing is most likely due to alternate mRNA:guide RNA (gRNA) alignment forming a hyphenated anchor; its having only a 2-bp proximal duplex helps explain the prevalence of unexpected editing in vivo. Such unexpected editing was not previously reported in vitro, presumably because the common use of artificially tight mRNA:gRNA base pairing precludes alternate alignments. The multicycle editing and unexpected editing presented in this paper bring in vitro reactions closer to reproducing the in vivo editing process.
RNA editing in Trypanosoma brucei and related organisms is a posttranscriptional maturation process where U residues (U's) are added and removed in sequential editing cycles, at up to many hundred separate sites in a single mRNA (reviewed in references 47 and 50). This editing is directed by mismatches in base pairing between the pre-mRNA and trans-acting guide RNAs (gRNAs) (whose pairing involves G:U as well as Watson-Crick interactions) (5). Unpaired U's in the pre-mRNA become deleted, while unpaired purines in the gRNA guide the insertion of U's into the pre-mRNA, to make the mRNA complementary to the gRNA. The gRNAs contain an anchor region that base pairs with a 3′ mature sequence in the substrate mRNA, forming an anchor duplex, whose upstream terminus specifies the editing site. The gRNA also has a central guiding region, which specifies the number of U's to be inserted or deleted at up to ∼10 editing sites, and a 3′ oligo(U) tether, which provides pairing with a not-yet-edited pre-mRNA sequence that consists largely of A's and G's. Multiple gRNAs are used sequentially across large editing domains (33).
The U insertion or U deletion cycle at each editing site involves three enzymatic reactions (5) catalyzed by proteins that have several nomenclatures (reviewed in references 30 and 50). First the mRNA is cleaved just upstream of the anchor duplex by an endonuclease (TbMP90 [KREN1] for U deletion, TbMP61 [KREN3] for U insertion [8, 54], or TbMP67 [KREPB2/KREN3] for U insertion with a gRNA in cis [9]). Then, at the newly generated 3′ end, unpaired U's are removed by a U exonuclease (3′-U-exo) (TbMP99 [KREPC2] [43] and/or TbMP100 [KREPC1] [25, 55] and/or possibly TbMP42 [KREPA3; band VI] [7]); alternatively, U's are added by a terminal U transferase (TUTase) (TbMP57 [KRET2] [2, 17]). Finally, the mRNA is rejoined by RNA ligases (34, 39, 40, 42) (TbMP52 [KREL1; band-IV] for U deletion and generally TbMP48 [KREL2; band V] for U insertion [15, 21, 43]). After completing an editing cycle, the anchor duplex can extend up to the next mismatch, and the next editing cycle can begin (5). Thus, editing would progress sequentially 3′ to 5′ across the mRNA (33), which is generally supported by analysis of partially edited mRNAs (for example, see reference 1).
These reaction cycles are catalyzed by ∼20S editing complexes that contain up to ∼20 identified proteins, including the above-noted enzymes and additional required factors that coordinate the reactions (reviewed in references 30 and 50). However, the relative stoichiometry of these proteins remains to be determined and might even vary in different cell lines (see references 30 and 56), potentially explaining why some preparations of editing complexes exhibit ∼20 major proteins (35, 50), while others exhibit only ∼7 major proteins (39, 43) yet are at least as active in catalyzing editing cycles (14, 15).
Models have generally envisioned that an editing complex moves processively along the pre-mRNA, sequentially acting on its numerous editing sites. Although this seems more efficient than the editing complex dissociating and rebinding between editing cycles, intriguing newer data indicating that the TbMP90 U-deletional and TbMP61 U-insertional endonucleases are present in different editing complexes (9, 36) suggest that these complexes dissociate from the mRNA:gRNA substrate during editing, at least between the highly intermingled U deletion and U insertion sites. It is even possible that editing complexes dissociate from the RNA between each editing cycle; however, until now, this has not been investigated.
The initial in vitro editing systems (24, 44, 45) utilized the 3′ portion of T. brucei ATPase 6 (A6) pre-mRNA (4), whose processing begins with a U deletion at editing site 1 (ES1) followed by a U insertion at ES2 (this ES2 processing was recreated in vitro by using an mRNA substrate already edited at ES1 [24]). Almost all subsequent studies have focused on these single A6 editing cycles, using gRNAs designed to direct either the ES1 U deletion cycle or the ES2 U insertion cycle (for examples, see references 14 and 15), or parts of such cycles (for examples, see references 22, 23, 29, and 30). Thus, those studies did not attempt to reproduce the progression of multicycle editing. Indeed, we are aware of no publication demonstrating more than one editing cycle catalyzed in vitro. Yet achieving multicycle editing would seem important in assessing whether editing complexes are progressive or dissociate between cycles and would represent progress toward recreating the complete editing process in vitro.
Although studies of in vitro editing have emphasized the accuracy of this process, a large fraction of mRNAs in vivo are “unexpectedly” edited, where sites appear incorrectly processed or not utilized in a strict 3′-to-5′ order or possibly were accurately edited but did not use the expected gRNA alignment (16, 18, 27, 37, 51-53). This unexpected editing seems surprisingly common in vivo, as it occurs in over 90% of steady-state T. brucei COIII mRNAs (16, 18) and frequently also in other mRNAs (1, 3, 27, 37, 51-53). Insightful sequence comparisons suggest that at least some of this unexpected editing arises from inappropriately aligned gRNAs, likely using anchor regions that are not perfect duplexes but have hyphenated pairing (27, 51-53), and that gRNAs might then progressively realign (27). Beyond these early investigations and a subsequent study showing that TUTase can add U's at what should be U deletion sites (56), unexpected editing has not been further investigated. Indeed, in vitro editing generally utilizes extensively base-paired substrates with only one possible editing site, which should disfavor such unexpected editing.
We here report achieving more than one cycle of editing in vitro. Two neighboring U deletion sites are acted upon in concert, with the second editing cycle substantially more productive, initiating more rapidly, and responsive to different gRNA features, relative to a single editing cycle. Our data indicate that this pre-mRNA utilizes the editing complex and gRNA in a processive manner and they do not dissociate between these editing cycles. Furthermore, the multicycle in vitro editing substrate, which is more like natural editing substrates, in having an appreciable region of mis-pairing, also supports unexpected editing, which appears very commonly in vivo.
MATERIALS AND METHODS
Cells and extract preparation.
From T. brucei procyclic strain TREU-667, traditional mitochondrial extract (∼2.5 × 1010 cell equivalents/ml) (19, 41) was prepared, and the editing complex was purified as described previously (39).
RNA substrates.
Pre-mRNAs and gRNAs are designated as described in Cruz-Reyes et al. (12, 14) and shown in Fig. 1 (see also Fig. 3). The mRNAs were synthesized without PCR as described previously (26), using 5 μM of cDNA oligonucleotide containing a T7 promoter annealed with 5 μM of T7 primer (10 min at 72°C and then slow cooled to 37°C) and incubated with 0.8 mM nucleoside triphosphate mix (Roche) and 250 U of T7 RNA polymerase (USB) for 2 h at 37°C. The oligonucleotides were as follows: T7 primer (5′-GTAATACGACTCACTATA-3′), cDNAs for mRNAs (5′-GATGCCAGGTAAGTATTCTA TAACTCC* ATAACACAAC TTTCCCTTTC TTCTCTCCTC CCCCTAACCT TTCCTATAGT GAGTCGTATT AC-3′, where “*” indicates AAA for m[2,4] and nothing for m[2,1]), and cDNAs for gRNAs (5′-AGGAAAGT* AATGGAGTTA TAGTATATCC TATAGTGAGT CGTATTAC-3′, where “*” indicates TAT for D31, ATC for D31a, TTATAA for D34UU, and TGT for D31c; also, for D31a, residue number 1 was a G). RNAs were gel isolated, and mRNAs were then 3′-end labeled as described previously (11).
FIG. 1.
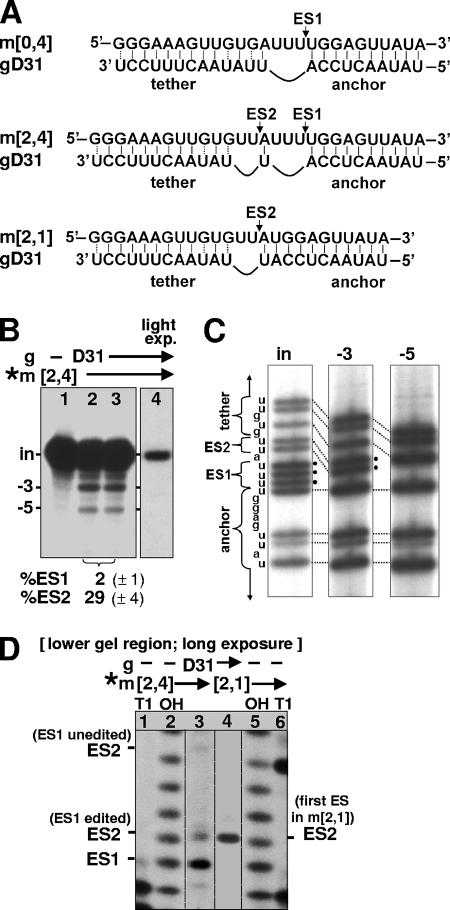
Two cycles of U deletion. (A) mRNAs (upper rows) and gRNA (lower rows) used in the in vitro editing reaction. The mRNAs are designated by “m” followed by the number of U's present at ES2 and ES1, respectively, while gRNAs for U deletion are designated by “gD” followed by their nucleotide length, as designated by Cruz-Reyes et al. (12, 14). The mRNA m[0,4] (which has no U's at ES2 and four U's at ES1) is a 72-nt segment from the 3′ region of the natural T. brucei A6 pre-mRNA (12) (it is basically the A6/TAG RNA described by Seiwert and Stuart [44] with its 5′ portion further shortened as described in Seiwert et al. [45]). gD31 is gD30CC (14) with an additional 3′ U, a simplified version of a natural gRNA that from m[0,4] directs deletion of the three unpaired U's (of the four U's present) at ES1 (14) (top). The mRNA m[2,4] contains an additional two U's at ES2 that do not pair with gD31, so this gRNA theoretically could direct deletion of the two U's at ES2 as well as the three U's at ES1 (middle). The mRNA m[2,1] (shown in Fig. 1D and 2) contains two U's at ES2 and one U at ES1, so it is effectively already edited at ES1; it fully base pairs with gD31 at ES1 and thus should exhibit U deletion of only the two unpaired U's at ES2 (bottom). The ES1 and ES2 cleavage sites are indicated by arrows. The lines at the 5′ and 3′ ends of the RNAs represent the following: for pre-mRNAs, 5′-GGAAAGGUUAGGGGGAGGAG AGAAGAAA and ACCUGGCAUC-3′, and for gRNAs, 5′-GGAUAUAC. (B) In vitro editing of radiolabeled (*) m[2,4], using gD31, showing input m[2,4] (in) and the −3 and −5 RNAs (that have lost three and five U's in one or two cycles of U deletion, respectively). Lanes 2 and 3 are duplicate reactions. The values of the measured extent (%) of editing at ES1 and ES2 (see Materials and Methods) are shown at the bottom of the panel along with the standard deviations of those values in parentheses. Lane 4 is a much lighter exposure of lane 3, selected to show the substrate for ES1 editing (the input RNA) as slightly more intense than the substrate for ES2 editing (the −3 band) in lane 3; yet the product from ES2 editing (the −5 band) is readily visible in lane 3, while the product from ES1 editing (the −3 band) is not observed in lane 4. (C) Sequencing (T tracks) of the major class of cDNAs cloned using RNA isolated from gel bands of the indicated sizes, as shown in panel B. The bands in this sequencing represent positions that were U's in the RNA. The anchor and tether regions and the U's to be deleted at ES1 and ES2 are indicated. Dotted lines indicate corresponding residues in the different-length molecules; those residues marked by black circles are deleted in the lane on the right. (D) Cleavage products from double-round U deletion reaction. The lower region of an editing gel, as shown in panel B, using the indicated RNAs (exposure ∼3-fold longer than is optimal for the upper region of the gel). Sizing markers were from each mRNA treated with RNase T1 or with hydroxide (lanes 1, 2, 5, and 6) to generate a G ladder and a nucleotide ladder of that RNA (12, 56). (Since the editing cleavage leaves a 5′ P and the markers a 5′ OH, the bands do not precisely align [references 12, 30, and 56 and references therein].) The cleavage at ES2 of m[2,4] that has already been edited at ES1 generates a band that is 1 nt longer than the band from the cleavage at ES1; the cleavage at ES2 of m[2,4] that is unedited at ES1 generates a band that is 4 nt longer than the band from the cleavage at ES1 (A).
FIG. 3.
Effect of gRNA pairing on the coupled editing cycle. (A) m[2,4] paired to gRNA variants that increase (gD31a and gD34uu) or decrease (gD31c) the single-stranded character adjoining ES2. Also shown is the measured extent of editing at ES2 and the standard deviations of those values. (B) Editing reactions as shown in Fig. 1B, using the indicated RNAs.
Editing reactions and sequencing analysis.
The mRNA (∼30 fmol) and gRNA (1.25 pmol) were preannealed as described previously (12, 13). Twenty-microliter U deletion reaction mixtures were in 10 mM KCl-MRB buffer (15) supplemented with 3 mM ADP (Sigma), 3 mM ATP (Sigma), 5 mM CaCl2, 20 U RNase inhibitor (Promega), and 10 mM dichlorodiphenyltrichloroethane (Invitrogen), plus 1 μg of mitochondrial extract (or an amount of purified editing complex [39] that has a similar level of ligase activity), and were incubated at 28°C for 45 min, unless otherwise indicated. Product RNAs were analyzed on 1-m-long, 9% polyacrylamide/7.5 M urea gels in Tris-borate-EDTA. To sequence editing products, the isolated gel bands were used for reverse transcription-PCR as described previously (11) with A6-RT (44) and T7A6 short primers (12, 45), and the products were cloned into pCR2.1-TOPO vector using the TOPO TA cloning kit (Invitrogen), analyzed with the Sequenase version 2.0 DNA sequencing kit (USB), using [α-32P]dATP and dideoxyadenosine triphosphate to terminate extension at each U, and resolved on 6% polyacrylamide/7.5 M urea gels in Tris-borate-EDTA.
Data analysis.
To calculate the percentage of editing, each autoradiogram was scanned using a FluorChem 8000 advanced fluorescence, chemiluminescence and visible light imaging system with AlphaEaseFC software and analyzed within the linear range. The editing extent at a site, (P)/[(S) + (P)], was calculated where P is the band intensity or summed band intensities of the product from editing at the site and S is the band intensity of the substrate for editing at the site (input mRNA for the first editing cycle and the −3 RNA for the second editing cycle in the double-round U deletion). Values were averaged for multiple replicated experiments and are given with their standard deviations.
RESULTS
U deletion in vitro.
We attempted double-round editing reactions in vitro using mRNA m[2,4] and gRNA gD31 (Fig. 1A, middle; see the legend for a description of the mRNA and gRNA designations). m[0,4] is the 3′ portion of the natural T. brucei A6 pre-mRNA, and m[2,4] is a derivative that additionally contains two U's at ES2 and thus has U deletion sites at both ES1 and ES2. gD31 is from a family of gRNAs commonly used to direct deletion in vitro of three U's at ES1 in m[0,4] (14) (Fig. 1A, top). With m[2,4], gD31 should direct the same −3 product in a first editing cycle, and this product might then be a substrate for deletion of the two U's at ES2 (Fig. 1A, middle), which would yield a final −5 U product from the two editing cycles. In the in vitro reaction with m[2,4] and gD31, both −3 and −5 products were indeed observed when catalyzed by T. brucei mitochondrial extract or by a purified editing complex (Fig. 1B, lanes 2 and 3, and data not shown). Sequence analysis confirmed that the major −3 RNA band represents a three-U deletion at ES1 and the major −5 RNA band represents that deletion plus an additional two-U deletion at ES2 (Fig. 1C). Thus, a double-round U deletion reaction has been achieved for the first time in vitro.
In the lower portion of the editing gel are faint bands representing small amounts of cleaved products from this pre-mRNA (which is 3′-end labeled) that were not rejoined into full-length molecules (Fig. 1D, lane 3). (These bands are best seen when the autoradiogram is exposed severalfold longer [Fig. 1D] than it is to examine the full-round editing reactions [Fig. 1B].) The strongest of these lower bands corresponds to pre-mRNA that was cleaved at the first editing site, which is ES1 in the double-round reaction using m[2,4] and gD31 (Fig. 1D, lane 3; sequence in Fig. 1A; reaction analogous to lanes 2 and 3 of Fig. 1B). When input RNA m[2,1] that already has the edited sequence at ES1 is used instead, its first (and only) editing site is ES2 (Fig. 1A), and RNA cleaved at that position is observed (Fig. 1D, lane 4). Notably, that ES2 cleavage band provides a size marker for RNA from the double-round reaction that has undergone a U deletion at ES1 in a first editing cycle and subsequently is cleaved at ES2, initiating the second editing cycle. Indeed, that mRNA fragment is also present (Fig. 1D, lane 3). There is only a much weaker band corresponding to the double-round mRNA cleaved at ES2 without ES1 having already been edited (Fig. 1D, lane 3). These observations are consistent with the double-round U deletion reaction generating −5 product mainly from faithful editing first at ES1 and then at ES2 (Fig. 1A). Furthermore, the substantially lower intensity of the mRNA cleaved at ES1 or ES2 (Fig. 1D), relative to the mRNAs that have been ligated to full-length molecules, attests to the efficient completion of these editing cycles, once initiated. Thus, analysis of the cleaved mRNA fragments supports the understanding of the double-round editing reaction.
When examining the complete editing cycles from the double-round reaction shown in Fig. 1B, it is striking that the second editing cycle was about 15-fold more efficient than the first editing cycle. (This is calculated from quantitation of the −5 versus the −3 RNA bands and of the −3 versus the input RNA bands, respectively [see Materials and Methods].) Fig. 1B shows the average values of the percent editing at ES1 and at ES2 (which generate the −3 RNA from the input RNA and the −5 product from its −3 substrate, respectively), as well as the standard deviations of these determinations scored from parallel reactions. Notably, the second cycle of editing appears much more efficient than all reported examples of single-round editing reactions (for examples, see references 14 and 45) (except those using an ∼100-fold enhanced gRNA [14, 54] and subsequent studies using this gRNA pairing). To visually confirm that the second cycle is much more efficient than the first, we compared Fig. 1B, lane 3, with a light exposure of the same gel lane (Fig. 1B, lane 4). The light exposure was selected so that it shows the substrate for the first editing cycle (the input RNA) approximately as intensely as the substrate for the second editing cycle (the −3 RNA) was shown in the original exposure. Yet the product from the first editing cycle (the −3 band) is not visible in the light exposure (lane 4), while the product from the second editing cycle (the −5 band) is readily visible in the original exposure (lane 3). We conclude that this second editing cycle, when following the first cycle, is markedly more efficient than the first editing cycle.
Two coupled cycles of editing.
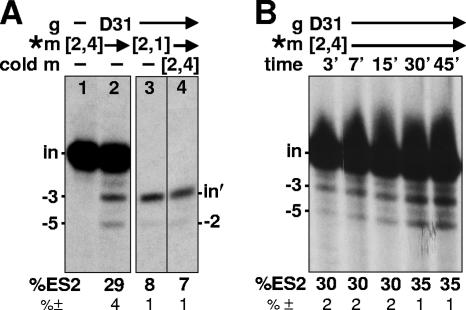
The second editing cycle shown in Fig. 1B could be more efficient than the first for at least two reasons: (i) the two cycles could be coupled, so that the second cycle occurs rapidly upon completion of the first, and/or (ii) editing at the second site could be extremely efficient by itself. To address these possibilities, the experiment shown in Fig. 2A examined the relative efficiencies of U deletion at ES2 when it is the second editing cycle in a double-round reaction (using m[2,4]; lane 2) versus when it is a single-round reaction (using m[2,1]; lane 3). (This m[2,1] substrate [Fig. 1A] has the sequence of the −3 U deletion product that is generated in the first editing cycle of the double-round reaction.) To compare the efficiencies, the gel from the single-round ES2 reaction (Fig. 2A, lane 3) is shown as a very light exposure so that the bands representing the substrate for editing at ES2 are similarly intense from the single-round reaction (the m[2,1] input mRNA, labeled “in′”; lane 3) and from the double-round reaction (the −3 band of lane 2). The band representing editing at ES2 is considerably more intense from the double-round reaction (the −5 band in lane 2) than from the single-round reaction (the −2 band in lane 3). This comparison reveals that U deletion at ES2 is considerably more efficient when its substrate is generated by a previous U deletion cycle in the same reaction (lane 2) than when it occurs by itself (lane 3). This finding strongly suggests that the second editing cycle is coupled to the first editing cycle in the double-round reaction.
FIG. 2.
Coupled U deletion cycles. (A) Editing reactions as shown in Fig. 1B, using the indicated RNAs. Lane 3 is a light exposure that shows the substrate for ES2 editing (the m[2,1] input mRNA [in′]) at approximately the same intensity as that of the substrate for ES2 editing in lane 2 (the −3 product from the editing of m[2,4] at ES1). Lane 4 is a normal exposure of a gel lane containing a reaction that used 3% of the input mRNA as radiolabeled (*) m[2,1] and 97% as unlabeled (cold) m[2,4], so its input RNA band is similar in intensity to that shown in lane 3. (B) Kinetics of the editing reaction, with parallel reactions terminated at the indicated times. Note that the substrate and product of ES2 editing in the double-round reaction (the −3 and −5 RNAs, respectively) accumulate in parallel, with an approximately constant percentage of ES2 editing observed at the various times, indicating that ES2 editing occurs rapidly, while ES1 editing occurs more slowly. The measured extent of editing at ES2 (see Materials and Methods) and the standard deviations of those values are shown at the bottom of both panels.
One potential caveat with the previously described experiment is that the single-round reaction with the m[2,1] (Fig. 2A, lane 3) might conceivably saturate the editing capacity of the editing complex in the in vitro reaction. We therefore set up a reaction with radiolabeled m[2,1] comprising only the approximate amount that was generated in the double-round reaction, with the balance of the input mRNA provided by unlabeled m[2,4] (Fig. 2A, lane 4). The quantitated efficiency of ES2 editing when it is the only editing site (in radiolabeled m[2,1]) is similar whether the input mRNA is entirely m[2,1] (lane 3) or mainly m[2,4] (lane 4). Finding that the efficiency of editing at ES2 is considerably higher when its substrate is generated by editing at the adjacent editing site in the same reaction (Fig. 2A, lane 2) than when it is conducted as a single-round reaction (lane 3, 4) confirms that the efficiency of editing at ES2 is augmented in the double-round reaction where it follows editing at the adjacent ES1. This reinforces that the two editing events are coupled.
If editing at ES2 is coupled to editing at ES1 in the double-round reaction, one might expect that it could occur rapidly, so that the percentage of the −3U substrate that is edited to the −5U final product might start out high and remain relatively constant over time. In contrast, if the protein complex released and rebound the mRNA between the two editing cycles, the percentage of editing in the second cycle should increase slowly over time, as is observed for single editing cycles (for examples, see references 11 and 45). As seen in the quantitation of a reaction time course (Fig. 2B), the extent of editing in the second cycle is high at the earliest time point and remains relatively constant at longer times of reaction. In contrast to this relatively constant ∼30 to 35% editing efficiency, the efficiency of editing at ES1 increases substantially over time (Fig. 2B), although it remains considerably less than one-tenth that at ES2. Thus, most editing at ES2 occurs rapidly following the editing of ES1 in that molecule, substantially faster than the shortest time point examined. We interpret these data (see Discussion) to indicate that editing at ES2 is closely coupled to that at ES1, rather than being established independently.
gRNA features in the coupled editing cycles.
We wanted to examine whether the second, coupled U deletion cycle is responsive to the same gRNA features that were earlier demonstrated to affect the single-round U deletion reaction (14). It has been shown not only that U deletion in a single editing cycle requires mRNA:gRNA base pairing in the anchor and tether regions but that its efficiency can be modulated up (or down) over a 100-fold range by increasing (or decreasing) the single-stranded character of the mRNA and/or the gRNA strands within a few nucleotides upstream of the editing cleavage site (14). To compare the importance of single-strand character in the second editing cycle, we examined analogous changes adjoining the ES2 site of the double-round substrate (Fig. 3). These reactions utilized variants of gD31 (Fig. 3A, row 1). Notably, substantial additional single-stranded character is provided by gD31a (Fig. 3A, row 2) or gD34uu (Fig. 3A, row 3), but those gRNAs did not increase the editing at ES2; if anything, they decreased the efficiency of this coupled editing cycle (Fig. 3A and B, lanes 2 to 4). Thus, while additional single-strand character greatly increases U deletion efficiency in the single-round reaction (14), it does not have this effect on the coupled editing cycle. Furthermore, tightening base pairing just upstream of ES2, using gD31c, had little effect on reaction efficiency, leaving it at least as high as with gD31a or gD34uu (Fig. 3A, row 4; compare to rows 2 and 3; Fig. 3B, lane 5), while an analogous change in a single-round reaction greatly decreased editing efficiency (14). Therefore, we conclude that the second cycle of U deletion in the double-round editing reaction does not exhibit the same gRNA requirements as a single-round U deletion reaction. This could be because the substrate RNA does not need to be independently recognized for the coupled editing cycle and such upstream single-strand character is important for that recognition.
DNA sequence analysis of partial products from the double-round U deletion in vitro.
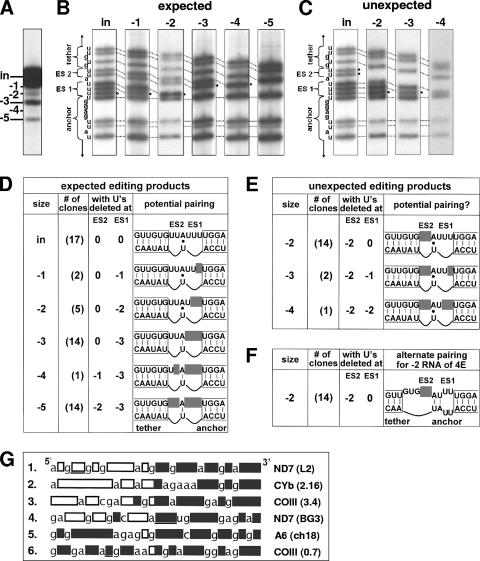
Sequencing of cDNAs cloned from isolated gel bands of the double-round U deletion reaction (Fig. 4A) showed a surprising diversity. Of 53 sequenced clones that exhibit editing, two-thirds had sequences expected if U deletion occurs first at ES1 and only subsequently at ES2 (Fig. 4B and D) and if editing progresses in a strictly 3′-to-5′ direction (1, 5) across the pre-mRNA, aligned to the gRNA as shown in Fig. 1A and 4D. However, one-third of the edited cDNAs had sequences that were unexpected from that understanding of editing, since U's were removed at ES2 before complete U removal at ES1 (Fig. 4C and E). Cleaved mRNAs consistent with such an unexpected order of editing were also observed (Fig. 1D). These editing products appear counterintuitive to the progressive editing model (Fig. 4E, diagram), where the anchor duplex should not extend up to ES2 until after editing at ES1 has been completed (Fig. 4D). Thus, processive editing using the mRNA:gRNA pairing scheme shown in Fig. 1A (and reproduced in Fig. 4D and E) should not initiate at ES2 to generate the 17 mRNAs reported in Fig. 4E, where ES2 is edited but ES1 has not been fully edited. Notably, these unexpected editing products, which include three quarters of the sequenced −2-size RNAs and half of the sequenced −4-size RNAs, as well as a smaller fraction of the sequenced −3-size RNAs, all show U deletion at ES2 without prior complete U deletion at ES1 and thus evidently reflect the same unexpected editing progression (compare Fig. 4D and E). However, the fact that all these molecules had the same U deletion at ES2 indicates they were not spurious but rather guided events. Since everything known about the cleavage that initiates the editing cycle indicates it occurs precisely at the upstream border of a duplex region (summarized in reference 49), the cleavage that initiated this common class of unexpected editing products was presumably guided by an alternate mRNA:gRNA pairing of a few residues flanking the same editing site. A pairing that could direct that cleavage and editing is shown in Fig. 4F (see also Discussion). This alternate pairing would generate a false anchor to direct cleavage at ES2 when ES1 is still unedited (Fig. 4F), and completion of this editing cycle would create an mRNA with sites that appear to have been edited out of order. Similarly, in the published sequences of mRNAs that were edited in vivo (16, 27, 51, 53), while some molecules are, as expected from faithful editing, progressing 3′ to 5′ along the pre-mRNA (Fig. 4G, rows 1 and 2), many molecules show unexpected editing patterns, including what appears to be such out-of-order progression between editing sites (Fig. 4G, rows 3 to 6). Thus, the assortment of unexpected editing products we observed for reactions with the double-round U deletion substrate (Fig. 4E and F) provides an in vitro prototype for the common unexpected editing that occurs in vivo.
FIG. 4.
Expected and unexpected edited sequences. (A) Gel of double-round U deletion reaction products from m[2,4] and gD31, showing the bands that were cloned as cDNAs. (B and C) Sequencing of cDNAs cloned from editing products of the indicated sizes and comparing to the input size RNA, expanding on the data shown in Fig. 1C to show minor as well as major classes of products. (Fig. 1C showed only the most abundant kind of sequence from the three strongest bands (the input [in], −3, and −5 bands.) The resultant sequences were expected (B) or unexpected (C) from 3′-to-5′ editing of the intended mRNA:gRNA pairing. (D and E) Summary of the sequencing data, both the expected (D) and unexpected (E) editing products. Of the cDNAs with sizes indicated in the first column, the number of clones indicated in parentheses in the second column exhibited U deletion at ES2 and ES1 of the number of residues indicated in the third and fourth columns. (The total number of sequenced clones in each size class does not strictly correspond to the RNA abundance in panel A, which demonstrates that there are fewer −2 mRNAs than −3 mRNAs; however, since many −2 mRNAs represent the unexpected kind of editing, we sequenced more of those clones [hence, more −2-size clones than −3-size clones were sequenced].) The final column shows the sequence of the mRNA (upper rows), with the gray boxes representing deleted U's; the lower rows show the gRNA aligned to maximize pairing with the mRNAs. That pairing would be expected (D) or unexpected (E) to generate the observed editing products. A potential A:U base pair between ES1 and ES2 should form (|) after all three U's at ES1 have been removed (and thus it would help guide the −4 and −5 products in panel D) but should not form otherwise (•); yet, pairing of that mRNA residue should be needed to guide the observed U deletion at ES2 that occurs without that U deletion at ES1 (E). The RNAs extend in 5′ and 3′ directions for an additional 6 nucleotides, as shown in Fig. 1A. Note that although the unexpected editing at ES2 arises at an impressively high frequency, it is considerably less favored than the expected editing at ES1. (F) An alternate mRNA:gRNA alignment that could direct U deletion at ES2 without U deletion at ES1, using a hyphenated anchor, which involves the normal 10-bp anchor duplex plus a 2-bp proximal duplex separated by a 2-nucleotide symmetric bulge. The tether duplex in this mRNA:gRNA alignment contains 9 of the normal 12 bp. (G) Examples of reported in vivo mRNAs that exhibit partial editing. The data in rows 1, 4, and 5 are taken from Koslowsky et al. (27), and the data in rows 2, 3, and 6 are taken from Decker and Sollner-Webb (16); the particular RNA is shown on the right. Letters represent nucleotides that do not become edited; boxes represent U's that should be edited in the mature mRNA and were either edited (gray fill) or not edited (white fill) in that particular sequenced molecule. The editing is mainly U insertions, with U deletions indicated by underlined boxes. Some molecules exhibit the expected 3′-to-5′ progression of editing (rows 1 and 2), while many other molecules reflect unexpected editing, including sites that appear to be edited out of order (rows 3 to 6).
DISCUSSION
Since its discovery two decades ago (3, 18, 46), there has been great progress in understanding trypanosome RNA editing, much of it from in vitro studies that reproduce a single cycle of U deletion or U insertion or parts of a single cycle (reviewed in references 30, 32, 43, 47, and 50). However, to study the in vivo progression of editing, where single mRNAs can undergo hundreds of editing cycles (18), it is also important to reproduce multicycle editing in vitro. To the best of our knowledge, that has not yet been demonstrated. We here report two consecutive cycles of U deletion catalyzed in vitro (Fig. 1B). We used a variant of the common A6 pre-mRNA that contains U's at both ES1 and ES2 (Fig. 1A) and a commonly used cognate gRNA to direct products that were visualized on gels and verified by sequencing cDNAs cloned from the product gel bands (Fig. 1C). Importantly, in this reaction, the second editing cycle is catalyzed about 15-fold more efficiently than the first cycle (Fig. 1B), and control studies reveal that ES2 itself, in a single-round reaction, is not a similarly favorable substrate (Fig. 2A); rather, the efficiency of the single-editing cycle arises from being part of a multiround editing reaction. This indicates that the editing complex acts on these sequential sites in a concerted manner, most likely because it can remain associated with the substrate RNA and does not need to dissociate and rebind between these sequential editing cycles.
In addition to being more efficient, the second editing cycle appears different from the initial editing cycle in at least two other ways. The first concerns the relative speed of the two editing cycles in the double-round reaction. The subsequent U deletion cycle at ES2 is already virtually at its maximal efficiency at the earliest time point (3 min) (Fig. 2B), while the efficiency of the initial editing cycle slowly increases over time (for at least 45 min) (Fig. 2B), much like what has been shown for a single-round editing reaction (11, 45). Thus, molecules that undergo this second cycle of editing generally do so rather quickly (<3 min), after the substantially slower first cycle has taken place; hence, the second U deletion cycle is coupled to the first. The rapid execution of the second editing cycle further suggests that the actual catalysis of in vitro U deletion is quite rapid and that the slower kinetics of the first U deletion cycle likely reflect the time required for the editing complex to productively associate with the substrate RNA. That would imply that such a de novo association step is not required for the second U deletion cycle, presumably because the editing complex can remain with the RNA between these sequential editing cycles. Another difference between the initial and second editing cycles is how gRNA features affect the U deletion efficiency (Fig. 3). While the U deletion efficiency in a single-round reaction is modulated over 100-fold depending on the extent of single-strand character within a few nucleotides upstream of the editing site (14), equivalent changes at the coupled second editing site do not show such effects (Fig. 3) and can even cause a small response in the opposite direction (e.g., gD31a) (Fig. 3). This single-strand character at the first editing site is thought to favor functional recognition by the editing complex (14, 49), so its relative unimportance at the coupled editing site implies that recognition by the editing complex is different. This could be expected if the editing complex does not dissociate from the substrate RNA between the sequential U deletion cycles; hence, it would not need to search out the second editing site de novo.
Although this concerted editing occurs whether catalyzed using mitochondrial extract or purified complex, suggesting that only components of the recognized editing complex and not separate factors are needed for the observed progression between adjacent editing sites, additional components could favor the progression. It also remains possible that between consecutive cycles of U deletion and U insertion, editing may not be concerted, as predicted if different kinds of editing complexes catalyze cleavage in U deletion and U insertion (9, 36, 54). In previous in vitro editing studies where the gRNA could have directed a U insertion cycle following either a previous U deletion cycle (45) or a previous U insertion cycle (24), only the first and not the second editing cycle was observed; however, the rather limited efficiency of in vitro U insertion may have precluded the detection of coupled cycles. We have also devoted considerable effort toward a double-round in vitro reaction involving one U deletion and one U insertion, but the results were inconclusive. Thus, further analyses beyond the scope of this paper will be required to discern whether concerted editing cycles can involve U insertion.
The most surprising aspect of RNA editing, besides its existence, is the very large percentage of in vivo-edited mRNAs that exhibit sequences which are unexpected from a 3′-to-5′ progression of editing across the pre-mRNA when paired as expected with its cognate gRNA (1, 3, 16, 27, 37, 51-53). For instance, ≥90% of steady-state T. brucei COIII mRNAs are unexpectedly edited (combined data from references 16 and 18). It is unclear whether these RNAs can later be edited to achieve the mature sequence (12, 27). The abundance of such unexpectedly edited products in vivo appears to correlate with the prevalence of editing in the genome of that species (16, 28, 51), likely reflecting the number of potential gRNAs that could mis-pair and possibly suggesting why U deletion/insertion editing is restricted to the mitochondrion, which has only a few genes and is not found in nuclei. These unexpectedly edited sequences in cellular RNA have been suggested to arise largely from editing using misaligned gRNAs and may involve hyphenated duplexes as anchor regions (27, 51-53). However, when analyzing in vivo RNAs, it is important to remember that T. brucei contains vast numbers of gRNAs, many not yet sequenced, and there are often multiple gRNAs with somewhat different sequences for a single mRNA region (for examples, see references 10 and 38), so it remains unclear which gRNA actually directed the unexpected editing events observed in vivo; hence, it remains a hypothesis whether a hyphenated duplex actually served as an anchor (53). Furthermore, if a hyphenated duplex does provide an anchor, it remains unclear how small the proximal duplex abutting the editing site can be and still have function. In vitro editing reactions, on the other hand, contain a single added gRNA and mRNA, considerably shorter than the natural RNAs, generally with a strongly pairing anchor and tether duplexes and only a small unpaired region in between (for examples, see references 14, 22, and 23) (Fig. 1A), which virtually preclude alternate pairing. Indeed, unexpected editing has not been the focus of any in vitro study. Now finding an unexpected editing product that is generated relatively frequently in vitro (Fig. 4C and E) (although not nearly as frequently as the expected editing product [compare to Fig. 4A]) indicates it is not spurious but instead was guided, evidently by the added gRNA. It thus is very likely that the frequent unexpected editing of the double-round substrate (Fig. 4C and E) was guided by alternate mRNA:gRNA pairing, as shown in Fig. 4F. Cleavage at ES2 would occur using a hyphenated anchor region with a proximal duplex of only two base pairs; this should form at least transiently, since the strands are held in proximity by abutting tight base pairing of the normal anchor duplex (Fig. 1A and 4F). Once this editing of ES2 has occurred (generating the −2 mRNAs sequenced in Fig. 4E), the mRNA:gRNA pairing could then realign to favor editing at ES1 and generate the −3 and −4 RNAs sequenced in Fig. 4E, analogous to the progressive gRNA realignment suggested to occur in vivo (27). One other report in the literature (31) appears to show the cleavage step of editing directed by a hyphenated anchor, with evidently a 4-base-pair proximal duplex separated from a longer tight duplex by a 1-nucleotide bulge, but this was not stressed or pursued. Notably, a hyphenated anchor with a proximal duplex of only 2 base pairs being sufficient to direct appreciable levels of full-cycle editing (Fig. 4F) could certainly contribute to the high frequency of unexpectedly edited products observed in growing trypanosomes.
Trypanosome mitochondria also exhibit several additional kinds of nonproductive editing-related reactions, whose in vitro study has aided in understanding the editing process. These include formation of gRNA:mRNA chimeras, originally thought to be editing intermediates (6) but subsequently found to be quite rare (38) and shown by in vitro analysis (20) to arise from mis-editing (24, 39, 45). Other nonproductive products arise from TUTase adding U's at U deletion sites, as demonstrated in vitro but which evidently also occurs in vivo (56). Just as the in vitro reproduction of faithful single rounds of U deletion or U insertion proved important to studying the editing cycle, it is hoped that an ability to reproduce in vitro faithful multicycle editing and unexpected editing will also lead to a better understanding of the complete editing processes.
Acknowledgments
We thank Paul Englund, Terry Shapiro, Julie Law, Catherine Huang, SoHee Lee, and Sean O'Hearn for helpful discussions, as well as Paul Englund and Julie Law for suggestions on the manuscript. We also thank the reviewers for helpful suggestions.
This work was supported by Public Health Service grant GM 34231 from the NIH.
Footnotes
Published ahead of print on 28 January 2008.
REFERENCES
- 1.Abraham, J. M., J. E. Feagin, and K. Stuart. 1988. Characterization of cytochrome c oxidase III transcripts that are edited only in the 3′ region. Cell 55267-272. [DOI] [PubMed] [Google Scholar]
- 2.Aphasizhev, R., I. Aphasizheva, and L. Simpson. 2003. A tale of two TUTases. Proc. Natl. Acad. Sci. USA 10010617-10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benne, R., J. Van den Burg, J. P. Brakenhoff, P. Sloof, J. H. Van Boom, and M. C. Tromp. 1986. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell 46819-826. [DOI] [PubMed] [Google Scholar]
- 4.Bhat, G. J., D. J. Koslowsky, J. E. Feagin, B. L. Smiley, and K. Stuart. 1990. An extensively edited mitochondrial transcript in kinetoplastids encodes a protein homologous to ATPase subunit 6. Cell 61885-894. [DOI] [PubMed] [Google Scholar]
- 5.Blum, B., N. Bakalara, and L. Simpson. 1990. A model for RNA editing in kinetoplastid mitochondria: “guide” RNA molecules transcribed from maxicircle DNA provide the edited information. Cell 60189-198. [DOI] [PubMed] [Google Scholar]
- 6.Blum, B., N. R. Sturm, A. M. Simpson, and L. Simpson. 1991. Chimeric gRNA-mRNA molecules with oligo(U) tails covalently linked at sites of RNA editing suggest that U addition occurs by transesterification. Cell 65543-550. [DOI] [PubMed] [Google Scholar]
- 7.Brecht, M., M. Niemann, E. Schluter, U. F. Muller, K. Stuart, and H. U. Goringer. 2005. TbMP42, a protein component of the RNA editing complex in African trypanosomes, has endo-exoribonuclease activity. Mol. Cell 17621-630. [DOI] [PubMed] [Google Scholar]
- 8.Carnes, J., J. R. Trotter, N. L. Ernst, A. Steinberg, and K. Stuart. 2005. An essential RNase III insertion editing endonuclease in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 10216614-16619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carnes, J., J. R. Trotter, A. Peltan, M. Fleck, and K. Stuart. 22 October 2007. RNA editing in Trypanosoma brucei requires three different editosomes. Mol. Cell. Biol. doi: 10.1128/MCB.01374-07. [DOI] [PMC free article] [PubMed]
- 10.Corell, R. A., J. E. Feagin, G. R. Riley, T. Strickland, J. A. Guderian, P. J. Myler, and K. Stuart. 1993. Trypanosoma brucei minicircles encode multiple guide RNAs which can direct editing of extensively overlapping sequences. Nucleic Acids Res. 214313-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Reyes, J., and B. Sollner-Webb. 1996. Trypanosome U-deletional RNA editing involves guide RNA-directed endonuclease cleavage, terminal U exonuclease, and RNA ligase activities. Proc. Natl. Acad. Sci. USA 938901-8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz-Reyes, J., L. N. Rusche, K. J. Piller, and B. Sollner-Webb. 1998. T. brucei RNA editing: adenosine nucleotides inversely affect U-deletion and U-insertion reactions at mRNA cleavage. Mol. Cell 1401-409. [DOI] [PubMed] [Google Scholar]
- 13.Cruz-Reyes, J., L. N. Rusche, and B. Sollner-Webb. 1998. Trypanosoma brucei U insertion and U deletion activities co-purify with an enzymatic editing complex but are differentially optimized. Nucleic Acids Res. 263634-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz-Reyes, J., A. Zhelonkina, L. Rusche, and B. Sollner-Webb. 2001. Trypanosome RNA editing: simple guide RNA features enhance U deletion 100-fold. Mol. Cell. Biol. 21884-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruz-Reyes, J., A. G. Zhelonkina, C. E. Huang, and B. Sollner-Webb. 2002. Distinct functions of two RNA ligases in active Trypanosoma brucei RNA editing complexes. Mol. Cell. Biol. 224652-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decker, C. J., and B. Sollner-Webb. 1990. RNA editing involves indiscriminate U changes throughout precisely defined editing domains. Cell 611001-1011. [DOI] [PubMed] [Google Scholar]
- 17.Ernst, N. L., B. Panicucci, R. P. Igo, Jr., A. K. Panigrahi, R. Salavati, and K. Stuart. 2003. TbMP57 is a 3′ terminal uridylyl transferase (TUTase) of the Trypanosoma brucei editosome. Mol. Cell 111525-1536. [DOI] [PubMed] [Google Scholar]
- 18.Feagin, J. E., J. M. Abraham, and K. Stuart. 1988. Extensive editing of the cytochrome c oxidase III transcript in Trypanosoma brucei. Cell 53413-422. [DOI] [PubMed] [Google Scholar]
- 19.Harris, M. E., D. R. Moore, and S. L. Hajduk. 1990. Addition of uridines to edited RNAs in trypanosome mitochondria occurs independently of transcription. J. Biol. Chem. 26511368-11376. [PubMed] [Google Scholar]
- 20.Harris, M. E., and S. L. Hajduk. 1992. Kinetoplastid RNA editing: in vitro formation of cytochrome b gRNA-mRNA chimeras from synthetic substrate RNAs. Cell 681091-1099. [DOI] [PubMed] [Google Scholar]
- 21.Huang, C. E., J. Cruz-Reyes, A. G. Zhelonkina, S. O'Hearn, E. Wirtz, and B. Sollner-Webb. 2001. Roles for ligases in the RNA editing complex of Trypanosoma brucei: band IV is needed for U-deletion and RNA repair. EMBO J. 204694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Igo, R. P., Jr., S. S. Palazzo, M. L. Burgess, A. K. Panigrahi, and K. Stuart. 2000. Uridylate addition and RNA ligation contribute to the specificity of kinetoplastid insertion RNA editing. Mol. Cell. Biol. 208447-8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igo, R. P. Jr., D. S. Weston, N. L. Ernst, A. K. Panigrahi, R. Salavati, and K. Stuart. 2002. Role of uridylate-specific exoribonuclease activity in Trypanosoma brucei RNA editing. Eukaryot. Cell 1112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kable, M. L., S. D. Seiwert, S. Heidmann, and K. Stuart. 1996. RNA editing: a mechanism for gRNA-specified uridylate insertion into precursor mRNA. Science 2731189-1195. [DOI] [PubMed] [Google Scholar]
- 25.Kang, X., K. Rogers, G. Gao, A. M. Falick, S. Zhou, and L. Simpson. 2005. Reconstitution of uridine-deletion precleaved RNA editing with two recombinant enzymes. Proc. Natl. Acad. Sci. USA 1021017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korenčić, D., D. Söll, and A. Ambrogelly. 2002. A one-step method for in vitro production of tRNA transcripts. Nucleic Acids Res. 30e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koslowsky, D. J., G. J. Bhat, L. K. Read, and K. Stuart. 1991. Cycles of progressive realignment of gRNA with mRNA in RNA editing. Cell 67537-546. [DOI] [PubMed] [Google Scholar]
- 28.Landweber, L. F., and W. Gilbert. 1994. Phylogenetic analysis of RNA editing: a primitive genetic phenomenon. Proc. Natl. Acad. Sci. USA 91918-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Law, J. A., C. E. Huang, S. F. O'Hearn, and B. Sollner-Webb. 2005. In Trypanosoma brucei RNA editing, band II enables recognition specifically at each step of the U insertion cycle. Mol. Cell. Biol. 252785-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Law, J. A., S. O'Hearn, and B. Sollner-Webb. 2007. In Trypanosoma brucei RNA editing, TbMP18 (band VII) is critical for editosome integrity and for both insertional and deletional cleavages. Mol. Cell. Biol. 27777-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawson, S. D., R. P. Igo, Jr., R. Salavati, and K. D. Stuart. 2001. The specificity of nucleotide removal during RNA editing in Trypanosoma brucei. RNA 71793-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madison-Antenucci, S., J. Grams, and S. L. Hajduk. 2002. Editing machines: the complexities of trypanosome RNA editing. Cell 108435-438. [DOI] [PubMed] [Google Scholar]
- 33.Maslov, D. A., and L. Simpson. 1992. The polarity of editing within a multiple gRNA-mediated domain is due to formation of anchors for upstream gRNAs by downstream editing. Cell 70459-467. [DOI] [PubMed] [Google Scholar]
- 34.McManus, M. T., M. Shimamura, J. Grams, and S. L. Hajduk. 2001. Identification of candidate mitochondrial RNA editing ligases from Trypanosoma brucei. RNA 7167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panigrahi, A. K., A. Schnaufer, N. L. Ernst, B. Wang, N. Carmean, R. Salavati, and K. Stuart. 2003. Identification of novel components of Trypanosoma brucei editosomes. RNA 9484-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panigrahi, A. K., N. L. Ernst, G. J. Domingo, M. Fleck, R. Salavati, and K. D. Stuart. 2006. Compositionally and functionally distinct editosomes in Trypanosoma brucei. RNA 121038-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Read, L. K., R. A. Corell, and K. Stuart. 1992. Chimeric and truncated RNAs in Trypanosoma brucei suggest transesterifications at non-consecutive sites during RNA editing. Nucleic Acids Res. 202341-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riley, G. R., R. A. Corell, and K. Stuart. 1994. Multiple guide RNAs for identical editing of Trypanosoma brucei apocytochrome b mRNA have an unusual minicircle location and are developmentally regulated. J. Biol. Chem. 2696101-6108. [PubMed] [Google Scholar]
- 39.Rusché, L. N., J. Cruz-Reyes, K. J. Piller, and B. Sollner-Webb. 1997. Purification of a functional enzymatic editing complex from Trypanosoma brucei mitochondria. EMBO J. 164069-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rusché, L. N., C. E. Huang, K. J. Piller, M. Hemann, E. Wirtz, and B. Sollner-Webb. 2001. The two RNA ligases of the Trypanosoma brucei RNA editing complex: cloning the essential band IV gene and identifying the band V gene. Mol. Cell. Biol. 21979-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabatini, R., B. Adler, S. Madison-Antenucci, M. McManus, and S. Hajduk. 1998. Biochemical methods for analysis of kinetoplastid RNA editing. Methods 1515-26. [DOI] [PubMed] [Google Scholar]
- 42.Schnaufer, A., A. K. Panigrahi, B. Panicucci, R. P. Igo, Jr., E. Wirtz, R. Salavati, and K. Stuart. 2001. An RNA ligase essential for RNA editing and survival of the bloodstream form of Trypanosoma brucei. Science 2912159-2162. [DOI] [PubMed] [Google Scholar]
- 43.Schnaufer, A., N. L. Ernst, S. S. Palazzo, J. O'Rear, R. Salavati, and K. Stuart. 2003. Separate insertion and deletion subcomplexes of the Trypanosoma brucei RNA editing complex. Mol. Cell 12307-319. [DOI] [PubMed] [Google Scholar]
- 44.Seiwert, S. D., and K. Stuart. 1994. RNA editing: transfer of genetic information from gRNA to precursor mRNA in vitro. Science 266114-117. [DOI] [PubMed] [Google Scholar]
- 45.Seiwert, S. D., S. Heidmann, and K. Stuart. 1996. Direct visualization of uridylate deletion in vitro suggests a mechanism for kinetoplastid RNA editing. Cell 84831-841. [DOI] [PubMed] [Google Scholar]
- 46.Shaw, J. M., J. E. Feagin, K. Stuart, and L. Simpson. 1988. Editing of kinetoplastid mitochondrial mRNAs by uridine addition and deletion generates conserved amino acid sequences and AUG initiation codons. Cell 53401-411. [DOI] [PubMed] [Google Scholar]
- 47.Simpson, L., S. Sbicego, and R. Aphasizhev. 2003. Uridine insertion/deletion RNA editing in trypanosome mitochondria: a complex business. RNA 9265-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson, L., R. Aphasizhev, G. Gao, and X. Kang. 2004. Mitochondrial proteins and complexes in Leishmania and Trypanosoma involved in U-insertion/deletion RNA editing. RNA 10159-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sollner-Webb, B., L. N. Rusche, and J. Cruz-Reyes. 2001. Ribonuclease activities of trypanosome RNA editing complex directed to cleave specifically at a chosen site. Methods Enzymol. 341154-174. [DOI] [PubMed] [Google Scholar]
- 50.Stuart, K. D., A. Schnaufer, N. L. Ernst, and A. K. Panigrahi. 2005. Complex management: RNA editing in trypanosomes. Trends Biochem. Sci. 3097-105. [DOI] [PubMed] [Google Scholar]
- 51.Sturm, N. R., and L. Simpson. 1990. Partially edited mRNAs for cytochrome b and subunit III of cytochrome oxidase from Leishmania tarentolae mitochondria: RNA editing intermediates. Cell 61871-878. [DOI] [PubMed] [Google Scholar]
- 52.Sturm, N. R., and L. Simpson. 1990. Kinetoplast DNA minicircles encode guide RNAs for editing of cytochrome oxidase subunit III mRNA. Cell 61879-884. [DOI] [PubMed] [Google Scholar]
- 53.Sturm, N. R., D. A. Maslov, B. Blum, and L. Simpson. 1992. Generation of unexpected editing patterns in Leishmania tarentolae mitochondrial mRNAs: misediting produced by misguiding. Cell 70469-476. [DOI] [PubMed] [Google Scholar]
- 54.Trotter, J. R., N. L. Ernst, J. Carnes, B. Panicucci, and K. Stuart. 2005. A deletion site editing endonuclease in Trypanosoma brucei. Mol. Cell 20403-412. [DOI] [PubMed] [Google Scholar]
- 55.Worthey, E. A., A. Schnaufer, I. S. Mian, K. Stuart, and R. Salavati. 2003. Comparative analysis of editosome proteins in trypanosomatids. Nucleic Acids Res. 316392-6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhelonkina, A. G., S. F. O'Hearn, J. A. Law, J. Cruz-Reyes, C. E. Huang, V. S. Alatortsev, and B. Sollner-Webb. 2006. T. brucei RNA editing: action of the U-insertional TUTase within a U-deletion cycle. RNA 12476-487. [DOI] [PMC free article] [PubMed] [Google Scholar]