Abstract
Steroid receptor coactivator 3 (SRC-3/AIB1/ACTR/NCoA-3) is a transcriptional coactivator for nuclear receptors including vitamin D receptor (VDR). Growth hormone (GH) regulates insulin-like growth factor I (IGF-I) expression, and IGF-I forms complexes with acid-labile subunit (ALS) and IGF-binding protein 3 (IGFBP-3) to maintain its circulating concentration and endocrine function. This study demonstrated that the circulating IGF-I was significantly reduced in SRC-3−/− mice with the C57BL/6J background. However, SRC-3 deficiency affected neither GH nor ALS expression. The low IGF-I level in SRC-3−/− mice was not due to the failure of IGF-I mRNA and protein synthesis but was a consequence of rapid degradation. The rapid IGF-I degradation was associated with drastically reduced IGFBP-3 levels. Because IGF-I and IGFBP-3 stabilize each other, SRC-3−/− mice were crossbred with the liver-specific transthyretin (TTR)-IGF-I transgenic mice to assess the relationship between reduced IGF-I and IGFBP-3. In SRC-3−/−/TTR-IGF-I mice, the IGF-I level was significantly increased over that in SRC-3−/− mice, but the IGFBP-3 level failed to increase proportionally, indicating that the low IGFBP-3 level is a responsible factor that limits the IGF-I level in SRC-3−/− mice. Furthermore, IGFBP-3 mRNA was reduced in SRC-3−/− mice. The IGFBP-3 promoter activity induced by vitamin D, through VDR, was diminished in SRC-3−/− cells, suggesting an important role of SRC-3 in VDR-mediated transactivation of the IGFBP-3 gene. In agreement with the role of SRC-3 in VDR function, the expression of several VDR target genes was also reduced in SRC-3−/− mice. Therefore, SRC-3 maintains IGF-I in the circulation through enhancing VDR-regulated IGFBP-3 expression.
Nuclear receptors are hormone-inducible transcription factors that require coactivators to mediate their transcriptional activities (34). Most nuclear receptor coactivators exist in cells at limiting concentrations, and their transcriptional activities are regulated by various signaling pathway-triggered posttranslational modifications (3, 32, 34). Accordingly, regulation of coactivator concentrations and activities appears to be one of the major means to control gene expression and metabolic homeostasis regulated by hormones and their cognate receptors. Conversely, coactivator dysfunction may cause a number of developmental and physiological disorders and life-threatening diseases (11, 34). In the p160 steroid receptor coactivator (SRC) family (34), disruption of the SRC-1 gene in mice results in partial resistance to steroid and thyroid hormones (31, 36); disruption of SRC-2 (TIF2 or GRIP1) impairs both male and female reproductive functions (5, 17); and disruption of SRC-3 (AIB1, RAC3, p/CIP, ACTR, or TRAM-1) retards somatic and mammary gland growth, reduces female reproductive function, and suppresses oncogene- and carcinogen-induced mammary tumorigenesis (9, 10, 30, 35). In addition, SRC-3 is overexpressed in human breast cancers, and overexpression of SRC-3 in the mouse mammary epithelium is sufficient to induce ductal carcinomas (1, 29). Therefore, studies to understand the biological functions and molecular mechanisms of nuclear receptor coactivators have been a recent focus in the field of molecular endocrinology.
The p160 SRC coactivators interact with nuclear receptors in a hormone-dependent manner and recruit protein acetyltransferases such as CBP, p300, and p/CAF and protein methyltransferases such as CARM1 and PRMT1 to the promoter of hormone-responsive genes. These SRC-recruited histone modification enzymes change the chromatin topology, facilitate general transcription factor assembly, and potentiate transcription (34). In cultured cells, overexpression of each SRC member increases most nuclear receptor-mediated transactivation, suggesting that each SRC can serve as a coactivator for multiple nuclear receptors (34). This notion is further supported by our findings showing partial redundancy of function between SRC-1 and SRC-2 in promoting Purkinje cell development in the cerebellum, spermatogenesis in the testis, and postnatal viability (17, 21). On the other hand, the distinct phenotypes observed in mice lacking SRC-1, SRC-2, or SRC-3 indicate that each SRC member has a specific biological function, presumably due to its preference for specific transcription factors and/or its temporal and spatial expression patterns (6, 7, 34, 35, 39). One of the unique phenotypes observed in SRC-3−/− mice is the significantly reduced insulin-like growth factor I (IGF-I) level in the circulation (35). However, the mechanisms responsible for SRC-3 control of IGF-I circulating levels remain to be characterized.
IGF-I, as a growth hormone (GH) target, plays an essential role in regulation of postnatal somatic growth. Inactivation of the IGF-I gene in mice severely impairs somatic growth after birth (8, 15, 24, 28). In the circulation, most IGF-I associates with IGF-binding protein 3 (IGFBP-3) and acid-labile subunit (ALS) to form ternary complexes, which remarkably increases IGF-I and IGFBP-3 stabilities in the circulation and efficiently maintains the reservoir of IGF-I and its endocrine function (8, 28). It has been demonstrated that overexpression of IGFBP-3 increases plasma IGF-I in transgenic mice (20). Conversely, IGF-I reduction decreases circulating IGFBP-3 without affecting its mRNA expression (37). These studies indicate that IGF-I and IGFBP-3 depend on each other for stability. These associated changes between IGF-I and IGFBP-3 make it difficult to assess which one is the initiative factor if both are reduced in the circulation.
The liver is the principal organ that produces circulating IGF-I and ALS (8, 28). ALS is exclusively expressed in the liver, and its expression is dependent on GH stimulation. GH also directly stimulates IGF-I mRNA expression in the liver and other tissues. IGFBP-3 is more broadly expressed in the kidney, liver, and other organs (8, 28). The mechanism for transcriptional regulation of IGFBP-3 is less clear, although some studies suggest that GH may indirectly regulate IGFBP-3 expression through enhancing IGF-I production (4, 26). Several studies have demonstrated that the IGFBP-3 promoter contains a functional vitamin D response element (VDRE) and that vitamin D-activated vitamin D receptor (VDR) binds to the VDRE and significantly enhances the transcriptional activity of the IGFBP-3 promoter (18, 23). This raises the possibility that SRC-3 serves as a coactivator of VDR to regulate IGFBP-3 expression.
In this study, we show that genetic ablation of SRC-3 in mice results in a significant decrease in circulating IGF-I levels. The IGF-I decrease is caused not by GH and IGF-I expression but by a rapid IGF-I clearance in the circulation. The rapid IGF-I degradation in SRC-3−/− mice is associated with a drastic reduction of serum IGFBP-3 levels that cannot be restored by transgenic IGF-I compensation. Furthermore, disruption of SRC-3 in mice decreases the expression of IGFBP-3 and several other known VDR target genes. SRC-3-deficient cells in culture are also unable to support vitamin D-induced IGFBP-3 promoter activity. These compelling findings demonstrate that SRC-3 plays an important role in maintenance of normal serum IGF-I levels through regulation of IGFBP-3 expression.
MATERIALS AND METHODS
Mice.
Animal protocols were approved by the Animal Care and Use Committee for Baylor College of Medicine. SRC-3−/− mice were initially generated in a 129SvEv/C57BL/6J genetic background as described previously (35). To reduce possible complications of different strain backgrounds, we backcrossed these mice with C57BL/6J mice for six generations. The transthyretin (TTR)-IGF-I-transgenic mice were generated in the C57BL/6J strain background as described previously (13). Double-heterozygous mice were produced by breeding the backcrossed male SRC-3−/− mice with the female TTR-IGF-I mice. Bigenic mice with combinatorial genotypes of SRC-3 and TTR-IGF-I were generated by one more generation of breeding. TTR-IGF-I mice used for all experiments were heterozygous to the IGF-I transgene. Mouse genotypes were analyzed by PCR using genomic DNA isolated from ear tips after proteinase K digestion and allele-specific primer pairs as described previously (13, 35). For growth analysis, body weights of wild-type (WT) and SRC-3−/− mice were measured weekly from postnatal day 3 (P3) to P90. The same groups of mice were weighed again at P180 and P365.
Mouse IGF-I and GH assay.
Blood samples were collected by periorbital puncture, and serum was isolated after the blood was clotted at 4°C. The concentration of total IGF-I in the serum was measured by using a radioimmunoassay (RIA) kit for rat IGF-I (Diagnostic Systems Laboratories, Webster, TX) as described previously (35). For measuring IGF-I levels in tissues, mice were anesthetized with avertin (2.5%, 0.3 ml/mouse) and their blood circulating system was perfused with 20 ml phosphate-buffered saline (PBS) from the left ventricle to the right ventricle. Tissues were dissected, frozen immediately in liquid nitrogen, and stored in a −80°C freezer. Frozen tissues were pulverized under liquid nitrogen and then collected into cold Eppendorf tubes. Each 100 mg of tissue powder was mixed with 0.5 ml of 1 M acetic acid, incubated on ice for 2 h, and then centrifuged at 600 × g for 10 min. Supernatant was saved, and the precipitate was extracted one more time. The combined supernatants were dried under a speed vacuum, and the dried samples were reconstituted in 200 μl of 50 mM Tris-HCl (pH 7.8) for each 100 mg of tissue. The IGF-I concentration in the reconstituted samples was assayed as described above and normalized to tissue weight. The concentration of mouse GH in the serum was measured by using a mouse/rat GH enzyme-linked immunosorbent assay kit (RPA551; Amersham Biosciences, Piscataway, NJ) with its primary antibody replaced by the rabbit anti-recombinant GH immunoglobulin G made in the Parlow laboratory (AFP5672099; 1:100,000 dilution in PBS with 3% bovine serum albumin [BSA]).
Analysis of IGF-I stability in vivo.
Mice were injected with recombinant human IGF-I (hIGF-I) (0.25 μg/g of body weight, intraperitoneally) after 30 μl of blood was collected from their tails. After hIGF-I injection, mice were bled over a time course. The concentration of hIGF-I in the serum was measured by using the hIGF-I-specific RIA kit (Diagnostic Systems Laboratories, Webster, TX).
Immunoblotting assay.
Mouse pituitaries were individually collected and homogenized in lysis buffer containing 50 mM Tris-HCl (pH 7.4), 0.1% sodium dodecyl sulfate (SDS), 2 μM phenylmethylsulfonyl fluoride, and 10 μg/ml leupeptin. The samples were sonicated and centrifuged. Pituitary lysate containing 2 μg of protein was separated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to a nitrocellulose membrane. The blot was probed with the rabbit anti-recombinant GH antibody produced in the Parlow laboratory and developed by using the Enhanced Chemiluminescence kit (Amersham Biosciences, Piscataway, NJ).
Ligand blot assay.
IGF-binding proteins were assayed by ligand blotting as performed previously (13). Briefly, 0.5 μl of serum was mixed with nonreducing sample buffer for SDS-PAGE and boiled for 5 min. The sample was separated by running a 10% SDS-PAGE minigel at 100 V and blotted onto a nitrocellulose membrane. The membrane was washed with saline, incubated in saline containing 3% NP-40 at 4°C for 30 min, blocked with saline containing 1% RIA-grade BSA (A-7888; Sigma, St. Louis, MO) at 4°C for 2 h, and washed with 0.1% Tween 20 in saline at 4°C for 10 min. The washed membrane was incubated with 5 × 105 cpm of 125I-hIGF-I in a sealed bag with 5 ml of saline containing 1% BSA and 0.1% Tween 20 overnight at 4°C. After incubation the membrane was washed twice with 0.1% Tween 20 in saline and then three times in saline before being exposed to X-ray film for 2 days.
RPA.
An RNase protection assay (RPA) was carried out by using the RPA III kit (Ambion, Austin, TX). Total RNA was prepared from liver and kidney by using Trizol reagent (Invitrogen, Carlsbad, CA) and hybridized with 32P-labeled riboprobes. The riboprobes for protecting IGF-I, ALS, IGFBP-3, and cyclophilin A mRNAs were described previously (13). The probe-protected samples were separated by running a 6% acrylamide denatured gel. The gels were dried and exposed to X-ray films at −80°C.
qPCR.
RNA samples were prepared from mouse livers and kidneys and mouse embryonic fibroblasts (MEFs). The relative concentrations of IGFBP-3, SRC-1, SRC-2, and SRC-3 mRNAs were measured by quantitative TaqMan real-time reverse transcription-PCR (qPCR) using the One-Step Master Mix reagent (Applied Biosystems, Foster City, CA) as described previously (21). The relative concentrations of calbindin D9K, calbindin 28K, CYP3A11, CYP24, P21, and type IIa Na-Pi cotransporter (NPT2) mRNAs were measured by qPCR using gene-specific primer pairs and matched universal mouse probe sets (Roche Applied Science, Mannheim, Germany). Specifically, the 5′-AGTTCCCCAGCCTCCTGA and the 3′-AACTTCTCCATCGCCATTCTT primers and probe 17 were used for calbindin D9K, the 5′-GACGGAAGTGGTTACCTGGA and the 3′-ATTTCCGGTGATAGCTCCAA primers and probe 17 were used for calbindin 28K, the 5′-TCAATATCTAAGGATGATGAATGGAA and the 3′-TCAATGACAGGGAACATCTCC primers and probe 56 were used for CYP3A11, the 5′-AACTGTACGCTGCTGTCACG and the 3′-AATCCACATCAAGCTGTTTGC primers and probe 78 were used for CYP24, the 5′-TCCACAGCGATATCCAGACA and the 3′-GGCACACTTTGCTCCTGTG primers and probe 9 were used for P21, and the 5′-CGAGGGTATAAAGAAGAGGGTCT and the 3′-GGCACCCACAATGAGTCCT primers and probe 60 were used for NPT2. All data were normalized to the 18S RNA concentration of the same sample.
Transfection assay.
Stable cultures of WT and SRC-3−/− MEFs were maintained in Dulbecco modified Eagle medium with 10% fetal bovine serum. For transient-transfection assays, MEFs were plated in six-well plates overnight and transfected with pGL3-basic, pGL3-BP3(−1901/+55), or pGL3-BP3(−3590/+55) plasmid containing the luciferase reporter by using the Fugene 6 transfection reagent (Roche Applied Science, Indianapolis, IN). All cells also were cotransfected with the pRSV-βGal expression plasmid, which served as a control for transfection efficiency. Transfected cells were treated with 10−8 M 1,25-dihyroxy-vitamin D3 [1,25-(OH)2D3] or 10−7 M 9-cis-retinoic acid (9-cis-RA) for 24 h. Cell lysates were prepared, and the luciferase activity was measured with a luciferase assay kit (Promega, Madison, WI). The β-galactosidase activity in the same amount of cell lysate also was measured. Relative luciferase units were normalized to the β-galactosidase activity.
RESULTS
SRC-3 deficiency causes growth retardation and low IGF-I levels without affecting GH synthesis and secretion.
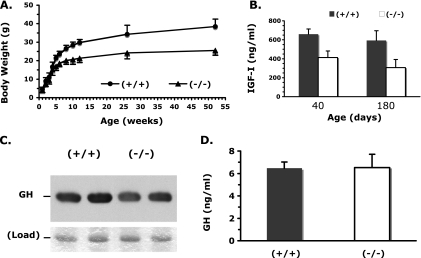
We have previously shown that SRC-3−/− young mice with a mixed 129SvEv and C57BL/6J strain background exhibited postnatal growth retardation that was accompanied by a significant reduction in serum IGF-I levels (35). In this study, these mice were backcrossed with C57BL/6J mice for six generations to reduce possible phenotypic influence from a mixed strain background. WT and SRC-3−/− mice for experiments were produced from the sixth generation of heterozygous breeding pairs. The average body weights between the backcrossed male SRC-3−/− and WT mice were similar up to P28, but male SRC-3−/− mice were significantly lighter than male WT mice after P35 (Fig. 1A). The average body weight of female SRC-3−/− mice was also significantly lower than that of female WT mice after P21 (data not shown). The lower body weight of SRC-3−/− mice was in accord with their reduced organ weight. The average weights of brain, pituitary, heart, liver, and kidney in 8-week-old SRC-3−/− mice were statistically lower than those of the same organs in age-matched WT mice (Table 1). In addition, SRC-3−/− mice displayed a shorter body length than age-matched WT mice (Table 1). The serum IGF-I levels in the backcrossed SRC-3−/− mice were significantly reduced as examined at P40 and P180 compared with age-matched WT mice (Fig. 1B). These results indicate that SRC-3 also regulates somatic growth and circulating IGF-I levels in mice with the backcrossed C57BL/6J strain background.
FIG. 1.
Body weight comparison and levels of IGF-I and GH in WT and SRC-3−/− mice. (A) Body weight comparison between male WT (+/+) and SRC-3−/− (−/−) mice over the period of 1 year (∼52 weeks). The average body weight of SRC-3−/− mice (n = 8) was similar to that of WT mice (n = 8) before the age of 5 weeks (P > 0.05, unpaired t test) but was significantly decreased below that of WT mice over 5 weeks of age (P < 0.01, unpaired t test). (B) Measurement of serum IGF-I levels in WT and SRC-3−/− male mice at ages of 40 days (10 mice for each group) and 180 days (seven mice for each group). The serum IGF-I levels were statistically higher in WT mice than in SRC-3−/− mice (P < 0.01 and 0.001 by t test at ages of 40 and 180 days, respectively). (C) Immunoblotting analysis of the pituitary extracts (2 μg of protein per lane) prepared from two mice for each group by using a GH-specific antibody (upper panel), showing comparable GH protein levels in the pituitaries of WT and SRC-3−/− mice. The lower panel shows a protein band revealed by R-250 Coomassie blue staining of the gel with identical sample loading. (D) Measurement of GH levels in male WT and SRC-3−/− mice. For each mouse, serum samples were prepared at three different time points (see Materials and Methods). There was no significant difference in the serum GH levels between WT (n = 10) and SRC-3−/− (n = 10) mice.
TABLE 1.
Organ weights and body lengths of SRC-3−/− micea
| Parameter | Mean value ± SD for mouse type
|
Significance of difference between mouse types (unpaired t test)b | |
|---|---|---|---|
| WT | KO | ||
| Wt (mg) of organ | |||
| Brain | 447 ± 26 | 393 ± 23 | VS |
| Pituitary | 1.50 ± 0.28 | 1.02 ± 0.10 | VS |
| Heart | 103 ± 10 | 85 ± 6 | VS |
| Liver | 1,024 ± 98 | 772 ± 72 | VS |
| Kidney | 292 ± 35 | 221 ± 40 | S |
| Spleen | 68 ± 8 | 70 ± 30 | NS |
| Body length (cm) | 8.88 ± 0.27 | 8.32 ± 0.29 | S |
Data were obtained from 8-week-old male WT (n = 8) and SRC-3-knockout (KO) (n = 5) mice.
VS, very significant (P < 0.01); S, significant (P < 0.05); NS, not significant (P > 0.05).
Since 75% of IGF-I in the circulation is produced in the liver in response to GH stimulation (37), we examined GH levels in the pituitary and serum. Immunoblotting analysis of samples from multiple mice revealed that GH protein levels in the pituitary extracts were comparable between WT and SRC-3−/− mice when samples with equal amount of protein were analyzed (Fig. 1C). Because of the pulsatile fashion of GH secretion, we performed the following procedure to obtain reliable measurement of serum GH levels. Blood samples were collected from male adult SRC-3−/− and age-matched WT mice at 8 a.m. on experimental day 1, 3 p.m. on day 14, and 10 p.m. on day 28, so that animals could recover after each bleeding. GH levels in individual samples were measured, and the average GH level for each mouse was calculated from the three samples collected at the three time points. Our assay demonstrated that the average serum GH levels in SRC-3−/− mice were similar to those in WT mice (Fig. 1D). These results indicate that disruption of SRC-3 in mice does not affect GH synthesis and secretion. Therefore, the growth deficit and lower IGF-I levels in SRC-3−/− mice are not caused by GH deficiency.
The serum IGF-I reduction in SRC-3−/− mice is not caused by lower IGF-I mRNA expression but by rapid IGF-I degradation.
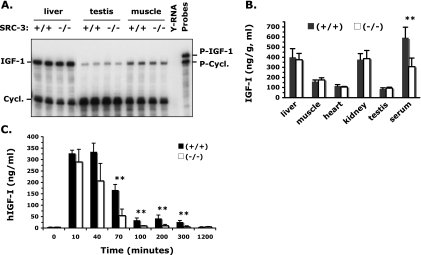
To examine whether the lower serum IGF-I levels in SRC-3−/− mice were caused by a reduction of IGF-I gene transcription, we measured the IGF-I mRNA levels by RPA. Interestingly, IGF-I mRNA was comparably expressed in WT and SRC-3−/− mice in all organs examined, including liver, testis, muscle, kidney, and brain (Fig. 2A and data not shown). To address whether SRC-3 deficiency would affect IGF-I protein synthesis, we measured the IGF-I protein levels in different tissues after the mouse circulation system was thoroughly flushed with PBS. Our assay revealed that the amounts of IGF-I protein in the liver, skeletal muscle, heart, kidney, and testis were similar in WT and SRC-3−/− mice (Fig. 2B). Again, we repeatedly observed a significant reduction of IGF-I concentration in the serum (Fig. 2B). These results indicate that inactivation of SRC-3 does not affect the transcription of IGF-I mRNA and the translation of IGF-I protein.
FIG. 2.
IGF-I expression and stability in SRC-3−/− mice. (A) RPAs showing comparable expression levels of IGF-I mRNA in liver, testis, and skeletal muscle of WT and SRC-3−/− mice. Five micrograms of liver RNA and 15-μg testis and muscle RNA samples prepared from two mice of each genotype group were analyzed. Yeast RNA (Y-RNA) was used as a negative control. The protected cyclophilin A (Cycl.) served as a control for RNA input. P-IGF-1, IGF-I probe; P-Cycl., cyclophilin A probe. (B) IGF-I levels in different tissues. A significantly lower level of IGF-I was observed only in the serum of SRC-3−/− mice compared with WT mice. Six mice were assayed for each genotype group. **, P < 0.01 by unpaired t test. (C) Degradation of human IGF-I in WT and SRC-3−/− mice. Recombinant hIGF-I was administrated at zero time, and serum levels of hIGF-I were measured before and after hIGF-I administration at indicated time points. Six mice were assayed for each genotype group. Note the much faster degradation of hIGF-I in SRC-3−/− mice than in WT mice. **, P < 0.01 by unpaired t test.
The above results logically prompted us to check IGF-I stability after its secretion into the blood circulation. In this experiment, hIGF-I was injected into WT and SRC-3−/− mice and the hIGF-I degradation profiles in mice were monitored by measuring the serum concentrations of hIGF-I using an RIA kit that specifically detects hIGF-I but not the endogenous mouse IGF-I (Fig. 2C). In WT mice, the serum hIGF-I concentration reached the maximum level in 10 min after injection. This maximum value was maintained for 40 min, and it dropped to 50% at 70 min after injection. About 10% of the injected IGF-I was maintained up to 5 h in the circulation of WT mice (Fig. 2C). In contrast, the injected hIGF-I was much more rapidly degraded in SRC-3−/− mice than in WT mice. In SRC-3−/− mice, a decrease in serum hIGF-I was first observed at 40 min after injection and an 80% decrease in serum hIGF-I was found at 70 min after injection. By 100 min, the injected hIGF-I became undetectable in SRC-3−/− mice (Fig. 2C). These results indicate that SRC-3 plays a critical role in IGF-I stabilization in the circulation and that disruption of SRC-3 significantly accelerates IGF-I degradation.
SRC-3 deficiency drastically decreases serum IGFBP-3 concentration.
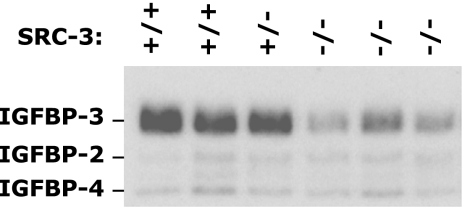
Since IGF-binding proteins play essential roles in stabilizing IGF-I in the circulation (8, 28), we performed ligand blot analysis using the 125I-labeled IGF-I to measure the levels of IGF-binding proteins in the circulation. In the serum of WT and SRC-3+/− mice, abundant IGFBP-3 proteins and low levels of IGFBP-2 and IGFBP-4 were clearly detected (Fig. 3). In the serum of SRC-3−/− mice, the levels of IGFBP-2 and IGFBP-4 were unaltered compared with those of WT and SRC-3+/− mice. However, the levels of IGFBP-3 in the serum of SRC-3−/− mice were drastically reduced compared with the levels of IGFBP-3 in the serum of WT and SRC-3+/− mice (Fig. 3). These results indicate that SRC-3 deficiency causes a significant reduction of serum IGFBP-3 concentration, which is associated with the accelerated degradation of IGF-I in SRC-3−/− mice.
FIG. 3.
Severely reduced serum IGFBP-3 protein in SRC-3−/− mice. Serum protein was separated by nonreducing SDS-PAGE and blotted onto a nitrocellulose membrane. IGF-binding proteins were detected by incubating the membrane with 125I-labeled IGF-I. Note that IGFBP-3 was significantly reduced but that IGFBP-2 and IGFBP-4 were unchanged in SRC-3−/− mice (n = 3) compared with WT and SRC-3+/− mice (n = 3).
Compensation of the circulating IGF-I with a liver-specific transgene in SRC-3−/− mice is unable to restore serum IGFBP-3 levels.
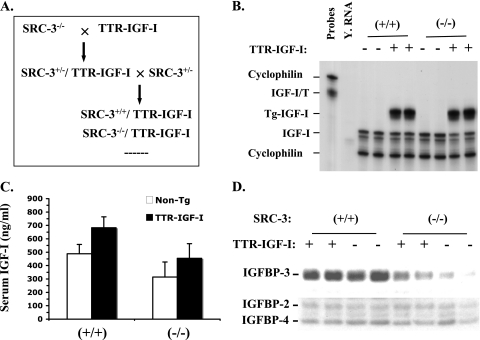
It has been shown that liver-specific IGF-I-deficient mice have a 75% reduction in serum IGF-I levels, which is associated with a drastic decrease in serum IGFBP-3 levels (38). To assess whether the low IGFBP-3 levels in SRC-3−/− mice were caused by IGF-I reduction in these mice, we investigated whether restoration of the circulating IGF-I levels in SRC-3−/− mice by expression of IGF-I from a liver-specific TTR-IGF-I transgene would rescue IGFBP-3 levels. In the TTR-IGF-I mouse line generated in our laboratory, the IGF-I transgene was driven by the liver-specific TTR enhancer/promoter and its liver-specific expression resulted in a 50% increase in biologically active IGF-I in the circulation (13). We then crossed TTR-IGF-I mice with SRC-3−/− mice and produced WT/TTR-IGF-I and SRC-3−/−/TTR-IGF-I bigenic mice for experimental analysis (Fig. 4A). RPA was performed to examine the expression of the TTR-IGF-I transgene in these bigenic mice. The transgenic IGF-I mRNA was abundantly and comparably expressed in the livers of WT/TTR-IGF-I and SRC-3−/−/TTR-IGF-I mice, and the transgene expression had no effect on the expression of the endogenous IGF-I gene in these mice compared with WT and SRC-3−/− mice (Fig. 4B). RIA was performed to measure the serum levels of IGF-I. The serum IGF-I levels were increased about 50% in WT/TTR-IGF-I mice compared with WT mice (P < 0.001, one-way analysis of variance [ANOVA]). The IGF-I levels in SRC-3−/−/TTR-IGF-I mice were also significantly elevated compared with SRC-3−/− mice (P < 0.001, one-way ANOVA), and it reached the average IGF-I level in WT mice. However, the average IGF-I level in SRC-3−/−/TTR-IGF-I mice was much lower than that in WT/TTR-IGF-I mice (P < 0.001, one-way ANOVA) (Fig. 4C). These results demonstrate that overexpression of the liver-specific TTR-IGF-I transgene can only partially elevate the circulating IGF-I levels in SRC-3−/−/TTR-IGF-I mice, suggesting that IGF-I in SRC-3−/−/TTR-IGF-I mice is still less stable than IGF-I in WT/TTR-IGF-I mice.
FIG. 4.
Generation and analysis of SRC-3−/−/TTR-IGF-I bigenic mice. (A) Breeding strategy for generation of the bigenic mice by crossing SRC-3−/− mice with the TTR-IGF-I transgenic mice. (B) Expression of the endogenous and transgenic IGF-I mRNA in the liver of WT (+/+), WT/TTR-IGF-I (+), SRC-3−/− (−/−), and SRC-3−/−/TTR-IGF-I (+) mice. RPA was performed with 15 μg of liver RNA isolated from two mice of each genotype group and with the probe (IGF-I/T) that could detect the endogenous (partial sequence protection) and the transgenic (full-length protection) IGF-I mRNA. (C) Serum IGF-I levels. Serum samples were prepared from age-matched WT (n = 20), WT/TTR-IGF-I (n = 23), SRC-3−/− (n = 20), and SRC-3−/−/TTR-IGF-I (n = 12) mice. Non-Tg, mice negative for the TTR-IGF-I transgene. One-way ANOVA indicates no significant difference (P > 0.05) in IGF-I levels between WT (+/+) and SRC-3−/−/TTR-IGF-I mice; all other comparisons between the two groups showed significant differences (P < 0.01). (D) Levels of IGFBP-3 in serum in WT (+/+), WT/TTR-IGF-I (+), SRC-3−/− (−/−), and SRC-3−/−/TTR-IGF-I (+) mice were analyzed by ligand blotting using 125I-labeled IGF-I. Samples from two mice of each genotype group were assayed. Note that the IGFBP-3 levels in SRC-3−/−/TTR-IGF-I and SRC-3−/− mice were significantly lower than those in WT/TTR-IGF-I and WT mice.
Next, we performed ligand blot assays to examine whether an up-regulation of IGF-I levels by the TTR-IGF-I transgene would also elevate the IGFBP-3 levels in the circulation of SRC-3−/−/TTR-IGF-I mice. Our analysis showed that serum IGFBP-3 levels were similar in WT and WT/TTR-IGF-I mice (Fig. 4D), suggesting that elevation of circulating IGF-I in WT mice does not further increase the concentration of circulating IGFBP-3. Importantly, the serum IGFBP-3 levels in SRC-3−/−/TTR-IGF-I mice were much lower than those in WT and WT/TTR-IGF-I mice, and they showed only a small increase compared with SRC-3−/− mice (Fig. 4D), indicating that compensation of IGF-I levels in the circulation system of SRC-3−/− mice does not efficiently rescue the serum IGFBP-3 levels.
SRC-3 deficiency affects IGFBP-3 mRNA expression.
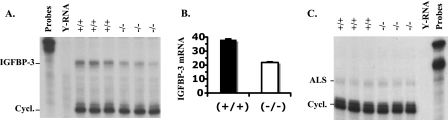
Since the low serum IGFBP-3 level was not a consequence of low serum IGF-I level in SRC-3−/− mice, we next investigated how SRC-3 deficiency affects IGFBP-3 mRNA expression. IGFBP-3 is expressed in multiple tissues, and the mechanisms regulating IGFBP-3 expression in vivo are currently less well understood. RPAs using total RNA samples prepared from the kidneys of multiple WT and SRC-3−/− mice revealed that the expression levels of IGFBP-3 mRNA were significantly reduced (Fig. 5A). Because IGFBP-3 is expressed only in the nonparenchymal cells in the liver (28) and could not be easily detected by RPA, we performed qPCR, a more sensitive assay, to measure the expression levels of IGFBP-3 in the liver. Our assay showed that the expression levels of IGFBP-3 in the liver were reduced about 50% in SRC-3−/− mice compared with WT mice (Fig. 5B). These results indicate that SRC-3 is required for IGFBP-3 expression in liver and kidney.
FIG. 5.
Analysis of IGFBP-3 and ALS expression. (A) Reduced IGFBP-3 expression in the kidney of SRC-3−/− mice. RPA was performed with 15 μg of RNA isolated from the kidneys of three WT and three SRC-3−/− mice. Yeast RNA (Y-RNA) was used as a negative control. The protected cyclophilin (Cycl.) RNA served as a loading control of total RNA input. (B) Reduced IGFBP-3 expression in the liver of SRC-3−/− mice. qPCR measurement of relative IGFBP-3 mRNA levels in the livers of WT and SRC-3−/− mice. The data were normalized to 18S RNA measurements of the same samples and are presented as averages ± standard deviations. RNA samples from six mice for each genotype group were measured in triplicate. The average concentration in SRC-3−/− liver is significantly lower than that in WT liver (P < 0.01, unpaired t test). (C) ALS mRNA expression in the liver of WT (+/+) and SRC-3−/− (−/−) mice. RPA was performed with 15 μg of RNA isolated from the livers of three WT and three SRC-3−/− mice.
Since the circulating IGF-I forms ternary complexes with both IGFBP-3 and ALS, we also examined ALS expression. ALS is regulated by GH and solely expressed in the liver. RPAs with a riboprobe complementary to ALS mRNA revealed that ALS mRNA was comparably expressed in the livers of WT and SRC-3−/− mice (Fig. 5C), suggesting that SRC-3 is not required for ALS gene expression.
SRC-3 enhances vitamin D-induced IGFBP-3 promoter activity.
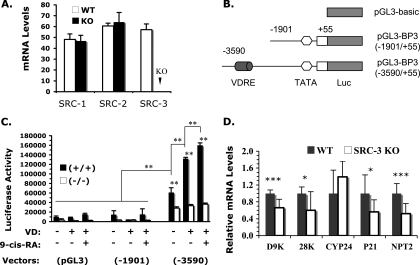
Recently, a functional VDRE has been identified in the IGFBP-3 promoter region (23). Since SRC-3 interacts with VDR and potentiates its transcriptional activity (22), we assessed the role of SRC-3 in VDR-dependent activation of the IGFBP-3 promoter by cellular transfection assays in the presence or absence of SRC-3. For this purpose, we developed MEFs from WT and SRC-3−/− mouse embryos and immortalized these cells by serial passages as described previously (33). Among members of the p160 SRC family, SRC-1 and SRC-2 were comparably expressed in WT and SRC-3−/− MEFs as measured by qPCR (Fig. 6A); SRC-3 was expressed in WT MEFs, but it was absent in SRC-3−/− MEFs as expected (Fig. 6A). In transfectional assays using these cells, we utilized the pGL3-basic vector containing only the luciferase coding sequence, the pGL3-BP3(−1901/+55) vector containing the promoter region of the IGFBP-3 gene from bp −1901 to +55 and the pGL3-BP3(−3590/+55) vector containing the promoter region of the IGFBP-3 gene from bp −3590 to +55. The functional VDRE mapped recently was located in the promoter region of the IGFBP-3 gene from bp −3590 to −1901 (Fig. 6B) (23). Transfection of the pGL3-basic and the pGL3-BP3(−1901/+55) vectors into WT and SRC-3−/− MEFs produced minimal luciferase activity regardless of the absence or presence of 1,25-(OH)2D3 and 9-cis-RA for activation of VDR and its heterodimerization partner retinoid X receptor (RXR) (Fig. 6C), suggesting that the promoter region of the IGFBP-3 gene from −1901 to +55 is not regulated by vitamin D and 9-cis-RA in WT and SRC-3−/− MEFs. In contrast, transfection of the pGL3-BP3(−3590/+55) vector into WT MEFs resulted in an increase in luciferase activity, indicating that the BP3(−3590/+55) region has promoter activity in these cells. More importantly, the promoter activity of the BP3(−3590/+55) region was significantly potentiated in WT MEFs by either vitamin D treatment or vitamin D and 9-cis-RA treatment, suggesting that the ligand-activated VDR/RXR strongly enhances the promoter activity of the BP3(−3590/+55) region containing a known functional VDRE (Fig. 6C). Nevertheless, transfection of the pGL3-BP3(−3590/+55) reporter into SRC-3−/− MEFs failed to exert an appreciable promoter activity, and treatment of these transfected SRC-3−/− MEFs with vitamin D or vitamin D plus 9-cis-RA was unable to increase the luciferase production. Therefore, the vitamin D-induced transcriptional activity of VDR on the IGFBP-3 promoter is severely compromised in MEFs lacking SRC-3. Collectively, these results suggest that SRC-3 is required for vitamin D-induced activation of the IGFBP-3 promoter.
FIG. 6.
SRC-3 is required for vitamin D-induced activation of the IGFBP-3 promoter and VDR target gene expression. (A) Real-time reverse transcription-PCR measurement of SRC-1, SRC-2, and SRC-3 mRNA levels in WT and SRC-3−/− (KO) MEFs. The data were normalized to 18S RNA levels in the same samples and are presented as averages ± standard deviations. (B) Luciferase (Luc) reporter DNA constructs. VDRE, a VDRE located in the 5′ regulatory region of the IGFBP-3 gene. (C) Transfection analysis. WT (+/+) and SRC-3−/− (−/−) MEFs were transfected with pGL3-basic, pGL3-BP3(−1901/+55), or pGL3-BP3(−3590/+55) luciferase reporter plasmid and treated with vitamin D (VD) or with VD and 9-cis-RA. Luciferase activity was normalized to the cotransfected β-galactosidase activity, expression of which was directed by a constitutively active cytomegalovirus promoter. The bar graph was generated by using data from one of the three experiments. Each experiment was done in triplicate. Statistical calculation was performed based on all three experiments with nine repeats. **, P < 0.01 by one-way ANOVA. (D) Relative expression levels of VDR target genes. The relative levels of calbindin D9K (D9K), calbindin 28K (28K), CYP24, P21, and NPT2 mRNAs were measured by qPCR using RNA samples prepared from kidneys of WT and SRC-3−/−-knockout (KO) mice (n = 5). Data were normalized to 18S RNA. The average relative levels of gene expression in WT mice were set as 1 unit. * and ***, P < 0.05 and P < 0.001, respectively, by unpaired t test.
Furthermore, if decreased IGFBP-3 expression was a result of reduced VDR function, the expression of other VDR target genes might also be affected. To test this possibility, we performed qPCR and measured the expression levels of several known VDR target genes, including calbindin D9K, calbindin 28K, CYP3A11, CYP24, P21, and NPT2 in the kidney (12, 16, 25, 40, 42). These assays revealed that the expression levels of calbindin D9K, calbindin 28K, P21, and NPT2 were significantly reduced in SRC-3−/− mice compared with WT mice. CYP24 expression showed no significant change (Fig. 6D). CYP3A11 expression was undetectable in the kidneys of both WT and SRC-3−/− mice (data not shown). These results indicate that SRC-3 deficiency selectively decreases the expression of certain VDR target genes.
DISCUSSION
The major goal of this study is to understand the mechanisms responsible for SRC-3 controlling the serum IGF-I concentration. We have shown that genetic ablation of SRC-3 causes a significant reduction of circulating IGF-I in backcrossed C57BL/6J SRC-3−/− mice, which is associated with postnatal growth retardation, indicating that the role of SRC-3 in regulation of circulating IGF-I and somatic growth in C57BL/6J mice is consistent with that observed in 129SvEv/C57BL/6J mice (35). Interestingly, SRC-3 deficiency does not significantly alter GH synthesis and secretion as well as IGF-I mRNA expression and protein production in tissues, suggesting that SRC-3 is not required for normal production of GH and GH-regulated IGF-I expression. We also have shown that the expression of ALS, a well-defined GH target gene in the liver, is not affected in SRC-3−/− mice. Thus, the low IGF-I levels in SRC-3−/− mice are due to neither GH deficiency nor IGF-I expression.
Instead, the low IGF-I levels in SRC-3−/− mice are associated with rapid IGF-I degradation and remarkably reduced IGFBP-3 levels in the circulation. Since previous studies have demonstrated that IGF-I and IGFBP-3 can stabilize each other and reduction of either one of them will decrease the level of the other one in the circulation (20, 37), it is important to understand which alteration, the low IGF-I or the low IGFBP-3, is the initial change and which one of them is a secondary effect. We have addressed this question by generating WT/TTR-IGF-I and SRC-3−/−/TTR-IGF-I bigenic mice. In these transgenic mice, the IGF-I transgene is expressed only in the liver (13). Our data have demonstrated that expression of TTR-IGF-I in the liver efficiently elevates serum IGF-I in both WT/TTR-IGF-I and SRC-3−/−/TTR-IGF-I mice. The average serum IGF-I level in SRC-3−/−/TTR-IGF-I mice is similar to that in nontransgenic WT mice. However, the serum IGF-I level in SRC-3−/−/TTR-IGF-I mice is still much lower than that in WT/TTR-IGF-I mice. Further analysis has revealed that compensation of IGF-I levels in SRC-3−/−/TTR-IGF-I mice only slightly increases serum IGFBP-3 levels and is unable to restore IGFBP-3 to normal levels in these mice. The small increase of IGFBP-3 may be attributed to the positive effect of elevated IGF-I on IGFBP-3 stability (20, 37). Since the serum IGFBP-3 levels are quite low in both SRC-3−/− and SRC-3−/−/TTR-IGF-I mice, these findings indicate that the low IGFBP-3 level is not a consequence but a cause of the low IGF-I level in SRC-3−/− mice.
After IGFBP-3 was identified as a limiting factor for IGF-I stability in SRC-3−/− mice, the expression of IGFBP-3 mRNA was further found to be decreased in all examined tissues including liver and kidney of SRC-3−/− mice. These results suggest that SRC-3 is required for IGFBP-3 expression. Recently, it has been demonstrated that the 5′ regulatory region of the IGFBP-3 gene contains a functional VDRE between −3296 and −3282. VDR showed strong binding to this VDRE both in vivo and under cell-free conditions in the presence of 1,25-(OH)2D3. More importantly, the 1,25-(OH)2D3-induced VDR recruitment to the VDRE site of the IGFBP-3 promoter significantly stimulated IGFBP-3 promoter activity and IGFBP-3 mRNA expression in LNCaP cells and other types of cells (18, 23). On the other hand, SRC-3 has been shown to be a transcriptional coactivator for VDR; SRC-3 interacts with VDR in a ligand-dependent manner and enhances 1,25-(OH)2D3-induced and VDR-dependent transcription in keratinocytes (22). Based on these studies, we reasoned that SRC-3 might serve as a coactivator for VDR to regulate IGFBP-3 expression. Indeed, we found that the VDR/RXR-mediated transcriptional activity of the IGFBP-3 promoter is dependent on the mapped VDRE and the presence of SRC-3 in MEFs. These results indicate that SRC-3 is required for 1,25-(OH)2D3-induced IGFBP-3 promoter activity and that this promoter activity is dependent on VDR binding to the VDRE in the IGFBP-3 promoter region.
In agreement with the SRC-3 coactivator activity in VDR-mediated IGFBP-3 expression, the expression of four out of five previously known VDR target genes in the kidney was found to be significantly reduced in SRC-3−/− mice. Since these genes are not solely regulated by VDR and SRC-3, it is not surprising to see differential effects of SRC-3 deficiency on the expression of different vitamin D-responsive genes. Overall, our data support the notion that SRC-3 is an in vivo coactivator for VDR. In addition, a previous study has shown that VDR-knockout mice were smaller and had 29% lower IGF-I levels in serum than did WT mice when fed a normal diet (27). In the future, it will be interesting to find out if the reduced IGF-I levels in VDR-knockout mice are also a consequence of reduced IGFBP-3 expression.
Although this study has demonstrated the activation functions of SRC-3 and VDR for the IGFBP-3 expression, other nuclear receptors might also be involved in SRC-3-regulated IGFBP-3 expression. It has been shown that activation of the RA receptor β (RARβ) by 9-cis-RA can induce IGFBP-3 expression in cultured cells and that the IGFBP-3 promoter contains active DR-8 RA response elements (2). Since SRC-3 is also a putative coactivator of RAR (19), it cannot be excluded that a partially decreased RAR function might also contribute to the reduced IGFBP-3 expression in SRC-3−/− mice. Furthermore, it has also been shown that the estrogen antagonist ICI 182780 could induce IGFBP-3 expression in breast cancer cells, suggesting that the function of estrogen receptor (ER) is to suppress IGFBP-3 expression. Since IGFBP-3 is reduced in SRC-3−/− mice, where the estrogen level and ER function are partially reduced (35, 41), the altered estrogen and ER function should not be responsible for the decrease in IGFBP-3 expression in SRC-3−/− mice. In addition, IGFBP-3 was found to interact with RXRα in the cell nucleus (14). However, it is unclear if SRC-3 is involved in the modulation of RXRα activity by IGFBP-3.
Although the three members of the SRC family are homologous to each other (34), SRC-1 and SRC-2 do not have the same role as SRC-3 in regulating somatic growth and IGF-I levels in the circulation. SRC-1- and SRC-2-knockout mice showed normal or nearly normal somatic growth and normal IGF-I and IGFBP-3 levels (5, 36). The present study also showed that although both SRC-1 and SRC-2 were comparably expressed in WT and SRC-3−/− MEFs, the vitamin D-stimulated IGFBP-3 promoter activity was still compromised in SRC-3−/− MEFs. Therefore, only SRC-3 in the SRC family plays a major role in regulating IGFBP-3 expression for maintenance of the circulating concentration and endocrine function of IGF-I. However, additional studies are required for understanding the underlying mechanisms governing the specificity of SRC-3 for IGFBP-3 expression in vivo.
Taken together, our findings have revealed a novel role for SRC-3 in regulation of IGFBP-3 and IGF-I concentrations in the circulation. SRC-3 serves as an in vivo transcriptional coactivator for VDR, and it enhances IGFBP-3 mRNA expression. Inactivation of SRC-3 causes down-regulation of IGFBP-3 mRNA expression, leading to a reduction of IGFBP-3 protein levels in the circulation. Consequently, the low IGFBP-3 levels shorten the half-life of IGF-I and cause a rapid degradation of IGF-I, resulting in low IGF-I levels in the circulation. Therefore, it appears that SRC-3 maintains normal circulating IGF-I levels and its endocrine function by regulating IGFBP-3 expression.
Acknowledgments
We thank Bert W. O'Malley for scientific discussion, Suoling Zhou for technical assistance, David Feldman and Lihong Peng for IGFBP-3 promoter/reporter plasmids, and Johan W. van Neck for mouse IGFBP-3 cDNA plasmid.
This work is partially funded by research grants from the National Institutes of Health to J. Xu. J. Xu is also a recipient of an American Cancer Society Scholar Award.
Footnotes
Published ahead of print on 22 January 2008.
REFERENCES
- 1.Anzick, S. L., J. Kononen, R. L. Walker, D. O. Azorsa, M. M. Tanner, X. Y. Guan, G. Sauter, O. P. Kallioniemi, J. M. Trent, and P. S. Meltzer. 1997. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277965-968. [DOI] [PubMed] [Google Scholar]
- 2.Chang, Y. S., J. Y. Cho, H. A. Cho, H. J. Kim, J. Chang, C. M. Ahn, S. K. Kim, and S. K. Kim. 2006. 9-Cis retinoic acid induces insulin-like growth factor binding protein-3 through DR-8 retinoic acid responsive elements. Cancer Biol. Ther. 5586-592. [DOI] [PubMed] [Google Scholar]
- 3.Chen, H., R. J. Lin, W. Xie, D. Wilpitz, and R. M. Evans. 1999. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell 98675-686. [DOI] [PubMed] [Google Scholar]
- 4.Chin, E., J. Zhou, J. Dai, R. C. Baxter, and C. A. Bondy. 1994. Cellular localization and regulation of gene expression for components of the insulin-like growth factor ternary binding protein complex. Endocrinology 1342498-2504. [DOI] [PubMed] [Google Scholar]
- 5.Gehin, M., M. Mark, C. Dennefeld, A. Dierich, H. Gronemeyer, and P. Chambon. 2002. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol. Cell. Biol. 225923-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han, S. J., F. J. Demayo, J. Xu, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 2006. Steroid receptor coactivators SRC-1 and SRC-3 differentially modulate tissue-specific activation functions of the progesterone receptor. Mol. Endocrinol. 2045-55. [DOI] [PubMed] [Google Scholar]
- 7.Han, S. J., J. Jeong, F. J. Demayo, J. Xu, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 2005. Dynamic cell type specificity of SRC-1 coactivator in modulating uterine progesterone receptor function in mice. Mol. Cell. Biol. 258150-8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones, J. I., and D. R. Clemmons. 1995. Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 163-34. [DOI] [PubMed] [Google Scholar]
- 9.Kuang, S. Q., L. Liao, S. Wang, D. Medina, B. W. O'Malley, and J. Xu. 2005. Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen-induced mammary tumorigenesis. Cancer Res. 657993-8002. [DOI] [PubMed] [Google Scholar]
- 10.Kuang, S. Q., L. Liao, H. Zhang, A. V. Lee, B. W. O'Malley, and J. Xu. 2004. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res. 641875-1885. [DOI] [PubMed] [Google Scholar]
- 11.Kuang, S. Q., L. Liao, H. Zhang, F. A. Pereira, Y. Yuan, F. J. DeMayo, L. Ko, and J. Xu. 2002. Deletion of the cancer-amplified coactivator AIB3 results in defective placentation and embryonic lethality. J. Biol. Chem. 27745356-45360. [DOI] [PubMed] [Google Scholar]
- 12.Li, Y. C., A. E. Pirro, and M. B. Demay. 1998. Analysis of vitamin D-dependent calcium-binding protein messenger ribonucleic acid expression in mice lacking the vitamin D receptor. Endocrinology 139847-851. [DOI] [PubMed] [Google Scholar]
- 13.Liao, L., R. K. Dearth, S. Zhou, O. L. Britton, A. V. Lee, and J. Xu. 2006. Liver-specific overexpression of the insulin-like growth factor-I enhances somatic growth and partially prevents the effects of growth hormone deficiency. Endocrinology 1473877-3888. [DOI] [PubMed] [Google Scholar]
- 14.Liu, B., H. Y. Lee, S. A. Weinzimer, D. R. Powell, J. L. Clifford, J. M. Kurie, and P. Cohen. 2000. Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-alpha regulate transcriptional signaling and apoptosis. J. Biol. Chem. 27533607-33613. [DOI] [PubMed] [Google Scholar]
- 15.Liu, J. P., J. Baker, A. S. Perkins, E. J. Robertson, and A. Efstratiadis. 1993. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 7559-72. [PubMed] [Google Scholar]
- 16.Liu, M., M. H. Lee, M. Cohen, M. Bommakanti, and L. P. Freedman. 1996. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 10142-153. [DOI] [PubMed] [Google Scholar]
- 17.Mark, M., H. Yoshida-Komiya, M. Gehin, L. Liao, M. J. Tsai, B. W. O'Malley, P. Chambon, and J. Xu. 2004. Partially redundant functions of SRC-1 and TIF2 in postnatal survival and male reproduction. Proc. Natl. Acad. Sci. USA 1014453-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matilainen, M., M. Malinen, K. Saavalainen, and C. Carlberg. 2005. Regulation of multiple insulin-like growth factor binding protein genes by 1α,25-dihydroxyvitamin D3. Nucleic Acids Res. 335521-5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenna, N. J., R. B. Lanz, and B. W. O'Malley. 1999. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 20321-344. [DOI] [PubMed] [Google Scholar]
- 20.Modric, T., J. V. Silha, Z. Shi, Y. Gui, A. Suwanichkul, S. K. Durham, D. R. Powell, and L. J. Murphy. 2001. Phenotypic manifestations of insulin-like growth factor-binding protein-3 overexpression in transgenic mice. Endocrinology 1421958-1967. [DOI] [PubMed] [Google Scholar]
- 21.Nishihara, E., H. Yoshida-Komiya, C. S. Chan, L. Liao, R. L. Davis, B. W. O'Malley, and J. Xu. 2003. SRC-1 null mice exhibit moderate motor dysfunction and delayed development of cerebellar Purkinje cells. J. Neurosci. 23213-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oda, Y., C. Sihlbom, R. J. Chalkley, L. Huang, C. Rachez, C. P. Chang, A. L. Burlingame, L. P. Freedman, and D. D. Bikle. 2003. Two distinct coactivators, DRIP/mediator and SRC/p160, are differentially involved in vitamin D receptor transactivation during keratinocyte differentiation. Mol. Endocrinol. 172329-2339. [DOI] [PubMed] [Google Scholar]
- 23.Peng, L., P. J. Malloy, and D. Feldman. 2004. Identification of a functional vitamin D response element in the human insulin-like growth factor binding protein-3 promoter. Mol. Endocrinol. 181109-1119. [DOI] [PubMed] [Google Scholar]
- 24.Powell-Braxton, L., P. Hollingshead, C. Warburton, M. Dowd, S. Pitts-Meek, D. Dalton, N. Gillett, and T. A. Stewart. 1993. IGF-I is required for normal embryonic growth in mice. Genes Dev. 72609-2617. [DOI] [PubMed] [Google Scholar]
- 25.Segawa, H., I. Kaneko, S. Yamanaka, M. Ito, M. Kuwahata, Y. Inoue, S. Kato, and K. Miyamoto. 2004. Intestinal Na-P(i) cotransporter adaptation to dietary P(i) content in vitamin D receptor null mice. Am. J. Physiol. Renal Physiol. 287F39-F47. [DOI] [PubMed] [Google Scholar]
- 26.Silha, J. V., and L. J. Murphy. 2002. Insights from insulin-like growth factor binding protein transgenic mice. Endocrinology 1433711-3714. [DOI] [PubMed] [Google Scholar]
- 27.Song, Y., S. Kato, and J. C. Fleet. 2003. Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J. Nutr. 133374-380. [DOI] [PubMed] [Google Scholar]
- 28.Stewart, C. E., and P. Rotwein. 1996. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol. Rev. 761005-1026. [DOI] [PubMed] [Google Scholar]
- 29.Torres-Arzayus, M. I., J. F. De Mora, J. Yuan, F. Vazquez, R. Bronson, M. Rue, W. R. Sellers, and M. Brown. 2004. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 6263-274. [DOI] [PubMed] [Google Scholar]
- 30.Wang, Z., D. W. Rose, O. Hermanson, F. Liu, T. Herman, W. Wu, D. Szeto, A. Gleiberman, A. Krones, K. Pratt, R. Rosenfeld, C. K. Glass, and M. G. Rosenfeld. 2000. Regulation of somatic growth by the p160 coactivator p/CIP. Proc. Natl. Acad. Sci. USA 9713549-13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss, R. E., J. Xu, G. Ning, J. Pohlenz, B. W. O'Malley, and S. Refetoff. 1999. Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone. EMBO J. 181900-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu, R. C., J. Qin, P. Yi, J. Wong, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 2004. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic responses to multiple cellular signaling pathways. Mol. Cell 15937-949. [DOI] [PubMed] [Google Scholar]
- 33.Xu, J. 2005. Preparation, culture, and immortalization of mouse embryonic fibroblasts, p. 28.1.1-28.1.8. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 5. John Wiley & Sons, Inc., New York, NY. [DOI] [PubMed] [Google Scholar]
- 34.Xu, J., and Q. Li. 2003. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol. Endocrinol. 171681-1692. [DOI] [PubMed] [Google Scholar]
- 35.Xu, J., L. Liao, G. Ning, H. Yoshida-Komiya, C. Deng, and B. W. O'Malley. 2000. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc. Natl. Acad. Sci. USA 976379-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu, J., Y. Qiu, F. J. DeMayo, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1998. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 2791922-1925. [DOI] [PubMed] [Google Scholar]
- 37.Yakar, S., J. L. Liu, B. Stannard, A. Butler, D. Accili, B. Sauer, and D. LeRoith. 1999. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 967324-7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yakar, S., C. J. Rosen, W. G. Beamer, C. L. Ackert-Bicknell, Y. Wu, J. L. Liu, G. T. Ooi, J. Setser, J. Frystyk, Y. R. Boisclair, and D. LeRoith. 2002. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Investig. 110771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye, X., S. J. Han, S. Y. Tsai, F. J. DeMayo, J. Xu, M. J. Tsai, and B. W. O'Malley. 2005. Roles of steroid receptor coactivator (SRC)-1 and transcriptional intermediary factor (TIF) 2 in androgen receptor activity in mice. Proc. Natl. Acad. Sci. USA 1029487-9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshizawa, T., Y. Handa, Y. Uematsu, S. Takeda, K. Sekine, Y. Yoshihara, T. Kawakami, K. Arioka, H. Sato, Y. Uchiyama, S. Masushige, A. Fukamizu, T. Matsumoto, and S. Kato. 1997. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 16391-396. [DOI] [PubMed] [Google Scholar]
- 41.Yuan, Y., L. Liao, D. A. Tulis, and J. Xu. 2002. Steroid receptor coactivator-3 is required for inhibition of neointima formation by estrogen. Circulation 1052653-2659. [DOI] [PubMed] [Google Scholar]
- 42.Zhou, C., M. Assem, J. C. Tay, P. B. Watkins, B. Blumberg, E. G. Schuetz, and K. E. Thummel. 2006. Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. J. Clin. Investig. 1161703-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]