Abstract
GP64, the major envelope glycoprotein of the Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV) budded virion, is important for host cell receptor binding and mediates low-pH-triggered membrane fusion during entry by endocytosis. In the current study, we examined the functional role of the AcMNPV GP64 transmembrane (TM) domain by replacing the 23-amino-acid GP64 TM domain with corresponding TM domain sequences from a range of viral and cellular type I membrane proteins, including Orgyia pseudotsugata MNPV (OpMNPV) GP64 and F, thogotovirus GP75, Lymantria dispar MNPV (LdMNPV) F, human immunodeficiency virus type 1 (HIV-1) GP41, human CD4 and glycophorin A (GpA), and influenza virus hemagglutinin (HA), and with a glycosylphosphatidylinositol (GPI) anchor addition sequence. In transient expression experiments with Sf9 cells, chimeric GP64 proteins containing either a GPI anchor or TM domains from LdMNPV F or HIV-1 GP41 failed to localize to the cell surface and thus appear to be incompatible with either GP64 structure or cell transport. All of the mutant constructs detected at the cell surface mediated hemifusion (outer leaflet merger) upon low-pH treatment, but only those containing TM domains from CD4, GpA, OpMNPV GP64, and thogotovirus GP75 mediated pore formation and complete membrane fusion activity. This supports a model in which partial fusion (hemifusion) proceeds by a mechanism that is independent of the TM domain and the TM domain participates in the enlargement or expansion of fusion pores after hemifusion. GP64 proteins containing heterologous TM domains mediated virion budding with dramatically differing levels of efficiency. In addition, chimeric GP64 proteins containing TM domains from CD4, GpA, HA, and OpMNPV F were incorporated into budded virions but were unable to rescue the infectivity of a gp64 null virus, whereas those with TM domains from OpMNPV GP64 and thogotovirus GP75 rescued infectivity. These results show that in addition to its basic role in membrane anchoring, the GP64 TM domain is critically important for GP64 trafficking, membrane fusion, virion budding, and virus infectivity. These critical functions were replaced only by TM domains from related viral membrane proteins.
The family Baculoviridae is a large family of double-stranded DNA viruses that are pathogenic for insects, primarily those in the order Lepidoptera but also including some insects in the orders Diptera and Hymenoptera. The family Baculoviridae currently includes the following two genera: Nucleopolyhedrovirus (NPV) and Granulovirus (16, 47). The NPV genus can be subdivided phylogenetically into two subgroups, which are referred to as group I and group II (12, 47). The virus Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV) is a member of the group I NPVs and is the type species for the Baculoviridae. In the infection cycle, NPVs such as AcMNPV produce two forms of virions, the occlusion-derived virus and the budded virus (BV). Occlusion-derived viruses initiate infection in the gut of a susceptible host and are responsible for the horizontal spread of the virus in an insect population, whereas BV are responsible for the spread of the infection from cell to cell in the individual larval host and in cell culture (1, 47).
BV enter cells via a clathrin-mediated, low-pH-dependent endocytic pathway (21, 51). During entry by endocytosis, the major envelope glycoprotein GP64 (group I NPVs) or F protein (group II NPVs) mediates membrane fusion. F proteins appear to have general structural and functional similarities to paramyxovirus F proteins. F proteins like those from Spodoptera exigua MNPV and Lymantria dispar MNPV (LdMNPV) are first expressed as a precursor (F0) and then cleaved into two disulfide-linked subunits (F1 and F2) by a cellular furin-like proprotein convertase (42, 53). The cleavage is essential for activation of the F protein to a fusogenic form (53). Unlike F proteins, GP64 is not cleaved by a protease. The GP64 proteins are highly conserved in the group I NPVs and appear to be similar in structure and function only to GP75 proteins from a subgroup of the orthomyxoviruses, the thogotoviruses (35). GP64 is a type I integral membrane protein that is present on the infected cell surface and in the virion as a homotrimer that is covalently associated by disulfide bonds (38). Interestingly, GP64 trimers are typically observed as two distinct electrophoretic forms (with different migration rates by nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]), but the two forms have the same mass, as determined by mass spectrometry (38). While preliminary data suggest that the two trimer forms may represent disulfide isomers (26), it is not known whether they differ in any functional properties. GP64 is involved in host cell receptor binding (8) and is both necessary and sufficient for mediating pH-dependent membrane fusion during viral entry (3, 52). In addition to its essential role in virus entry in group I NPVs, GP64 is also necessary for efficient budding and production of infectious virions (34, 37). The AcMNPV GP64 protein is posttranslationally modified by palmitoylation, and by glycosylation at multiple sites. However, neither the palmitoylation site nor any single glycosylation site is necessary for GP64 synthesis or transport, BV production or infectivity, or membrane fusion activity (15, 54). GP64 is also posttranslationally modified by phosphorylation (27, 50), and little is known of the structural or functional implications of this modification. Using mutagenesis, two important hydrophobic regions were identified within the GP64 ectodomain (33). One, a putative fusion peptide, is located between amino acids (aa) 220 and 230 of Orgyia pseudotsugata MNPV (OpMNPV) GP64, and amino acid substitutions that reduced the hydrophobic nature of the local domain disrupted the fusion activity of the GP64 protein. The second hydrophobic region lies within a highly conserved 4-3 heptad repeat (residues 299 to 346) that is predicted to form an amphipathic alpha-helix (33). Replacement of two heptad repeat leucines with either alanine or proline resulted in disruption of oligomerization and fusion function. In addition, peptide inhibition studies of AcMNPV GP64 suggest that the hydrophobic 4-3 heptad repeat plays a direct role in membrane fusion (18). GP64 proteins have very short predicted cytoplasmic tail domains (CTD), ranging from approximately 3 to 8 aa in length. Experimental analysis showed that the 7-aa CTD of AcMNPV GP64 is not essential for production of infectious BV, but removal of the CTD results in a measurable reduction in budding efficiency (37).
In addition to anchoring the protein in the membrane, the transmembrane (TM) domains of many viral envelope fusion proteins play an important role in membrane fusion. Replacement of the TM domain of the influenza virus hemagglutinin (HA) protein with a glycosylphosphatidylinositol (GPI) anchor resulted in a protein that mediated hemifusion (17) and fusion pore formation, but an apparent block in pore enlargement was observed (17, 25). In addition, although some heterologous TM domains can substitute functionally for the native TM of human immunodeficiency virus type 1 (HIV-1) GP41 (31, 40), vesicular stomatitis virus G (36), or HA (28), specific amino acid sequences within the TM domains are required to induce efficient fusion (7, 10, 29, 40). Baculovirus GP64s have predicted TM domains of 16 to 23 aa. Unlike the highly conserved ectodomain amino acid sequences (which are approximately 80% identical), the sequences of the TM domains can be highly variable, ranging from approximately 13 to 100% identity. Beyond the most fundamental role in anchoring GP64 in the membrane, the biological function(s) of the GP64 TM domain has not been examined.
In this study, we investigated the functional role of the TM domain of the AcMNPV GP64 protein. We constructed chimeric proteins in which the TM domain of GP64 was replaced by equivalent sequences from other TM proteins and examined cell surface localization, membrane fusion, virion budding, and virus infectivity. Our results indicate that the specific amino acid sequence of the GP64 TM domain is critical for the function of GP64.
MATERIALS AND METHODS
Cells, transfections, and infections.
Spodoptera frugiperda (Sf9) cells and the cell line Sf9Op1D, which constitutively expresses the OpMNPV GP64 protein (43), were cultured at 27°C in TNMFH medium (13) containing 10% fetal bovine serum (FBS). Transfections were carried out using the CaPO4 precipitation method as described earlier (2). For viral infection, virus was incubated with cells for 1 h, and afterwards, cells were washed once in TNMFH. Times postinfection (p.i.) were calculated from the time the viral inoculum was added.
Mutagenesis and construction of plasmids and bacmids.
The promoter region and open reading frame (ORF) of the AcMNPV gp64 gene were cloned into plasmid pGEM3Z (Promega) to generate plasmid pGEM3ZGP64, which was used as a target for mutagenesis. The TM domain replacement mutants were generated using the slim-site mutation method (6) and the PCR primers shown in Table 1. For this protocol, the first-round PCR primers were FftX, FrtX, RFs, and RRs, and the second-round PCR primers were BftX, BrtX, RFs, and BrcX (where X represents the source of the TM domain). The transient expression plasmids, named pBieY plasmids (Y represents different modified GP64 genes), were constructed by subcloning the PCR-amplified fragments of the 599-bp AcMNPV ie-1 promoter (primers Acie1pF [AATAATGAGCTCATCGATGTCTTTGTGATGCG] and Acie1pR [AATAATTCTAGAAGTCACTTGGTTGTTCACGA]) and the 218-bp GP64 poly(A) signal (primers GP64pAF [AATAAGAATTCATGTAATAATAAAAATTGTATCA] and GP64pAR [AATAATAAGCTTCACACTCGCTATTTGGAA]) into pBlue SK(−) to generate plasmid pBiepA. The pBieY plasmids were subsequently generated by subcloning the different mutant GP64 ORFs, which were PCR amplified from the mutant pGEM3ZGP64 (primers Ac64oF [AATAATTCTAGAATGGTAAGCGCTATTGTTT] and Ac64oR [AATAATGAATTCTTAATATTGTCTATTACGGTTTC]), into the XbaI/EcoRI sites of pBiepA. All pBieY plasmids were purified using a DNA Maxiprep kit (Marligen Biosciences, Inc.). In order to construct recombinant baculoviruses expressing the modified GP64 proteins, GP64 constructs in pGEM3ZGP64 were digested with KpnI and EcoRI to excise the fragment containing the GP64 promoter region and GP64 ORF, and the excised fragments were subcloned into the KpnI and EcoRI sites of the pFastBac1 plasmid (Invitrogen). The modified gp64 genes were then inserted into the polyhedrin locus of the bacmid (AcMNPV gp64 null bacmid]vAc64−]) by Tn7-mediated transposition as described previously (23). All constructs were confirmed by restriction enzyme digestion and DNA sequencing.
TABLE 1.
PCR primers used for production of TM-substituted GP64 mutants
| Primer | Sequence (5′ to 3′) |
|---|---|
| FftCD4 | ATGGCCCTGATTGTGCTGGGTGGCGTCGCCGGCCTCAGAAACCGTAATAGACAA |
| BftCD4 | CTGCTTTTCATTGGGCTAGGCATCTTCTTCTGTGTCAGAAACCGTAATAGACAA |
| BrcCD4 | GAGGCCGGCGACGCCACC |
| FftGpA | ATAACACTCATTATTTTTGGGGTGATGGTGTTAGAAACCGTAATAGACAA |
| BftGpA | ATTGGAACGATCCTCTTAATTTCTTACGGTATTCGCAGAAACCGTAATAGACAA |
| BrcGpA | AACACCAGCCATCACCCC |
| FftHA | TGGATCCTGTGGATTTCCTTTGCCATATCATGCTTTAGAAACCGTAATAGACAA |
| BftHA | TTGCTTTGTGTTGTTTTGCTGGGGTTCATCATGAGAAACCGTAATAGACAA |
| BrcHA | AAAGCATGATATGGCAAAGG |
| FftGP41 | ATATTCATAATGATAGTAGGAGGCTTAATAGGAAGAAACCGTAATAGACAA |
| BftGP41 | TTAAGAATAGTTTTTGCTGTGCTTTCTATAATAAGAAACCGTAATAGACAA |
| BrcGP41 | TCCTATTAAGCCTCCTAC |
| FftLdF | GCGGTGGTGGCGTGCGTCGTTCTGTTCCTGAGAAACCGTAATAGACAA |
| BftLdF | GTGGCGCTGTTGCTGTTTCGCATTTACAGAAACCGTAATAGACAA |
| BrcLdF | CAGGAACAGAACGACGCA |
| FftOpF | ATGCTGGTGTACGTGCTGCTGGGCGTGGGTGTCAGAAACCGTAATAGACAA |
| BftOpF | TTGAGCGCGCTTTGCGTCGTTGGCGCCTATAGAAACCGTAATAGACAA |
| BrcOpF | GACACCCACGCCCAGCAG |
| FftOpGP64 | TTTATGCTGGGGCACGGGTTCACCTTTGTGTTGATTAGAAACCGTAATAGACAA |
| BftOpGP64 | GTGGGCGTCATCCTGTTTTTGGTTTGCATGTTGAGAAACCGTAATAGACAA |
| BrcOpGP64 | AATAATCAACACAAAGGTGAACCCG |
| FftGP75 | TTGCTATATGGCAATATCGGTGTGTACTTGTTAATTAGAAACCGTAATAGACAA |
| BftGP75 | GCTTTCGCTTTTGTGCTATTGATCAGACTAATTAGAAACCGTAATAGACAA |
| BrcGP75 | AATTAACAAGTACACACC |
| FftGPI | GCAGCGGCCCCGGCTCCTGGTGCTCCTCTGCTCCCTAGAAAC CGTAATAG ACAA |
| BftGPI | CTGCCAGCCCTCGCAGCACGACTGCTCCCACCAGCACTCAGAAACCGTAATAGACAA |
| BrcGPI | AGGGAGCAGAGGAGCACCAGGAGC |
| RFs | AGAAACCGTAATAGACAA |
| FrtCD4 | GAGGCCGGCGACGCCCCCCAGCACAATCAGGGCCATCGAAGTCAATTTAGCGGC |
| BrtCD4 | GACACAGAAGAAGATGCCTAGCCCAATGAAAAGCAGGAGGCCGGCGACGCCACC |
| FrtGpA | AACACCAGCCATCACCCCAAAAATAATGAGTGTTATCGAAGTCAATTTAGCGGC |
| BrtGpA | GCGAATACCGTAAGAAATTAAGAGGATCGTTCCAATAACACCAGCCATCACCCC |
| FrtHA | AAAGCATGATATGGCAAAGGAAATCCACAGGATCCACGAAGTCAATTTAGCGGC |
| BrtHA | CATGATGAACCCCAGCAAAACAACACACAAAGCAAAAAGCATGATATGGCAAAGG |
| FrtGP41 | TCCTATTAAGCCTCCTACTATCATTATGAATATCGAAGTCAATTTAGCGGC |
| BrtGP41 | TATTATAGAAAGCACAGCAAAAACTATTCTTAATCCTATTAAGCCTCCTAC |
| FrtLdf | CAGGAACAGAACGACGCAC GCCACCACCGCCGAAGTCAATTTAGCGGC |
| BrtLdf | GTAAATGCGAAACAGCAACAGCGCCACCAGGAACAGAACGACGCA |
| FrtOpF | GACACCCACGCCCAGCAGCACGTACACCAGCATCGAAGTCAATTTAGCGGC |
| BrtOpf | ATAGGCGCCAACGACGCAAAGCGCGCTCAAGACACCCACGCCCAGCAG |
| FrtOpGP64 | AATCAACACAAAGGTGAACCCGTGCCCCAGCATAAACGAAGTCAATTTAGCGGC |
| BrtOpGP64 | CAACATGCAAACCAAAAACAGGATGACGCCCACAATAATCAACACAAAGGTGAACCCG |
| FrtGP75 | AATTAACAAGTACACACCGATATTGCCATATAGCAACGAAGTCAATTTAGCGC |
| BrtGP75 | AATTAGTCTGATCAATAGCACAAAAGCGAAAGCAATTAACAAGTACACACCG |
| FrtGPI | CAGAGGAGCACCAGGAGCCGGGGCCGCTGC GCCCGACGAAGTCAATTTAGCGGC |
| BrtGPI | TGGTGGGAGCAGTCGTGCTGCGAGGGCTGGCAGCAGCAGAGGGAGCAGAGGAGCACCAGGAGC |
| RRs | CGAAGTCAATTTAGCGC |
cELISA and syncytium formation assays.
For analysis of cell surface localization of GP64 proteins by cell surface enzyme-linked immunosorbent assays (cELISAs), Sf9 cells were transfected with plasmids, incubated for 24 h, and then fixed in glutaraldehyde, and cell surface-localized GP64 was detected with monoclonal antibody (MAb) AcV5 as described previously (54). Briefly, transfected cells in 12-well plates (Corning Inc.) were rinsed once in phosphate-buffered saline (PBS; pH 7.4) and fixed in 0.5% glutaraldehyde for 10 min at room temperature (RT) so that cells were not permeabilized. Fixed cells were washed once with PBS and blocked by incubation for 2 h in PBS containing 1% gelatin at 27°C. Cells were then incubated with MAb AcV5 (hybridoma culture supernatant diluted 1:25 in PBS containing 0.5% gelatin) for 45 min at 27°C. Cells were washed three times in PBS and then incubated with a secondary goat anti-mouse antibody conjugated to beta-galactosidase (diluted 1:750 in PBS containing 0.5% gelatin) for 45 min at 27°C. Cells were washed five times in PBS and then incubated at 37°C in 2-mg/ml chlorophenol red-beta-d-galactopyranoside (CPRG) in a solution of PBS containing 2 mM MgCl2 and 1% bovine serum albumin. After addition of the substrate, the absorbance at an optical density of 570 nm was determined using an ELISA plate reader.
For membrane fusion activity assays, Sf9 cells were transfected with plasmids expressing wild-type (wt) or modified forms of GP64 in 12-well plates. At 24 h posttransfection (p.t.), TNMFH medium was removed, and cells were washed once with PBS at pH 7.4. The PBS at pH 7.4 was then replaced with PBS at pH 5.0. After 3 min of incubation, cells were washed again with PBS at pH 7.4 and then returned to TNMFH. After 4 h of incubation at 27°C, cells were fixed with methanol for 10 min and stained with a HEMA3 stain (Fisher Scientific Company LLC), and the number of nuclei found in syncytia was scored. The criterion for identification of syncytia was the presence of at least five nuclei in one syncytial mass. Five randomly selected fields were evaluated for each construct.
RBC labeling and dye transfer assays.
Sheep red blood cells (RBCs; HemoState Laboratories) were colabeled with the lipid probe octadecyl rhodamine B chloride (R18) and the aqueous dye calcein-AM (Molecular Probes, Invitrogen) and used for dye transfer assays as described previously (49), with minor modifications. In brief, 10 μl of R18 (2 mM in ethanol) was added to RBCs (1% hematocrit in 10 ml PBS) with gentle shaking. The mixture was incubated for 30 min at RT in the dark, and then 20 ml of 7.5% FBS-Dulbecco's modified Eagle's medium was added to the suspension to absorb unbound probe. After a 20-min incubation at RT in the dark, the RBC suspension was washed five times in 40 ml of PBS and resuspended in 1.25 ml of PBS. A 5-μl aliquot of calcein-AM (4 mM in dimethyl sulfoxide) was added to 1 ml R18-labeled RBCs, and the suspension was incubated for 1 h at 37°C in the dark. The unbound calcein-AM was also absorbed using 20 ml of 7.5% FBS-Dulbecco's modified Eagle's medium for 20 min, and the colabeled cells were washed four times with PBS. The double-labeled RBCs were suspended in 200 ml PBS and used within 1 h.
To analyze hemifusion and fusion pore formation by wt GP64 and GP64 mutants, an R18 and calcein transfer assay was performed. At 24 h p.t., the transfected Sf9 cells were incubated with R18- and calcein-labeled RBCs for 20 min at RT for binding. After unbound RBCs were removed by three washes with PBS (pH 7.4), the cells were incubated with PBS at pH 5.0 for 3 min at RT, washed in PBS at pH 7.4, and transferred to TNMFH medium. After incubation for 20 min at 27°C, hemadsorption and the transfer of fluorescence were observed with a phase-contrast microscope and an epifluorescence microscope, respectively. Five randomly selected fields were scored for dye transfer.
Immunofluorescence analysis.
Sf9 cells were plated in 12-well plates (4 × 105 cells/well) and transfected with plasmids expressing either wt or mutant GP64 proteins. At 24 h p.t., cells were fixed with methanol or 4% paraformaldehyde in PBS (pH 7.4). Cells were washed with PBS and then incubated with a blocking buffer (1% gelatin in PBS, pH 7.4) at 27°C for 2 h. After being washed with PBS, the cells were incubated with a primary anti-GP64 MAb (AcV1 or AcV5; 1:25 dilution in PBS) at 27°C for 1 h. Cells were washed three times with PBS (pH 7.4) and incubated for 1 h with Alexa Fluor 488-goat anti-mouse immunoglobulin G (IgG; Molecular Probes, Invitrogen) at a 1:750 dilution in PBS at 27°C. After washing of cells five times with PBS (pH 7.4), fluorescence was observed with an Olympus IX70 epifluorescence microscope.
Viral growth curves.
To generate viral growth curves, Sf9 cells (1 × 106) were infected in triplicate with each virus (vAcGP64wt, vAcGP64TM-OpGP64, or vAcGP64TM-gp75) in six-well plates at a multiplicity of infection (MOI) of 5. After a 1-h incubation, the inoculum was removed and exchanged with TNMFH. Supernatants were collected at the indicated times p.i., and the titers of all supernatants were determined by the 50% tissue culture infective dose on Sf9 cells (39).
Budding assay and measurement of GP64 incorporation into virions.
For measurement of virus budding efficiency and the relative amounts of GP64 (wt or modified) incorporated into BV, we performed the following assays. Viruses were amplified and titrated in Sf9OP1D cells. The viruses (vAcGP64wt, vAcGP64ko [GP64 knockout], vAcGP64TM-CD4, vAcGP64TM-GpA, vAcGP64TM-HA, vAcGP64TM-OpF, vAcGP64TM-OpGP64, and vAcGP64TM-GP75) were used to infect Sf9 cells (5 × 106) at an MOI of 5. After inoculation and incubation for 1 h, the cells were washed once with TNMFH and further incubated at 27°C. At 15 h p.i., cells were washed once and starved by incubation in 2 ml methionine-free Grace's medium (Grace's−met; Invitrogen) for 1 h. At 16 h p.i., the medium was replaced with 2.2 ml of Grace's−met containing 200 μCi of 35S-EasyTag Express protein labeling mix (1,175.0 Ci/mmol; Perkin-Elmer Life Sciences). At 30 h p.i., 0.8 ml of TNMFH was added. The supernatants were harvested at 40 h p.i., cleared of cell debris by brief centrifugation (10 min at 3,000 × g and 4°C), and then loaded onto a 25% sucrose cushion and centrifuged at 80,000 × g for 90 min at 4°C in an SW60 rotor. Virus pellets were resuspended in 200 μl Laemmli buffer (4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.04% bromophenol blue, 0.125 M Tris, pH 6.8) containing a cocktail of protease inhibitors (Complete; Roche Applied Science) and electrophoresed in SDS-10% polyacrylamide gels. Dried gels were exposed on a phosphorimager screen and scanned on a Molecular Dynamics phosphorimager. Quantification of individual bands was performed with the ImageQuant TL software package (Amersham).
Western blot analysis.
Reducing and nonreducing SDS-PAGE was performed with SDS-6% or 10% polyacrylamide slab gels as described previously (37). Following transfer of proteins to polyvinylidene difluoride membranes (Millipore), blots were blocked in a 4% milk-Tris-buffered saline-Tween 20 solution and processed as previously described (54). MAbs AcV5 and B12D5 were used at a dilution of 1:1,000. Immunoreactive proteins were visualized using alkaline phosphatase-conjugated anti-mouse IgG antibody and nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP; Promega).
RESULTS
Expression and intracellular processing of GP64 chimeras.
To determine whether the TM domain of the AcMNPV GP64 protein is required for virus infectivity and membrane fusion, we replaced the TM domain of GP64 with analogous regions from eight type 1 viral or eukaryotic membrane proteins and examined the functions of the resulting proteins. For these studies, we selected the TM domains from six viral envelope proteins and two cellular membrane proteins, and we also replaced the GP64 TM domain with a GPI anchor signal. The selected viral TM domains were from the baculovirus OpMNPV GP64 and F proteins, the LdMNPV F protein, influenza virus HA (X:31 strain), thogotovirus GP75, and HIV-1 GP41. TM domains from cellular membrane proteins included those from human GpA and human CD4. The GPI anchor signal was from human melanotransferrin (p97). A summary of the chimeric GP64 constructs is shown in Fig. 1. To characterize the function of GP64 proteins containing substitutions of the TM domain, the substituted proteins were transiently expressed from plasmids in Sf9 cells. At 24 h posttransfection, the expressed proteins were all detected by Western blot analysis of proteins from reducing SDS-PAGE gels (Fig. 2B). The oligomeric forms (trimer I, trimer II, and dimer) of all mutants except GP64GPI were detected by Western blot analysis of proteins from nonreducing gels, and their profiles appeared similar to those of oligomers of wt GP64 (Fig. 2A, lane 1 versus lanes 2 to 9). The GP64GPI construct was expressed but appeared to be defective for oligomerization, since the characteristic trimer forms were not observed in nonreducing gels. However, the monomer was readily detected in reducing gels (Fig. 2B, lane 10), indicating that the protein was expressed and stable.
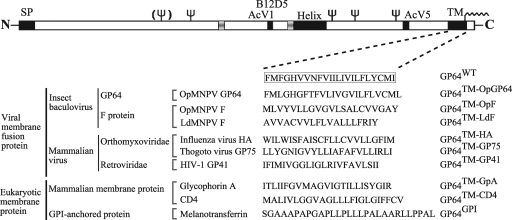
FIG. 1.
Schematic representation of wt GP64 protein and GP64 chimeras containing substitutions of the GP64 TM domain. The GP64 TM domain sequence (GP64) is shown as a boxed sequence, and the TM domain replacements are shown below. The origins of the sequences used for TM domain replacements are indicated to the left of each sequence. The schematic at the top shows the positions of several major features of the GP64 protein, including five predicted glycosylation sites (fork symbols) and an acylation site (wave symbol). SP, signal peptide; AcV1, B12D5, and AcV5, MAb epitopes; TM, transmembrane domain; helix, predicted amphipathic alpha-helix.
FIG. 2.
Analysis of oligomerization of GP64 chimeras containing TM domain substitutions. Sf9 cells were transfected with expression plasmids encoding wt and GP64 mutants. Cells were lysed 24 h after transfection under nonreducing (A) or reducing (B) conditions. GP64 proteins were resolved by SDS-PAGE and Western blotting using MAb AcV5. The positions of oligomeric forms of GP64 (trimers I and II and dimer) are identified on the right.
Two of the mutant GP64 constructs, GP64TM-LdF and GP64TM-OpF, were detected at lower levels than those of the other constructs (Fig. 2). These observations suggest that GP64 substitution mutants GP64TM-LdF and GP64TM-OpF may be synthesized at a lower rate or may be less stable.
Cell surface localization and conformation.
We next examined the cellular transport of the GP64 chimeras. Using a cELISA protocol, we measured the relative cell surface level of each chimera and compared that to the level detected from wt GP64. We first established a linear range of GP64 detection at the cell surface by titrating levels of wt GP64. By transfecting Sf9 cells with decreasing quantities of the plasmid that expressed wt GP64 and performing cELISA analysis, we established a standard curve for GP64 detection. We then compared the relative level of cell surface GP64 to the standard curve, as shown in Fig. 3A.
FIG. 3.
Analysis of relative cell surface levels of GP64 chimeras. (A) Relative cell surface levels of chimeric GP64 proteins containing TM domain substitutions were measured by cELISA, using MAb AcV5. Values represent the means from triplicate transfections and are normalized to that for 7.5 μg of plasmid DNA expressing wt GP64. Error bars represent the standard deviations from the means. The amount of DNA used per transfection is indicated below the graph. (B and C) Immunofluorescence analysis of cell surface levels of GP64 mutants, using MAb AcV5 (B) or AcV1 (C). Cell surface GP64 was detected in transfected Sf9 cells fixed with 4% paraformaldehyde (B [left] and C), and total cell GP64 was detected in Sf9 cells fixed with methanol (B [right]). Cells in panel C were transfected with plasmids expressing the following constructs: a, mock transfected; b, wt GP64; c, GP64TM-CD4; d, GP64TM-GpA; e, GP64TM-HA; f, GP64TM-OpF; g, GP64TM-OpGP64; and h, GP64TM-GP75.
For all the substituted GP64 proteins, cell surface levels were lower than that of wt GP64. Constructs GP64TM-OpGP64 and GP64TM-GP75 were detected at approximately 33% of the level of wt GP64, while constructs GP64TM-CD4, GP64TM-GpA, and GP64TM-HA were detected at the surface at relatively low levels of only 5 to 11% of the wt GP64 level. Chimeras containing TM domains from LdMNPV F (GP64TM-LdF) and HIV-1 GP41 (GP64TM-GP41) or containing the GPI anchor (GP64GPI) were found to have the lowest levels of surface localization, at approximately 1.2 to 2.8% of the wt GP64 level, which was not significantly different from the background of mock-transfected cells. To confirm the results from cELISA analysis, with particular attention to the constructs that were not detected above background, we used indirect immunofluorescence analysis to examine total cell and cell surface localization of each GP64 construct. As shown in Fig. 3B, the GP64TM-LdF, GP64TM-GP41, and GP64GPI chimeras were readily detected by MAb AcV5 when cells were permeabilized by fixation with methanol, but no fluorescence was detected at the cell surface (paraformaldehyde fixation). This, combined with cELISA data, suggests that these three constructs were either absent from or only very poorly transported to the plasma membrane and that cell surface levels were perhaps below the sensitivity of immunofluorescence detection. The other TM substitution constructs were readily detected in both permeabilized and nonpermeabilized cells by MAb AcV5 (Fig. 3B). In addition, all of the constructs detected at the cell surface were also detected by MAb AcV1 (Fig. 3C). Because the AcV1 antibody recognizes a conformational epitope present in the native, neutral-pH form of GP64 but not in conformations that result from exposure to low pH (14, 55), these results indicate that these TM substitutions are in a conformation equivalent to the native, neutral-pH conformation of wt GP64.
Fusion activity of GP64 chimeras.
The fusion activity of the GP64 chimeras with TM domain substitutions was measured by determining the syncytium formation efficiency. The fusion phenotype of each mutant GP64 was analyzed by comparison with wt GP64 localized to the cell surface at the same level. The fusion activities measured for mutants GP64TM-OpGP64 and GP64TM-GP75 were similar to that of wt GP64 (Fig. 4B). In contrast, constructs GP64TM-CD4 and GP64TM-GpA had only 3.5% and 0.28% of the wt GP64 fusion activity, respectively. Additionally, although syncytia were occasionally observed in GP64TM-CD4- or GP64TM-GpA-transfected cells, each syncytium observed contained many fewer nuclei than any given syncytium observed from wt GP64 (Fig. 4A). No syncytia were found in cells transfected with constructs GP64TM-HA and GP64TM-OpF (Fig. 4A, panels GP64TM-HA and GP64TM-OpF). Additionally, the GP64 chimeras that were defective for cell surface localization (GP64TM-LdF, GP64TM-GP41, and GP64GPI) were also defective for fusion in the syncytium formation assay (Fig. 4A).
FIG. 4.
Analysis of membrane fusion activities of chimeric GP64 proteins by syncytium formation assay. (A) Sf9 cells were transfected with plasmids expressing wt or chimeric GP64 proteins and exposed to pH 5.0 for 3 min at 24 h p.t. Cells were observed and photographed by phase-contrast microscopy (magnification, ×200) 4 h after exposure to pH 5. (B) Relative efficiencies of syncytium formation were calculated from representative random fields in which the numbers of nuclei in syncytia were divided by the number of total cells. A syncytial mass was defined as a mass containing five or more nuclei. For each sample, five fields were examined. The efficiency of syncytium formation for each construct was normalized to that of Sf9 cells transfected with 7.5 μg plasmid expressing wt GP64 (100%). The means and standard deviations of triplicate transfections are shown.
Hemifusion and pore formation.
Biological membrane fusion requires a dramatic restructuring of lipid bilayers. Lipid mixing is thought to proceed in two sequential steps. In the first step, merger of the contacting, i.e., proximal, monolayers (outer leaflets) results in an intermediate stage, termed hemifusion. In the second step, merger of the distal monolayers (inner leaflets) results in pore formation and full fusion (46). Pore formation is then followed by enlargement or widening of the pore. To determine the effect of the TM substitutions on the first step of the fusion process, we used a fluorescent-dye transfer assay to identify hemifusion and to distinguish between hemifusion and pore formation. Dually labeled RBCs (containing R18 and calcein dyes) were bound to Sf9 cells that were previously transfected with and expressing the GP64 chimeras. Membrane fusion was initiated by exposure of the RBC-bound Sf9 cells to a low pH (pH 5.0) for 3 min. R18 redistribution from RBCs to Sf9 cell membranes in the absence of calcein transfer to the Sf9 cell cytosol indicates merger of the outer membrane leaflets (hemifusion) and the absence of pore formation. All of the GP64s with substituted TMs, except for GP64TM-LdF, GP64TM-GP41, and GP64GPI (which do not efficiently localize to the cell surface), induced the transfer of R18 from RBCs to Sf9 cells (Fig. 5A). However, the R18 dye transfer efficiency was variable. For constructs GP64TM-OpGP64 and GP64TM-GP75, the R18 transfer was similar to that of wt GP64, while constructs GP64TM-CD4, GP64TM-GpA, GP64TM-HA, and GP64TM-OpF induced R18 transfer at levels lower than that observed for wt GP64 (expressed at the cell surface at similar or lower levels). This indicated that the replacement of the TM domain of GP64 by that of CD4, GpA, HA, or the OpMNPV F protein had a substantial negative effect on hemifusion by GP64.
FIG. 5.
Analysis of hemifusion and fusion pore formation by chimeric GP64 proteins. RBCs that were dually labeled with membrane-restricted (R18) and cytosolic (calcein-AM) dyes were bound to Sf9 cells transiently expressing chimeric GP64 proteins with TM domain substitutions. The bound cells were exposed to acidic PBS (pH 5.0) for 3 min to induce fusion and examined after a 20-min incubation period. (A) Fluorescence microscopy showing either membrane dye transfer (R18; left panels) or cytosolic dye transfer (calcein-AM; right panels). Each GP64 chimeric construct (or control) used for transfection is indicated at the right. (B) Efficiencies of lipid dye (R18) transfer (black bars) and pore formation (hatched bars). The efficiency of lipid dye transfer was calculated as a percentage from the number of R18-transferred cells for chimeric GP64s relative to that for wt GP64 (7.5 μg DNA). The efficiency of pore formation was calculated as a percentage from the number of calcein-transferred Sf9 cells relative to the number of R18-transferred Sf9 cells.
To characterize the ability of transfected cells to form aqueous fusion pores, the same cells were further examined for the transfer of the small soluble fluorescent dye calcein from the RBCs to Sf9 cells. The ratio of calcein-transferred Sf9 cells to R18-transferred Sf9 cells was used as a measure of fusion pore formation ability (Fig. 5B). The efficiencies of pore formation by GP64TM-OpGP64 (96.51%) and GP64TM-GP75 (92.94%) were similar to that of wt GP64 (98.31% with 7.5 μg DNA and 94.4% with 0.75 μg DNA). However, pore formation efficiencies of GP64TM-CD4 (31.2%) and GP64TM-GpA (12.71%) were significantly lower. No transfer was observed with Sf9 cells expressing GP64TM-HA and GP64TM-OpF, indicating that although these constructs are capable of mediating hemifusion, no fusion pore formation was detected (Fig. 5A). No fusion pore formation was detected from chimeras GP64TM-LdF, GP64TM-GP41, and GP64GPI, as expected, since these constructs did not localize to the cell surface.
Chimeras GP64TM-OpGP64 and GP64TM-GP75 rescue an AcMNPV gp64 null bacmid.
To determine whether the TM substitution constructs could rescue gp64 null AcMNPV, each chimeric gp64 gene was inserted into the polyhedrin locus of a gp64 null bacmid, using standard Tn7-based transposition (23) with a donor plasmid that also contains a GUS reporter gene. As a positive control, the wt AcMNPV gp64 gene was inserted into the same donor plasmid. A donor plasmid containing no gp64 gene served as a negative control. The resulting bacmids were named vAcGP64ko (no gp64 gene), vAcGP64wt (wt gp64 reinserted), vAcGP64TM-CD4, vAcGP64TM-GpA, vAcGP64TM-HA, vAcGP64TM-GP41, vAcGP64TM-LdF, vAcGP64TM-OpF, vAcGP64TM-OpGP64, vAcGP64TM-GP75, and vAcGP64GPI, where the superscript indicates the source of the TM domain (Fig. 1). Following the insertion of each gp64 gene, bacmid DNA from each construct was used to transfect Sf9 cells, and cells were scored for the spread of infection and the generation of infectious virions by a transfection-infection assay (20, 24). Transfection of Sf9 cells with gp64 null bacmids carrying the mutant GP64 genes which contained the TM domains from OpMNPV GP64 and thogotovirus GP75 resulted in rescue of the gp64 null AcMNPV and in production of infectious virions (Fig. 6A, panels p and r). In contrast, the gp64 null bacmids carrying the mutant genes which contained the TM domains from the other viral and eukaryotic integral membrane proteins, or with the GPI signal, did not produce infectious virions (Fig. 6A). Because GP64TM-GP41, GP64TM-LdF, and GP64GPI were not efficiently transported to the cell surface (Fig. 3A), they were not expected to rescue virus infectivity. As a control to ensure that all mutant proteins were expressed in Sf9 cells after transfection of the bacmids into Sf9 cells, the bacmid-transfected cells were examined by Western blot analysis for the presence of the chimeric GP64 proteins. We detected expression of all of the mutant proteins in the bacmid-transfected cells (data not shown). Also, to confirm that these results were not due to other lethal mutations in the bacmid, all of the bacmids expressing the chimeric GP64 proteins were transfected into Sf9Op1D cells, which constitutively express the wt OpMNPV GP64 protein. In each case, infectious virions were produced (data not shown), indicating that the lack of virion production in Sf9 cells was due to lack of a functional GP64 protein, not to a second-site mutation.
FIG. 6.
Analysis of viral replication in Sf9 cells. (A) Transfection-infection assay. The top panels represent Sf9 cells transfected with the indicated bacmid constructs, incubated for 96 h at 27°C, and then stained for GUS activity. At 96 h p.t., the supernatants were removed and transferred to a new monolayer of Sf9 cells that were subsequently incubated for 96 h and stained for GUS activity (bottom panels). (B) Virus one-step growth curves generated from an infection time course. BV stocks generated from bacmids vAcGP64wt, vAcGP64TM-OpGP64, and vAcGP64TM-GP75 were used to infect Sf9 cells (MOI of 5), the supernatants were removed at the indicated time points, and infectious virus yields were determined by the 50% tissue culture infective dose. The data points indicate averages for infections performed in triplicate, and error bars represent standard deviations.
To more thoroughly characterize the ability of GP64TM-OpGP64 and GP64TM-GP75 to rescue the gp64 null virus, we performed one-step growth curve studies. Infectious virus production from viruses vAcGP64TM-OpGP64 and vAcGP64TM-GP75 was similar to that from vAcGP64wt, with no dramatic differences at any time p.i. (Fig. 6B).
Budding efficiency and incorporation of chimeric GP64s into virions.
GP64 plays an important role in virion budding, as budding is severely reduced in the absence of GP64 (37). To examine possible functional roles of the TM domain in virion budding, we examined the efficiency of virion budding of all GP64 TM substitution constructs that were localized to the cell surface. Each virus expressing a TM-substituted GP64 was amplified and titrated in Sf9Op1D cells and then used to infect Sf9 cells. The infected Sf9 cells were pulse labeled with [35S]methionine, and progeny virions were purified. The relative levels of labeled progeny virions that accumulated in cell culture supernatants (see Materials and Methods) were estimated by quantifying the label from the major capsid protein VP39, as described previously (37). Comparisons with a virus expressing wt GP64 revealed that two viruses, vAcGP64TM-OpGP64 and vAcGP64TM-GP75, budded with a similar or even higher efficiency (110% and 140%). Other mutant viruses (vAcGP64TM-CD4, vAcGP64TM-GpA, vAcGP64TM-HA, and vAcGP64TM-OpF) produced substantially less BV than the virus expressing wt GP64, with virion production decreased approximately 90% (Fig. 7C). Because lower BV production is correlated with lower cell surface levels of these GP64 constructs (Fig. 3A), this may explain the reduction in budding. However, the robust budding by viruses vAcGP64TM-OpGP64 and vAcGP64TM-GP75, which also had moderately reduced GP64 surface localization (Fig. 3A), suggests that the TM domain plays a role in budding efficiency.
FIG. 7.

Analysis of budding efficiency and incorporation of chimeric GP64 proteins into BV. (A) Viruses expressing TM domain-substituted GP64 chimeras were pulse labeled with [35S]methionine and BV purified as described in Materials and Methods. Labeled viral proteins were analyzed by SDS-PAGE and phosphorimager analysis. GP64 constructs are indicated above the lanes, and the positions of GP64 and VP39 proteins are indicated on the right. Each lane represents a virus purified from an equivalent volume of cell culture supernatant. (B) Western blot analysis of unlabeled virions prepared as described for panel A and challenged with MAb B12D5 (Note that MAb B12D5 reacts with AcMNPV GP64 but not with OpMNPV GP64). Each lane represents an approximately equal amount of purified virions. (C) Virion budding efficiencies were calculated by comparisons of measurements of labeled major capsid protein in each virus expressing a chimeric GP64 construct. All data were normalized to the value for a virus construct expressing wt GP64 (100%). (D) The efficiency of GP64 incorporation into virions was calculated for each virus expressing a chimeric TM-substituted GP64 protein. Molar ratios of GP64 to VP39 were calculated for virions of each virus, based on 16 methionine residues (mature wt GP64 and GP64TM-OpGP64), 15 methionine residues (vAcGP64TM-CD4, vAcGP64TM-GpA, and vAcGP64TM-HA), 14 methionine residues (vAcGP64TM-OpF and vAcGP64TM-GP75), or 9 methionine residues (VP39). The means and standard deviations determined from triplicate experiments are shown.
In addition to the effects of the GP64 TM domain on virion production, we also examined the effects of TM substitutions on the incorporation of GP64 into virions. As shown in Fig. 7A, the labeled TM-substituted GP64 proteins were assembled into virions, and the presence of the GP64 TM chimeras in the purified virions was confirmed by Western blot analysis using MAb B12D5 (Fig. 7B), which is specific for an AcMNPV GP64 epitope (33). As a measure of the relative quantity of chimeric GP64 in each virion, the ratio of GP64 to the major capsid protein VP39 was determined for each virion preparation. Since the mutant GP64 proteins and the VP39 protein contain different numbers of methionine residues (see the legend to Fig. 7) and the amount of incorporated label is proportional to the number of methionine residues, protein labeling data were adjusted to represent relative molar quantities of the two proteins (Fig. 7D). The ratio of GP64 to VP39 in the virus (vAcGP64wt) expressing the wt GP64 protein was 0.61 (Fig. 7D). Comparison of this ratio to those for viruses vAcGP64TM-CD4, vAcGP64TM-GpA, vAcGP64TM-HA, vAcGP64TM-OpF, vAcGP64TM-OpGP64, and vAcGP64TM-GP75 indicated that the GP64/VP39 ratio was moderately higher for each of the viruses containing a heterologous TM domain (Fig. 7D). Although the differences are not dramatic, in all cases replacement of the GP64 TM domain with that of another viral or membrane protein resulted in an increase (13 to 40%) in the concentration of GP64 in the virion. Together, these data indicate that while the GP64 TM domain substantially affects the efficiency of virion production, the assembly of GP64 into virions does not require a specific sequence in the TM domain.
DISCUSSION
The role of the TM domain in membrane fusion has been studied for several different viral fusion proteins, including influenza virus HA, vesicular stomatitis virus G, HIV-1 GP41, Semliki Forest virus F protein, and severe acute respiratory syndrome coronavirus spike protein (4, 7, 17, 19, 28, 31, 36). However, the precise role of the TM domain in the membrane fusion process is still not well understood. In the baculovirus group I NPVs, the GP64 protein is essential for the entry of BV. In previous studies, deletion of 14 aa from the C terminus of AcMNPV GP64 (removing the 7-aa cytoplasmic tail and 7 aa of the predicted TM domain) resulted in shedding of the truncated GP64 protein from the cell membrane and in a loss of fusion activity (37). Because of the loss of membrane anchoring and deletion of the CTD in those studies, it was not possible to ascribe any role to the TM domain, except perhaps that of anchoring. Thus, the significance of the GP64 TM domain in the virus infection cycle was unknown.
Compared with the highly conserved GP64 ectodomain, the TM domains from the currently available baculovirus GP64 sequences are variable in length (16 to 23 aa) and amino acid sequence similarity. Amino acid identities for GP64 ectodomains range from 74 to 100%, whereas those for the GP64 TM domains range from 13 to 100%. In this report, the functional role of the AcMNPV GP64 TM domain was investigated by replacing the TM domain of AcMNPV GP64 with TM domains from other viral or cellular TM proteins. All TM-substituted GP64s, except for GP64GPI, were expressed and trimerized, although two constructs (GP64TM-LdF and GP64TM-OpF) were detected at much lower levels. Compared with that of the wt GP64 construct, cell surface detection was substantially reduced for all TM substitution constructs, suggesting that, in general, the TM substitutions negatively affected GP64 transport to the cell surface. GP64GPI, which contains the GPI anchor signal of human melanotransferrin, did not appear to trimerize (Fig. 2A), and little or no protein was transported to the cell surface. Insect cells are able to express fully functional recombinant GPI-anchored proteins on the cell surface (22), and human melanotransferrin (p97) is synthesized correctly and transported to the cell surface in Sf9 cells (9). Since heterologous GPI cleavage/attachment signal peptides are not recognized or processed as efficiently as those derived from native proteins (9), we propose that GP64GPI may be processed incorrectly or misfolded. Our data do not suggest any requirement for the TM domain for stable expression or trimerization and transport, since previous studies have shown that a soluble form of GP64 (with a deletion of the TM domain and CTD) can be expressed stably and transported through the secretory pathway (38).
In addition to GP64GPI, two additional constructs, GP64TM-LdF and GP64TM-GP41, failed to localize efficiently to the cell surface. While both appeared to trimerize in a manner similar to that of wt GP64 (Fig. 2A), these proteins were not readily detected on the cell surface above the background (Fig. 3A and B). GP64TM-LdF, which contains the predicted 19-aa TM domain from the LdMNPV F protein (41), had the shortest TM domain used in this study. However, since a truncated AcMNPV GP64 TM domain of 19 aa can mediate fusion and rescue virus replication (37), it is unlikely that the reduction in length of the TM domain resulted in the functional defect of GP64TM-LdF.
Replacement of the AcMNPV GP64 TM domain with that of OpMNPV GP64 or thogotovirus GP75, both of which are homologues of AcMNPV GP64, had a moderately negative effect on cell surface levels (a reduction of about 60% compared with the wt AcMNPV GP64 TM) (Fig. 3A) but no effect on either fusion activity, virus infectivity, assembly, or budding efficiency. These two TM domains differ dramatically in their degrees of similarity to the AcMNPV GP64 TM domain. While the OpMNPV GP64 TM domain is 61% identical with that of AcMNPV, the thogotovirus GP75 TM domain has only 17% identity. Although the closely related OpMNPV GP64 protein can readily rescue gp64 null AcMNPV (34, 37), the very distantly related GP75 protein was unable to rescue gp64 null AcMNPV replication (24). In the current study, we found that the AcMNPV GP64 TM domain could be replaced with that of GP75 without a loss of function, indicating a high degree of functional conservation in the TM domains of GP64 and GP75, even though the sequences are not highly conserved.
When the TM domain of GP64 was replaced with those of the heterologous human membrane proteins CD4 and GpA, the chimeric proteins were detected at the cell surface, but membrane fusion activity was severely impaired in both constructs (Fig. 4). Expression of wt GP64 at similar levels (at the cell surface) resulted in robust membrane fusion (Fig. 3A and 4A). Interestingly, the TM domains of CD4 and GpA contain a conserved GXXXG motif. Because GpA is known to form a dimer through the GXXXG motif (30), the dimerization activity may interfere with proper trimerization of GP64 or interaction between trimers during fusion, causing the observed defect.
The precise defect in the membrane fusion mechanism of the TM replacement mutants remains to be determined. It is noteworthy that all of the chimeric GP64s that were transported to the cell surface induced hemifusion (Fig. 5). Some chimeric GP64s were competent to form fusion pores (GP64TM-OpGP64, GP64TM-GP75, GP64TM-CD4, and GP64TM-GpA), while no fusion pores were detected for others (GP64TM-HA and GP64TM-OpF) (Fig. 5). This result supports a popular current model of membrane fusion in which it was proposed (28) that outer leaflet fusion (hemifusion) is a TM domain-independent process that is mediated by the ectodomain, leading to the hemifusion intermediate. Conformational changes in the ectodomain subsequently cause the TM domain to destabilize the hemifusion intermediate and induce fusion pore formation followed by pore expansion. Within the context of the model, the fusion protein forms a ring at the point of hemifusion (5, 32), and even after the formation of a small pore, this ring blocks lipid movement. Lipids are inserted into the ring as the fusion protein trimers move apart, and this is observed as both pore enlargement and lipid dye movement (5, 11, 48). Since a GP64-induced fusion pore was estimated to be composed of 5 to 10 trimers (26, 44), the capacity of chimeric GP64 trimers to associate with (and dissociate from) each other may depend on the amino acid sequence of the TM domain. In this model, the observed decrease in efficiency of hemifusion and pore formation (observed as differences in lipid dye and cytoplasmic dye transfer in the current experiments) may result from defects in the normal interactions between GP64 trimers. We might speculate that the very inefficient formation of large syncytia by GP64 chimeras GP64TM-CD4 and GP64TM-GpA might suggest the presence of a functional defect in the stages after the formation of initial fusion pores, perhaps preventing efficient pore enlargement.
To analyze the effects of TM domain mutations in the context of viral infection, the genes encoding the wt and TM-substituted GP64s were inserted into the polyhedrin locus of a gp64 null AcMNPV genome. The viruses carrying GP64TM-OpGP64 and GP64TM-GP75 showed no detectable change in production of infectious virions in one-step growth curve experiments (Fig. 6B). However, none of the other TM substitution mutants rescued the infectivity of gp64 null AcMNPV (Fig. 6A), even though two of the constructs (GP64TM-CD4 and GP64TM-GpA) were functional for membrane fusion. The defect in infectivity for these chimeras may be caused by a reduction in fusion efficiency but also may be caused by inefficient budding. The viruses expressing TM substitution constructs GP64TM-CD4 and GP64TM-GpA showed a 90 to 95% decrease in virion production (Fig. 7C). Interestingly, the chimeras containing TM domains from related proteins (GP64TM-OpGP64 and GP64TM-GP75) mediated virion budding at levels similar to or even higher than that from the control virus expressing wt AcMNPV GP64 (Fig. 7C). Thus, the dramatic differences in BV production among these TM-substituted GP64 proteins suggest that the specific amino acid sequence of the TM domain is an important determinant of budding efficiency. Recent studies revealed that residues in the HA TM domain are important for localization of the protein to membrane subdomains (lipid rafts). Substitutions of those residues caused HA to become distributed randomly at the cell surface and decreased virus budding (45). The GP64 TM domain may similarly affect localization in the membrane, although GP64 was not identified in lipid rafts in a prior study (54).
In addition to examining the effect of the TM domain on the production of BV, we also asked whether the GP64 TM domain affected GP64 incorporation into virions. Viruses expressing wt and chimeric GP64 proteins were compared. We measured the GP64/VP39 ratio as an indicator of the relative level of GP64 in each virion. Surprisingly, the GP64/VP39 ratios of viruses GP64TM-CD4, GP64TM-GpA, GP64TM-HA, GP64TM-OpF, GP64TM-OpGP64, and GP64TM-GP75 were similar to or moderately increased in comparison with that of the control virus expressing wt GP64 (Fig. 7D). Since none of these constructs failed to be incorporated into the virion, we can conclude that the TM domain alone does not specify virion targeting but does have a moderate effect on the level of GP64 found in virions and thus may serve to regulate the GP64 concentration in the virion. Because these ratios were derived from viruses that, in some cases, had differing cell surface levels of GP64 (Fig. 3A and B) or a substantially reduced budding efficiency (Fig. 7C), we cannot rule out the possibility that the concentration of GP64 on progeny virions may be affected by the quantity of GP64 available on the membrane or by the rate of virion budding. Further studies will be necessary to specifically address these complex issues.
In combination, the data presented in this study clearly demonstrate that the AcMNPV GP64 TM domain serves a critical function. Some, but not all, heterologous TM domains can replace that of AcMNPV GP64 in its various functions in the infection cycle. While TM domains from both closely and distantly related viral envelope proteins (OpMNPV GP64 and thogotovirus GP75) substituted for all functions examined, many of the TM domains from unrelated proteins severely and negatively affected the function of GP64. Substitution of the TM domain did not substantially affect GP64 expression, trimerization, or the ability of GP64 trimers to incorporate into virions. However, TM domain substitutions showed that the TM domain was critical for protein trafficking to the plasma membrane, membrane fusion, and virion budding. The importance of the TM domain in these functions may be related to either TM domain interactions with lipids in the cell and virion membranes or interactions with cellular proteins or between and among GP64 trimers. Such interactions are likely important during virion budding and during the complex process of pH-triggered membrane fusion. In future studies, we will examine critical residues or features of the TM domain and the interactions that mediate these fundamental processes. Such studies will enhance our understanding of molecular mechanisms of membrane fusion during virus entry as well as the production of progeny virions by budding from the plasma membrane.
Acknowledgments
We thank Gerrit Heetderks, Jian Zhou, and Guoxun Li for technical assistance and comments on the manuscript.
This work was supported by NIH grant AI33657 and BTI project 1255.
Footnotes
Published ahead of print on 23 January 2008.
REFERENCES
- 1.Blissard, G. W. 1996. Baculovirus-insect cell interactions. Cytotechnology 2073-93. [DOI] [PubMed] [Google Scholar]
- 2.Blissard, G. W., and G. F. Rohrmann. 1991. Baculovirus gp64 gene expression: analysis of sequences modulating early transcription and transactivation by IE1. J. Virol. 655820-5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blissard, G. W., and J. R. Wenz. 1992. Baculovirus GP64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J. Virol. 666829-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broer, R., B. Boson, W. Spaan, F. L. Cosset, and J. Corver. 2006. Important role for the transmembrane domain of severe acute respiratory syndrome coronavirus spike protein during entry. J. Virol. 801302-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chernomordik, L. V., V. A. Frolov, E. Leikina, P. Bronk, and J. Zimmerberg. 1998. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell Biol. 1401369-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu, J., P. E. March, R. Lee, and D. Tillett. 2004. Site-directed, ligase-independent mutagenesis (SLIM): a single-tube methodology approaching 100% efficiency in 4 h. Nucleic Acids Res. 32e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleverley, D. Z., and J. Lenard. 1998. The transmembrane domain in viral fusion: essential role for a conserved glycine residue in vesicular stomatitis virus G protein. Proc. Natl. Acad. Sci. USA 953425-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hefferon, K., A. Oomens, S. Monsma, C. Finnerty, and G. Blissard. 1999. Host cell receptor binding by baculovirus GP64 and kinetics of virion entry. Virology 258455-468. [DOI] [PubMed] [Google Scholar]
- 9.Hegedus, D. D., T. A. Pfeifer, D. A. Theilmann, M. L. Kennard, R. Gabathuler, W. A. Jefferies, and T. A. Grigliatti. 1999. Differences in the expression and localization of human melanotransferrin in lepidopteran and dipteran insect cell lines. Protein Expr. Purif. 15296-307. [DOI] [PubMed] [Google Scholar]
- 10.Helseth, E., U. Olshevsky, D. Gabuzda, B. Ardman, W. Haseltine, and J. Sodroski. 1990. Changes in the transmembrane region of the human immunodeficiency virus type 1 gp41 envelope glycoprotein affect membrane fusion. J. Virol. 646314-6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12627-661. [DOI] [PubMed] [Google Scholar]
- 12.Herniou, E. A., J. A. Olszewski, J. S. Cory, and D. R. O'Reilly. 2003. The genome sequence and evolution of baculoviruses. Annu. Rev. Entomol. 48211-234. [DOI] [PubMed] [Google Scholar]
- 13.Hink, W. F. 1970. Established insect cell line from the cabbage looper, Trichoplusia ni. Nature 226466-467. [DOI] [PubMed] [Google Scholar]
- 14.Hohmann, A. W., and P. Faulkner. 1983. Monoclonal antibodies to baculovirus structural proteins: determination of specificities by Western blot analysis. Virology 125432-444. [DOI] [PubMed] [Google Scholar]
- 15.Jarvis, D. L., L. Wills, G. Burow, and D. A. Bohlmeyer. 1998. Mutational analysis of the N-linked glycans on Autographa californica nucleopolyhedrovirus gp64. J. Virol. 729459-9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jehle, J. A., G. W. Blissard, B. C. Bonning, J. S. Cory, E. A. Herniou, G. F. Rohrmann, D. A. Theilmann, S. M. Thiem, and J. M. Vlak. 2006. On the classification and nomenclature of baculoviruses: a proposal for revision. Arch. Virol. 1511257-1266. [DOI] [PubMed] [Google Scholar]
- 17.Kemble, G. W., T. Danieli, and J. M. White. 1994. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell 76383-391. [DOI] [PubMed] [Google Scholar]
- 18.Kingsley, D. H., A. Behbahani, A. Rashtian, G. W. Blissard, and J. Zimmerberg. 1999. A discrete stage of baculovirus GP64-mediated membrane fusion. Mol. Biol. Cell 104191-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao, M., and M. Kielian. 2005. The conserved glycine residues in the transmembrane domain of the Semliki Forest virus fusion protein are not required for assembly and fusion. Virology 332430-437. [DOI] [PubMed] [Google Scholar]
- 20.Lin, G., and G. W. Blissard. 2002. Analysis of an Autographa californica multicapsid nucleopolyhedrovirus lef-11 knockout virus: LEF-11 is essential for viral DNA replication. J. Virol. 762770-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long, G., X. Pan, R. Kormelink, and J. M. Vlak. 2006. Functional entry of baculovirus into insect and mammalian cells is dependent on clathrin-mediated endocytosis. J. Virol. 808830-8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longacre, S., K. N. Mendis, and P. H. David. 1994. Plasmodium vivax merozoite surface protein 1 C-terminal recombinant proteins in baculovirus. Mol. Biochem. Parasitol. 64191-205. [DOI] [PubMed] [Google Scholar]
- 23.Luckow, V. A., S. C. Lee, G. F. Barry, and P. O. Olins. 1993. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J. Virol. 674566-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lung, O., M. Westenberg, J. M. Vlak, D. Zuidema, and G. W. Blissard. 2002. Pseudotyping Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV): F proteins from group II NPVs are functionally analogous to AcMNPV GP64. J. Virol. 765729-5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markosyan, R. M., F. S. Cohen, and G. B. Melikyan. 2000. The lipid-anchored ectodomain of influenza virus hemagglutinin (GPI-HA) is capable of inducing nonenlarging fusion pores. Mol. Biol. Cell 111143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markovic, I., H. Pulyaeva, A. Sokoloff, and L. V. Chernomordik. 1998. Membrane fusion mediated by baculovirus gp64 involves assembly of stable gp64 trimers into multiprotein aggregates. J. Cell Biol. 1431155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maruniak, J. W., and M. D. Summers. 1981. Autographa californica nuclear polyhedrosis virus phosphoproteins and synthesis of intracellular proteins after virus infection. Virology 10925-34. [DOI] [PubMed] [Google Scholar]
- 28.Melikyan, G. B., S. Lin, M. G. Roth, and F. S. Cohen. 1999. Amino acid sequence requirements of the transmembrane and cytoplasmic domains of influenza virus hemagglutinin for viable membrane fusion. Mol. Biol. Cell 101821-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melikyan, G. B., R. M. Markosyan, M. G. Roth, and F. S. Cohen. 2000. A point mutation in the transmembrane domain of the hemagglutinin of influenza virus stabilizes a hemifusion intermediate that can transit to fusion. Mol. Biol. Cell 113765-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mingarro, I., P. Whitley, M. A. Lemmon, and G. von Heijne. 1996. Ala-insertion scanning mutagenesis of the glycophorin A transmembrane helix: a rapid way to map helix-helix interactions in integral membrane proteins. Protein Sci. 51339-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyauchi, K., J. Komano, Y. Yokomaku, W. Sugiura, N. Yamamoto, and Z. Matsuda. 2005. Role of the specific amino acid sequence of the membrane-spanning domain of human immunodeficiency virus type 1 in membrane fusion. J. Virol. 794720-4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monck, J. R., and J. M. Fernandez. 1992. The exocytotic fusion pore. J. Cell Biol. 1191395-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monsma, S. A., and G. W. Blissard. 1995. Identification of a membrane fusion domain and an oligomerization domain in the baculovirus GP64 envelope fusion protein. J. Virol. 692583-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monsma, S. A., A. G. P. Oomens, and G. W. Blissard. 1996. The GP64 envelope fusion protein is an essential baculovirus protein required for cell-to-cell transmission of infection. J. Virol. 704607-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morse, M. A., A. C. Marriott, and P. A. Nuttall. 1992. The glycoprotein of Thogoto virus (a tick-borne orthomyxo-like virus) is related to the baculovirus glycoprotein gp64. Virology 186640-646. [DOI] [PubMed] [Google Scholar]
- 36.Odell, D., E. Wanas, J. Yan, and H. P. Ghosh. 1997. Influence of membrane anchoring and cytoplasmic domains on the fusogenic activity of vesicular stomatitis virus glycoprotein G. J. Virol. 717996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oomens, A. G. P., and G. W. Blissard. 1999. Requirement for GP64 to drive efficient budding of Autographa californica multicapsid nucleopolyhedrovirus. Virology 254297-314. [DOI] [PubMed] [Google Scholar]
- 38.Oomens, A. G. P., S. A. Monsma, and G. W. Blissard. 1995. The baculovirus GP64 envelope fusion protein: synthesis, oligomerization, and processing. Virology 209592-603. [DOI] [PubMed] [Google Scholar]
- 39.O'Reilly, D. R., L. K. Miller, and V. A. Luckow. 1992. Baculovirus expression vectors, a laboratory manual. W. H. Freeman and Co., New York, NY.
- 40.Owens, R. J., C. Burke, and J. K. Rose. 1994. Mutations in the membrane-spanning domain of the human immunodeficiency virus envelope glycoprotein that affect fusion activity. J. Virol. 68570-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearson, M. N., C. Groten, and G. F. Rohrmann. 2000. Identification of the Lymantria dispar nucleopolyhedrovirus envelope fusion protein provides evidence for a phylogenetic division of the Baculoviridae. J. Virol. 746126-6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearson, M. N., R. L. Russell, and G. F. Rohrmann. 2002. Functional analysis of a conserved region of the baculovirus envelope fusion protein, LD130. Virology 30481-88. [DOI] [PubMed] [Google Scholar]
- 43.Plonsky, I., M. S. Cho, A. G. P. Oomens, G. W. Blissard, and J. Zimmerberg. 1999. An analysis of the role of the target membrane on the gp64-induced fusion pore. Virology 25365-76. [DOI] [PubMed] [Google Scholar]
- 44.Plonsky, I., and J. Zimmerberg. 1996. The initial fusion pore induced by baculovirus GP64 is large and forms quickly. J. Cell Biol. 1351831-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda, M., G. P. Leser, C. J. Russell, and R. A. Lamb. 2003. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc. Natl. Acad. Sci. USA 10014610-14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamm, L. K., J. Crane, and V. Kiessling. 2003. Membrane fusion: a structural perspective on the interplay of lipids and proteins. Curr. Opin. Struct. Biol. 13453-466. [DOI] [PubMed] [Google Scholar]
- 47.Theilmann, D. A., G. W. Blissard, B. Bonning, J. Jehle, D. R. O'Reilly, G. F. Rohrmann, S. Thiem, and J. M. Vlak. 2005. Baculoviridae, p. 177-185. In H. V. Van Regenmortel, D. H. L. Bishop, M. H. Van Regenmortel, and C. M. Fauquet (ed.), Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, New York, NY.
- 48.Tse, F. W., A. Iwata, and W. Almers. 1993. Membrane flux through the pore formed by a fusogenic viral envelope protein during cell fusion. J. Cell Biol. 121543-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ujike, M., K. Nakajima, and E. Nobusawa. 2004. Influence of acylation sites of influenza B virus hemagglutinin on fusion pore formation and dilation. J. Virol. 7811536-11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volkman, L. E., and P. A. Goldsmith. 1984. Budded Autographa californica NPV 64K protein: further biochemical analysis and effects of postimmunoprecipitation sample preparation conditions. Virology 139295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volkman, L. E., and P. A. Goldsmith. 1985. Mechanism of neutralization of budded Autographa californica nuclear polyhedrosis virus by a monoclonal antibody: inhibition of entry by adsorptive endocytosis. Virology 143185-195. [DOI] [PubMed] [Google Scholar]
- 52.Volkman, L. E., P. A. Goldsmith, R. T. Hess, and P. Faulkner. 1984. Neutralization of budded Autographa californica NPV by a monoclonal antibody: identification of the target antigen. Virology 133354-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westenberg, M., H. Wang, W. F. IJkel, R. W. Goldbach, J. M. Vlak, and D. Zuidema. 2002. Furin is involved in baculovirus envelope fusion protein activation. J. Virol. 76178-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, S. X., Y. Han, and G. W. Blissard. 2003. Palmitoylation of the Autographa californica multicapsid nucleopolyhedrovirus envelope glycoprotein GP64: mapping, functional studies, and lipid rafts. J. Virol. 776265-6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou, J., and G. W. Blissard. 2006. Mapping the conformational epitope of a neutralizing antibody (AcV1) directed against the AcMNPV GP64 protein. Virology 352427-437. [DOI] [PMC free article] [PubMed] [Google Scholar]