Abstract
Developing an immunotherapy to keep human immunodeficiency virus type 1 (HIV-1) replication suppressed while discontinuing highly active antiretroviral therapy (HAART) is an important challenge. In the present work, we evaluated in vitro whether dendritic cells (DC) electroporated with gag mRNA can induce HIV-specific responses in T cells from chronically infected subjects. Monocyte-derived DC, from therapy-naïve and HAART-treated HIV-1-seropositive subjects, that were electroporated with consensus codon-optimized HxB2 gag mRNA efficiently expanded T cells, secreting gamma interferon (IFN-γ) and interleukin 2 (IL-2), as well as other cytokines and perforin, upon restimulation with a pool of overlapping Gag peptides. The functional expansion levels after 1 week of stimulation were comparable in T cells from HAART-treated and treatment-naïve patients and involved both CD4+ and CD8+ T cells, with evidence of bifunctionality in T cells. Epitope mapping of p24 showed that stimulated T cells had a broadened response toward previously nondescribed epitopes. DC, from HAART-treated subjects, that were electroporated with autologous proviral gag mRNA equally efficiently expanded HIV-specific T cells. Regulatory T cells did not prevent the induction of effector T cells in this system, whereas the blocking of PD-L1 slightly increased the induction of T-cell responses. This paper shows that DC, loaded with consensus or autologous gag mRNA, expand HIV-specific T-cell responses in vitro.
Studies of immune responses generated in human immunodeficiency virus type 1 (HIV-1)-infected individuals suggest that CD8+ T cells play an important role in the defense against the virus. In acute HIV infection, the appearance of HIV-specific CD8+ T cells is associated with a decline in viremia (11, 32). More-direct evidence for the role of CD8+ T cells in viral control is deduced from studies of simian immune deficiency virus (SIV)-infected rhesus macaques in which the depletion of the CD8+ T cells results in an increase of the viral load and rapid disease progression (41, 55), although this is not always the case (35). Among HIV-infected humans, long-term nonprogressors (LTNP) with an undetectable viral load have higher levels of multifunctional HIV-specific CD8+ T cells in comparison to patients with rapidly progressive disease (53). Conversely, the HIV-specific CD8+ T cells from rapid progressors release low levels of interleukin 2 (IL-2) and high levels of gamma interferon (IFN-γ), they have a reduced proliferative capacity, and their perforin expression is impaired or exhausted (42, 69). Moreover, during primary and chronic infection, viral escape mutations are often observed as a consequence of immunological pressure mediated by SIV- and HIV-specific CD8+ T cells (3, 12, 20, 23, 50). During this process of viral adaptation, all the previous variants are stored as proviral DNA (46).
Although current highly active antiretroviral therapy (HAART) may suppress viral replication and protect against disease progression, it is unable to eliminate the proviral latent reservoir. Moreover, as a consequence of low or absent HIV antigenic stimulation, HIV-1-specific cytotoxic T lymphocyte (CTL) responses tend to wane during HAART (16, 39). Therapy interruption invariably results in a viral rebound to pretreatment levels, indicating that no protective immunity has been built up during therapy (38). On the other hand, the partial immune reconstitution, induced by HAART, opens a window of opportunity to boost T-cell immunity by therapeutic vaccination. Clearly, it is not sufficient to enhance the response against the circulating virus. To minimize the risk of escape, it is equally important that immune responses against the entire latent reservoir are activated (49).
Dendritic cells (DC) are the most powerful antigen-presenting cells (APC) that can stimulate effective immune responses both in vitro and in vivo (5, 9, 62). In the context of DC-based immunotherapy, many groups have used DC expressing HIV antigens (e.g., pulsed with peptides, transduced with different vectors, or loaded with apoptotic infected cells) to stimulate memory (19, 34, 59, 69) or even primary (13, 14, 33, 63, 66, 67) CD8+ T cells in vitro. In vivo, SIV-specific CD8+ and CD4+ T-cell responses were induced in macaques using DC expressing SIV antigen (63). Finally, Lu and Andrieu and Lu et al. (36, 37) showed that DC pulsed with chemically inactivated autologous virus specifically stimulated HIV-specific immune responses in vitro and in vivo in cells of HIV-1-seropositive individuals.
Recently, we (47, 48, 61) and others (9, 15, 22, 28, 40, 54, 57) have shown that transfection with mRNA is more effective than mRNA lipofection, peptide pulsing, or viral transduction to generate primary (65) and memory (57) responses. Furthermore, we demonstrated that DC from treatment-naïve HIV-1-seropositive subjects can efficiently be transfected with HIV gag and env mRNA, derived either from consensus subtype B or autologous viral or proviral HIV, and that these DC readily trigger autologous CD4+ and CD8+ T cells to release IFN-γ and IL-2 in a short-term ex vivo enzyme-linked immunospot (ELISPOT) assay (60).
Our previous study (60) considered only the direct ex vivo immune responses of untreated HIV-1-seropositive persons, who have, by definition, a rather damaged immune system (42). Therefore, with the ultimate aim to develop an immunotherapy based on DC, we decided to evaluate the responses of treatment-naïve and HAART-treated HIV-1-seropositive persons after 1 week of stimulation with electroporated DC. Besides IFN-γ production, other parameters were also evaluated, such as a series of other cytokines, measured in various ways (by ELISPOT, microbead assay, and intracellular cytometry), and the potential influence of regulatory T cells (Treg) on the response. Finally, because HIV escapes very easily from the immune system, we also investigated if it is possible to use autologous proviral gag mRNA and to broaden the immune response.
MATERIALS AND METHODS
Study population.
EDTA-anticoagulated blood samples (100 ml) were obtained from 75 HIV-1-seropositive individuals (designated P1 to P75) recruited at the Clinical Department of the Institute of Tropical Medicine of Antwerp according to institutional guidelines and after obtaining informed consent. The demographic and clinical information of these patients is summarized in Table 1. Seventeen of these HIV-1-seropositve individuals were antiretroviral therapy-naïve (P1 to P17) with a CD4+ T-cell count above 300 cells/μl. The other 58 received HAART (P18 to P75) and had an undetectable viral load for at least 1 year and CD4 T-cell counts above 300 cells/μl. HLA typing of some individuals was done at the Antwerp Blood Transfusion Center.
TABLE 1.
Demographic and clinical data of treatment-naive and HAART-treated HIV-1-seropositive individuals
| Patient no. | Actual cell count (cells/μl)
|
Nadir CD4+ T cell count (cells/μl) | Viral load (copies/ml) | No. of mo since diagnosisa | No. of mo on HAART | Viral subtypeb | |
|---|---|---|---|---|---|---|---|
| CD4+ T | CD8+ T | ||||||
| P1 | 348 | 1,066 | 348 | 82,000 | 17 | 0 | ND |
| P2 | 1,186 | 1,779 | 714 | <50 | 7 | 0 | ND |
| P3 | 770 | 2,275 | 744 | 525,000 | 11 | 0 | B |
| P4 | 283 | 756 | 177 | 46,000 | 72 | 0 | ND |
| P5 | 622 | 1,726 | 501 | 16,700 | 111 | 0 | ND |
| P6 | 390 | 617 | 390 | 130,000 | 5 | 0 | ND |
| P7 | 502 | 1,065 | 440 | 230,000 | 32 | 0 | ND |
| P8 | 394 | 1,392 | 96,300 | 58 | 0 | ND | |
| P9 | 323 | 908 | 184 | 5,900 | 25 | 0 | ND |
| P10 | 579 | 1,092 | 475 | 56,100 | 10 | 0 | B |
| P11 | 466 | 1,759 | 425 | 84,800 | 31 | 0 | ND |
| P12 | 423 | 733 | 423 | 17,600 | 12 | 0 | ND |
| P13 | 626 | 1,878 | 444 | 6,590 | 8 | 0 | ND |
| P14 | 201 | 1,692 | 201 | 8,470 | 92 | 0 | ND |
| P15 | 595 | 828 | 407 | 17,200 | 31 | 0 | B |
| P16 | 753 | 1,020 | 610 | 1,400 | 39 | 0 | ND |
| P17 | 588 | 719 | 444 | 112,000 | 19 | 0 | B |
| P18 | 359 | 425 | 220 | <50 | 98 | 97 | A |
| P19 | 1,083 | 877 | 211 | <50 | 204 | 99 | A/G |
| P20 | 809 | 2,417 | 274 | <50 | 143 | 54 | ND |
| P21 | 262 | 676 | 150 | <50 | 150 | 136 | B |
| P22 | 655 | 585 | 89 | <50 | 126 | 124 | ND |
| P23 | 441 | 864 | 49 | <50 | U | 128 | C |
| P24 | 522 | 3,325 | 167 | <50 | 208 | 180 | ND |
| P25 | 382 | 300 | 40 | <50 | 141 | 85 | H |
| P26 | 312 | 720 | 205 | <50 | 193 | 183 | B |
| P27 | 552 | 1,357 | 16 | <50 | 70 | 68 | B |
| P28 | 460 | 2,297 | 174 | <50 | 207 | 46 | C |
| P29 | 429 | 2,056 | 91 | <50 | 88 | 33 | B |
| P30 | 815 | 990 | 277 | <50 | 186 | 32 | B |
| P31 | 1,023 | 1,023 | 316 | <50 | 119 | 79 | ND |
| P32 | 432 | 445 | 214 | <50 | 52 | 51 | ND |
| P33 | 735 | 490 | 283 | <50 | 137 | 127 | ND |
| P34 | 499 | 921 | 121 | <50 | 115 | 109 | ND |
| P35 | 1,106 | 1,136 | 207 | <50 | U | 150 | D/F |
| P36 | 460 | 1,080 | 167 | <50 | 46 | 27 | B |
| P37 | 387 | 336 | 126 | <50 | 64 | 62 | ND |
| P38 | 570 | 668 | 289 | <50 | 197 | 120 | B |
| P39 | 1,475 | 826 | 535 | <50 | 132 | 74 | ND |
| P40 | 954 | 619 | 307 | <50 | 58 | 55 | ND |
| P41 | 855 | 1,054 | 309 | <50 | 189 | 95 | ND |
| P42 | 1,188 | 764 | 207 | <50 | 190 | 171 | ND |
| P43 | 553 | 1,107 | 100 | <50 | 135 | 63 | ND |
| P44 | 1,214 | 2,802 | 179 | <50 | 187 | 186 | B |
| P45 | 306 | 408 | 93 | <50 | U | 130 | ND |
| P46 | 623 | 2,492 | 202 | <50 | 148 | 14 | ND |
| P47 | 826 | 1,625 | 192 | <50 | 64 | 32 | ND |
| P48 | 860 | 980 | 224 | <50 | 131 | 74 | ND |
| P49 | 1,179 | 1,457 | 106 | <50 | 171 | 34 | B |
| P50 | 986 | 1,096 | 292 | <50 | 229 | 33 | D |
| P51 | 875 | 636 | 429 | <50 | 145 | 35 | ND |
| P52 | 312 | 639 | 111 | <50 | 46 | 45 | ND |
| P53 | 582 | 1,187 | 25 | <50 | 148 | 22 | B |
| P54 | 533 | 1,484 | 240 | <50 | 49 | 39 | ND |
| P55 | 579 | 1,055 | 265 | <50 | 223 | 71 | ND |
| P56 | 314 | 605 | 281 | <50 | 43 | 30 | ND |
| P57 | 646 | 1,025 | 319 | <50 | 197 | 58 | B |
| P58 | 820 | 1,710 | 232 | <50 | 83 | 31 | ND |
| P59 | 703 | 869 | 59 | <50 | 216 | 94 | B |
| P60 | 675 | 970 | 121 | <50 | 117 | 41 | B |
| P61 | 492 | 446 | 25 | <50 | 111 | 13 | B |
| P62 | 962 | 2,627 | 315 | <50 | U | 67 | A |
| P63 | 429 | 627 | 20 | <50 | 120 | 27 | A |
| P64 | 345 | 954 | 142 | <50 | 159 | 59 | A |
| P65 | 473 | 1,112 | 53 | <50 | U | 98 | ND |
| P66 | 897 | 818 | 80 | <50 | U | 33 | ND |
| P67 | 400 | 1,300 | 267 | <50 | U | 19 | ND |
| P68 | 588 | 1,288 | 109 | <50 | U | 105 | ND |
| P69 | 1,090 | 616 | 248 | <50 | U | 42 | ND |
| P70 | 931 | 698 | 346 | <50 | 202 | 128 | B |
| P71 | 528 | 2,182 | 68 | <50 | 194 | 193 | D/F |
| P72 | 476 | 527 | 20 | <50 | U | 119 | ND |
| P73 | 732 | 1,586 | 19 | <50 | 240 | 205 | ND |
| P74 | 575 | 1,878 | 18 | <50 | 115 | 114 | ND |
| P75 | 635 | 1,117 | 167 | <50 | 55 | 50 | B |
U, unknown.
ND, not determined.
In vitro generation of MO-derived DC.
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque gradient separation (Amersham Biosciences, Freiburg, Germany). Monocytes (MO) were isolated from PBMC by magnetic isolation using CD14 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. About 8 × 106 to 10 × 106 MO were obtained from 100 × 106 PBMC with consistent purity of more than 90%. MO-derived immature DC (iDC) were generated as described previously (51) in RPMI (Cambrex, Verviers, Belgium) supplemented with 2.5% pooled human serum (PHS), granulocyte-macrophage colony-stimulating factor, and IL-4. In all experiments, maturation of iDC was induced on day 6 using a mixture of IL-1β, IL-6, prostaglandin E2 and tumor necrosis factor alpha (TNF-α) as described previously (48). MO-depleted PBMC, further referred to as peripheral blood lymphocytes (PBL), were cryopreserved (48).
Plasmid DNA constructs.
The pGEM4Z/hHxB-2-gag/A64 plasmid (pGEMhgag), generated by the Laboratory of Physiology and Immunology in Brussels, was used to prepare a “humanized” (codon-optimized) mRNA (further referred to as h-HxB2 gag) encoding the HxB2 HIV-1 Gag protein (10). The original humanized cDNA was provided by Bernard Verrier (Biomérieux, Lyon, France).
Gag from wild-type (wt)-HxB2 was amplified and subcloned in the pGEM4Z/A64 vector and used to prepare wt-HxB2 gag mRNA.
Amplification of autologous proviral gag mRNA derived from PBMC.
Proviral DNA was extracted and amplified from 10 × 106 PBMC from HIV-1-seropositive blood donors as described previously (60). Amplified gag DNA products were purified using a QiaQuick PCR purification kit (Qiagen, Chatsworth, CA), in vitro transcribed using a T7 mMessage mMachine in vitro transcription kit, and then polyadenylated using a poly(A) tailing kit (Ambion, Cambridgeshire, United Kingdom) according to the manufacturer's instructions. RNA was stored at −80°C in small aliquots.
Peptides.
Peptides corresponding to the Gag sequence of consensus HIV-1 HxB2 were kindly provided by the National Institutes of Health AIDS Research Reagent Program (NIH, Germantown, MD). These peptides consisted of 15-mers overlapping by 11 amino acids. For most experiments, all Gag peptides (n = 121) were pooled together (HxB2 Gag peptide pool).
Induction of HIV-1-specific T cells using DC transfected with gag mRNA.
Electroporation of in vitro-transcribed mRNA encoding h-HxB2 gag, wt-HxB2 gag, or autologous proviral gag (aut-gag) was performed as described previously (48). After electroporation, iDC were cultured in fresh complete medium (including 2.5% PHS and cytokines for DC maturation) for 24 h. For T-cell stimulation, transfected mature DC were cocultured with autologous PBL (ratio 1:10) in RPMI supplemented with 2.5% PHS. After 7 days of culture, cells were always analyzed in IFN-γ, IL-2, and perforin ELISPOT assays (Diaclone, Besançon, France). Briefly, freshly thawed PBL as well as PBL stimulated with electroporated autologous DC were incubated at a concentration of 2.5 × 105 cells/well with the Gag peptide pool (2 μg/ml) in anti-human IFN-γ, IL-2, or perforin (Diaclone) antibody-coated 96-well plates. For the detection of spots, biotin-labeled anti-human IFN-γ, IL-2, or perforin was used. Spots were counted using an automated ELISPOT reader (AID, Strassberg, Germany).
In some experiments CD4+ and CD8+ T cells were purified from in vitro-stimulated PBL by positive selection, according to the manufacturer's instructions (Miltenyi), and were further analyzed by ELISPOT assay as described above.
ICS.
To evaluate the CD4+ and CD8+ T-cell responses in this system, intracellular cytokine staining (ICS) was performed besides the ELISPOT assay. Briefly, thawed PBL or PBL stimulated for 1 week with electroporated autologous DC were incubated at a concentration of 1 × 106 cells/well with the Gag peptide pool (2 μg/ml), monensin A (5 μg/ml; Sigma-Aldrich, Bornem, Belgium), and brefeldin A (5 μg/ml; Sigma-Aldrich) for 6 h. An unstimulated and positive control (Staphylococcus enterotoxin B, 1 μg/ml; Sigma-Aldrich) was included in each assay. Following incubation, the cells were washed and stained with surface antibodies CD3 (Pacific Blue) and CD8 (APC-Hy7) (Becton Dickinson, BD Biosciences, Erembodegem, Belgium). The cells were washed and fixed using the cytofix/cytoperm kit (BD Biosciences). Following fixation, cells were washed and incubated for 10 min with perm buffer. Afterward they were stained with antibodies against intracellular markers: IFN-γ (phycoerythrin [PE]-cy7), IL-2 (fluorescein isothiocyanate), perforin (PE) (BD Biosciences). The samples were analyzed on a Cyflow ML (Partec GmbH, Münster, Germany), and data analysis was performed using FlowJo version 7.2.2 (TreeStar, San Carlos, CA).
Multiplex fluorescent bead immunoassay.
In some experiments, 50 μl supernatant was taken from the ELISPOT assay to measure IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, and IL-12p70 by a multiplex fluorescent bead immunoassay, according to the manufacturer's instructions (Bender Medsystems, Vienna, Austria). Afterward the samples were measured on a FACScan (BD Biosciences).
Treg immuno-phenotyping and functionality.
Phenotyping of naturally occurring Treg was performed on thawed PBL and in vitro-stimulated PBL by four-color flow cytometry. The following monoclonal antibodies were used: anti-Forkhead Box P3 (FoxP3) PE, anti-CD25 allophycocyanin (e-Bioscience, San Diego, CA), anti-CD3 fluorescein isothiocyanate, and anti-CD4 peridinin chlorophyll (BD Biosciences). PBL were first stained with monoclonal antibodies against surface markers and then subjected to intracellular staining for FoxP3 per the manufacturer's directions. Analyses were performed using FACSCalibur and CellQuest software (BD Biosciences).
In order to assess the role of Treg, CD25+ cells were removed from the PBL using anti-CD25 beads, according to the manufacturer's instructions (Miltenyi Biotec). Afterward, unfractionated PBL and CD25-depleted PBL were cocultured with h-HxB2 gag mRNA-electroporated DC (DC h-HXB2 gag). After 1 week, the ELISPOT response and proliferative responses of both PBL populations toward the HxB2 Gag peptide pool were compared. To measure proliferation, an [3H]thymidine assay was performed (43).
In addition, blocking experiments were performed to evaluate the role of potentially inhibitory mediators. Antibodies against IL-10 (10 μg/ml), transforming growth factor β (TGF-β) (10 μg/ml; both from Sigma, Bornem, Belgium), cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4) (10 μg/ml; BD Biosciences), or PD-L1 (10 μg/ml; e-Biosciences) were added either during DC-PBL coculture (induction phase) or during the incubation of stimulated PBL with the Gag peptide pool in an ELISPOT assay (effector phase).
Epitope mapping.
Freshly thawed PBL and PBL stimulated for 1 week with DC h-HxB2 gag were screened for specific T-cell responses in the IFN-γ ELISPOT assay by incubation with single peptides (10 μg/ml), corresponding to HxB2 p24 peptides 34 to 76. As a positive control, responses toward the pool of all these p24 peptides and toward the entire Gag pool were also measured.
Statistical analyses.
Statistical analysis was performed with SPSS software (Chicago, IL) or GraphPad Prism software (GraphPad Software, Inc., San Diego, CA). Paired variables were compared using a Kruskal-Wallis test and corrected for multiple comparisons using Dunn's test, P values of ≤0.05 were considered significant. Spearman's rank coefficient was used to determine the correlation between two variables.
RESULTS
Codon-optimized HxB2 gag mRNA-electroporated DC efficiently stimulate cytokine secretory capacity by autologous T cells from treatment-naïve and HAART-treated HIV-1-seropositive individuals.
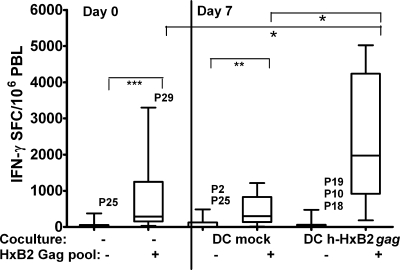
To investigate if mature MO-derived DC (MO-DC) from HIV-infected individuals could efficiently expand autologous effector T cells, we cocultured PBL from 12 treatment-naïve (P1 to P12) and from 12 HAART-treated (P18 to P29) HIV-1-seropositive individuals with either mock-electroporated or codon-optimized h-HxB2 gag mRNA-electroporated mature DC (DC mock or DC h-HxB2 gag, respectively) for 1 week. Thereafter, these DC-stimulated PBL were subjected to the ELISPOT assay, using medium only and the pool of HxB2 Gag peptides in parallel in the same experiments. As a baseline control, PBL from the same patients, which had been freshly frozen, were thawed after 1 week and simultaneously assayed with and without Gag peptides (thus representing day 0 responses). Figure 1 summarizes the results from all 24 patients. Their freshly thawed PBL showed a significant (P < 0.001) IFN-γ response upon triggering with a Gag peptide pool (response on day 0). Further, PBL cocultured with DC h-HxB2 gag induced significantly more IFN-γ spot-forming cells (SFC) compared to either freshly thawed (P < 0.05) or DC mock-stimulated day 7 PBL (P < 0.05). Remarkably, the PBL response to Gag peptides after 7 days of coculture with DC mock remained unchanged compared to the day 0 PBL response (Fig. 1). A very similar picture was observed in IL-2 and perforin ELISPOT assays (data not shown). In all experiments, these controls were carried out, showing very similar results. To avoid overloaded figures, only the responses to HxB2 Gag peptides in freshly thawed PBL (day 0) and in PBL after coculture with gag mRNA-electroporated DC (day 7) are shown.
FIG. 1.
Induction of HIV-1 Gag-specific T-cell responses by autologous DC, electroporated with codon-optimized HxB2 gag mRNA from chronically HIV-1-infected subjects. The graph shows the box plots of IFN-γ SFC per 106 PBL from 24 chronically HIV-1-infected individuals, including 12 treatment-naïve (P1 to P12) and 12 HAART (P18 to P29) patients. Freshly thawed PBL were incubated in IFN-γ ELISPOT assays with medium only or with the HxB2 Gag peptide pool (day 0 coculture, absence of DC [−] or HxB2 Gag pool, absent [−] or present [+], respectively). The other thawed PBL were first stimulated for 1 week with autologous DC mock or h-HxB2 gag-electroporated DC (DC h-HxB2 gag). Afterward, these DC-stimulated PBL were restimulated in ELISPOT assays with either medium or the HxB-2 Gag peptide pool (day 7: HxB2 Gag pool, − or +). The horizontal lines within the boxes indicate the median SFC values, the boxes' top and bottom borders represent the 25th through 75th percentiles, and the vertical lines end at the 10th and 90th percentiles. The P values of statistically significant differences between groups are indicated as P ≤ 0.05 (*), P ≤ 0.01 (**), and P ≤ 0.001 (***).
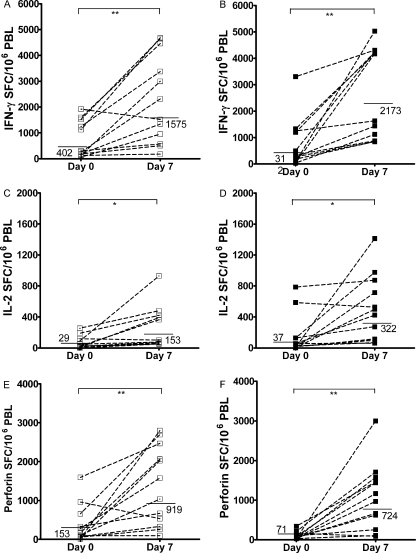
Next, all day 0 and day 7 responses of PBL from the 12 treatment-naïve individuals (P1 to P12) were compared to those of the 12 HAART-treated individuals (P18 to P29) in ELISPOT assays for IFN-γ (Fig. 2A and B), IL-2 (Fig. 2C and D), and perforin (Fig. 2E and F). As can be seen in Table 1, these two patient groups were carefully matched for absolute CD4+ T-cell count (P = 0.9). The results depicted in Fig. 2 show that DC h-HxB2 gag induced an expansion of responding PBL from most treatment-naïve and HAART-treated individuals. The responses of freshly thawed PBL as well as the responses after 1 week of stimulation with DC to the Gag peptide pool were similar in HAART-treated and treatment-naïve individuals (P > 0.4 for IFN-γ and P > 0.3 for perforin). However, IL-2 SFC levels after DC stimulation were significantly higher in PBL from HAART-treated than those in PBL from treatment-naïve individuals (P < 0.02).
FIG. 2.
Secretion of IFN-γ, IL-2, and perforin by DC h-HXB2 gag-induced HIV-1-specific T cells from therapy-naïve versus HAART patients. Summary of HIV-1 Gag-specific IFN-γ (A and B), IL-2 (C and D), and perforin (E and F) ELISPOT assays triggered in the direct ex vivo ELISPOT (day 0) or induced after 1 week of in vitro stimulation with DC h-HxB2 gag in 12 treatment-naïve (P1 to 12; panels A, C, and E) and 12 HAART-treated (P18 to 29; B, D, and F) HIV-1-infected individuals whose absolute CD4+ T-cell counts/μl blood matched. The horizontal lines indicate the geometric mean SFC values. The P values of statistically significant differences between day 0 and day 7 responses are as indicated in the legend for Fig. 1.
A closer look at day 0 IFN-γ responses reveals a bimodal distribution, in that 5 out of 12 of therapy-naïve individuals and 4 out of 12 of HAART-treated individuals had clearly higher amounts of IFN-γ SFC triggered. Interestingly, three out of these five and two out of these four, respectively, also had higher IL-2 and perforin ELISPOT assay responses. However, clear correlations between results from the ELISPOT assay, on one hand, and viral load, absolute CD4+, and CD8+ T-cell count at the time of blood drawing or nadir CD4+ T-cell count, on the other hand, could not be found, either by performing Spearman's rank testing on the entire therapy-naïve and HAART-treated groups or by comparing the subgroups with initially high or low responses.
This first set of experiments demonstrates that it is possible to amplify responses to Gag peptides in PBL from both therapy-naïve and HAART-treated HIV-1-infected individuals, using autologous mature MO-DC electroporated with h-HxB2 gag mRNA.
Evaluation of additional DC-induced cytokines in PBL, using multiplex fluorescent bead immunoassay.
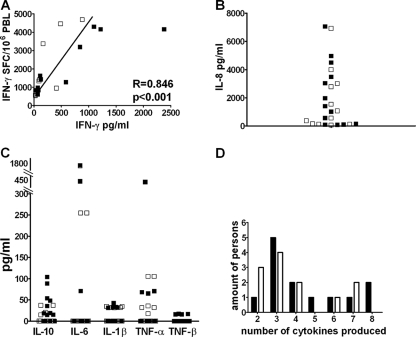
Because IFN-γ, IL-2, and perforin do not provide a complete picture of T-cell function (25, 70), other cytokines that are secreted during the restimulation of DC h-HxB2 gag-expanded PBL were also measured. This was done for 12 therapy-naïve patients (P2 to P8, P10, P12 to P14, and P17) and 13 HAART-treated (P18 to 20, P22 to P26, P28, and P30 to P33) HIV-1-seropositive individuals. Depending on availability, supernatants from either the IL-2 (19 cases) or IFN-γ (6 cases) ELISPOT assays were used. IFN-γ in the supernatants correlated (R = 0.8; P < 0.001) with the amount of SFC detected in the IFN-γ ELISPOT assays (Fig. 3A), confirming the reliability and sensitivity of the fluorescent bead assay.
FIG. 3.
Production of various cytokines by DC h-HxB2 gag-induced PBL from therapy-naïve and HAART patients. The supernatants of the ELISPOT assays from DC h-HxB2 gag-stimulated PBL, restimulated with the Gag peptide pool, were used to perform the cytometric bead assay. Cells from 13 HAART-treated HIV-1-seropositive subjects (P18 to 20, P22 to P26, P28, and P30 to P33 [black squares]) and 12 therapy-naïve HIV-1-seropositive subjects (P2 to P8, P10, P12 to P14, and P17 [open squares]) were used. (A) The number of IFN-γ SFC in the ELISPOT assay (y axis) correlates with the concentration of IFN-γ secreted in the supernatant measured by cytometric beads (x axis; in pg/ml). (B) Secretion of IL-8 in pg/ml. (C) Secretion of IL-6, IL-10, TNF-α, TNF-β, and IL-1β. (D) The number of cytokines secreted by cells from each HIV-1-seropositive individual. HAART-treated individuals are represented by black bars, and treatment-naïve individuals are represented by white bars.
Whereas in none of the cultures could IL-12p70, IL-4, or IL-5 be detected, IL-8 was secreted by PBL from all individuals in massive amounts (Fig. 3B). IL-10, IL-1-β, TNF-α, IL-6, and TNF-β were secreted by 12 donors out of 25 in measurable and variable amounts (Fig. 3C). Figure 3D summarizes the number of cytokines (besides IL-8) that were secreted by PBL from each donor. No significant differences in the numbers of cytokines produced were found between therapy-naïve and HAART-treated patients, and the number of cytokines produced did not correlate with viral load, absolute CD4+ and CD8+ T-cell count, or nadir CD4+ T-cell count.
Clearly, at least in some individuals, coculturing PBL with h-HxB2 gag DC resulted in the capacity to secrete several cytokines, but no significant qualitative or quantitative differences in secreted cytokines were observed between PBL from therapy-naïve and HAART-treated individuals.
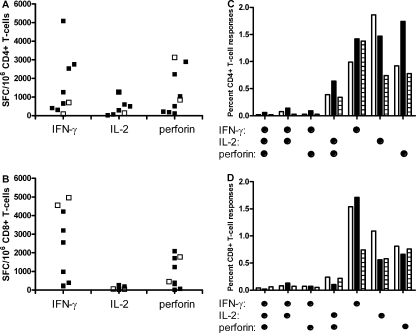
Induction of CD4+ and CD8+ T-cell responses using HxB2 gag mRNA-electroporated DC.
Since both CD4+ and CD8+ T cells are presumed to be important for protective immune responses (44), it was analyzed if DC h-HxB2 gag could induce responses in both T subsets from treatment-naïve (P15 and P16) and HAART-treated individuals (P32 to P35 and P70 to P72). After coculture with DC, a part of the PBL was restimulated with the peptide pool and intracellularly stained for IFN-γ, IL-2, and perforin. From the other part, CD4+ and CD8+ T cells were isolated by positive magnetic selection and separately restimulated with the peptide pool in ELISPOT assays. Comparing both assay results reveals that CD4+ T cells contained and produced IFN-γ, IL-2, and perforin (Fig. 4A and C), whereas CD8+ T cells also contained IFN-γ, IL-2, and perforin but secreted only IFN-γ and perforin (Fig. 4B and D). Further, after 1 week of stimulation with DC and restimulation with peptide pool, a small fraction of T cells were bifunctional, and this was most pronounced in the CD4+ T cells. These data indicate that h-HxB2 gag mRNA-electroporated DC result in both class I and class II restricted immune responses but with some differences in cytokine profiles between the CD4 and CD8 responses.
FIG. 4.
Induction of HIV-1-specific CD4+- and CD8+-mediated T-cell responses by DC h-HxB2 gag. PBL from HIV-infected subjects were cocultured with autologous DC h-HxB2 gag for 1 week. Afterward, from one part of the PBL, CD4+ (A) and CD8+ (B) T cells were isolated and restimulated with the Gag peptide pool in IFN-γ, IL-2, and perforin ELISPOT assays. The resulting levels of IFN-γ, IL-2, and perforin SFC per million responder cells are shown for CD4+ (A) and CD8+ (B) T cells. The experiments were done for seven HAART-treated individuals (P15 to 18 and P70 to P72 [black squares]) and for two treatment-naïve individuals (P62 and P63 [open squares]). The other part of the PBL were left unfractionated, similarly restimulated with a Gag peptide pool, and stained intracellularly for IFN-γ, IL-2, and perforin (P70 to 72). The CD4+ (C) and CD8+ (D) T-cell response to Gag is composed of multiple functional subpopulations. Underneath the graph, the respective (combinations of) functions are shown (dots denote IFN-γ, IL-2, and/or perforin positivity). The bars in the graph represent the percentages of positive cells within the CD4 and CD8 T cells. White bars are for P72, black bars for P71, and striped bars for P72.
Influence of Treg and PD/PD-L1 interaction on DC-induced immune responses.
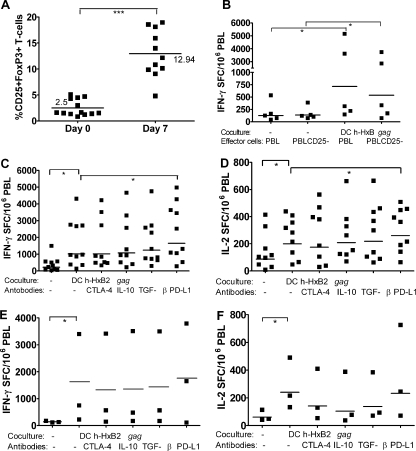
In a myeloma tumor model, similarly generated DC were shown to increase the frequency of functional Treg, defined as CD4+ CD25+ FoxP3+ T cells, which were able to effectively suppress T-cell responses (6). In our system, we also observed that the frequency of cells with the Treg phenotype increased during coculture by an average of 10% in all experiments (Fig. 5A). Since these presumed Treg obviously did not completely prevent the expansion of HIV-1-specific T cells, it was investigated to what extent they were truly functional as negative regulators.
FIG. 5.
Investigation of the potential role of Treg as mediators of suppression. (A) Increase of Treg phenotype during coculture. The frequency of CD25+ FoxP3+ cells was assessed within the CD3+ CD4+ T cells by fluorescence-activated cell sorter on nonstimulated PBL (day 0) and on PBL that were stimulated for 1 week with DC h-HxB2 gag (day 7). The experiments were done for HAART patients P41 to P48 and P65 to P69. (B) Effect of preliminary removal of CD25+ cells. Complete PBL or CD25+ cell-depleted PBL were cocultured with autologous DC h-HxB2 gag. After 1 week, the PBL were restimulated with the Gag peptide pool in an IFN-γ ELISPOT assay. As a control, freshly thawed PBL and CD25+ cell-depleted PBL (PBLCD25-) from the same patients, not cocultured with DC (indicated as absence [−] of coculture), were directly incubated in ELISPOT assays in parallel experiments. The experiments were done for HAART patients P36 to P40. The horizontal lines indicate the mean SFC values. (C and D) Effect of antibodies against mediators of suppression during the induction phase. PBL were cocultured for 1 week with autologous DC h-HxB2 gag in the absence (-) or presence of antibodies against CTLA-4, IL-10, TGF-β, or PD-L1. Afterward, PBL were restimulated with the Gag peptide pool in IFN-γ (C) or IL-2 (D) ELISPOT assays in the absence of antibodies. As a control, non-DC-stimulated PBL were directly incubated in ELISPOT assays in parallel experiments. In the graph, the levels of IFN-γ SFC per million responder cells are shown. The experiments were done for HAART patients P41 to P45 and P65 to 69. The horizontal lines indicate the mean SFC values induced. (E and F) Effect of antibodies against mediators of suppression during the effector phase. DC h-HxB2 gag were cocultured for 1 week with autologous PBL. Afterward, the PBL were restimulated with the HxB2 Gag peptide pool in an IFN-γ (E) or IL-2 (F) ELISPOT assay in the absence (-) or presence of antibodies against CTLA-4, PD-L1, IL-10, or TGF-β. The experiments were done for HAART patients P46 to P48. The horizontal lines indicate the mean SFC values. Horizontal lines are geometrical mean values. The P values are as indicated in the legend for Fig. 1.
First, the effect of in vitro CD25+ cell depletion before coculture on proliferative and Gag-specific responses was examined in PBL from patients under HAART (P36 to P40 and P73 to 75). Using a standardized CD25 depletion kit, a 20-fold reduction in the frequency of CD25-expressing CD4+ T cells to less than 0.5% of PBL was obtained. Afterward, CD25+ cell-depleted PBL and unfractionated PBL were cocultured with DC h-HxB2 gag in parallel experiments. After 1 week, the frequency of Treg was increased in both cultures (8.2% in unfractionated and 4.2% in CD25+ cell-depleted PBL). However, the increase of IFN-γ SFC (Fig. 5B) and the proliferation rate (Table 2) after DC stimulation were rather similar in CD25+ cell-depleted PBL and in unfractionated PBL.
TABLE 2.
HIV Gag-specific proliferative responsesa
| Patient no. | PBL (cpm)
|
CD25-depleted PBL (cpm)
|
||
|---|---|---|---|---|
| − | DC h-HxB2 gag | − | DC h-HxB2 gag | |
| P73 | 186 | 17,219 | 130 | 6,190 |
| P74 | 328 | 4,920 | 520 | 4,680 |
| P75 | 263 | 4,471 | 444 | 5,332 |
Freshly thawed unfractionated PBL or CD25-depleted PBL were cocultured for 1 week alone (−) or with h-HxB2 gag mRNA-electroporated DC (DC h-HxB2 gag). The last day of the coculture, [3H]thymidine was added.
In order to determine whether cells with the Treg phenotype and/or a related suppressive mechanism were operational, neutralizing antibodies against mediators of suppressors were added during the coculture (Fig. 5C and D, induction phase) or during the ELISPOT assay (Fig. 5E and F, effector phase).
As shown in Fig. 5C and D, DC-stimulated PBL responded to Gag peptide pool restimulation with 1,022 IFN-γ and 199 IL-2 SFC (geometrical mean value) in the absence of blocking antibodies. When anti-CTLA-4 or anti-IL-10 had been added during coculture, similar numbers of SFC were obtained. In contrast, the numbers of SFC induced after the addition of anti-TGF-β (1,232 IFN-γ [P = 0.07] and 218 IL-2 SFC [P = 0.06]) or anti-PD-L1 (1,651 IFN-γ [P = 0.002] and 260 IL-2 SFC [P = 0.01]) were higher than the control values, but the difference was only statistically significant with anti-PD-L1. Adding the same blocking antibodies during the effector phase (P46 to P48) clearly failed to improve IFN-γ and IL-2 responses, as shown in Fig. 5E and F.
These experiments indicate that stimulation with DC results in an increase of CD4+ T cells with a Treg phenotype but without a clear suppressive function, in either the induction or the effector phase. Our results confirm, however, that PDL-1-PD-1 interaction has a negative effect during the induction phase.
Epitope mapping suggests broadening of the T-cell response after stimulation with gag mRNA-electroporated DC.
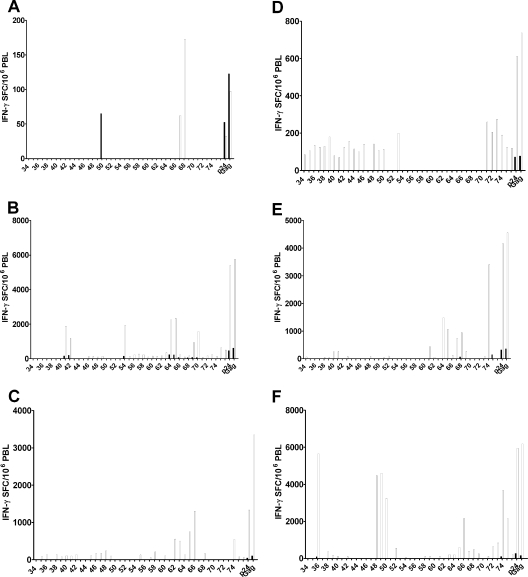
Of all HAART-treated individuals included in this study, seven who are infected with HIV-1 subtype B and seven infected with nonsubtype B turned out to have matching absolute amounts of CD4+ T-cell counts. In these 14 individuals, we compared the IFN-γ responses in freshly thawed and in DC h-HxB2 gag-cocultured PBL. Unexpectedly, there was no difference between subtype B and nonsubtype B individuals. A tentative explanation for the remarkably good response in PBL from nonsubtype B-infected patients could be that, besides an expansion of preexisting Gag-specific T-cell clones, a broader immune response is also induced with gag-electroporated DC.
To investigate this hypothesis, six HAART patients, three infected with HIV-1 subtype B (P59 to P61) and three with subtype A (P62 to P64), were selected for measurements of IFN-γ T-cell responses against single peptides of p24 (peptides 34 to 76), which contain the highest CD8 T-cell epitope density (2, 8). IFN-γ SFC against individual and pooled peptides were assessed in nonstimulated PBL and DC h-HxB2 gag-stimulated PBL side by side. Positive responses were defined as explained in the legend for Fig. 6, based on reference 48. According to these stringent criteria, nonstimulated PBL sometimes failed to respond to any of the single peptides of Gag p24, although an initial response (usually small) to the pools of p24 and Gag peptides was always present. These limited baseline responses corresponded to known epitopes associated with respective HLA types (Los Alamos database). Conversely, after 1 week of stimulation with gag mRNA-electroporated DC, PBL from all patients showed clear-cut responses against several epitopes. Not surprisingly, preexisting (day 0) responses against single peptides were always amplified after 1 week of DC stimulation. Importantly, however, DC stimulation also induced SFC against peptides which were below the detection limit at day 0, apparently including epitopes not previously associated with the HLA type. For example, P60 triggered responses against peptides 41 to 42, related to Cw*01, and against peptides 64 to 65, related to B*08, in freshly thawed PBL. After stimulation with DC, SFC against the same peptides were amplified, and responses were induced against additional peptides, i.e., 46 to 49, 54 to 63, and 69 to 76, which were not known to be associated with these HLA alleles (Fig. 6B). Clearly, DC electroporated with h-HxB2 gag mRNA result in an apparently broader immune response.
FIG. 6.
Epitope mapping of p24 in nonstimulated and DC h-HxB2 gag-stimulated PBL. Freshly thawed PBL (black bars) or PBL cocultured for 1 week with DC h-HxB2 gag (white bars) were stimulated with single peptides representing p24 (34 to 76) or with the pool of peptides encompassing whole p24 (p24) or whole Gag (Gag). The first peptide is number 34 (PIVQNLQGQMVHQAI), and the last one is 76 (YKTLRAEQASQEVKN). A positive response was defined as a minimum of 50 SFC/million responder cells, a ratio of a minimum of 4 between the responses of T cells stimulated with DC mock and with DC h-HxB2 gag, and in addition, more than three times the standard deviation above the mock-stimulated mean. This experiment was done for six HAART donors (P59 [A], P60 [B], P61 [C], P62 [D], P63 [E], and P64 [F]).
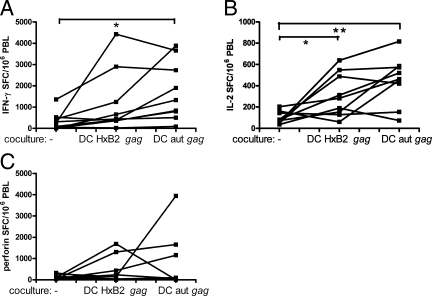
Stimulatory capacity of autologous proviral gag mRNA-electroporated DC versus wt-HxB2 gag mRNA-electroporated DC toward T cells from HAART-treated individuals.
Previously, we showed that autologous viral as well as proviral-derived gag mRNA DC from treatment-naïve HIV-1-infected persons readily triggered IFN-γ- and IL-2-secreting T cells in direct ex vivo ELISPOT assays (60). Here it was investigated whether DC, from HAART-treated HIV-1-infected persons, electroporated with autologous proviral gag mRNA could increase the level of IFN-γ-, IL-2-, and perforin-secreting T cells (P49 to P57). For comparison in this particular set of experiments, the “wt” (i.e., not codon-optimized) HxB2 was used, because the gag sequence of the autologous virus is also “wt.” Clearly, as shown in Fig. 7, PBL specifically stimulated with either wt-HxB2 or autologous proviral gag mRNA and restimulated with the Gag peptide pool showed increased IFN-γ (P > 0.05 and P < 0.05, respectively) and IL-2 (P < 0.05 and P < 0.01, respectively) SFC compared to freshly thawed PBL directly stimulated with the peptide pool. The response of perforin SFC was not significantly increased. The geometric mean increases of the responses after coculture with autologous gag mRNA compared to those after coculture with wt-HxB2 gag mRNA were slightly (but not significantly) higher for IFN-γ (10.4 versus 5.0) and IL-2 (4.4 versus 2.9) but not for perforin (1.2 in both cases).
FIG. 7.
HIV-specific T cells can be expanded by autologous proviral gag mRNA-electroporated DC. MO-DC from nine HIV-1-seropositive subjects under HAART (P49 to 57) were electroporated with mRNA encoding wt-HxB2 (DC HxB2 gag) or autologous proviral gag (DC aut gag). These DC were matured and cocultured for 1 week with autologous PBL. Afterward, these PBL were stimulated with the HxB2 Gag peptide pool (day 7), and their responses were compared with those of freshly thawed PBL directly stimulated with the HxB-2 Gag peptide pool (day 0). The results are shown as individual data points representing the proportion of PBL from that person that secrete IFN-γ (A), IL-2 (B), or perforin (C). The P values of statistically significant differences between groups are as indicated in the legend for Fig. 1.
DISCUSSION
In the present work, in vitro proof of principle is provided for the use of gag mRNA-electroporated mature MO-DC as a possible strategy to improve T-cell immunity under HAART. In agreement with the literature, only low levels of IFN-γ and perforin were triggered and no IL-2 was triggered with Gag peptides during a direct ex vivo ELISPOT assay of PBL from most chronically infected individuals, irrespective of whether they were treatment naïve or successfully HAART treated (31, 64). However, after 1 week of stimulation with consensus B gag mRNA-electroporated DC, PBL from both groups responded with high numbers of IFN-γ, IL-2, and perforin SFC. This result is encouraging since it shows that, besides the easily triggered IFN-γ, important regulatory cytokines, such as IL-2, and the lytic capacity (perforin) can be enhanced by gag mRNA-electroporated DC. Although the IFN-γ and perforin responses in HAART and treatment-naïve patients were comparable, the amount of IL-2-secreting T cells was significantly higher in HAART-treated individuals, implying that proliferative responses may be restored to a larger extent in the treated group.
Both CD8+ and CD4+ T cells from the infected individuals responded to DC-mediated induction, pointing to efficient class I and II presentation, even in the absence of additional major histocompatibility complex class II targeting. As shown previously, gag mRNA-electroporated DC efficiently secrete Gag p24 protein, which might be processed through the exogenous class II pathway (65, 60). In view of further therapeutic applications, it is encouraging that CD4+ T cells could efficiently be induced by DC to produce IFN-γ, perforin, and especially IL-2, since vigorously proliferating and type 1 cytokine-secreting memory CD4+ T cells are needed to support protective HIV-specific CTL (44).
Besides IFN-γ and IL-2, other cytokines play an important role in HIV-specific immune response (4, 27, 45). Recently, Betts et al. demonstrated an association between polyfunctional T-cells and the “elite controller” status (7). Whereas Betts et al. measured the expression of multiple cytokines and effector molecules directly ex vivo by intracellular staining, we examined T-cell functionality after 1 week of DC stimulation in three complementary ways. Besides the ELISPOT, measuring the frequency of T cells secreting particular individual factors (IFN-γ, IL-2, and perforin), we used multiparametric flow cytometry to evaluate the intracellular production of the same factors and a cytometric bead assay to measure additional cytokines in the supernatant. Obviously, gag mRNA-electroporated DC induction, combined with Gag peptide restimulation, resulted in the production of several cytokines in PBL from our population of chronically infected individuals, who failed to spontaneously control the virus. Interestingly, in the PBL of two HAART-treated persons who showed significant direct ex vivo IFN-γ, IL-2, and perforin ELISPOT responses, DC induction resulted in cells that secreted seven or eight cytokines after restimulation, as evaluated by the cytometric beads. Moreover, comparing ELISPOT and ICS results at the T-cell subset level, indicated that gag mRNA DC-stimulated CD4+ T cells produce and secrete all three studied factors (IFN-γ, IL-2, and perforin), with some evidence of bifunctionality (IL-2 and perforin coexpression) at the single-cell level. However, gag mRNA DC-stimulated CD8+ T cells from HIV-infected subjects failed to secrete IL-2, although it was present intracellularly, pointing to a selective secretion defect. In general, it seems promising that our gag mRNA DC induce T cells that can secrete several cytokines, although true polyfunctionality at the single-cell level was limited.
Besides h-HxB2 gag mRNA-electroporated DC, DC electroporated with wt-HxB-2 gag mRNA or autologous proviral gag mRNA were also able to efficiently induce T cells to secrete IFN-γ, IL-2, and perforin. Importantly, the responses induced with autologous proviral gag mRNA DC were comparable with those induced by HxB2 gag mRNA, confirming extensive cross-reactivity between autologous and consensus subtype B sequences. Furthermore, from our previous work, it is known that the transcribed autologous proviral gag mRNA reflects a spectrum of quasispecies (60), which is important to induce a broad enough response to cover the endogenous repertoire and to possibly prevent escape.
In the same context, it is shown here that the induction of T cells with consensus B gag mRNA DC not only resulted in the expansion of preexisting clones but also increased the breadth of the response, even in three donors infected with subtype A. This is in accordance with a recent study showing extensive cross-reactivity among subtypes A, B, and C in untreated HIV-1 infected individuals (24). Furthermore, we also demonstrate that after 1 week of DC stimulation, responses were induced toward epitopes that were previously not known to be restricted by the particular HLA molecules of the respective patients. Presumably this is because the previously described associations were performed in direct ex vivo ELISPOT assays (21, 30), whereas we included an induction phase with electroporated DC. The use of transfected DC is probably responsible for the broadening of the immune response, because they present epitopes other than DC that take up antigen via the normal way. These transfected DC that present other epitopes can be responsible for the priming of new T cells or, probably more likely, for strongly increasing the frequency of T cells that on day 0 (corresponding to direct ex vivo) remained below the detection limit. All these results are encouraging, because if they can be reproduced in vivo, virus escape after treatment interruption might become more difficult.
The exact role of Treg in HIV remains controversial. They could be detrimental by impairing T-cell responses, thus facilitating viral persistence, or conversely, rather protective by limiting immune hyper-activation (56). Moreover, the PD-1/PD-L1 or -L2 pathway may also constitute a negative regulator of HIV-specific immune responses, but again, the exact importance of this pathway remains to be clarified (29). Studies in regular PBMC cultures from HIV-1 untreated and treated individuals have shown that the “natural Treg” exert suppressive activity on the proliferation of T cells triggered by p24 protein (1, 18, 31, 64). However, recently Kinter et al. showed that Treg suppression was much more pronounced in cultures from LTNP than in those from progressors (31). Moreover, Banerjee et al. (6) pointed out that inflammatory cytokine-treated MO-DC (generated similarly in our study) are the most effective Treg inducers in vitro (even in CD25+ cell-depleted PBL) and that DC-induced Treg from both healthy donors and myeloma patients effectively suppressed T-cell responses in allogeneic mixed leukocyte reactions.
In our present study, 1 week of stimulation with DC also increased the frequency of cells with a Treg phenotype. To identify the role of Treg in our system, depletion and blocking experiments were done. In contrast to the findings mentioned above, natural Treg depletion failed to enhance subsequent proliferative T-cell responses in our model. Of note also, all our patients were chronic progressors under treatment, a population that has not yet extensively been studied with regard to Treg. Secondly, the effect of induced Tregs was determined by adding neutralizing antibodies against CTLA-4, IL-10, and TGF-β. Blocking these presumed Treg mediators (26) did not significantly improve the effector T-cell function either, suggesting that induced CD25+ FoxP3+ CD4+ T cells lack a suppressive function during DC-mediated T-cell stimulation.
Recently, several groups showed that PD-1 was upregulated on PBMC from treatment-naïve HIV-1 infected individuals and that blocking the PD-1/PD-L1 pathway partly restored the effector function (17, 68). In addition, it was found that the expression of PD-L1 remained elevated on cells from HAART-treated HIV-1-infected individuals (52), whereas the PD-1 level was not different on those from LTNP (58, 68). In our model, a significant but limited improvement of effector function was observed if anti-PD-L1 was present during the induction phase.
In conclusion, we show that DC from HAART-treated individuals, electroporated with gag mRNA, efficiently expand HIV-specific T cells that secrete IFN-γ, IL-2, perforin, and other cytokines. This strategy induces not only CD8+ T cells but also CD4+ T cells, which selectively secrete IL-2, and some degree of bifunctionality. Although there is an accumulation of CD4+ T cells with a Treg phenotype, they apparently did not suppress the response, but blocking the PD-1/PD-L1 pathway slightly improved effector functions. Consensus B (h-HxB2) gag mRNA-electroporated DC could induce T cells from patients infected with either subtype B viruses or non-B viruses to respond to a broader repertoire of HIV-1 epitopes compared to ex vivo freshly thawed T cells. Moreover, mRNA derived from autologous provirus could also be efficiently used to induce T-cell responses. All these results together open perspectives for the development of an immunotherapy in HIV-infected individuals under HAART based on DC electroporated with mRNA encoding Gag and possibly other viral proteins, derived from either consensus or autologous proviral sequences.
Acknowledgments
We thank the AIDS reference clinic (ARC) and laboratory (ARL) of our institute for the recruitment of HIV-1-seropositive persons for this study and the collection of samples. We also thank Nathalie Cools for the skillful help with performing experiments on a polychromatic flow cytometer. We are also grateful to the Antwerp Blood Transfusion Center for HLA typing of some samples, to Bernard Verrier for providing the codon-optimized Gag construct, to Kris Thielemans for providing the pGEM4Z/hHxB-2-gag/A64 plasmid, and to the NIH AIDS Research and Reference Reagent Program for providing the peptides.
This work was supported by grants from the Fund for Scientific Research Flanders (FWO), GOA (FA20000/802) of the University of Antwerp, and IUAP (Inter-University Attraction Poles, P6/41 of the Belgian government). E. Van Gulck is a predoctoral fellow of the Institute for Science and Technology (IWT), Flanders.
Footnotes
Published ahead of print on 30 January 2008.
REFERENCES
- 1.Aandahl, E. M., J. Michaelsson, W. J. Moretto, F. M. Hecht, and D. F. Nixon. 2004. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 782454-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addo, M. M., X. G. Yu, E. S. Rosenberg, B. D. Walker, and M. Altfeld. 2002. Cytotoxic T-lymphocyte (CTL) responses directed against regulatory and accessory proteins in HIV-1 infection. DNA Cell Biol. 21671-678. [DOI] [PubMed] [Google Scholar]
- 3.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407386-390. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill, F. R., and F. Burke. 1989. The cytokine network. Immunol. Today 10299-304. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee, D. K., M. V. Dhodapkar, E. Matayeva, R. M. Steinman, and K. M. Dhodapkar. 2006. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood 1082655-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 1074781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betts, M. R., K. Yusim, and R. A. Koup. 2002. Optimal antigens for HIV vaccines based on CD8+ T response, protein length, and sequence variability. DNA Cell Biol. 21665-670. [DOI] [PubMed] [Google Scholar]
- 9.Boczkowski, D., S. K. Nair, D. Snyder, and E. Gilboa. 1996. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 184465-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bojak, A., J. Wild, L. Deml, and R. Wagner. 2002. Impact of codon usage modification on T cell immunogenicity and longevity of HIV-1 gag-specific DNA vaccines. Intervirology 45275-286. [DOI] [PubMed] [Google Scholar]
- 11.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 686103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3205-211. [DOI] [PubMed] [Google Scholar]
- 13.Brown, K., W. Gao, S. Alber, A. Trichel, M. Murphey-Corb, S. C. Watkins, A. Gambotto, and S. M. Barratt-Boyes. 2003. Adenovirus-transduced dendritic cells injected into skin or lymph node prime potent simian immunodeficiency virus-specific T cell immunity in monkeys. J. Immunol. 1716875-6882. [DOI] [PubMed] [Google Scholar]
- 14.Carbonneil, C., A. Aouba, M. Burgard, S. Cardinaud, C. Rouzioux, P. Langlade-Demoyen, and L. Weiss. 2003. Dendritic cells generated in the presence of granulocyte-macrophage colony-stimulating factor and IFN-alpha are potent inducers of HIV-specific CD8 T cells. AIDS 171731-1740. [DOI] [PubMed] [Google Scholar]
- 15.Chassin, D., M. Andrieu, W. Cohen, B. Culmann-Penciolelli, M. Ostankovitch, D. Hanau, and J. G. Guillet. 1999. Dendritic cells transfected with the nef genes of HIV-1 primary isolates specifically activate cytotoxic T lymphocytes from seropositive subjects. Eur. J. Immunol. 29196-202. [DOI] [PubMed] [Google Scholar]
- 16.Chun, T. W., and A. S. Fauci. 1999. Latent reservoirs of HIV: obstacles to the eradication of virus. Proc. Natl. Acad. Sci. USA 9610958-10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443350-354. [DOI] [PubMed] [Google Scholar]
- 18.Eggena, M. P., B. Barugahare, N. Jones, M. Okello, S. Mutalya, C. Kityo, P. Mugyenyi, and H. Cao. 2005. Depletion of regulatory T cells in HIV infection is associated with immune activation. J. Immunol. 1744407-4414. [DOI] [PubMed] [Google Scholar]
- 19.Engelmayer, J., M. Larsson, A. Lee, M. Lee, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 2001. Mature dendritic cells infected with canarypox virus elicit strong anti-human immunodeficiency virus CD8+ and CD4+ T-cell responses from chronically infected individuals. J. Virol. 752142-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans, D. T., D. H. O'Connor, P. Jing, J. L. Dzuris, J. Sidney, J. da Silva, T. M. Allen, H. Horton, J. E. Venham, R. A. Rudersdorf, T. Vogel, C. D. Pauza, R. E. Bontrop, R. DeMars, A. Sette, A. L. Hughes, and D. I. Watkins. 1999. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 51270-1276. [DOI] [PubMed] [Google Scholar]
- 21.Geldmacher, C., J. R. Currier, E. Herrmann, A. Haule, E. Kuta, F. McCutchan, L. Njovu, S. Geis, O. Hoffmann, L. Maboko, C. Williamson, D. Birx, A. Meyerhans, J. Cox, and M. Hoelscher. 2007. CD8 T-cell recognition of multiple epitopes within specific Gag regions is associated with maintenance of a low steady-state viremia in human immunodeficiency virus type 1-seropositive patients. J. Virol. 812440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilboa, E., and J. Vieweg. 2004. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol. Rev. 199251-263. [DOI] [PubMed] [Google Scholar]
- 23.Goulder, P. J., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3212-217. [DOI] [PubMed] [Google Scholar]
- 24.Gupta, S. B., C. T. Mast, N. D. Wolfe, V. Novitsky, S. A. Dubey, E. G. Kallas, M. Schechter, B. Mbewe, E. Vardas, P. Pitisuttithum, D. Burke, D. Freed, R. Mogg, P. M. Coplan, J. H. Condra, R. S. Long, K. Anderson, D. R. Casimiro, J. W. Shiver, and W. L. Straus. 2006. Cross-clade reactivity of HIV-1-specific T-cell responses in HIV-1-infected individuals from Botswana and Cameroon. J. Acquir. Immune Defic. Syndr. 42135-139. [DOI] [PubMed] [Google Scholar]
- 25.Harari, A., F. Vallelian, P. R. Meylan, and G. Pantaleo. 2005. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J. Immunol. 1741037-1045. [DOI] [PubMed] [Google Scholar]
- 26.Hryniewicz, A., A. Boasso, Y. Edghill-Smith, M. Vaccari, D. Fuchs, D. Venzon, J. Nacsa, M. R. Betts, W. P. Tsai, J. M. Heraud, B. Beer, D. Blanset, C. Chougnet, I. Lowy, G. M. Shearer, and G. Franchini. 2006. CTLA-4 blockade decreases TGF-β, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood 1083834-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacob, C. O. 1992. Tumor necrosis factor and interferon gamma: relevance for immune regulation and genetic predisposition to autoimmune disease. Semin. Immunol. 4147-154. [PubMed] [Google Scholar]
- 28.Kavanagh, D. G., D. E. Kaufmann, S. Sunderji, N. Frahm, S. Le Gall, D. Boczkowski, E. S. Rosenberg, D. R. Stone, M. N. Johnston, B. S. Wagner, M. T. Zaman, C. Brander, E. Gilboa, B. D. Walker, and N. Bhardwaj. 2006. Expansion of HIV-specific CD4+ and CD8+ T cells by dendritic cells transfected with mRNA encoding cytoplasm- or lysosome-targeted Nef. Blood 1071963-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keir, M. E., L. M. Francisco, and A. H. Sharpe. 2007. PD-1 and its ligands in T-cell immunity. Curr. Opin. Immunol. 19309-314. [DOI] [PubMed] [Google Scholar]
- 30.Kiepiela, P., K. Ngumbela, C. Thobakgale, D. Ramduth, I. Honeyborne, E. Moodley, S. Reddy, C. de Pierres, Z. Mncube, N. Mkhwanazi, K. Bishop, M. van der Stok, K. Nair, N. Khan, H. Crawford, R. Payne, A. Leslie, J. Prado, A. Prendergast, J. Frater, N. McCarthy, C. Brander, G. H. Learn, D. Nickle, C. Rousseau, H. Coovadia, J. I. Mullins, D. Heckerman, B. D. Walker, and P. Goulder. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 1346-53. [DOI] [PubMed] [Google Scholar]
- 31.Kinter, A. L., R. Horak, M. Sion, L. Riggin, J. McNally, Y. Lin, R. Jackson, A. O'Shea, G. Roby, C. Kovacs, M. Connors, S. A. Migueles, and A. S. Fauci. 2007. CD25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific CD8+ T cells in vitro. AIDS Res. Hum. Retrovir. 23438-450. [DOI] [PubMed] [Google Scholar]
- 32.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lapenta, C., S. M. Santini, M. Logozzi, M. Spada, M. Andreotti, T. Di Pucchio, S. Parlato, and F. Belardelli. 2003. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-alpha. J. Exp. Med. 198361-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsson, M., D. T. Wilkens, J. F. Fonteneau, T. J. Beadle, M. J. Merritt, R. G. Kost, P. A. Haslett, S. Cu-Uvin, N. Bhardwaj, D. F. Nixon, and B. L. Shacklett. 2002. Amplification of low-frequency antiviral CD8 T cell responses using autologous dendritic cells. AIDS 16171-180. [DOI] [PubMed] [Google Scholar]
- 35.Ling, B., R. S. Veazey, M. Hart, A. A. Lackner, M. Kuroda, B. Pahar, and P. A. Marx. 2007. Early restoration of mucosal CD4 memory CCR5 T cells in the gut of SIV-infected rhesus predicts long term non-progression. AIDS 212377-2385. [DOI] [PubMed] [Google Scholar]
- 36.Lu, W., and J.-M. Andrieu. 2001. In vitro human immunodeficiency virus eradication by autologous CD8+ T cells expanded with inactivated-virus-pulsed dendritic cells. J. Virol. 758949-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu, W., L. C. Arraes, W. T. Ferreira, and J. M. Andrieu. 2004. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat. Med. 101359-1365. [DOI] [PubMed] [Google Scholar]
- 38.Maserati, R., A. Foli, L. Tomasoni, L. Sighinolfi, F. Maggiolo, D. Sacchini, M. Di Pietro, D. Bertelli, C. Tinelli, and F. Lori. 2007. Effects of structured treatment interruptions on metabolic, anthropometric, immunologic, and quality of life outcomes in HIV-positive adults on HAART. Curr. HIV Res. 5337-343. [DOI] [PubMed] [Google Scholar]
- 39.McMichael, A. J., and R. E. Phillips. 1997. Escape of human immunodeficiency virus from immune control. Annu. Rev. Immunol. 15271-296. [DOI] [PubMed] [Google Scholar]
- 40.Melhem, N. M., X. D. Liu, D. Boczkowski, E. Gilboa, and S. M. Barratt-Boyes. 2007. Robust CD4+ and CD8+ T cell responses to SIV using mRNA-transfected DC expressing autologous viral Ag. Eur. J. Immunol. 372164-2173. [DOI] [PubMed] [Google Scholar]
- 41.Metzner, K. J., X. Jin, F. V. Lee, A. Gettie, D. E. Bauer, M. Di Mascio, A. S. Perelson, P. A. Marx, D. D. Ho, L. G. Kostrikis, and R. I. Connor. 2000. Effects of in vivo CD8+ T cell depletion on virus replication in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Exp. Med. 1911921-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 31061-1068. [DOI] [PubMed] [Google Scholar]
- 43.Nair, M. P., R. Pottathil, E. P. Heimer, and S. A. Schwartz. 1988. Immunoregulatory activities of human immunodeficiency virus (HIV) proteins: effect of HIV recombinant and synthetic peptides on immunoglobulin synthesis and proliferative responses by normal lymphocytes. Proc. Natl. Acad. Sci. USA 856498-6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pantaleo, G., and R. A. Koup. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10806-810. [DOI] [PubMed] [Google Scholar]
- 45.Paul, W. E. 1989. Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell 57521-524. [DOI] [PubMed] [Google Scholar]
- 46.Persaud, D., Y. Zhou, J. M. Siliciano, and R. F. Siliciano. 2003. Latency in human immunodeficiency virus type 1 infection: no easy answers. J. Virol. 771659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponsaerts, P., G. Van den Bosch, N. Cools, A. Van Driessche, G. Nijs, M. Lenjou, F. Lardon, C. Van Broeckhoven, D. R. Van Bockstaele, Z. N. Berneman, and V. F. Van Tendeloo. 2002. Messenger RNA electroporation of human monocytes, followed by rapid in vitro differentiation, leads to highly stimulatory antigen-loaded mature dendritic cells. J. Immunol. 1691669-1675. [DOI] [PubMed] [Google Scholar]
- 48.Ponsaerts, P., V. F. Van Tendeloo, N. Cools, A. Van Driessche, F. Lardon, G. Nijs, M. Lenjou, G. Mertens, C. Van Broeckhoven, D. R. Van Bockstaele, and Z. N. Berneman. 2002. mRNA-electroporated mature dendritic cells retain transgene expression, phenotypical properties and stimulatory capacity after cryopreservation. Leukemia 161324-1330. [DOI] [PubMed] [Google Scholar]
- 49.Pope, M. 2003. Dendritic cells as a conduit to improve HIV vaccines. Curr. Mol. Med. 3229-242. [DOI] [PubMed] [Google Scholar]
- 50.Price, D. A., P. J. Goulder, P. Klenerman, A. K. Sewell, P. J. Easterbrook, M. Troop, C. R. Bangham, and R. E. Phillips. 1997. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA 941890-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romani, N., S. Gruner, D. Brang, E. Kampgen, A. Lenz, B. Trockenbacher, G. Konwalinka, P. O. Fritsch, R. M. Steinman, and G. Schuler. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 18083-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosignoli, G., A. Cranage, C. Burton, M. Nelson, A. Steel, B. Gazzard, F. Gotch, and N. Imami. 2007. Expression of PD-L1, a marker of disease status, is not reduced by HAART in aviraemic patients. AIDS 211379-1381. [DOI] [PubMed] [Google Scholar]
- 53.Rowland-Jones, S., R. Tan, and A. McMichael. 1997. Role of cellular immunity in protection against HIV infection. Adv. Immunol. 65277-346. [PubMed] [Google Scholar]
- 54.Saeboe-Larssen, S., E. Fossberg, and G. Gaudernack. 2002. mRNA-based electrotransfection of human dendritic cells and induction of cytotoxic T lymphocyte responses against the telomerase catalytic subunit (hTERT). J. Immunol. Methods 259191-203. [DOI] [PubMed] [Google Scholar]
- 55.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283857-860. [DOI] [PubMed] [Google Scholar]
- 56.Sempere, J. M., V. Soriano, and J. M. Benito. 2007. T regulatory cells and HIV infection. AIDS Rev. 954-60. [PubMed] [Google Scholar]
- 57.Strobel, I., S. Berchtold, A. Gotze, U. Schulze, G. Schuler, and A. Steinkasserer. 2000. Human dendritic cells transfected with either RNA or DNA encoding influenza matrix protein M1 differ in their ability to stimulate cytotoxic T lymphocytes. Gene Ther. 72028-2035. [DOI] [PubMed] [Google Scholar]
- 58.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 121198-1202. [DOI] [PubMed] [Google Scholar]
- 59.Tsunetsugu-Yokota, Y., Y. Morikawa, M. Isogai, A. Kawana-Tachikawa, T. Odawara, T. Nakamura, F. Grassi, B. Autran, and A. Iwamoto. 2003. Yeast-derived human immunodeficiency virus type 1 p55gag virus-like particles activate dendritic cells (DCs) and induce perforin expression in Gag-specific CD8+ T cells by cross-presentation of DCs. J. Virol. 7710250-10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Gulck, E. R., P. Ponsaerts, L. Heyndrickx, K. Vereecken, F. Moerman, A. De Roo, R. Colebunders, G. Van den Bosch, D. R. Van Bockstaele, V. F. Van Tendeloo, S. Allard, B. Verrier, C. Maranon, G. Hoeffel, A. Hosmalin, Z. N. Berneman, and G. Vanham. 2006. Efficient stimulation of HIV-1-specific T cells using dendritic cells electroporated with mRNA encoding autologous HIV-1 Gag and Env proteins. Blood 1071818-1827. [DOI] [PubMed] [Google Scholar]
- 61.Van Tendeloo, V. F., P. Ponsaerts, F. Lardon, G. Nijs, M. Lenjou, C. Van Broeckhoven, D. R. Van Bockstaele, and Z. N. Berneman. 2001. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood 9849-56. [DOI] [PubMed] [Google Scholar]
- 62.Van Tendeloo, V. F., C. Van Broeckhoven, and Z. N. Berneman. 2001. Gene-based cancer vaccines: an ex vivo approach. Leukemia 15545-558. [DOI] [PubMed] [Google Scholar]
- 63.Villamide-Herrera, L., R. Ignatius, M. A. Eller, K. Wilkinson, C. Griffin, E. Mehlhop, J. Jones, S. Y. Han, M. G. Lewis, S. Parrish, T. C. Vancott, J. D. Lifson, S. Schlesinger, J. R. Mascola, and M. Pope. 2004. Macaque dendritic cells infected with SIV-recombinant canarypox ex vivo induce SIV-specific immune responses in vivo. AIDS Res. Hum. Retrovir. 20871-884. [DOI] [PubMed] [Google Scholar]
- 64.Weiss, L., V. Donkova-Petrini, L. Caccavelli, M. Balbo, C. Carbonneil, and Y. Levy. 2004. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood 1043249-3256. [DOI] [PubMed] [Google Scholar]
- 65.Weissman, D., H. Ni, D. Scales, A. Dude, J. Capodici, K. McGibney, A. Abdool, S. N. Isaacs, G. Cannon, and K. Kariko. 2000. HIV gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human in vitro primary immune response. J. Immunol. 1654710-4717. [DOI] [PubMed] [Google Scholar]
- 66.Wilson, C. C., W. C. Olson, T. Tuting, C. R. Rinaldo, M. T. Lotze, and W. J. Storkus. 1999. HIV-1-specific CTL responses primed in vitro by blood-derived dendritic cells and Th1-biasing cytokines. J. Immunol. 1623070-3078. [PubMed] [Google Scholar]
- 67.Yoshida, A., R. Tanaka, T. Murakami, Y. Takahashi, Y. Koyanagi, M. Nakamura, M. Ito, N. Yamamoto, and Y. Tanaka. 2003. Induction of protective immune responses against R5 human immunodeficiency virus type 1 (HIV-1) infection in hu-PBL-SCID mice by intrasplenic immunization with HIV-1-pulsed dendritic cells: possible involvement of a novel factor of human CD4+ T-cell origin. J. Virol. 778719-8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang, J. Y., Z. Zhang, X. Wang, J. L. Fu, J. Yao, Y. Jiao, L. Chen, H. Zhang, J. Wei, L. Jin, M. Shi, G. F. Gao, H. Wu, and F. S. Wang. 2007. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood 1094671-4678. [DOI] [PubMed] [Google Scholar]
- 69.Zhao, X. Q., X. L. Huang, P. Gupta, L. Borowski, Z. Fan, S. C. Watkins, E. K. Thomas, and C. R. Rinaldo, Jr. 2002. Induction of anti-human immunodeficiency virus type 1 (HIV-1) CD8+ and CD4+ T-cell reactivity by dendritic cells loaded with HIV-1 X4-infected apoptotic cells. J. Virol. 763007-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zimmerli, S. C., A. Harari, C. Cellerai, F. Vallelian, P. A. Bart, and G. Pantaleo. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA 1027239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]