Abstract
Hepatitis C virus (HCV) is a major cause of liver disease in humans. The CD81 tetraspanin is necessary but not sufficient for HCV penetration into hepatocytes, and it was recently reported that the tight junction protein claudin-1 is a critical HCV entry cofactor. Here, we confirm the role of claudin-1 in HCV entry. In addition, we show that claudin-6 and claudin-9 expressed in CD81+ cells also enable the entry of HCV pseudoparticles derived from six of the major genotypes. Whereas claudin-1, -6, and -9 function equally well as entry cofactors in endothelial cells, claudin-1 is more efficient in hepatoma cells. This suggests that additional cellular factors modulate the ability of claudins to function as HCV entry cofactors. Our work has generated novel and essential means to investigate the mechanism of HCV penetration into hepatocytes and the role of the claudin protein family in HCV dissemination, replication, and pathogenesis.
Hepatitis C virus (HCV) affects 2% of the human population, causing chronic hepatitis, cirrhosis, and hepatocellular carcinoma (19, 28). The envelope glycoproteins E1 and E2 form heterodimers on the surface of infectious HCV particles and restrict viral tropism to hepatocytes (23). The CD81 tetraspanin was identified as the first putative HCV receptor by its ability to bind specifically to a soluble form of E2 (18). Subsequently, it was shown that CD81 expression is necessary but not sufficient for entry into target cells because numerous human cell lines, including several hepatoma cells, are CD81+ and resistant to HCV entry (1). Also, several human and nonhuman cell lines modified to express CD81 did not acquire an entry-permissive phenotype (2, 6, 10). These observations indicated that additional cofactors are recruited into the virion-receptor complex to mediate HCV internalization, trafficking, or fusion in hepatocytes. A significant time lag between virus attachment and internalization, as well as a lack of endocytosis signals on CD81, further supported this premise (16, 21).
Several cellular factors play a role in HCV entry, including high-density lipoprotein receptor SR-B1, low-density lipoprotein receptor (LDLR), and glycosaminoglycans (1, 5, 12). SR-BI was initially implicated in HCV entry by its ability to associate with soluble E2 (20). Subsequently, it was shown that high-density lipoprotein enhances HCV entry (7, 24). LDLR also promotes HCV entry through E2 interactions with lipoproteins (26). These and other observations suggest that lipoprotein receptors may facilitate HCV entry by modulating the lipid content of target cell membranes. More recently, Evans et al. (8) reported that the expression of the tight junction protein claudin-1 is associated with the susceptibility of human hepatoma cell lines to cell culture-replicating HCV (HCVcc), as well as retroviral particles pseudoytped with HCV E1E2 (HCV pseudoparticle [HCVpp]) which authentically recapitulate all stages of HCV entry (2, 10, 16). The silencing of claudin-1 expression impaired HCV replication in hepatoma cells. The expression of claudin-1 in a CD81+ human cell line reversed its resistance to HCV entry.
Here, we investigated the abilities of human claudins, including claudin-1, -2, -4, -6, and -9, amplified from hepatoma or liver mRNAs to act as HCV entry cofactors. We confirmed the role of claudin-1 in HCV entry and found that claudin-6 and claudin-9 also allowed robust entry into CD81+ human endothelial cells. In these cells, the three claudins functioned equally well with envelope glycoproteins derived from six major genotypes, including subtypes 1a, 1b, 2a, 2b, 3a, 4, 5, and 6. Claudin-6 and -9, however, appeared to function poorly in hepatoma cells compared to claudin-1. Our work provides critical, novel tools to elucidate the molecular basis of claudin function in HCV entry and to determine the role of this family of proteins in HCV dissemination, replication, and pathogenesis.
MATERIALS AND METHODS
Cells and DNAs.
The cell lines acquired through the ATCC included 293T, HeLa, Hep3B, and HepG2, and cells were cultured as recommended. Huh-7 and Huh-7.5 cells were provided by C. Rice (Rockefeller University, NY) (14), NKNT3 cells were provided by I. Fox (University of Nebraska Medical Center, Omaha, NE) (6), and H1H cells were provided by R. Chowdhurry (Albert Einstein College of Medicine, Bronx, NY). Primary hepatocytes were provided by S. Strom (University of Pittsburgh, Pittsburgh, PA) through the NIH's liver tissue procurement and distribution system and cultured in serum-free Waymouth's medium.
The coding sequences for claudin-1, -2, -4, and -6 were amplified from Huh-7.5 mRNA using one-step reverse transcription PCR (Qiagen), and claudin-9 was PCR amplified from a liver cDNA library (Clontech). The cDNAs were cloned into the pQCXIN retroviral expression vector (Clontech). Vesicular stomatitis virus (VSV) G pseudoparticles (VSVpp) were generated and used to transduce cells as described previously (16). Cells were maintained under G418 selection (1 mg/ml).
Immunoblotting.
Cells (5 × 106) were lysed, and Western blotting was performed as previously described by us (16). The membranes were probed using the following antibodies: Invitrogen antibodies JAY.8 rabbit polyclonal antibody against claudin-1 (1:500), 12H12 monoclonal antibody (MAb) against claudin-2 (1:500), and 3E2C1 MAb against claudin-4 (1:500) and Santa Cruz antibodies C20 goat polyclonal antibody against claudin-6 (1:200) and C20 goat polyclonal antibody against claudin-9 (1:200). Staining was revealed with a horseradish peroxidase (HRP)-conjugated donkey anti-rabbit or sheep anti-mouse immunoglobulin G antibody (1:10,000; Amersham Biosciences) or an HRP-conjugated rabbit anti-goat antibody (1:2,000; Invitrogen) and developed using Western lightning chemiluminescence reagent Plus (PerkinElmer). To control for the amount of protein loaded per well, the membranes were stripped with a Re-Blot Western blot kit (Chemicon International) and reprobed with Santa Cruz HRP-conjugated MAb H-235 against β-tubulin (1:1,000) or C2 against actin (1:200).
Pseudoparticle and virus production.
Human immunodeficiency virus type 1 pseudoparticles comprising HCV E1E2, VSV G, or human T-cell leukemia virus type 1 (HTLV-1) gp46/gp21 were generated as described previously (16). Envelope glycoprotein sequences derived from subtypes 2a, 3a, 4, 5, and 6 were previously described (13), as were envelopes from subtypes 1a, 1b, and 2b (3). HCVcc encoding Renilla luciferase (HCVcc-Rluc) were generated by using the construct FL-J6/JFH-5′C19Rluc2AUbi provided by C. Rice. Briefly, full-length HCV RNA was synthesized by in vitro transcription of a linearized template using a RiboMAX large-scale RNA production system T7 (Promega) and purified with an RNeasy mini kit (Qiagen). Virus was generated by transfecting Huh-7.5 cells with the RNA, using Lipofectamine 2000.
Infection and inhibition of entry.
Target cells (5 × 103) were infected with pseudoparticles or HCVcc and resuspended in passive lysis buffer (Promega) 48 h postinfection. The luciferase activity (in relative light units [RLU]) was measured after cell lysates were mixed with a luciferase substrate (Promega). For determining inhibition of entry, cells were infected with various pseudoparticles in the presence of a 1:400 dilution of a neutralizing serum from a subtype 1b HCV-positive (HCV+) individual, 1 μg/ml of anti-CD81 MAb JS-81 (Pharmingen), or 25 nM bafilomycin A1 (Sigma). The respective mock inhibitors included an HCV serum, a mouse immunoglobulin G1, and dimethyl sulfoxide.
RNA interference assays.
Primary hepatocytes, Huh-7.5 cells, and Huh-7.5 cells expressing claudin-6 or claudin-9 were transfected using the Lipofectamine RNAiMax protocol (Invitrogen) with Dharmacon On-Targetplus Smartpool small interfering RNAs (siRNAs) directed against claudin-1 (L-017369-00), claudin-2 (L-020781-00), claudin-4 (L-013612-00), claudin-6 (L-015883-01), claudin-9 (L-014125-01), insulin-degrading enzyme (IDE) (L-005899-00), or nonspecific Smartpool control siRNA (D-001810-10). After 72 h, the cells were infected with pseudoparticles or with HCVcc. The luciferase activity in cell lysates was measured 48 h postinfection.
RESULTS
Claudin-1 is an HCV entry cofactor in hepatoma cells and primary hepatocytes.
In order to study claudins in HCV entry, we performed PCR amplification of sequences encoding claudin-1 through -9 using mRNAs from Huh-7.5 hepatoma cells or human liver cDNAs. We were able to amplify coding sequences for claudin-1, -2, -3, -4, -6, -7, and -9. Claudin-5 and -8 could not be amplified from either source. The expression patterns of the amplified claudins were investigated by Western blotting in several HCV-permissive hepatoma cell lines, as well as in primary hepatocytes (4). Claudin-1 was detected in all HCV-permissive hepatoma cells, including Huh-7, Huh-7.5, Hep3B, and HepG2-CD81 cells, as well as in primary hepatocytes (Fig. 1). Claudin-2 and -4 were found in all cell lines except Hep3B, and claudin-2 was also found in hepatocytes (Fig. 1). Claudin-3, -6, -7, and -9 could not be detected by Western blotting in any of the test cells (data not shown). None of the amplified claudins were detectable in human peripheral blood mononuclear cells (PBMC). These results prompted us to verify the role of claudin-1, -2, and -4 in HCV entry into hepatoma cells and hepatocytes.
FIG. 1.
Expression of various claudins in bona fide HCV target cells. Human hepatoma or primary cells, as indicated at the top of the blots (Hepat., hepatocytes), were analyzed by Western blotting. Membranes were probed using antibodies against the proteins indicated to the right of the blots. To control for the amount of protein loaded per well, membranes were stripped with a Re-Blot Western blot kit (Chemicon International) and reprobed with antibodies against β-tubulin or actin for PBMC and hepatocytes.
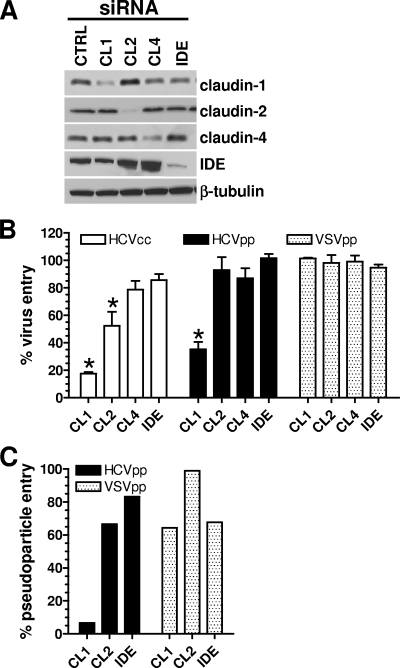
RNA interference was used to silence claudin-1, -2, or -4 expression in Huh-7.5 cells, and the silencing was confirmed by Western blotting (Fig. 2A). A pool of siRNAs targeting claudin-1 inhibited infection with replicating HCVcc-Rluc by ∼85% compared to inhibition with a nonspecific, control siRNA (Fig. 2B). There was also a small but statistically significant inhibition of HCVcc-Rluc replication by the siRNA pool targeting claudin-2. In contrast, silencing the expression of claudin-4 or IDE (a cell surface marker) had no effect on viral replication. Using the HCVpp system, we found that single-round entry into Huh-7.5 cells was significantly silenced only by the suppression of claudin-1. The inhibition of claudin-1 expression, but not claudin-2 or IDE, also very strongly suppressed HCVpp entry into primary hepatocytes from three independent donors (Fig. 2C and data not shown). The entry of VSVpp into either Huh-7.5 cells or primary hepatocytes was not affected by any of the siRNAs (Fig. 2B and C). Our data therefore confirm that claudin-1 is an important cofactor for HCV entry into hepatoma cells and hepatocytes. In contrast, claudin-2, -3, -4, -7, -11, -12, -15, -17, and -23 are inactive according to our results and those of Evans et al. and Zheng et al. (8, 29).
FIG. 2.
Claudin-1 cofactor activity. (A) Huh-7.5 cells were transfected with control siRNAs (CTRL) or siRNAs directed against claudin-1 (CL1), claudin-2 (CL2), claudin-4 (CL4), or IDE. Expression of the targeted proteins was verified by Western blotting using appropriate antibodies, indicated to the right of the blots. Membranes were stripped with a Re-Blot Western blot kit (Chemicon International) and reprobed with a MAb against β-tubulin. (B) Cells were infected 48 h posttransfection with HCVcc-Rluc, HCVpp subtype 1a, or VSVpp. (C) Primary hepatocytes from a single donor were transfected with siRNAs targeting the claudins indicated along the x axis and infected with HCVpp or VSVpp. Luciferase activity (in RLU) was measured in cell lysates 48 h postinfection, and percent virus or pseudoparticle entry was calculated relative to the RLU obtained with the control siRNAs. For panel B, the values are the averages of the results of four independent experiments ± the standard deviations, whereas the values in panel C are from a representative experiment. *, statistically significant reduction of virus or pseudoparticle entry compared to entry with IDE; P < 0.0002 as calculated by the two-tailed paired t test.
Expression of claudin-1, -6, and -9 allows HCVpp entry into human cells.
Next, we determined whether other claudins could act as HCV entry cofactors. CD81+ 293T kidney endothelial cells, which do not endogenously express claudin-1, -2, -4, -6, and -9, were transduced by retroviral vectors encoding these proteins. The various claudins were readily detected by Western blotting following selection using drugs (data not shown). Because we currently lack antibodies to the extracellular domains of claudin-1, -2, -4, -6, and -9, we were unable to quantify their cell surface expression. However, we used immunofluorescence labeling followed by confocal microscopy analyses to reveal the subcellular localization of the various claudins. Claudin-1, -6 and -9 appeared to localize to the plasma membrane, whereas claudin-2 and -4 appeared to concentrate in intracellular compartments (data not shown). Our observations suggest that the claudins are routed to different subcellular compartments.
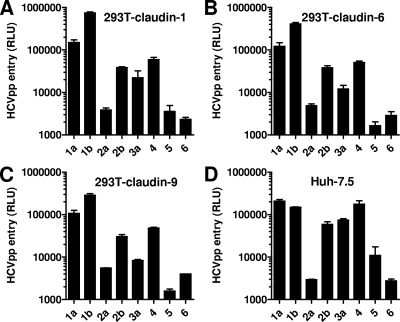
HCVpp were generated with HCV envelope glycoproteins derived from subtypes 1a, 1b, 2a, 2b, 3a, 4, 5, and 6. Pseudoparticles were also generated with VSV G and HTLV-1 gp46/gp21. HCVpp entry levels were quantified in parallel for Huh-7.5 cells and claudin-expressing 293T cell derivatives. The expression in 293T endothelial cells of claudin-1, as well as claudin-6 or claudin-9, resulted in robust pseudoparticle entry mediated by envelope glycoproteins from all eight subtypes (Fig. 3A to C) (3). Both the absolute and relative entry levels of the various pseudoparticles were similar to those observed in Huh-7.5 cells (Fig. 3D). 293T cells expressing claudin-2 or claudin-4 remained resistant to all HCVpp (data not shown). Also, the entry of VSVpp and HTLV-1pp was not modified by the expression of any of the five claudins (data not shown).
FIG. 3.
Entry of HCVpp into endothelial cells expressing various human claudins. 293T cells stably expressing claudin-1 (A), claudin-6 (B), or claudin-9 (C) or Huh-7.5 cells (D) were infected with HCVpp comprising E1E2 envelope glycoproteins derived from the HCV subtypes indicated along the x axis. Luciferase activity (in RLU) in cell lysates was measured 48 h postinfection. Background levels of entry of various HCVpp into 293T cells transduced by a control vector were 100 to 500 RLU and were subtracted from RLU values obtained for the claudin-positive cell lines for every experiment. Values are the means ± standard deviations of the results of three independent experiments performed with different viral stocks.
HCVpp entry into claudin-expressing 293T cells remains dependent on E1E2, CD81, and endosomal acidification.
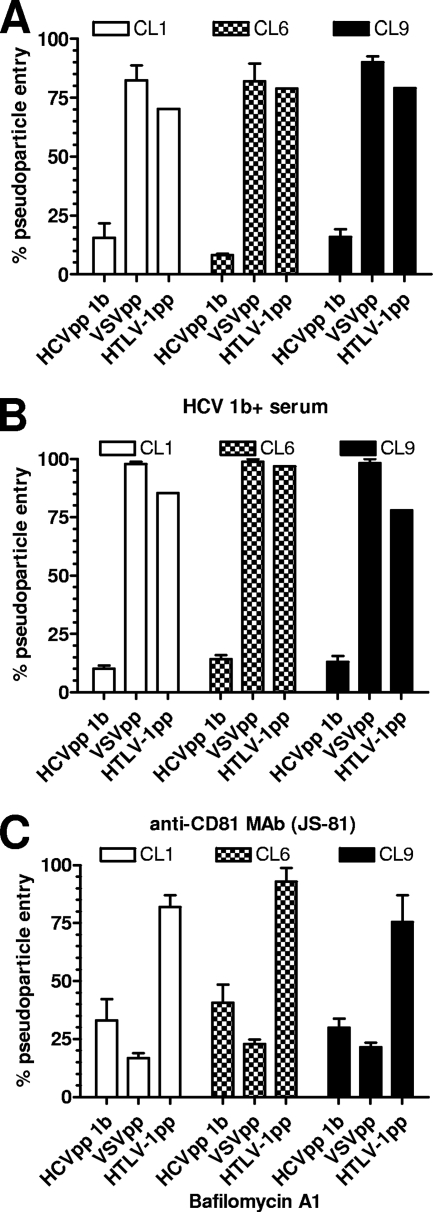
The pathway of HCVpp entry into claudin-expressing 293T cells was verified by using several specific inhibitors. HCVpp entry into 293T cells expressing claudin-1, -6, or -9 was significantly inhibited by serum from an HCV+ patient, an anti-CD81 MAb, and the lysosomotropic agent bafilomycin A1 (Fig. 4C). VSVpp and HTLV-1pp entry into the various claudin-positive 293T cells was not affected by these agents, except for inhibition of VSVpp by bafilomycin A1, which was expected. These results demonstrate that HCVpp entry into claudin-expressing 293T cells was still contingent upon HCV envelope glycoproteins, CD81, and endosomal acidification, as in bona fide target cells.
FIG. 4.
HCVpp entry remains dependent on E1E2, CD81, and endosomal acidification. 293T cells expressing claudin-1 (CL1), -6 (CL6), or -9 (CL9) were infected with HCVpp subtype 1b, VSVpp, or HTLV-1pp in the presence of a neutralizing serum from a subtype 1b HCV+ individual (A), anti-CD81 MAb JS-81 (Pharmingen) (B), bafilomycin A1 (Sigma) (C), or appropriate mock inhibitors (not shown). The percent pseudoparticle entry was calculated with the following equation: (RLU with inhibitor)/(RLU with mock inhibitor) × 100. All values are the means ± standard deviations of the results of four independent experiments. Inhibition of HCVpp entry into cells expressing claudin-1, -6, and -9 was statistically significant since P values were ≤0.004 as calculated by two-tailed paired t tests.
Claudin-1, -6, and -9 confer various HCVpp permissivities to human hepatoma cells.
We also tested the abilities of various claudins to enable HCVpp entry into other human cells, in particular, two CD81+ hepatoma cell lines that are resistant to HCV entry. H1H and NKNT3 cells were transduced with retroviral vectors expressing claudin-1, -2, -4, -6, or -9, which they do not express endogenously. Following drug selection, the exogenous claudins were detected by Western blotting. The expression levels of the claudins were comparable between the different cell lines (data not shown). Thus, claudin-1 was similarly expressed in 293T, H1H, and NKNT3 cells, and the same could be said of claudin-6. Claudin-9 was expressed more strongly in 293T than in NKNT3 cells but within the same order of magnitude. We could not show claudin-9 expression in H1H cells even though they were transduced with the same pseudoviral stocks as all the other cell lines and were drug resistant. Presently, it is not clear how claudin-9 transgene expression is silenced in these cells. Currently, we are unable to ascertain whether the expression levels of different claudins are similar or not.
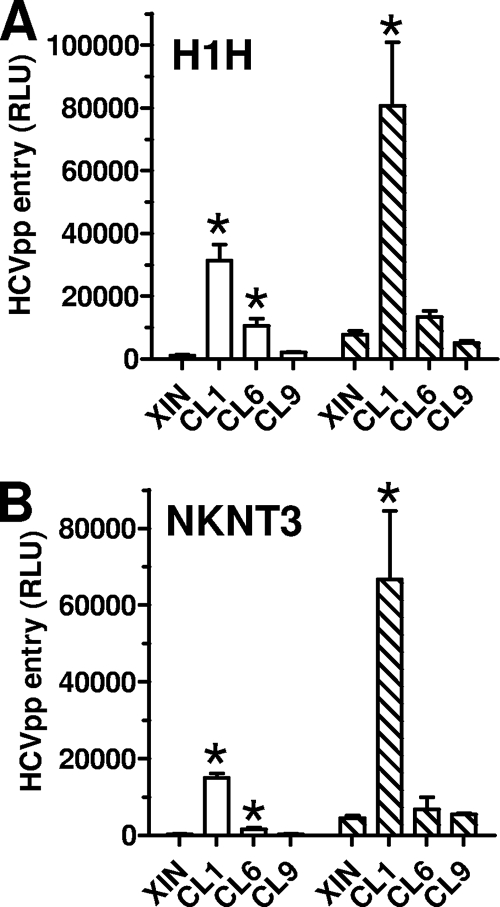
We then tested the abilities of claudin-1, -6, and -9 to enable HCVpp entry into the modified H1H and NKNT3 cells. The absolute luciferase values for the control pseudoparticles, VSVpp and HTLV-1pp, were 10- to 100-fold lower in these cells than in Huh-7.5 and 293T cells. Nonetheless, we were able to detect robust HCVpp entry into H1H and NKNT3 cells expressing claudin-1. The entry mediated by claudin-6 was low and detectable only for subtype 1a HCVpp. No entry was detected into the claudin-9-positive NKNT3 cells, and there was no expression of claudin-9 in the H1H cells (Fig. 5). The expression of claudin-2 and -4 in H1H or NKNT3 cells also did not result in any HCVpp entry (data not shown). VSVpp and HTLV-1pp entry into H1H and NKNT3 cells was not affected by the expression of the various claudins (data not shown). Overall, these results indicate that claudin-6 and -9 function poorly or not at all as HCV entry cofactors in hepatoma cells.
FIG. 5.
HCVpp entry into claudin-expressing hepatoma cell lines. Parental (XIN) and claudin-1-expressing (CL1), claudin-6-expressing (CL6), and claudin-9-expressing (CL9) H1H (A) or NKNT3 (B) cells were infected with HCVpp bearing subtype 1a (white bars) or 1b (hatched bars) envelope glycoproteins. Values are the means ± standard deviations of the results of four independent experiments. *, statistically significant increases in HCVpp entry compared to its entry in cells transduced by vector alone; P values were ≤0.04 as calculated by the two-tailed paired t test.
Inefficient trans-complementation by claudin-6 and -9 of target cells in which claudin-1 expression is blocked.
In light of the poor cofactor activities of claudin-6 and -9 in the H1H and NKNT3 hepatoma cell lines, we asked whether the reduction in HCVcc and HCVpp entry resulting from claudin-1 silencing could be overcome by claudin-6 and claudin-9 expression. To this end, we transduced Huh-7.5 cells with retroviral vectors expressing one or the other of these proteins (data not shown). Populations expressing either claudin-6 or claudin-9 were then transfected with siRNAs targeting claudin-1 and infected with either HCVcc or HCVpp 1b. We did not detect any entry of HCVcc above what we observed in the control cells (Fig. 6). For HCVpp, a small but statistically significant level of entry into both the claudin-6- and the claudin-9-expressing Huh-7.5 cell derivatives was observed. There was no effect on the entry of VSVpp from either the anticlaudin-1 siRNA or claudin-6 or -9 expression. Finally, we note that cotransfection of Huh-7.5 parental cell lines or the claudin-6 and -9 derivatives with siRNAs targeting both claudin-1 and claudin-6 or claudin-9 did not further decrease HCVpp entry (data not shown). Together, these observations indicate that only claudin-1 is an efficient HCV entry cofactor in hepatoma cells.
FIG. 6.
Claudin-6 and claudin-9 are weak HCVpp entry cofactors in Huh-7.5 cells. Huh-7.5 cell derivatives expressing a neomycin resistance marker (XIN), claudin-6, or claudin-9 were transfected by siRNAs targeting claudin-1 and infected with HCVcc-Rluc, HCVpp, or VSVpp. Luciferase activity (in RLU) was measured in cell lysates 48 h postinfection, and percent viral entry was calculated relative to the RLU obtained with the control siRNAs. Values are the averages ± standard deviations of the results of four independent experiments. *, statistically significant increase in virus or pseudoparticle entry compared to entry into XIN control cells; P < 0.02 as calculated by the two-tailed paired t test. α, anti.
DISCUSSION
In this report, we confirmed that the tight junction protein claudin-1 is an HCV entry cofactor and extended this observation to claudin-6 and claudin-9. While our manuscript was under review, a similar report was published by Zheng et al. (29). To date, therefore, it has been established that claudin-1, -6, and 9, but not claudin-2, -3, -4, -7, -11, -12, -15, -17, and -23, can enable HCVpp entry into CD81+ cells. Whereas claudin-6 and -9 are highly related, with identical first extracellular loops, they are phylogenetically closer to claudin-3 and -4 than to claudin-1 (22). Claudin-1 is most closely related to claudin-7. Claudin-2 is more closely related to claudin-6 and -9 than to claudin-1. Moreover, we have found that the residue in position 32 of the first extracellular loop is not vital for claudin-6 or claudin-9 function, whereas it is critical for claudin-1 function (T.D., unpublished results). The abilities of claudin-1, -6, and -9 to function as HCV entry cofactors therefore cannot be fully explained by primary structure homologies, suggesting that they either share a common three-dimensional motif or interact with additional cellular components in order to allow HCV entry.
Claudin-1, -6, and -9 enabled HCVpp entry into 293T cells to the same degree. The three claudins facilitated entry mediated by envelope glycoproteins derived from eight different HCV subtypes. The entry into claudin-expressing 293T cells was similar to the entry measured in permissive Huh-7.5 hepatoma cells. Together, these observations led us to propose that claudins are HCV entry cofactors shared by most if not all HCV isolates. Our observations also lead us to assume that the three claudins are expressed at similar levels in the 293T cell derivatives, though we currently have no way of ascertaining this by Western blotting or flow cytometry. However, we did determine that claudin-1 was similarly expressed in 293T, H1H, and NKNT3 cells and that the same could be said for claudin-6. Claudin-9 expression was stronger in 293T than in NKNT3 cells, and it was not detectable in H1H cells. Based on these similar expression levels, we expected HCVpp entry into H1H and NKNT3 cells expressing claudin-6 or -9 to be comparable within an order of magnitude to its entry into the claudin-1 derivatives, but this was not the case.
Instead, we observed extremely weak entry of subtype 1a and no entry of subtype 1b HCVpp into claudin-6-positive H1H and NKNT3 cells. We did not detect the entry of either subtype into claudin-9-positive NKNT3 cells. Moreover, we observed no HCVcc entry and only marginal HCVpp entry into claudin-6- or -9-positive Huh-7.5 cells in which claudin-1 expression was silenced. In other words, claudin-6 and -9 could not effectively trans-complement permissive hepatoma cells for claudin-1 function in HCV entry. Based on these observations, we strongly favor the hypothesis that additional cellular factors modulate the cofactor activities of claudins. Either different molecules interact with claudin-1, -6, and -9 or a single factor interacts with all three claudins but with various affinities. Cells such as 293T cells would express either all of the additional factors or saturating amounts of the single factor. Hepatoma cells would express a factor that preferentially interacts with claudin-1 or has the highest affinity for claudin-1. These additional factors are probably part of the bona fide entry pathway mediated by HCV envelope glycoproteins, since claudins didn't appear to modify the requirement for CD81 and endosomal acidification. We do not exclude the possibility that these modulators may be already-identified entry molecules, such as SR-B1 or LDLR. Indeed, the interplay between CD81, lipoprotein binding proteins, glycosaminoglycans, and claudins remains to be elucidated.
HCV entry into hepatocytes occurs in the context of a complex liver architecture. The apical surface of hepatocytes is restricted to bile canaliculi and is delineated by tight and other intercellular junctions, which keep bile from escaping into the circulation (11, 25). The backbone of tight junction strands is made of claudins (9, 17). We found claudin-1, -6, and -9 to localize in strands along intercellular contacts, whereas claudin-2 and -4 appeared to be more intracellular. Moreover, we did not observe any significant colocalization of CD81 and claudin-1 in the absence of virus (T.D., unpublished results). We previously reported a significant lag between virus attachment and internalization (16) and now propose that it corresponds to the time required for the HCV-receptor complex to migrate from the lateral surface of hepatocytes, which it encounters first after crossing the sinusoidal endothelium (27), to tight junctions. There, it would interact with claudins in order to exploit the endocytic pathways associated with these structures (15).
The precise role of claudins in HCV dissemination and pathogenesis remains to be determined. Our limited investigation of claudin expression patterns detected claudin-1, -2, and -4 in permissive hepatoma cells, whereas claudin-1 and -2 were found in hepatocytes. Claudin-6 and -9 expression was not detected by Western blotting in any of the HCV target cells that we investigated, even though their mRNAs were present in Huh-7.5 and liver cells. Note that Zheng et al. (29) reported the presence of claudin-6 mRNA in Huh-7 cells and claudin-9 mRNA in PBMC. Our results confirm that the presence of mRNA transcripts does not necessarily correlate with protein expression. The expression of various claudins in the intact normal liver, as well as the regulation of expression during liver regeneration, inflammation, and various pathological states, remains to be fully investigated. The findings reported here provide novel and critical tools with which to investigate the molecular mechanism of HCV entry, to further understand HCV pathogenesis, and most importantly, to assist the development of novel classes of antiviral agents.
Acknowledgments
We thank Phyllis Novikoff and the Albert Einstein College of Medicine analytical imaging facility for help with immunofluorescence microscopy. We are grateful to C. Rice for his generous gift of the FL-J6/JFH-5′C19Rluc2AUbi construct.
This work was supported by NIH grant AI060390 and the Burroughs Wellcome Investigators in Pathogenesis of Infectious Diseases fund. This work was also supported in part by NIAID Centers for AIDS Research grant AI051519 to Albert Einstein College of Medicine.
Footnotes
Published ahead of print on 30 January 2008.
REFERENCES
- 1.Bartosch, B., and F. L. Cosset. 2006. Cell entry of hepatitis C virus. Virology 3481-12. [DOI] [PubMed] [Google Scholar]
- 2.Bartosch, B., J. Dubuisson, and F. L. Cosset. 2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J. Exp. Med. 197633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertaux, C., D. Daelemans, L. Meertens, E. G. Cormier, J. F. Reinus, W. J. Peumans, E. J. Van Damme, Y. Igarashi, T. Oki, D. Schols, T. Dragic, and J. Balzarini. 2007. Entry of hepatitis C virus and human immunodeficiency virus is selectively inhibited by carbohydrate-binding agents but not by polyanions. Virology 36640-50. [DOI] [PubMed] [Google Scholar]
- 4.Bertaux, C., and T. Dragic. 2006. Different domains of CD81 mediate distinct stages of hepatitis C virus pseudoparticle entry. J. Virol. 804940-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocquerel, L., C. Voisset, and J. Dubuisson. 2006. Hepatitis C virus entry: potential receptors and their biological functions. J. Gen. Virol. 871075-1084. [DOI] [PubMed] [Google Scholar]
- 6.Cormier, E. G., F. Tsamis, F. Kajumo, R. J. Durso, J. P. Gardner, and T. Dragic. 2004. CD81 is an entry coreceptor for hepatitis C virus. Proc. Natl. Acad. Sci. USA 1017270-7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreux, M., T. Pietschmann, C. Granier, C. Voisset, S. Ricard-Blum, P. E. Mangeot, Z. Keck, S. Foung, N. Vu-Dac, J. Dubuisson, R. Bartenschlager, D. Lavillette, and F. L. Cosset. 2006. High density lipoprotein inhibits hepatitis C virus-neutralizing antibodies by stimulating cell entry via activation of the scavenger receptor BI. J. Biol. Chem. 28118285-18295. [DOI] [PubMed] [Google Scholar]
- 8.Evans, M. J., T. von Hahn, D. M. Tscherne, A. J. Syder, M. Panis, B. Wolk, T. Hatziioannou, J. A. McKeating, P. D. Bieniasz, and C. M. Rice. 2007. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446801-805. [DOI] [PubMed] [Google Scholar]
- 9.Furuse, M., and S. Tsukita. 2006. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 16181-188. [DOI] [PubMed] [Google Scholar]
- 10.Hsu, M., J. Zhang, M. Flint, C. Logvinoff, C. Cheng-Mayer, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. USA 1007271-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kojima, T., T. Yamamoto, M. Murata, H. Chiba, Y. Kokai, and N. Sawada. 2003. Regulation of the blood-biliary barrier: interaction between gap and tight junctions in hepatocytes. Med. Electron Microsc. 36157-164. [DOI] [PubMed] [Google Scholar]
- 12.Koutsoudakis, G., A. Kaul, E. Steinmann, S. Kallis, V. Lohmann, T. Pietschmann, and R. Bartenschlager. 2006. Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J. Virol. 805308-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavillette, D., A. W. Tarr, C. Voisset, P. Donot, B. Bartosch, C. Bain, A. H. Patel, J. Dubuisson, J. K. Ball, and F. L. Cosset. 2005. Characterization of host-range and cell entry properties of the major genotypes and subtypes of hepatitis C virus. Hepatology 41265-274. [DOI] [PubMed] [Google Scholar]
- 14.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309623-626. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda, M., A. Kubo, M. Furuse, and S. Tsukita. 2004. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J. Cell Sci. 1171247-1257. [DOI] [PubMed] [Google Scholar]
- 16.Meertens, L., C. Bertaux, and T. Dragic. 2006. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J. Virol. 8011571-11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita, K., M. Furuse, K. Fujimoto, and S. Tsukita. 1999. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. USA 96511-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pileri, P., Y. Uematsu, S. Campagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282938-941. [DOI] [PubMed] [Google Scholar]
- 19.Raza, S. A., G. M. Clifford, and S. Franceschi. 2007. Worldwide variation in the relative importance of hepatitis B and hepatitis C viruses in hepatocellular carcinoma: a systematic review. Br. J. Cancer 961127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarselli, E., H. Ansuini, R. Cerino, R. M. Roccasecca, S. Acali, G. Filocamo, C. Traboni, A. Nicosia, R. Cortese, and A. Vitelli. 2002. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 215017-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tscherne, D. M., C. T. Jones, M. J. Evans, B. D. Lindenbach, J. A. McKeating, and C. M. Rice. 2006. Time- and temperature-dependent activation of hepatitis C virus for low-pH-triggered entry. J. Virol. 801734-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Itallie, C. M., and J. M. Anderson. 2006. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68403-429. [DOI] [PubMed] [Google Scholar]
- 23.Voisset, C., and J. Dubuisson. 2004. Functional hepatitis C virus envelope glycoproteins. Biol. Cell 96413-420. [DOI] [PubMed] [Google Scholar]
- 24.Voisset, C., A. Op de Beeck, P. Horellou, M. Dreux, T. Gustot, G. Duverlie, F. L. Cosset, N. Vu-Dac, and J. Dubuisson. 2006. High-density lipoproteins reduce the neutralizing effect of hepatitis C virus (HCV)-infected patient antibodies by promoting HCV entry. J. Gen. Virol. 872577-2581. [DOI] [PubMed] [Google Scholar]
- 25.Wakabayashi, Y., P. Dutt, J. Lippincott-Schwartz, and I. M. Arias. 2005. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc. Natl. Acad. Sci. USA 10215087-15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wunschmann, S., H. M. Muller, C. S. Stipp, M. E. Hemler, and J. T. Stapleton. 2006. In vitro interaction between hepatitis C virus (HCV) envelope glycoprotein E2 and serum lipoproteins (LPs) results in enhanced cellular binding of both HCV E2 and LPs. J. Infect. Dis. 1941058-1067. [DOI] [PubMed] [Google Scholar]
- 27.Yanez-Mo, M., R. Tejedor, P. Rousselle, and F. Sanchez-Madrid. 2001. Tetraspanins in intercellular adhesion of polarized epithelial cells: spatial and functional relationship to integrins and cadherins. J. Cell Sci. 114577-587. [DOI] [PubMed] [Google Scholar]
- 28.Younossi, Z., J. Kallman, and J. Kincaid. 2007. The effects of HCV infection and management on health-related quality of life. Hepatology 45806-816. [DOI] [PubMed] [Google Scholar]
- 29.Zheng, A., F. Yuan, Y. Li, F. Zhu, P. Hou, J. Li, X. Song, M. Ding, and H. Deng. 2007. Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus. J. Virol. 8112465-12471. [DOI] [PMC free article] [PubMed] [Google Scholar]