Abstract
Hepatitis C virus (HCV) chronic infection is characterized by low-level or undetectable cellular immune responses against HCV antigens. HCV proteins have been shown to affect various intracellular events and modulate immune responses, although the precise mechanisms used to mediate these effects are not fully understood. In this study, we have examined the effect of HCV proteins on the modulation of major histocompatibility complex (MHC) class II expression and other functions important for antigen presentation in humans. Expression of an HCV1-2962 genomic clone (HCV-FL) in human fibrosarcoma cells (HT1080) inhibited gamma interferon (IFN-γ)-induced upregulation of human leukocyte antigen-DR (HLA-DR) cell surface expression. Furthermore, inhibition of promoter activities of MHC class II transactivator (CIITA), IFN-γ-activated site (GAS), and HLA-DR was observed in IFN-γ-inducible HT1080 cells expressing HCV-FL by in vitro reporter assays. Exposure of human monocyte-derived dendritic cells (DCs) to cell culture-grown HCV (HCVcc) genotype 1a (clone H77) or 2a (clone JFH1) significantly inhibited DC maturation and was associated with the production of IL-10. Furthermore, DCs exposed to HCVcc were impaired in their functional ability to stimulate antigen-specific CD4-positive (CD4+) and CD8+ T-cell responses. Taken together, our results indicated that HCV can have direct and/or indirect inhibitory effects on antigen-presenting cells, resulting in reduction of antigen-specific T-cell activation. These effects may account for or contribute to the low overall level of immunogenicity of HCV observed in chronically infected patients.
Hepatitis C virus (HCV) infection is one of the major causes of chronic liver disease worldwide. In the early phase of acute infection, HCV continues to replicate in the liver, overcoming innate and acquired immunity in a majority of infected humans. Sustained, vigorous, and multiepitope-specific CD4-positive (CD4+) and CD8+ T-cell responses are essential for spontaneous HCV clearance (26). Infected cells display peptides derived from viral antigens bound to major histocompatibility complex (MHC)/human leukocyte antigen (HLA) receptors at the cell surface and are recognized by T cells. Generation of HCV-specific CD4+ T cells occurs after stimulation by MHC class II/viral peptide complexes and facilitates the induction of HCV-specific CD8+ T-cell responses and optimal antibody production. CD8+ T cells recognize and attack infected cells expressing viral peptides in the context of MHC class I surface molecules.
Dendritic cells (DCs) are important for the initiation of T-cell responses to foreign antigens. The antigen uptake and presentation capacities of DCs enable them to prime and activate naïve T cells. Immature DCs are optimally phagocytic and pinocytose antigens. However, DCs must be activated to mature before serving as efficient antigen-presenting cells (APCs). Chronic HCV infection has been shown to affect the allostimulatory function of DCs (3). Monocyte-derived DCs from patients with chronic HCV infection do not respond to maturation stimuli, maintaining their immature phenotype (1). The presence of HCV genomic sequences in DCs could be documented for HCV carriers (4), although DCs may not support HCV replication. DC-SIGN and DC-SIGNR are two closely related membrane-associated C-type lectins. DC-SIGN is expressed on some DCs, while DC-SIGNR is localized to certain endothelial cell populations, including hepatic sinusoidal endothelial cells. Interactions of HCV envelope glycoprotein E2 with DC-SIGN and DC-SIGNR may contribute to the capture and delivery of virus to the liver by DCs (29, 39).
Several HLA polymorphisms are associated with viral clearance or persistence (21, 34, 35). The HLA-DR molecules are pivotal for the adaptive immune system, as they guide the development and activation of CD4+ T helper cells (41, 42). This complex gene expression profile is controlled almost exclusively by the CIITA, a global regulator for the expression of genes involved in antigen presentation (10, 30). Differential activation of independent promoters that drive expression of the gene encoding CIITA ultimately determines the exquisitely regulated pattern of MHC gene expression. Transcription of CIITA is driven by a large regulatory region that contains four distinct promoters known as pI, pII, pIII, and pIV (36). Cytokine regulation by CIITA is complex and may reflect both direct and indirect mechanisms of T-cell development and differentiation (37). The promoter pIV is induced by gamma interferon (IFN-γ), and a conserved 300-bp promoter-proximal region is sufficient for the activation. This region contains three regulatory elements, GAS, an E box, and an IFN-regulatory factor element (IRF-E), all of which are required for the induction of pIV. The main function of HLA-DR molecules encoded by one of the three MHC class II alleles is to present processed antigens which are derived primarily from exogenous sources to CD4+ T lymphocytes (22). Therefore, HLA-DR molecules are critical for the initiation of the antigen-specific immune response. Constitutive expression of MHC class II molecules is confined to professional APCs of the immune system, while MHC class II molecule expression can be induced by a variety of immune regulators in nonprofessional APCs.
MHC class II proteins are important for the initiation of immune responses and are essential for specific recognition of foreign antigens by the immune system. Thus, an interaction of DCs with HCV particles may play an important role in immunopathogenesis. In this study, we have examined the role of HCV proteins and infectious HCV in suppression of APC function/T-cell activation. IFN-γ is the most potent inducer of MHC class II expression in many cell types (8). Our results indicate that cells endogenously expressing HCV proteins from an HCV1-2962 (FL) construct or those exposed to cell culture-grown HCV (HCVcc) perturb HLA-DR cell surface expression. The inhibition of MHC class II cell surface expression was evident on IFN-γ-induced HT1080 cells and on professional antigen-presenting monocyte-derived DCs. A deficiency in expression of HLA-DR molecules was further observed to be associated with the negative regulation of CIITA, GAS, and the HLA-DR promoters at the transcriptional level, induction of interleukin-10 (IL-10) in DCs, and inhibition of DC-mediated antigen-specific T-cell activation.
MATERIALS AND METHODS
Cells and transfections.
The human fibrosarcoma epithelial-like HT1080 cell line was a gift from George Stark (Cleveland Clinic Foundation, Cleveland, OH). HT1080 cells were transfected with pCI-neo-HCV1-2962 plasmid DNA (HCV-FL) from genotype 1a (clone H77) by use of Lipofectamine (Life Technologies, Inc., Rockville, MD). Stable cell colonies were selected using G418 and pooled to avoid artifactual results from clonal variation, as previously described (5). Parental HT1080 cells were used in parallel as a control. HT1080 cells, and their transfected derivatives, were maintained in Dulbecco's modified Eagle medium containing 10% fetal calf serum with a lower dose of the selection antibiotic (1 μg of puromycin/ml or 400 μg of G418/ml).
Generation of cell culture-grown HCV.
HCV genotype 1a (clone H77; HCV1) was grown in immortalized human hepatocytes (IHH) as recently described (25). Virus growth was measured from cell culture supernatant filtered through a 0.45-um-pore-size cellulose acetate membrane (Nalgene, Rochester, NY) by fluorescent focus-forming assay from serial dilutions. HCV titer was calculated as \∋7ε105 focus-forming units/ml. HCV genotype 2a (clone JFH1; HCV2) was grown in Huh-7.5 cells as previously described (25).
Exposure of DCs to cell culture-grown HCV.
Immature DCs were prepared from human monocytes treated with granulocyte-macrophage colony-stimulating factor and IL-4 for 6 days (protocol approved by the Internal Review Board, Saint Louis University), as described earlier (48). Immature DCs (1 × 105/ml) in RPMI 1640 supplemented with 10% fetal bovine serum and 2 mM l-glutamine were seeded in plastic tubes. The next day, DCs were exposed for 6 h to 104 focus-forming units of HCVcc or conditioned medium (CM) from mock-infected hepatocytes as a negative control and incubated for 16 h. Subsequently, a maturation cocktail (MC) (containing a final concentration of 2 ng/ml IL-1β, 1,000 U/ml IL-6, 10 ng/ml tumor necrosis factor alpha, and 1 μg/ml prostaglandin E2 [PGE2]) was added and the DC cultures were incubated for 48 h more to induce maturation. IL-10 was added in culture as a negative control for inhibition of DC maturation. DCs were subjected to fluorescence-activated cell sorter (FACS) analysis for phenotypic characterization.
Flow cytometry.
Cell surface expression of costimulatory molecules or HLA-DR was quantified by flow cytometry. Transfected HT1080 cells or DCs exposed to HCVcc were grown in six-well plates (5 × 106 cells) or plastic tubes (1 × 105 cells), respectively. Cells were treated with 0.1 mM EDTA for detaching and harvesting, washed with phosphate-buffered saline (PBS), and stained with fluorochrome-tagged antibodies to HLA-DR peridinin-chlorophyll-protein complex, CD80 fluorescein isothiocyanate, CD14 Pac Blue, CD40 allophycocyanin, CD83 phycoerythrin, and CD3 Alexa 700 (BD Pharmingen, San Diego, CA). For staining, cells were incubated with 10 μl of monoclonal antibody in a total volume of 50 μl (2% fetal bovine serum -PBS) for 30 min at 4°C in the dark, washed, and resuspended in 250 μl of PBS containing 1% paraformaldehyde. Cells were gated according to their size (forward light scatter) and granularity (side light scatter) using a Guava Easysite (Guava Technology, Hayward, CA) or Becton Dickinson flow cytometer. Surface marker expression on gated cells was analyzed using FlowJo (Tree Star) and CellQuest (BD Immunocytometry Systems) software. The Statistica program was used for analyses of variations.
Luciferase reporter assay.
HT1080 cells (1 × 106) were seeded 24 h before transfection. Cells were transfected with 1 μg of CIITA or DRA promoter in a luciferase reporter construct (38) (kindly provided by Jenny P.-Y. Ting). The GAS-luciferase construct (Stratagene) was also used in this study. Cells were transfected together with reporter construct and 1 μg HCV protein expression plasmid or empty vector as a negative control. At 24 h after transfection, 500 U/ml IFN-γ was added to the cells and incubated for another 24 h. Cells were detached with PBS containing 0.1 mM EDTA, harvested, and lysed with RLU lysis buffer (Promega, Madison, WI) by incubation for 30 min at room temperature. After clarification by centrifugation, the supernatant was subjected to a luciferase reporter assay using a luminometer (Opticomp II; MGM Instruments). A cytomegalovirus-β-galactosidase plasmid construct was used for transfection efficiency as described previously (40). HCV core protein expression in transfected cells was also detected by Western blot analysis using specific antibodies.
Enzyme-linked immunosorbent assay for cytokines.
Levels of secreted cytokines IL-12 (p70) and IL-10 in human DC culture medium were measured using a BD OptEIA set human cytokine kit following the supplier's protocol (BD Biosciences, San Jose, CA). Briefly, microwells were coated with capture antibody and incubated overnight at 4°C. Plates were blocked and incubated with standard, sample, and control. Detection antibody and streptavidin-horseradish peroxidase reagents were added to each well. Finally, substrate solution was added and the mixture was incubated for color development and stopped by the addition of H2SO4 and read in an enzyme immunoassay reader. Cytokine concentrations were determined by comparison with a standard curve generated using highly purified recombinant cytokine. All samples were assayed in duplicate, and data are expressed as picograms per milliliter.
DC function.
The experimental design for the experiment was similar to that of work previously described (15, 49). For each condition, 4 × 104 DCs were resuspended in a total volume of 200 μl RPMI medium, RPMI medium plus recombinant human IL-10 (BD Biosciences), control hepatocyte culture medium, or supernatants from HCV-infected hepatocytes. Control hepatocyte culture media were included as conditioned medium from either uninfected IHH (CM1 medium) or Huh-7.5 cells (CM2 medium), and virus-containing supernatants were generated by infecting these cells with HCV1 (clone H77) or HCV2 (clone JFH1), respectively, at a multiplicity of infection of approximately 0.5. The DCs and the various conditioned media were incubated for 48 h in the presence or absence of 10 μg/ml Mycobacterium tuberculosis culture filtrate (Mycobacteria Research Laboratories, Colorado State University, Fort Collins, CO). DCs were then washed twice with fresh RPMI medium and irradiated before addition of 1 × 106 autologous peripheral blood mononuclear cells labeled with carboxyfluoroscein succinimidyl ester (CFSE) (Vybrant CFDA-SE; Molecular Probes/Invitrogen, Eugene, OR). Cultures (1 ml) were incubated for 7 days at 37°C and 5% CO2, after which the cells were restimulated for 2 h with phorbol myristate acetate and ionomycin (Sigma Aldrich, Saint Louis, MO) and counted. Cells were surface stained with anti-CD3 peridinin-chlorophyll-protein complex, anti-human IFN-γ allophycocyanin, anti-human CD4 Alexa 700, and anti-human CD8 Pac Blue (BD Biosciences), permeabilized and fixed for intracellular IFN-γ staining, and analyzed by flow cytometry. Absolute numbers of antigen-specific CD3+/CD4+/CFSElo/IFN-γ+ cells were calculated by multiplying the frequency of each subset by the total number of viable cells.
RESULTS
HCV polyprotein expression inhibits MHC class II upregulation in IFN-γ-treated HT1080 cells.
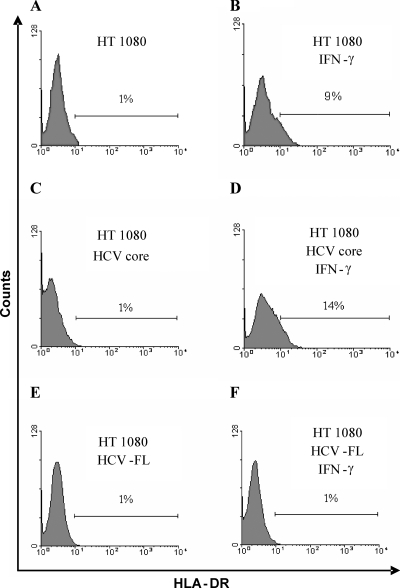
HT1080 cells were generated from fibrosarcoma tissue and originally subcultivated under conditions that eliminate the growth of fibroblasts and favor that of epithelial cells. HT1080 cells are sensitive to the induction of HLA-DR molecules by IFNs. IFN-γ activates antigen presentation by several mechanisms, including transcriptional upregulation of HLA-DR molecules and proteases involved in the immunoproteasome that are important for processing antigenic peptide. We have examined whether the presence of HCV proteins perturbs the upregulation of HLA-DR molecules in IFN-γ-treated HT1080 cells. For this, HT1080 cells were stably transfected with plasmid DNA encoding a partial or HCV-FL genomic region, resulting in transfected cells expressing HCV proteins (5). Mock-transfected cells were used in parallel as negative controls. Cells were treated with IFN-γ (500 U/ml) as an inducer of HLA-DR expression and analyzed by flow cytometry. Treatment of mock-transfected HT1080 cells with IFN-γ caused an upregulation of HLA-DR surface expression (Fig. 1A and B). HT1080 cells expressing HCV core protein also responded to IFN-γ similarly, with upregulation of HLA-DR molecules (Fig. 1C and D). However, HT1080 cells transfected with HCV-FL genomic region did not upregulate HLA-DR expression after stimulation with IFN-γ (Fig. 1E and F). The present results indicated that inhibition of HLA-DR expression is associated with HCV polyprotein expression in IFN-γ-treated HT1080 cells.
FIG. 1.
Inhibition of HLA-DR expression by HCV polyprotein in IFN-γ-treated HT1080 cells. (A and B) Mock-transfected HT1080 cells (A) or IFN-γ-treated cells (B) were used as a negative and positive controls, respectively, for assays of inducible HLA-DR expression. (C to F) HT1080 cells transfected with HCV core or HCV-FL were incubated in the absence (C and E) or presence (D and F) of IFN-γ (500 U/ml) for 48 h. Cells were subjected to FACS analysis of surface expression of HLA-DR using specific antibodies. Similar results of percentages of cells positive for HLA-DR expression (within 2% variations) were observed in triplicate experiments.
HCV protein expression downregulates CIITA, GAS, and HLA-DR promoter activities.
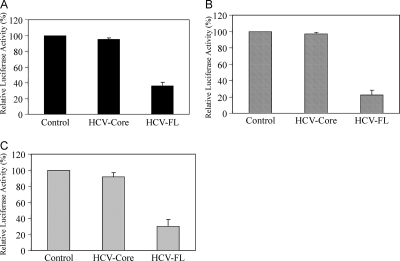
MHC class II expression is exquisitely controlled at a cell type-specific level and is precisely fine tuned and regulated, primarily by CIITA (41). Indeed, CIITA is the primary factor activating the expression of the class II genes necessary for the exogenous pathway of antigen processing and presentation (16). CIITA is a highly regulated non-DNA-binding coactivator that exhibits a remarkable degree of specificity for MHC class II genes. CIITA expression is regulated mainly at the level of transcription. CIITA is involved in both constitutive and inducible class II gene expression. The regulation of antigen expression by CIITA is complex and differs in various cell types, depending on the relative activities of CIITA promoters (12). We determined whether IFN-γ could act directly to induce CIITA gene transcription in HT1080 cells. For this, the IFN-γ-inducible promoter of the human CIITA gene was used to drive expression of the luciferase reporter gene. The CIITA-luciferase plasmid construct was transiently transfected into HT1080 cells and induced with IFN-γ, and luciferase activity levels were determined (Fig. 2A). A very low level of basal transcriptional activity of the reporter construct was detected in the absence of IFN-γ, while a five- to sixfold increase in induction of CIITA promoter activity was observed upon stimulation with IFN-γ (data not shown). HT1080 cells expressing HCV core displayed CIITA promoter induction similar to that seen with the vector control, while HT1080 cells transfected with the HCV-FL construct were found to have markedly lower levels of IFN-γ-induced CIITA promoter activity. An analysis of GAS (Fig. 2B) and HLA-DR (Fig. 2C) promoters exhibited similar results, demonstrating that HCV core protein did not have inhibitory effects, whereas HCV-FL expression did inhibit promoter activity in IFN-γ-treated HT1080 cells.
FIG. 2.
Expression of HCV-FL downregulates CIITA, GAS, and HLA-DR promoter activities. HT1080 cells were transfected with plasmid DNA containing CIITA (A), GAS (B), or HLA-DR (C) promoter with a luciferase reporter construct together with empty vector (control), HCV core, or HCV-FL from genotype 1a with Lipofectamine 2000 (Invitrogen). After 48 h of transfection, cells were incubated with IFN-γ (500 U/ml) for 24 h, and luciferase gene expression was analyzed. Results represent triplicate analyses and are shown with standard errors.
HCV inhibits the maturation of dendritic cells.
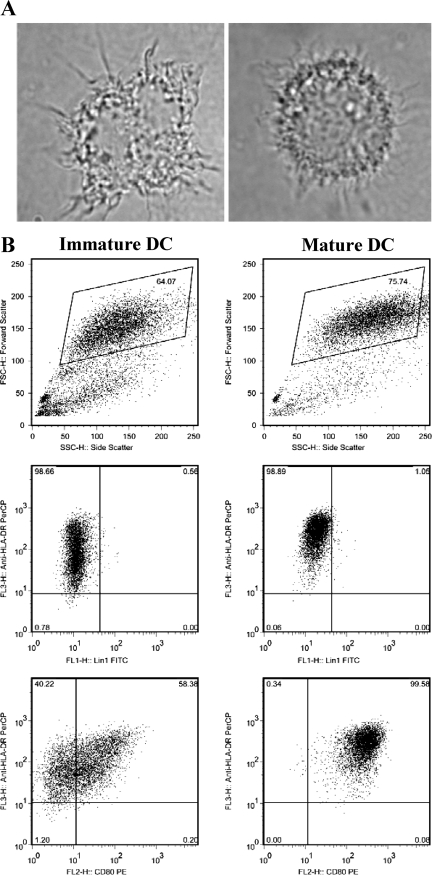
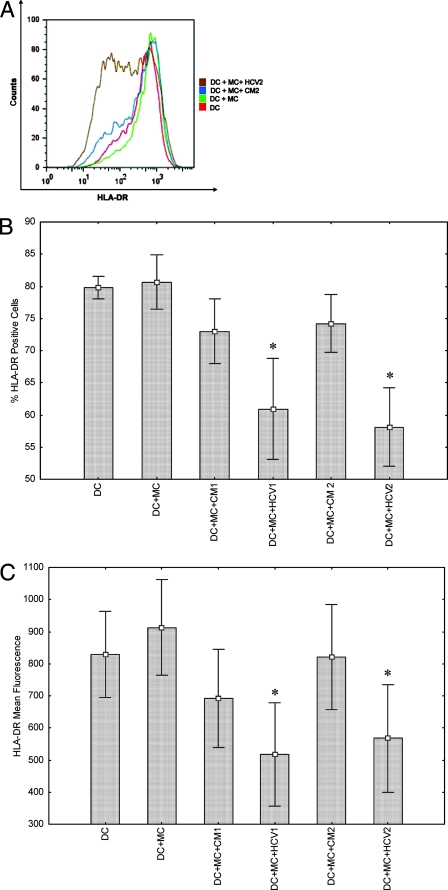
We focused our studies in the above-described experiments using HT1080 cells to understand the molecular mechanisms controlling MHC class II-inducible expression as a model system. However, HT1080 cells are not representative of APCs involved in the initial induction of immune functions. Next, we have focused on studies of DCs, which are important for the initiation of immune responses to foreign antigens, because of their competence in the capture and presentation of antigen to T cells. After antigen internalization, the DCs themselves undergo a process of maturation, migration, and relocation (7). During maturation, cell morphology alters and DCs upregulate MHC class II and a variety of costimulatory molecules. A typical characterization profile of immature and mature DCs used in our studies is shown (Fig. 3). HCVcc inhibited DC maturation even though HCV core and NS4a expression were observed in 0.7% and 0.5% of DCs, respectively (data not shown). DCs exposed to HCVcc were examined for cell surface expression of HLA-DR by FACS analysis. As shown with a representative preparation of DCs (Fig. 4A), immature DCs expressed HLA-DR, and the level of expression increased to some degree with MC treatment. However, supernatants from HCV-infected hepatocytes markedly inhibited HLA-DR expression on these DCs. Despite culture conditions which minimized the increases in HLA-DR expression with DC maturation, components from the supernatants collected from HCV-infected cells suppressed HLA-DR expression below the levels detected at baseline in immature DCs. MC-induced HLA-DR expression was also reduced in DCs incubated with IL-10 (not shown). MC-induced DCs incubated with CM from uninfected IHH displayed a trend for a lower mean fluorescence of HLA-DR staining compared with cells incubated with MC alone. The percentages of HLA-DR-positive cells and mean HLA-DR fluorescence obtained in similar experiments using DCs from five different healthy volunteers are shown (Fig. 4B and C). Percentages of both HLA-DR-positive cells and mean fluorescence intensity were significantly reduced following incubation with HCVcc. Statistical analyses of percentages of HLA-DR-positive cells and HLA-DR mean fluorescence gave P values of <0.05 by Wilcoxon matched-pair test in comparisons of DCs incubated with virus (HCV1 or HCV2) to DCs incubated with control medium (CM1 or CM2).
FIG. 3.
Characterization of immature and mature human DCs. Monocytes were treated for 6 days with IL-4 and granulocyte-macrophage colony-stimulating factor and then either rested in the same medium for 1 more day (immature DC) or treated with activation cocktail (IL-1β, IL-6, tumor necrosis factor alpha, and PGE2) for 1 more day (mature DC). (A) Light microscopic views (×100 magnification) of two monocyte-derived DCs are shown. (B) Granularity and surface expression results for the indicated markers (HLA-DR, Lin1, and CD80) in immature and mature DCs are also shown.
FIG. 4.
Expression of HLA-DR on cell surface upon exposure of human monocyte-derived DCs to HCV. FACS analysis and a typical expression profile of HLA-DR on the cell surface of monocyte-derived DCs from a healthy volunteer, and after stimulation with MC as a positive control are shown. (A) DCs were treated separately with conditioned medium (CM2) from hepatocytes as a mock control or exposed to cell culture-grown HCV2. (B and C) FACS analysis showing inhibition of HLA-DR expression on cell surface upon exposure of DCs from five healthy volunteers to cell culture-grown HCV genotype 1a or genotype 2a. Results are presented as percentages of positive cells (B) and mean fluorescence intensity (C). HLA-DR expression was analyzed using immature DCs, cells treated with MC, and cells treated with MC and CM from IHH (CM1) or Huh-7.5 (CM2) and HCVcc genotype 1a (HCV1) or genotype 2a (HCV2). *, P < 0.05 by Wilcoxon matched-pair test in comparison with DCs treated with MC alone.
During maturation, DCs upregulate MHC, adhesion, and costimulatory molecules, including CD40, CD80, and CD83. DCs from the five different healthy volunteers (Fig. 4B and C) were examined for MC-induced costimulatory molecule expression after incubation with supernatants from HCV2-infected hepatocytes (Table 1). DCs expressed increased levels of maturation markers CD40, CD80, and CD83 upon incubation with MC. In contrast, DCs incubated with HCVcc expressed lower MC-induced levels of these markers. Interestingly, conditioned medium from mock-infected hepatocytes also had a negative effect on these additional markers of DC maturation but to a much lower extent. The differences in the percentages of CD40-, CD80-, or CD83-positive cells were consistently lower in DCs incubated with HCV, reflecting a less mature phenotype.
TABLE 1.
Expression of costimulatory molecules in DCs determined by FACS analysesa
| Costimulatory molecule | % (± SE) of indicated molecule
|
|||
|---|---|---|---|---|
| DC | DC + MC | DC + MC + CM2 | DC + MC + HCV2 | |
| CD40b | 49.3 ± 4 | 64.7 ± 5 | 61.1 ± 6 | 50.9 ± 8 |
| CD80c | 19.2 ± 5 | 41.4 ± 7 | 37.6 ± 8 | 24.5 ± 7 |
| CD83b | 29.1 ± 7 | 45.5 ± 11 | 39.3 ± 11 | 24.6 ± 9 |
Data represent results obtained with different individual donors.
For DC + MC versus DC + MC + HCV2, P < 0.05; for DC + MC + CM2 versus DC + MC + HCV2, P < 0.05.
For DC + MC versus DC + MC + HCV2, P < 0.05; for DC + MC + CM2 versus DC + MC + HCV2, P = 0.07.
Cytokine expression in DCs exposed to HCV.
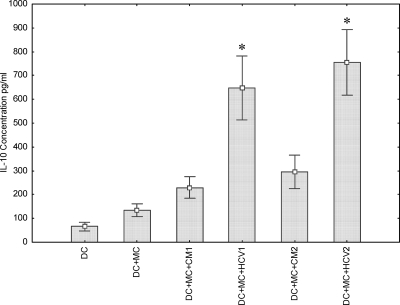
DCs regulate virus-specific immune responses that are crucial for virus eradication through the production of stimulatory and suppressive cytokines. Th1 cytokines are required for host antiviral immune responses, while Th2 cytokines can inhibit the development of these effector mechanisms. IL-12 produced by DCs can bias for the development of Th1 responses. IL-10 production by DCs, in contrast, is associated with tolerogenic DCs (28). The ability of HCVcc to influence cytokine secretion by DCs has yet to be explored. We investigated the effects of HCVcc on IL-12 (p70) and IL-10 secretion by use of MC-induced human DCs. Monocyte-derived DCs from five different healthy volunteers were induced with IL-1β, TNF, IL-6, and PGE2 in the presence of HCVcc, and IL-10/IL-12 secretion results were analyzed. DCs stimulated with the MC did not produce IL-12 (p70) in the presence or absence of HCVcc (data not shown). This result was probably related to the finding that PGE2 inhibits IL-12 production (24). On the other hand, a significant increase in IL-10 secretion was observed in MC-induced DC treated with HCVcc (Fig. 5). Mock- or HCV-infected hepatocyte medium from either IHH (CM1) or Huh-7.5 cells (CM2) did not contain a detectable quantity of IL-10 (data not shown). Therefore, culture medium of HCV-infected hepatocytes used as a source of HCVcc did not induce IL-12 production but instead induced IL-10 secretion from DCs.
FIG. 5.
IL-10 secretion is increased in HCV-exposed DC cultures. An enzyme-linked immunosorbent assay-based cytokine assay was performed using culture medium from cells incubated alone or treated with MC, conditioned medium from naïve cells (CM1 or CM2), and cell culture-grown HCV1 or HCV2. *, P < 0.05 by the Wilcoxon matched-pair test in comparisons of CM- and HCV-treated DCs.
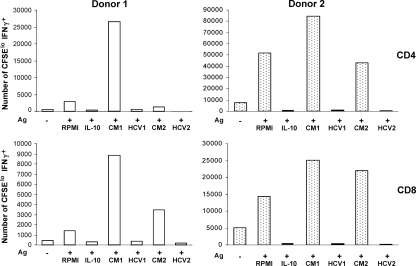
Incubation of DCs with HCVcc impairs antigen-specific T-cell activation.
To determine the functional capacity of human DCs exposed to HCV, we used an assay to measure the ability of DCs to stimulate proliferation of and effector cytokine secretion in autologous T cells. DCs from purified protein derivative-positive donors were incubated for 48 h with M. tuberculosis culture filtrate antigens in the presence or absence of HCV-containing hepatocyte culture medium. By flow cytometry, we were able to determine for each condition the number of T cells (CD4+ or CD8+) that had proliferated and that secreted IFN-γ upon restimulation (Fig. 6). For both donors, the presence of HCV in the DC supernatants effectively inhibited the proliferation and cytokine secretion of mycobacterium-specific T cells. As indicated, this was observed for both genotypes of HCVcc (HCV1 and HCV2). On the other hand, conditioned medium from IHH (CM1) and Huh-7.5 cells (CM2), used to support HCV growth, enhanced proliferation and cytokine secretion. This could have been due to the differences in composition of culture media for two different cell types used in this study and the presence of a number of secretory components in the conditioned medium of the hepatocytes (6). Furthermore, the effect of the use of HCV-containing hepatocyte culture medium seemed to be broad and at least as potent as the addition of recombinant exogenous IL-10, since both the subsets of effector T cells were inhibited by coculturing with HCV. These results provided a clear indication that HCV inhibits the antigen presentation function and T-cell-stimulatory capacity of DCs.
FIG. 6.
DC function is impaired in the presence of HCV. Human monocyte-derived dendritic cells from purified protein derivative-positive donors were fed mycobacterial culture filtrate antigen (Ag) in the absence or presence of HCV1, HCV2, or conditioned media from uninfected hepatocytes (CM1 or CM2). After 48 h, the DCs were washed and incubated with CFSE-labeled autologous peripheral blood mononuclear cells for 7 days. Subsets of CD3+ lymphocytes (CD4+ or CD8+) were analyzed by flow cytometry for dilution of CFSE and intracellular staining of IFN-γ. Based on cell counts in the 7-day cultures, the absolute numbers of each subset that had proliferated and secreted IFN-γ were calculated.
DISCUSSION
In this study, we investigated the regulation of IFN-γ-inducible HLA-DR antigen expression in HT1080 cells stably expressing HCV proteins as a model cell line. HT1080 cells have been extensively used for studies of IFN-γ signaling (33). Our results demonstrated that polyprotein expression from an HCV-FL construct inhibits HLA-DR expression in IFN-γ-treated HT1080 cells. Transient expression of the HCV-FL also downregulated CIITA, GAS, and HLA-DR promoter activities in IFN-γ-treated HT1080 cells, although we do not know at this time the mechanisms for downregulation. CIITA is a global regulator for the expression of genes involved in antigen presentation (10). The presence of HCV proteins leads to the downregulation of MHC class II antigen presentation on the cell surface. Our results further provided direct evidence that monocyte-derived DCs exposed to HCVcc have decreased expression of several markers of DC maturation, secrete significant amounts of IL-10, and are significantly impaired in their ability to present antigen. Our observations are in agreement with earlier reports suggesting that monocyte-derived DCs from chronically HCV-infected patients do not respond to maturation stimulus antigens and maintain their immature phenotype (1, 3). Together, these results suggest that the negative effects of HCV on APC function could lead to reduced immunogenicity in vivo.
A maturation defect in monocyte-derived DCs generated from chronic HCV-infected donors has been reported earlier (1, 3, 27). Distinct and contrasting dysfunction features of plasmacytoid and myeloid DC subsets were also observed during chronic HCV infection (2). Myeloid DCs derived from persons with chronic HCV infection displayed defects in IL-12 p70 production that were related to IL-10 activity and that could be overcome by treatment of the DCs with CD40L and IFN-γ (17). However, these observations fail to explain why HCV infection is not characterized by general immunosuppression. Subsequent studies of chronically HCV-infected chimpanzees (44) or humans (32) did not suggest a defect in DCs. A significant level of HCV protein expression or virus replication was not observed upon incubation of DCs with HCV. Infected hepatocytes might be secreting unknown constituent(s) and while in circulation may be diluted enough not to cause a significant generalized effect on DCs. On the other hand, HCV has been shown to infect not only liver cells but also extrahepatic tissues, including DCs (20, 31). Virus replication may occur in only a proportion of DCs in patients with chronic HCV infection, allowing the uninfected DCs to normally present non-HCV antigens to T cells (45). This would explain how patients with chronic hepatitis C exhibit a selective deficit of anti-HCV immunity with preservation of normal immune response to unrelated antigens.
Cellular immune responses are critical for the clearance of HCV (19). Failure to mount a potent and broad T-cell repertoire response results in persistent HCV infection. It has been suggested that HCV subverts cellular immunity by inducing IL-10, which in turn inhibits the activation of DC and development of Th1 cells (9). This may or may not be a direct effect from the culture medium of hepatocytes harboring HCV growth or HCV interacting with the DC surface. IL-10 can be produced by monocytes, DCs, and certain subsets of CD4+ T regulatory cells. It is possible that IL-10 induced in DCs by HCV or factors produced by HCV-infected hepatocytes may play a role in downregulating HCV-specific immunity in patients (14). The presence of DCs in the liver facilitates presentation of viral antigens to both CD4+ and CD8+ T-cell populations. Incubation of DC with HCVcc displayed an immunosuppressive effect upon M. tuberculosis culture filtrate with respect to the induction of CD4+ and CD8+ T-cell responses. Thus, our studies also provided evidence that HCV can interfere with antigen presentation, which could be a critical mechanism for HCV persistence. HCV has developed multiple strategies to escape immunity and persist within the host. A lower CD8+ T-cell response induced during HCV infection may be dependent upon reduced activation of CD4+ T cells. The importance of CD4+ T cells in cytotoxic T lymphocyte activity has been shown with chronic lymphocytic choriomeningitis virus infection in mice (50). Viral persistence is facilitated by the presence of unresponsive CD8+ T cells because of the absence of an efficient CD4+ response. Therefore, downregulation of APC function associated with IL-10 production, especially by intrahepatic infiltrating DCs, may lead to a crippled immune system that is incapable of responding with an appropriate immune response against HCV. Further work should elucidate the mechanisms by which specific HCV proteins regulate MHC class II expression and inhibit DC maturation and the interplay of these effects for viral persistence and pathogenesis.
Acknowledgments
We thank Jenny P.-Y. Ting for providing CIITA and HLA-DR promoters and Lin Cowick for preparation of the manuscript.
This work was supported by research grant AI068769 from the National Institutes of Health.
Footnotes
Published ahead of print on 23 January 2008.
REFERENCES
- 1.Auffermann-Gretzinger, S., E. B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 973171-3176. [DOI] [PubMed] [Google Scholar]
- 2.Averill, L., W. M. Lee, and N. J. Karandikar. 2007. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin. Immunol. 12340-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bain, C., A. Fatmi, F. Zoulim, J. P. Zarski, C. Trepo, and G. Inchauspe. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120512-524. [DOI] [PubMed] [Google Scholar]
- 4.Bain, C., and G. Inchauspe. 2001. Dendritic cells and hepatitis C virus. Pathol. Biol. 49464-465. [DOI] [PubMed] [Google Scholar]
- 5.Basu, A., K. Meyer, R. B. Ray, and R. Ray. 2001. Hepatitis C virus core protein modulates the interferon induced transacting factors of JAK/STAT signaling pathway but does not affect the activation of interferon-stimulated genes. Virology 288379-390. [DOI] [PubMed] [Google Scholar]
- 6.Basu, A., K. Meyer, K. Lai, K. Saito, A. Di Bisceglie, L Grosso, R. B. Ray, and R. Ray. 2006. Microarray analyses and molecular profiling of Stat3 signaling pathway induced by hepatitis C virus core protein in human hepatocytes. Virology 349347-358. [DOI] [PubMed] [Google Scholar]
- 7.Bell, D., J. W. Young, and J. Banchereau. 1999. Dendritic cells. Adv. Immunol. 72255-324. [DOI] [PubMed] [Google Scholar]
- 8.Boss, J. M. 1997. Regulation of transcription of MHC class II genes. Curr. Opin. Immunol. 9107-113. [DOI] [PubMed] [Google Scholar]
- 9.Brady, M. T., A. J. MacDonald, A. G. Rowan, and K. H. Mills. 2003. Hepatitis C virus non-structural protein 4 suppresses Th1 responses by stimulating IL-10 production from monocytes. Eur. J. Immunol. 333448-3457. [DOI] [PubMed] [Google Scholar]
- 10.Chang, C. H., and R. A. Flavell. 1995. Class II transactivator regulates the expression of multiple genes involved in antigen presentation. J. Exp. Med. 181765-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reference deleted.
- 12.Chou, S. D., A. N. Khan, W. J. Magner, and T. B. Tomasi. 2005. Histone acetylation regulates the cell type specific CIITA promoters, MHC class II expression and antigen presentation in tumor cells. Int. Immunol. 171483-1494. [DOI] [PubMed] [Google Scholar]
- 13.Reference deleted.
- 14.Della Bella, S., A. Crosignani, A. Riva, P. Presicce, A. Benetti, R. Longhi, M. Podda, and M. L. Villa. 2007. Decrease and dysfunction of dendritic cells correlate with impaired hepatitis C virus-specific CD4+ T-cell proliferation in patients with hepatitis C virus infection. Immunology 121283-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Vallière, S., G. Abate, A. Blazevic, R. M. Heuertz, and D. F. Hoft. 2005. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect. Immun. 736711-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drozina, G., J. Kohoutek, N. Jabrane-Ferrat, and B. M. Peterlin. 2005. Expression of MHC II genes. Curr. Top. Microbiol. Immunol. 290147-170. [DOI] [PubMed] [Google Scholar]
- 17.Fan, Z., X. L. Huang, P. Kalinski, S. Young, and C. R. Rinaldo, Jr. 2007. Dendritic cell function during chronic hepatitis C virus and human immunodeficiency virus type 1 infection. Clin. Vaccine Immunol. 141127-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reference deleted.
- 19.Fowler, N. L., J. Torresi, D. C. Jackson, L. E. Brown, and E. J. Gowans. 2003. Immune responses in hepatitis C virus infection: the role of dendritic cells. Immunol. Cell Biol. 8163-66. [DOI] [PubMed] [Google Scholar]
- 20.Goutagny, N., A. Fatmi, V. De Ledinghen, F. Penin, P. Couzigou, G. Inchauspe, and C. Bain. 2003. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J. Infect. Dis. 1871951-1958. [DOI] [PubMed] [Google Scholar]
- 21.Höhler, T., G. Gerken, A. Notghi, P. Knolle, R. Lubjuhn, H. Taheri, P. M. Schneider, K. H. Meyer zum Büschenfelde, and C. Rittner. 1997. MHC class II genes influence the susceptibility to chronic active hepatitis C. J. Hepatol. 27259-264. [DOI] [PubMed] [Google Scholar]
- 22.Holling, T. M., E. Schooten, and P. J. van Den Elsen. 2004. Function and regulation of MHC class II molecules in T-lymphocytes of mice and men. Hum. Immunol. 65282-290. [DOI] [PubMed] [Google Scholar]
- 23.Reference deleted.
- 24.Kaliński, P., P. L. Vieira, J. H. Schuitemaker, E. C. de Jong, and M. L. Kapsenberg. 2001. Prostaglandin E (2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 973466-3469. [DOI] [PubMed] [Google Scholar]
- 25.Kanda, T., A. Basu, R. Steele, T. Wakita, J. S. Ryerse, R. Ray, and R. B. Ray. 2006. Generation of infectious hepatitis C virus in immortalized human hepatocytes. J. Virol. 804633-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanto, T., and N. Hayashi. 2006. Immunopathogenesis of hepatitis C virus infection: multifaceted strategies subverting innate and adaptive immunity. Intern. Med. J. 45183-191. [DOI] [PubMed] [Google Scholar]
- 27.Kanto, T., N. Hayashi, T. Takehara, T. Tatsumi, N. Kuzushita, A. Ito, Y. Sasaki, A. Kasahara, and M. Hori. 1999. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J. Immunol. 1625584-5591. [PubMed] [Google Scholar]
- 28.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High level IL-12 production by murine dendritic cells: up-regulation via MHC class II and CD40 molecules and down-regulation by IL-4 and IL-10. J. Exp. Med. 184741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai, W. K., P. J. Sun, J. Zhang, A. Jennings, P. F. Lalor, S. Hubscher, J. A. McKeating, and D. H. Adams. 2006. Expression of DC-SIGN and DC-SIGNR on human sinusoidal endothelium: a role for capturing hepatitis C virus particles. Amer. J. Pathol. 169200-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LeibundGut-Landmann, S., J. M. Waldburger, M. Krawczyk, L. A. Otten, T. Suter, A. Fontana, H. Acha-Orbea, and W. Reith. 2004. Specificity and expression of CIITA, the master regulator of MHC class II genes. Eur. J. Immunol. 341513-1525. [DOI] [PubMed] [Google Scholar]
- 31.Lerat, H., F. Berby, M. A. Trabaud, O. Vidalin, M. Major, C. Trépo, and G. Inchauspé. 1996. Specific detection of hepatitis C virus minus strand RNA in hematopoietic cells. J. Clin. Investig. 97845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longman, R. S., A. H. Talal, I. M. Jacobson, M. L. Albert, and C. M. Rice. 2004. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood 1031026-1029. [DOI] [PubMed] [Google Scholar]
- 33.Mao, C., D. Davies, I. M. Kerr, and G. R. Stark. 1993. Mutant human cells defective in induction of major histocompatibility complex class II genes by interferon gamma. Proc. Natl. Acad. Sci. USA 902880-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKiernan, S. M., R. Hagan, M. Curry, G. S. McDonald, N. Nolan, J. Crowley, J. Hegarty, E. Lawlor, and D. Kelleher. 2000. The MHC is a major determinant of viral status, but not fibrotic stage, in individuals infected with hepatitis C. Gastroenterology 1181124-1130. [DOI] [PubMed] [Google Scholar]
- 35.Minton, E. J., D. Smillie, K. R. Neal, W. L. Irving, J. C. Underwood, V. James, and Members of the Trent Hepatitis C Virus Study Group. 1998. Association between MHC class II alleles and clearance of circulating hepatitis C virus. J. Infect. Dis. 17839-44. [DOI] [PubMed] [Google Scholar]
- 36.Muhlethaler-Mottet, A., L. A. Otten, V. Steimle, and B. Mach. 1997. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 162851-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel, D. R., L. W. Park, J. S. Sofi, T. S. Gourley, G. Hangoc, M. H. Kaplan, and Chang. 2005. Constitutive expression of CIITA directs CD4 T cells to produce Th2 cytokines in the thymus. Cell. Immunol. 23330-40. [DOI] [PubMed] [Google Scholar]
- 38.Piskurich, J. F., Y. Wang, M. W. Linhoff, L. C. White, and J. P. Ting. 1998. Identification of distinct regions of 5′ flanking DNA that mediate constitutive, IFN-gamma, STAT1, and TGF-beta-regulated expression of the class II transactivator gene. J. Immunol. 160233-240. [PubMed] [Google Scholar]
- 39.Pöhlmann, S., J. Zhang, F. Baribaud, Z. Chen, G. J. Leslie, G. Lin, A. Granelli-Piperno, R. W. Doms, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 774070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray, R. B., L. M. Lagging, K. Meyer, R. Steele, and R. Ray. 1995. Transcriptional regulation of cellular and viral promoters by the hepatitis C virus core protein. Virus Res. 37209-220. [DOI] [PubMed] [Google Scholar]
- 41.Reith, W., S. LeibundGut-Landmann, and J. M. Waldburger. 2005. Regulation of MHC class II gene expression by the class II transactivator. Nat. Rev. Immunol. 5793-806. [DOI] [PubMed] [Google Scholar]
- 42.Reith, W., and B. Mach. 2001. The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 19331-373. [DOI] [PubMed] [Google Scholar]
- 43.Reference deleted.
- 44.Rollier, C., J. A. Drexhage, B. E. Verstrepen, E. J. Verschoor, R. E. Bontrop, G. Koopman, and J. L. Heeney. 2003. Chronic hepatitis C virus infection established and maintained in chimpanzees independent of dendritic cell impairment. Hepatology 38851-858. [DOI] [PubMed] [Google Scholar]
- 45.Sarobe, P., J. J. Lasarte, A. Zabaleta, L. Arribillaga, A. Arina, I. Melero, F. Borras-Cuesta, and J. Prieto. 2003. Hepatitis C virus structural proteins impair dendritic cell maturation and inhibit in vivo induction of cellular immune responses. J. Virol. 7710862-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reference deleted.
- 47.Reference deleted.
- 48.Thurner, B., C. Roder, D. Dieckmann, M. Heuer, M. Kruse, A. Glaser, P. Keikavoussi, E. Kampgen, A. Bender, and G. Schuler. 1999. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods 2231-15. [DOI] [PubMed] [Google Scholar]
- 49.Worku, S., and D. F. Hoft. 2003. Differential effects of control and antigen-specific T cells on intracellular mycobacterial growth. Infect. Immun. 711763-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1882205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]