Abstract
Bovine spongiform encephalopathy (BSE), the prion disease in cattle, was widely believed to be caused by only one strain, BSE-C. BSE-C causes the fatal prion disease named new variant Creutzfeldt-Jacob disease in humans. Two atypical BSE strains, bovine amyloidotic spongiform encephalopathy (BASE, also named BSE-L) and BSE-H, have been discovered in several countries since 2004; their transmissibility and phenotypes in humans are unknown. We investigated the infectivity and human phenotype of BASE strains by inoculating transgenic (Tg) mice expressing the human prion protein with brain homogenates from two BASE strain-infected cattle. Sixty percent of the inoculated Tg mice became infected after 20 to 22 months of incubation, a transmission rate higher than those reported for BSE-C. A quarter of BASE strain-infected Tg mice, but none of the Tg mice infected with prions causing a sporadic human prion disease, showed the presence of pathogenic prion protein isoforms in the spleen, indicating that the BASE prion is intrinsically lymphotropic. The pathological prion protein isoforms in BASE strain-infected humanized Tg mouse brains are different from those from the original cattle BASE or sporadic human prion disease. Minimal brain spongiosis and long incubation times are observed for the BASE strain-infected Tg mice. These results suggest that in humans, the BASE strain is a more virulent BSE strain and likely lymphotropic.
Overwhelming evidence indicates that bovine spongiform encephalopathy (BSE), a prion disease that has been detected in several hundred thousand cattle in the United Kingdom and many other countries since the 1980s, has been transmitted to humans through the consumption of prion-contaminated beef, causing a prion disease named variant Creutzfeldt-Jakob disease (vCJD) (5, 19, 24). Over 200 cases of vCJD have been reported around the world (19). In 2004, two types of bovine prion disease that differ from the original BSE, now named classical BSE (BSE-C), were reported (3, 8). The two atypical BSE types were associated with prion protein (PrP) scrapie isoforms (PrPSc) that after protease digestion, displayed distinct electrophoretic mobility or ratios of the PrPSc glycoforms different from those of BSE-C (3, 8). Currently, a total of at least 36 cases of these two atypical BSE types have been reported for cattle older than 8 years (5; M. Caramelli, unpublished data). The two atypical BSE types are identified as BSE-H and bovine amyloidotic spongiform encephalopathy (BASE, also named BSE-L); the “L” and “H” identify the higher and lower electrophoretic positions, respectively, of their protease-resistant PrPSc isoforms (7). The bovine phenotype and the PrPSc molecular features of BASE have previously been described in detail (8). The histopathology of BASE and the PrP immunostaining pattern of BASE strains are characterized by the presence of prion amyloid plaques and a more rostral distribution of the PrPSc, which at variance with BSE-C is present in the cerebral cortex, including the hippocampus, but is underrepresented in the brain stem (8). These phenotypic features and PrPSc characteristics resemble a subtype of sporadic Creutzfeldt-Jakob disease (sCJD) named sCJDMV2, which affects subjects who are methionine (M)/valine (V) heterozygous at codon 129 of the PrP gene, and it is associated with PrPSc identified as type 2 (15). This similarity has raised the question of whether sCJDMV2 is not sporadic but acquired from the consumption of BASE strain-contaminated meat (5, 8). To begin to investigate the transmissibility to humans and the “human” disease phenotype of BASE, including the involvement of the lymphoreticular system, we have inoculated brain homogenates from BASE-affected cattle to transgenic (Tg) mice expressing normal human PrP with Met at codon 129 (HuPrP-129M) in a mouse PrP-ablated background [Tg(HuPrP)] (13). The inoculated Tg mice were examined for attack rates and the disease phenotype, including the presence and characteristics of protease-resistant PrPSc in the brain and spleen and the histopathology, along with the PrPSc topography and pattern of deposition in the brain.
MATERIALS AND METHODS
Transgenic mice.
Transgenic mice expressing human PrP-129M [Tg(HuPrP)] were reported previously (13). The Tg40 line that expresses human PrP-129M at the wild-type level in the mouse PrP-ablated background was used in this study. Intracerebral (i.c.) inoculation of Tg mice and the monitoring of symptoms were performed as described previously (13). The mice were sacrificed 2 or 3 days after the appearance of symptoms or at death, and the brains and spleens were taken. The brains were sliced sagittally, with half frozen for immunochemical studies and the other half either fixed in formalin for histological and immunohistochemical staining or frozen for histoblot analysis (see below). Total PrP as well as proteinase K (PK)-resistant PrPSc was determined by immunoblotting in sodium dodecyl sulfate (SDS)-polyacrylamide gels as described below. This study was conducted with approvals from the Institutional Review Board and the Institutional Animal Care and Use Committee.
Immunoblotting, histology, histoblotting, and immunohistochemistry.
Frozen brain or spleen tissues were homogenized in 2 volumes of cold phosphate-buffered saline to obtain 33% (wt/vol) crude homogenate for storage in aliquots at −80°C. The frozen 33% crude homogenate was thawed at 4°C for 2 h and diluted to 10% (wt/vol) with the lysis buffer (final concentration, 100 mM Tris, 10 mM EDTA, 100 mM NaCl, 0.5% sodium deoxycholate, 1.0% NP-40, pH 8.0). After incubation at room temperature for 15 min, the 10% homogenate was subjected to sonication with the Ultrasonic Dismembrator 100 (Fisher Scientific) for 3 min. The sonicated 10% homogenate was treated with 100 μg/ml PK (Roche Diagnostics GmbH, Mannheim, Germany) for 30 min at 37°C and denatured by being boiled at 100°C for 10 min after being mixed with an equal volume of 2× sample buffer (200 mM Tris-HCl, pH 6.8, 2% SDS, 40% glycerol, 0.04% Coomassie blue G-250, 2% β-mercaptoethanol). The enrichment of PrPSc by precipitation with sodium phosphotungstate (NaPTA) was performed virtually as previously reported (18), and special care and efforts were taken to ensure that the pellets were completely resuspended each time. Proteins were separated by precast 10 to 20% gradient Tris-Tricine gel (Bio-Rad), transferred to a polyvinylidene difluoride membrane, and subjected to Western blot analysis with monoclonal antibody (MAb) 8H4, 6H4, or 3F4 in conjunction with horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin G Fc antibody (GE Healthcare, Buckinghamshire, United Kingdom) as described previously (13). The blots were developed with the ECL Western blotting detection reagent (GE Healthcare Amersham, Buckinghamshire, United Kingdom) and exposed to X-ray films. The blots were digitized by scanning the film. To determine the precise molecular weights of the bands, the digitized blots were analyzed by image acquisition and analysis software (UVP, Upland, CA) that automatically detects the midpoint of the band and calculates the molecular weight based on the sizes of the unglycosylated PK-resistant PrP fragments of sCJDMM1 and sCJDMM2; the values were statistically analyzed by Matlab 7.0 software (MathWorks, Natick, MA). To determine the glycoform ratios of PK-resistant PrPSc fragments, each PrP band on the digitized blots was quantified with UN-SCAN-IT software (Silk Scientific, Orem, UT); the values from duplicate blots were analyzed with Excel software to calculate the averages and standard deviations and to create the column chart.
Histological staining with hematoxylin and eosin (H&E) and immunohistochemical staining with 3F4 were performed as reported previously (13). Histoblot analysis was performed mostly as described previously (20), with the following modifications: the cryosections were 12 μm thick, and the sections were treated with 100 μg/ml of proteinase K for 4 h at 37°C, incubated with monoclonal antibody 3F4 (1:10,000 dilution) overnight at 4°C, followed by incubation with alkaline phosphatase-conjugated goat anti-mouse secondary antibody (1:500; DAKO), and developed with BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium solutions (Sigma).
RESULTS
To assess the transmissibility of BASE in humans, two BASE isolates (8) were used to intracerebrally inoculate 30 Tg40 mice that express normal levels of human PrP-129M. More than half of the inoculated mice (18/30) became infected, as determined by the presence of protease-resistant PrPSc, with average incubation times of 649 ± 34 days for BASE isolate 1 and 595 ± 28 days for BASE isolate 2, respectively (Table 1). Ten of the 18 infected mice that could be examined showed clear clinical signs of disease (Table 1), including hunched backs, ruffled fur, lethargy, occasional wobbling, and rigid tails. These signs were best detected in the younger mice, because in mice older than 24 months, the signs became difficult to distinguish from aging-related changes.
TABLE 1.
BASE transmission in Tg(HuPrP) mice
| Inoculum | Attack rate as determined by:
|
Incubation time (days) | ||
|---|---|---|---|---|
| Clinical signs | Presence of PrPSc | Spongiform degeneration | ||
| BASE-1 | 4/15 | 9/15 | 1 (focal)/8 | 649 ± 34 |
| BASE-2 | 6/15 | 9/15 | 1 (focal)/11 | 595 ± 28 |
| sCJDMM1 | 10/10 | 9/10 | 4/4 | 263 ± 13a |
| sCJDMM2 | 9/9 | 9/9 | 7/7 | 267 ± 17 |
Reported previously (13).
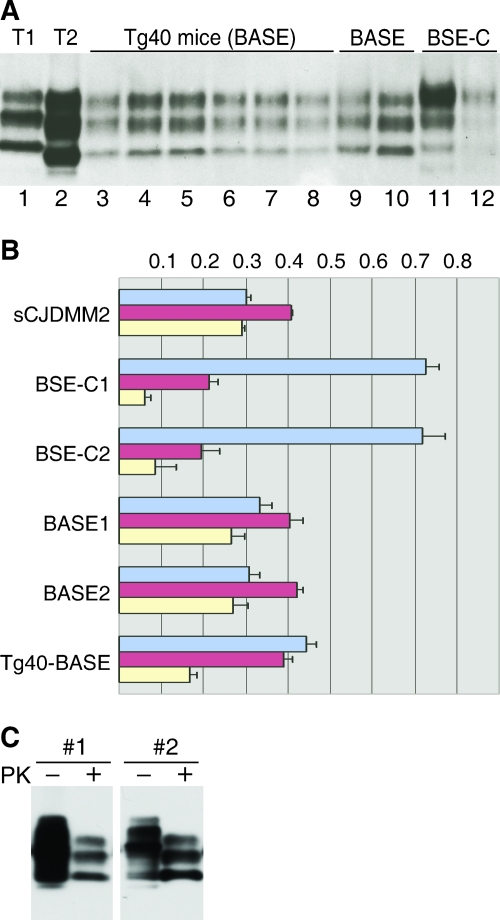
All the Tg40 mice were examined for the presence of PK-resistant PrPSc in the brain by immunoblot analysis both directly and after enrichment with NaPTA precipitation. Such immunoblot analysis with three monoclonal antibodies (3F4, 6H4, and 8H4) to various PrP regions (12, 14, 25) showed that all 18 BASE strain-infected Tg40 mice accumulated comparable amounts of PK-resistant PrPSc in the brain (Fig. 1A, Table 1, and data not shown). The electrophoretic mobility of PK-resistant PrPSc fragments from all the BASE strain-infected Tg40 mice was indistinguishable from that of the PK-resistant PrPSc present in either the BASE strain inoculum or sCJDMM2, which contains type 2 PrPSc (Fig. 1A). The PK-resistant PrPSc fragments associated with both the BASE strain-infected Tg40 mice and the BASE isolates migrated slightly faster than those of BSE-C as originally reported (8). Measurements with software that automatically calculates the midpoint of the bands revealed a difference of 0.29 ± 0.12 kDa in gel mobility between the unglycosylated PK-resistant PrPSc bands of the BASE strain (native as well as from the Tg40 mice) and BSE-C.
FIG. 1.
Immunoblots and glycoform ratios of PK-resistant PrPSc from sCJD-affected, BASE strain-infected Tg(HuPrP) mice, the BASE strain inocula, and BSE-C and of PK-resistant PrPSc from the spleens of BASE strain-infected Tg(HuPrP) mice. (A) Immunoblot of PK-resistant PrPSc in the brain. Lanes 1 and 2, type 1 (sCJDMM1) (T1) and type 2 (sCJDMM2) (T2) sCJD, respectively; lanes 3 to 6, Tg(HuPrP) (Tg40) mice infected with BASE isolate 1 inoculum; lanes 7 to 8, Tg40 mice infected with BASE isolate 2 inoculum; lane 9, BASE isolate 1; lane 10, BASE isolate 2; lanes 11 and 12, two BSE-C isolates. All brain homogenates were treated with 100 μg/ml of PK for 30 min at 37°C and processed for immunoblot analysis with MAb 8H4. Five microliters of 10% brain homogenate was loaded for lanes 3 to 10. (B) Glycoform ratios of PK-resistant PrPSc in the brain. The upper (diglycosylated) (blue), middle (mostly monoglycosylated) (red), and lower (unglycosylated) (yellow) bands of PK-resistant PrPSc from BASE strain-infected Tg40 mice, the BASE strain, and BSE-C were quantified after optical scanning of duplicate immunoblots for panel A. Error bars indicate standard deviations. (C) PK-resistant PrPSc in the spleen. Ten milligrams of spleen tissue each from two of the BASE strain-infected Tg(HuPrP) (Tg40) mice (#1 and #2) was homogenized, PrPSc enriched by NaPTA precipitation, and either treated (+) or not treated (−) with 100 μg/ml of PK for 30 min at 37°C, followed by electrophoresis in a 10 to 20% Tris-Tricine SDS-polyacrylamide gradient gel and immunoblot analysis with MAb 8H4.
The glycoform ratio of PrPSc in isolates from the BASE strain-infected Tg40 mice was slightly different from that of the BASE isolates (Fig. 1B), and both were quite different from that of BSE-C (Fig. 1B). The monoglycosylated form was the most prominent species in the BASE strain inocula, where the glycoform ratio (diglycosylated-to-monoglycosylated-to-unglycosylated) is 32:41:27, whereas the diglycosylated form was slightly more intense than the monoglycosylated form in BASE strain-infected Tg40 mice, where the glycoform ratio is 44:39:17 (Fig. 1B). In contrast, the diglycosylated form accounted for over 70% of the total PrPSc in BSE-C (glycoform ratio of 72:20:8).
PrPSc in the spleen was also examined after NaPTA enrichment for all 30 BASE strain-inoculated Tg40 mice. PK-resistant PrPSc was readily detected in the spleens of four mice (Fig. 1C), all of which also contained PK-resistant PrPSc in the brain. The electrophoretic mobility of the spleen PrPSc was similar to that of the brain PrPSc. The glycoform ratio of the spleen PrPSc was different from that of the brain and was characterized by the prominence of the monoglycosylated and unglycosylated forms (Fig. 1C), but the glycoform ratio may have been affected by the NaPTA enrichment. In contrast, none of the nine Tg40 mice inoculated with sCJDMM1 had detectable PK-resistant PrPSc in the spleen after NaPTA enrichment (data not shown).
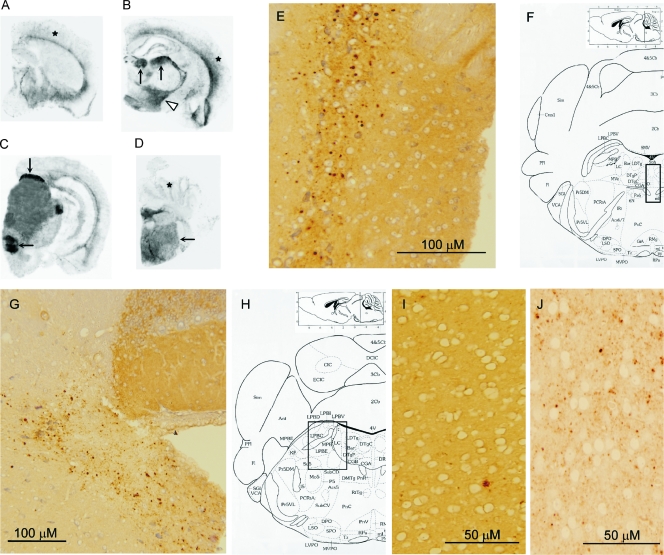
None of the 12 BASE strain-infected Tg40 mice examined showed prominent and consistent histopathological changes related to prion diseases (Fig. 2A). Focal, ambiguous spongiform degeneration was observed for two mice. No PrP amyloid plaques were observed in BASE strain-infected Tg40 mice. Histoblot analysis with MAb 3F4 showed a very distinct and selective distribution of PrPSc (Fig. 3A to D). Particular nuclei or groups of adjacent periventricular nuclei in the thalamus, hypothalamus, and brain stem were intensely immunostained for PrPSc (Fig. 3B to D). In contrast, PrPSc appeared to be overall less intense in the cerebral and cerebellar cortices (Fig. 3A to D). Immunohistochemical staining of paraffin-embedded brain tissue with 3F4 revealed PrP deposits in 5 of the 11 BASE strain-infected Tg40 mice examined. PrPSc deposits that stained intensely in the histoblots consisted of relatively large and well-circumscribed granules (Fig. 3E and G). Fine granular or small plaque-like aggregate patterns were occasionally seen in inferior regions of the cerebral cortex and in the thalamus (Fig. 3I and data not shown). In contrast, widespread, mostly fine-granular staining was detected in the cerebral cortex of symptomatic Tg40 mice inoculated with sCJDMM1 brain homogenate (Fig. 3J).
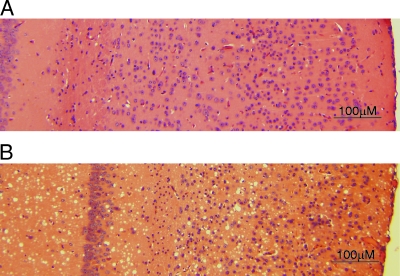
FIG. 2.
Histopathology (with H&E) of BASE strain-infected and sCJDMM1-infected Tg(HuPrP) mice. (A) No consistent pathology was detected in the cerebral cortex as well as subcortical brain regions of symptomatic and immunoblot-positive BASE strain-infected Tg(HuPrP) (Tg40) mice. (B) In contrast, Tg40 mice inoculated with sCJDMM1 brain homogenate showed widespread spongiform degeneration.
FIG. 3.
Histoblot analysis and immunohistochemistry of BASE strain-infected and sCJDMM1-infected Tg(HuPrP) mice. (A to D) The histoblot analysis revealed preferential immunostaining of the PrPSc in the dorsal thalamic nuclei (arrows in panel B), along with hypothalamic nuclei (arrowhead) and brain stem nuclei (arrows in panels C and D), while PrPSc in the cerebral and cerebellar cortices (stars in panels A, B, and D) was mostly limited to the deep and inferior cortical regions. (E to J) The PrP immunostaining (E and G) of the intensely PrP-reactive brain stem nuclei in histoblot analysis (boxed regions in panels F and H) revealed coarse PrP granules, while the PrP immunostain in the cerebral cortex (I) was minimal and characterized mostly by a plaque-like pattern. In contrast, widespread fine-granular PrP immunostaining was observed in the cerebral cortex of symptomatic Tg40 mice following inoculation of sCJDMM1 brain homogenates (J). Monoclonal antibody 3F4 was used for all the staining.
The histopathological features of the BASE strain-inoculated Tg40 mice were quite different from those observed following inoculation with brain homogenates from the two forms of sCJD, sCJDMM1 and sCJDMM2. The sCJDMM1-inoculated Tg40 mice had widespread spongiform degeneration in the cerebrum (Fig. 2B) and moderate apoptosis of neuronal cells without spongiform degeneration in the cerebellum (13). Widespread spongiform degeneration was also seen in Tg40 mice inoculated with sCJDMM2 brain homogenate (data not shown).
DISCUSSION
We have shown that 60% of our Tg40 mice (in an inbred FVB background) that express normal levels of human PrP-129M became infected 20 to 22 months after i.c. inoculation with 0.3 mg of brain tissue from the two BASE isolates, suggesting a titer of approximately 3 50% infective dose units per milligram of brain tissue in the Tg40 line. An approximately 20% attack rate has been reported for the Tg650 line (in a mixed 129/Sv × C57BL/6 background) after i.c. inoculation with 2 mg brain tissues from BSE-C-infected cattle (2). It is noteworthy that the Tg650 mice express human PrP-129M at five to eight times the normal level, and high PrP levels are known to increase prion transmissibility (9, 17, 22). Inefficient BSE-C transmissions (0 to 30%) in Tg mouse lines of other genetic backgrounds expressing human PrP-129M at one or two times the normal level have also been reported by different groups (1, 4). Although it is difficult to compare results from different mouse lines, these findings suggest that the BASE strain has higher transmissibility than BSE-C does for humanized Tg mice with PrP-129M and possibly for humans with PrP-129MM. The BASE strain also appears to be more virulent than BSE-C in bovinized Tg mice, since the incubation time for the BASE strain is 185 ± 12 days, whereas that for BSE-C is 230 ± 7 days (7). Nevertheless, compared with the 100% attack rate and incubation times of ∼9 months for sCJDMM1 and sCJDMM2 in the Tg40 line (Table 1), the 60% attack rate and unusually long incubation times (20 to 22 months) for the BASE strain in the same Tg line suggest that the transmission barrier from the BASE strain to humans with PrP-129MM is still quite significant.
PK-resistant PrPSc was also detected in the spleen in 4 out of 18 BASE strain-infected Tg40 mice. In contrast, no spleen involvement could be demonstrated for the Tg40 mice following i.c. inoculation with human PrPSc from sCJDMM1. This is the first report of the presence of PrPSc in the spleens of humanized Tg mice after i.c. inoculation with a BSE strain, suggesting that the BASE strain, like BSE-C, where at least in vCJD-infected subjects PrPSc and prion infectivity have been detected in spleens and tonsils (6, 11), is intrinsically lymphotropic. Therefore, lymphoid tissues of BASE strain-infected individuals might also carry prion infectivity.
The gel mobility of the PK-resistant PrPSc recovered from the BASE strain-inoculated Tg40 mice was consistently slightly faster than the mobility of BSE-C, as originally reported for the BASE strain (8). The computed difference in gel mobilities between BASE and BSE-C PrPSc is 0.29 ± 0.12 kDa, corresponding to 2 to 4 amino acid residues. In contrast, the gel mobilities of the PK-resistant PrPSc species from the BASE strain, BASE strain-infected Tg40 mice, and sCJDMM2, which was used as representative of human PrPSc of type 2, were indistinguishable. This finding suggests that the PK-resistant PrPSc electrophoretic heterogeneity between the BASE strain and BSE-C falls well within the 7-amino-acid variability of the N terminus (positions 92 to 99) that is consistently found in PK-resistant PrPSc of type 2 (16). Therefore, despite their minor but distinct variability in gel mobility, both the BASE strain and BSE-C PrPSc species appear to belong to the PrPSc of type 2. However, the PrPSc glycoform ratios of BASE strain-infected Tg40 mice and the BASE strain inocula display a small but statistically significant difference (Fig. 1). Therefore, PrPSc in BASE strain-infected human subjects may be expected to display a different glycoform ratio from that of the BASE strain. It is worth noting that the electrophoretic characteristics of the PK-resistant PrPSc of some human prion strains has been faithfully reproduced by our Tg40 line as well as by other humanized mouse lines (10, 13, 21).
Two distinct histopathological and PrP immunohistochemical phenotypes have been reported following BSE-C inoculation: one reproduced the distinctive features of vCJD with the “florid” plaques that intensely immunostained for PrP, and the other was reminiscent of sCJDMM1, with prominent spongiform degeneration and no plaque PrP immunostaining (1, 23). The brain histopathology, the PrPSc distribution, and the PrP immunostaining pattern of BASE strain-inoculated Tg40 mice were definitely distinct from such features described above (1, 23), further supporting the notion that BASE and classical BSE are associated with two distinct prion strains (8).
The relatively easy transmission of BASE to humanized Tg mice indicates that effective cattle prion surveillance should be maintained until the extent and origin of this and other atypical forms of BSE are fully understood.
Acknowledgments
This study was supported by Public Health Service grants (AG014359 [P.G.] from the National Institute of Aging and NS052319 [Q.K.] from the National Institute of Neurological Disorders and Stroke) and by an award to P.G. from the Charles S. Britton Fund.
We are grateful to Diane Kofsky, Phyliss Scalzo, Carrie Harris, and Kay Edmonds for their assistance.
Footnotes
Published ahead of print on 30 January 2008.
REFERENCES
- 1.Asante, E. A., J. M. Linehan, M. Desbruslais, S. Joiner, I. Gowland, A. L. Wood, J. Welch, A. F. Hill, S. E. Lloyd, J. D. Wadsworth, and J. Collinge. 2002. BSE prions propagate as either variant CJD-like or sporadic CJD-like prion strains in transgenic mice expressing human prion protein. EMBO J. 216358-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Béringue, V., O. Andreoletti, A. Le Dur, R. Essalmani, J. L. Vilotte, C. Lacroux, F. Reine, L. Herzog, A. G. Biacabe, T. Baron, M. Caramelli, C. Casalone, and H. Laude. 2007. A bovine prion acquires an epidemic bovine spongiform encephalopathy strain-like phenotype on interspecies transmission. J. Neurosci. 276965-6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biacabe, A. G., J. L. Laplanche, S. Ryder, and T. Baron. 2004. Distinct molecular phenotypes in bovine prion diseases. EMBO Rep. 5110-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop, M. T., P. Hart, L. Aitchison, H. N. Baybutt, C. Plinston, V. Thomson, N. L. Tuzi, M. W. Head, J. W. Ironside, R. G. Will, and J. C. Manson. 2006. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol. 5393-398. [DOI] [PubMed] [Google Scholar]
- 5.Brown, P., L. M. McShane, G. Zanusso, and L. Detwiler. 2006. On the question of sporadic or atypical bovine spongiform encephalopathy and Creutzfeldt-Jakob disease. Emerg. Infect. Dis. 121816-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce, M. E., I. McConnell, R. G. Will, and J. W. Ironside. 2001. Detection of variant Creutzfeldt-Jakob disease infectivity in extraneural tissues. Lancet 358208-209. [DOI] [PubMed] [Google Scholar]
- 7.Buschmann, A., A. Gretzschel, A. G. Biacabe, K. Schiebel, C. Corona, C. Hoffmann, M. Eiden, T. Baron, C. Casalone, and M. H. Groschup. 2006. Atypical BSE in Germany—proof of transmissibility and biochemical characterization. Vet. Microbiol. 117103-116. [DOI] [PubMed] [Google Scholar]
- 8.Casalone, C., G. Zanusso, P. Acutis, S. Ferrari, L. Capucci, F. Tagliavini, S. Monaco, and M. Caramelli. 2004. Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease. Proc. Natl. Acad. Sci. USA 1013065-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer, M., T. Rulicke, A. Raeber, A. Sailer, M. Moser, B. Oesch, S. Brandner, A. Aguzzi, and C. Weissmann. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 151255-1264. [PMC free article] [PubMed] [Google Scholar]
- 10.Hill, A. F., M. Desbruslais, S. Joiner, K. C. Sidle, I. Gowland, J. Collinge, L. J. Doey, and P. Lantos. 1997. The same prion strain causes vCJD and BSE. Nature 389448-450. [DOI] [PubMed] [Google Scholar]
- 11.Hill, A. F., R. J. Butterworth, S. Joiner, G. Jackson, M. N. Rossor, D. J. Thomas, A. Frosh, N. Tolley, J. E. Bell, M. Spencer, A. King, S. Al-Sarraj, J. W. Ironside, P. L. Lantos, and J. Collinge. 1999. Investigation of variant Creutzfeldt-Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet 353183-189. [DOI] [PubMed] [Google Scholar]
- 12.Kascsak, R. J., R. Rubenstein, P. A. Merz, M. Tonna-DeMasi, R. Fersko, R. I. Carp, H. M. Wisniewski, and H. Diringer. 1987. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J. Virol. 613688-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong, Q., S. Huang, W. Zou, D. Vanegas, M. Wang, D. Wu, J. Yuan, H. Bai, M. Zheng, H. Deng, K. Chen, A. L. Jenny, K. O'Rourke, E. D. Belay, L. B. Schonberger, R. B. Petersen, M. S. Sy, S. G. Chen, and P. Gambetti. 2005. Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. J. Neurosci. 257944-7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korth, C., B. Stierli, P. Streit, M. Moser, O. Schaller, R. Fischer, W. Schulz-Schaeffer, H. Kretzschmar, A. Raeber, U. Braun, F. Ehrensperger, S. Hornemann, R. Glockshuber, R. Riek, M. Billeter, K. Wüthrich, and B. Oesch. 1997. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 39074-77. [DOI] [PubMed] [Google Scholar]
- 15.Parchi, P., A. Giese, S. Capellari, P. Brown, W. Schulz-Schaeffer, O. Windl, I. Zerr, H. Budka, N. Kopp, P. Piccardo, S. Poser, A. Rojiani, N. Streichemberger, J. Julien, C. Vital, B. Ghetti, P. Gambetti, and H. Kretzschmar. 1999. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann. Neurol. 46224-233. [PubMed] [Google Scholar]
- 16.Parchi, P., W. Zou, W. Wang, P. Brown, S. Capellari, B. Ghetti, N. Kopp, W. J. Schulz-Schaeffer, H. A. Kretzschmar, M. W. Head, J. W. Ironside, P. Gambetti, and S. G. Chen. 2000. Genetic influence on the structural variations of the abnormal prion protein. Proc. Natl. Acad. Sci. USA 9710168-10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prusiner, S. B., M. Scott, D. Foster, K. M. Pan, D. Groth, C. Mirenda, M. Torchia, S. L. Yang, D. Serban, G. A. Carlson, P. C. Hoppe, D. Westaway, and S. J. DeArmond. 1990. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63673-686. [DOI] [PubMed] [Google Scholar]
- 18.Safar, J., H. Wille, V. Itri, D. Groth, H. Serban, M. Torchia, F. E. Cohen, and S. B. Prusiner. 1998. Eight prion strains have PrP(Sc) molecules with different conformations. Nat. Med. 41157-1165. [DOI] [PubMed] [Google Scholar]
- 19.Seitz, R., F. von Auer, J. Blumel, R. Burger, A. Buschmann, K. Dietz, M. Heiden, W. E. Hitzler, H. Klamm, T. Kreil, H. Kretzschmar, M. Nübling, R. Offergeld, G. Pauli, V. Schottstedt, P. Volkers, and I. Zerr. 2007. Impact of vCJD on blood supply. Biologicals 3579-97. [DOI] [PubMed] [Google Scholar]
- 20.Taraboulos, A., K. Jendroska, D. Serban, S. L. Yang, S. J. DeArmond, and S. B. Prusiner. 1992. Regional mapping of prion proteins in brain. Proc. Natl. Acad. Sci. USA 897620-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Telling, G. C., M. Scott, J. Mastrianni, R. Gabizon, M. Torchia, F. E. Cohen, S. J. DeArmond, and S. B. Prusiner. 1995. Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 8379-90. [DOI] [PubMed] [Google Scholar]
- 22.Tremblay, P., Z. Meiner, M. Galou, C. Heinrich, C. Petromilli, T. Lisse, J. Cayetano, M. Torchia, W. Mobley, H. Bujard, S. J. DeArmond, and S. B. Prusiner. 1998. Doxycyline control of prion protein transgene expression modulates prion disease in mice. Proc. Natl. Acad. Sci. USA 9512580-12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadsworth, J. D., E. A. Asante, M. Desbruslais, J. M. Linehan, S. Joiner, I. Gowland, J. Welch, L. Stone, S. E. Lloyd, A. F. Hill, S. Brandner, and J. Collinge. 2004. Human prion protein with valine 129 prevents expression of variant CJD phenotype. Science 3061793-1796. [DOI] [PubMed] [Google Scholar]
- 24.Ward, H. J. T., D. Everington, S. N. Couseus, B. Smith-Bathgate, M. Leitch, S. Cooper, C. Heath, R. S. G. Knight, P. G. Smith, and R. G. Will. 2006. Risk factors for variant Creutzfeldt-Jakob disease: a case-control study. Ann. Neurol. 59111-120. [DOI] [PubMed] [Google Scholar]
- 25.Zanusso, G., D. Liu, S. Ferrari, I. Hegyi, X. Yin, A. Aguzzi, S. Hornemann, S. Liemann, R. Glockshuber, J. C. Manson, P. Brown, R. B. Petersen, P. Gambetti, and M. S. Sy. 1998. Prion protein expression in different species: analysis with a panel of new MAbs. Proc. Natl. Acad. Sci. USA 958812-8816. [DOI] [PMC free article] [PubMed] [Google Scholar]