Abstract
A characteristic feature in the immune response to many persistent viral infections is the dysfunction or deletion of antigen-specific T cells (exhaustion). This down-regulation of virus-specific T-cell response represents a critical control mechanism that exists within T-cell activation pathways to prevent lethal disease by inappropriate responses against disseminating virus infections. However, the molecular mechanisms by which the immune system determines whether to mount a full response to such infections remain largely unexplored. Here, we have established that in the murine lymphocytic choriomeningitis virus (LCMV) model, induction of the T-cell receptor signaling inhibitor molecule E3 ligase Cbl-b is critically involved in this decision. In particular, our data revealed that Cbl-b controls the program responsible for T-cell tolerance (exhaustion) induction during a chronic viral infection. Thus, Cbl-b−/− mice infected with a low dose of LCMV Docile mount a strong CD8+ T-cell response that rapidly clears the infection, and the animals remain healthy; in contrast, down-regulation of the epitope-specific CD8+ T-cell population in persistently infected Cbl-b−/− mice, compared to that in chronically infected B6 mice, was significantly delayed, and this was associated with increased morbidity and eventual death in nearly 20% of the animals. Interestingly, infection of Cbl-b−/− mice with a moderate virus dose resulted in rapid death with 100% mortality by 7 to 8 days after infection, caused by a dysregulated antiviral T-cell response, whereas the infected B6 mice survived and remained healthy. In conclusion, our results suggest that Cbl-b is critically involved in T-cell exhaustion and prevention of lethal disease.
Persistent viral infections in human and animal models are associated with a failure of the host immune response to generate and sustain functional CD8+ and CD4+ T-cell populations as well as antibodies to neutralize infectivity (for reviews, see references 10, 12, 22, 26, 42, 60, and 62). From an evolutionary viewpoint, down-regulation of the immune response represents an adaptive mechanism that limits the damage caused by overaggressive T cells and influences antiviral immunity and the outcome of a viral infection. To explain the chronic course of viral infections in human and animal populations, a variety of mechanisms associated with a virus-specific defect in immunity have been proposed, including mechanisms of immune evasion from immunological pressure and exhaustion (deletion or functional inactivation) of virus-specific T cells. In particular, evasion mechanisms utilized to various degrees by different RNA viruses adapted to generate genomic diversity can be exploited to evade T-cell immune recognition and impact the course of infection (12, 13, 57, 59, 61, 63). Two pertinent mechanisms of immune evasion, mutational escape of virus-specific T-cell epitopes and inhibition of antigen processing and peptide presentation by expression of certain viral proteins, enjoy substantial experimental and clinical evidence (2, 12, 49). On the other hand, impairment of virus-specific T-cell responses, observed to various degrees in individuals with chronic infections, can be a consequence of virus-specific CD8+ or CD4+ T-cell exhaustion (functional defect or physical elimination). This phenomenon, originally described for the murine lymphocytic choriomeningitis virus (LCMV) model, represents a general T-cell-regulatory mechanism to limit T-cell-mediated tissue damage during an infection (38). Indeed, increasing experimental and clinical evidence from studies of chronic infections in human and other model systems strongly support the concept that this regulatory mechanism of adaptive immunity is an important contributor to the inability of the host to eliminate persisting pathogens (4, 18, 19, 27, 50).
Features that distinguish partial or complete failure of T-cell responses that provide transient or lack of control of infection are not fully defined. In particular, the molecular mechanisms underlying virus-specific T-cell exhaustion in a chronically infected host remain largely elusive. Our understanding at the molecular level of this important issue is further complicated by the fact that T-cell exhaustion apparently reflects a composite of regulatory checkpoints that together place T cells in a continuum from dysfunctional to nonfunctional to preterminal stages. However, guided from evidence in different experimental models of T-cell tolerance, we predict that there are many defects involved in the exhausted cells, including disrupted T-cell receptor (TCR) signaling, up-regulation of inhibitor molecules, down-regulation of costimulator proteins, up-regulation of ubiquitin ligases, and defects in accessory and cytokine signals. How these regulatory checkpoint mechanisms affect the program of T-cell exhaustion is yet to be fully elucidated. E3 ubiquitin ligases, such as the Casitas B-cell lymphoma (Cbl) family of proteins (c-Cbl, Cbl-b, and Cbl-3), GRAIL, Itch, and Nedd4, are signaling adaptors and have been implicated in induction of T-cell tolerance by regulating the ubiquitination and degradation of downstream effectors of T-cell anergy (3, 5, 21, 30, 31, 40, 51). In particular, c-Cbl and Cbl-b, two homologous members of the Cbl family of proteins, are differentially expressed in thymocytes and mature T cells and play a predominant role in thymocyte development (c-Cbl) or in peripheral T-cell activation (Cbl-b) (5, 24, 41). Biochemical analyses revealed that ubiquitination of protein kinase C θ (PKC-θ) and phospholipase C-γ1 (PLC-γ1) by Cbl-b contributes to immunological synapse disintegration, which attenuates TCR signaling. In addition, Cbl-b-mediated ubiquitination can regulate PLC-γ1 and Ca2+ flux in anergic cells by reduced phosphorylation, therefore inhibiting the activity of this proximal TCR signaling molecule (21, 25). Thus, Cbl-b E3 ligase activity defines the threshold for a TCR-induced calcium response that is central for determining the anergic or responsive status of T cells. Participation of Cbl-b in regulating peripheral tolerance and anergy of T cells in vivo has been demonstrated in an experimental model of peptide-induced tolerance (25). However, the importance of Cbl-b in regulating the T-cell response to acute and persistent infections has not yet been defined. In this report, we examine the contribution of E3 ligase Cbl-b in T-cell exhaustion during chronic infection by using the mouse model of LCMV infection, which is a unique experimental system to study the dynamics of virus-host interactions in the context of acute and chronic viral infections. The data suggest an important role for the E3 ligase Cbl-b in modulating T-cell function during chronic viral infection and define a specific mechanism of T-cell exhaustion.
MATERIALS AND METHODS
Mice and animal experiments.
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). LCMV glycoprotein (GP)-specific CD8+-TCR-transgenic (P14) (44, 48) and Cbl-b−/− mice were described previously (5). Experiments were performed with relatively young (8- to 12-week-old) mice. Animals were kept and experiments were performed in accordance with institutional animal welfare guidelines.
Viruses.
LCMV Docile (isolated from an LCMV UBC carrier mouse), as a plaque-purified second passage virus (47), was obtained from C. J. Pfau (Troy, NY). LCMV titers were determined with an immunological focus assay (8).
Flow cytometry and functional analysis.
Phenotypic analysis of cell surface markers, intracellular cytokine staining, and major histocompatibility complex class I (MHC-I) tetramer staining were performed as previously described (43, 65). All antibodies were purchased from BD-Pharmingen (San Jose, CA) or eBioscience (San Diego, CA), except for granzyme B (Caltag, Carlsbad, CA) and Bcl-xL (Gene Tex, Inc., San Antonio, TX).
Experiments utilized Db/GP133-41 (KAVYNFATM), Db/NP396-404 (FQPQNGQFI), or Kb/GP34-43 (AVYNFATCGI) phycoerythrin (PE)- or allophycocyanin-conjugated tetramers. Single-cell suspensions prepared from spleens or lymphocytes isolated from different tissues were stained with tetramer and antibody for CD8 (clone 53-6.72) alone or in combination with staining for activation- and differentiation-specific cell markers, in fluorescence-activated cell sorter buffer (phosphate-buffered saline with 1% bovine serum albumin and 0.2% sodium azide). After staining for 1 h at 4°C, cells were fixed in phosphate-buffered saline containing 0.1% paraformaldehyde, acquired on a FACSContor flow cytometer (Becton-Dickinson, San Jose, CA). Data were analyzed using CellQuest software.
For intracellular cytokine staining, lymphocytes were cultured in 96-well, flat-bottom plates at 1 × 106 cells/well in 200 μl RPMI 1640 (Gibco) supplemented with 10% fetal calf serum in the presence or absence of peptide at a concentration of 1 μg/ml. To quantitate total virus-specific CD8+ T-cell responses, virus-infected dendritic-cell (DC2.4, kindly provided by K. Rock, Boston, MA) (54) stimulators were used in combination with intracellular cytokine staining. A total of 1 × 106 effector cells were incubated with 4 × 105 DC2.4 cells, uninfected or infected 48 h previously with LCMV at a multiplicity of infection of 0.5. Stimulations were performed for 6 h at 37°C, in the presence of 10 units/well of murine interleukin 2 (IL-2) and 1 μg/well brefeldin A (BD-Pharmingen). The cells were then surface stained for CD8α and subsequently permeabilized (Cytofix/Cytoperm kit; BD-Pharmingen) and stained with fluorescein isothiocyanate-conjugated antibody against gamma interferon (IFN-γ) (clone XMG1.2), or PE-conjugated antibody against tumor necrosis factor alpha (TNF-α) or IL-2 (clone MP6-XT22 or JES6-5H4, respectively). For intracellular granzyme B staining, cells were directly surface labeled with anti-CD8 and anti-MHC-I tetramers, permeabilized, and labeled with anti-granzyme B (clone GB12) or mouse immunoglobulin G1 (IgG1) isotype control. Intracellular CTLA-4 staining was performed by direct (ex vivo) intracellular staining of lymphocytes as described above and was combined with surface staining with antibody against CD8 and MHC-I tetramers. Analysis of Bcl-2 or Bcl-xL expression in virus-specific CD8+ T-cell populations was performed as previously described (64). Stained cells were washed, fixed, and acquired as described above.
Analysis of the cytotoxic T-cell response.
Direct ex vivo cytotoxic T-lymphocyte (CTL) activity was determined by a 5 or 18 h 51Cr release assay as described previously (65). Lymphocytes were incubated with virus-infected MC57G cells at an effector-to-target (E:T) ratio ranging from 1:1 to 100:1. E:T ratios shown are corrected for the number of Db/GP133-41 and Db/NP396-404 tetramer-positive cells in the total virus-specific CTL population.
Depletion of CD8+ T cells and neutralization of IFN-γ or TNF-α in vivo.
LCMV Docile (104 PFU given intravenously [i.v.])-infected Cbl-b−/− mice were depleted of CD8+ T cells by intraperitoneal injection of purified anti-CD8 (YTS169) antibody on day 5.5 postinfection (p.i.) as previously described (37). For neutralization of cytokines in vivo, infected Cbl-b−/− mice were injected on days 4 and 6 with 125 μg of anti-TNF-α (TN3-19.12; eBioscience) (53) or 0.5 mg of anti-IFN-γ (XMG1.2) antibody. Control animals were treated with functional-grade purified Armenian hamster IgG or rat IgG1 isotype.
Adoptive immunization.
Recipient B6 mice infected i.v. with 102 or 2 × 106 PFU of LCMV Docile were transfused with 104 splenocytes containing an equivalent number of P14 or P14-Cbl-b−/− T cells. Alternatively, CD8+-TCR-transgenic cells were transferred into the neonatal carrier mice.
Proliferation of T cells in response to peptide stimulation.
Transgenic splenocytes (5 × 104/well) were cultured with irradiated (30 Gy) splenocytes (5 × 105/well) from B6 mice in the given concentrations of peptides in Iscove's modified Dulbecco's medium supplemented with 10% fetal calf serum for 72 h. Proliferation of T cells was determined by incorporation of [3H]thymidine (1 μCi/well) during the last 6 to 8 h of culture.
DTH reaction.
Virus-specific delayed-type hypersensitivity (DTH) was determined as local swelling after subcutaneous inoculation of virus in the footpad as described previously (39).
Biochemical and histological analysis of viral hepatitis and cytokine detection.
Liver injury was monitored by immunohistochemical analysis and measurement of serum alanine aminotransferase (sALT) and serum aspartate aminotransferase (sAST) activity following the manufacturer's protocol (Pointe Scientific Inc., MI). Liver tissues harvested from infected mice were embedded in optimum cutting temperature compound, snap-frozen in a dry ice-2-methyl-butane bath, sectioned, air dried, and fixed in 10% acetone. Sections from each tissue specimen were stained with hematoxylin and eosin and subjected to gross and microscopic pathological analyses.
RESULTS
Cbl-b ubiquitin ligase regulates virus-specific CD8+ T-cell expansion and antiviral function during acute and chronic infections.
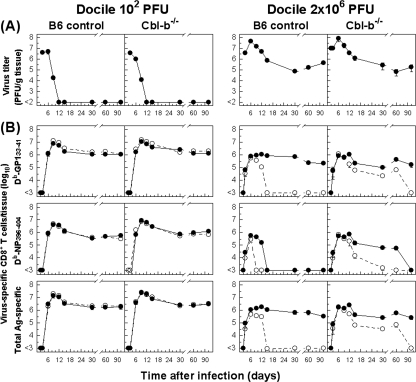
To examine the relevance of Cbl-b in regulating virus-specific immune responses, we asked whether elimination of the Cbl-b activity would affect the course of an acute or chronic infection and the kinetics of a virus-specific CD8+ T-cell response. Cbl-b−/− and B6 mice were injected with a low or high dose of LCMV Docile (102 or 2 × 106 PFU), known to cause acute or chronic infection in adult mice, and T-cell response and viral control were monitored (Fig. 1). As expected, infection of B6 mice with 102 PFU of Docile induced a robust CD8+ T-cell response primarily directed to the dominant Db/GP133-41 and Db/NP396-404 epitopes (accounting for almost 50% of the expanded CD8+ T-cell population), which resulted in rapid virus control from the spleen and other tissues, with viral titers declining below detectable levels by day 9 p.i. (Fig. 1). The ensuing contraction phase, after virus elimination, shown by numerical reduction of antigen-specific CD8+ T cells, was followed by the memory phase, during which epitope-specific CD8+ T cells persisted at stable levels while retaining a functional phenotype. In marked contrast, virus-specific CD8+ T cells became exhausted during persistent infection with Docile (2 × 106 PFU). This was illustrated by progressive functional inactivation (e.g., loss of IFN-γ production) and physical elimination that differentially affected virus-specific CD8+ T-cell populations (65). Double staining of CD8+ T cells for IFN-γ and tetramers confirmed that the exhausted tetramer-positive cells are the same as those that do not produce IFN-γ (data not shown). When Cbl-b−/− mice were infected with a low dose of Docile, their reaction was similar to that of control B6 mice, as the Cbl-b−/− mice mounted a strong but slightly superior (three- to fivefold) CD8+ T-cell response as revealed by the higher peak in epitope-specific CD8+ T-cell population expansion and memory level; this resulted in rapid control of the infection with slightly accelerating kinetics. In contrast, when Cbl-b−/− mice were infected with a high dose of Docile, the initial expansion of virus-specific CD8+ T cells did not increase above the levels detected in naive infected B6 mice, but we observed a dramatic delay in their functional impairment (loss of IFN-γ production). Strikingly, the physical elimination of the population of NP396-404-epitope-specific CD8+ T cells in chronically infected Cbl-b−/− mice was also greatly delayed for several weeks. This transient relief in virus-specific CD8+ T-cell inhibition in the absence of Cbl-b function, however, did not result in full viral control, because virus persisted at high levels over the 90 days of this analysis. The delay in functional exhaustion of CD8+ T cells in Cbl-b−/− mice was also seen when we determined the ability of CD8+ T-cell populations to produce TNF-α or IL-2 in response to antigen stimulation, but the proportion of cells producing this cytokine was lower than the proportion of cells producing IFN-γ (data not shown). An illustration of the distinct pattern of expansion and function of GP133-41 or NP396-404 tetramer-positive CD8+ T-cell populations in relation to their capacity to produce IFN-γ in Cbl-b−/− and control B6 mice during the course of an acute infection or chronic infection is presented in Fig. 2. In other experiments, we found that a second challenge of primed (102 PFU of Docile) mice 70 days p.i. with a high dose (106 PFU) resulted in early proliferation of a virus-specific CD8+ T-cell population, but their proportional expansion kinetics for Cbl-b−/− and control B6 mice, measured in spleen, peripheral lymph nodes (PLN), liver, or bone marrow (BM), were indistinguishable (data not shown). Furthermore, loss of Cbl-b did not significantly affect the immunodominance hierarchy of the CD8+ T-cell response to acute LCMV infection. However, similar to the case with immunodominant CD8+ T-cell epitopes, Cbl-b−/− mice with chronic infection exhibited a delay in functional exhaustion of CD8+ T cells specific to subdominant epitopes (Kb/NP205-212 or Db/GP92-101) (data not shown).
FIG. 1.
Cbl-b inactivation delays virus-specific CD8+ T-cell exhaustion. (A) Cbl-b−/− or B6 mice were infected with 102 or 2 × 106 PFU of LCMV Docile, and virus titers in spleens were measured at the indicated times. Data shown are means ± standard errors of the means (SEM) of log10 PFU/g of tissue for five mice. (B) Parallel total numbers of antigen-specific CD8+ T cells (sums of GP133-41-specific and NP396-404-specific cells indicated as total antigen specific) or specific for GP133-41 or NP396-404 peptide were determined by staining with MHC-I tetramers (•). Virus-specific CD8+ T cells were tested for their ability to produce IFN-γ following stimulation of cells with peptide or with virus-infected DC2.4 cells (○). Data shown are means ± SEM of log10 virus-specific T cells per spleen for 5 to 10 mice.
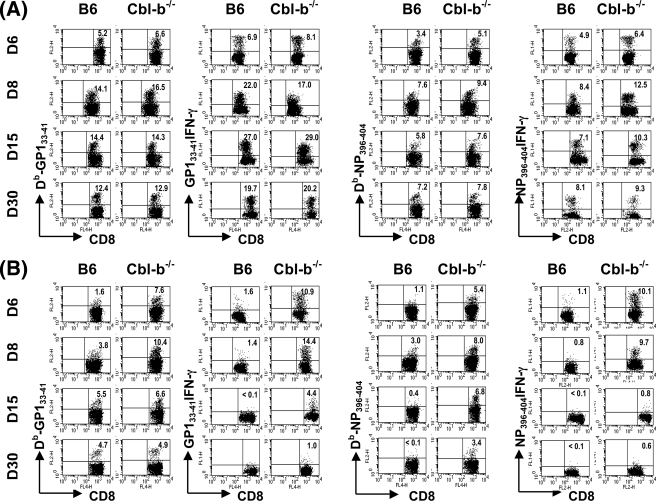
FIG. 2.
Profiles of virus-specific CD8+ T-cell expansion and functional inactivation during acute or chronic infection of Cbl-b−/− and control B6 mice. Cbl-b−/− or B6 mice were infected with 102 (A) or 2 × 106 (B) PFU of LCMV Docile, and the percentages of virus-specific CD8+ T cells in spleens at the indicated days was assessed by staining with Db/GP133-41 or Db/NP396-404 tetramers and antibody against CD8α. Virus-specific CD8+ T cells producing IFN-γ following stimulation with peptide were determined with concurrent analyses. Plots shown are gated on live cells. The percentages of CD8+ T cells staining positive for Db/GP133-41 or Db/NP396-404 tetramer or for IFN-γ are indicated in the upper corners of the corresponding panels. Results are representative of several separate experiments.
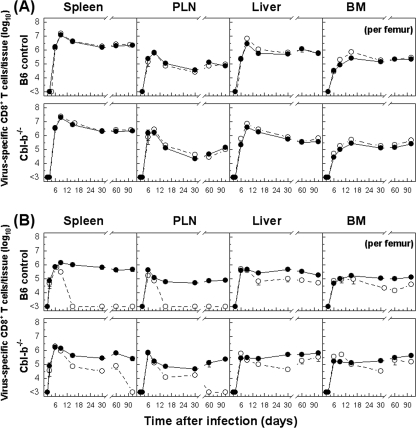
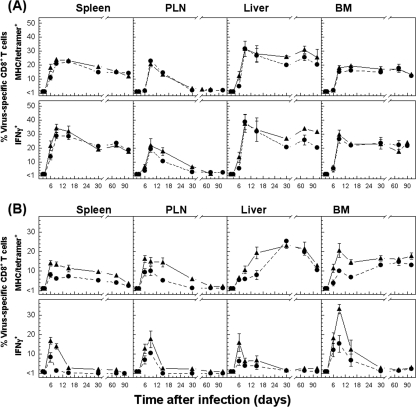
There was also a substantial expansion of the CD8+ T-cell population with antiviral functions in other tissues such as PLN, liver, and BM, but both acutely infected Cbl-b−/− mice and control B6 mice displayed comparable kinetics patterns for T-cell expansion and activation, similar to those observed for the spleen (Fig. 3). Furthermore, in mice with chronic infection, the initial expansion of virus-specific CD8+ T cells analyzed in different tissues (PLN, liver, and BM) was increased with similar kinetics, but in Cbl-b−/− mice, the diminished ability of antigen-specific T cells to produce cytokines (clearly seen in the PLN) was significantly inhibited, and the physical elimination of NP396-404-specific CD8+ T cells was delayed, as observed for the spleen (Fig. 3 and data not shown). To further examine whether virus-specific CD8+ T-cell populations preferentially accumulated or were retained in tissues of infected Cbl-b−/− and B6 mice, we evaluated the recruitment kinetics of specific CD8+ T cells by calculating the percentages of tetramer- and IFN-γ-positive T cells within the lymphocyte population in each tissue as a function of time (Fig. 4). Determined as percentages of CD8+ T cells, tetramer (sum of GP133-41- and NP396-404-specific cells) or IFN-γ (total virus-specific)-positive cells were more abundant during an acute infection than during a persistent infection. Unlike the acutely infected mice (102 PFU), for which the percentages of virus-specific CD8+ T cells were comparable, persistently infected Cbl-b−/− mice showed levels of functional virus-specific CD8+ T cells in different tissues that were profoundly higher than those of wild-type-infected mice. Thus, expression of E3 ligase Cbl-b is essential to virus-specific CD8+ T-cell exhaustion during chronic infection.
FIG. 3.
Kinetics of virus-specific CD8+ T-cell responses in tissues of Cbl-b−/− and B6 mice with acute or chronic infection. Cbl-b−/− or B6 mice were infected with 102 (A) or 2 × 106 (B) PFU of LCMV Docile, and total numbers of virus-specific CD8+ T cells (sums of GP133-41 and NP396-404 peptide-specific T cells) were determined by staining with Db/GP133-41 and Db/NP396-404 tetramers (•). The total numbers of virus-specific CD8+ T cells from tissues were tested for their ability to produce IFN-γ following short-term culture with virus-infected DC2.4 cells on the indicated days after infection (○). Data shown are mean ± SEM log10 virus-specific T cells per tissue for 5 to 10 mice.
FIG. 4.
Tissue-specific kinetics of virus-specific CD8+ T-cell responses expressed as percentages of CD8+ T cells (tetramer or IFN-γ positive) in Cbl-b−/− and B6 mice with acute or chronic infection. The percentages of total CD8+ T cells specific for GP133-41 and NP396-404 determined by tetramer staining in indicated tissues were calculated for acute (102 PFU) (panel A) or chronic (2 × 106 PFU) (panel B) infection with LCMV Docile of Cbl-b−/− (▴) or B6 (•) mice. In addition, the percentages of CD8+ T cells producing IFN-γ following short-term culture with virus-infected DC2.4 cells are indicated. Data shown are mean ± SEM log10 virus-specific T cells per tissue for 5 to 10 mice.
Role of Cbl-b in regulating virus-specific CD8+ CTL activity.
To better characterize virus-specific CD8+ T-cell populations during an acute or chronic LCMV infection of Cbl-b−/− mice or B6 controls, we compared the antiviral CTL activities of antigen-specific T cells in the spleen and liver. When we performed direct ex vivo CTL assays, we observed that antigen-specific T cells from acutely infected Cbl-b−/− mice exhibited a slightly enhanced ex vivo CTL activity, measured in virus-infected target cells at identical E:T ratios; this difference in CTL activity was more clearly seen on days 7 and 16 p.i. in both tissues (Fig. 5A). Interestingly, antigen-specific CD8+ T cells in spleens of Cbl-b−/− mice with chronic infection retained substantial cytolytic activity in the spleen assayed by days 7, 9, and 16 p.i. (Fig. 5B), whereas those from chronically infected B6 controls gradually lost their activity. This enhanced CTL activity in chronically infected Cbl-b−/− mice was more dramatic when we determined specific lysis after 18 h (Fig. 5B, lower panels). In livers of chronically infected mice, in which the T-cell exhaustion proceeds with delayed kinetics, we did not observe significant differences in CTL activity at days 7 and 9 after infection, although at day 16, virus-specific CD8+ T cells from Cbl-b−/− mice exhibited a slightly increased CTL activity. In addition, we found that, consistent with the improved CTL activity detected in infected Cbl-b−/− mice, the proportional contribution of granzyme B-positive cells within the Db/GP133-41 or Db/NP396-404 tetramer-positive CD8+ T-cell populations was consistently higher in Cbl-b−/− mice than in B6 mice with acute or chronic infection. Notably, intracellular granzyme B levels, expressed as mean fluorescence intensities, were in general elevated in virus-specific CD8+ T cells from mice with chronic infection compared to those from mice with acute infection. However, we determined that this expression was further up-regulated in antigen-specific CD8+ T cells from chronically infected Cbl-b−/− mice compared to B6 controls (data not shown).
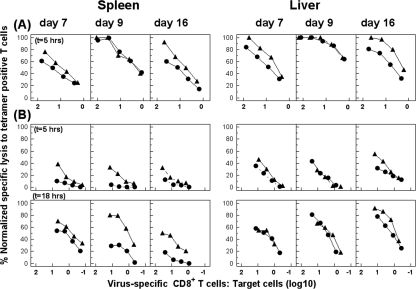
FIG. 5.
Enhanced CTL activity in Cbl-b−/− mice with acute or chronic infection. Cbl-b−/− (▴) or B6 (•) mice were infected with 102 (A) or 2 × 106 (B) PFU of LCMV Docile and lymphocytes isolated from tissues at the indicated times. Direct ex vivo CTL activity was measured on virus-infected MC57G targets at an E:T ratio of 100:1 to 1:1 and for an incubation period of 5 or 18 h (t) as indicated. The E:T cell values shown are corrected for the number of Db/GP133-41 and Db/NP396-404 tetramer-positive cells in the total virus-specific CTL population. Lysis of untreated target cells was usually ≤10% (5-h assay) or ≤20% (18-h assay) at the highest E:T ratio. However, in certain cases, nonspecific lysis exceeding the 10% or 20% levels was subtracted from corresponding lysis values. Results are representative of two separate experiments.
Effects of Cbl-b deficiency on the relative expression of Bcl-2 family members by virus-specific CD8+ T cells during chronic viral infection.
A role of Bcl-2 family members in survival of virus-specific CD8+ T-cell populations during chronic infection has been described previously (64). Therefore, we determined whether altered expression profiles of antiapoptotic Bcl-2 or Bcl-xL molecules may explain the delayed physical elimination of antigen-specific CD8+ T-cell populations, in particular, elimination of NP396-404-specific T cells, in Cbl-b−/− mice with chronic infection. As shown in Fig. 6, the levels of Bcl-2 found on GP133-41- or NP396-404-specific CD8+ T cells from acutely infected Cbl-b−/− or B6 mice decreased transiently around the time of the peak T-cell response (day 9) to levels lower than those in naive (CD8+-CD44low) T cells but increased later (day 16) to a level similar to or even slightly higher than that found in naive T cells. However, the Bcl-2 expression profiles for infected Cbl-b−/− mice and B6 controls were similar. In contrast, these changes were not observed for functionally unresponsive GP133-41- or NP396-404-specific CD8+ T cells from persistently infected mice (2 × 106 PFU), which had high Bcl-2 levels on days 9 and 16 p.i. Notably, the rapid elimination of NP396-404-specific CD8+ T cells by day 16 p.i. in chronically infected B6 mice precludes direct comparison in their Bcl-2 expression levels at this time point between the different genotypes. However, we determined that NP396-404-specific CD8+ T cells resistant to apoptotic death in Cbl-b−/− mice expressed remarkably high Bcl-2 levels. In addition, functionally deficient GP133-41-specific CD8+ T cells from infected Cbl-b−/− or B6 mice showed expression profiles of Bcl-xL comparable to those of Bcl-2. Interestingly, the Bcl-xL expression in NP396-404-specific CD8+ T cells remained elevated over the level of naive CD8+ T cells on day 9 after acute infection and further increased on day 16. In general, we consistently observed that NP396-404-specific CD8+ T cells expressed Bcl-xL levels much higher than those in GP133-41-specific T cells, but the expression profiles were similar for the two genotypes.
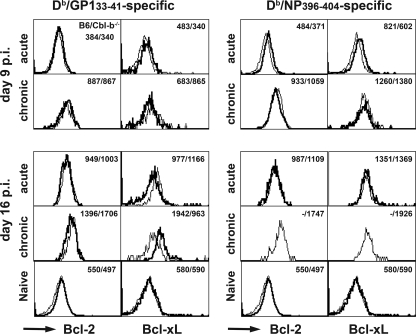
FIG. 6.
Bcl-2 or Bcl-xL expression in virus-specific CD8+ T-cell populations generated in Cbl-b−/− or B6 mice with acute or chronic infection. Spleen cells from Cbl-b−/− (thin lines) or B6 (bold lines) mice infected with 102 or 2 × 106 PFU of LCMV Docile 9 or 16 days prior were stained for intracellular Bcl-2 or Bcl-xL by using directly conjugated PE or allophycocyanin antibody after surface staining with anti-CD8α and Db/GP133-41 or Db/NP396-404 tetramers. Spleen cells from naive Cbl-b−/− or B6 mice were stained for CD8, CD44, and intracellular Bcl-2 or Bcl-xL. The histograms are gated on CD8 and Db/GP133-41 or Db/NP396-404 tetramer-binding T cells (infected) or CD8+-CD44low (naïve), and the numbers are the mean fluorescence intensities for Bcl-2 or Bcl-xL staining of B6 or Cbl-b−/− mice. Data are for two mice per group and are representative of two experiments.
Phenotypic analysis of antigen-specific CD8+ T cells during acute and chronic infections.
We next profiled the phenotypic changes in virus-specific CD8+ T-cell populations between infected Cbl-b−/− and B6 mice. At days 6, 9, 20, 30, and 50 p.i. (102 or 2 × 106 PFU), we did not observe any apparent differences in expression profiles of several activation markers, including CD11a, CD25 (IL-2 receptor alpha [IL2Rα]), CD122 (IL2Rβ), CD44, CD62L, and Ly-6C, on virus-specific CD8+ T-cell populations between the genotypes (65; data not shown). Since combined deficiency in Cbl-b and c-Cbl has been reported to impair antigen-induced down-modulation of TCRs in naive CD4 T cells in vitro (41), we also compared the surface expression levels of TCRs and CD8 molecules on virus-specific CD8+ T-cell populations during acute or chronic infection of Cbl-b−/− and B6 mice. At days 9 and 16 p.i., the expression profiles of TCRs and CD8 in antigen-specific CD8+ T cells were indistinguishable in both genotypes (data not shown). However, during acute infection, we recorded a marked decrease in CD8 expression levels on both gated lymphocyte populations (total) or on antigen-specific CD8+ T cells that was not observed in chronically infected mice. This suggests that differences in the steady-state expression profiles of TCRs or CD8 cannot explain the relative resistant of virus-specific CD8+ T cells to exhaustion in chronically infected Cbl-b−/− mice.
There is increasing evidence that several signaling pathways are associated with T-cell exhaustion during persistent viral infection. In particular, inducible or constitutive expression of inhibitory molecules by virus-specific T cells are critically involved in regulating not only T-cell expansion and function during an acute viral infection but also seem to be key to effective inhibition of their antiviral function during persistent infection. Thus, it remains possible that, although loss of Cbl-b significantly delays (but does not prevent) virus-specific CD8+ T-cell exhaustion, activation of other inhibitory pathways may compensate for the loss of Cbl-b-mediated control of antiviral T-cell activity in chronically infected mice. To test this possibility, we next determined the expression levels of known or potential inhibitory receptors PD-1, CTLA-4 (CD152), LAG-3 (CD223), 2B4 (CD244), and KLRG1 (data not shown). At day 9 p.i., expression of PD-1, LAG-3, and 2B4 on virus-specific CD8 T cells increased markedly over the basal level of naive CD8+ T cells after both infections but more strongly after chronic infection at this time point. However, by day 16 p.i., the expression of these receptors was low during acute infection, consistent with viral clearance. In contrast, during chronic infection PD-1, LAG-3, and 2B4 remained highly expressed on populations of both GP133-41- and NP396-404-specific CD8+ T cells. KLRG1 expression by CD8+ T cells was low and remained low in chronically infected mice compared to virus-specific CD8+ T cells generated after an acute infection. Overall, our study revealed similar expression profiles of these inhibitory molecules, except for CTLA-4, for both genotypes. It is interesting that CTLA-4 protein levels on antigen-specific CD8+ T cells generated during chronic infection increased more rapidly in Cbl-b−/− mice compared to those in B6 controls. Thus, at day 9 p.i., virus-specific CD8+ T cells from Cbl-b−/− mice expressed high CTLA-4 levels that remained high at the later time point (day 16); in contrast, CTLA-4 expression on antigen-specific CD8+ T cells from B6 mice was low at day 9 p.i. but increased by day 16 p.i. to similar steady-state levels in both genotypes. It is worthwhile to emphasize that although the biological significance of this early CTLA-4 expression in the regulation of CD8+ T-cell response during chronic infection is unclear, it is tempting to hypothesize that it represents a compensatory inhibitory mechanism in response to Cbl-b deficiency. Future studies are aimed at investigating the role of this early CTLA-4 expression in regulating CD8+ T cells during chronic infection. This study is guided also from data in the literature suggesting that expression of Cbl-b is necessary for the function of CTLA-4 (28).
Cbl-b expression on virus-specific CD8+ T cells defines the threshold of activation and functional impairment (exhaustion) during chronic infection.
In addition to regulating peripheral CD4+ and CD8+ T-cell signaling, Cbl-b plays roles in regulating the activation of B cells and other hematopoietic cells (e.g., mast cells) (20, 52, 55). To examine, therefore, the specific role of Cbl-b expression in virus-specific CD8+ T-cell exhaustion, we employed an adoptive transfer system with transgenic LCMV-specific CD8+ T cells (P14-Thy1.1) injected into virus-infected congenic B6-Thy1.2 recipient mice (Fig. 7A). An optimal number (103) of P14 cells sufficient or deficient in Cbl-b were transferred i.v. into C57BL/6 mice that subsequently were infected with a high (2 × 106 PFU) or low (102 PFU) dose of LCMV Docile. In addition, as a more stringent model for chronic infection, P14 cells were transferred into neonatal B6 carrier mice harboring very high viral titers in virtually all the organs. Both P14 cell populations (P14-Cbl-b+/+ or P14-Cbl-b−/−) transferred into acutely infected recipients proliferated vigorously in response to a low dose of infection and with similar kinetics, and the majority (60 to 80%) exhibited effector function (IFN-γ production). Interestingly, whereas the proportion of TNF-α expression in the P14-Cbl-b+/+ cell population on day 6 p.i. was relatively low, its expression was significantly increased in P14-Cbl-b−/− cells. However, this discrepancy in cytokine production was not sustained, and we found that both donor cell populations produced TNF-α with similar frequencies when analyzed at later time points after infection (data not shown). As expected, injection of P14-Cbl-b−/− or P14-Cbl-b+/+ cells into the chronically infected mice (2 × 106 PFU) resulted in their substantial proliferation and expansion, and the kinetics did not differ significantly between the donor cell populations. However, consistent with the delay in polyclonal CD8+ T-cell exhaustion observed for chronically infected Cbl-b−/− mice (presented in Fig. 1), P14-Cbl-b−/− cells exhibited an enhanced resistance to functional exhaustion (loss of IFN-γ production) compared to P14-Cbl-b+/+ cells as revealed by the delayed kinetics of their functional inactivation. Similarly, there was a significant delay in functional exhaustion of P14-Cbl-b−/− cells that were transferred into recipients with established persistent infection (neonatal carriers), but we did not find significant differences in the kinetics of physical elimination of both donor populations, which was completed by day 50 after cell transfer. The discrepancy in donor T-cell population survivals observed for adult and neonatal mice that were chronically infected was an anticipated outcome and is likely a reflection of the various levels of viral loads detected in tissues of the recipient mice (Fig. 7A, lower panels). We found that the proliferation of P14-Cbl-b−/− cells in vitro also occurred at a slightly lower level of cognate peptide (GP133-41) stimulation (Fig. 7B). To investigate this further, we used carboxyfluorescein diacetate ester (CFSE)-labeled P14-Cbl-b−/− or P14-Cbl-b+/+ (Thy1.1) cells transferred into chronically infected recipients (Thy1.2). Although the in vivo proliferation of P14-Cbl-b−/− cells occurs at slightly higher rates, the peak levels of both donor populations in the spleen and liver at day 4 after transfer were indistinguishable (Fig. 7C and D). However, P14-Cbl-b−/− cells were superior to P14-Cbl-b+/+ donor cells in their capacity to express antiviral function (IFN-γ production) following antigenic stimulation. This was apparent by the fact that although virtually the entire population of proliferating P14-Cbl-b−/− cells expressed an effector phenotype already on day 1 after transfer, the percentage of IFN-γ-positive P14-Cbl-b+/+ cells was low at this time point and increased with delayed kinetics during the course of infection (Fig. 7C). With these data taken together, we concluded that Cbl-b plays a critical role in direct regulation of virus-specific CD8+ T-cell functions during chronic infection by defining a threshold for TCR-induced activation and expression of antiviral functions.
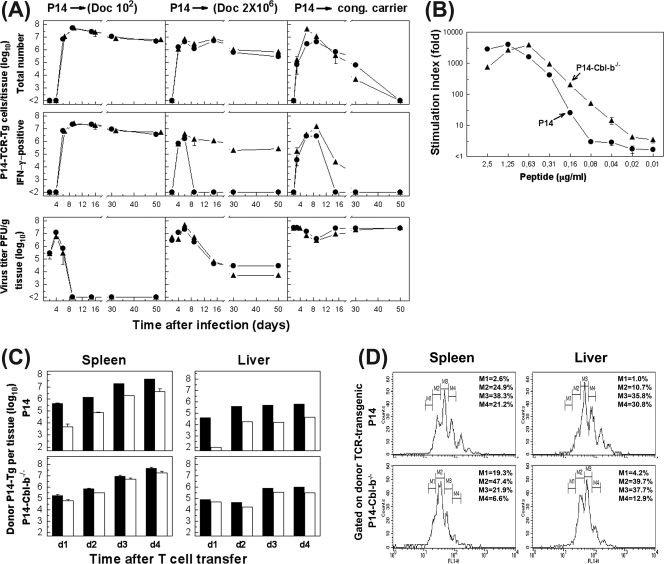
FIG. 7.
Cbl-b expression on virus-specific CD8+ T-cell populations regulates their functional impairment during chronic viral infection. (A) B6 mice (Thy1.2) were transfused with P14-Thy1.1 (•) or Cbl-b−/−-P14-Thy1.1 (▴) CD8+ TCR transgenic cells and subsequently infected with 102 (left panels) or 2 × 106 (middle panels) PFU of LCMV Docile (Doc). Alternatively, CD8+ TCR-transgenic cells were transferred into the neonatal virus carrier mice (cong. carrier; right panels). Expansion and functional properties (IFN-γ production) of donor cells in spleen were monitored for a period of 50 days after cell transfer. Virus-specific CD8+ TCR-transgenic cells (P14-TCR-Tg) were determined by triple staining with H-2Db tetramers, CD8, and Thy.1.1 antibody alone or combined with intracellular IFN-γ staining following stimulation of cells with GP133-41 peptide. Virus titers in the spleen of recipients were determined at the indicated times (lower panels). Data shown are mean ± SEM log10 virus-specific T cells per tissue for three to five mice. (B) P14-Thy1.1 (•) or Cbl-b−/−-P14-Thy1.1 (▴) cells (105/well) from spleens of naive mice were stimulated with the given concentration of GP133-41 peptide for 3 days. Proliferation was determined by incorporation of [3H]thymidine pulsed during the last 6 h of culture. Stimulation indices were calculated in relation to proliferation in medium control. (C) P14-Thy.1.1 or Cbl-b−/−-P14-Thy1.1 cells (5 × 106 cells) were labeled with CFSE and transferred into neonatal carriers. Expansion and functional properties (IFN-γ production) of donor cells in spleens or livers were monitored for a period of 4 days (d) after cell transfer. Columns indicate total numbers of donor transgenic cells determined by staining with Db/GP133-41 tetramer, CD8, and Thy.1.1 antibody (filled bars) or total numbers of IFN-γ-positive cells (open bars) following peptide stimulation in spleens and livers of recipients. Data shown are mean ± SEM log10 virus-specific T cells per tissue for three to five mice. (D) Representative proliferation profile of CFSE-labeled virus-specific donor CD8+ T cells in the spleen and liver analyzed on day 3 after cell transfer. The percentage of dividing cells based on the CFSE expression levels is indicated for each donor population. M1 through M4 indicate cell populations with discrete numbers of divisions.
Induction of Cbl-b is critically involved in T-cell exhaustion and prevention of lethal disease.
Immune cell exhaustion is a multistep process in which virus-specific T cells dependent on the viral load in the infected host progressively lose their antiviral capacity (cytokine secretion and cytotoxicity) before their eventual physical elimination. We next asked whether infection of Cbl-b−/− mice at various doses alters the outcome of infection (Fig. 8A). As predicted from our previous studies, naive B6 mice infected with different doses of Docile (102, 104, or 2 × 106 PFU) survived and did not exhibit any overt signs of illness. In striking contrast, ablation of Cbl-b altered dramatically the outcome of infection. When we infected Cbl-b−/− mice with a low dose, they behaved like the B6 controls, producing a strong CD8+ T-cell response that resolved the infection, and the mice remained healthy. In contrast, when Cbl-b−/− mice were infected with a moderate dose of infection (104 PFU), all mice died by day 8 p.i. Before death (the last 48 to 72 h), they developed a wasting disease with severe clinical signs, including hunched backs, ruffled coat, reduced mobility, and unresponsiveness, which are all characteristic symptoms of virus-specific T-cell-mediated pathology. Challenge of Cbl-b−/− mice with a high virus dose (2 × 106 PFU) that causes T-cell exhaustion and chronic infection dramatically reduced lethality but did not entirely prevent immune-mediated pathology. We regularly observed an increased morbidity in the several experiments performed, and this was associated with transient loss of body weight between days 6 and 20 and occasional death in some of the infected mice (20%). The requirement for CD8+ T cells in the lethal pathology was confirmed by the absence of signs of disease and lethality in Cbl-b−/− mice in which CD8+ T cells were depleted by antibody treatment (Fig. 8A, lower panels). To investigate the possibility that the increased morbidity of Cbl-b−/− mice is caused by the sustained levels of effector cytokines produced by persistent antigen-specific CD8+ T cells, we tested the effect of treating Cbl-b−/− mice infected with a moderate dose of Docile (104 PFU) with specific antibody capable of neutralizing TNF-α or IFN-γ in vivo. To mitigate potential confounding effects of TNF-α or IFN-γ neutralization in vivo on induction of antiviral T-cell response and perhaps on the magnitude of viral load in the onset of infection, we treated the mice on days 4 and 6 p.i., at a time period at which the induction of antiviral T cells is far advanced. In conclusion, this antibody treatment did not prevent the lethal pathology, and the cumulative mortality of mice was indistinguishable between anti-TNF-α or anti-IFN-γ-treated mice compared to animals without treatment or injected with isotype-control antibody. This suggests that the lethal organ pathology in infected Cbl-b−/− mice is not solely caused by sustained or overproduction of these inflammatory cytokines.
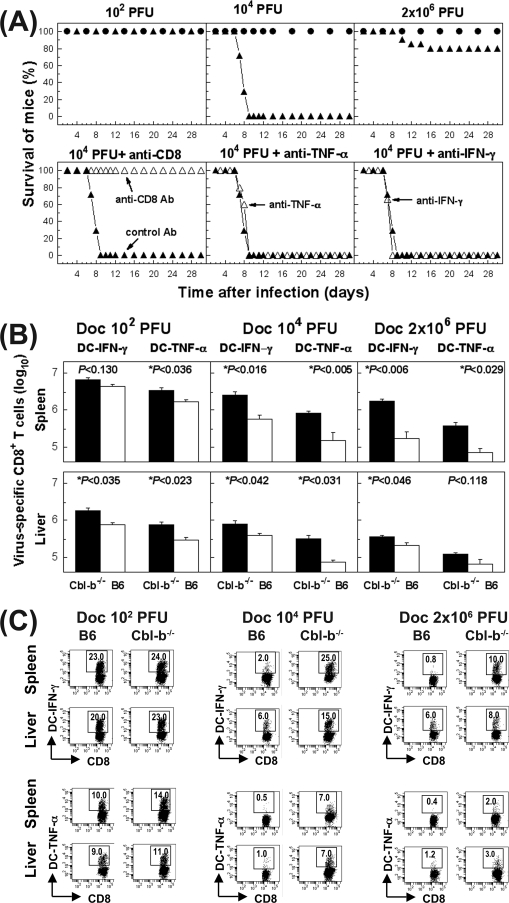
FIG. 8.
Dysregulated virus-specific CD8+ T-cell response in Cbl-b−/− mice causes lethal pathology. (A) The upper panels show results for B6 (•) or Cbl-b−/− (▴) mice infected with a low (102 PFU), moderate (104 PFU), or high (2 × 106 PFU) dose of LCMV Docile; these animals were monitored for survival. Data represent a cohort of 10 mice for each mouse genotype for two experiments. The lower panels show results for Cbl-b−/− mice infected with 104 PFU of LCMV Docile; the mice were either depleted of CD8+ T cells by antibody (Ab) treatment on day 5.5 p.i. or injected with anti-TNF-α or anti-IFN-γ (Δ). For a control, mice were injected with isotype control antibody on days 4 and 6 p.i. (▴). Data represent a cohort of three to five mice for each group. (B) Kinetics of virus-specific CD8+ T-cell responses and their functional properties (IFN-γ and TNF-α production) in Cbl-b−/− and control B6 mice that were infected with different doses (102, 104, or 2 × 106 PFU) of Docile (Doc). Total numbers of virus-specific CD8+ T cells on day 7 p.i. in the spleen or liver were determined by staining for IFN-γ (filled bars) or TNF-α (open bars) following stimulation with virus-infected DC2.4 cells (DC). Statistical significance is indicated by an asterisk and the P value indicated above the column. Data shown are mean ± SEM log10 virus-specific T cells per spleen for five mice. (C) Representative profile of virus-specific CD8+ T cells positive for IFN-γ or TNF-α in the spleen or liver on day 7 p.i. determined by intracellular staining following stimulation of cells with virus-infected DC2.4 cells (DC). The percentages of CD8+ T cells staining positive for IFN-γ or TNF-α are indicated in the upper corners of the corresponding panels.
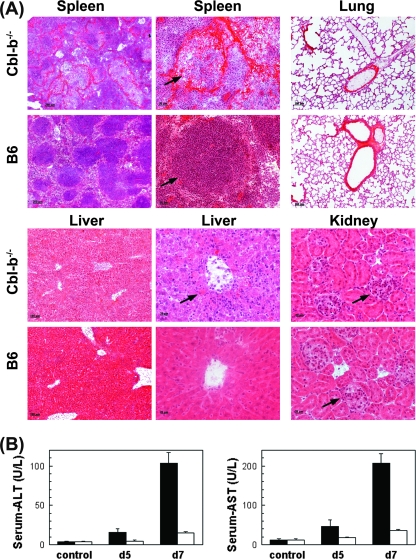
To further investigate the mechanisms responsible for the morbidity and lethality in infected Cbl-b−/− mice, we analyzed virus-specific CD8+ T-cell expansion under different virus challenge conditions (102, 104, or 2 × 106 PFU of Docile) (Fig. 8B). Cbl-b−/− and control B6 mice exhibited similar patterns of GP133-41- and NP396-404-specific CD8+ T-cell expansion as detected by MHC-I tetramers at days 5 and 7 p.i. There was a marked decrease in the number of Db/GP133-41 and, more profoundly, Db/NP396-404 tetramer-positive cells in mice infected with a moderate or high dose of infection in comparison to the number in mice infected with a low dose of infection, but in general, the levels of virus-specific CD8+ T cells detected were indistinguishable between Cbl-b−/− and wild-type mice (data not shown). Interestingly, whereas the majority of virus-specific CD8+ T cells in B6 mice infected with a moderate or high dose were functionally exhausted (loss of IFN-γ and TNF-α production), infected Cbl-b−/− mice produced fully functional virus-specific CD8+ T cells (Fig. 8B and C). These results suggest that Cbl-b is primarily operating under conditions of increased and sustained high levels of antigenic stimulation, and the inhibitory effects of Cbl-b expression in effector T-cell function are involved in regulating immune-mediated pathology. Since we observed a defect in down-regulation of cytokine production by virus-specific CD8+ T cells in Cbl-b−/− mice, we propose that dysregulated cytokine production may be a potential mediator of death, although not necessarily a crucial once, because anti-TNF-α or anti-IFN-γ treatment was not effective in preventing animal death. We also further investigated the virus-induced pathology in Cbl-b−/− mice by examining the pathological process in different tissues by histology (Fig. 9A). In particular, histological examination of organ sections from infected Cbl-b−/− (104 PFU) mice on day 7 p.i. (preterminal stage) indicated pathological changes in the liver and spleen, but no other tissues, such as lungs, intestines, kidneys, or brains. In the spleens of infected Cbl-b−/− mice, we observed extensive destruction of the lymphoid follicles, which is a characteristic symptom of virus-specific T-cell-mediated pathology, whereas infected B6 mice showed minimal pathological alterations in the spleen lymphoid structure. In the liver, inflammatory cells consisting mostly of lymphocytes were found distributed over the entire liver parenchyma and were associated with signs of extensive liver cell destruction. This was reflected by a drastic increase in transaminase activities in serum for AST or ALT (Fig. 9B). Development of inflammation was also observed, especially in the liver of B6 mice, but it was more focal, with prominent formation of periportal mononuclear and lymphocytic infiltrates, and the extent of cell destruction was significantly reduced compared to the case in mutant mice. Thus, histological examination revealed extensive pathology in virally infected Cbl-b−/− mice that was largely confined to the liver, with less involvement of other tissues.
FIG. 9.
Immune-mediated pathology in Cbl-b−/− mice infected with a moderate dose of LCMV Docile. (A) Cbl-b−/− or B6 mice were infected with 104 PFU of LCMV Docile, and spleen, lung, liver, and kidney tissues recovered on day 7 p.i. were fixed in acetone, sectioned, and stained with hematoxylin and eosin. For spleens and livers, low- and high-magnification images are presented. Arrows in the micrographs indicate intact and destroyed lymphoid follicles in B6 and Cbl-b−/− infected mice (for spleens), inflammatory reaction in Cbl-b−/− mice associated with extensive destruction of cells centered on portal tract in a hepatic lobus (for livers), and glomeruli with scattered mononuclear cell infiltrates in both infected Cbl-b−/− and B6 mice (for kidneys). (B) Liver-specific enzyme activity (sALT and sAST) in Cbl-b−/− (filled bars) or B6 (open bars) mouse sera collected on days 5 and 7 p.i. with 104 PFU of Docile was measured. Control serum values from uninfected mice are indicated (control). Data shown are mean ± SEM enzymatic activity levels (U/liter) for three to six individual mice.
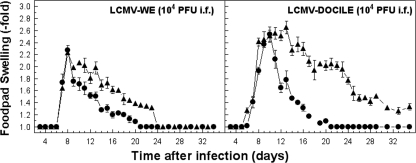
Finally, to investigate further the role of Cbl-b in regulating cytokine production in vivo, we used a classic model of virus-induced CD8+ and CD4+ T-cell-mediated inflammation, DTH, that was detected as footpad swelling following local virus inoculation (39). Although the initial phase of DTH reaction was indistinguishable between Cbl-b−/− and wild-type mice, the afferent plateau phase was substantially higher in Cbl-b−/− mice (Fig. 10). This finding suggests that Cbl-b expression is important for regulating stimulation of cytokine secretion at sites of virus infection and inflammatory reaction in the footpad.
FIG. 10.
Cbl-b ablation affects the kinetics of virus-induced DTH reaction. Results are virus-specific footpad swelling reactions in Cbl-b−/− (▴) or control B6 (•) mice following LCMV infection with 104 PFU of WE or Docile in the footpad. Virus-specific swelling reactions were monitored by measuring the increase in thickness of the infected foot compared to that of the uninfected foot (i.f.). Data points represent means ± SEM for five mice.
DISCUSSION
T-cell exhaustion is an effective way to preserve immunity in the face of chronic exposure of the host to a pathogen. Indeed, there is increasing evidence that T cells with impaired function represent a common feature of many chronic infections, and this inactivation represents an adaptive mechanism that is critical for preventing overt immune-mediated tissue damage but on the other hand is harmful to optimal antiviral immunity. The fact that T-cell impairment and deletion is not an all-or-nothing phenomenon, but rather the level of T-cell exhaustion not only in a given infection but also in a particular tissue compartment varies from partial loss of certain functional properties (e.g., cytokine secretion or cytotoxicity) or complete dysfunction to even physical elimination of virus-specific T-cell populations suggests that adaptation of T cells to chronic infection may involve alterations in multiple pathways. At the cellular level, critical factors that may affect the level of T-cell exhaustion include the level of antigen stimulation, as judged by the duration and magnitude of infection, and the availability of CD4+ T-cell help to promote an effective antiviral CD8+ T-cell response (9, 35, 36, 56). Indeed, epidemiological studies have revealed that in latent viral infections, in which the level of antigen exposure is low, virus-specific T cells with a functional phenotype are frequently detected at high frequencies (15, 23, 58). In contrast, in infections associated with high levels of replicating virus and thus high levels of antigen exposure, T cells deficient in a broad range of functions are frequently detected, and this is related to the chronic course of infection. At the molecular level, defects involved in the exhausted cells include the alteration of TCR signaling by inducible inhibitory cell surface receptors, ubiquitin ligases, or defects in cytokine signaling pathways as well as reversible or permanent modifications in DNA (e.g., gene methylation or histone acetylation) that are associated with silencing of effector T-cell functions. In this study, we have evaluated defects in exhausted T cells that involve actions of the E3 ligase Cbl-b. The data strongly suggest that Cbl-b-regulated virus-specific T-cell activation and function represent a critical regulatory checkpoint mechanism that ensures a balanced response to extracellular TCR signals and protects the host from the deleterious effects of chronic immune activation in persistent infections.
A critical issue raised by the data in this report is related to the molecular mechanisms that link E3 ubiquitin ligase Cbl-b with signaling pathways that, in a productive response, ensure a balanced response to antigen stimulation signals and protect host tissues from deleterious effects. We propose that, as is consistent with several previous studies with T-cell tolerance models, the functional deficit of virus-specific T cells in chronically LCMV-infected mice ensues as a consequence of the frequent and intense signal delivered simultaneously by several virus-infected antigen-presenting cells surrounding the same virus-specific CD8+ T cells. This can result in improperly balanced intracellular signals, leading to an antiviral T-cell deficit. Alternatively, the frequency of TCR engagement is too high; following stimulation, a refractory period when lymphocytes cannot be restimulated may exist (32), and additional signals delivered during this period could be inhibitory. It is well documented that selective silencing of virus-specific CD8+ T-cell functions, such as proliferation, cytokine production, cytotoxicity, or even physical elimination by programmed apoptotic death, are likely regulated by distinct signaling pathways. However, as T-cell exhaustion is a dynamic process in which T-cell functions are lost progressively in relation to antigenic load, alterations in several combined signaling pathways may be involved to increase the spectrum of functional defects. It is of interest to consider that in all infections, including the murine LCMV model, TCR engagement initially leads to activation and proliferation of antigen-specific T cells expressing a functional phenotype and that T-cell exhaustion is a consequence of chronic cell stimulation associated with persistence of the pathogen. This indicates that during an infection, there is proper antigen-specific T-cell stimulation and intact signaling for full expression of effector functions, a scenario that differs from the classical model of anergy induced when a T cell is stimulated via TCR in the absence of appropriate costimulation (16).
Regardless of the fate of activated virus-specific CD8+ T cells and the outcome of infection (acute or chronic), TCR-peptide-MHC and optimal costimulator receptor engagement signals T-cell activation and proliferation. In a productive T-cell response, the well-studied signaling program involving proximal and intermediate TCR signaling transducers and distal transcriptional modulators leads to induction of genes essential to CD8+ T-cell effector function. Central to this activation program is the induction of the calcium-calcineurin-nuclear factors of activated cells (NFAT) pathway, which induces expression of several cytokine genes and many other T-cell activation-induced proteins. We envisage that under conditions of chronic antigen stimulation, as suggested by current models of anergy, initiation and implementation of CD8+ T-cell exhaustion proceed through a complex multistep process that involves stimulation of the calcium-activated signaling pathway, perhaps in the absence or altered induction of other pathways, such as those involving Ras-mitogen-activated protein kinase, PKC, or inhibitor of NF-κB (IκB) kinases. This ultimately leads to unbalanced activation and nuclear localization of different sets of transcription factors that can direct transcription of alternate sets of genes responsible for the attenuation in TCR signaling that characterizes anergic T cells. At this point, the current models of T-cell anergy suggest that nuclear translocation of NFAT in the absence of AP1 and perhaps other transcriptional partners or of certain homo- or heterodimer complexes of NFAT proteins directs the transcriptional activation of these anergy-associated genes, which include not only those encoding E3 ligases but also those encoding tyrosine phosphatase, inhibitor cell receptors, proteins involved in intracellular trafficking, and transcriptional repressors (6, 11, 33). Thus, one consequence of sustained Ca2+-calcineurin signaling by continuous peptide-MHC-TCR engagement is up-regulation of the E3 ligases, including Cbl-b, Itch, GRAIL, and Tsg101, which participate in causing substantial decreases of activity levels of PLC-γ1 and PKC-θ, two key factors in the TCR signaling pathway. This can cause substantial delay and attenuation of TCR signaling and eventually leads to the inhibition of T-cell effector functions. Although we did not evaluate in this study which molecular signaling pathway(s) is altered in the exhausted T cells, the data that demonstrate diminished inhibition of antiviral function in the absence of Cbl-b are nevertheless compatible with the model described above as concerns the involvement of anergy-associated genes (e.g., the E3 ubiquitin ligase gene) in this process. Interestingly, under conditions of moderate antigen stimulation (transient viral persistence) that usually lead to generation of partially exhausted CD8+ T cells (impaired in production of cytokines such as IL-2, IFN-γ, and TNF-α, which is dependent on calcineurin phosphatase activity but not cytotoxicity), an alteration in nuclear NFAT2 translocation has been recently proposed for this selective defect (1). Although the underlying mechanism for this signaling impairment is not defined in the study described above, it is interesting to speculate that defective activation of NFAT2, perhaps in combination with other NFAT members whose function is altered, is responsible for the complete deficit in cytokine production and cytotoxicity by virus-specific CD8+ T cells at the preterminal stage of their exhaustion, as observed in mice harboring very high viral loads (e.g., infection of normal or CD4-deficient mice with 107 PFU of Docile) (38, 65). Consistent with this view, T cells doubly deficient in calcium-regulated NFATc (NFAT1 and NFAT2) remain capable of TCR-mediated activation but have impaired effector functions (such as reduced cytokine secretion, effector molecule expression, and allogeneic cytotoxicity) (45). In contrast, gene-targeted disruption of individual NFAT proteins showed only mild alterations in immune functions. A similar molecular scenario likely operates in exhausted virus-specific CD4+ T cells during a chronic infection. Finally, it should be noted that inhibitory immunoreceptors (e.g., PD-1 and CTLA-4) and cytokine receptor signals (e.g., IL-2R, IL-7R, and IL-15R) also play a key role in modulating T-cell functions during persistent viral infection. In particular, PD-1 expression on virus-specific T cells in chronic infections has been associated with T-cell exhaustion and disease progression (17, 34, 46). Furthermore, inhibition of the PD-1/PDL1 inhibitory pathway can enhance the function of partially exhausted CD8+ T cells (7). Similarly, antibody treatment against IL-10 or IL-10R can enhance antiviral functions and ameliorate the effects of T-cell exhaustion on virus control (14). Ultimately, the role of activating and inhibitory molecules and cytokine signaling in T-cell exhaustion requires further investigation at the cellular and biochemical levels because of the clinical implications of the results.
Another aspect in our study regards the manifestation of lethal organ pathology linked to the hyperactivation of virus-specific T cells in infected Cbl-b−/− mice. The lethal disease that developed in these mice may be attributed to cytokine toxicity by excessive cytokine production and destruction of liver cells by virus-specific cytotoxic CD8+ T cells. Although the contribution of these effector mechanisms in clinical outcome requires further investigation, our results suggest that the key factor for lethal outcome may be the lack of down-regulation of effector functions in response to increased viral load in Cbl-b−/− mice. It important to note that the pathological picture observed in our model study markedly differs from that observed in studies in which immune mice or TCR-transgenic mice were treated with peptides (25, 29). In the latter case, the death of mice within a few hours after peptide challenge is associated with development of characteristic symptoms of septic shock, in which the important role is played by bacterially induced cytokines, including TNF-α and IL-6. A massive release of mucin in the intestinal lumen and goblet cells and massive lung inflammation with edemas were the characteristic pathological changes observed in these studies but were not seen in our analysis. On the basis of our data on antibody treatment in vivo, overproduction of TNF-α or IFN-γ in infected Cbl-b−/− mice cannot account for the organ pathology and animal death solely. However, these data do not discount the possibility that severe organ injury in Cbl-b deficiency is induced due to intense activation of multiple CD8+ T-cell effector mechanisms, including cytotoxicity and additional TNF-α or IFN-γ proinflammatory cytokine production. Thus, it is possible that early during infection with a dose (e.g., 104 PFU) that generates moderate viral loads producing conditions that do not drive rapid virus-specific T-cell exhaustion, signals from positive costimulatory pathways outweigh those from negative pathways. In this case, the inhibitory function of Cbl-b might be more critical to mitigate effects of organ injury induced by effector CD8+ T cells. However, later during chronic infection, the increased expression of other inhibitory pathways might gradually shift the balance toward inhibition of effector T-cell functions, and this may attenuate the immune-mediated pathology. This scenario may reconcile the persistence of virus-specific CD8+ T-cell functions with viral persistence.
In conclusion, the challenge for future studies is to define the complex network of altered signaling pathways that determines the different fates of virus-specific CD8+ T cells during chronic infection. As T cells, in the face of persistent antigen stimulation, initially develop the functional features of a productive immune response and later undergo a gradual loss of effector functions and eventually are eliminated, it is our hypothesis that T-cell adaptation to chronic infection is a dynamic process with sequential and coordinated participation of many key molecular players and signaling pathways that are responsible for establishing T-cell tolerance in various existing models. It is our expectation that the present study will provide a further step toward elucidating the underlying mechanisms of T-cell exhaustion in the context of a chronic viral infection and may also help to define measures to prevent physical deletion of virus-specific T cells during persistent viral infections, which is a critical aspect for developing immunotherapeutic strategies to curtail such infections. In this context, these findings are expected to have implications for immunotherapy of persistent infections aimed to increase antiviral effector mechanisms to promote viral control. Thus, transient inhibition of E3 ubiquitin ligase activity during established chronic infections may provide an amenable strategy to promote viral clearance by avoiding T-cell-mediated organ pathology.
Acknowledgments
This work was supported by NIH grant AI42114 to D.M.
We are grateful to Rhea-Beth Markowitz for critical reading of the manuscript.
Footnotes
Published ahead of print on 16 January 2008.
REFERENCES
- 1.Agnellini, P., P. Wolint, M. Rehr, J. Cahenzli, U. Karrer, and A. Oxenius. 2007. Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proc. Natl. Acad. Sci. USA 1044565-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcami, A., and U. H. Koszinowski. 2000. Viral mechanisms of immune evasion. Trends Microbiol. 8410-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anandasabapathy, N., G. S. Ford, D. Bloom, C. Holness, V. Paragas, C. Seroogy, H. Skrenta, M. Hollenhorst, C. G. Fathman, and L. Soares. 2003. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity 18535-547. [DOI] [PubMed] [Google Scholar]
- 4.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 19263-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmaier, K., C. Krawczyk, I. Kozieradzki, Y. Y. Kong, T. Sasaki, A. Oliveira-dos-Santos, S. Mariathasan, D. Bouchard, A. Wakeham, A. Itie, J. Le, P. S. Ohashi, I. Sarosi, H. Nishina, S. Lipkowitz, and J. M. Penninger. 2000. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 403211-216. [DOI] [PubMed] [Google Scholar]
- 6.Bandyopadhyay, S., N. Soto-Nieves, and F. Macian. 2007. Transcriptional regulation of T cell tolerance. Semin. Immunol. 19180-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439682-687. [DOI] [PubMed] [Google Scholar]
- 8.Battegay, M., S. Cooper, A. Althage, J. Banziger, H. Hengartner, and R. M. Zinkernagel. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods 33191-198. [DOI] [PubMed] [Google Scholar]
- 9.Battegay, M., D. Moskophidis, A. Rahemtulla, H. Hengartner, T. W. Mak, and R. M. Zinkernagel. 1994. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J. Virol. 684700-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertoletti, A., and A. J. Gehring. 2006. The immune response during hepatitis B virus infection. J. Gen. Virol. 871439-1449. [DOI] [PubMed] [Google Scholar]
- 11.Borde, M., R. A. Barrington, V. Heissmeyer, M. C. Carroll, and A. Rao. 2006. Transcriptional basis of lymphocyte tolerance. Immunol. Rev. 210105-119. [DOI] [PubMed] [Google Scholar]
- 12.Borrow, P., and G. M. Shaw. 1998. Cytotoxic T-lymphocyte escape viral variants: how important are they in viral evasion of immune clearance in vivo? Immunol. Rev. 16437-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen, D. G., and C. M. Walker. 2005. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 436946-952. [DOI] [PubMed] [Google Scholar]
- 14.Brooks, D. G., M. J. Trifilo, K. H. Edelmann, L. Teyton, D. B. McGavern, and M. B. Oldstone. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 121301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callan, M. F. 2003. The evolution of antigen-specific CD8+ T cell responses after natural primary infection of humans with Epstein-Barr virus. Viral Immunol. 163-16. [DOI] [PubMed] [Google Scholar]
- 16.Choi, S., and R. H. Schwartz. 2007. Molecular mechanisms for adaptive tolerance and other T cell anergy models. Semin. Immunol. [DOI] [PMC free article] [PubMed]
- 17.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443350-354. [DOI] [PubMed] [Google Scholar]
- 18.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, Jr., I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 7410249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 755550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustin, S. E., C. B. Thien, and W. Y. Langdon. 2006. Cbl-b is a negative regulator of inflammatory cytokines produced by IgE-activated mast cells. J. Immunol. 1775980-5989. [DOI] [PubMed] [Google Scholar]
- 21.Heissmeyer, V., F. Macian, S. H. Im, R. Varma, S. Feske, K. Venuprasad, H. Gu, Y. C. Liu, M. L. Dustin, and A. Rao. 2004. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 5255-265. [DOI] [PubMed] [Google Scholar]
- 22.Hilleman, M. R. 2002. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine 203068-3087. [DOI] [PubMed] [Google Scholar]
- 23.Holtappels, R., M. F. Pahl-Seibert, D. Thomas, and M. J. Reddehase. 2000. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J. Virol. 7411495-11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, F., Y. Kitaura, I. Jang, M. Naramura, H. H. Kole, L. Liu, H. Qin, M. S. Schlissel, and H. Gu. 2006. Establishment of the major compatibility complex-dependent development of CD4+ and CD8+ T cells by the Cbl family proteins. Immunity 25571-581. [DOI] [PubMed] [Google Scholar]
- 25.Jeon, M. S., A. Atfield, K. Venuprasad, C. Krawczyk, R. Sarao, C. Elly, C. Yang, S. Arya, K. Bachmaier, L. Su, D. Bouchard, R. Jones, M. Gronski, P. Ohashi, T. Wada, D. Bloom, C. G. Fathman, Y. C. Liu, and J. M. Penninger. 2004. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity 21167-177. [DOI] [PubMed] [Google Scholar]
- 26.Klenerman, P., and A. Hill. 2005. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 6873-879. [DOI] [PubMed] [Google Scholar]
- 27.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 1911499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, D., I. Gal, C. Vermes, M. L. Alegre, A. S. Chong, L. Chen, Q. Shao, V. Adarichev, X. Xu, T. Koreny, K. Mikecz, A. Finnegan, T. T. Glant, and J. Zhang. 2004. Cutting edge. Cbl-b: one of the key molecules tuning CD28- and CTLA-4-mediated T cell costimulation. J. Immunol. 1737135-7139. [DOI] [PubMed] [Google Scholar]
- 29.Liu, F., R. Feuer, D. E. Hassett, and J. L. Whitton. 2006. Peptide vaccination of mice immune to LCMV or vaccinia virus causes serious CD8 T cell-mediated, TNF-dependent immunopathology. J. Clin. Investig. 116465-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, Y. C., J. Penninger, and M. Karin. 2005. Immunity by ubiquitylation: a reversible process of modification. Nat. Rev. Immunol. 5941-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loeser, S., and J. M. Penninger. 2007. Regulation of peripheral T cell tolerance by the E3 ubiquitin ligase Cbl-b. Semin. Immunol. 19206-214. [DOI] [PubMed] [Google Scholar]
- 32.Lombardi, G., S. Sidhu, R. Batchelor, and R. Lechler. 1994. Anergic T cells as suppressor cells in vitro. Science 2641587-1589. [DOI] [PubMed] [Google Scholar]
- 33.Macian, F. 2005. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 5472-484. [DOI] [PubMed] [Google Scholar]
- 34.Maier, H., M. Isogawa, G. J. Freeman, and F. V. Chisari. 2007. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J. Immunol. 1782714-2720. [DOI] [PubMed] [Google Scholar]
- 35.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 688056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moskophidis, D., M. Battegay, M. van den Broek, E. Laine, U. Hoffmann-Rohrer, and R. M. Zinkernagel. 1995. Role of virus and host variables in virus persistence or immunopathological disease caused by a non-cytolytic virus. J. Gen. Virol. 76381-391. [DOI] [PubMed] [Google Scholar]
- 37.Moskophidis, D., S. P. Cobbold, H. Waldmann, and F. Lehmann-Grube. 1987. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J. Virol. 611867-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moskophidis, D., F. Lechner, H. Pircher, and R. M. Zinkernagel. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362758-761. [DOI] [PubMed] [Google Scholar]
- 39.Moskophidis, D., and F. Lehmann-Grube. 1989. Virus-induced delayed-type hypersensitivity reaction is sequentially mediated by CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA 863291-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller, D. L. 2004. E3 ubiquitin ligases as T cell anergy factors. Nat. Immunol. 5883-890. [DOI] [PubMed] [Google Scholar]
- 41.Naramura, M., I. K. Jang, H. Kole, F. Huang, D. Haines, and H. Gu. 2002. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat. Immunol. 31192-1199. [DOI] [PubMed] [Google Scholar]
- 42.Neumann-Haefelin, C., H. E. Blum, F. V. Chisari, and R. Thimme. 2005. T cell response in hepatitis C virus infection. J. Clin. Virol. 3275-85. [DOI] [PubMed] [Google Scholar]
- 43.Ou, R., S. Zhou, L. Huang, and D. Moskophidis. 2001. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 758407-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oxenius, A., R. M. Zinkernagel, and H. Hengartner. 1998. CD4+ T-cell induction and effector functions: a comparison of immunity against soluble antigens and viral infections. Adv. Immunol. 70313-367. [DOI] [PubMed] [Google Scholar]
- 45.Peng, S. L., A. J. Gerth, A. M. Ranger, and L. H. Glimcher. 2001. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity 1413-20. [DOI] [PubMed] [Google Scholar]
- 46.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2032281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfau, C. J., J. K. Valenti, D. C. Pevear, and K. D. Hunt. 1982. Lymphocytic choriomeningitis virus killer T cells are lethal only in weakly disseminated murine infections. J. Exp. Med. 15679-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pircher, H., K. Burki, R. Lang, H. Hengartner, and R. M. Zinkernagel. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature 342559-561. [DOI] [PubMed] [Google Scholar]
- 49.Pircher, H., D. Moskophidis, U. Rohrer, K. Burki, H. Hengartner, and R. M. Zinkernagel. 1990. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature 346629-633. [DOI] [PubMed] [Google Scholar]
- 50.Reignat, S., G. J. Webster, D. Brown, G. S. Ogg, A. King, S. L. Seneviratne, G. Dusheiko, R. Williams, M. K. Maini, and A. Bertoletti. 2002. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J. Exp. Med. 1951089-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schartner, J. M., C. G. Fathman, and C. M. Seroogy. 2007. Preservation of self: an overview of E3 ubiquitin ligases and T cell tolerance. Semin. Immunol. 19188-196. [DOI] [PubMed] [Google Scholar]
- 52.Shao, Y., C. Yang, C. Elly, and Y. C. Liu. 2004. Differential regulation of the B cell receptor-mediated signaling by the E3 ubiquitin ligase Cbl. J. Biol. Chem. 27943646-43653. [DOI] [PubMed] [Google Scholar]
- 53.Sheehan, K. C., N. H. Ruddle, and R. D. Schreiber. 1989. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J. Immunol. 1423884-3893. [PubMed] [Google Scholar]
- 54.Shen, Z., G. Reznikoff, G. Dranoff, and K. L. Rock. 1997. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 1582723-2730. [PubMed] [Google Scholar]
- 55.Sohn, H. W., H. Gu, and S. K. Pierce. 2003. Cbl-b negatively regulates B cell antigen receptor signaling in mature B cells through ubiquitination of the tyrosine kinase Syk. J. Exp. Med. 1971511-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomsen, A. R., A. Nansen, S. O. Andreasen, D. Wodarz, and J. P. Christensen. 2000. Host factors influencing viral persistence. Philos. Trans. R. Soc. Lond. B 3551031-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18861-926. [DOI] [PubMed] [Google Scholar]
- 58.van Leeuwen, E. M., I. J. ten Berge, and R. A. van Lier. 2007. Induction and maintenance of CD8+ T cells specific for persistent viruses. Adv. Exp. Med. Biol. 590121-137. [DOI] [PubMed] [Google Scholar]
- 59.Vossen, M. T., E. M. Westerhout, C. Soderberg-Naucler, and E. J. Wiertz. 2002. Viral immune evasion: a masterpiece of evolution. Immunogenetics 54527-542. [DOI] [PubMed] [Google Scholar]
- 60.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 785535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wieland, S. F., and F. V. Chisari. 2005. Stealth and cunning: hepatitis B and hepatitis C viruses. J. Virol. 799369-9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao, Y., X. Dong, and Y. H. Chen. 2002. Neutralizing antibodies mechanism of neutralization and protective activity against HIV-1. Immunol. Res. 25193-200. [DOI] [PubMed] [Google Scholar]
- 63.Xu, X. N., G. R. Screaton, and A. J. McMichael. 2001. Virus infections: escape, resistance, and counterattack. Immunity 15867-870. [DOI] [PubMed] [Google Scholar]
- 64.Zhou, S., R. Ou, L. Huang, and D. Moskophidis. 2002. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection. J. Virol. 76829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou, S., R. Ou, L. Huang, G. E. Price, and D. Moskophidis. 2004. Differential tissue-specific regulation of antiviral CD8+ T-cell immune responses during chronic viral infection. J. Virol. 783578-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]