Abstract
Epstein-Barr virus (EBV)-associated, undifferentiated type of nasopharyngeal carcinoma (NPC) is characterized by intensive leukocyte infiltration. Interaction between the infiltrating cells and the tumor cells has been considered crucial for NPC development. Recruitment of the infiltrates can be directed by certain chemokines present in the NPC tissues. It is unknown whether and how EBV lytic infection regulates expression of the chemokines. Using an antibody array, we first found that several chemokines secreted from EBV-infected NPC cells are increased upon EBV reactivation into the lytic cycle, and interleukin-8 (IL-8) is the chemokine upregulated most significantly and consistently. Further studies showed that the EBV lytic transactivator Zta is a potent inducer of IL-8 in NPC cells, augmenting secreted and intracellular IL-8 proteins, as well as IL-8 RNA. Zta upregulates Egr-1, a cellular transcription factor that has been involved in upregulation of IL-8, but the Zta-induced IL-8 expression is independent of Egr-1. The ability of Zta to transactivate the IL-8 promoter is important for the induction of IL-8, and we have identified two Zta-responsive elements in the promoter. Zta can bind to these two elements in vitro and can also be recruited to the IL-8 promoter in vivo. DNA-binding-defective Zta mutants can neither activate the IL-8 promoter nor induce IL-8 production. In addition, Zta-expressing NPC cells exert enhanced chemotactic activity that is mainly mediated by IL-8. Since IL-8 may contribute to not only leukocyte infiltration but also multiple oncogenic processes, the present study provides a potential link between EBV lytic infection and pathogenesis of NPC.
Recent studies have recognized that a chronic inflammatory microenvironment can be an incubator for cancer development (2, 51). The local inflammation with recurrent destruction-reconstruction of tissues results in frequent DNA damage and the accumulation of genomic aberrations, which facilitates the initiation of tumor cells. In addition, a complex network of inflammatory mediators, produced by infiltrating immune cells and cancer or precancer cells, may promote the growth, survival, angiogenesis, invasion, and metastasis of tumors. Among the inflammatory mediators, several cytokines and chemokines, such as tumor necrosis factor, interleukin-1 (IL-1), IL-6 and IL-8, have been documented for their potent roles in tumorigenesis (2, 5).
Undifferentiated carcinoma, the most frequent histological type of nasopharyngeal carcinoma (NPC) in areas of endemicity, is closely associated with Epstein-Barr virus (EBV) infection (61). Notably, this type of NPC exhibits several inflammation-like features in the tumor tissues, including intensive leukocyte infiltration, abundant expression of inflammatory cytokines, and constitutive activation of inflammation-associated transcription factors (16, 35, 42). In the inflammation-like microenvironment, the interaction between infiltrating immune cells and tumor cells may be crucial for the development of NPC. The interaction can be mediated by several inflammatory chemokines or cytokines (42, 70). Another way of the interaction may involve cell contact through ligand-receptor binding (1). For example, tumor-infiltrating T cells may provide a survival signal to NPC cells through CD40-CD40 ligand interaction, preventing the tumor cells from CD95-triggered apoptosis (63). In addition, a clinical report has correlated the highly intratumoral infiltration of certain T cells with poor prognosis of NPC, supporting an impact of the immune infiltrates on NPC progression (57).
Being an initial step to establish the inflammation-like microenvironment of NPC, recruitment of infiltrating immune cells can be directed by certain chemotactic factors. Expression of several chemokines has been demonstrated in NPC tumors, including IL-8, macrophage inflammatory proteins (MIPs), macrophage chemoattractant proteins (MCPs), and RANTES (11, 70, 75). Considering that EBV may promote chemokine production in B lymphocytes (49), an issue is raised as to how EBV infection of the epithelial tumor cells contributes to the production of chemokines and the recruitment of leukocytes in NPC. Previous studies have focused on the effects of EBV latent infection and revealed that viral latent membrane protein 1 (LMP1) is a chemokine inducer. LMP1 can upregulate IL-8, RANTES, and MCP-1 in epithelial cells, mainly through a NF-κB-mediated mechanism (11, 22, 75). Since LMP1 protein is not always detected in NPC biopsies (23, 76), it is worth examining whether other EBV gene products may be also involved in the regulation of chemokines.
Several clues have indicated that EBV reactivation into the lytic cycle plays certain roles in development of NPC. Elevated antibody titers against EBV lytic antigens, representing EBV reactivation in vivo, correlate with advanced cancer stages, poor prognosis, or tumor recurrence of NPC (21, 34). The serologic marker of EBV reactivation also serves as a risk factor of NPC (17). In addition, the EBV lytic cycle can be induced in vitro by extracts of some foodstuffs or plants that have been associated with a high incidence of NPC (9, 66). Although EBV infection is predominantly latent in NPC tumors, a small subset of the tumor cells may exhibit sporadic lytic EBV infection (20, 55, 59). There is still a question as to how the lytically infected cells exert their contribution to the whole NPC tumors.
During EBV reactivation, Zta and Rta are the two immediate-early transactivators that essentially induce the expression cascade of downstream lytic genes (24). The ectopic expression of either Zta or Rta has been shown to sufficiently initiate the EBV lytic cycle (24, 32, 60). Through binding to the Zta-responsive element (ZRE) and the Rta-responsive element (RRE) in the target promoters, respectively, Zta and Rta can stimulate the transcription of many EBV lytic genes (25, 38, 47, 58). Apart from the viral genes, Zta and Rta can regulate several cellular genes whose promoters contain ZRE and RRE, respectively, suggesting that certain cellular activities may be affected by the EBV lytic proteins (13, 26, 37, 45, 54). Notably, previous studies have shown that Zta and Rta can augment production of inflammatory cytokines such as IL-6 and IL-10, implying an association of the EBV lytic cycle with inflammatory events (46, 54).
The present study aims to explore whether and how EBV lytic infection regulates chemokine expression in NPC cells. Using an antibody array, we first found that several chemokines secreted from EBV-infected NPC cells are increased upon EBV reactivation into the lytic cycle, and IL-8 is the chemokine upregulated most significantly and consistently. Further studies indicated that Zta is a potent transactivator of the IL-8 gene. Two Zta-responsive sites reside within the IL-8 promoter, and Zta can bind to the promoter both in vitro and in vivo. In addition, Zta-expressing NPC cells exert enhanced chemotactic activity that is mainly mediated by IL-8. Considering that IL-8 may contribute to not only leukocyte infiltration but also multiple oncogenic processes such as tumor growth, angiogenesis, and metastasis, we describe here a potential link between EBV lytic infection and tumorigenesis of NPC.
MATERIALS AND METHODS
Cell lines.
NPC-TW01 and HONE-1 are two EBV-negative NPC cell lines. The EBV-converted cell line, NA, was established by in vitro infection of NPC-TW01 cells with the recombinant Akata EBV (14). The tetracycline-inducible, Zta-expressing cell clone, HONE-tetonZ, was generated from HONE-1 cells in a previous study (52). All of the cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (HyClone) at 37°C with 5% CO2. For those studies examining chemokine secretion from the cell lines, cells were cultured in serum-free medium for up to 48 h before the culture supernatants were collected.
Induction of EBV reactivation or Zta expression.
For induction of the lytic cycle, EBV-infected NA cells were activated with 12-O-tetradecanoylphorbol-13-acetate (TPA; 40 ng/ml) and sodium n-butyrate (3 mM) for 36 h. For induction of Zta expression in the tetracycline-inducible system, HONE-tetonZ cells were treated with doxycycline (1 μg/ml) for 36 h. All of the inducing chemicals were purchased from Sigma.
Plasmids.
The simian virus 40 promoter-driven, Zta- or Rta-expressing plasmids have been described in our previous study (13). Plasmids expressing Zta with deletion of amino acids 27 to 53 (d27/53) or amino acids 52 to 78 (d52/78), or with mutations within the DNA-binding domain, have also been used previously (27, 28). A series of pIL-8-Luc, the reporter plasmids with deletion or site-directed mutation of the IL-8 promoter, were constructed by inserting PCR-amplified IL-8 promoter fragments into pGL2-basic vector (Promega) at the 5′ KpnI site and the 3′ NheI site. (The inserted promoter fragments or mutated sites are illustrated in Fig. 6.) All of the plasmids were purified by using a DNA-midi kit (iNtRON Biotechnology).
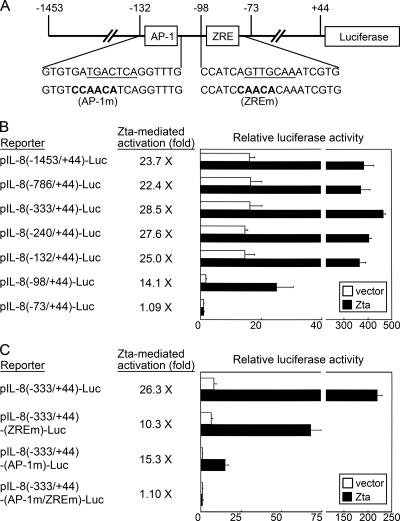
FIG. 6.
The IL-8 promoter contains two responsive sites for Zta-induced activation. (A) The IL-8 promoter used in the reporter gene assay is illustrated. The DNA sequences of AP-1 and ZRE sites in the promoter are shown underlined. Mutations introduced into these two sites (AP-1m and ZREm) are also indicated. (B) NPC-TW01 cells were cotransfected with the indicated reporter plasmids (a series of pIL-8-Luc) and the effecter plasmids (vector plasmid or a Zta-expressing plasmid). At 36 h posttransfection, the luciferase assay was performed. The Zta-induced fold activation for each reporter (luciferase activity for the Zta transfectant versus that for its vector control) is provided. Shown is a representative result from three independent experiments. (C) A similar reporter gene assay was performed by using the pIL-8(−333/+44)-Luc-derived plasmids carrying mutations at ZRE and/or AP-1 sites. The experimental procedures and the way of data presentation are the same as that in panel B.
Transfection with plasmid DNA or siRNA.
When overnight-cultured cells in six-well plates were 90% confluent, transfection was performed by using Lipofectamine 2000 reagent (Invitrogen). For each well, 10 μl of Lipofectamine 2000 was mixed with 4 μg of DNA or 500 pmol small interfering RNA (siRNA) and then applied to the cells according to the manufacturer's instructions. After 4 h of incubation, the cells were washed and cultured in serum-free medium for further experiments. The Egr-1-targeted siRNA (5′-AGCAAAUUUCAAUUGUCCUGGGAGA-3′) and a control siRNA with comparable GC content were purchased from Invitrogen.
Chemokine antibody array and ELISA.
The serum-free culture supernatants from target cells were collected, and cell debris was removed by centrifugation. Expression profiles of chemokines in the supernatants were analyzed by using human chemokine antibody array (RayBiotech) according to its protocol. To quantify the secreted IL-8, the culture supernatants were subjected to enzyme-linked immunosorbent assay (ELISA) by using DuoSet ELISA development system (R&D systems) according to the manufacturer's instruction.
Immunoblotting assay.
Cells were lysed in the sample buffer (3% sodium dodecyl sulfate [SDS], 1.6 M urea, 4% β-mercaptoethanol), resolved in a SDS-10% polyacrylamide gel, and electrotransferred onto Hybond-C extra membranes (Amersham). The blots were preincubated with TBST (50 mM Tris-HCl [pH 7.4], 0.15 M NaCl, 0.05% Tween 20) containing 5% skim milk at room temperature for 1 h and then reacted with primary antibodies at 4°C overnight. After being washed with TBST, the blots were reacted with horseradish peroxidase-conjugated secondary antibodies (Amersham) at room temperature for 1 h. The blots were then washed with TBST and developed with Western Lightning chemiluminescence reagent (Perkin-Elmer). The primary antibodies used in this assay included 4F10 (anti-Zta), 467 (anti-Rta), 88A9 (anti-BMRF1), 6217 (anti-IL-8; R&D Systems), C-19 (anti-Egr-1; Santa Cruz Biotechnology), and C4 (anti-β-actin; Chemicon).
RT-PCR.
Total RNAs were extracted by using REzol C&T reagent (PROtech) according to the manufacturer's instructions. The RNAs were then subjected to reverse transcription (RT) reaction by using Superscript III reverse transcriptase (Invitrogen) and the RT primer oligo(dT)15 (Roche). The RT products were then examined for the expression of IL-8 and β-actin in the PCR analysis. The primers for IL-8 were 5′-CAGTTTTGCCAAGGAGTGCTAAAG-3′ (forward) and 5′-AACTTCTCCACAACCCTCTGCAC-3′ (reverse), with an annealing temperature of 50°C, and the PCR product is 208 bp. The primers for β-actin were 5′-GAGCACAGAGCCTCGCCTTT-3′ (forward) and 5′-AGATGGGCACAGTGTGGGTG-3′ (reverse), with an annealing temperature of 60°C, and the PCR product is 555 bp.
Reporter gene assay.
Cells cultured in six-well plates were cotransfected each well with 2 μg of reporter plasmid (with a luciferase gene driven by various IL-8 promoters) and 2 μg of effector plasmid (the Zta-expressing plasmid or the control vector). At 36 h posttransfection, the cells were harvested and subjected to the luciferase assay by using a Bright-Glo assay kit (Promega) according to the manufacturer's instructions. Each assay was carried out in duplicate, and the whole set of the experiments was performed three times independently. Representative results from these experiments are shown here.
EMSA.
Cell nuclei were harvested after incubation of cells with a hypotonic buffer (10 mM HEPES-KOH [pH 7.9], 10 mM KCl, 0.1 mM EDTA) and lysed in a lysis buffer (20 mM HEPES-KOH [pH 7.9], 0.4 M NaCl, 1 mM EDTA, and 10% glycerol, with protease inhibitors). The electrophoretic mobility shift assay (EMSA) probes were double-stranded oligonucleotides, 32P end labeled by using T4 polynucleotide kinase (New England Biolabs). Each 20-μl binding mixture contained 4 μg of nuclear extracts, 4 nM concentrations of labeled probes, 20 mM HEPES (pH 7.9), 50 mM KCl, 2.5 mM MgCl2, 0.5 mM EDTA, 1 mM dithiothreitol, 10% glycerol, and 50 ng of poly(dI-dC)/μl. In the indicated experiments, 400 nM unlabeled oligonucleotides were used for competition assay, and 0.6 μg of antibodies were added for supershift assay. The binding mixture was incubated at 37°C for 30 min and then electrophoresed in a 6% native polyacrylamide gel with 0.5× Tris-borate-EDTA buffer. The gel was dried and exposed on X-ray films. The DNA sequences of oligonucleotides used as EMSA probes or competitors are shown in Fig. 7A.
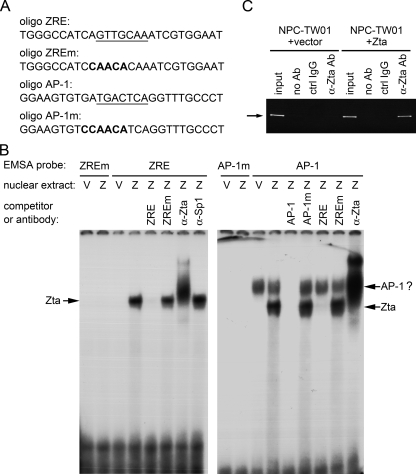
FIG. 7.
Zta binds to the IL-8 promoter both in vitro and in vivo. (A) DNA sequences of the probes and the competitors used in the EMSA study are provided. Oligonucleotide (oligo) ZRE represents the IL-8 promoter region −102 to −76, and oligonucleotide AP-1 represents the region −136 to −110 of the IL-8 promoter. The AP-1 and ZRE sites in the promoter are shown underlined. (B) Nuclear extracts from NPC-TW01 cells transfected with the vector plasmid (V) or a Zta-expressing plasmid (Z) were examined in the EMSA study. The DNA probes, competitors, and antibodies used in the experiment are indicated. The shifted bands representing DNA-binding of Zta or possible AP-1 transcription factors are indicated by arrows. (C) NPC-TW01 cells transfected with the vector plasmid or a Zta-expressing plasmid were subjected to a ChIP assay for detection of Zta's binding to the IL-8 promoter in vivo. Zta proteins were immunoprecipitated by an anti-Zta antibody, and the presence of the IL-8 promoter DNA in the precipitants was detected by nested PCR. The expected size of PCR products is indicated by an arrow. PCR detection of the IL-8 promoter DNA from inputs was used as the positive control. Immunoprecipitation without antibody (no Ab) or using a control immunoglobulin G antibody (ctrl IgG) was included as the negative control.
Chromatin immunoprecipitation (ChIP) assay.
Cells were treated with 1% formaldehyde at 37°C for 10 min, washed three times with ice-cold phosphate-buffered saline, and lysed in a lysis buffer (1% SDS, 10 mM EDTA, and 50 mM Tris-HCl [pH 8.0], with protease inhibitors). The cell lysates were sonicated and 10-fold diluted in a dilution buffer (0.01% SDS, 1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl [pH 8.0], 150 mM NaCl, 2 mM dithiothreitol, and 200 μg of salmon sperm DNA/ml, with protease inhibitors). The lysates were reacted with an anti-Zta antibody (AZ-69; Argene) or a control mouse immunoglobulin (R&D Systems) at 4°C overnight, followed by incubation with salmon sperm DNA and protein G-agarose beads (Upstate) at 4°C for 1 h. The beads were then washed four times with low-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8.0], 150 mM NaCl), four times with high-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8.0], 500 mM NaCl), four times with LiCl washing buffer (0.25 M LiCl, 1% NP-40, 1% sodium deoxycholate, 1 mM EDTA, 10 mM Tris-HCl [pH 8.0]), and twice with TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]). The immunoprecipitated complexes were eluted with elution buffer (1% SDS, 0.1 M NaHCO3), treated with 5 M NaCl at 65°C for 4 h, and digested by using proteinase K. The released DNA fragments were purified by phenol-chloroform extraction and then subjected to PCR detection of the IL-8 promoter. The first round of PCR was performed as 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min, using the primers 5′-GGGGTACCAAATTGTGGAGCTTCAGT-3′ (forward) and 5′-GGGCTAGCTTGTGTGCTCTGCTGTCT-3′ (reverse). The nested PCR was performed as 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min, using the primers 5′-GGGGTACCGTGTGATGACTCAGGTTTGCCCTGAG-3′ (forward) and 5′-GGGCTAGCTTGTGTGCTCTGCTGTCT-3′ (reverse). The product of nested PCR is 176 bp. The PCR primers were also used for the construction of the pIL-8-Luc plasmids mentioned above, so every primer contains a short 5′ adapter sequence (underlined).
Chemotaxis assay.
Human whole blood from healthy donors was provided from Taiwan Blood Service Foundation (Tainan Blood Center). Blood cells were separated by using Ficoll-Paque Plus density centrifugation (GE Healthcare). Granulocytes above the bottommost erythrocyte layer were collected, and contaminating erythrocytes were lysed with BD PharmLyse (Becton Dickinson). The obtained cellular fraction was high in granulosity, as confirmed by forward and side scatter on flow cytometry. Transwells (6.5-mm diameter and 5-μm pore size; Corning) were used in 24-well plates for the chemotaxis assay. Cell culture supernatants (600 μl/well) were applied to the lower chambers of transwells, while purified granulocytes (106 cells/well) were added to the upper chambers and spun onto the porous membrane at 1,200 rpm for 3 min. After incubation at 37°C with 5% CO2 for 30 min, the number of granulocytes migrating into the lower chambers was determined by using a CyQuant NF cell proliferation kit (Invitrogen). In the indicated experiments, neutralizing antibodies (purchased from R&D systems) against IL-8, MIP-1β, or growth-regulated oncogene (GRO) were added to the cell culture supernatants at a final concentration 1 μg/ml. The data are expressed as chemotaxis index, representing x-fold chemoattraction compared to the culture supernatants from vector control cells. All assays were carried out in duplicate.
RESULTS
Several chemokines are upregulated upon EBV reactivation in NPC cells.
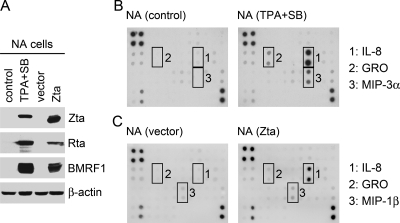
First, we analyzed chemokine expression profiles of an EBV-infected NPC cell line, NA, before and after induction of the EBV lytic cycle. Two approaches were utilized to trigger EBV reactivation. One was treatment with TPA and sodium n-butyrate, and the other was transfection with a plasmid expressing the EBV lytic transactivator Zta. Figure 1A shows that both ways efficiently induced expression of EBV lytic genes such as Zta, Rta, and BMRF1. The cell culture supernatants were then subjected to chemokine analysis by using an antibody-based array. Among the 38 chemokines that were examined, IL-8, GRO, and MIP-3α were increased from chemically activated NA cells compared to the chemokine secretion from untreated cells (Fig. 1B). Zta-transfected NA cells also secreted more IL-8 than the vector-transfected cells, while secretion of GRO and MIP-1β was just slightly increased from the Zta-expressing cells (Fig. 1C). Therefore, among the chemokines upregulated upon EBV reactivation in NA cells, the induction of IL-8 is associated with EBV lytic infection most significantly and consistently.
FIG. 1.
Several chemokines are upregulated upon EBV reactivation in NPC cells. (A) The EBV lytic cycle was induced by either treatment with TPA and sodium butyrate (TPA+SB) or transfection with a Zta-expressing plasmid. Meanwhile untreated cells (control) or vector plasmid-transfected cells were used as controls. Expression of EBV lytic proteins (Zta, Rta, and BMRF1) and cellular β-actin was examined in an immunoblotting assay. (B) Culture supernatants from untreated (control) or chemically activated (TPA+SB) NA cells were subjected to analysis of chemokine expression profiles by using an antibody array. The upregulated chemokines (IL-8, GRO, and MIP-3α) are indicated. (C) Culture supernatants from the NA cells transfected with vector plasmid or Zta-expressing plasmid were analyzed for their chemokine expression profiles. The upregulated chemokines (IL-8, GRO, and MIP-1β) are indicated.
Several chemokines are upregulated by EBV Zta or Rta in NPC cells.
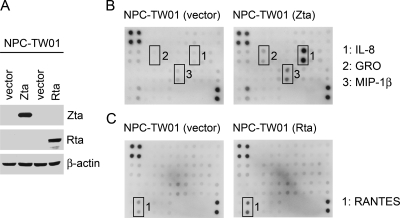
Zta and Rta are potent transcriptional activators during EBV reactvation, regulating not only viral lytic genes but also certain cellular genes. Next, we tested whether Zta or Rta alone can affect chemokine expression in an EBV-negative NPC cell line, NPC-TW01 (Fig. 2A). Ectopic expression of Zta prominently induced IL-8 and also upregulated GRO and MIP-1β at a lower level (Fig. 2B). On the other hand, Rta had little effect on the chemokine expression, except that it slightly increased the secretion of RANTES (Fig. 2C). Since IL-8 was the most upregulated chemokine from NPC cells consistently by either EBV reactivation (Fig. 1) or Zta expression (Fig. 2B), we focused on Zta-mediated induction of IL-8 in the following studies.
FIG. 2.
Several chemokines are upregulated by EBV Zta or Rta in NPC cells. (A) EBV-negative NPC-TW01 cells were transfected with the indicated plasmids (vector or plasmids expressing Zta or Rta), and the expression of Zta, Rta and β-actin was examined in an immunoblotting assay. The expression profiles of chemokines in the cell culture supernatants were analyzed by using an antibody array. Compared to the vector control experiments, the Zta-upregulated (B) and Rta-upregulated (C) chemokines are also indicated.
Zta induces IL-8 expression in NPC cells.
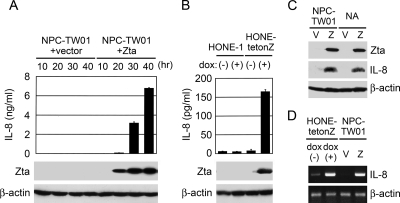
To confirm Zta-induced expression of IL-8, ELISA was performed to quantify IL-8 in the culture supernatants of NPC cells with or without Zta expression. Figure 3A shows time-dependent induction of IL-8 from NPC-TW01 cells transiently transfected with a Zta-expressing plasmid but not from the cells transfected with a vector plasmid. Using HONE-tetonZ, a tetracycline-inducible, Zta-expressing NPC cell line, we also detected increased secretion of IL-8 upon induction of Zta by doxycycline, while treatment of the parental cell line HONE-1 with doxycycline did not upregulate IL-8 (Fig. 3B). The intracellular levels of IL-8 proteins were also increased by ectopic expression of Zta in both EBV-negative and -positive NPC cells, as demonstrated by an immunoblotting assay (Fig. 3C). In addition, RT-PCR analysis showed Zta-mediated augmentation of IL-8 RNA in both HONE-tetonZ and NPC-TW01 cells, suggesting that Zta induces transcription of the IL-8 gene (Fig. 3D).
FIG. 3.
Zta induces IL-8 expression in NPC cells. (A) NPC-TW01 cells were transfected with the vector plasmid or a Zta-expressing plasmid. At indicated time posttransfection (10 to 40 h), the cell culture supernatants were collected and quantified for IL-8 secretion by using ELISA, while Zta and β-actin proteins in the cell lysates were detected in an immunoblotting assay. (B) HONE-tetonZ cells and the parental HONE-1 cells were treated with (+) or without (−) doxycycline (dox). The culture supernatants were subjected to quantification of IL-8 by using ELISA, and the cell lysates were analyzed for the expression of Zta and β-actin in an immunoblotting assay. (C) EBV-negative NPC-TW01 cells and EBV-infected NA cells were transfected with vector plasmid (V) or a Zta-expressing plasmid (Z). IL-8, Zta, and β-actin proteins in the cell lysates were detected by using an immunoblotting assay. (D) HONE-tetonZ cells were treated with (+) or without (−) doxycycline (dox), and NPC-TW01 cells were transfected with the vector plasmid (V) or a Zta-expressing plasmid (Z). RNAs were extracted from the cells and subjected to RT-PCR assay for the detection of IL-8 and β-actin transcripts.
Zta-mediated induction of IL-8 is independent of early growth response-1 (Egr-1).
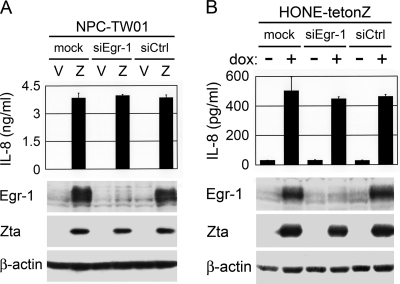
Our previous study indicated that Zta can induce expression of Egr-1, a cellular transcription factor regulating diverse biologic functions (13). Since Egr-1 has been reported to be involved in induction of IL-8 (31), we wondered whether Zta upregulates IL-8 through Egr-1. In NPC-TW01 cells transfected with a Zta-expressing plasmid, Egr-1-targeted siRNA efficiently knocked down Zta-induced Egr-1 expression but did not affect Zta-induced IL-8 production (Fig. 4A). A similar result was also observed in HONE-tetonZ cells, where siRNA-directed inhibition of Egr-1 expression did not interfere Zta-mediated induction of IL-8 (Fig. 4B). Therefore, Zta can upregulate IL-8 in NPC cells in an Egr-1-independent manner.
FIG. 4.
Zta-mediated induction of IL-8 is independent of Egr-1. (A) NPC-TW01 cells transfected with the vector plasmid (V) or a Zta-expressing plasmid (Z) were cotransfected with no siRNA (mock), Egr-1-targeted siRNA (siEgr-1), or a control siRNA (siCtrl). The culture supernatants were subjected to quantification of IL-8 by using ELISA, and the cell lysates were analyzed for the expression of Egr-1, Zta, and β-actin in an immunoblotting assay. (B) HONE-tetonZ cells were transfected with no siRNA (mock), Egr-1-targeted siRNA (siEgr-1) or a control siRNA (siCtrl) and treated with (+) or without (−) doxycycline (dox). The culture supernatants and cell lysates were subjected to the same experiments described in panel A.
Transactivation activity of Zta is important for induction of IL-8.
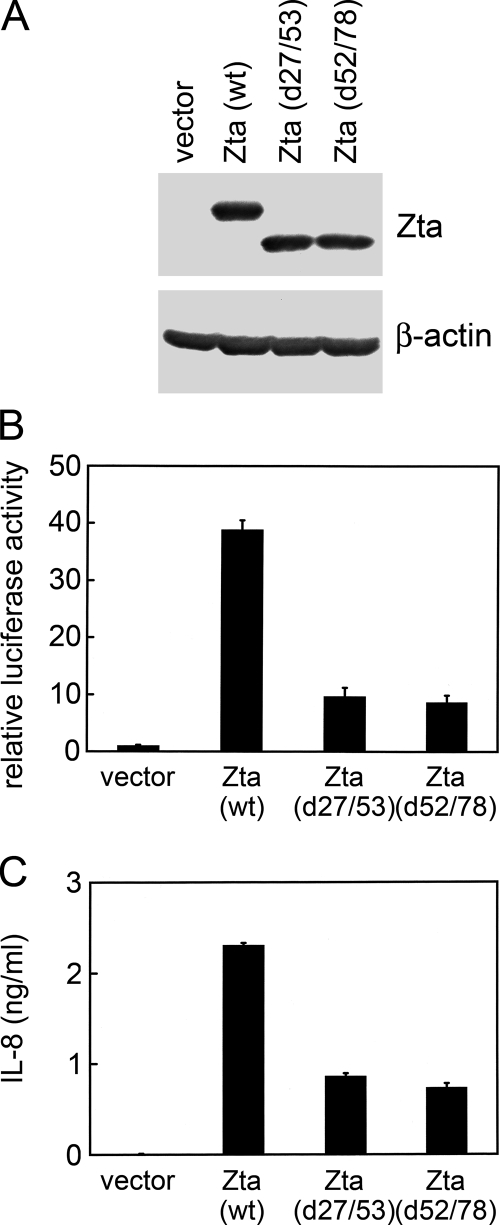
Since Zta increased IL-8 RNA (Fig. 3D), it is possible that Zta-induced IL-8 production occurs via transactivation of the IL-8 promoter. In a reporter gene assay, wild-type Zta activated the IL-8 promoter spanning positions −1453 to + 44 of the IL-8 gene (Fig. 5B). To examine whether the transactivation activity of Zta is important for induction of IL-8, we used two Zta mutants, d27/53 and d52/78, with deletions in the N-terminal transactivation domain (Fig. 5A). Both of the Zta mutants largely lose their transactivation functions, though they retain the DNA-binding ability (27). Compared to the wild-type protein, these two Zta mutants showed impaired abilities to activate the IL-8 promoter (Fig. 5B). The induction of IL-8 secretion by the Zta mutants was also significantly lower than the induction by wild-type Zta (Fig. 5C). Hence, transactivation of the IL-8 promoter could be a major mechanism for Zta to upregulate IL-8.
FIG. 5.
Transactivation activity of Zta is important for induction of IL-8. (A) NPC-TW01 cells were transfected with the vector plasmid or plasmids expressing wild-type (wt) Zta or Zta mutants with deletion of amino acids 27 to 53 (d27/53) or amino acids 52 to 78 (d52/78). The expression of Zta and β-actin was examined in an immunoblotting assay. (B) NPC-TW01 cells were cotransfected with the indicated effector plasmids (vector plasmid or plasmids expressing wild-type Zta or Zta mutants) and an IL-8 promoter-driven reporter plasmid, pIL-8(−1453/+44)-Luc. After transfection, the cells were harvested and subjected to the luciferase assay. (C) NPC-TW01 cells were transfected with the indicated expression plasmids, and IL-8 in the cell culture supernatants was quantified by using ELISA.
The IL-8 promoter contains two responsive sites for Zta-mediated activation.
Reporter gene assays were carried out to further identify Zta-targeted sites in the IL-8 promoter. Mapping through serial 5′ deletion of the IL-8 promoter, we found two Zta-responsive regions in the promoter. Deletion of the promoter region from positions −132 to −98 not only significantly diminished the basal promoter activity but also reduced the extent of Zta-mediated activation, and additional removal of its downstream region from positions −98 to −73 completely abolished the responsiveness to Zta (Fig. 6B). An AP-1 element is recognized within the promoter region from positions −132 to −98, and a putative ZRE is located within the region between positions −98 and −73 (Fig. 6A). In the site-directed mutagenesis study, disruption of either the AP-1 element or the ZRE reduced Zta-mediated activation of the IL-8 promoter, while dual mutation of both sites completely abolished the responsiveness to Zta (Fig. 6C). According to the result in Fig. 6C, we concluded that the AP-1 site contributes to both basal promoter activity and Zta-induced transactivation, while the ZRE contributes to the residual responsiveness of the IL-8 promoter to Zta.
Zta binds to the IL-8 promoter both in vitro and in vivo.
Since both the AP-1 element and the ZRE are potential Zta-binding sites (15, 26, 50), we performed EMSA to confirm the binding in vitro. Figure 7B shows that both the ZRE and the AP-1 probes can form a new complex in the presence of Zta. The Zta-DNA interaction was further verified, since the Zta-binding complexes were competed by specific ZRE and were supershifted by anti-Zta antibody (Fig. 7B). We also observed a Zta-independent complex formed with the AP-1 probe, and it was competed for by AP-1 sequence but not by ZRE (Fig. 7B, right panel). This complex is likely to be binding of cellular AP-1 transcription factors, which may account for the contribution of the AP-1 site to basal activity of the IL-8 promoter in our reporter gene assays (Fig. 6C). To further demonstrate that Zta can be recruited to the IL-8 promoter in vivo, we carried out a ChIP assay. The presence of DNA of the IL-8 promoter was detected in the immunoprecipitant from Zta-expressing cells by using anti-Zta antibody, but not in the precipitants from vector control cells or by using control antibody or no antibody (Fig. 7C), indicating binding of Zta to the IL-8 promoter in vivo.
DNA-binding-defective Zta mutants fail to induce IL-8.
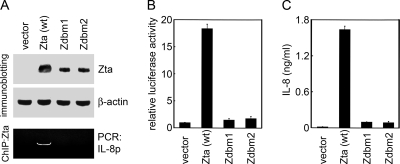
We also examined the effects of two DNA-binding-defective Zta mutants, Zdbm1 and Zdbm2, which contain mutations within the DNA-binding domain (28). The two Zta mutants were not recruited to the IL-8 promoter in a ChIP assay (Fig. 8A). They failed to transactivate the IL-8 promoter (Fig. 8B) and showed a significant impairment in ability to induce IL-8 production (Fig. 8C). The results confirm that the DNA-binding ability of Zta is essential for the induction of IL-8.
FIG. 8.
DNA-binding-defective Zta mutants fail to induce IL-8. (A) NPC-TW01 cells were transfected with the vector plasmid or plasmids expressing wild-type (wt) Zta or DNA-binding-defective Zta mutants, Zdbm1 and Zdbm2. The expression of Zta and β-actin was examined in an immunoblotting assay. A ChIP assay was performed to immunoprecipitate Zta and to detect the IL-8 promoter by PCR. (B) NPC-TW01 cells were cotransfected with a reporter plasmid pIL-8(−1453/+44)-Luc and the indicated effector plasmids (vector plasmid or plasmids expressing wild-type Zta or Zta mutants). The transfected cells were then harvested and subjected to the luciferase assay. (C) NPC-TW01 cells were transfected with the indicated expression plasmids, and IL-8 in the cell culture supernatants was quantified by using ELISA.
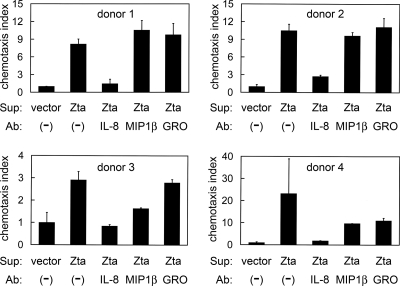
Zta-induced IL-8 exerts chemotactic activity.
A chemotaxis assay was performed to examine whether the Zta-induced IL-8 is functionally active. Neutrophil-predominant granulocytes were used as target cells because IL-8 is a potent chemoattractant for neutrophils, which express the highest level of IL-8 receptors (4, 19). Compared to the culture supernatants from vector-transfected cells, the conditioned medium from Zta-expressing NPC-TW01 cells prominently attracted more granulocytes, though extent of the enhanced chemotaxis varied among the four donors (Fig. 9). Neutralization of IL-8 significantly inhibited Zta-induced chemotaxis in all cases, while neutralizing antibodies against MIP-1β or GRO exerted only minor inhibitory effect in two cases (Fig. 9), indicating that IL-8 is a major contributor to Zta-enhanced chemotactic activity.
FIG. 9.
Zta-induced IL-8 exerts chemotactic activity. Cell culture supernatants (Sup) of NPC-TW01 cells transfected with the vector plasmid or a Zta-expressing plasmid were applied to the lower chambers of transwells, while purified granulocytes were added to the upper chambers. In indicated experiments, neutralizing antibodies (Ab) against IL-8, MIP-1β, or GRO were pre-added to the culture supernatants. Migration of granulocytes to the lower chambers was measured, and the chemotaxis index was calculated to represent x-fold chemoattraction by comparison with the culture supernatants from vector-transfected cells. Shown are the results using granulocytes from four donors.
DISCUSSION
In this study, EBV reactivation in NPC cells was associated with the induction of certain chemokines, among which IL-8 was upregulated most significantly and consistently (Fig. 1). The lytic protein Zta was found to be a potent inducer of IL-8, increasing IL-8 at both protein and RNA levels and activating the IL-8 promoter (Fig. 2, 3, and 5). We have identified two Zta-responsive sites in the IL-8 promoter (Fig. 6) and confirmed that Zta can bind to the promoter both in vitro and in vivo (Fig. 7). In addition, Zta enhanced chemotactic activity mainly through the induced IL-8 (Fig. 9). Therefore, the present study provides a clue that EBV lytic infection may contribute to the inflammation-like microenvironment of NPC by the upregulation of chemokines.
The IL-8 promoter contains two major regulatory elements, the AP-1 and NF-κB sites, which have been involved in the induction of IL-8 by various stimulators (22, 62, 68, 74). In the present study, the AP-1 site also contributes to Zta-mediated activation of the IL-8 promoter in NPC cells (Fig. 6C). Moreover, downstream of the AP-1 site, we have identified a ZRE (Fig. 6C), which has been recognized as a C/EBP site previously (73). Consistent with previous studies showing Zta's binding to the DNA with AP-1- or C/EBP-like sequences (15, 50), our EMSA results demonstrated that Zta can bind to the AP-1 element and the ZRE of the IL-8 promoter (Fig. 7B). The responsiveness of the IL-8 promoter to Zta was completely abolished when both the AP-1 and the ZRE were disrupted (Fig. 6C), supporting the importance of the two elements for the Zta-mediated transactivation.
Zta mutants defective in DNA binding or transactivation exhibited impaired abilities to induce IL-8 production (Fig. 5 and 8), indicating that binding to and transactivation of the IL-8 promoter could be a major mechanism for Zta to upregulate IL-8. Meanwhile, other mechanisms may be also involved in the Zta-induced IL-8 expression. One possible mechanism we tested is the role of Egr-1. It has been reported that Egr-1 is essential for amyloid peptide-induced IL-8 expression in monocytic cells; the induction of IL-8 can be blocked by Egr-1-targeted siRNA (31). However, putative Egr-1 binding site has not been recognized within the IL-8 promoter (72). Our previous study found that Zta can upregulate Egr-1 (13), so we wondered whether Egr-1 plays any role in Zta-induced IL-8 expression. Our result did not support the hypothesis, showing that Egr-1 is dispensable for the IL-8 induction by Zta (Fig. 4). Considering that Egr-1 can regulate many other inflammatory chemokines and cytokines (18, 29, 31), it is still of interest to study whether Zta can affect inflammatory events through the mediation of Egr-1.
Although several clues have suggested a link between EBV reactivation and NPC, it is largely unknown how the small subset of lytically infected tumor cells contributes to cancer development. Secretion of soluble factors is a plausible way for these cells to exert their effects on the whole tumors. A clue supporting the notion has come from a SCID mouse model of EBV-associated lymphoproliferative disease, where the lytic infection is required for efficient tumor outgrowth of early-passage EBV-transformed B cells (39). The requirement could be attributed to the lytic-cycle-induced secretion of B-cell growth factors (IL-6 and IL-10) and an angiogenesis factor VEGF (39, 40, 46). Focusing on the study of NPC, we found that EBV lytic infection and the lytic protein Zta can upregulate several secreted chemokines, especially IL-8. By initiating or enhancing leukocyte infiltration, the lytic-cycle-induced chemokines may contribute to an inflammation-like microenvironment, where the interaction between immune infiltrates and tumor cells is crucial for NPC development (1, 63). The contribution possibly occurs not only in the developed NPC tumors but also at the precancer stage where an inflammation-like microenvironment predisposes precancerous cells to tumor formation (2, 51), which may account for how EBV reactivation serves as a risk factor before the onset of NPC (17).
Our chemotaxis assay showed that the chemokines induced by Zta in NPC cells are functional to attract neutrophil-predominant granulocytes and that IL-8 is a major contributor to the enhanced chemotactic activity (Fig. 9). Neutrophils are among the immune cells first invoked into inflamed tissues, and they can produce a variety of chemokines with potentials to direct sequential recruitment of other leukocytes (64). The essential roles of neutrophils in the establishment of local leukocyte infiltration have been demonstrated in several models of inflammation (8, 77). Therefore, by initial recruitment of neutrophils, Zta-induced IL-8 may trigger the subsequent influx of leukocytes in NPC. Notably, neutrophil infiltration promoted by tumor-derived IL-8 has been linked to the poor prognosis of bronchioloalveolar carcinoma and to increased genetic instability of Mutatect tumors (6, 33), suggesting that the IL-8-attracted neutrophils may contribute to tumorigenesis in some cases.
The infiltrating cells in NPC tumors consist mainly of lymphocytes, while granulocytes, monocytes, and natural killer cells exist as a minor population (35, 42). We have considered whether Zta-induced IL-8 can directly recruit lymphocytes, since an IL-8 receptor CXCR1 can be expressed on certain subsets of CD8+ T cells, including the effector/cytotoxic cells and the activated central memory cells (30, 36, 69). However, the chemotaxis was not observed in our preliminary study (data not shown), possibly because the IL-8 concentration in the culture supernatants was too low to attract T cells which express IL-8 receptors at a lower level than neutrophils (19, 30, 36). IL-8 receptors have also been detected on monocytes and natural killer cells (7, 43). Thus far, we have not ruled out chemotactic effects of IL-8 on infiltrating lymphocytes and other immune cells in NPC tumors in vivo.
In addition to the functions for chemoattraction, IL-8 plays multiple roles in cancer development. Some tumor cells can express IL-8 receptors and utilize IL-8 as an autocrine growth factor (56, 65). Notably, IL-8 receptors have been detected on NPC tumor cells, and the expression of one receptor CXCR1 correlates with a shorter survival rate of the patients, supporting a contribution of IL-8/CXCR1 to NPC (41). IL-8 is also a well-documented angiogenesis factor, and its expression has been associated with the level of vascularization in many tumors, including NPC (3, 48, 67, 75). Moreover, NPC is a highly metastatic cancer, and IL-8 may be involved in the phenotype since it can promote tumor invasion or metastasis through induction of certain metalloproteinases (44, 53). Interestingly, GRO, a chemokine that is weakly but consistently upregulated by EBV reactivation and by Zta expression (Fig. 1 and 2), also exerts similar functions in autocrine-driven tumor growth and angiogenesis (12, 71). Therefore, the present study showing Zta-mediated upregulation of oncogenic chemokines has pointed a promising direction to explore the link between EBV lytic infection and pathogenesis of NPC.
Furthermore, we notice that IL-8 is a converged target gene of gammaherpesviruses in both latent and lytic infection states. EBV utilizes the lytic protein Zta and the latent protein LMP1 to induce IL-8 expression, while Kaposi's sarcoma-associated herpesvirus (KSHV) can upregulate IL-8 by either the lytic protein K15 or the latent protein K13 (10, 22, 68, 75). Since KSHV-associated Kaposi's sarcoma also exhibits several inflammation-like features, induction of IL-8 is likely to be critical for the virus-mediated “inflammatory tumorigenesis”; thus, the oncogenic gammaherpesviruses have evolved several strategies to achieve the same effect. In many cancer models, anti-IL-8 neutralizing antibodies and IL-8-specific antisense oligonucleotides have been proven effective to inhibit tumor growth through the reduction of cell proliferation, angiogenesis, or metastasis (3, 44, 67). Therefore, blockage of IL-8 or IL-8 receptors may be considered a potential therapeutic approach for treating NPC or other inflammation-related malignancies.
Acknowledgments
We thank Erik Flemington for providing plasmids expressing Zta mutants.
This study was supported by the National Health Research Institutes (CL-095-PP-16 and CL-096-PP-13) and the National Science Council (NSC96-2320-B-400-002-MY3).
Footnotes
Published ahead of print on 30 January 2008.
REFERENCES
- 1.Agathanggelou, A., G. Niedobitek, R. Chen, J. Nicholls, W. Yin, and L. S. Young. 1995. Expression of immune regulatory molecules in Epstein-Barr virus-associated nasopharyngeal carcinomas with prominent lymphoid stroma: evidence for a functional interaction between epithelial tumor cells and infiltrating lymphoid cells. Am. J. Pathol. 1471152-1160. [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal, B. B., S. Shishodia, S. K. Sandur, M. K. Pandey, and G. Sethi. 2006. Inflammation and cancer: how hot is the link? Biochem. Pharmacol. 721605-1621. [DOI] [PubMed] [Google Scholar]
- 3.Arenberg, D. A., S. L. Kunkel, P. J. Polverini, M. Glass, M. D. Burdick, and R. M. Strieter. 1996. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J. Clin. Investig. 972792-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baggiolini, M., P. Imboden, and P. Detmers. 1992. Neutrophil activation and the effects of interleukin-8/neutrophil-activating peptide 1 (IL-8/NAP-1). Cytokines 41-17. [PubMed] [Google Scholar]
- 5.Balkwill, F. 2004. Cancer and the chemokine network. Nat. Rev. Cancer 4540-550. [DOI] [PubMed] [Google Scholar]
- 6.Bellocq, A., M. Antoine, A. Flahault, C. Philippe, B. Crestani, J. F. Bernaudin, C. Mayaud, B. Milleron, L. Baud, and J. Cadranel. 1998. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am. J. Pathol. 15283-92. [PMC free article] [PubMed] [Google Scholar]
- 7.Bishayi, B., and A. K. Samanta. 1996. Identification and characterization of specific receptor for interleukin-8 from the surface of human monocytes. Scand. J. Immunol. 43531-536. [DOI] [PubMed] [Google Scholar]
- 8.Bonder, C. S., M. N. Ajuebor, L. D. Zbytnuik, P. Kubes, and M. G. Swain. 2004. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J. Immunol. 17245-53. [DOI] [PubMed] [Google Scholar]
- 9.Bouvier, G., M. Hergenhahn, A. Polack, G. W. Bornkamm, G. de The, and H. Bartsch. 1995. Characterization of macromolecular lignins as Epstein-Barr virus inducer in foodstuff associated with nasopharyngeal carcinoma risk. Carcinogenesis 161879-1885. [DOI] [PubMed] [Google Scholar]
- 10.Brinkmann, M. M., M. Pietrek, O. Dittrich-Breiholz, M. Kracht, and T. F. Schulz. 2007. Modulation of host gene expression by the K15 protein of Kaposi's sarcoma-associated herpesvirus. J. Virol. 8142-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buettner, M., B. Meyer, S. Schreck, and G. Niedobitek. 2007. Expression of RANTES and MCP-1 in epithelial cells is regulated via LMP1 and CD40. Int. J. Cancer 1212703-2710. [DOI] [PubMed] [Google Scholar]
- 12.Caunt, M., L. Hu, T. Tang, P. C. Brooks, S. Ibrahim, and S. Karpatkin. 2006. Growth-regulated oncogene is pivotal in thrombin-induced angiogenesis. Cancer Res. 664125-4132. [DOI] [PubMed] [Google Scholar]
- 13.Chang, Y., H. H. Lee, Y. T. Chen, J. Lu, S. Y. Wu, C. W. Chen, K. Takada, and C. H. Tsai. 2006. Induction of the early growth response 1 gene by Epstein-Barr virus lytic transactivator Zta. J. Virol. 807748-7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang, Y., C. H. Tung, Y. T. Huang, J. Lu, J. Y. Chen, and C. H. Tsai. 1999. Requirement for cell-to-cell contact in Epstein-Barr virus infection of nasopharyngeal carcinoma cells and keratinocytes. J. Virol. 738857-8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang, Y. N., D. L. Dong, G. S. Hayward, and S. D. Hayward. 1990. The Epstein-Barr virus Zta transactivator: a member of the bZIP family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J. Virol. 643358-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, H., J. M. Lee, Y. Zong, M. Borowitz, M. H. Ng, R. F. Ambinder, and S. D. Hayward. 2001. Linkage between STAT regulation and Epstein-Barr virus gene expression in tumors. J. Virol. 752929-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chien, Y. C., J. Y. Chen, M. Y. Liu, H. I. Yang, M. M. Hsu, C. J. Chen, and C. S. Yang. 2001. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N. Engl. J. Med. 3451877-1882. [DOI] [PubMed] [Google Scholar]
- 18.Cho, S. J., M. J. Kang, R. J. Homer, H. R. Kang, X. Zhang, P. J. Lee, J. A. Elias, and C. G. Lee. 2006. Role of early growth response-1 (Egr-1) in interleukin-13-induced inflammation and remodeling. J. Biol. Chem. 2818161-8168. [DOI] [PubMed] [Google Scholar]
- 19.Chuntharapai, A., J. Lee, C. A. Hebert, and K. J. Kim. 1994. Monoclonal antibodies detect different distribution patterns of IL-8 receptor A and IL-8 receptor B on human peripheral blood leukocytes. J. Immunol. 1535682-5688. [PubMed] [Google Scholar]
- 20.Cochet, C., D. Martel-Renoir, V. Grunewald, J. Bosq, G. Cochet, G. Schwaab, J. F. Bernaudin, and I. Joab. 1993. Expression of the Epstein-Barr virus immediate-early gene, BZLF1, in nasopharyngeal carcinoma tumor cells. Virology 197358-365. [DOI] [PubMed] [Google Scholar]
- 21.de-Vathaire, F., H. Sancho-Garnier, H. de-The, C. Pieddeloup, G. Schwaab, J. H. Ho, R. Ellouz, C. Micheau, M. Cammoun, Y. Cachin, and G. de-The. 1988. Prognostic value of EBV markers in the clinical management of nasopharyngeal carcinoma (NPC): a multicenter follow-up study. Int. J. Cancer 42176-181. [DOI] [PubMed] [Google Scholar]
- 22.Eliopoulos, A. G., N. J. Gallagher, S. M. Blake, C. W. Dawson, and L. S. Young. 1999. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J. Biol. Chem. 27416085-16096. [DOI] [PubMed] [Google Scholar]
- 23.Fahraeus, R., H. L. Fu, I. Ernberg, J. Finke, M. Rowe, G. Klein, K. Falk, E. Nilsson, M. Yadav, P. Busson, T. Tursz, and B. Kallin. 1988. Expression of Epstein-Barr virus-encoded proteins in nasopharyngeal carcinoma. Int. J. Cancer 42329-338. [DOI] [PubMed] [Google Scholar]
- 24.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 193080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flemington, E., and S. H. Speck. 1990. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J. Virol. 641227-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flemington, E., and S. H. Speck. 1990. Epstein-Barr virus BZLF1 trans activator induces the promoter of a cellular cognate gene, c-fos. J. Virol. 644549-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flemington, E. K., A. M. Borras, J. P. Lytle, and S. H. Speck. 1992. Characterization of the Epstein-Barr virus BZLF1 protein transactivation domain. J. Virol. 66922-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flemington, E. K., J. P. Lytle, C. Cayrol, A. M. Borras, and S. H. Speck. 1994. DNA-binding-defective mutants of the Epstein-Barr virus lytic switch activator Zta transactivate with altered specificities. Mol. Cell. Biol. 143041-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu, M., X. Zhu, J. Zhang, J. Liang, Y. Lin, L. Zhao, M. U. Ehrengruber, and Y. E. Chen. 2003. Egr-1 target genes in human endothelial cells identified by microarray analysis. Gene 31533-41. [DOI] [PubMed] [Google Scholar]
- 30.Gasser, O., A. Missiou, C. Eken, and C. Hess. 2005. Human CD8+ T cells store CXCR1 in a distinct intracellular compartment and up-regulate it rapidly to the cell surface upon activation. Blood 1063718-3724. [DOI] [PubMed] [Google Scholar]
- 31.Giri, R. K., S. K. Selvaraj, and V. K. Kalra. 2003. Amyloid peptide-induced cytokine and chemokine expression in THP-1 monocytes is blocked by small inhibitory RNA duplexes for early growth response-1 messenger RNA. J. Immunol. 1705281-5294. [DOI] [PubMed] [Google Scholar]
- 32.Grogan, E., H. Jenson, J. Countryman, L. Heston, L. Gradoville, and G. Miller. 1987. Transfection of a rearranged viral DNA fragment, WZhet, stably converts latent Epstein-Barr viral infection to productive infection in lymphoid cells. Proc. Natl. Acad. Sci. USA 841332-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haqqani, A. S., J. K. Sandhu, and H. C. Birnboim. 2000. Expression of interleukin-8 promotes neutrophil infiltration and genetic instability in mutatect tumors. Neoplasia 2561-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henle, W., J. H. Ho, G. Henle, J. C. Chau, and H. C. Kwan. 1977. Nasopharyngeal carcinoma: significance of changes in Epstein-Barr virus-related antibody patterns following therapy. Int. J. Cancer 20663-672. [DOI] [PubMed] [Google Scholar]
- 35.Herait, P., G. Ganem, M. Lipinski, C. Carlu, C. Micheau, G. Schwaab, G. De-The, and T. Tursz. 1987. Lymphocyte subsets in tumour of patients with undifferentiated nasopharyngeal carcinoma: presence of lymphocytes with the phenotype of activated T cells. Br. J. Cancer 55135-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hess, C., T. K. Means, P. Autissier, T. Woodberry, M. Altfeld, M. M. Addo, N. Frahm, C. Brander, B. D. Walker, and A. D. Luster. 2004. IL-8 responsiveness defines a subset of CD8 T cells poised to kill. Blood 1043463-3471. [DOI] [PubMed] [Google Scholar]
- 37.Ho, C. H., C. F. Hsu, P. F. Fong, S. K. Tai, S. L. Hsieh, and C. J. Chen. 2007. Epstein-Barr virus transcription activator Rta upregulates decoy receptor 3 expression by binding to its promoter. J. Virol. 814837-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holley-Guthrie, E. A., E. B. Quinlivan, E. C. Mar, and S. Kenney. 1990. The Epstein-Barr virus (EBV) BMRF1 promoter for early antigen (EA-D) is regulated by the EBV transactivators, BRLF1 and BZLF1, in a cell-specific manner. J. Virol. 643753-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong, G. K., M. L. Gulley, W. H. Feng, H. J. Delecluse, E. Holley-Guthrie, and S. C. Kenney. 2005. Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. J. Virol. 7913993-14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong, G. K., P. Kumar, L. Wang, B. Damania, M. L. Gulley, H. J. Delecluse, P. J. Polverini, and S. C. Kenney. 2005. Epstein-Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J. Virol. 7913984-13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horikawa, T., Y. Kaizaki, H. Kato, M. Furukawa, and T. Yoshizaki. 2005. Expression of interleukin-8 receptor A predicts poor outcome in patients with nasopharyngeal carcinoma. Laryngoscope 11562-67. [DOI] [PubMed] [Google Scholar]
- 42.Huang, Y. T., T. S. Sheen, C. L. Chen, J. Lu, Y. Chang, J. Y. Chen, and C. H. Tsai. 1999. Profile of cytokine expression in nasopharyngeal carcinomas: a distinct expression of interleukin 1 in tumor and CD4+ T cells. Cancer Res. 591599-1605. [PubMed] [Google Scholar]
- 43.Inngjerdingen, M., B. Damaj, and A. A. Maghazachi. 2001. Expression and regulation of chemokine receptors in human natural killer cells. Blood 97367-375. [DOI] [PubMed] [Google Scholar]
- 44.Inoue, K., J. W. Slaton, B. Y. Eve, S. J. Kim, P. Perrotte, M. D. Balbay, S. Yano, M. Bar-Eli, R. Radinsky, C. A. Pettaway, and C. P. Dinney. 2000. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin. Cancer Res. 62104-2119. [PubMed] [Google Scholar]
- 45.Jones, R. J., S. Dickerson, P. M. Bhende, H. J. Delecluse, and S. C. Kenney. 2007. Epstein-Barr virus lytic infection induces retinoic acid-responsive genes through induction of a retinol-metabolizing enzyme, DHRS9. J. Biol. Chem. 2828317-8324. [DOI] [PubMed] [Google Scholar]
- 46.Jones, R. J., W. T. Seaman, W. H. Feng, E. Barlow, S. Dickerson, H. J. Delecluse, and S. C. Kenney. 2007. Roles of lytic viral infection and IL-6 in early versus late passage lymphoblastoid cell lines and EBV-associated lymphoproliferative disease. Int. J. Cancer 1211274-1281. [DOI] [PubMed] [Google Scholar]
- 47.Kenney, S., E. Holley-Guthrie, E. C. Mar, and M. Smith. 1989. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J. Virol. 633878-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitadai, Y., K. Haruma, K. Sumii, S. Yamamoto, T. Ue, H. Yokozaki, W. Yasui, Y. Ohmoto, G. Kajiyama, I. J. Fidler, and E. Tahara. 1998. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am. J. Pathol. 15293-100. [PMC free article] [PubMed] [Google Scholar]
- 49.Klein, S. C., D. Kube, H. Abts, V. Diehl, and H. Tesch. 1996. Promotion of IL8, IL-10, TNF alpha, and TNF beta production by EBV infection. Leuk. Res. 20633-636. [DOI] [PubMed] [Google Scholar]
- 50.Kouzarides, T., G. Packham, A. Cook, and P. J. Farrell. 1991. The BZLF1 protein of EBV has a coiled coil dimerisation domain without a heptad leucine repeat but with homology to the C/EBP leucine zipper. Oncogene 6195-204. [PubMed] [Google Scholar]
- 51.Lu, H., W. Ouyang, and C. Huang. 2006. Inflammation, a key event in cancer development. Mol. Cancer Res. 4221-233. [DOI] [PubMed] [Google Scholar]
- 52.Lu, J., S. Y. Chen, H. H. Chua, Y. S. Liu, Y. T. Huang, Y. Chang, J. Y. Chen, T. S. Sheen, and C. H. Tsai. 2000. Upregulation of tyrosine kinase TKT by the Epstein-Barr virus transactivator Zta. J. Virol. 747391-7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luca, M., S. Huang, J. E. Gershenwald, R. K. Singh, R. Reich, and M. Bar-Eli. 1997. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am. J. Pathol. 1511105-1113. [PMC free article] [PubMed] [Google Scholar]
- 54.Mahot, S., A. Sergeant, E. Drouet, and H. Gruffat. 2003. A novel function for the Epstein-Barr virus transcription factor EB1/Zta: induction of transcription of the hIL-10 gene. J. Gen. Virol. 84965-974. [DOI] [PubMed] [Google Scholar]
- 55.Martel-Renoir, D., V. Grunewald, R. Touitou, G. Schwaab, and I. Joab. 1995. Qualitative analysis of the expression of Epstein-Barr virus lytic genes in nasopharyngeal carcinoma biopsies. J. Gen. Virol. 761401-1408. [DOI] [PubMed] [Google Scholar]
- 56.Masood, R., J. Cai, A. Tulpule, T. Zheng, A. Hamilton, S. Sharma, B. M. Espina, D. L. Smith, and P. S. Gill. 2001. Interleukin 8 is an autocrine growth factor and a surrogate marker for Kaposi's sarcoma. Clin. Cancer Res. 72693-2702. [PubMed] [Google Scholar]
- 57.Oudejans, J. J., H. Harijadi, J. A. Kummer, I. B. Tan, E. Bloemena, J. M. Middeldorp, B. Bladergroen, D. F. Dukers, W. Vos, and C. J. Meijer. 2002. High numbers of granzyme B/CD8-positive tumour-infiltrating lymphocytes in nasopharyngeal carcinoma biopsies predict rapid fatal outcome in patients treated with curative intent. J. Pathol. 198468-475. [DOI] [PubMed] [Google Scholar]
- 58.Quinlivan, E. B., E. A. Holley-Guthrie, M. Norris, D. Gutsch, S. L. Bachenheimer, and S. C. Kenney. 1993. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 211999-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raab-Traub, N., and K. Flynn. 1986. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 47883-889. [DOI] [PubMed] [Google Scholar]
- 60.Ragoczy, T., L. Heston, and G. Miller. 1998. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J. Virol. 727978-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 62.Roebuck, K. A., L. R. Carpenter, V. Lakshminarayanan, S. M. Page, J. N. Moy, and L. L. Thomas. 1999. Stimulus-specific regulation of chemokine expression involves differential activation of the redox-responsive transcription factors AP-1 and NF-κB. J. Leukoc. Biol. 65291-298. [DOI] [PubMed] [Google Scholar]
- 63.Sbih-Lammali, F., B. Clausse, H. Ardila-Osorio, R. Guerry, M. Talbot, S. Havouis, L. Ferradini, J. Bosq, T. Tursz, and P. Busson. 1999. Control of apoptosis in Epstein Barr virus-positive nasopharyngeal carcinoma cells: opposite effects of CD95 and CD40 stimulation. Cancer Res. 59924-930. [PubMed] [Google Scholar]
- 64.Scapini, P., J. A. Lapinet-Vera, S. Gasperini, F. Calzetti, F. Bazzoni, and M. A. Cassatella. 2000. The neutrophil as a cellular source of chemokines. Immunol. Rev. 177195-203. [DOI] [PubMed] [Google Scholar]
- 65.Schadendorf, D., A. Moller, B. Algermissen, M. Worm, M. Sticherling, and B. M. Czarnetzki. 1993. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J. Immunol. 1512667-2675. [PubMed] [Google Scholar]
- 66.Shao, Y. M., S. Poirier, H. Ohshima, C. Malaveille, Y. Zeng, G. de The, and H. Bartsch. 1988. Epstein-Barr virus activation in Raji cells by extracts of preserved food from high risk areas for nasopharyngeal carcinoma. Carcinogenesis 91455-1457. [DOI] [PubMed] [Google Scholar]
- 67.Sparmann, A., and D. Bar-Sagi. 2004. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 6447-458. [DOI] [PubMed] [Google Scholar]
- 68.Sun, Q., H. Matta, G. Lu, and P. M. Chaudhary. 2006. Induction of IL-8 expression by human herpesvirus 8 encoded vFLIP K13 via NF-κB activation. Oncogene 252717-2726. [DOI] [PubMed] [Google Scholar]
- 69.Takata, H., H. Tomiyama, M. Fujiwara, N. Kobayashi, and M. Takiguchi. 2004. Cutting edge: expression of chemokine receptor CXCR1 on human effector CD8+ T cells. J. Immunol. 1732231-2235. [DOI] [PubMed] [Google Scholar]
- 70.Tang, K. F., S. Y. Tan, S. H. Chan, S. M. Chong, K. S. Loh, L. K. Tan, and H. Hu. 2001. A distinct expression of CC chemokines by macrophages in nasopharyngeal carcinoma: implication for the intense tumor infiltration by T lymphocytes and macrophages. Hum. Pathol. 3242-49. [DOI] [PubMed] [Google Scholar]
- 71.Wang, B., D. T. Hendricks, F. Wamunyokoli, and M. I. Parker. 2006. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 663071-3077. [DOI] [PubMed] [Google Scholar]
- 72.Worden, B., X. P. Yang, T. L. Lee, L. Bagain, N. T. Yeh, J. G. Cohen, C. Van Waes, and Z. Chen. 2005. Hepatocyte growth factor/scatter factor differentially regulates expression of proangiogenic factors through Egr-1 in head and neck squamous cell carcinoma. Cancer Res. 657071-7080. [DOI] [PubMed] [Google Scholar]
- 73.Wu, G. D., E. J. Lai, N. Huang, and X. Wen. 1997. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter: the role of Oct-1 as a transcriptional repressor. J. Biol. Chem. 2722396-2403. [PubMed] [Google Scholar]
- 74.Xu, L., and I. J. Fidler. 2000. Acidic pH-induced elevation in interleukin 8 expression by human ovarian carcinoma cells. Cancer Res. 604610-4616. [PubMed] [Google Scholar]
- 75.Yoshizaki, T., T. Horikawa, R. Qing-Chun, N. Wakisaka, H. Takeshita, T. S. Sheen, S. Y. Lee, H. Sato, and M. Furukawa. 2001. Induction of interleukin-8 by Epstein-Barr virus latent membrane protein-1 and its correlation to angiogenesis in nasopharyngeal carcinoma. Clin. Cancer Res. 71946-1951. [PubMed] [Google Scholar]
- 76.Young, L. S., C. W. Dawson, D. Clark, H. Rupani, P. Busson, T. Tursz, A. Johnson, and A. B. Rickinson. 1988. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J. Gen. Virol. 691051-1065. [DOI] [PubMed] [Google Scholar]
- 77.Zhou, J., S. A. Stohlman, D. R. Hinton, and N. W. Marten. 2003. Neutrophils promote mononuclear cell infiltration during viral-induced encephalitis. J. Immunol. 1703331-3336. [DOI] [PubMed] [Google Scholar]