Abstract
Reovirus cell entry is mediated by attachment to cell surface carbohydrate and junctional adhesion molecule A (JAM-A) and internalization by β1 integrin. The β1 integrin cytoplasmic tail contains two NPXY motifs, which function in recruitment of adaptor proteins and clathrin for endocytosis and serve as sorting signals for internalized cargo. As reovirus infection requires disassembly in the endocytic compartment, we investigated the role of the β1 integrin NPXY motifs in reovirus internalization. In comparison to wild-type cells (β1+/+ cells), reovirus infectivity was significantly reduced in cells expressing mutant β1 integrin in which the NPXY motifs were altered to NPXF (β1+/+Y783F/Y795F cells). However, reovirus displayed equivalent binding and internalization levels following adsorption to β1+/+ cells and β1+/+Y783F/Y795F cells, suggesting that the NPXY motifs are essential for transport of reovirus within the endocytic pathway. Reovirus entry into β1+/+ cells was blocked by chlorpromazine, an inhibitor of clathrin-mediated endocytosis, while entry into β1+/+Y783F/Y795F cells was unaffected. Furthermore, virus was distributed to morphologically distinct endocytic organelles in β1+/+ and β1+/+Y783F/Y795F cells, providing further evidence that the β1 integrin NPXY motifs mediate sorting of reovirus in the endocytic pathway. Thus, NPXY motifs in the β1 integrin cytoplasmic tail are required for functional reovirus entry, which indicates a key role for these sequences in endocytosis of a pathogenic virus.
Virus entry into host cells requires interactions between viral capsid constituents and receptors expressed on the cell surface. Many viruses utilize distinct cellular molecules to mediate the functions of attachment and internalization (36). Mammalian reoviruses are nonenveloped, double-stranded RNA (dsRNA) viruses that infect a broad range of hosts in nature (55). Reoviruses are pathogenic in newborn mice, infecting most organs, including the central nervous system, heart, and liver (65). Reoviruses first attach to the cell surface by binding to carbohydrate (4), which is α2,3- or α2,6-linked sialic acid for serotype 3 reovirus strains (19, 46, 47). All reovirus serotypes bind to proteinaceous receptor junctional adhesion molecule A (JAM-A) to mediate high-affinity attachment (5, 14, 50). Following receptor binding, reovirus is internalized into cells via a mechanism involving β1 integrin (34).
The process by which reovirus enters cells is poorly understood. Data gathered from thin-section electron microscopy (EM) images (11, 12, 52, 59) and video fluorescence microscopy (24) suggest that reovirus is internalized by clathrin-dependent endocytosis. However, these studies were performed using imaging techniques that did not address the role of clathrin in productive reovirus infection. Within minutes of internalization, reovirus undergoes stepwise disassembly, which in fibroblasts is dependent on acidic pH (35, 59) and endosomal cysteine-containing proteases cathepsins B and L (23). Viral disassembly leads to generation of well-defined intermediates, the first of which is the infectious subvirion particle (ISVP) (12, 17, 57, 59). ISVPs are characterized by the loss of outer capsid protein σ3 and cleavage of outer capsid protein μ1 to form particle-associated fragments δ and φ. Following formation of ISVPs, σ1 is shed and the μ1 cleavage fragments undergo conformational rearrangement, yielding the ISVP* (15, 16). ISVP*s penetrate endosomes to deliver transcriptionally active viral cores into the cytoplasm (40, 42). Although the precise mechanism of membrane penetration is not well understood, ISVP*s are thought to breach the endosomal membrane by a process that requires μ1-mediated formation of small pores (1). The cellular compartment in which reovirus disassembly takes place is unknown, yet cathepsins B and L generally reside in late endosomes or lysosomes (62), suggesting that reovirus disassembly occurs in these endocytic organelles.
Integrins are heterodimeric cell surface molecules consisting of α and β subunits (32). Integrin heterodimers mediate cell adhesion to the extracellular matrix, regulate cell trafficking, and transduce both inside-out and outside-in signaling events (31). In addition to reovirus, several other pathogenic microorganisms use the adhesion and signaling properties of various integrins to bind or enter host cells, including adenovirus (αvβ3 and αvβ5) (72), cytomegalovirus (α2β1, α6β1, αvβ3) (25), echovirus (α2β1) (6), rotavirus (αvβ3, α2β1, α4β1) (27, 30), West Nile virus (αvβ3) (20), and Yersinia pseudotuberculosis (β1) (33). Little is known about the internalization and the endocytic trafficking of β1 integrin and its cognate cargo. The β1 integrin cytoplasmic domain is approximately 47 amino acids in length and contains two Asn-Pro-any amino acid-tyrosine (NPXY) motifs (51), with tyrosine residues at amino acid positions 783 and 795 (51). NPXY motifs are potential sites of tyrosine phosphorylation (53) and serve as endocytic sorting signals that can be recognized by adaptor proteins and clathrin (8, 9, 37, 38, 43). The function of NPXY motifs in cargo internalization was first deduced in studies of familial hypercholesterolemia, a disorder in which low-density lipoprotein (LDL) cholesterol levels are elevated, leading to premature atherosclerosis (22). This disease results from a tyrosine-to-phenylalanine mutation in the NPXY motif of the LDL receptor (22). The NPXY-to-F mutation renders the receptor incapable of recognition by adaptor protein disabled 2 (Dab2), which is engaged by adaptor protein 2 (AP-2) for clathrin-mediated endocytosis (38).
To determine the function of the β1 integrin NPXY motifs in reovirus entry and infection, we used β1−/− cells stably expressing either wild-type β1 integrin or β1 integrin with altered NPXY motifs. Mutation of the β1 integrin NPXY motifs to NPXF prevents tyrosine phosphorylation but does not affect β1 integrin surface expression, the capacity of β1 to form heterodimers with α integrin partners, fibronectin assembly, or cell adhesion (54). However, β1 integrin NPXY-to-F mutation results in increased focal contacts, shorter actin filaments, and impaired cell motility (54). We found that reovirus particles enter into β1+/+ cells via a chlorpromazine-sensitive mechanism and are delivered to vesicles that morphologically resemble endosomes and lysosomes, while a chlorpromazine-resistant mechanism is utilized in cells with altered NPXY motifs that do not lead to productive infection. These findings indicate that the β1 integrin NPXY motifs are required for functional reovirus entry and provide new insights into mechanisms by which β1 integrin delivers cargo to the endocytic compartment.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
Spinner-adapted murine L929 (L) cells were grown in either suspension or monolayer cultures in Joklik's modified Eagle's minimal essential medium (Sigma-Aldrich, St. Louis, MO) supplemented to contain 5% fetal bovine serum, 2 mM l-glutamine, 100 U of penicillin per ml, 100 U of streptomycin per ml, and 0.25 mg amphotericin per ml (Gibco Invitrogen Corp., Grand Island, NY). GD25, GD25β1A, GD25β1AY783F, GD25β1AY795F, and GD25β1AY783F/Y795F cells (69, 70) were obtained from Deane Mosher (University of Wisconsin, Madison) and maintained in Dulbecco's minimal essential medium (Gibco Invitrogen) supplemented to contain 10% fetal bovine serum, 100 U of penicillin per ml, and 100 U of streptomycin per ml. Medium for all GD25β1A cells was supplemented to contain 10 μg of puromycin (Sigma-Aldrich) per ml to maintain β1 integrin expression (69, 70).
Reovirus strain type 1 Lang (T1L) is a laboratory stock. Working stocks of virus were prepared by plaque purification and passage using L cells (66). Purified virions were generated from second-passage L-cell lysate virus stocks. Virus was purified from infected cell lysates by Freon extraction and CsCl gradient centrifugation as described previously (26). Bands corresponding to the density of reovirus particles (1.36 g/cm3) were collected and dialyzed against virion storage buffer (150 mM NaCl, 15 mM MgCl2, 10 mM Tris [pH 7.4]). The reovirus particle concentration was determined from the equivalence of 1 unit of optical density at 260 nm to 2.1 × 1012 particles (58). The viral titer was determined by fluorescent focus assay (4). ISVPs were generated by the treatment of 2 × 1011 particles with 200 μg of α-chymotrypsin (Sigma-Aldrich) in a 100-μl volume of virion storage buffer at 37°C for 30 min (2). Reactions were terminated by the addition of 1.0 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich).
Immunoglobulin G (IgG) fractions of rabbit antisera raised against T1L and type 3 Dearing (71) were purified by protein A Sepharose as described previously (4). Fluorescently conjugated secondary Alexa antibodies were obtained from Molecular Probes (Invitrogen, San Diego, CA). R-phycoerythrin-conjugated secondary antibodies were obtained from BD Biosciences Pharmingen (Franklin Lakes, NJ). Murine β1 integrin-specific monoclonal antibody (MAb) MAB1997 (Chemicon, Temecula, CA) and JAM-A-specific MAb H202-106-7-4 (provided by Beat Imof, Université de Genève) were used to assess cell surface expression of β1 integrin and JAM-A by flow cytometry. IgG controls used for flow cytometry were purchased from Chemicon.
Flow cytometric analysis.
Cells were detached from plates using phosphate-buffered saline (PBS)-EDTA (20 mM EDTA), washed, and centrifuged at 1,000 × g to form a pellet. Cells were resuspended with antibodies in PBS-bovine serum albumin (BSA) (Sigma-Aldrich) (5% BSA) and incubated at 4°C for 1 h. Cells were washed twice and incubated with an appropriate secondary antibody conjugated to R-phycoerythrin (BD Biosciences Pharmingen) at 4°C for 1 h. Cells were washed, resuspended in PBS, and analyzed by flow cytometry. Reovirus binding to cells was analyzed by adsorbing cells with T1L particles at 4°C for 1 h. Cells were washed and stained with reovirus polyclonal antisera and an appropriate secondary antibody conjugated to Alexa 546 (Invitrogen) and analyzed by flow cytometry.
Fluorescent focus assay of viral infection.
Cells plated in 24-well plates (Costar; Corning Life Sciences, Corning, NY) were either untreated or treated with 5 μg per ml chlorpromazine (Alexis Biochemicals, Lausen, Switzerland) at 37°C for 3 h. Cells were adsorbed with virions or ISVPs in incomplete medium (without serum and antibiotics) at 4°C for 1 h. Inocula were removed, cells were washed, and complete medium with or without chlorpromazine was added. Infected cells were incubated at 37°C for 20 h to allow a single cycle of viral replication. Cells were fixed with cold, absolute methanol at −20°C for at least 30 min. Fixed cells were washed with PBS, incubated with PBS-BSA (5% BSA) for at least 15 min, and incubated with reovirus-specific polyclonal antiserum (1:500) in PBS containing 0.5% Triton X-100 (TX-100) at room temperature for 1 h. Cells were washed twice and incubated with an Alexa Fluor 488- or 546-labeled anti-rabbit IgG (1:1,000) in PBS-TX-100 (0.5% TX-100) at room temperature for 1 h. Cells were washed twice and visualized by indirect immunofluorescence at a magnification of ×20 using an Axiovert 200 fluorescence microscope (Carl Zeiss, New York, NY). Infected cells (fluorescent focus units [FFU]) were identified by diffuse cytoplasmic fluorescence staining that was excluded from the nucleus. Reovirus-infected cells were quantified by counting random fields in equivalently confluent monolayers for three to five fields of view for triplicate wells (4).
Confocal imaging of reovirus internalization.
Cells plated on coverslips in 24-well plates (Costar) were adsorbed with reovirus virions in incomplete medium and incubated at 4°C for 1 h. Cells were washed and fixed or nonadherent reovirus was aspirated and replaced with complete medium and incubated at 37°C. At various intervals, cells were washed with PBS and fixed with 10% formalin (Laboratory Supply Company, Louisville, KY) for 20 min, followed by washing with PBS. Cells were treated with 1% TX-100 for 5 min and incubated with PBS-BGT (PBS, 0.5% BSA, 0.1% glycine, and 0.05% Tween 20) for 10 min. Cells were incubated with reovirus-specific polyclonal antiserum (1:500) in PBS-BGT for 1 h, washed with PBS-BGT, and incubated with donkey anti-rabbit immunoglobulin conjugated to Alexa Fluor 488 (Molecular Probes) (1:1,000) to visualize reovirus and phalloidin conjugated to Alexa Fluor 546 (Molecular Probes) (1:100) to visualize actin for 1 h in PBS-BGT. Cell nuclei were visualized by incubating cells with To-Pro 3 conjugated to Alexa Fluor 642 (Molecular Probes) (1:1,000) in PBS-BGT for 20 min. Cells were washed extensively with PBS-BGT, and coverslips were removed from wells and placed on slides using Aqua-Poly/Mount mounting medium (Polysciences, Inc., Warrington, PA). Images were captured using a Zeiss LSM 510 Meta laser-scanning confocal microscope. Three-dimensional image reconstructions were generated from multiple images collected in the Z plane and compiled using Zeiss imaging software.
Quantitation of virus internalization.
Virus internalization was quantified as a function of pixel intensity using Metamorph software (Universal Imagining, Downingtown, PA). Confocal images were collected and analyzed for internalized reovirus particles by capturing multiple images in the Z plane and determining the center of the cell based on cell thickness as analyzed using Zeiss imaging software. Cells analyzed for quantitative purposes were selected at random and counted by an observer blinded to the conditions of the experiment. The intracellular space was outlined using the trace tool to exclude the plasma membrane, which was identified by intense actin staining. The region measurement function was used to quantify intensity of green pixels in the trace region for each cell.
Confocal imaging of transferrin internalization.
Cells were plated on coverslips in 24-well plates and incubated at 37°C overnight in complete medium. Following incubation in incomplete medium at 37°C for 30 min, cells were untreated or treated with 5 μg per ml chlorpromazine at 37°C for 3 h. Cells were incubated with 2.5 μg per ml Alexa Fluor 488-conjugated transferrin (Molecular Probes) in the presence or absence of 5 μg per ml chlorpromazine in incomplete medium at 37°C for 10 min. The medium was removed, and cells were washed once with PBS, once with an acid wash (0.2 M acetic acid, 0.5 M NaCl in distilled H2O [diH2O]), and twice with PBS. Cells were fixed with 10% formalin and prepared for confocal microscopy. Transferrin internalization was quantified using Metamorph software (Universal Imaging) as described for virus internalization.
EM.
Cells plated in 60-mm dishes (Costar) were prechilled at 4°C for 1 h, adsorbed with reovirus virions in incomplete medium, and incubated at 4°C for 1 h. Cells were washed twice with PBS, and cells were harvested or nonadherent virus was aspirated and replaced with warm, complete medium and incubated at 37°C. At 10-min intervals, cells were harvested on ice in 1 ml of PBS using a cell lifter (Costar) and transferred to a 1.5-ml microcentrifuge tube. Cells were pelleted at 1,000 × g at 4°C for 5 min. The supernatant was removed, and 1 ml of 2% glutaraldehyde was placed over the cell pellet. Cells were incubated at room temperature for 90 min. Glutaraldehyde was removed and replaced with fresh 2% glutaraldehyde and incubated overnight at 4°C. Cells were washed three times in PBS, transferred to 1% osmium tetroxide in diH2O for 1 h, and washed three times in diH2O. Cells were stained en bloc in 1% aqueous uranyl acetate for 1 h and washed three times in diH2O. Cells were dehydrated by using a graded series of ethanol washes and by increasing the incubation times in each solution starting with 30% for 5 min, followed by two washes with 50% for 5 min, 70% for 5 min, 95% for 10 min, and absolute ethanol for 15 min. Cells were passed through propylene oxide, transferred to a 1:1 araldite:propylene oxide mixture, and embedded in Araldite embedding medium (Electron Microscopy Sciences, Hatfield, PA). Ultra-thin serial sections (50 to 60 nm) were obtained using a Leica UCT Ultracut microtome (Leica Microsystems, Vienna, Austria), transferred to Formvar-coated grids, and examined using a Phillips CM10 transmission EM (FEI Company, Hillsboro, OR) equipped with an Advantage Plus Digital charge-coupled-device system for CM10 transmission EM (Advanced Microscopy Techniques, Danvers, MA).
Reovirus localization with lysosomal markers.
Cells were plated on coverslips in 24-well plates and incubated with 75 nM Lysotracker-Red DND-99 dye (Molecular Probes) in incomplete medium at 37°C for 30 min. Cells were prechilled at 4°C for 1 h, adsorbed with reovirus virions in incomplete medium, and incubated at 4°C for 1 h. Cells were washed on ice three times with PBS, nonadherent reovirus was removed, and complete medium was added. Following incubation at 37°C for various intervals, cells were washed with PBS, fixed with 10% formalin, and prepared for confocal immunofluorescence microscopy. Images were analyzed for colocalization of Lysotracker-Red DND-99 dye and reovirus virions using ImageJ software (version 1.37v; W. S. Rasband, National Institutes of Health, Bethesda, MD) Colocalize RGB plug-in.
Statistical analysis.
Means for at least triplicate samples were compared by using unpaired Student's t test (Microsoft Excel, Redmond, WA). P values of <0.05 were considered to be statistically significant.
RESULTS
Reovirus infection is diminished in cells expressing β1 integrin with altered NPXY motifs.
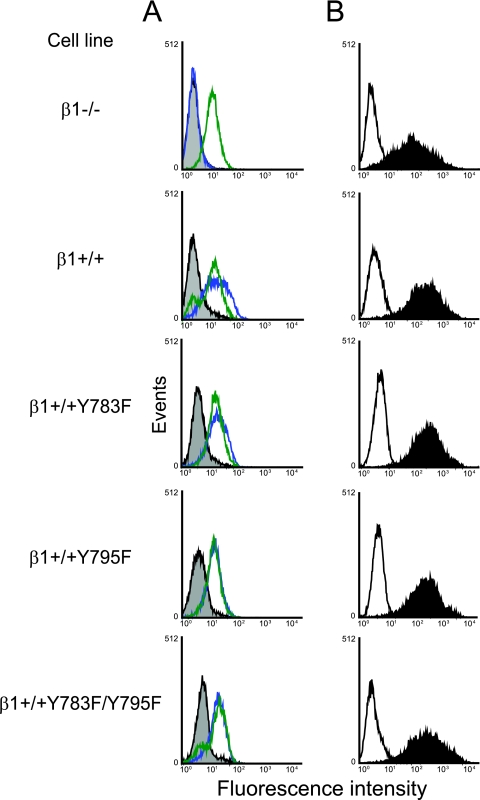
To determine whether the NPXY motifs in the cytoplasmic domain of β1 integrin are required for functional reovirus entry, we tested cells with mutations in the β1 integrin NPXY motifs for the capacity to support reovirus infection. GD25 (β1−/−) cells are embryonic stem cells derived from β1-null mouse embryos, while GD25β1A (β1+/+) cells stably express full-length, wild-type β1 integrin (70). GD25β1AY783F (β1+/+Y783F), GD25β1AY795F (β1+/+Y795F), and GD25β1AY783F/Y795F (β1+/+Y783F/Y795F) cells stably express β1 integrin in which the tyrosine residues of the NPXY motifs at amino acid positions 783 and 795 have been substituted with phenylalanine (69, 70). Cell surface expression of β1 integrin by β1−/−, β1+/+, β1+/+Y783F, β1+/+Y795F, and β1+/+Y783F/Y795F cells was assessed using flow cytometry (Fig. 1A). With the exception of β1−/− cells, these cell lines expressed equivalent levels of cell surface β1 integrin. Importantly for our studies of reovirus infection, these cells also expressed equivalent levels of cell surface JAM-A (Fig. 1A). To test for attachment of reovirus to cells expressing wild-type and mutant β1 integrin, cells were incubated with reovirus particles, and binding was detected using flow cytometry (Fig. 1B). Reovirus bound equivalently to all cell types, suggesting that reovirus attachment is not affected by mutation of the β1 integrin NPXY motifs to NPXF.
FIG. 1.
Reovirus exhibits equivalent binding to β1−/−, β1+/+, and β1+/+ cells with altered NPXY motifs. (A) GD25 (β1−/−), GD25β1A (β1+/+), GD25β1AY783F (β1+/+Y783F), GD25β1AY795F (β1+/+Y795F), and GD25β1AY783F/Y795F (β1+/+Y783F/Y795F) cells were detached from plates using 20 mM EDTA, washed, collected by centrifugation, and incubated with antibodies specific for either murine β1 integrin (blue), murine JAM-A (green), or an IgG control antibody (gray). Cell surface expression of these molecules was detected by flow cytometry. Data are expressed as fluorescence intensity. (B) β1−/−, β1+/+, β1+/+Y783F, β1+/+Y795F, and β1+/+Y783F/Y795F cells were detached from plates using 20 mM EDTA, washed, collected by centrifugation, and incubated with 104 particles per cell of reovirus T1L in incomplete medium (filled) or incomplete medium alone (open) at 4°C for 1 h to allow attachment. Cells were washed and incubated with reovirus-specific antiserum. Reovirus binding was detected by flow cytometry. Data are expressed as fluorescence intensity.
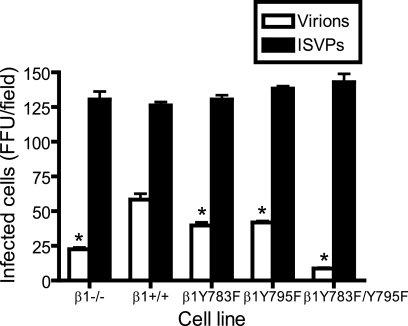
To determine whether mutations in the β1 integrin NPXY motifs alter reovirus infection, cells were adsorbed with reovirus virions or ISVPs, and infectivity was scored by indirect immunofluorescence (Fig. 2). In comparison to β1+/+ cells, β1−/−, β1+/+Y783F, β1+/+Y795F, and β1+/+Y783F/Y795F cells were less susceptible to infection by reovirus virions (Fig. 2). While reovirus infection was significantly reduced in β1+/+Y783F and β1+/+Y795F cells, infection was most dramatically diminished in β1+/+Y783F/Y795F cells, to an extent even greater than in β1−/− cells (Fig. 2). ISVPs, which bind to JAM-A (5) but bypass a requirement for endocytosis and disassembly (2, 59), were capable of infecting all cell types (Fig. 2). Equivalent infection by ISVPs suggests that the block to reovirus infection in cells expressing mutant β1 integrin occurs subsequent to viral attachment but preceding capsid disassembly.
FIG. 2.
Reovirus infection is diminished in cells with altered β1 integrin NPXY motifs. β1−/−, β1+/+, β1+/+Y783F, β1+/+Y795F, and β1+/+Y783F/Y795F cells were adsorbed with T1L virions or ISVPs at an MOI of 1 FFU per cell at 4°C for 1 h. Cells were washed with PBS, incubated in complete medium at 37°C for 20 h, fixed, and stained by indirect immunofluorescence. Infected cells were quantified by counting cells exhibiting cytoplasmic staining in five visual fields of equivalently confluent monolayers for triplicate samples. The results are expressed as mean FFU per visual field for triplicate samples. Error bars indicate standard deviations. These data are representative of results of three individual experiments performed in triplicate. *, P < 0.05 in comparison to β1+/+ cells.
Reovirus virions are internalized into the cytoplasm of β1+/+Y783F/Y795F cells.
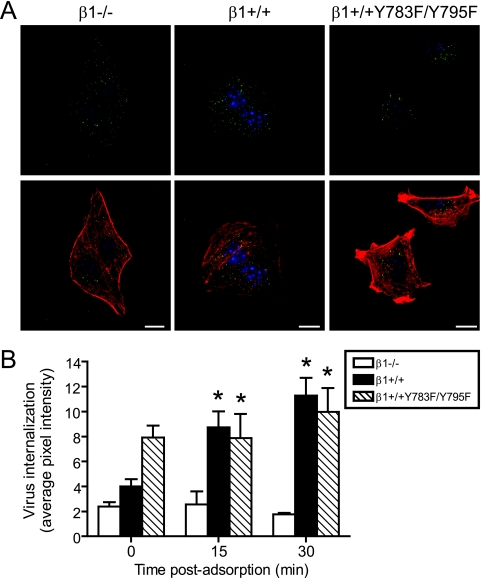
To determine whether the block to reovirus infection in β1+/+Y783F/Y795F cells is due to a defect in internalization, β1−/−, β1+/+, and β1+/+Y783F/Y795F cells were adsorbed with reovirus particles at 4°C for 1 h to allow virus binding and then incubated at 37°C to allow internalization over a time course concurrent with reovirus entry. At 0, 15, and 30 min, cells were fixed, stained using reovirus-specific antiserum, and examined for reovirus particles by confocal immunofluorescence microscopy. Representative confocal micrographic images of β1−/−, β1+/+, and β1+/+Y783F/Y795F cells infected with reovirus and fixed at 30 min postadsorption are shown in Fig. 3A. Although reovirus particles were observed in the cytoplasm of all cell types, there was a notable decrease in β1−/− cells, as described previously (34). Surprisingly, reovirus particles were internalized into both β1+/+ and β1+/+Y783F/Y795F cells (Fig. 3A). To determine the distribution of reovirus particles internalized into β1+/+ and β1+/+Y783F/Y795F cells, multiple confocal micrograph images in the Z plane were collected and examined using Z-stack analysis and three-dimensional image reconstructions (data not shown). Comparisons of the two cell types revealed that reovirus particles were distributed throughout the nonnuclear compartment of β1+/+Y783F/Y795F cells in a pattern similar to that for β1+/+ cells. To directly quantify the fluorescence intensity of internalized reovirus particles, confocal micrographs were obtained over a time course and analyzed using Metamorph imaging software. The average pixel intensity representing reovirus particles was significantly greater in β1+/+ and β1+/+Y783F/Y795F cells than in β1−/− cells (Fig. 3B). These findings indicate that reovirus particles are internalized into β1+/+ and β1+/+Y783F/Y795F cells, and in both cases, the number of particles internalized exceeds that for β1−/− cells.
FIG. 3.
Reovirus is internalized into β1+/+ and β1+/+Y783F/Y795F cells. β1−/−, β1+/+, and β1+/+Y783F/Y795F cells were adsorbed with 5 × 104 particles per cell of T1L virions and incubated at 4°C for 1 h. Nonadherent virus was removed, warm medium was added, and cells were incubated at 37°C. Cells were fixed over a time course, stained for reovirus (green), actin (red), and DNA (blue), and imaged using confocal immunofluorescence microscopy. (A) Representative digital fluorescence images of cells fixed at 30 min postadsorption are shown. Scale bars, 10 μm. (B) Fluorescent particles internalized into the cytoplasm were analyzed to determine the average pixel intensity of fluorescent particles per cell. Fluorescent particles localized at the cell periphery were excluded from the analysis. The results are expressed as the average pixel intensity per cell for five cells at each time point. Error bars indicate standard errors of the means. *, P < 0.05 in comparison to β1−/− cells.
Reovirus uptake into β1+/+ cells is blocked by chlorpromazine.
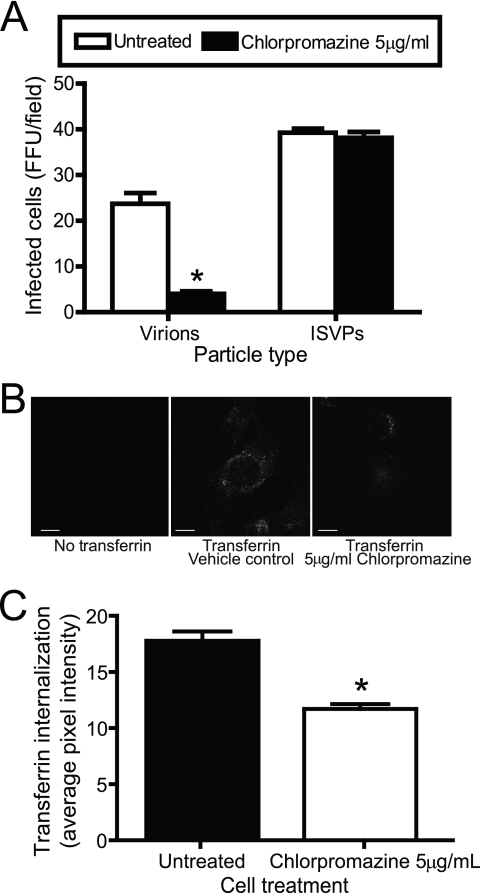
To determine the mechanism required for functional reovirus entry, β1+/+ cells were pretreated with chlorpromazine, which inhibits clathrin assembly at the plasma membrane by interfering with AP-2 localization to membranes and forcing clathrin lattices to form on endosomal membranes (67). Treatment of cells with chlorpromazine blocks clathrin-dependent endocytosis of a number of viruses, including hepatitis C virus (7), polyomavirus JC virus (49), and vesicular stomatitis virus (60). Cells were infected with reovirus virions or ISVPs in the presence or absence of chlorpromazine and scored for infection by indirect immunofluorescence (Fig. 4A). Treatment of cells with 5 μg per ml of chlorpromazine decreased viral infectivity by greater than 80% following adsorption with virions (Fig. 4A). However, infectivity was unaffected by chlorpromazine treatment following adsorption with ISVPs, suggesting that diminished infectivity by reovirus is caused by a block to entry. Failure of chlorpromazine to inhibit infection by ISVPs also indicates that the concentration of chlorpromazine used in these experiments does not affect viral attachment or the capacity of cells to support the reovirus replication cycle. As a control, chlorpromazine treatment of β1+/+ cells diminished the uptake of transferrin (Fig. 4B and C), which is internalized by clathrin-dependent endocytosis (29, 48). Chlorpromazine also blocked reovirus infection and transferrin uptake in experiments using HeLa cells and L cells (data not shown). Thus, reovirus virions use a chlorpromazine-sensitive pathway, most likely clathrin-mediated endocytosis, to enter cells.
FIG. 4.
Uptake of reovirus and transferrin into β1+/+ cells is chlorpromazine sensitive. (A) β1+/+ cells were pretreated with 5 μg of chlorpromazine per ml for 3 h, adsorbed with T1L virions or ISVPs at an MOI of 1 FFU per cell, and incubated at 4°C for 1 h. Cells were washed, complete medium with or without chlorpromazine was added, and cells were incubated at 37°C for 20 h. Cells were fixed and stained by indirect immunofluorescence. Infected cells were quantified by counting cells exhibiting cytoplasmic staining in three visual fields of equivalently confluent monolayers for triplicate samples. The results are expressed as mean FFU per visual field for triplicate samples. Error bars indicate standard deviations. These data are representative of results of three independent experiments performed in triplicate. *, P < 0.05 in comparison to untreated cells. (B) β1+/+ cells were pretreated with 5 μg per ml of chlorpromazine for 3 h and incubated with 2.5 μg per ml Alexa Fluor 488-conjugated transferrin in the presence or absence of 5 μg per ml chlorpromazine in incomplete medium at 37°C for 10 min. The medium was removed, and cells were washed, fixed, and imaged by confocal microscopy. Representative digital fluorescence images of untreated and chlorpromazine-treated cells incubated with transferrin are shown. Scale bars, 10 μm. (C) Untreated and chlorpromazine-treated cells were analyzed for fluorescent transferrin internalized into the cytoplasm to determine the average pixel intensity of transferrin per cell. Fluorescent signal localized at the cell periphery was excluded from the analysis. The results are expressed as the average pixel intensity per cell for 10 cells. Error bars indicate standard errors of the means. *, P < 0.05 in comparison to untreated cells.
β1 integrin NPXY motifs are required for functional reovirus uptake.
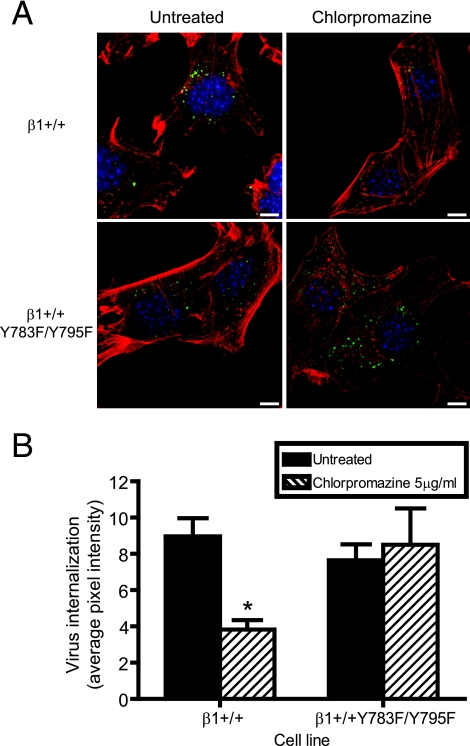
To determine the role of the β1 integrin NPXY motifs in reovirus endocytosis, β1+/+ and β1+/+Y783F/Y795F cells were either treated with chlorpromazine or left untreated, adsorbed with reovirus particles at 4°C for 1 h to allow virus binding, and incubated at 37°C to allow internalization. At 0 and 20 min postadsorption, cells were fixed, stained using reovirus-specific antiserum, and examined by confocal immunofluorescence microscopy. Representative confocal micrographic images of β1+/+ and β1+/+Y783F/Y795F cells at 20 min postadsorption are shown in Fig. 5A. The fluorescence intensities of reovirus particles internalized into β1+/+ and β1+/+Y783F/Y795F cells were quantified by averaging the pixel intensities for representative confocal micrographs by using Metamorph imaging software (Fig. 5B). In β1+/+ cells treated with chlorpromazine, the average pixel intensity representing reovirus particles was significantly diminished in comparison to that in untreated β1+/+ cells (Fig. 5B). However, chlorpromazine treatment did not diminish the pixel intensity in β1+/+Y783F/Y795F cells, indicating that reovirus internalization into β1+/+Y783F/Y795F cells is not inhibited by chlorpromazine (Fig. 5B). These data demonstrate that the reovirus uptake pathway in β1+/+ cells is chlorpromazine sensitive, whereas reovirus internalization into β1+/+Y783F/Y795F cells is mediated by an alternative uptake mechanism that is not sensitive to chlorpromazine and does not give rise to productive infection.
FIG. 5.
β1 integrin NPXY motifs are required for functional reovirus internalization. β1+/+ and β1+/+Y783F/Y795F cells were pretreated with 5 μg per ml of chlorpromazine at 37°C for 3 h, adsorbed with 5 × 104 particles per cell of T1L virions in incomplete medium, and incubated at 4°C for 1 h. Cells where washed, complete medium with or without chlorpromazine was added, and cells were incubated at 37°C for 20 min. Cells were fixed, stained for reovirus (green), actin (red), and DNA (blue), and imaged using confocal immunofluorescence microscopy. (A) Representative digital fluorescence images of untreated and chlorpromazine-treated cells at 20 min postadsorption are shown. Scale bars, 10 μm. (B) Fluorescent particles internalized into the cytoplasm were analyzed to determine the average pixel intensity per cell. Fluorescent particles localized at the cell periphery were excluded from the analysis. The results are expressed as the average pixel intensity per cell for five cells. Error bars indicate standard errors of the means. *, P < 0.05 in comparison to untreated cells.
Intracellular trafficking of reovirus virions.
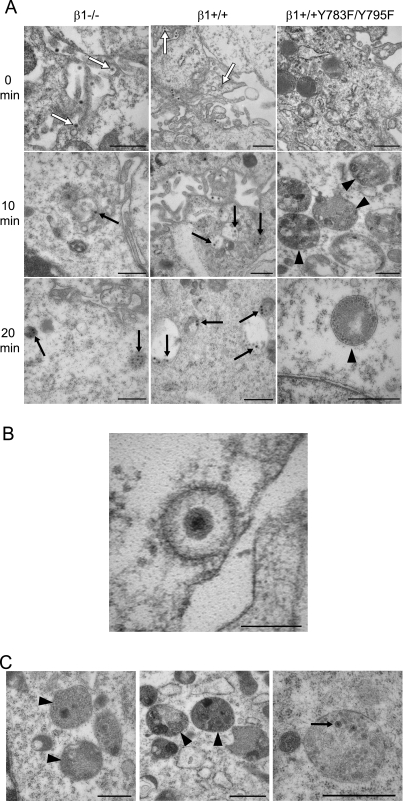
To define the fate of reovirus virions in the endocytic pathway during internalization and disassembly, we performed an ultrastructural analysis of reovirus-infected cells. β1−/−, β1+/+, and β1+/+Y783F/Y795F cells were adsorbed with reovirus particles at 4°C for 1 h to allow virus binding, incubated at 37°C over a 30-min time course to allow internalization, and analyzed by transmission EM (Fig. 6). Fewer reovirus particles were detected in β1−/− cells than in β1+/+ and β1+/+Y783F/Y795F cells at all time points examined, consistent with the quantitative confocal microscopy data. In β1+/+ cells, and to a much lesser extent in β1−/− cells, reovirus particles were clearly visible in coated-pit and coated-vesicle structures at 0 min postadsorption (Fig. 6A, white arrows, and Fig. 6B). At 10, 20, and 30 min postadsorption, the majority of reovirus particles (Fig. 6A, black arrows, and data not shown) appeared in structures that morphologically resemble early endosomes, which have an electron-lucent appearance, late endosomes, which appear larger and more electron dense, and primary lysosomes, which are small, electron-dense, membrane-bound vesicles. In β1+/+Y783F/Y795F cells, few reovirus particles were observed in coated-pit structures or structures that resemble endosomes. Instead, the majority of reovirus particles in β1+/+Y783F/Y795F cells localized in electron-dense, membrane-bound vesicular structures that morphologically resemble secondary lysosomes or phagolysosomes, which contain a notable amount of heterogeneous material (Fig. 6A and C, black arrowheads). These structures were present in both uninfected and infected β1−/− and β1+/+Y783F/Y795F cells, yet there were a greater number of these structures in the infected β1+/+Y783F/Y795F cells (Fig. 6A). These findings suggest that NPXY-to-F mutations in the β1 integrin cytoplasmic tail lead to delivery of reovirus virions to an endocytic compartment incapable of either supporting functional capsid disassembly or allowing release of uncoated particles into the cytoplasm.
FIG. 6.
Ultrastructural analysis of reovirus internalization. (A) β1−/−, β1+/+, and β1+/+Y783F/Y795F cells were adsorbed with 105 particles per cell of T1L virions at 4°C for 1 h, washed, and fixed or incubated in complete medium at 37°C. At 10-min intervals, cells were washed, collected by centrifugation, fixed, and stained for EM. Representative images at 0, 10, and 20 min postadsorption are shown. Reovirus virions localized in coated pits and coated vesicles are indicated by white arrows. Reovirus virions localized in structures that resemble early and late endosomes and primary lysosomes are indicated by black arrows. Secondary lysosomes are indicated by black arrowheads. Scale bars, 500 nm. (B) Representative image of reovirus virion in a coated pit structure in β1+/+ cells at 0 min postadsorption. Scale bar, 100 nm. (C) Representative images of organelles resembling secondary lysosomes containing reovirus particles in β1+/+Y783F/Y795F cells at 10, 20, and 30 min postadsorption (left, center, and right, respectively). Reovirus virions are indicated by black arrows. Secondary lysosomes are indicated by black arrowheads. Scale bars, 500 nm.
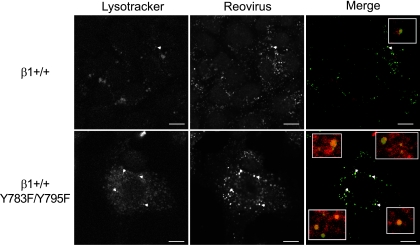
In a complementary approach to assess the intracellular distribution of reovirus particles following internalization into β1+/+ and β1+/+Y783F/Y795F cells, both cell types were pretreated with the lysosomal-specific marker, Lysotracker-Red DND-99 dye, adsorbed with reovirus virions, and examined by confocal immunofluorescence microscopy (Fig. 7 and data not shown). Reovirus particles appeared in vesicles marked by Lysotracker-Red DND-99 dye in both β1+/+ and β1+/+Y783F/Y795F cells, yet substantially more viral particles were localized to these structures in β1+/+Y783F/Y795F cells at 40 min postadsorption than in β1+/+ cells at that time point (Fig. 7, white arrowheads). These data suggest that while reovirus particles are transported to a lysosomal compartment in both β1+/+ and β1+/+Y783F/Y795F cells, virions in β1+/+ cells egress this compartment to initiate an infectious cycle, while virions in β1+/+Y783F/Y795F cells are trapped and fail to productively infect the cell.
FIG. 7.
Intracellular transport of reovirus particles is altered in β1+/+Y783F/Y795F cells. β1+/+ and β1+/+Y783F/Y795F cells were pretreated with Lysotracker-Red DND-99 dye (Lysotracker), adsorbed with 5 × 104 particles per cell of T1L virions (Reovirus), and incubated at 4°C for 1 h. Nonadherent virus was removed, warm medium was added, and cells were incubated at 37°C. Cells were fixed over a time course and stained for reovirus. Images were analyzed for colocalization using ImageJ (version 1.37v) Colocalize RGB plug-in. Representative digital fluorescence images pseudocolored as monochromatic images or fluorescence images of cells fixed at 40 min postadsorption are shown. Lysotracker-Red DND-99 dye is colored red; reovirus is colored green. White arrowheads indicate areas of colocalization, which are magnified in the insets. Scale bars, 10 μm.
DISCUSSION
The goal of this study was to gain a better understanding of mechanisms by which β1 integrin mediates reovirus internalization. The data demonstrate a function for the β1 integrin NPXY motifs in reovirus endocytosis and endocytic transport. Tyrosine-to-phenylalanine mutation of the β1 integrin NPXY motifs leads to an aberrant pathway of reovirus internalization that does not yield infectious progeny. EM analysis indicates that reovirus virions are internalized into β1+/+Y783F/Y795F cells yet localize in organelles that morphologically resemble secondary lysosomes rather than in endosomes and lysosomes as they do in β1+/+ cells. Reovirus internalization into β1+/+ cells is mediated by a chlorpromazine-sensitive pathway, most likely clathrin-dependent endocytosis, yet particles internalized into β1+/+Y783F/Y95F cells enter by a different route. Collectively, these data indicate that β1 integrin serves to deliver reovirus to the endocytic compartment required to complete subsequent steps in the viral life cycle.
Findings presented in this report provide new insights into mechanisms by which reovirus enters into cells. After attachment to carbohydrate (4, 19, 46, 47) and JAM-A (5, 14, 50), reovirus is internalized into the endocytic pathway by β1 integrin (34). Our results indicate that this process is dependent on signals elicited by the NPXY motifs in the β1 integrin cytoplasmic tail. Since chlorpromazine diminishes reovirus entry and infection in β1+/+ cells, but does not affect reovirus uptake into β1+/+Y783F/Y795F cells, it is likely that the β1 integrin NPXY motifs recruit the endocytic machinery to deliver reovirus to the endocytic organelle required for capsid disassembly and release of the viral core into the cytoplasm.
NPXY motifs recruit clathrin and adaptor proteins and serve as cargo recognition motifs to direct the delivery of cargo to endosomes and lysosomes (10, 37). For example, NPXY motifs recruit AP-2 to the cell surface by directly interacting with the AP-2 μ2 subunit (9). The β2 subunit of AP-2 then binds clathrin subunits (45) and initiates clathrin assembly at the plasma membrane, which leads to accumulation of clathrin at the cell surface for clathrin-mediated endocytosis (44). NPXY motifs also recruit Dab2 (38, 44), which directly interacts with NPXY motifs via the Dab2 phosphotyrosine binding domain (37). Dab2 induces clathrin-mediated endocytosis by binding either to clathrin (37) or to AP-2 (39). Dab2 recognizes either phosphorylated or nonphosphorylated tyrosines of NPXY motifs (37, 38). Therefore, in β1+/+Y783F/Y795F-infected cells, Dab2 could potentially engage β1 integrin with NPXY-to-F mutations and mediate reovirus internalization but direct particles to a nonfunctional endocytic compartment. Since reovirus infection requires acid-dependent proteolytic disassembly (2, 23, 59), virus delivery to an intracellular site other than an endosomal compartment containing the appropriate pH (59) and proteolytic enzymes (23) could result in the failure of reovirus particles to uncoat and penetrate into the cytoplasm. Our experiments using Lysotracker-Red DND-99 dye suggest that reovirus virions distribute to a lysosomal compartment in both β1+/+ and β1+/+Y783F/Y795F cells (Fig. 7). However, this marker does not distinguish between primary and secondary lysosomes, which appear morphologically distinct (Fig. 6). Additional experiments are required to define the intracellular transport pathways traversed by reovirus following uptake into wild-type and mutant cells.
Since phenylalanine residues cannot be phosphorylated, our findings with cells expressing β1 integrin with NPXY-to-F mutations in the cytoplasmic tail raise the possibility that reovirus interactions with β1 integrin lead to tyrosine phosphorylation of the NPXY motifs. In support of this hypothesis, v-src transformation of β1+/+ cells but not β1+/+Y783F/Y795F cells results in phosphorylation of β1 integrin (53), suggesting that the NPXY tyrosine residues can serve as substrates for phosphorylation. It is possible that phosphorylation of the β1 integrin NPXY motifs is required for postattachment signaling events required for reovirus endocytosis or other steps in the virus life cycle. For example, phosphorylation of the NPXY tyrosine residues is required for autophosphorylation of focal adhesion kinase (68). Thus, reovirus-β1 integrin interactions may induce phosphorylation and activation of integrin-linked signaling pathways.
It is also possible that the β1 integrin NPXY motifs mediate functional reovirus internalization by an indirect mechanism. The NPXY motifs in the β1 integrin cytoplasmic tail are separated by eight residues that form a tight β turn (3, 63). This region serves as a docking site for phosphotyrosine binding domain-containing proteins (61), including talin (13) and integrin cytoplasmic domain-associated protein 1-α (18). In addition, two of the intervening residues are threonines at positions 788 and 789, and Thr788 is notably essential for integrin activation and ligand engagement (41). Therefore, mutation of the NPXY motifs may result in structural alterations that affect the β1 integrin cytoplasmic tail and preclude engagement of the cytosolic molecules required for reovirus uptake or sorting within the endocytic pathway.
β1+/+Y783F/Y795F cells are surprisingly less permissive for reovirus infection than β1−/− cells (Fig. 2). Since both β1−/− and β1+/+Y783F/Y795F cells express JAM-A on the cell surface, it is possible that reovirus infection of these cells occurs via attachment to JAM-A and internalization via a non-β1-integrin-dependent mechanism, resulting in a low level of infection. However, in the case of β1+/+Y783F/Y795F cells, it is possible that virus interactions with the mutant form of β1 integrin are preferred to non-β1 integrin internalization routes, resulting in delivery of virions to a nonproductive entry pathway as a consequence of the NPXY-to-F mutations. Thus, these data do not exclude the possibility that reovirus utilizes coreceptors other than β1 integrin to internalize into cells after binding to JAM-A. Additionally, it is possible that reovirus internalization may occur via both clathrin-dependent and clathrin-independent mechanisms, which has been reported for influenza virus (56).
Following adsorption to β1+/+Y783F/Y795F cells, reovirus virions localize in electron-dense, membrane-bound vesicular structures that morphologically resemble secondary lysosomes or phagolysosomes (Fig. 6). These structures are present in both uninfected and infected β1−/− and β1+/+Y783F/Y795F cells at all time points analyzed. However, they are not apparent in either uninfected or infected β1+/+ cells. These observations suggest that the lack of β1 integrin signaling or recycling has a detrimental effect on endocytic transport that alters the morphology of endocytic organelles. As probed by analysis of reovirus internalization, these alterations are associated with impairments in the sorting or processing of cargo in the endocytic compartment.
β1 integrin NPXY motifs are required for efficient uptake of Y. pseudotuberculosis by clathrin-mediated endocytosis (28, 64). However, mutation of the NPXY motifs to NPXF does not affect internalization of Y. pseudotuberculosis; instead, mutation to NPXA leads to abolished Y. pseudotuberculosis uptake. Transgenic mice expressing β1 integrin with NPXY-to-F mutations are viable (21), whereas those expressing NPXY-to-A mutations recapitulate the phenotype of β1-null mice and arrest in embryogenesis (21). These data indicate that there are key functional differences mediated by the nature of β1 integrin NPXY motif alterations. In contrast to studies of Y. pseudotuberculosis, we found that mutation of NPXY to NPXF results in a nonfunctional route of internalization for reovirus, suggesting that the β1 integrin NPXY motifs play an important regulatory role in endocytic uptake and transport in the endocytic pathway. Our findings, coupled with previous findings made with Y. pseudotuberculosis (28, 64), provide support for the idea that β1 integrin NPXY motifs are required for multiple functions in the process of endocytosis. Understanding mechanisms by which integrins engage the endocytic machinery to mediate endocytosis of microbes should provide a useful avenue to investigate integrin cell biology and lead to the identification of new targets for anti-infective drug development.
Acknowledgments
We thank Pranav Danthi and members of the Dermody laboratory for advice and technical assistance. We thank Elvin Woodruff for performing the EM studies and Agnes Fogo and Anne Kenworthy for assistance in interpretation of the EM images. We thank the Nashville Veterans Hospital Flow Cytometry Core for flow cytometric analysis. Confocal microscopy experiments were performed in part through use of the VUMC Cell Imaging Shared Resource. We thank Deane Mosher (University of Wisconsin, Madison) for providing GD25 cell lines. We thank Beat Imof (Université de Genève) for providing mJAM-A-specific MAb H202-106-7-4.
This research was supported by Public Health Service awards T32 AI007281 (M.S.M.), T32 HL07751 (B.A.M.), T32 HL07526 (A.D.), T32 AI07611 (E.M.J.), R01 AI32539, the Vanderbilt Research Council (M.S.M.), a Veterans Affairs Merit Award (R.Z.), and the Elizabeth B. Lamb Center for Pediatric Research. Additional support was provided by Public Health Service awards P30 CA68485 for the Vanderbilt-Ingram Cancer Center and P60 DK20593 for the Vanderbilt Diabetes Research and Training Center.
Footnotes
Published ahead of print on 23 January 2008.
REFERENCES
- 1.Agosto, M. A., T. Ivanovic, and M. L. Nibert. 2006. Mammalian reovirus, a nonfusogenic nonenveloped virus, forms size-selective pores in a model membrane. Proc. Natl. Acad. Sci. USA 10316496-16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baer, G. S., and T. S. Dermody. 1997. Mutations in reovirus outer-capsid protein σ3 selected during persistent infections of L cells confer resistance to protease inhibitor E64. J. Virol. 714921-4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal, A., and L. M. Gierasch. 1991. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell 671195-1201. [DOI] [PubMed] [Google Scholar]
- 4.Barton, E. S., J. L. Connolly, J. C. Forrest, J. D. Chappell, and T. S. Dermody. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 2762200-2211. [DOI] [PubMed] [Google Scholar]
- 5.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104441-451. [DOI] [PubMed] [Google Scholar]
- 6.Bergelson, J. M., M. P. Shepley, B. M. Chan, M. E. Hemler, and R. W. Finberg. 1992. Identification of the integrin VLA-2 as a receptor for echovirus 1. Science 2551718-1720. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard, E., S. Belouzard, L. Goueslain, T. Wakita, J. Dubuisson, C. Wychowski, and Y. Rouille. 2006. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J. Virol. 806964-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boll, W., H. Ohno, Z. Songyang, I. Rapoport, L. C. Cantley, J. S. Bonifacino, and T. Kirchhausen. 1996. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 155789-5795. [PMC free article] [PubMed] [Google Scholar]
- 9.Boll, W., I. Rapoport, C. Brunner, Y. Modis, S. Prehn, and T. Kirchhausen. 2002. The μ2 subunit of the clathrin adaptor AP-2 binds to FDNPVY and Yppφ sorting signals at distinct sites. Traffic 3590-600. [DOI] [PubMed] [Google Scholar]
- 10.Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72395-447. [DOI] [PubMed] [Google Scholar]
- 11.Borsa, J., B. D. Morash, M. D. Sargent, T. P. Copps, P. A. Lievaart, and J. G. Szekely. 1979. Two modes of entry of reovirus particles into L cells. J. Gen. Virol. 45161-170. [DOI] [PubMed] [Google Scholar]
- 12.Borsa, J., M. D. Sargent, P. A. Lievaart, and T. P. Copps. 1981. Reovirus: evidence for a second step in the intracellular uncoating and transcriptase activation process. Virology 111191-200. [DOI] [PubMed] [Google Scholar]
- 13.Calderwood, D. A., R. Zent, R. Grant, D. J. Rees, R. O. Hynes, and M. H. Ginsberg. 1999. The Talin head domain binds to integrin β subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 27428071-28074. [DOI] [PubMed] [Google Scholar]
- 14.Campbell, J. A., P. Shelling, J. D. Wetzel, E. M. Johnson, G. A. R. Wilson, J. C. Forrest, M. Aurrand-Lions, B. Imhof, T. Stehle, and T. S. Dermody. 2005. Junctional adhesion molecule A serves as a receptor for prototype and field-isolate strains of mammalian reovirus. J. Virol. 797967-7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandran, K., D. L. Farsetta, and M. L. Nibert. 2002. Strategy for nonenveloped virus entry: a hydrophobic conformer of the reovirus membrane penetration protein μ1 mediates membrane disruption. J. Virol. 769920-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandran, K., J. S. Parker, M. Ehrlich, T. Kirchhausen, and M. L. Nibert. 2003. The delta region of outer-capsid protein μ1 undergoes conformational change and release from reovirus particles during cell entry. J. Virol. 7713361-13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang, C. T., and H. J. Zweerink. 1971. Fate of parental reovirus in infected cell. Virology 46544-555. [DOI] [PubMed] [Google Scholar]
- 18.Chang, D. D., C. Wong, H. Smith, and J. Liu. 1997. ICAP-1, a novel β1 integrin cytoplasmic domain-associated protein, binds to a conserved and functionally important NPXY sequence motif of β1 integrin. J. Cell Biol. 1381149-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chappell, J. D., V. L. Gunn, J. D. Wetzel, G. S. Baer, and T. S. Dermody. 1997. Mutations in type 3 reovirus that determine binding to sialic acid are contained in the fibrous tail domain of viral attachment protein σ1. J. Virol. 711834-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu, J. J., and M. L. Ng. 2004. Interaction of West Nile virus with αvβ3 integrin mediates virus entry into cells. J. Biol. Chem. 27954533-54541. [DOI] [PubMed] [Google Scholar]
- 21.Czuchra, A., H. Meyer, K. R. Legate, C. Brakebusch, and R. Fassler. 2006. Genetic analysis of β1 integrin “activation motifs” in mice. J. Cell Biol. 174889-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis, C. G., M. A. Lehrman, D. W. Russell, R. G. Anderson, M. S. Brown, and J. L. Goldstein. 1986. The J. D. mutation in familial hypercholesterolemia: amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell 4515-24. [DOI] [PubMed] [Google Scholar]
- 23.Ebert, D. H., J. Deussing, C. Peters, and T. S. Dermody. 2002. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 27724609-24617. [DOI] [PubMed] [Google Scholar]
- 24.Ehrlich, M., W. Boll, A. Van Oijen, R. Hariharan, K. Chandran, M. L. Nibert, and T. Kirchhausen. 2004. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118591-605. [DOI] [PubMed] [Google Scholar]
- 25.Feire, A. L., H. Koss, and T. Compton. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA 10115470-15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furlong, D. B., M. L. Nibert, and B. N. Fields. 1988. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J. Virol. 62246-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerrero, C. A., E. Mendez, S. Zarate, P. Isa, S. Lopez, and C. F. Arias. 2000. Integrin αvβ3 mediates rotavirus cell entry. Proc. Natl. Acad. Sci. USA 9714644-14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gustavsson, A., A. Armulik, C. Brakebusch, R. Fassler, S. Johansson, and M. Fallman. 2002. Role of the β1-integrin cytoplasmic tail in mediating invasin-promoted internalization of Yersinia. J. Cell Sci. 1152669-2678. [DOI] [PubMed] [Google Scholar]
- 29.Harding, C., J. Heuser, and P. Stahl. 1983. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 97329-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hewish, M. J., Y. Takada, and B. S. Coulson. 2000. Integrins α2β1 and α4β1 can mediate SA11 rotavirus attachment and entry into cells. J. Virol. 74228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110673-687. [DOI] [PubMed] [Google Scholar]
- 32.Hynes, R. O. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 6911-25. [DOI] [PubMed] [Google Scholar]
- 33.Isberg, R. R., and J. M. Leong. 1990. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 60861-871. [DOI] [PubMed] [Google Scholar]
- 34.Maginnis, M. S., J. C. Forrest, S. A. Kopecky-Bromberg, S. K. Dickeson, S. A. Santoro, M. M. Zutter, G. R. Nemerow, J. M. Bergelson, and T. S. Dermody. 2006. β1 integrin mediates internalization of mammalian reovirus. J. Virol. 802760-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maratos-Flier, E., M. J. Goodman, A. H. Murray, and C. R. Kahn. 1986. Ammonium inhibits processing and cytotoxicity of reovirus, a nonenveloped virus. J. Clin. Investig. 78617-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsh, M., and A. Helenius. 2006. Virus entry: open sesame. Cell 124729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra, S. K., P. A. Keyel, M. J. Hawryluk, N. R. Agostinelli, S. C. Watkins, and L. M. Traub. 2002. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 214915-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris, S. M., and J. A. Cooper. 2001. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic 2111-123. [DOI] [PubMed] [Google Scholar]
- 39.Morris, S. M., M. D. Tallquist, C. O. Rock, and J. A. Cooper. 2002. Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J. 211555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nibert, M. L., A. L. Odegard, M. A. Agosto, K. Chandran, and L. A. Schiff. 2005. Putative autocleavage of reovirus μ1 protein in concert with outer-capsid disassembly and activation for membrane permeabilization. J. Mol. Biol. 345461-474. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson, S., D. Kaniowska, C. Brakebusch, R. Fassler, and S. Johansson. 2006. Threonine 788 in integrin subunit beta1 regulates integrin activation. Exp. Cell Res. 312844-853. [DOI] [PubMed] [Google Scholar]
- 42.Odegard, A. L., K. Chandran, X. Zhang, J. S. Parker, T. S. Baker, and M. L. Nibert. 2004. Putative autocleavage of outer capsid protein μ1, allowing release of myristoylated peptide μ1N during particle uncoating, is critical for cell entry by reovirus. J. Virol. 788732-8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohno, H., J. Stewart, M. C. Fournier, H. Bosshart, I. Rhee, S. Miyatake, T. Saito, A. Gallusser, T. Kirchhausen, and J. S. Bonifacino. 1995. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 2691872-1875. [DOI] [PubMed] [Google Scholar]
- 44.Oleinikov, A. V., J. Zhao, and S. P. Makker. 2000. Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin. Biochem. J. 347613-621. [PMC free article] [PubMed] [Google Scholar]
- 45.Owen, D. J., Y. Vallis, B. M. Pearse, H. T. McMahon, and P. R. Evans. 2000. The structure and function of the β2-adaptin appendage domain. EMBO J. 194216-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pacitti, A., and J. R. Gentsch. 1987. Inhibition of reovirus type 3 binding to host cells by sialylated glycoproteins is mediated through the viral attachment protein. J. Virol. 611407-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paul, R. W., A. H. Choi, and P. W. K. Lee. 1989. The α-anomeric form of sialic acid is the minimal receptor determinant recognized by reovirus. Virology 172382-385. [DOI] [PubMed] [Google Scholar]
- 48.Pearse, B. M. 1982. Coated vesicles from human placenta carry ferritin, transferrin, and immunoglobulin G. Proc. Natl. Acad. Sci. USA 79451-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pho, M. T., A. Ashok, and W. J. Atwood. 2000. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J. Virol. 742288-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prota, A. E., J. A. Campbell, P. Schelling, J. C. Forrest, T. R. Peters, M. J. Watson, M. Aurrand-Lions, B. Imhof, T. S. Dermody, and T. Stehle. 2003. Crystal structure of human junctional adhesion molecule 1: implications for reovirus binding. Proc. Natl. Acad. Sci. USA 1005366-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reszka, A. A., Y. Hayashi, and A. F. Horwitz. 1992. Identification of amino acid sequences in the integrin β1 cytoplasmic domain implicated in cytoskeletal association. J. Cell Biol. 1171321-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin, D. H., D. B. Weiner, C. Dworkin, M. I. Greene, G. G. Maul, and W. V. Williams. 1992. Receptor utilization by reovirus type 3: distinct binding sites on thymoma and fibroblast cell lines result in differential compartmentalization of virions. Microb. Pathog. 12351-365. [DOI] [PubMed] [Google Scholar]
- 53.Sakai, T., R. Jove, R. Fassler, and D. F. Mosher. 2001. Role of the cytoplasmic tyrosines of β1A integrins in transformation by v-src. Proc. Natl. Acad. Sci. USA 983808-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakai, T., Q. Zhang, R. Fassler, and D. F. Mosher. 1998. Modulation of β1A integrin functions by tyrosine residues in the β1 cytoplasmic domain. J. Cell Biol. 141527-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schiff, L. A., M. L. Nibert, and K. L. Tyler. 2007. Orthoreoviruses and their replication, p. 1853-1915. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 56.Sieczkarski, S. B., and G. R. Whittaker. 2002. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J. Virol. 7610455-10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silverstein, S. C., C. Astell, D. H. Levin, M. Schonberg, and G. Acs. 1972. The mechanism of reovirus uncoating and gene activation in vivo. Virology 47797-806. [DOI] [PubMed] [Google Scholar]
- 58.Smith, R. E., H. J. Zweerink, and W. K. Joklik. 1969. Polypeptide components of virions, top component and cores of reovirus type 3. Virology 39791-810. [DOI] [PubMed] [Google Scholar]
- 59.Sturzenbecker, L. J., M. L. Nibert, D. B. Furlong, and B. N. Fields. 1987. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J. Virol. 612351-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun, X., V. K. Yau, B. J. Briggs, and G. R. Whittaker. 2005. Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells. Virology 33853-60. [DOI] [PubMed] [Google Scholar]
- 61.Trub, T., W. E. Choi, G. Wolf, E. Ottinger, Y. Chen, M. Weiss, and S. E. Shoelson. 1995. Specificity of the PTB domain of Shc for β turn-forming pentapeptide motifs amino-terminal to phosphotyrosine. J. Biol. Chem. 27018205-18208. [DOI] [PubMed] [Google Scholar]
- 62.Turk, V., B. Turk, and D. Turk. 2001. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 204629-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ulmer, T. S., B. Yaspan, M. H. Ginsberg, and I. D. Campbell. 2001. NMR analysis of structure and dynamics of the cytosolic tails of integrin αIIb β3 in aqueous solution. Biochemistry 407498-7508. [DOI] [PubMed] [Google Scholar]
- 64.Van Nhieu, G. T., E. S. Krukonis, A. A. Reszka, A. F. Horwitz, and R. R. Isberg. 1996. Mutations in the cytoplasmic domain of the integrin β1 chain indicate a role for endocytosis factors in bacterial internalization. J. Biol. Chem. 2717665-7672. [DOI] [PubMed] [Google Scholar]
- 65.Virgin, H. W., K. L. Tyler, and T. S. Dermody. 1997. Reovirus, p. 669-699. In N. Nathanson (ed.), Viral pathogenesis. Lippincott-Raven, New York, NY.
- 66.Virgin, H. W., IV, R. Bassel-Duby, B. N. Fields, and K. L. Tyler. 1988. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J. Virol. 624594-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1231107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wennerberg, K., A. Armulik, T. Sakai, M. Karlsson, R. Fassler, E. M. Schaefer, D. F. Mosher, and S. Johansson. 2000. The cytoplasmic tyrosines of integrin subunit β1 are involved in focal adhesion kinase activation. Molec. Cell Biol. 205758-5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wennerberg, K., R. Fassler, B. Warmegard, and S. Johansson. 1998. Mutational analysis of the potential phosphorylation sites in the cytoplasmic domain of integrin β1A: requirement for threonines 788-789 in receptor activation. J. Cell Sci. 1111117-1126. [DOI] [PubMed] [Google Scholar]
- 70.Wennerberg, K., L. Lohikangas, D. Gullberg, M. Pfaff, S. Johansson, and R. Fassler. 1996. β1 integrin-dependent and -independent polymerization of fibronectin. J. Cell Biol. 132227-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wetzel, J. D., J. D. Chappell, A. B. Fogo, and T. S. Dermody. 1997. Efficiency of viral entry determines the capacity of murine erythroleukemia cells to support persistent infections by mammalian reoviruses. J. Virol. 71299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvb3 and αvb5 promote adenovirus internalization but not virus attachment. Cell 73309-319. [DOI] [PubMed] [Google Scholar]