Abstract
The Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) is an oncogenic protein which has previously been shown to engage the NF-κB, stress-activated MAP kinase, phosphatidylinositol 3-kinase (PI 3-kinase), and extracellular-regulated kinase (ERK)-MAPK pathways. In this study, we demonstrate that LMP1 activates ERK-MAPK in epithelial cells via the canonical Raf-MEK-ERK-MAPK pathway but in a Ras-independent manner. In agreement with the results of a previous study (B. A. Mainou, D. N. Everly, Jr., and N. Raab-Traub, J. Virol. 81:9680-9692, 2007), we show that the ability of LMP1 to activate ERK-MAPK mapped to its CTAR1 domain, the TRAF binding domain previously implicated in PI 3-kinase activation. A role for ERK-MAPK in LMP1-induced epithelial cell motility was identified, as LMP1-expressing cells displayed increased rates of haptotactic migration compared to those of LMP1-negative cells. These data implicate the ERK-MAPK pathway in LMP1-induced effects associated with transformation, suggesting that this pathway may contribute to the oncogenicity of LMP1 through its ability to promote cell motility and to enhance the invasive properties of epithelial cells.
Most of what is known about the biology of Epstein-Barr virus (EBV) relates to its interaction with cells of the B lymphoid lineage. EBV was originally identified on the basis of its association with Burkitt's lymphoma, and the B lymphotropic nature of the virus is confirmed by its ability to readily infect and transform normal resting B cells in vitro (47). The virus, however, can also infect epithelial cells, and this interaction can result in malignant transformation, as exemplified by the consistent association of EBV with nasopharyngeal carcinoma (NPC) and with a proportion of gastric adenocarcinomas (44, 47, 53). The EBV-induced transformation of B cells in vitro is associated with the expression of two nonpolyadenylated RNAs (EBER1 and EBER2), along with a set of latent viral gene products consisting of six EBV nuclear antigens (EBNA1, EBNA2, EBNA3A, EBNA3B, EBNA3C, and EBNA leader protein) and three latent membrane proteins (LMP1, LMP2A, and LMP2B) (67). However, more restricted patterns of latent gene expression are observed in NPC and in EBV-positive gastric adenocarcinomas, where only the EBERs, EBNA1, and LMP2A are consistently expressed and the expression of LMP1 is either variable or absent (44, 67). While the association of EBV with various lymphoid and epithelial malignancies is well documented, the precise contribution of virus infection to the etiology of these tumors remains unknown.
LMP1 is the major transforming protein of EBV, being absolutely essential for B-cell transformation (28) and behaving as a classical oncogenic protein in rodent fibroblast transformation assays (61). LMP1 induces the expression of lymphocyte activation antigens (62, 63) and antiapoptotic proteins (Bcl-2, TNFAIP3, and MCL1) (20, 32, 64) and stimulates the secretion of cytokines such as interleukin-10 (IL-10), among others (58). When expressed in epithelial cells, LMP1 induces the expression of IL-6 and IL-8 (10, 12). It can transform certain established epithelial cell lines and block the terminal differentiation of the cells (7, 8, 15, 23), and it induces hyperkeratosis when expressed in transgenic mouse epidermis (65). LMP1 also induces the expression of the epidermal growth factor (EGF) receptor (41, 42, 43) and matrix metalloproteinases (35, 54, 59) and can down-regulate the expression of E-cadherin (16), findings which suggest that LMP1 can modulate signaling pathways that regulate the motile and invasive properties of epithelial cells. This characteristic is of particular relevance given that LMP1-positive NPC tumors are reportedly more aggressive than their LMP1-negative counterparts (24).
LMP1 functions as a constitutively activated member of the tumor necrosis factor receptor superfamily, activating a number of signaling pathways in a ligand-independent manner (18, 30). LMP1 activates both the canonical and noncanonical NF-κB pathways (37, 49), can induce AP-1 and p38/ATF2 activity (11, 12, 13, 29, 50, 60), and has been shown previously to activate phosphatidylinositol 3-kinase (PI 3-kinase)/Akt kinase (2, 9, 39).
Although the activation of the NF-κB, stress-activated protein kinase, and PI 3-kinase cascade pathways accounts for most of the transforming functions of LMP1, additional unidentified signaling pathways may also participate in LMP1-mediated growth transformation. In this regard, the activation of the classical extracellular-regulated kinase (ERK)-MAPK pathway has been reported to contribute to LMP1-mediated effects (35). Indeed, a recent study has identified a critical role for ERK-MAPK in Rat-1 fibroblast transformation by LMP1 (40), underscoring the importance of ERK-MAPK in the oncogenic effects of LMP1. The ERK-MAPK family of protein kinases regulates many different biological processes by virtue of their ability to target multiple effector proteins (51). As such regulators, ERK1 and ERK2 are involved in diverse cellular processes such as proliferation, differentiation, cytoskeletal remodeling, and cell motility. Here, we demonstrate that LMP1 activation of the ERK-MAPK pathway is responsible for LMP1-induced cell motility and haptotactic migration.
MATERIALS AND METHODS
Cell lines and tissue culture.
The human epithelial cell line SCC12F (45) and a clone of SCC12F cells stably expressing LMP1 were cultured as described previously (8). HeLa cell clones stably expressing LMP1 have been described previously (10). A single HeLa MTLM clone was induced to express LMP1 after the addition of 25 μM ZnSO4. HeLa cells were cultured in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum (FCS), 2 mM glutamine, and a penicillin-streptomycin solution (Sigma) supplemented with 0.5 μg of mycophenolic acid/ml, 25 μg of xanthine/ml, and 10 μg of hypoxanthine/ml as previously described (10). Rhek-1 cells and clones stably expressing LMP1 were cultured as described previously (10).
MDCK cells stably expressing LMP1 and mutant derivatives were generated by retroviral transduction with pLXSN-based expression vectors containing wild-type LMP1 (pLXSN-LMP1) or mutant LMP1 proteins defective in the CTAR1 domain (pLXSN-AAA-LMP1), the CTAR2 domain (pLXSN-378stop-LMP1), or both the CTAR1 and CTAR2 domains (pLXSN-AAA/378Stop-LMP1). These mutant proteins have been described previously (12). The cell-permeable MEK1 inhibitor U0126 (Promega) was dissolved in dimethyl sulfoxide (DMSO) and diluted to a final concentration of 30 μM. Recombinant human EGF (Sigma) was used at a final concentration of 100 ng/ml.
Immunoblotting analysis.
Standard immunoblotting procedures (9) were used to detect LMP1 (with mouse CS1-4 at 1:50), phosphorylated ERK1/2 (p-ERK1/2; with rabbit anti-p-ERK1/2 at 1:1,000 [Cell Signaling Technology]), ERK-MAPK (with rabbit anti-ERK at 1:1,000 [Cell Signaling Technology]), phosphorylated Akt (p-AKT; with rabbit anti-p-AKT at 1:1,000 [Cell Signaling Technology]), Akt (with rabbit anti-Akt at 1:1,000 [Santa Cruz]), phosphorylated c-Raf (p-c-Raf; with rabbit anti-p-c-Raf at 1:1,000 [Cell Signaling Technology]), phosphorylated MEK1/2 (p-MEK1/2; with rabbit anti-p-MEK1/2 at 1:1,000 [Cell Signaling Technology]), phosphorylated p90RSK (p-p90RSK; with rabbit anti-p-p90RSK at 1:1,000 [Cell Signaling Technology]), and β-actin (with mouse anti-β-actin at 1:20,000 [Sigma]).
Ras activity assays.
Ras activity was assayed using a commercially available kit according to the instructions of the manufacturer (Upstate Biotechnology). Briefly, cells were serum starved for 24 h prior to lysis in an NP-40-based lysis buffer. The total cell lysate (400 μg) was immunoprecipitated with a glutathione S-transferase (GST) fusion protein comprising the Ras binding domain of c-Raf1 for 90 min at 4°C. Immune complexes were subjected to three washes and finally resuspended in 40 μl of gel sample buffer. Samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with an antibody to Ras. Bound antibody was detected using anti-mouse horseradish peroxidase-conjugated secondary antibody and visualized by enhanced chemiluminescence with a kit from Amersham Biosciences.
Immunofluorescence staining for ERK-MAPK and polymerized actin in adherent cells.
Control and LMP1-expressing MDCK cells were cultured in medium containing 0.5% FCS for 24 h prior to recovery as single-cell suspensions and maintained in suspension for 60 min. Cells (104/100 μl) were plated onto slides coated with fibronectin (10 μg/ml) and allowed to adhere for 10 min. After fixation in 4% paraformaldehyde, cells were permeabilized in 0.5% Triton X-100 (ICN Biochemicals) and stained with antiserum specific for active ERK-MAPK (Promega) followed by Alexa Fluor 488-conjugated anti-rabbit or anti-mouse antibodies (Molecular Probes). To visualize cellular actin, fixed and permeabilized cells were incubated with 0.5-μg/ml tetramethyl rhodamine isocyanate-conjugated phalloidin (Sigma) for 60 min. Slides were mounted with DABCO and viewed at a magnification of ×400 with a Nikon eclipse E600 microscope and the Nikon Act-1 program.
Transwell migration assays.
Serum-starved cells were recovered as single-cell suspensions, and 5 × 104 cells were seeded into the upper well of a transwell migration chamber (Corning) coated with 10 μg of fibronectin/ml. After 16 h of incubation at 37°C, the wells were fixed in 30% methanol and stained with 1% crystal violet. The percentage of cell migration was determined after photographing representative fields and counting the number of stained (migrating) cells. The extent of cell migration was quantitated by dissolving stained membranes in 0.2% Triton X-100 and measuring the absorbance at 550 nm.
RESULTS
The stable expression of LMP1 results in constitutive activation of the ERK-MAPK pathway.
The ability of LMP1 to activate the ERK-MAPK pathway in epithelial cell lines stably expressing LMP1 was investigated. A panel of cell lines including Rhek-1 (46), SCC12F (45), and MDCK (38) cells was examined. The origin and derivation of these cell lines and their LMP1-expressing counterparts have been detailed elsewhere (7, 10). To analyze the effects of LMP1 on ERK-MAPK activation, cell lysates from serum-starved cells were probed with an antiserum specific for the dual phosphorylated active forms of ERK1/2 (p-ERK1/2) (33). Immunoblotting with LMP1-specific antibodies confirmed the expression of LMP1 only in Rhek-1, SCC12F, and MDCK cells transduced to express LMP1 (Fig. 1A, B, and C, lower panels). The probing of the same cell lysates for p-ERK1/2 demonstrated increased basal ERK1/2 phosphorylation in cells expressing LMP1 (Fig. 1A, B, and C, upper panels) that was comparable to that in control cells treated with EGF (Fig. 1D). Reprobing of the immunoblots with antiserum to total ERK1/2 (Fig. 1A, B, and C, middle panels) confirmed the presence of equal amounts of ERK1/2 in control and LMP1-expressing cells, excluding the possibility that the increased basal ERK1/2 phosphorylation observed in LMP1-expressing cells was due to an increase in the pool of total ERK1/2. The specificity of ERK1/2 activation was evidenced by the ability of the U0126 MEK inhibitor to block both basal and LMP1-induced ERK1/2 phosphorylation without affecting the levels of either LMP1 or total ERK1/2 (Fig. 1A, B, and C).
FIG. 1.
Stable LMP1 expression induces the constitutive activation of ERK1/2 in LMP1-expressing epithelial cell lines. (A to C) Immunoblot analyses demonstrating the expression of p-ERK1/2 (upper panels), total ERK1/2 (middle panels), and LMP1 (lower panels) in control and LMP-expressing cells in the presence (+ U0126) or absence (untreated) of the MEK inhibitor U0126. Representative analyses for SCC12F (A), Rhek-1 (B), and MDCK (C) cells are shown. (D) Rhek-1, SCC12F, and MDCK cells stimulated with EGF (+) or left untreated (−) were included as a positive control. The increases (n-fold) in ERK-MAPK phosphorylation are indicated in parentheses. Numbers on the left of the blots are molecular weight markers. p-p44, phosphorylated p44; p-p42, phosphorylated p42.
Effects of inducible LMP1 expression on the ERK-MAPK pathway.
The constitutively active nature of LMP1 can obscure the impact of this molecule on cell signaling pathways. To address this issue, we examined whether ERK1/2 phosphorylation occurred in response to transient LMP1 expression in HeLa cells carrying a metallothionien-regulatable LMP1 gene. While negligible expression of LMP1 was observed in uninduced HeLa MTLM cells, treatment with 25 μM ZnSO4 rapidly induced LMP1 protein, with peak expression being observed at around 6 h posttreatment (Fig. 2). The probing of the same cell lysates for p-ERK1/2 confirmed that the activation of ERK1/2 coincided temporally with the induction of LMP1 and that LMP1 expression did not increase the total amount of ERK1/2.
FIG. 2.
LMP1 activates ERK1/2 via MEK in HeLa MTLM cells. Immunoblot analyses demonstrated the expression of LMP1, p-ERK1/2, ERK1/2, p-c-Raf, p-MEK1/2, and p-p90RSK in HeLa MTLM cells induced to express LMP1 (HeLa-LMP1) after the addition of 25 μM ZnSO4. HeLa cells stimulated with EGF (100 ng/ml) were included as a positive control. The increases (n-fold) in ERK-MAPK, c-Raf, MEK1/2, and p90RSK phosphorylation are indicated in parentheses. p-p44, phosphorylated p44; p-p42, phosphorylated p42.
To examine whether the ability of LMP1 to activate ERK1/2 followed the canonical Raf-MEK-ERK-MAPK pathway, the same panel of lysates from induced and uninduced HeLa MTLM cells were subjected to immunoblotting with antiserum specific for the phosphorylated forms of c-Raf and MEK1/2. The activation of ERK1/2 in the canonical ERK-MAPK cascade involves the activation of Ras by tyrosine kinase-coupled growth factor receptors (50). Once activated, Ras recruits and activates the upstream MAPK kinase kinase (MAPKKK) c-Raf, which in turn phosphorylates and activates the MAPK kinase MEK. As shown in Fig. 2, the induced expression of LMP1 resulted in the phosphorylation of both c-Raf (observed as a significant mobility shift in the c-Raf protein) and MEK1/2. Interestingly, the maximal phosphorylation of c-Raf did not coincide temporally with the phosphorylation of MEK1/2 or ERK1/2, indicating that c-Raf activation may not be involved in the first wave of MEK-ERK-MAPK activation. The phosphorylation of p90RSK, a putative downstream target of ERK1/2, mirrored that of ERK1/2, providing evidence that the ERK1/2 induced by LMP1 is functionally active.
LMP1 activation of the ERK-MAPK pathway is independent of Ras.
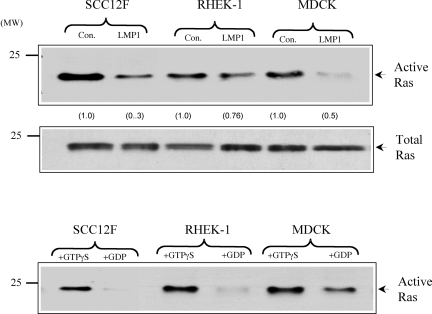
Given the slight discrepancy in c-Raf, MEK, and ERK-MAPK phosphorylations, we next sought to examine the effects of LMP1 on c-Ras activity. In the canonical Raf-MEK-ERK pathway, the Ras small GTPase activates ERK-MAPK by engaging c-Raf. We examined the effect of LMP1 on Ras activity by using an immunoprecipitation assay with a GST fusion protein comprising the Ras binding domain of c-Raf. The presence of “active” Ras was determined by probing immunoprecipitates with a Ras-specific antibody. As a specificity control, lysates were treated with the nonhydrolyzable analogue of GTP, GTPγS, and excess GDP to activate and inhibit Ras activity, respectively. As shown in Fig. 3 (lower panel), lysates from control cells treated with GTPγS showed increased Ras activity whereas this activity was completely abolished in SCC12F and Rhek-1 lysates treated with a 100-fold excess of GDP; GDP treatment of MDCK lysates reduced rather than abolished Ras activity. The analysis of lysates from control and LMP1-expressing cells revealed that the levels of Ras activity were consistently lower in all epithelial cell lines expressing LMP1. Ras expression levels in control and LMP1-expressing epithelial cells were similar (Fig. 3, middle panel), ruling out the possibility that LMP1 expression was associated with reduced levels of the Ras protein.
FIG. 3.
LMP1 does not stimulate endogenous c-Ras activity. (A) SCC12F, Rhek-1, and MDCK cells or their LMP1-expressing counterparts were cultured in medium containing 0.5% FCS for 24 h prior to analysis. Equal amounts of total cell lysates (300 μg) were subjected to immunoprecipitation with a GST fusion protein containing the Ras binding domain of c-Raf. The levels of active GTP-bound Ras were determined after immunoblotting of the immunoprecipitates with a monoclonal antibody to Ras (upper panel). The increases (n-fold) in c-Ras activity are indicated in parentheses. Immunoblotting of total cell lysates from control (con.) and LMP1-expressing cells with an antibody to Ras confirmed the presence of equal amounts of endogenous Ras in control and LMP1-expressing cells (middle panel). As an internal assay control, lysates generated from control SCC12F, Rhek-1, and MDCK cells were treated with GTPγS or a 100-fold excess of GDP to activate or inhibit endogenous Ras activity, respectively (lower panel). Numbers on the left of the blots are molecular weight markers.
LMP1 induces ERK-MAPK through the proximal TRAF binding domain, CTAR1.
To gain further insight into the mechanism by which LMP1 induces the activation of the ERK-MAPK pathway, we sought to identify which domain of LMP1 was responsible for this activation. MDCK cells stably expressing wild-type LMP1 and LMP1 mutant proteins defective in the CTAR1 domain (AAA-LMP1), the CTAR2 domain (378stop-LMP1), or both domains (AAA/378stop-LMP1) (12) were generated by retroviral transduction. Immunoblot analyses confirmed that wild-type and mutant LMP1 proteins were expressed in all transduced cells at broadly similar levels (Fig. 4A). To examine which of the LMP1 mutant proteins was responsible for ERK-MAPK activation, total cell lysates from serum-starved cells were subjected to immunoblotting for p-ERK1/2. This analysis confirmed the ability of wild-type LMP1 to activate the ERK-MAPK pathway in MDCK cells and identified the CTAR1 domain of LMP1 as being responsible for this activation. Only minimal levels of p-ERK1/2 were observed in AAA-LMP1-expressing cells, whereas cells expressing 378stop-LMP1 had levels of p-ERK1/2 comparable to those observed in cells expressing wild-type LMP1 (Fig. 4A). As expected, cells expressing AAA/378stop-LMP1 showed only low levels of ERK1/2 phosphorylation, ruling out contributions of the amino terminus and transmembrane-spanning regions of LMP1 to this effect. Reprobing with an antibody to ERK1/2 demonstrated the presence of equal amounts of these proteins in all cell lines, confirming that LMP1 expression does not influence the levels of ERK1/2 protein expression.
FIG. 4.
The CTAR1 domain of LMP1 is responsible for ERK-MAPK and Akt activation. (A) Immunoblot analyses of MDCK control cells and cells expressing wild-type and mutant LMP1 proteins for LMP1 (upper panel), p-ERK1/2 (upper middle panel), total ERK1/2 (middle panel), p-Akt (lower middle panel), and Akt (bottom panel). Control cells stimulated with 100 ng of EGF/ml for 5 min were included as positive controls. The increases (n-fold) in ERK-MAPK and Akt phosphorylation are indicated in parentheses. p-p44, phosphorylated p44; p-p42, phosphorylated p42. (B) Control and LMP1-expressing cells were cultured in medium containing 0.5% FCS and either a vehicle control (DMSO) (upper panel), 20 μM U0126 (upper middle panel), or 20 μM SB203580 (lower middle panel) for 24 h prior to being probed with antiserum specific for p-ERK1/2 or total ERK1/2 (bottom panel). The decreases (n-fold) in ERK-MAPK phosphorylation are indicated in parentheses.
The probing of the same panel of cell lysates with antiserum specific for p-Akt demonstrated that the CTAR1 domain of LMP1, in addition to activating ERK-MAPK, is responsible for engaging the PI 3-kinase/Akt pathway. High levels of serine 473-phosphorylated Akt compared to levels in control MDCK cells were observed only in MDCK cells expressing wild-type LMP1 and 378stop-LMP1 proteins (Fig. 4A). Control EGF-treated MDCK cells were used as a positive control and displayed high levels of serine 473-phosphorylated Akt. Reprobing with an antibody to Akt confirmed the presence of equal amounts of Akt protein in all cell lines (Fig. 4A).
The specificity of wild-type LMP1- and 378stop-LMP1-induced ERK1/2 phosphorylation was established using the U0126 MEK inhibitor. Whereas the treatment of parental MDCK cells and MDCK cells expressing wild-type and mutant LMP1 proteins with DMSO had no effect on the ability of wild-type LMP1 or the 378stop LMP1 mutant form to induce ERK-MAPK phosphorylation, treatment with 20 μM U0126 significantly inhibited this activation; the effect on ERK1 phosphorylation appeared to be more dramatic than that on ERK2 phosphorylation (Fig. 4B). The specificity of this effect was confirmed by the inability of SB203850, a selective cell-permeable inhibitor of p38, to influence LMP1-induced ERK1/2 phosphorylation (Fig. 4B). The probing of the immunoblots with an antibody to ERK1/2 confirmed the loading of equal amounts of protein (Fig. 4B).
LMP1-induced ERK-MAPK activation promotes epithelial cell motility and invasion.
In addition to their role in cell proliferation, members of the ERK-MAPK family regulate epithelial cell motility and invasion (57). ERK1 and ERK2 regulate cell movement by coordinating actin filament dynamics and focal adhesion turnover. Putative targets of ERK1/2 action are myosin light-chain kinase and a focal adhesion-associated calpain that regulates focal adhesion stability by cleaving focal adhesion kinase (25, 57). Additionally, ERK1 and ERK2 regulate the production and secretion of matrix metalloproteinase and the remodeling of the extracellular matrix (7). In a previous study, Li and colleagues demonstrated a role for ERK-MAPK in LMP1-mediated cell invasion through the increase of matrix metalloproteinase production (35). As this study had highlighted an ability of LMP1 to promote epithelial cell invasion through the extracellular matrix (35), we next examined whether LMP1 could promote the migration of MDCK cells and determined whether the ERK-MAPK pathway was required for this process. In this assay, the ability of cells to migrate through a fibronectin-coated polycarbonate membrane was assayed under serum-free conditions to exclude any contributions from soluble growth factors or cytokines. Results from a representative experiment depicted in Fig. 5A show that LMP1-expressing MDCK cells displayed significantly higher rates of haptotactic migration than control LMP1-negative cells over 16 h, and this finding was confirmed in triplicate assays (Fig. 5B). The ability of LMP1 to promote migration mapped, like ERK-MAPK activation, to the CTAR1 domain (Fig. 4). Thus, MDCK cells expressing the 378stop-LMP1 mutant protein displayed significantly higher rates of migration than either control cells or cells expressing either AAA-LMP1 or AAA/378stop-LMP1 (Fig. 5).
FIG. 5.
MDCK cells expressing LMP1 show increased rates of motility in transwell migration assays. (A) MDCK control cells and cells expressing wild-type (WT) and mutant LMP1 proteins were seeded (in triplicate) into fibronectin-coated transwell chambers. Sixteen hours after plating, the number of migrating cells was determined by visualization after fixation and staining with 1% crystal violet. Representative membranes are shown. Magnification, ×100. (B) Sixteen hours after plating, the number of cells migrating through the fibronectin-coated polycarbonate membrane was determined after fixation and staining with 1% crystal violet. After solubilization in 0.1% Triton X-100, the relative number of migrated cells was evaluated by measuring the absorbance at 595 nm. Data presented are the averages of triplicate results. Error bars indicate standard deviations.
Active ERK-MAPK is translocated to the leading edges of adhering epithelial cells.
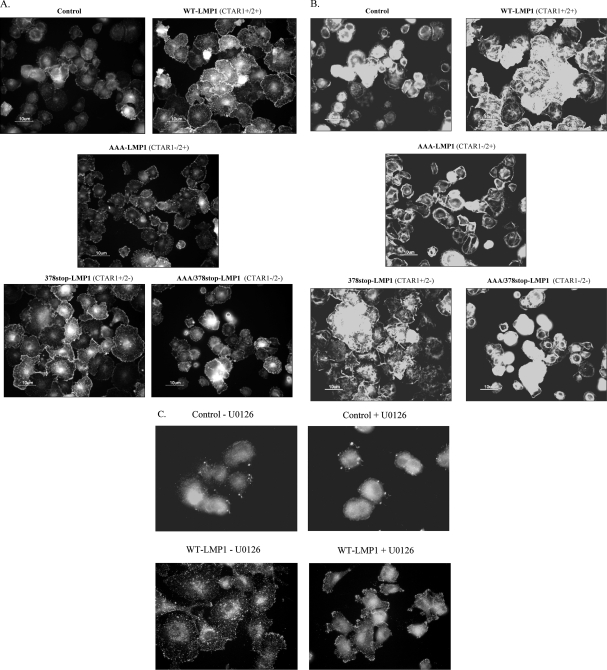
As ERK-MAPK activity appeared to be essential for LMP1 to enhance cell migration and invasion, we next examined whether membrane targeting of ERK-MAPK occurred in response to cell adhesion. MDCK cells expressing wild-type or mutant LMP1 proteins were serum starved for 16 h, recovered as single-cell suspensions, and plated onto fibronectin-coated slides. Ten minutes after plating, cells were fixed and stained with a polyclonal rabbit antiserum specific for active ERK-MAPK (Promega) or rhodamine-conjugated phalloidin to visualize actin filament reorganization. MDCK cells expressing wild-type LMP1 or 378stop-LMP1 showed increased rates of attachment, and spreading onto fibronectin and immunostaining revealed increased amounts of active p-ERK-MAPK in lamellapodia at the leading edges of spreading cells (Fig. 6A). The staining of the same cells with phalloidin (Fig. 6B) demonstrated robust actin stress fiber formation in wild-type LMP1- and 378stop-LMP1-expressing cells, indicating ERK-MAPK-mediated cytoskeletal remodeling.
FIG. 6.
LMP1 promotes ERK translocation to the leading edges of adhering cells. (A) MDCK control cells and cells expressing wild-type (WT) or mutant LMP1 protein were plated onto fibronectin, and after fixation, adherent cells were immunostained with an antiserum to active ERK-MAPK. (B) Cells were analyzed as described for panel A except that they were stained with tetramethyl rhodamine isocyanate-conjugated phalloidin to visualize polymerized actin. (C) Cells were analyzed as described for panel A except that MDCK control cells and cells expressing wild-type LMP1 were plated onto fibronectin in the presence (+U0126) or absence (−U0126) of 20 μM U0126 and stained for active p-ERK-MAPK. Magnification, ×400.
To examine the effects of ERK-MAPK inhibition on these responses, cells were resuspended in serum-free medium containing 20 μM U0126 for 60 min prior to being plated onto fibronectin. Results from a representative experiment presented in Fig. 6C show that ERK-MAPK inhibition, while not inhibiting cell attachment per se, had a profound effect on cell spreading. U0126 treatment severely impaired the membrane targeting of active ERK-MAPK in LMP1-expressing cells, with ERK-MAPK localizing to large focal adhesions at protrusive sites at the cell membrane. This pattern was in marked contrast to that in untreated cells, where active ERK-MAPK was uniformly distributed at the leading edges of spreading cells.
DISCUSSION
The oncogenic properties of LMP1 are a consequence of its ability to constitutively engage signaling pathways that impact different cellular processes, including cell growth, survival, and differentiation. While previous work has clearly defined the contributions of the NF-κB, Jun N-terminal protein kinase/p38, and PI 3-kinase pathways to these effects, the role of the ERK-MAPK pathway in the oncogenic effects of LMP1 has, until recently, remained controversial. Mainou and colleagues have recently identified a role for ERK-MAPK in the LMP1-mediated transformation of Rat-1 fibroblasts in vitro (40). In epithelial cells, LMP1 activation of the ERK-MAPK pathway appears to be cell type specific. In an early study, LMP1 failed to induce ERK-MAPK activation in 293 kidney epithelial cells (29), whereas studies performed with squamous epithelial cells have demonstrated an ability of LMP1 to engage the ERK-MAPK pathway (35, 36). We set out to more carefully define the contribution of LMP1 to ERK-MAPK activation and have found that this pathway has profound phenotypic effects consistent with the oncogenic function of LMP1.
Our data confirm and extend results from previous studies showing that LMP1 promotes the invasive growth of canine (MDCK) and human (NP-69 and TW04) epithelial cell lines (31, 35, 36). In addition to demonstrating an ERK-MAPK-dependent effect of LMP1 on cell motility and invasion, we have partly delineated the mechanism by which LMP1 activates the ERK-MAPK pathway and have identified the domain of LMP1 responsible for this activation. Using phosphorylation state-specific antiserum recognizing the phosphorylated form of active ERK1/2 (p-ERK1/2), we showed that the basal level of ERK1/2 activity was significantly elevated in a number of epithelial cell lines stably expressing LMP1. That this activity was not due to the effects of long-term LMP1 expression was supported by findings showing that ERK-MAPK activity was induced in response to the transient expression of LMP1. Although we did not perform in vitro kinase assays to assess ERK1/2 activity, numerous studies have demonstrated a strong correlation between ERK1/2 phosphorylation, the nuclear translocation of ERK1/2, and increased ERK-MAPK activity (19, 34). Further confirmation that LMP1-induced ERK1/2 phosphorylation was associated with increased ERK1/2 activity was established as p90RSK, a putative downstream target of ERK1/2, was phosphorylated in response to LMP1 expression. While these data suggest that LMP1 activates ERK-MAPK signaling directly, an indirect contribution from LMP1-induced effects such as the secretion of soluble cytokines or growth factors or the modulation of adhesion receptor signaling cannot be excluded.
Although our studies clearly demonstrate that LMP1 is able to activate the ERK-MAPK pathway in epithelial cells, the mechanism(s) involved in this activation is still unresolved. Many growth factor (receptor tyrosine kinase) and adhesion receptors recruit adapter molecules (SHC and Grb2) to the “activated” receptor complex, which couples the receptors to the Ras small GTPase. Ras then binds to and sequentially activates c-Raf (a MAPKKK) and MEK1/2 (a MAPK kinase), culminating in ERK-MAPK activation. Using phosphorylation state-specific antiserum, we were able to demonstrate that in the inducible HeLa MTLM cell system, c-Raf and MEK1/2 phosphorylation coincided temporally with the induction of LMP1. These findings, coupled with our ability to block LMP1-induced ERK1/2 phosphorylation with U0126, a specific inhibitor of MEK1/2, strongly support the notion that LMP1 induces ERK1/2 activation through MEK1/2. However, we were unable to identify a role for Ras activation in this phenomenon, which is surprising given that dominant-negative N17Ras has previously been shown to block the ability of LMP1 to transform Rat-1 fibroblasts (48). This result suggests that in LMP1-expressing epithelial cells, as opposed to rodent fibroblasts, the ability of LMP1 to engage the ERK-MAPK pathway involves Ras-independent activation of c-Raf. Such a scenario is not without precedent, as Ras-independent activation of c-Raf has been reported previously and can be mediated via either classical and atypical protein kinase C isoforms or certain classes of receptor tyrosine kinases (4, 5, 52). Alternatively, c-Raf may not be the only upstream MAPKKK responsible for LMP1-mediated MEK1/2 activation. Previous work from our laboratory has identified a role for the serine/threonine kinase Tpl-2 in LMP1-mediated NF-κB activation (14), and as Tpl-2 can also couple with the ERK-MAPK pathway (6), it is possible that this kinase mediates the ability of LMP1 to activate the ERK-MAPK cascade by directly phosphorylating MEK1/2.
Other oncogenic herpesviruses encode terminal membrane proteins that activate ERK-MAPK, suggesting that this pathway is important for the virus life cycle and that aberrant or inappropriate activation contributes to tumorigenesis. Thus, the herpesvirus saimiri-encoded StpC protein engages the ERK-MAPK pathway via binding to Ras (27), while the Kaposi's sarcoma-associated herpesvirus-encoded K15 membrane protein activates this pathway through mechanisms involving src family kinases and TRAF2 (3). It is thus possible that TRAF2 mediates the ability of LMP1 to activate ERK-MAPK and that this may be a consequence of the ability of TRAF2 to interact with Tpl-2, a complex we have previously shown to be important for LMP1-induced NF-κB activation (6, 14). The expression of LMP1 in TRAF2-deficient murine fibroblasts or B cells results in normal levels of NF-κB activation, suggesting that the interaction of TRAF2 with LMP1 is not essential for the NF-κB pathway but may be responsible for engaging other signaling pathways (37, 66). In agreement with the results of a study by Mainou and colleagues (40), we found that the LMP1-induced activation of ERK-MAPK maps to the CTAR1 domain of LMP1, and thus, it may be that the interaction of TRAF2 with this site mediates this effect. Future studies using knockout murine cells as well as RNA interference approaches will directly address the role of TRAF2 and Tpl-2 in LMP1-induced ERK-MAPK activation.
Although ERK-MAPK activation is associated with proliferative responses in most cell types, ERK-MAPK also functions to regulate cell motility and migration (25, 56). In this study, we demonstrate that the ability of LMP1 to activate ERK-MAPK is both necessary and sufficient to promote cell spreading, motility, and haptotactic migration. In transwell migration assays, LMP1-expressing MDCK cells showed increased rates of motility and migrated through a fibronectin matrix more efficiently than control cells.
We also demonstrate that the CTAR1 domain of LMP1 is responsible for these effects, as both wild-type LMP1 and the CTAR1-positive mutant form (378stop-LMP1) not only induced ERK-MAPK activity but also promoted the intracellular targeting of active ERK-MAPK to newly formed focal adhesions and lamellapodia in adhering cells. That the membrane targeting of ERK-MAPK is important for LMP1-induced effects on cell spreading and motility was borne out by our finding that U0126 treatment severely inhibited ERK-MAPK translocation to membrane ruffles and prevented efficient cell spreading. As cell spreading relies on the activity of the small Rho GTPases, this effect is most likely attributed to the inhibition of Rac GTPase activity, an established target of ERK-MAPK that is known to promote membrane ruffling and cell motility (1, 56).
Previous work has implicated integrin engagement and the activation of c-src in the plasma membrane targeting of activated ERK-MAPK to focal adhesions, suggesting that the effects of LMP1 may be mediated through these pathways (17). Future studies will determine whether LMP1 induces ERK-MAPK activity directly, through the direct binding of effector molecules that engage the ERK-MAPK pathway, or induces this pathway indirectly by altering the activation state of integrins, so-called “inside-out” signaling.
As deregulated integrin signaling is associated with constitutive ERK-MAPK activation and this contributes to aberrant growth and differentiation (19, 21, 22), it is possible that LMP1 induces a hyperkeratotic state and perturbs keratinocyte differentiation by disrupting normal integrin signaling. Indeed, deregulated or constitutive ERK-MAPK signaling plays an important role in the pathogenesis of many epithelial disorders and is implicated in the etiology of both benign and malignant epithelial diseases. In these disorders, ERK-MAPK is chronically activated as a result of overexpression or deregulated signaling from certain classes of integrin receptors (26). As many of these features are characteristic of epithelial cells expressing LMP1 (7, 8, 65), the possibility that some of these phenotypic effects are the result of the chronic activation of the ERK-MAPK pathway clearly warrants further examination. Whether this results from the effects of LMP1 on integrin receptor signaling is presently unknown, although our unpublished observations suggest that LMP1 does indeed modulate integrin receptor expression and signaling in human keratinocytes. Whatever the underlying mechanism, the ability of LMP1-induced ERK-MAPK activation to promote cell motility and migration is likely to have clinically relevant consequences and may account for the observation that LMP1-positive NPC tumors are both more aggressive and more metastatic than their LMP1-negative counterparts. The clinical development of ERK-MAPK (MEK) inhibitors is a focus of much interest, particularly in conjunction with genetic-based patient selection (e.g., B-raf mutations). Such inhibitors may also be of clinical benefit in the context of LMP1-positive EBV-associated tumors.
Acknowledgments
This work was supported by program funding from Cancer Research UK and the European Commission's FP6 Life Sciences Health Programme (INCA project LSHC-CT-2005-018704).
Footnotes
Published ahead of print on 16 January 2008.
REFERENCES
- 1.Belguise, K., N. Kersual, F. Galtier, and D. Chalbos. 2005. FRA-1 expression level regulates proliferation and invasiveness of breast cancer cells. Oncogene 241434-1444. [DOI] [PubMed] [Google Scholar]
- 2.Brennan, P., A. M. Mehl, M. Jones, and M. Rowe. 2002. Phosphatidylinositol 3-kinase is essential for the proliferation of lymphoblastoid cells. Oncogene 211263-1271. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann, M. M., M. Glenn, L. Rainbow, A. Kieser, C. Henke-Gendo, and T. F. Schulz. 2003. Activation of mitogen-activated protein kinase and NF-kappaB pathways by a Kaposi's sarcoma-associated herpesvirus K15 membrane protein. J. Virol. 779346-9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, C., and A. J. Sytkowski. 2004. Erythropoietin regulation of Raf-1 and MEK: evidence for a Ras-independent mechanism. Blood 10473-80. [DOI] [PubMed] [Google Scholar]
- 5.Corbit, K. C., N. Trakul, E. M. Eves, B. Diaz, M. Marshall, and M. R. Rosner. 2003. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J. Biol. Chem. 27813061-13068. [DOI] [PubMed] [Google Scholar]
- 6.Das, S., J. Cho, I. Lambertz, A. A. Kelliher, A. G. Eliopoulos, K. Du, and P. N. Tsichlis. 2005. Tpl2/cot signals activate ERK, JNK, and NF-kappaB in a cell-type and stimulus-specific manner. J. Biol. Chem. 28023748-23757. [DOI] [PubMed] [Google Scholar]
- 7.Dawson, C. W., A. B. Rickinson, and L. S. Young. 1990. Epstein-Barr virus latent membrane protein inhibits human epithelial cell differentiation. Nature 344777-780. [DOI] [PubMed] [Google Scholar]
- 8.Dawson, C. W., A. G. Eliopoulos, S. M. Blake, R. Barker, and L. S. Young. 2000. Identification of functional differences between prototype Epstein-Barr virus-encoded LMP1 and a nasopharyngeal carcinoma-derived LMP1 in human epithelial cells. Virology 272204-207. [DOI] [PubMed] [Google Scholar]
- 9.Dawson, C. W., G. Tramountanis, A. G. Eliopoulos, and L. S. Young. 2003. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J. Biol. Chem. 2783694-3704. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos, A. G., M. Stack, C. W. Dawson, K. M. Kaye, L. Hodgkin, S. Sihota, M. Rowe, and L. S. Young. 1997. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-kappaB pathway involving TNF receptor-associated factors. Oncogene 142899-2916. [DOI] [PubMed] [Google Scholar]
- 11.Eliopoulos, A. G., and L. S. Young. 1998. LMP1 structure and signal transduction. Oncogene 161731-1742. [DOI] [PubMed] [Google Scholar]
- 12.Eliopoulos, A. G., N. J. Gallagher, S. M. Blake, C. W. Dawson, and L. S. Young. 1999. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J. Biol. Chem. 27416085-16096. [DOI] [PubMed] [Google Scholar]
- 13.Eliopoulos, A. G., S. M. Blake, J. E. Floettmann, M. Rowe, and L. S. Young. 1999. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J. Virol. 731023-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eliopoulos, A. G., C. Davies, S. M. Blake, P. Murray, S. Najafipour, P. N. Tsichlis, and L. S. Young. 2002. The oncogenic protein kinase Tpl-2/Cot contributes to Epstein-Barr virus-encoded latent infection membrane protein 1-induced NF-κB signaling downstream of TRAF2. J. Virol. 764567-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fahraeus, R., L. Rymo, J. S. Rhim, and G. Klein. 1990. Morphological transformation of human keratinocytes expressing the LMP gene of Epstein-Barr virus. Nature 345447-449. [DOI] [PubMed] [Google Scholar]
- 16.Fahraeus, R., W. Chen, P. Trivedi, G. Klein, and B. Obrink. 1992. Decreased expression of E-cadherin and increased invasive capacity in EBV-LMP-transfected human epithelial and murine adenocarcinoma cells. Int. J. Cancer 52834-838. [DOI] [PubMed] [Google Scholar]
- 17.Fincham, V. J., M. James, M. C. Frame, and S. J. Winder. 2000. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 192911-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gires, O., U. Zimber-Strobl, R. Gonnella, M. Ueffing, G. Marschall, R. Zeidler, D. Pich, and W. Hammerschmidt. 1997. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 166131-6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haase, I., R. M. Hobbs, M. R. Romero, S. Broad, and F. M. Watt. 2001. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J. Clin. Investig. 108527-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson, S., M. Rowe, C. Gregory, D. Croom-Carter, F. Wang, R. Longnecker, E. Kieff, and A. B. Rickinson. 1991. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell 651107-1115. [DOI] [PubMed] [Google Scholar]
- 21.Hobbs, R. M., and F. M. Watt. 2003. Regulation of interleukin-1alpha expression by integrins and epidermal growth factor receptor in keratinocytes from a mouse model of inflammatory skin disease. J. Biol. Chem. 27819798-19807. [DOI] [PubMed] [Google Scholar]
- 22.Hobbs, R. M., V. Silva-Vargas, R. Groves, and F. M. Watt. 2004. Expression of activated MEK1 in differentiating epidermal cells is sufficient to generate hyperproliferative and inflammatory skin lesions. J. Investig. Dermatol. 123503-515. [DOI] [PubMed] [Google Scholar]
- 23.Hu, L. F., F. Chen, X. Zheng, I. Ernberg, S. L. Cao, B. Christensson, G. Klein, and G. Winberg. 1993. Clonability and tumorigenicity of human epithelial cells expressing the EBV encoded membrane protein LMP1. Oncogene 81575-1583. [PubMed] [Google Scholar]
- 24.Hu, L. F., F. Chen, Q. F. Zhen, Y. W. Zhang, Y. Luo, X. Zheng, G. Winberg, I. Ernberg, and G. Klein. 1995. Differences in the growth pattern and clinical course of EBV-LMP1 expressing and non-expressing nasopharyngeal carcinomas. Eur. J. Cancer 31A658-660. [DOI] [PubMed] [Google Scholar]
- 25.Huang, C., K. Jacobson, and M. D. Schaller. 2004. MAP kinases and cell migration. J. Cell Sci. 1174619-4628. [DOI] [PubMed] [Google Scholar]
- 26.Janes, S. M., and F. M. Watt. 2006. New roles for integrins in squamous-cell carcinoma. Nat. Rev. Cancer 6175-183. [DOI] [PubMed] [Google Scholar]
- 27.Jung, J. U., and R. C. Desrosiers. 1995. Association of the viral oncoprotein STP-C488 with cellular ras. Mol. Cell. Biol. 156506-6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaye, K. M., K. M. Izumi, and E. Kieff. 1993. An Epstein-Barr virus that expresses only the first 231 LMP1 amino acids efficiently initiates primary B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 909150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieser, A., E. Kilger, O. Gires, M. Ueffing, W. Kolch, and W. Hammerschmidt. 1997. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 166478-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilger, E., A. Kieser, M. Baumann, and W. Hammerschmidt. 1998. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 171700-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, K. R., T. Yoshizaki, H. Miyamori, K. Hasegawa, T. Horikawa, M. Furukawa, S. Harada, M. Seiki, and H. Sato. 2000. Transformation of Madin-Darby canine kidney (MDCK) epithelial cells by Epstein-Barr virus latent membrane protein 1 (LMP1) induces expression of Ets1 and invasive growth. Oncogene 191764-1771. [DOI] [PubMed] [Google Scholar]
- 32.Laherty, C. D., H. M. Hu, A. W. Opipari, F. Wang, and V. M. Dixit. 1992. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J. Biol. Chem. 26724157-24160. [PubMed] [Google Scholar]
- 33.Leevers, S. J., and C. J. Marshall. 1992. Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J. 11569-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lenormand, P., J. M. Brondello, A. Brunet, and J. Pouyssegur. 1998. Growth factor-induced p42/p44 MAPK nuclear translocation and retention requires both MAPK activation and neosynthesis of nuclear anchoring proteins. J. Cell Biol. 142625-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, L. T., J. P. Peng, H. C. Chang, and W. C. Hung. 2003. RECK is a target of Epstein-Barr virus latent membrane protein 1. Oncogene 228263-8270. [DOI] [PubMed] [Google Scholar]
- 36.Lo, A. K., D. P. Huang, K. W. Lo, Y. L. Chui, H. M. Li, J. C. Pang, and S. W. Tsao. 2004. Phenotypic alterations induced by the Hong Kong-prevalent Epstein-Barr virus-encoded LMP1 variant (2117-LMP1) in nasopharyngeal epithelial cells. Int. J. Cancer 109919-925. [DOI] [PubMed] [Google Scholar]
- 37.Luftig, M., E. Prinarakis, T. Yasui, T. Tsichritzis, E. Cahir-McFarland, J. Inoue, H. Nakano, T. W. Mak, W. C. Yeh, X. Li, S. Akira, N. Suzuki, S. Suzuki, G. Mosialos, and E. Kieff. 2003. Epstein-Barr virus latent membrane protein 1 activation of NF-kappaB through IRAK1 and TRAF6. Proc. Natl. Acad. Sci. USA 10015595-15600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madin, S. H., and N. B. Darby, Jr. 1958. Established kidney cell lines of normal adult bovine and ovine origin. Proc. Soc. Exp. Biol. Med. 98574-576. [DOI] [PubMed] [Google Scholar]
- 39.Mainou, B. A., D. N. Everly, Jr., and N. Raab-Traub. 2005. Epstein-Barr virus latent membrane protein 1 CTAR1 mediates rodent and human fibroblast transformation through activation of PI3K. Oncogene 246917-6924. [DOI] [PubMed] [Google Scholar]
- 40.Mainou, B. A., D. N. Everly, Jr., and N. Raab-Traub. 2007. Unique signaling properties of CTAR1 in LMP1-mediated transformation. J. Virol. 819680-9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller, W. E., H. S. Earp, and N. Raab-Traub. 1995. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J. Virol. 694390-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, W. E., G. Mosialos, E. Kieff, and N. Raab-Traub. 1997. Epstein-Barr virus LMP1 induction of the epidermal growth factor receptor is mediated through a TRAF signaling pathway distinct from NF-κB activation. J. Virol. 71586-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller, W. E., J. L. Cheshire, and N. Raab-Traub. 1998. Interaction of tumor necrosis factor receptor-associated factor signaling proteins with the latent membrane protein 1 PXQXT motif is essential for induction of epidermal growth factor receptor expression. Mol. Cell. Biol. 182835-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raab-Traub, N. 2002. Epstein-Barr virus in the pathogenesis of NPC. Semin. Cancer Biol. 12431-441. [DOI] [PubMed] [Google Scholar]
- 45.Rheinwald, J. G., and M. A. Beckett. 1981. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 411657-1663. [PubMed] [Google Scholar]
- 46.Rhim, J. S., J. B. Park, and G. Jay. 1989. Neoplastic transformation of human keratinocytes by polybrene-induced DNA-mediated transfer of an activated oncogene. Oncogene 41403-1409. [PubMed] [Google Scholar]
- 47.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2628. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 48.Roberts, M. L., and N. R. Cooper. 1998. Activation of a ras-MAPK-dependent pathway by Epstein-Barr virus latent membrane protein 1 is essential for cellular transformation. Virology 24093-99. [DOI] [PubMed] [Google Scholar]
- 49.Saito, N., G. Courtois, A. Chiba, N. Yamamoto, T. Nitta, N. Hironaka, M. Rowe, N. Yamamoto, and S. Yamaoka. 2003. Two carboxyl-terminal activation regions of Epstein-Barr virus latent membrane protein 1 activate NF-kappaB through distinct signaling pathways in fibroblast cell lines. J. Biol. Chem. 27846565-46575. [DOI] [PubMed] [Google Scholar]
- 50.Schultheiss, U., S. Puschner, E. Kremmer, T. W. Mak, H. Engelmann, W. Hammerschmidt, and A. Kieser. 2001. TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1. EMBO J. 205678-5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seger, R., and E. G. Krebs. 1995. The MAPK signaling cascade. FASEB J. 9726-735. [PubMed] [Google Scholar]
- 52.Sozeri, O., K. Vollmer, M. Liyanage, D. Frith, G. Kour, G. E. Mark III, and S. Stabel. 1992. Activation of the c-Raf protein kinase by protein kinase C phosphorylation. Oncogene 72259-2262. [PubMed] [Google Scholar]
- 53.Takada, K. 2000. Epstein-Barr virus and gastric carcinoma. Mol. Pathol. 53255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeshita, H., T. Yoshizaki, W. E. Miller, H. Sato, M. Furukawa, J. S. Pagano, and N. Raab-Traub. 1999. Matrix metalloproteinase 9 expression is induced by Epstein-Barr virus latent membrane protein 1 C-terminal activation regions 1 and 2. J. Virol. 735548-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reference deleted.
- 56.Vial, E., E. Sahai, and C. J. Marshall. 2003. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell 467-79. [DOI] [PubMed] [Google Scholar]
- 57.Vial, E., and J. Pouyssegur. 2004. Regulation of tumor cell motility by ERK mitogen-activated protein kinases. Ann. N. Y. Acad. Sci. 1030208-218. [DOI] [PubMed] [Google Scholar]
- 58.Vockerodt, M., D. Pinkert, S. Smola-Hess, A. Michels, R. M. Ransohoff, H. Tesch, and D. Kube. 2005. The Epstein-Barr virus oncoprotein latent membrane protein 1 induces expression of the chemokine IP-10: importance of mRNA half-life regulation. Int. J. Cancer 114598-605. [DOI] [PubMed] [Google Scholar]
- 59.Wakisaka, N., and J. S. Pagano. 2003. Epstein-Barr virus induces invasion and metastasis factors. Anticancer Res. 232133-2138. [PubMed] [Google Scholar]
- 60.Wan, J., L. Sun, J. W. Mendoza, Y. L. Chui, D. P. Huang, Z. J. Chen, N. Suzuki, S. Suzuki, W. C. Yeh, S. Akira, K. Matsumoto, Z. G. Liu, and Z. Wu. 2004. Elucidation of the c-Jun N-terminal kinase pathway mediated by Estein-Barr virus-encoded latent membrane protein 1. Mol. Cell. Biol. 24192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, D., D. Liebowitz, and E. Kieff. 1985. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 43831-840. [DOI] [PubMed] [Google Scholar]
- 62.Wang, D., D. Liebowitz, and E. Kieff. 1988. The truncated form of the Epstein-Barr virus latent-infection membrane protein expressed in virus replication does not transform rodent fibroblasts. J. Virol. 622337-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, D., D. Liebowitz, F. Wang, C. Gregory, A. Rickinson, R. Larson, T. Springer, and E. Kieff. 1988. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J. Virol. 624173-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, S., M. Rowe, and E. Lundgren. 1996. Expression of the Epstein Barr virus transforming protein LMP1 causes a rapid and transient stimulation of the Bcl-2 homologue Mcl-1 levels in B-cell lines. Cancer Res. 564610-4613. [PubMed] [Google Scholar]
- 65.Wilson, J. B., W. Weinberg, R. Johnson, S. Yuspa, and A. J. Levine. 1990. Expression of the BNLF-1 oncogene of Epstein-Barr virus in the skin of transgenic mice induces hyperplasia and aberrant expression of keratin 6. Cell 611315-1327. [DOI] [PubMed] [Google Scholar]
- 66.Xie, P., B. S. Hostager, and G. A. Bishop. 2004. Requirement for TRAF3 in signaling by LMP1 but not CD40 in B lymphocytes. J. Exp. Med. 199661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young, L. S, and A. B. Rickinson. 2004. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer 4757-768. [DOI] [PubMed] [Google Scholar]