Abstract
Degradation of de novo-generated adeno-associated virus type 5 (AAV5) Rep52 and capsid proteins is part of the limited target specificity displayed by adenovirus type 5 E4Orf6-E1B-55k as part of a cullin 5-containing E3 ligase complex. Both Rep and capsid proteins can be found in the ligase complex, and their presence is dependent on interaction between E4Orf6 and elongins B and C. Degradation of AAV5 proteins can be inhibited by a dominant-negative ubiquitin that prevents chain elongation or by small interfering RNA directed against cullin 5.
The E4Orf6 protein is one of five adenovirus (Ad) gene products (together with E1A, E1B-55k, E2A, and VA RNA) required for efficient adeno-associated virus (AAV) virus production (2, 4, 18). The E4Orf6 protein plays a number of critical roles essential for Ad infection, having effects primarily on viral and cellular RNA export and cellular DNA repair pathways (5, 12, 14). At least one aspect of the mechanism of its action is as a component of an E3 ligase complex (11). E4Orf6, together with E1B-55k, has been shown to cause the ubiquitinylation of cellular p53 and Mre11 and their subsequent degradation, which promotes efficient Ad replication (11, 16). E4Orf6 and E1B-55k form an E3 ligase complex with the cellular adaptor proteins elongin B and elongin C, cullin 5, and the ring finger protein Rbx1 (11, 13). Elongins B and C are thought to bring the substrate recognition molecules E4Orf6 and E1B-55k into association with cullin 5 (3, 7, 11, 19). The contact points between E4Orf6 and elongins B and C occur through two essential BC box motifs (3). Interestingly, the E4Orf6-E1B-55k complex isolated from infected cells is larger than predicted from its known minimal components, and it has been shown to contain additional proteins (7).
The E4Orf6 protein has been shown to be required for the conversion of AAV type 2 (AAV2) genomic single-stranded DNA into the double-stranded DNA replication intermediate (6, 14, 17, 18), and its role in degrading Mre11 has recently been shown to also be important for AAV2 replication in an as-yet-undetermined manner (14). In addition to these important functions, we have recently shown that E4Orf6 together with E1B-55k can promote the degradation of de novo-generated AAV5 capsid and small Rep proteins (9). We have also shown that one of the important roles that VA RNA plays in promoting AAV5 infection is to overcome the effect that E4Orf6-E1B-55k has in reducing the accumulated levels of AAV5 proteins (9).
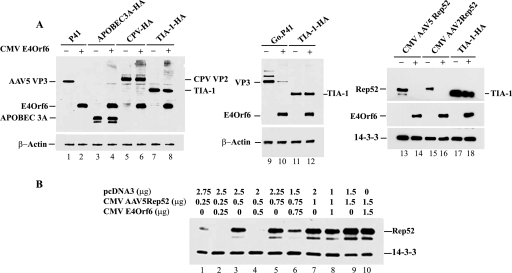
E4Orf6-E1B-55k-dependent degradation shows limited target specificity.
Only three cellular proteins have previously been shown to be targets of the E4Orf6-E1B-55k E3 ligase complex: Mre11, p53, and DNA ligase IV (1, 12, 14, 16). While we have previously demonstrated E4Orf6-E1B-55k-dependent degradation of AAV proteins (9), our current analysis indicates that the target number of this complex remains small. As shown in Fig. 1, in experiments in which E4Orf6-E1B-55k directed the degradation of de novo-generated AAV5 capsid proteins as detected by immunoblotting following transient transfection of E1A- and E1B-55k-expressing 293 cells (Fig. 1A, lanes 1 and 2), other randomly chosen, transiently expressed cellular proteins, including APOBEC 3A (Fig. 1A, lanes 3 and 4), canine parvovirus VP2 (Fig. 1A, lanes 5 and 6), the RNA processing factors TIA-1 (Fig. 1A, lanes 7 and 8, 11 and 12, and 17 and 18) and SMN (data not shown), and APOBEC 3G (data not shown), as well as endogenous actin (Fig. 1A) and 14-3-3 (Fig. 1A and B), were found to be resistant to degradation. Thus, AAV Rep and Cap proteins joined part of a restricted target population of proteins degraded in a manner directed by Ad type 5 (Ad5) E4Orf6-E1B-55k. Interestingly, the capsid proteins of the highly related Go.1 AAV (Fig. 1A, lanes 9 and 10) and the small Rep proteins of AAV2 (Fig. 1A, lanes 15 and 16) and AAV5 (Fig. 1A, lanes 13 and 14) were also degraded in an E4Orf6-E1B-55k-dependent manner. The insertion of a premature termination codon in the N-amino terminal region of E4Orf6 abrogated its ability to direct the loss of AAV5 proteins (data not shown).
FIG. 1.
The degradative effects of E4Orf6 show limited target specificity and act in a dose-dependent fashion. (A) Immunoblot following electrophoresis in a denaturing 10% polyacrylamide gel (9), using anti-HA antibody (clone HA-7; Sigma, St. Louis, MO), anti-AAV5 capsid antibody (B1; American Research Products, Inc., Belmont, MA), or anti-AAV Rep antibody (259.5; American Research Products, Inc., Belmont, MA) (top panel) or anti-actin or anti-14-3-3 antibody (Santa Cruz, Santa Cruz, CA) (bottom panel) of protein extracts taken 48 h posttransfection of 293 cells (9) with 1 μg/60-mm2 dish of either a capsid protein-expressing AAV5 minimal capsid gene plasmid (P41 [7]) (lanes 1 and 2), an HA-tagged APOBEC 3A expression construct (APOBEC 3A-HA, gift of M. D. Weitzman) (lanes 3 and 4), a construct expressing HA-tagged canine parvovirus VP2 (CPV-HA) (lanes 5 and 6), a construct expressing HA-tagged TIA-1 (TIA-1-HA, gift of B. Blencowe) (lanes 7 and 8, 11 and 12, and17 and 18), a plasmid expressing the Go.1-AAV capsid proteins (10; lanes 9 and 10), the AAV5 Rep52 protein (cytomegalovirus [CMV] AAV5Rep52, lanes 13 and 14), or the AAV2 Rep52 protein (CMV AAV2Rep52, lanes 15 and 16), together with 1 μg of either a plasmid expressing HA-tagged E4Orf6 (E4Orf6-HA) (+ lanes) or empty vector pSK (Invitrogen, Carlsbad, CA) (− lanes). The total amount of DNA in each transfection was brought to 2 μg/60-mm2 dish with the bacterial plasmid pcDNA3.1. The positions of the individual proteins are indicated. (B) Immunoblot following electrophoresis in a denaturing 10% polyacrylamide gel using anti-Rep antibody and antibody to 14-3-3, of protein extracts taken 48 h posttransfection of 293 cells with increasing amounts of CMV AAV5Rep52 alone (lanes 1, 3, 5, 7, and 9), or increasing amounts of CMV AAV5Rep52 plus increasing amounts of CMV E4Orf6 (lanes 2, 4, 6, 8, and 10), as shown. The total amount of DNA in each transfection was brought to 3 μg/60-mm2 dish with the bacterial plasmid pcDNA3.1. The positions of the individual proteins are indicated.
Degradation of AAV5 Rep52 (Fig. 1B), as well as AAV5 capsid proteins (data not shown), was dependent, in a dose-dependent manner, upon the amount of DNA used in the transient transfection. At high concentrations of Rep52- and E4Orf6-expressing plasmid DNA, degradation was not apparent (Fig. 1B, compare lanes 7 to 10 with lanes 1 to 6). These results suggested that degradation depended upon both the relative and the absolute levels of participating proteins. It is not yet clear, however, whether this indicates that the cellular degradative machinery can be saturated at the high levels of protein expression for which degradation cannot be demonstrated or whether overexpression by cotransfection leads to dominant-negative E4Orf6-E1B-55k complexes that lack cellular components required for ubiquitinylation. Supplementation of additional E1B-55k-expressing plasmid to transfections in 293 cells of high amounts of E4Orf6 and Rep52 did not result in increased degradation of Rep52 (data not shown).
E4orf6-E1B-55k-directed degradation of de novo-generated AAV5 capsid proteins requires both BC box motifs.
It has been previously shown that E4Orf6 function within the E3 ligase complex depends upon its interaction with elongins B and C, via motifs of E4Orf6 surrounding positions 46 and 122. A number of mutations within these sites that affect degradation have been characterized (3). Single-amino-acid mutations of leucine to glycine in BC box 1 (L47G), and leucine to serine in BC box 2 (L122S), have been shown to have little effect on degradation of p53 (3), while double mutations changing leucine and cysteine to glycine and valine in BC box 1 (L47G/C51V), and leucine and cysteine to serine and methionine in BC box 2 (L122S/C126M), abrogated E4Orf6-E1B-55k-dependent degradation of p53 (3). Consistent with these findings, we found that the same single-amino-acid mutations in either of the E4Orf6 BC box motifs (L47G or L122S) had little effect on the ability of E4Orf6 to participate with E1B-55k in directing the degradation of AAV5 capsid proteins transiently coexpressed in 293 cells, as assayed by immunoblotting (Fig. 2, compare lanes 4 and 5 with lanes 1 and 2). Mutations that changed two amino acids in BC box 1 (L47G/C51V) had a modest effect on E4Orf6-E1B-55k-dependent degradation of AAV5 capsid proteins (Fig. 2, compare lane 6 to lanes 1 and 2), while the double mutations in BC box 2 (L122S/C126M), or double mutations in both boxes 1 and 2 (L47G/C51V+L122S/C126M) more significantly impaired E4Orf6-E1B-55k-dependent degradation (Fig. 2, compare lanes 7 and 8 with lanes 1 and 2). An E4Orf6 mutant previously characterized as lacking a functional nuclear retention signal (NRS) and unable to degrade p53 (R240E/R241E [13]) was also significantly impaired in its ability to direct the degradation of AAV5 capsid proteins (Fig. 2, compare lane 9 with lane 10), suggesting that a functional NRS in E4Orf6 was critical for its ability to degrade AAV5 capsid proteins. Consistent with this finding, an E4Orf6 mutant previously suggested to lack a putative nuclear export signal and able to degrade p53 (lL90AI92A [13]), retained its ability to direct degradation of capsid proteins (Fig. 2, compare lane 3 to lanes 1 and 2).
FIG. 2.
E4orf6-E1B-55k-directed degradation of de novo-generated AAV5 capsid proteins requires both BC box motifs. An immunoblot following electrophoresis in a denaturing 10% polyacrylamide gel, using either anti-AAV5 capsid antibody (top panel), antibody to Ad5 E1B-55k (obtained from A. J. Berk, UCLA) (middle panel), or anti-tubulin (clone TUB2.1; Sigma Co., St. Louis, MO) and anti-HA antibody (bottom panel) of protein extracts taken 48 h posttransfection of 293 cells (9) with a 1 μg/well of a six-well dish of a minimal AAV5 P41-driven AAV5 capsid protein-expressing plasmid (9) by itself (lane 10) or together with 1 μg/well of a six-well dish of a plasmid expressing either wild-type E4Orf6 (E4Orf6; lane 1), wild-type E4Orf6 tagged with HA (E4Orf6-HA; lane 2), or various HA-tagged E4Orf6 mutants as described in the text (lanes 3 to 9). The final DNA concentration in all transfections was kept constant using the parent bacterial plasmid. The positions of the individual proteins are indicated.
E4Orf6 and E1B-55k form a cullin 5-containing E3 ligase complex together with de novo-generated AAV5 capsid proteins.
Following transient coexpression of AAV5 capsid proteins and wild-type hemagglutinin (HA)-tagged E4Orf6 in 293 cells, both E4Orf6 and E1B-55k could be immunoprecipitated under nondenaturing extraction conditions (8) using α-capsid antibody, and the sequestered levels of E1B-55k were greater if degradation was inhibited with the proteasome inhibitor MG132 (Fig. 3A, lanes 1 and 2). These results suggested that the E4Orf6 and E1B-55k proteins form a stable complex along with de novo-generated capsid proteins prior to proteasomal degradation. Complex formation was dependent on the addition of E4Orf6, suggesting that E1B-55k and AAV5 capsid proteins may not interact directly (Fig. 3A, lanes 9 and 10); however, such E1B-55k-containing complexes were seen to be relatively less abundant in the presence of the HA-tagged E4Orf6 mutant previously mentioned (Fig. 3A, lanes 5 and 6), in which a single amino acid in BC box 2 was altered and which moderately affected degradation of capsid proteins (L122S) (Fig. 2, lane 5). These complexes were undetectable in the presence of either the HA-tagged E4Orf6 mutant which lacks a functional NRS (R240E/R241E) (Fig. 3A, lanes 3 and 4) or that in which two amino acids within BC box 2 were altered (Fig. 3A, lanes 7 and 8) and for which degradation of AAV5 capsid proteins was more severely abrogated (L122S/C126M) (Fig. 2, lanes 9 and 7, respectively). Whether interaction between AAV5 capsid proteins and E4Orf6 can occur without participation of E1B-55k (in which case the inability of E4Orf6R240E/R241E HA to bind AAV5 capsid protein [Fig. 3A, lanes 3 and 4] may play a role in its inability to degrade this substrate [Fig. 2, lane 9]), or whether these results reflect the inability to isolate a less stable complex, is currently being investigated. Additionally, it remains unclear whether the immunoreactive material at the top of the gel in Fig. 3A is aggregated E1B-55k. Although impaired in its ability to direct degradation, the E4Orf6 BC box 2 mutant L122S/C126M was still able to associate with the capsid proteins both in the presence and in the absence of MG132 (Fig. 3A, lanes 7 and 8), suggesting that interaction with elongins and the subsequent inclusion of the E1B-55k protein into the complex was critical for degradation of AAV5 capsid proteins.
FIG. 3.
E4Orf6 and E1b-55k form a cullin 5-containing E3 ligase complex together with de novo-generated AAV5 capsid proteins. (A) Immunoblot following electrophoresis in a denaturing 10% polyacrylamide gel, using either antibody to E1B-55k (top panel) or HA (bottom panel) of immunoprecipitations (IP) (8), using anti-AAV5 capsid antibody, of protein extracts taken at 48 h posttransfection of 293 cells with 1 μg/well of a six-well dish of the minimal AAV5 P41-driven AAV5 capsid protein expressing plasmid either by itself (lanes 9 and 10) or together with 1 μg/well of a six-well dish of either a plasmid expressing wild-type E4Orf6 tagged with HA (lanes 1 and 2) or various HA-tagged E4Orf6 mutants as described in the text (lanes 3 to 8), either in the presence of 10 μM MG132 (+) or with dimethyl sulfoxide vehicle control (−). The final DNA concentration in all transfections was kept constant using the parent bacterial plasmid. The positions of individual proteins are indicated. WB, Western blot. (B) Immunoblot following electrophoresis in a denaturing 10% polyacrylamide gel, using either using either anti-AAV5 capsid antibody and anti-actin antibody (top panel), antibody to cullin 5 (clone H-300, sc13014; Santa Cruz, Inc., Santa Cruz, CA) (middle panel), or antibody to tubulin and HA (bottom panel) of protein extracts taken 48 h posttransfection of 293 cells. Transfections were performed with either 1 μg/well of a six-well dish of the minimal P41-driven AAV5 capsid protein-expressing plasmid (lanes 1 and 2) or 1 μg/well of a six-well dish of this plasmid together with 1 μg/well of a six-well dish of a plasmid expressing wild-type E4Orf6 tagged with HA (lanes 3 and 4), either with (lanes 2 and 4) or without (lanes 1 to 3) additional transfection of 50 pmol of siRNA to cullin 5a (37574; Santa Cruz, Inc., Santa Cruz, CA) using the siRNA transfection reagent (sc-29528; Santa Cruz, Inc., Santa Cruz, CA), 24 h before transfection with the above-mentioned plasmids. The final DNA concentration in all transfections was kept constant by using the parent bacterial plasmid. The locations of the individual proteins are shown.
The E4Orf6-E1B-55k E3 ligase complex that ubiquitinylates p53 and Mre11 utilizes cullin 5, which acts as a scaffold protein bringing E4Orf6, which is linked to elongins B and C, to an E2 ubiquitin-conjugating enzyme (7, 11, 19). Similarly, in our cotransfections, when cullin 5 was depleted by small interfering RNA (siRNA) treatment, E4Orf6 was no longer able to bring about the degradation of AAV5 capsid proteins (Fig. 3B, compare lanes 4 with lanes 3). These results confirmed that degradation of de novo-generated AAV capsid proteins occurs in an E3 ligase complex containing E4Orf6, E1B-55k, and cullin 5.
E4Orf6-E1B-55k-dependent degradation of AAV5 capsid and Rep52 proteins requires ubiquitin chain elongation.
E4Orf6-E1B-55k-dependent degradation of de novo-generated AAV5 capsid proteins and Rep52 is inhibited by both MG132 and lactacystin (9), suggesting that it occurs in a proteasome-dependent manner, which typically is mediated by ubiquitinylation of target proteins. As shown, E4Orf6-E1B-55k-dependent degradation of both AAV5 capsid proteins (Fig. 4, compare lane 4 to lane 3) and Rep52 (Fig. 4, compare lane 8 to lane 7) was inhibited by a dominant negative, lysineless ubiquitin UBR7 (15), which prevents the ubiquitin chain elongation required for targeting to, and processing by, the proteasome.
FIG. 4.
E4Orf6-E1B-55k-directed degradation of de novo-generated AAV5 capsid and Rep52 proteins requires ubiquitin chain elongation. An immunoblot following electrophoresis in a denaturing 10% polyacrylamide gel, using either using either anti-AAV5 capsid antibody and anti-actin antibody (top panel) or antibody to HA (bottom panel) of protein extracts taken 48 h posttransfection of 293 cells with 1 μg/well of a six-well dish of the minimal P41-driven AAV5 capsid protein expressing plasmid (lanes 1 to 4), or 1 μg/well of a six-well dish of a plasmid expressing AAV5 Rep52 (lanes 5 to 8), or 1 μg/well of a six-well dish of either of these plasmids together with 1 μg/well of a six-well dish of a plasmid expressing wild-type E4Orf6 tagged with HA (lanes 3 and 4 and lanes 7 and 8, respectively), either with (lanes 2, 4, 6, and 8) or without (lanes 1, 3, 5, and 7) additional cotransfection of a 1 μg/well of a six-well dish of plasmid expressing the dominant negative, lysineless ubiquitin UBR7 described in the text. The final DNA concentration in all transfections was kept constant using the parent bacterial plasmid. The positions of the individual proteins are indicated.
In summary, we have demonstrated that E4Orf6-E1B-55k-dependent degradation of AAV5 proteins was mediated by a ubiquitinylating, cullin 5-containing, E3 ligase complex similar to that previously demonstrated to degrade Mre11 and p53. The BC box motifs of E4Orf6, and hence the interactions between E4Orf6 and cellular elongins B and C, are crucial for E3 activity. Similar BC box motifs have been found for the E4Orf6 proteins of other Ad serotypes, and E4Orf6 from Ad4, Ad9, and Ad12 can direct degradation of AAV5 proteins in 293 cells, although the activity of Ad12 is less robust (K. D. Farris, R. Nayak, and D. J. Pintel, unpublished data). The E3 ligase complex likely brings about the degradation of AAV5 proteins via the addition of ubiquitin moieties onto the AAV5 substrate proteins, and a more detailed characterization of this process is currently being pursued.
E4Orf6-E1B-55k dependent degradation of Mre11 and p53 (11, 13, 14, 16), as well as an E4Orf6-E1B-55k-dependent degradative function required for RNA export (19), is necessary for efficient replication of Ad5 (16). E4Orf6-E1B-55k-dependent degradation of Mre11 has recently also been shown to enhance AAV2 replication (14); however, there are likely to be multiple mechanisms by which E4Orf6 supplies help to AAV replication. How can E4Orf6-E1B-55k-dependent degradation of AAV proteins be reconciled with their role as a helper functions? We have previously shown that the enhancement of translation that VA RNA provides as part of its helper function is necessary to restore AAV5 protein levels to those necessary for viral replication. It may be that E4Orf6-E1B-55k-dependent degradation of AAV5 proteins by E4Orf6 may be merely a byproduct of its role in targeting the degradation of a cellular protein necessary for viral replication. In this scenario, perhaps only the required levels of AAV5 proteins, and not the cellular target whose degradation is required for viral replication, become restored by VA RNA activity. Alternatively, it may be that AAV has evolved to rely on E4Orf6 and E1B-55k as regulators of its own gene expression. If unopposed, VA RNA might enhance excessive amounts of viral Rep and Cap at inappropriate times, which might be detrimental to infection. Another possibility might be that E4Orf6-E1B-55k activity in this regard has evolved to aid Ad replication in the presence of AAV, targeting the degradation of AAV proteins as a protective measure to temper AAV expression during AAV/Ad coinfection. Whatever its role in promoting AAV infection, E4Orf6-E1B-55k-dependent degradation of AAV5 proteins is likely to be an important facet of AAV biology.
Acknowledgments
We thank Lisa Burger for expert technical assistance. We thank Shih-Ching Lo, Mark Hannink, Matt Weitzman, Chris Lorson, Arnold Berk, and Benjamin Blencowe for reagents.
This work was supported by Public Health Service grants RO1 AI46458 and RO1 AI56310 from the National Institute of Allergy and Infectious Diseases to D.J.P.
Footnotes
Published ahead of print on 23 January 2008.
REFERENCES
- 1.Baker, A., K. J. Rohleder, L. A. Hanakahi, and G. Ketner. 2007. Adenovirus E4 34k and E1b 55k oncoproteins target host DNA ligase IV for proteasomal degradation. J. Virol. 817034-7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berns, K. I., and C. Giraud. 1996. Biology of adeno-associated virus. Curr. Top. Microbiol. Immunol. 2181-23. [DOI] [PubMed] [Google Scholar]
- 3.Blanchette, P., C. Y. Cheng, Q. Yan, G. Ketner, D. A. Ornelles, T. Dobner, R. C. Conaway, J. W. Conaway, and P. E. Branton. 2004. Both BC-box motifs of adenovirus protein E4orf6 are required to efficiently assemble an E3 ligase complex that degrades p53. Mol. Cell. Biol. 249619-9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowles, D., J. E. Rabinowitz, and R. J. Samulski. 2006. The genus Dependovirus, p. 15-24. In J. Kerr et al. (ed.), Parvoviruses. Hodder Arnold, London, United Kingdom.
- 5.Dosch, T., F. Horn, G. Schneider, F. Kratzer, T. Dobner, J. Hauber, and R. H. Stauber. 2001. The adenovirus type 5 E1B-55K oncoprotein actively shuttles in virus-infected cells, whereas transport of E4orf6 is mediated by a CRM1-independent mechanism. J. Virol. 755677-5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrari, F. K., T. Samulski, T. Shenk, and R. J. Samulski. 1996. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J. Virol. 703227-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harada, J. N., A. Shevchenko, A. Shevchenko, D. C. Pallas, and A. J. Berk. 2002. Analysis of the adenovirus E1B-55K-anchored proteome reveals its link to ubiquitination machinery. J. Virol. 769194-9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller, C. L., and D. J. Pintel. 2001. The NS2 protein generated by the parvovirus minute virus of mice is degraded by the proteasome in a manner independent of ubiquitin chain elongation or activation. Virology 285346-355. [DOI] [PubMed] [Google Scholar]
- 9.Nayak, R., and D. J. Pintel. 2007. Positive and negative effects of adenovirus type 5 helper functions on adeno-associated virus type 5 (AAV5) protein accumulation govern AAV5 virus production. J. Virol. 812205-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu, J., F. Cheng, and D. Pintel. 2006. Molecular characterization of caprine adeno-associated virus (AAV-Go.1) reveals striking similarity to human AAV5. Virology 356208-216. [DOI] [PubMed] [Google Scholar]
- 11.Querido, E., P. Blanchette, Q. Yan, T. Kamura, M. Morrison, D. Boivin, W. G. Kaelin, R. C. Conaway, J. W. Conaway, and P. E. Branton. 2001. Degradation of p53 by adenovirus E4orf6 and E1B55K proteins occurs via a novel mechanism involving a Cullin-containing complex. Genes Dev. 153104-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Querido, E., R. C. Marcellus, A. Lai, R. Charbonneau, J. G. Teodoro, G. Ketner, and P. E. Branton. 1997. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J. Virol. 713788-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Querido, E., M. R. Morrison, H. Chu-Pham-Dang, S. W.-L. Thirlwell, D. Boivin, and P. E. Branton. 2001. Identification of three functions of the adenovirus e4orf6 protein that mediate p53 degradation by the E4orf6-E1B55K complex. J. Virol. 75699-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz, R. A., J. A. Palacios, G. D. Cassell, S. Adam, M. Giacca, and M. D. Weitzman. 2007. The Mre11/Rad50/Nbs1 complex limits adeno-associated virus transduction and replication. J. Virol. 8112936-12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheaff, R. J., J. D. Singer, J. Swanger, M. Smitherman, J. M. Roberts, and B. E. Clurman. 2000. Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol. Cell 5403-410. [DOI] [PubMed] [Google Scholar]
- 16.Stracker, T. H., C. T. Carson, and M. D. Weitzman. 2002. Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature 418348-352. [DOI] [PubMed] [Google Scholar]
- 17.Ward, P. 2006. Replication of adeno-associated virus DNA, p. 189-212. In J. Kerr et al. (ed.), Parvoviruses. Hodder Arnold, London, United Kingdom.
- 18.Weitzman, M. D. 2006. The parvovirus life cycle: an introduction to molecular interactions important for infection, p. 143-156. In J. Kerr et al. (ed.), Parvoviruses. Hodder Arnold, London, United Kingdom.
- 19.Woo, J. L., and A. J. Berk. 2007. Adenovirus ubiquitin-protein ligase stimulates viral late mRNA nuclear export. J. Virol. 81575-587. [DOI] [PMC free article] [PubMed] [Google Scholar]