Abstract
Despite extensive evidence of cell signaling alterations induced by human immunodeficiency virus type 1 (HIV-1) in vitro, the relevance of these changes to the clinical and/or immunologic status of HIV-1-infected individuals is often unclear. As such, mapping the details of cell type-specific degradation of immune function as a consequence of changes to signaling network responses has not been readily accessible. We used a flow cytometric-based assay of signaling to determine Janus kinase/signal transducers and activators of transcription (Jak/STAT) signaling changes at the single-cell level within distinct cell subsets from the primary immune cells of HIV-1-infected donors. We identified a specific defect in granulocyte-macrophage colony-stimulating factor (GM-CSF)-driven Stat5 phosphorylation in the monocytes of HIV-1+ donors. This inhibition was statistically significant in a cohort of treated and untreated individuals. Ex vivo Stat5 phosphorylation levels varied among HIV-1+ donors but did not correlate with CD4+ T-cell counts or HIV-1 plasma viral load. Low Stat5 activation occurred in HIV-1-infected donors despite normal GM-CSF receptor levels. Investigation of mitogen-activated protein kinase (MAPK) pathways, also stimulated by GM-CSF, led to the observation that lipopolysaccharide-stimulated extracellular signal-regulated kinase phosphorylation is enhanced in monocytes. Thus, we have identified a specific, imbalanced monocyte signaling profile, with inhibition of STAT and enhancement of MAPK signaling, associated with HIV-1 infection. This understanding of altered monocyte signaling responses that contribute to defective antigen presentation during HIV-1 infection could lead to immunotherapeutic approaches that compensate for the deficiency.
The hallmark of uninhibited human immunodeficiency virus type 1 (HIV-1) infection is a well-documented, progressive immune cell dysfunction. Widespread T-cell activation, elevated inflammatory cytokine secretion, and hypergammaglobulinemia, to name a few examples, prominently occur in infected individuals. HIV-1 may affect immune cell function directly by inducing cell signaling through binding to cell surface receptors (8, 41) or by manipulating the cellular response to physiologic stimuli. For example, HIV-1-infected lymphocytes exhibit an hyperactive response to TCR stimulation, in a tat-dependent manner (30), that leads to potentiated, reflexively inappropriate signaling events that drive T-cell dysfunction. As part of the systemic changes induced by HIV-1, it is possible there will be alterations in intracellular signaling networks or feedback mechanisms that lead to dysfunctional immune cell responses to cytokines, further contributing to HIV-1 pathology in the host. Thus, direct or indirect effects upon a normal “set point” of immune cell homeostasis would lead to aberrant cell signaling and consequential immune dysfunction.
The Janus kinase/signal transducers and activators of transcription (Jak/STAT) pathways are essential for signaling by cytokines, including such important intracellular mediators as interferons (IFNs), interleukins, and colony-stimulating factors, as well as certain hormones (reviewed in reference 12). A recently described genetic mutation in humans that results in Stat1 deficiency is associated with fatal disseminated viral infection (14), highlighting the importance of STAT signaling for antiviral immunity. Among a diverse group of pathogens, HIV-1 has been shown to alter Jak/STAT signaling, although the exact mechanism is unclear, with some studies showing STAT hyperactivation (2, 4) and others reporting inhibition of STAT-driven transcriptional activity (26). Such paradoxical effects may be explained, at least in part, by effects upon distinct target cell types, different viral strains, or alternative pathways used in these studies. Thus, in the absence of a cell-by-cell analysis of signaling biochemistry, clear interpretation of the underlying mechanistic disorders driven by HIV-1 is difficult to formalize.
The development of flow cytometry-based approaches to the identification of aberrant signaling profiles, via measurement of phosphorylation of signaling intermediates (termed “phospho-flow”), has allowed for accelerated association of network topologies with disease states (19-22). Our previous work led to the realization that triggering cells to respond to environmental stimuli, such as cytokine or drug action, and the activation phenotypes associated with such perturbations, allows for clearer resolution of the underlying networks of protein activation states and allows for more distinct classification of signaling-associated disease outcomes (25). Signaling is a dynamic event, and as such static views of basal states would be considered insufficient for determination of a network's structure, therefore rendering correlations to clinical outcomes less meaningful. Phospho-flow is particularly well suited to address cell signaling in the context of HIV-1 disease because it can simultaneously discern multiple signaling events in an individual cell within complex cellular populations. The ability to monitor cell signaling in primary patient samples such as peripheral blood mononuclear cells (PBMC), allowed us to measure signaling pathway activation ex vivo. This novel approach obviates the use of potentially biased extended in vitro-cultured cells and allows for more physiologic interpretations in situations, for instance, where immune action depends on natural context for study of antigen presenting cells. In addition, phospho-flow technology allowed us to closely monitor signaling states within HIV-1-infected individuals, correlated to disease stage and treatment.
Here we compare the phosphorylation, and hence the activation status, of three Stat proteins (Stat1, Stat3, and Stat5) in response to a panel of cytokine stimuli, within three immune cell populations (T cells, B cells, and monocytes) from HIV-1-infected and uninfected pediatric subjects. We previously observed gender and ethnic differences in HIV-1-specific immunity in this cohort (35). Since we have also observed varied IFN-induced STAT signaling among racial groups of HCV-infected donors (18), we explored whether immune cell signaling also varied among ethnically diverse groups of HIV-1-infected subjects. The results of the initial phospho-flow screen then led us to investigate mitogen-activated protein kinase (MAPK) pathways. We identified an imbalanced signaling profile in response to model stimuli within the immune cells of HIV-1-infected pediatric subjects, with defective STAT activation and enhanced MAPK signaling. This cross-sectional study represents an critical step toward linking signaling changes found in HIV-1-infected individuals to recognized immune dysfunctions that occur in HIV-1 infection and disease progression.
MATERIALS AND METHODS
Clinical samples, isolation, storage, and thawing of primary cells.
Peripheral blood samples were obtained from perinatally HIV-1-infected and exposed-but-uninfected (EU) pediatric subjects (both groups born to HIV-1+ mothers) monitored at the pediatric HIV clinic at Jacobi Medical Center, Bronx, NY. The race and ethnic background of this cohort is reflective of the population of the Bronx, NY, where the residents are predominantly African-American (35.6%) and Hispanic (40.4%) (2000 census). The absolute total lymphocyte count, absolute CD4+ T-cell count and percentage, HIV-1 plasma viral load (Amplicor HIV-1 monitor; Roche Diagnostic Systems), and clinical data, including antiretroviral drug therapy history and adherence assessments, were recorded for samples from the HIV-1-infected patients. Peripheral blood samples from HIV-1-infected adults were obtained from an ongoing study of compartmental shedding in chronically infected adults conducted at San Mateo County Medical Center and Health Department. Peripheral blood samples from uninfected adults were obtained from the Stanford University Blood Bank. Institutional review boards at Jacobi, San Mateo County site, University of California at San Francisco (UCSF), and Stanford University approved this study. PBMC were isolated via density gradient separation (Ficoll-Paque Plus; Amersham Biosciences AB, Uppsala, Sweden). PBMC were pelleted and resuspended in either 90% fetal bovine serum (HyClone, South Logan, UT) or 90% human AB serum (Irvine Scientific, Irvine, CA) and 10% dimethyl sulfoxide (Sigma, St. Louis, MO) and then stored in liquid nitrogen. The blood samples from infected and uninfected pediatric subjects were handled and processed in parallel.
For signaling studies, PBMC were thawed in RPMI with 5% human AB serum, counted, pelleted, resuspended at 5 × 106 ml cells per ml, and allowed to rest at 37°C for 2 h in a 5% CO2 tissue culture incubator. PBMC isolated from buffy coat preparations from uninfected adults (Stanford University Blood Bank) were similarly isolated and stored in multiple aliquots from the same donor, which served as internal controls to test for the integrity of the stimulation and staining procedures. In separate experiments, repeat testing on multiple aliquots of the same normal donor showed low variance of phospho-flow responses (n = 4, σ2 < 0.1).
Stimulation, fixation, and permeabilization for detection of cell signaling.
PBMC were stimulated with vehicle or 4 ng of granulocyte-macrophage colony-stimulating factor (GM-CSF)/ml, 20 ng of IFN-γ/ml, 10 ng of interleukin-3 (IL-3)/ml, 20 ng of IL-7/ml, 100 ng of IL-10/ml, and 1 μg of lipopolysaccharide/ml (LPS) (from E. coli; Sigma, St. Louis, MO) and phorbol myristic acetate (PMA)-ionomycin (50 ng/ml and 1 μM, respectively; Sigma) for 12 min at 37°C. All stimuli were titrated to maximum signaling activity. All of the cytokines except GM-CSF were obtained from Peprotech (Rocky Hill, NJ). Clinical-grade GM-CSF (Sargramostim; Immunex, Seattle, WA) was obtained from the Stanford University Hospital Pharmacy. At the indicated times, paraformaldehyde (PFA; Electron Microscopy Services, Fort Washington, PA) was added to a 2% final concentration for 10 min at room temperature to arrest signaling activity. Cells were washed and permeabilized with 90% ice-cold methanol for 10 min on ice until staining for flow cytometry.
Cell surface and intracellular phospho-specific flow cytometry.
Directly conjugated antibodies against human CD3 (UCHT1), CD4 (RPA-T4), CD8 (RPA-T8), CD16 (3G8), CD20 (cytoplasmic, H1), CD33 (P7.6), and CD116 (GM-CSF receptor α chain, M5D12) were obtained from Becton-Dickinson (BD; San Jose, CA). Unconjugated anti-IL-3/IL-5/GM-CSF receptor β chain was obtained from Chemicon (Temecula, CA) and goat anti-mouse-phycoerythrin conjugate was obtained from Molecular Probes (Eugene, OR). Surface marker staining, both before and after the addition of methanol, was compared to the results obtained on unfixed, unpermeabilized cells. Since we determined that the traditional marker for monocytes, CD14 (M5E2), was not stable under methanol treatment, we identified monocytes by using CD33, a member of the sialic acid-binding immunoglobulin-like lectin (SIGLEC) family (reviewed in reference 9). In untreated cells, we confirmed that the two markers identified the same population and that the CD33+ cells were GM-CSFrα positive (Fig. 1A and B). We also confirmed the stability of CD33 and CD3 under different fixation-permeabilization conditions (Fig. 1C). Although CD33 also stains peripheral blood dendritic cells (16), this cell type comprises ca. 0.1% of all PBMC and is likely to make a minor contribution to the gated populations.
FIG. 1.
CD33 and CD14 recognize overlapping populations of cells within human PBMC. (A) Cells from adult HIV-1-negative donors were either fixed with 2% PFA, PFA fixed and permeabilized with 90% methanol (PFA/MeOH), or left untreated (live) prior to staining for flow cytometry with anti-CD14 and anti-CD33 antibody. (B) Untreated PBMC were stained with anti-CD33 (FMO, fluorescence minus one control), or anti-CD33 and anti-CD116 (GM-CSF receptor, alpha chain) antibody. (C) Cells treated as in panel A were stained with anti-CD3 and anti-CD33 antibody. The percentage of live events is shown next to each gate. Representative results of three independent experiments are shown.
PFA-fixed, methanol-permeabilized PBMC were washed twice and then resuspended in fluorescence-activated cell sorting staining buffer (phosphate-buffered saline plus 0.5% bovine serum albumin) and stained in final volume 100 μl for 30 min at room temperature. Antibodies obtained from BD were against phospho-Stat1(Y701), phospho-Stat3(Y705), phospho-Stat5(Y694), phospho-p38(T180/Y182), and extracellular signal-regulated kinase 1/2 (ERK1/2; pT202/pY204). Rabbit anti-phospho-p44/42 MAPK (T202/Y204) was obtained from Cell Signaling Technologies (Danvers, MA), and goat anti-rabbit-Alexa 647 conjugate was obtained from Molecular Probes (Eugene, OR).
Fluorescence-activated cell sorting acquisition and data analysis.
Events were acquired on a FACScalibur or an LSR II (BD). To minimize day-to-day variation in cytometer settings, control beads with fluorescence in all channels (Spherotech, Lake Forest, IL) were tested at the beginning of each acquisition run. Fluorescence values varied by <10% of target values. Flow cytometry data was analyzed with FlowJo software (Tree Star, Ashland, OR), Microsoft XL (Microsoft, Redmond, WA), and Prism 4.0 (GraphPad Software, San Diego, CA). Heat maps were generated with Spotfire DecisionSite (Tibco, Palo Alto, CA).
Purification of monocytes.
Anti-human CD33 microbeads were obtained from Miltenyi Biotec (Auburn, CA) and used according to the manufacturer's instructions. The purity was determined by flow cytometry and was >90% CD33+.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Isolated CD33+ cells were lysed in IPLS buffer containing 50 mM Tris-HCl (pH 8), 120 mM NaCl, 5 mM EDTA, and 0.5% (wt/vol) NP-40 and supplemented with fresh 1× protease inhibitor cocktail (Calbiochem, San Diego, CA). The protein concentration of each lysate was determined by using either a BCA assay (Pierce, Rockford, IL) or by Nanodrop technology. Lysates, matched for protein concentration, were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% Tris-glycine gels (Bio-Rad) before being transferred to a nitrocellulose membrane. Membranes were immunoblotted with STAT5-specific polyclonal antibodies (1:1,000) overnight at 4°C and visualized by chemiluminescence.
Quantitative reverse transcription-PCR.
After stimulation for the indicated times, PBMC were lysed with TRIzol (Invitrogen, Carlsbad, CA). RNA was isolated and treated with RNase-free DNase (amplification grade; Invitrogen) according to the manufacturer's instructions. cDNA was synthesized from 500 ng of RNA using oligo(dT) primers and Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). Real-time reverse transcription-PCR was performed on an Applied Biosystems (Foster City, CA) 7900 HT Fast Real-Time PCR system with a compatible Sybr green PCR master mix (Applied Biosystems). As a control for RNA quantity, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was detected by using specific primers: 5′-TGGGCTACACTGAGCACCAG-3′ (sense) and 5′-GGGTGTCGCTGTTGAAGTCA-3′ (antisense). The primer sets for human CIS1 (5′-TCCTCTGCGTTCAGGGACCT-3′ [sense] and 5′-ACACTAGGCGCATCCTCCTT-3′ [antisense]) and pim-1 (5′-CGAGCATGACGAAGAGATCAT-3′ [sense] and 5′-TCGAAGGTTGGCCTATCTGA-3′ [antisense]) were made to order by Operon Technologies (Huntsville, AL). Each of the PCR assays were run in duplicate, and the gene copy numbers were estimated from standard curves, using the S.D.S. software supplied with the PCR cycler.
RESULTS
Phospho-flow screening identifies multiple unresponsive STAT signaling nodes associated with advanced HIV-1 disease.
HIV-1 is known to alter cell signaling, including the Jak/STAT pathway, although with conflicting results reported (2, 26). We monitored cell signaling in primary patient samples, via phospho-flow cytometry, in an attempt to resolve whether cell subset signaling differences could be an explanation for the differences observed. We stimulated PBMC ex vivo (derived from uninfected and HIV-1-infected pediatric subjects) with each of the cytokines IL-7, IL-10, GM-CSF, and IFN-γ. We assayed for increases in phospho-Stat1, phospho-Stat3, and phospho-Stat5 (pStat1, pStat3, and pStat5, respectively) and represented this as a ratio of induction over baseline levels (fold change) as the readout. In aggregate form, this information was then represented as heat maps, as described by Eisen et al. (15). We confirmed that STAT phosphorylation in uninfected PBMC correlated with increased target gene transcription (mean 14.5-fold increase in cytokine-inducible SH2-domain protein [CIS], a Stat5 target gene, after GM-CSF stimulation; A. Lee et al., unpublished results).
As a first step toward determining whether HIV-1-associated cell signaling changes might be affected by disease state, we compared STAT activation in the group of HIV-1+ pediatric subjects that had robust responses to highly active antiretroviral therapy (HAART), i.e., low viral load and high CD4+ T-cell counts (categorized as “+T” for HIV-1+ on treatment), versus pediatric subjects with advanced HIV-1 disease, i.e., high viral load and low CD4 counts (categorized as “+A” for advanced HIV-1+). Table 1 lists the clinical and demographic data for the pediatric subjects screened.
TABLE 1.
Clinical and demographic features of HIV-infected and exposed, uninfected pediatric subjects
| Figure | Status | Donor identification no. | CD4% | CD4 ABSa | Viral load (copies/ml) | Treatmentb | Age (mo)c | Sex | Raced |
|---|---|---|---|---|---|---|---|---|---|
| Fig. 2 | Advanced HIV+ | 0802 | 4 | 62 | 527,298 | 111 | F | NHB | |
| 0408 | 4 | 100 | 150,000 | d4T, 3TC, ddI EC, ATV | 124 | M | NHB | ||
| 1903 | 11 | 251 | 24,008 | PTI on ABC/ZDV/3TC | 143 | M | H | ||
| Treated HIV+ | 1013 | 35 | 617 | 364 | d4T, ddI, EFV | 161 | M | H | |
| 1007 | 36 | 831 | <400 | ABC/ZDV/3TC and NFV | 171 | F | H | ||
| 0303 | 48 | 815 | <50 | ZDV, 3TC, LPV/RTV | 139 | F | NHB | ||
| 2602 | 48 | 1,401 | 135 | d4T, ddI, EFV | 93 | F | NHB | ||
| HIV− | 1006U | 84 | M | H | |||||
| 1813U | 37 | M | NHB | ||||||
| 2212U | 55 | M | H | ||||||
| Fig. 3 | HIV+ | 319 | 4 | 67 | 5,245 | 74 | M | NHB | |
| 610 | 6 | 19 | 511,000 | ABC/ZDV/3TC, ddI EC, ATV | 187 | F | NHB | ||
| 718 | 17 | 444 | 183,083 | 164 | M | H | |||
| 1310 | 18 | 542 | 6,630 | 112 | F | NHB | |||
| 1013 | 18 | 595 | 60,300 | 92 | F | H | |||
| 1819 | 22 | 251 | 10,100 | ZDV/3TC + LPV/RTV | 105 | F | H | ||
| 1007 | 25 | 1,067 | 10,439 | d4T/3TC | 35 | F | H | ||
| 1019 | 26 | 418 | 3,134 | 63 | M | NHB | |||
| 1001 | 31 | 620 | 6,358 | 58 | F | NHB | |||
| 416 | 31 | 856 | 6,390 | d4T/3TC | 53 | M | H | ||
| 1303 | 31 | 583 | 111,682 | 68 | F | NHB | |||
| 302 | 32 | 695 | 14,715 | 50 | F | NHB | |||
| 510 | 34 | 1,153 | <50 | ABC/ZDV/3TC, TDF, ATV/RTV | 187 | F | NHB | ||
| 103 | 36 | 912 | 31,116 | d4T/3TC | 110 | M | NHB | ||
| 1316 | 44 | 2400 | 521 | d4T/3TC | 20 | F | H | ||
| 1302 | 49 | 1,776 | 33,854 | 3TC, LPV/RTV, TDF | 108 | F | NHB | ||
| HIV− | 1018U | 12 | M | H | |||||
| 1006U | 16 | F | H | ||||||
| 1103U | 69 | M | NHB | ||||||
| 2212U | 49 | M | H | ||||||
| 1023U | 53 | F | NHB | ||||||
| 0402U | 57 | M | H | ||||||
| 0316U | 52 | F | H | ||||||
| 1823U | 171 | M | NHB | ||||||
| 0118U | 91 | M | H | ||||||
| 1302U | 87 | F | NHB | ||||||
| 0323U | 107 | M | NHB |
Absolute CD4+ T-cell count in cells/mm3.
Medication and treatment abbreviations: 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; d4T, stavudine; ddI EC, didanosine, enteric coated; EFV, efavirenz; LPV/RTV, lopinavir/ritonavir; RTV, ritonavir; TDF, tenofovir; ZDV, zidovudine. PTI, partial treatmentinterruption.
Figure 2: P = 0.23, NS, for Student t test of ages of HIV+ (n = 16) versus HIV− (n = 11) pediatric subjects.
H, Hispanic; NHB, non-Hispanic black.
As shown in Fig. 2A, we identified B cells (CD20+), monocytes (CD33+), and T lymphocytes (CD3+ CD4+ and CD3+ CD4− [i.e., CD8+]) within fixed and permeabilized PBMC by standard surface marker characterization and gating. The phospho-protein fold changes for each immune cell type, in response to the panel of cytokines, are shown in a heat map representation in Fig. 2B. Of the 60 unique signaling nodes (combination of cell type, stimulus, and phospho-protein) surveyed in these experiments, we identified three with very high fold changes (≥3 log2 fold change, highlighted in red) in uninfected (control) pediatric subjects. Interestingly, comparison of these signaling nodes across the donor categories showed “normal” STAT activation in HAART-treated, HIV-1-infected pediatric subjects (+T columns) and the absence of STAT phosphorylation in pediatric subjects with advanced HIV-1 disease (+A columns). Figure 2C shows the underlying data in histogram form for the three nodes with the highest fold change in uninfected pediatric subjects, compared across the different donor groups. In total, these results indicated that in advanced HIV-1 disease, multiple STATs in different immune cell types had lost responsiveness to upstream stimuli, whereas in HIV-1-infected donors with a good response to treatment, Jak/STAT signaling was preserved.
FIG. 2.
Flow cytometry screening reveals loss of STAT responsiveness in multiple immune cell types in advanced HIV-1 infection. Clinical and demographic data for the donors shown in Table 1. PBMC were stimulated ex vivo with cytokines, fixed, permeabilized, and stained for surface and intracellular phospho-epitope-specific flow cytometry as described in Materials and Methods. Briefly, PBMC were left untreated or exposed to 10 ng of IL-7/ml, 100 ng of IL-10/ml, 4 ng of GM-CSF/ml, or 20 ng of IFN-γ/ml for 12 min at 37°C; fixed with 2% PFA; permeabilized with 90% methanol; and stained for surface and phospho-specific markers. (A) Surface staining and gating hierarchy of fixed and permeabilized cells. B cells were identified as CD20+, monocytes were identified as CD33+, and T lymphocytes were identified as CD3+ CD4+ and CD3+ CD4−. The percentage of events is shown for each gate. One representative result from 10 experiments is shown for an HIV-1− pediatric donor. (B) Comparison of phospho-Stat1, -Stat3, and -Stat5 levels in immune cell populations for (i) HIV-1− pediatric subjects, (ii) treated HIV-1+ pediatric subjects with a good response, and (iii) pediatric subjects with advanced HIV-1 infection. A heat map representation of the fold change in phospho-protein levels after cytokine stimulation, normalized to unstimulated controls, is shown. The fold change was converted to log2 and colored according to the scale shown. Each quadrant shows the results for gated cell type (CD8+ and CD4+ T lymphocytes, B cells, and monocytes). Each column is labeled according to donor group (“−” for HIV-1−, “+T” for HIV-1+ with good treatment response, “+A” for HIV-1+ with advanced HIV-1+). Each row shows the ex vivo stimulus added (ns = no stimulus control). Three signaling nodes (cell type-stimulus-phosphoprotein combination) with high fold changes (>3 log2 fold change) in the HIV-1− donors are outlined in red. One representative of three experiments is shown. (C) Histogram data underlying the highlighted nodes outlined in the heat map representation shown in panel B. Nonstimulated cells are shown as gray histograms, while stimulated cells are shown in colors corresponding to the heat map scale. The median fluorescence intensities for the stimulated cells are shown. For cell populations with two peaks, the median fluorescence intensities for the higher peak are shown.
Directed phospho-flow testing reveals a significant HIV-1-associated decrease in GM-CSF-stimulated Stat5 activation in monocytes.
The initial phospho-flow screen indicated that in pediatric subjects with advanced HIV-1 disease, there was marked inhibition of Stat1 activation in monocytes and Stat5 activation in both T lymphocytes and monocytes (Fig. 2C). In order to determine the statistical significance of our initial observations, we repeated phospho-flow testing on these particular signaling nodes, selecting a random sample of uninfected and HIV-1-infected donor PBMC from among >2,000 potential samples contained within the Jacobi Medical Center database. We tested for signaling differences among ethnic groups within the HIV-1-infected pediatric cohort and found that cytokine-driven STAT phosphorylation did not differ significantly between Hispanics and non-Hispanic blacks (E. R. Sharp et al., unpublished results). Clinical and demographic data for the selected donors are shown in Table 1. In this larger sample, IL-7 stimulation of pStat5 in CD3+ T lymphocytes and IFN-γ activation of pStat1 in CD33+ monocytes did not significantly differ between uninfected and infected pediatric donors (Fig. 3A and B, respectively). In contrast, phosphorylation of Stat5 after GM-CSF stimulation was significantly inhibited in monocytes from HIV-1-infected pediatric subjects (P = 0.03 by two-tailed Student t test, Fig. 3C). Only 2 of the 16 HIV+ donors in this sample had very advanced HIV disease (<10% CD4+ T cells, Table 1), arguing against selection bias influencing these results. Titration of GM-CSF at up to 20 ng/ml did not correct the HIV-associated inhibition of Stat5 phosphorylation (A. Lee and E. R. Sharp, data not shown).
FIG. 3.
Identification of a specific inhibition of cytokine-driven Stat5 activation in monocytes associated with HIV-1 infection. Clinical and demographic data for the donors shown in Table 1. PBMC were stimulated and fixed as in Fig. 2. The data were compiled from 10 independent experiments. (Top panel) Representative surface staining showing the populations gated for the phospho-protein fold change (f-c) results shown in panels A to D. (A) IL-7-stimulated phospho-Stat5 f-c in CD3+ T lymphocytes. (P = NS by two-tailed Student t test). (B) IFN-γ-stimulated phospho-Stat1 f-c in CD33+ monocytes (P = NS). (C) GM-CSF-stimulated phospho-Stat5 f-c in CD33+ monocytes (*, P < 0.05). (D) IL-3-stimulated phospho-Stat5 in f-c CD33+ monocytes (**, P < 0.01). (Bottom panel) Heat map summary of mean fold changes for repeated experiments shown in panels A to D. The color scale is as described for Fig. 2.
To delineate whether this was a GM-CSF pathway-specific inhibition of the phosphorylation of Stat5, we examined IL-3-stimulated Stat5 activation in CD33+ monocytes. GM-CSF, IL-3, and IL-5 are closely related members of the βc cytokine family, with common β receptor subunits that signal through the activation of Jak2 and Stat5. IL-7, in contrast, signals through Jak3 and Stat5 (31). We found that IL-3-mediated activation of Stat5 was also inhibited in the CD33+ monocytes of HIV-1+ pediatric subjects (P = 0.006, Fig. 3D). Our observation that Stat5 activation is inhibited in response to IL-3 and GM-CSF in CD33+ monocytes, but not by IL-7 in CD3+ T lymphocytes, suggests that HIV-1 infection is associated with a cell type- and pathway-specific inhibition of Jak2 and Stat5 activation and that whatever feedback or regulatory mechanism that results in this “nonresponsiveness” is enabled specifically in monocytes.
Lack of correlation between clinical parameters and GM-CSF-mediated Stat5 phosphorylation.
We noted the broad range in GM-CSF-stimulated pStat5 responses among the HIV-1-infected donors (0.8- to 8.7-fold change, Fig. 3C) and explored whether clinical or virologic parameters correlated with Stat5 responsiveness. As shown in Fig. 4, there was no detectable correlation between either CD4% or plasma viremia and GM-CSF-stimulated pStat5 fold change (Fig. 4A and B, respectively). Since previous reports have described correction of HIV-1-related immune dysfunction in response to antiretroviral therapy (1), we also compared pStat5 responses in HAART-treated pediatric subjects versus patients that had been off HAART for at least 6 weeks. Although there was a trend toward higher pStat5 responses in treated compared to untreated pediatric subjects (mean, 4.9 versus 2.7, respectively, Fig. 4C), the differences were not statistically significant (P = 0.08). Taken together, these results suggest that low pStat5 responses are the result of complex factors, beyond HIV-1 disease progression and viral burden. In agreement with prior reports, we found that HIV-1-associated immune dysfunction (in this case low Stat5 phosphorylation) generally improved with antiretroviral treatment.
FIG. 4.
Comparison of Stat5 activation with clinical, virologic, and treatment status of HIV-1+ pediatric subjects. (A) GM-CSF-stimulated phospho-Stat5 fold change in monocytes versus %CD4+ of peripheral T cells. (P = 0.40, NS, by two-tailed Student t test). (B) Phospho-Stat5 fold change versus log10 HIV-1 viral load (P = 0.50, NS). (C) Phospho-Stat5 fold change for treated versus untreated HIV-1+ pediatric subjects (mean = 4.9 and 2.7, respectively, P = 0.08 [NS]).
GM-CSF receptor levels are not affected by HIV-1.
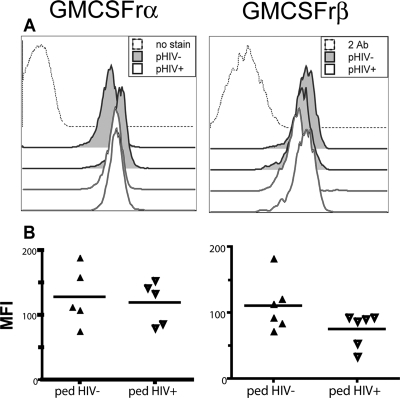
Our results left open the possibility that reduced GM-CSF receptor levels could result in reduced signaling through Jak2/Stat5. Since ligand binding to receptor immediately precedes Jak2 phosphorylation, we compared GM-CSF receptor levels in uninfected and HIV-1-infected donors. The complete GM-CSF receptor consists of an α/β heterodimer, with the α chain conferring ligand specificity, while the β chain is responsible for signal transduction (11). Since the α chain is found in excess, we determined the levels of both receptor subunits on monocytes by flow cytometry. GM-CSF receptor α and β levels did not differ significantly between uninfected and HIV-1-infected donors (n = 5 for each, P = not significant [NS], Fig. 5). This finding supports a conclusion that a specific intracellular mechanism is responsible for the lack of responsiveness in these cells.
FIG. 5.
HIV-1 does not significantly affect GM-CSF receptor levels. GM-CSF receptor α (left column) and β (right column) chain levels as determined by flow cytometry in monocytes from uninfected and HIV-1-infected pediatric subjects. (A) Representative histograms of receptor staining from two HIV-1− and two HIV-1+ pediatric subjects. Only events from CD33+ cells are shown. Controls (dotted histograms) were unstained samples for the α chain and secondary antibody only for the β chain. (B) Summary of MFI (median fluorescence intensities) for all donors (P = NS).
HIV-1-associated potentiation of ERK phosphorylation in monocytes.
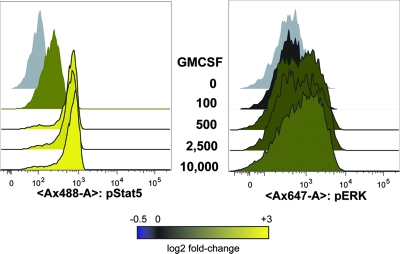
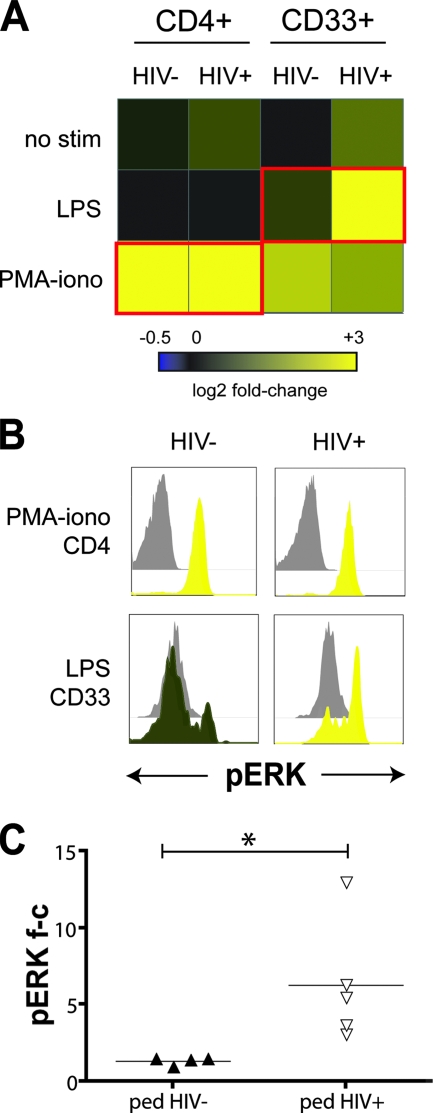
As a growth factor for myeloid cells, GM-CSF provides a pleiotropic stimulus for multiple signaling pathways, including Jak/STAT, PI-3 kinase, Akt, p38 MAPK, and Ras-raf-ERK (reviewed in reference 13). As a result of our observation that HIV-1 infection blocks GM-CSF signaling through Stat5, we hypothesized that other GM-CSF signaling arms, such as the MAPKs p38 and ERK, might be enhanced in a compensatory manner. We first tested if we could detect GM-CSF stimulation of ERK phosphorylation with flow cytometry. In normal adult blood donors, GM-CSF was a very potent stimulus for Stat5 phosphorylation (6.6-fold change at 0.5 ng/ml) but relatively weak for ERK phosphorylation (<2-fold change at 0.5 to 10 ng/ml, Fig. 6). We therefore expanded our phospho-flow screen to include other known stimuli for ERK and p38 phosphorylation, separately and in combination with GM-CSF. Comparison of MAPK signaling between HIV-1-infected and uninfected pediatric subjects revealed a striking HIV-1-associated enhancement of ERK phosphorylation in monocytes in response to bacterial LPS (mean fold change of 6.3 versus 1.4, P = 0.04, Fig. 7) and the combination of GM-CSF plus LPS (mean fold change of 3.3 versus 1.3, P = 0.02). These stimuli also resulted in tumor necrosis factor alpha (TNF-α) secretion by PBMC and purified monocytes from both uninfected and HIV-infected donors (n = 12; A. Lee, data not shown), arguing for the physiological relevance of the signaling results.
FIG. 6.
GM-CSF more potently activates Stat5 compared to ERK in normal adult donors. PBMC from HIV-1-negative adult donors were stimulated, fixed, and stained as in Fig. 2. Phospho-Stat5 and phospho-ERK were simultaneously detected with antibodies conjugated to Alexa-488 and Alexa-647, respectively. Histogram data are shown for CD33+ cells only. The GM-CSF concentrations used in the titration (0 to 10,000 pg/ml) in the center of the figure are shown at the same level as the corresponding histogram data. The data shown are representative of two independent experiments with similar results.
FIG. 7.
Enhanced ERK phosphorylation in monocytes of HIV-1+ pediatric subjects. (A) Heat map of flow cytometry analysis of ERK phosphorylation in PBMC from HIV-1− and HIV-1+ pediatric subjects. Cells were stimulated ex vivo with 1 μg of LPS/ml or 50 ng of PMA/ml and 1 μM ionomycin, for 12 min. (B) Histogram data underlying the heat maps outlined in red in panel A. Phospho-ERK levels are shown for CD4+ T cells stimulated with PMA-ionomycin (top row) and for CD33+ monocytes stimulated with LPS (bottom row). Unstimulated controls are shown in gray. One representative result from four experiments is shown. (C) Summary of phospho-ERK fold-changes for all donors. The data were compiled from four independent experiments (*, P < 0.05).
In contrast, ERK activation in response to phorbol ester treatment with PMA-ionomycin did not significantly differ in HIV-1 (P = NS, Fig. 7 and data not shown). These results indicate that HIV-1 enhances the monocyte signaling response to a natural innate immunity ligand such as LPS, with the potential to increase host inflammatory responses to pathogens. Thus, HIV-1 induced reduction of signaling potential is related to antiviral immunity (as represented by pStat5), while increasing signaling related to inflammatory responses (as represented by pERK) in monocytes, a cell type that HIV-1 interacts with extensively throughout the course of infection.
DISCUSSION
This study delineates the effects of in vivo HIV-1 infection on the signaling behavior of single immune cells interacting within a complex cellular milieu. Interrogation of phospho-epitopes within pathogen-altered intracellular signaling networks at the primary cell level allowed linkage of network states with virologic and clinical parameters. In this cross-sectional study, our comparison of the Jak/STAT pathways within prominent peripheral blood immune cell populations revealed a specific defect in GM-CSF-stimulated Stat5 phosphorylation within monocytes associated with HIV-1 infection. Expansion of the phospho-flow screen to include other phospho-proteins activated by GM-CSF led to the observation that LPS-stimulated ERK phosphorylation was enhanced in HIV-1 infection. Reduced signaling potential of “antiviral” STAT pathways, and enhanced signaling of “inflammatory” MAPK pathways are consistent with long-standing observations of monocyte dysfunction in HIV-1 infection, including reduced antigen uptake and hyperactive inflammatory cytokine secretion (5, 39). These results provide a specific molecular basis for the prior observations of dysfunction.
In order to put these results into context, it may be helpful to organize the complex body of literature on HIV-1 and Jak/STAT signaling into studies of pathway activation state (i.e., activated or depressed signaling) and studies of pathway responsiveness (i.e., the ability to activate in response to an upstream stimulus). Activation state studies have shown increased phospho-Stat5 after in vitro HIV-1 infection or exposure of both CD4+ T-cell and monocytic cell lines (24) and constitutive activation of a truncated form of Stat5 (Stat5Δ) in a majority of HIV-1-infected donors (4). In contrast, studies of Jak/STAT responsiveness in HIV-1 have shown a reduced ability to phosphorylate Stat5 after IL-2 stimulation of CD8+ T cells from HIV-1-infected adults (26), as well as after GM-CSF stimulation of monocyte-derived macrophages infected with HIV-1 in vitro (43). Preexisting activation through viral antigen stimulation or immune activation may desensitize the pathway to further stimulation. This could occur through negative feedback mechanisms, for instance, as mediated by the suppressor of cytokine signaling (SOCS) family (17, 29, 36), among others. Except for donors with advanced HIV-1 infection, our results were not consistent with a systemic decrease in Jak/STAT responsiveness across all cell types and pathways but rather a specific inhibition of Jak2/Stat5 signaling in response to related βc cytokines (GM-CSF and IL-3) in monocytes. Since our results are based on blood samples obtained from HIV-1-infected donors, we both validate and extend the significance of similar observations based on in vitro model infection systems (43), providing here a physiologic and in vivo characterization of the effect.
By studying a cohort of perinatally HIV-1-infected pediatric subjects and their exposed, uninfected controls, we were able to take advantage of closely matched socioeconomic status and environment. In addition, the drawing, processing, and storage of the blood samples were performed completely in parallel, providing ideal controls for sample handling conditions. Our results are not limited to pediatric disease but bear significance to HIV-1 in general. In separate experiments, we found that GM-CSF induced Stat5 phosphorylation was significantly reduced in HIV-infected adults compared to uninfected controls (67% of control, n = 8 [HIV+], n = 4 [HIV−], P = 0.02).
In addition to the advantage of monitoring cell signaling in primary immune cells ex vivo, phospho-flow screening allowed the integration of signaling results with the clinical and virologic parameters of the HIV-1-infected donors under study. In the initial comparison of treated HIV-1+ pediatric subjects (low VL and high CD4s) versus those with advanced HIV-1 disease (high VL and low CD4s), STAT activation was normal in the former group, whereas the latter group could not activate multiple STATs, suggesting a correlation between severe disease and nonresponsive STATs. However, repeat testing in a larger cohort of HIV-1-infected pediatric subjects did not reveal a clear relationship between low Stat5 activation and HIV-1 disease state, as measured by CD4% or HIV-1 viral burden. We also noted improvement in low Stat5 responses associated with HAART, as seen with other HIV-1-related immune dysfunctions, although the difference was not statistically significant (Fig. 4C).
We also tested our HIV-1-infected pediatric subjects for differences in signaling among different ethnic groups, taking advantage of the ethnic diversity reflected in this urban cohort. We did not find significant differences in ex vivo-stimulated STAT phosphorylation between Hispanics and African-Americans, despite an earlier demonstration of variable HIV-1-specific immunity in this cohort (35). Our results also show that reduced Stat1 responses to IFN stimulation in blacks, compared to whites, in the context of HCV infection (18) does not extend to other viruses such as HIV-1. As such, it is obvious the different disease contexts, as well as different ethnic groups, preclude a direct comparison of studies.
Testing the idea that a GM-CSF signaling block at Stat5 might lead to enhanced signaling of other GM-CSF-stimulated pathways led to a second significant finding, namely, that MAPK signaling in monocytes is potentiated in HIV-1 in response to specific natural ligands of innate immunity, but not to broadly active, small molecule inducers of cell signaling. As with the STAT activation observations, we found that the HIV-1-related increase in MAPK activity was specific, i.e., only in response to LPS or the combination of LPS and GM-CSF. MAPK signaling is essential for inflammatory cytokine secretion, and monocytes play a key role in secretion of these molecules. The data supports a hypothesis that HIV-1 increases the ERK response in monocytes specifically, in order to promote aberrant inflammatory cytokine secretion in response to Toll-like receptor (TLR) signals.
We did not find evidence for reduced GM-CSF receptor levels in HIV-1 as a potential explanation for low phosphorylation of Stat5 (Fig. 4). In addition, HIV-1 infection was not associated with lower total Stat5 levels by Western blotting (E. R. Sharp et al., unpublished results), arguing that the low pStat5 response we observed resulted from a reduction in steady-state phosphorylation. Although we considered the possibility that a reduction in the fold increase in pStat5 after stimulation might arise from elevated baseline phosphorylation due to chronic activation in the setting of HIV-1 infection, we did not find any significant difference in basal pStat5 between HIV-1− and HIV-1+ (P = 0.81, n = 11 [HIV-1−], n = 14 [HIV-1+]). The recent observation that HIV-1 Nef induces SOCS, which negatively regulates pStat1 and pStat3 activation in B cells (32) is potentially relevant to our findings. However, an upregulation in negative feedback mechanisms should result in generalized inhibition of STAT signaling, not a specific inhibition of βc cytokine-driven Stat5 activation, as we observed. Thus, a simple explanation of upregulation of broadly active SOCS inhibition is not the cause, but the results do not obviate a mechanism involving cell type specific utilization of SOCS.
Exposure to several HIV-1 proteins is known to result in ERK hyperphosphorylation. Nef exposure resulted in ERK activation in CD4+ T cells (33), increasing target cell activation and infectivity (44), and endothelial cells (38). HIV-1 gp120 binding to CCR5 on macrophages resulted in the activation of both ERK and phosphatidylinositol-3 kinase, which were required for gp120-stimulated TNF-α secretion (27). Extrapolating from the work above, one might predict that HIV-1 infection would result in elevated ERK phosphorylation ex vivo. However, we found that basal pERK levels were nearly equivalent (P = 0.56, n = 6 [HIV-1−], n = 10 [HIV-1+]), and instead we observed significant enhancement of LPS-stimulated ERK phosphorylation by HIV-1 (Fig. 7). LPS stimulation of ERK in monocytes signals through TLR4, whereas HIV-1 signals through TLR7/8 (3, 28), raising the possibility that HIV-1 infection enhances ERK activation by providing additional or complementary TLR signals (40).
What are the implications for the altered signaling responses we have demonstrated in this group of HIV-1-infected pediatric subjects? Although full-length Stat5 binds to the HIV-1 LTR and increases viral production in CD4+ T cells (34), a constitutively activated, truncated form of Stat5 is present in HIV-1-infected individuals and negatively regulates HIV-1 expression (10). Although we cannot distinguish isoforms with the phospho-specific Stat5 antibody, reduction in pStat5 is likely to counteract the antiviral effect of truncated Stat5. Variability in GM-CSF-mediated pStat5 response in HIV-1+ donors (Fig. 3C) may have contributed to observed differences in effects of GM-CSF therapy on HIV-1 viral load in clinical trials (7, 23). Nonresponsiveness to GM-CSF in a proportion of subjects has been observed in these and other studies of HIV-infected and uninfected adults.
MAPKs, including ERK, are integral for inflammatory cytokine secretion in response to a variety of stimuli. Enhancement of LPS-stimulated ERK phosphorylation by HIV-1 represents a possible mechanism for increasing inflammatory cytokine secretion, thus contributing to the development of generalized immune activation. Notably, HIV-1 gp120 may cause TNF-α secretion from macrophages in a CCR5- and Lyn (a member of the src family kinases)-dependent manner (37). Although “traditional” CD14+ monocytes lack CCR5 (42), CD16+ monocytes in HIV-1 have elevated CCR5 expression (up to 40% CCR5 in HIV-1+ donors) (16). It will be of interest to determine whether the enhancement of LPS-stimulated ERK in monocytes we have identified here depends on CCR5.
The potentiation of LPS-stimulated signaling in HIV-1 infection is of special interest, in light of the recent work by Brenchley et al. demonstrating a strong correlation between elevated serum LPS levels (resulting from gut microbial translocation) and HIV-1-related chronic immune activation (6). Chronic in vivo LPS stimulation also led to reduced ability of monocytes to secrete TNF-α ex vivo. The potentiation of LPS-induced ERK phosphorylation may represent a strategy used by HIV-1 to counter the desensitizing effects of chronic LPS stimulation and maintain persistent immune activation through inflammatory cytokine secretion.
In summary, we have applied phospho-specific flow cytometry to determine the extent of altered cell signaling profiles in HIV-1-infected individuals. In this cross-sectional study, we identified a significant defect in Stat5 activation and enhancement of ERK signaling within the monocytes of HIV-1-infected pediatric subjects, with intriguing implications for the role of monocytes in the immunopathogenesis of HIV-1 infection. These findings could guide further investigations into long-described immune cell dysfunctions that occur in HIV-1 infection but have eluded understanding at a mechanistic level. In the era of “personalized medicine,” one can envision how phospho-epitope-specific analysis could be applied to larger and/or longitudinal studies to further bridge our understanding of how HIV-related signaling abnormalities may affect disease progression and clinical outcomes.
Acknowledgments
This research was supported by grants from the NIAID (K08 AI067064 to A.W.L.), UCSF AIDS Biology Program of the AIDS Research Institute, NIH grant AI060379 (to D.F.N.), and grant CH05-SMCHC-612 from the University of California HIV/AIDS Research Program (to D.M.I.).
We thank J. Fortin and other members of the Nolan Laboratory for phospho-flow training and helpful advice, the Center for AIDS Research at Stanford University School of Medicine for the maintenance of the biosafety level 3 facility where parts of this study were performed, and C. Macaubas and E. Mellins (Department of Pediatrics, Stanford University School of Medicine) for their assistance with non-HIV-exposed pediatric subject samples.
Footnotes
Published ahead of print on 23 January 2008.
REFERENCES
- 1.Autran, B., G. Carcelain, T. S. Li, C. Blanc, D. Mathez, R. Tubiana, C. Katlama, P. Debre, and J. Leibowitch. 1997. Positive effects of combined antiretroviral therapy on CD4+ T-cell homeostasis and function in advanced HIV disease. Science 277112-116. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramanian, A., R. K. Ganju, and J. E. Groopman. 2006. Signal transducer and activator of transcription factor 1 mediates apoptosis induced by hepatitis C virus and HIV envelope proteins in hepatocytes. J. Infect. Dis. 194670-681. [DOI] [PubMed] [Google Scholar]
- 3.Beignon, A. S., K. McKenna, M. Skoberne, O. Manches, I. DaSilva, D. G. Kavanagh, M. Larsson, R. J. Gorelick, J. D. Lifson, and N. Bhardwaj. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Investig. 1153265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bovolenta, C., L. Camorali, A. L. Lorini, S. Ghezzi, E. Vicenzi, A. Lazzarin, and G. Poli. 1999. Constitutive activation of STATs upon in vivo human immunodeficiency virus infection. Blood 944202-4209. [PubMed] [Google Scholar]
- 5.Breen, E. C., A. R. Rezai, K. Nakajima, G. N. Beall, R. T. Mitsuyasu, T. Hirano, T. Kishimoto, and O. Martinez-Maza. 1990. Infection with HIV is associated with elevated IL-6 levels and production. J. Immunol. 144480-484. [PubMed] [Google Scholar]
- 6.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 121365-1371. [DOI] [PubMed] [Google Scholar]
- 7.Brites, C., M. J. Gilbert, D. Pedral-Sampaio, F. Bahia, C. Pedroso, A. P. Alcantara, M. D. Sasaki, J. Matos, B. Renjifo, M. Essex, J. B. Whitmore, J. M. Agosti, and R. Badaro. 2000. A randomized, placebo-controlled trial of granulocyte-macrophage colony-stimulating factor and nucleoside analogue therapy in AIDS. J. Infect. Dis. 1821531-1535. [DOI] [PubMed] [Google Scholar]
- 8.Cicala, C., J. Arthos, S. M. Selig, G. Dennis, Jr., D. A. Hosack, D. Van Ryk, M. L. Spangler, T. D. Steenbeke, P. Khazanie, N. Gupta, J. Yang, M. Daucher, R. A. Lempicki, and A. S. Fauci. 2002. HIV envelope induces a cascade of cell signals in non-proliferating target cells that favor virus replication. Proc. Natl. Acad. Sci. USA 999380-9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crocker, P. R., J. C. Paulson, and A. Varki. 2007. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7255-266. [DOI] [PubMed] [Google Scholar]
- 10.Crotti, A., M. Lusic, R. Lupo, P. M. Lievens, E. Liboi, G. D. Chiara, M. Tinelli, A. Lazzarin, B. K. Patterson, M. Giacca, C. Bovolenta, and G. Poli. 2007. Naturally occurring C-terminally truncated STAT5 is a negative regulator of HIV-1 expression. Blood 1095380-5389. [DOI] [PubMed] [Google Scholar]
- 11.Crowe, S. M., and A. Lopez. 1997. GM-CSF and its effects on replication of HIV-1 in cells of macrophage lineage. J. Leukoc. Biol. 6241-48. [DOI] [PubMed] [Google Scholar]
- 12.Darnell, J. E., Jr., I. M. Kerr, and G. R. Stark. 1994. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 2641415-1421. [DOI] [PubMed] [Google Scholar]
- 13.de Groot, R. P., P. J. Coffer, and L. Koenderman. 1998. Regulation of proliferation, differentiation and survival by the IL-3/IL-5/GM-CSF receptor family. Cell Signal. 10619-628. [DOI] [PubMed] [Google Scholar]
- 14.Dupuis, S., E. Jouanguy, S. Al-Hajjar, C. Fieschi, I. Z. Al-Mohsen, S. Al-Jumaah, K. Yang, A. Chapgier, C. Eidenschenk, P. Eid, A. Al Ghonaium, H. Tufenkeji, H. Frayha, S. Al-Gazlan, H. Al-Rayes, R. D. Schreiber, I. Gresser, and J. L. Casanova. 2003. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 33388-391. [DOI] [PubMed] [Google Scholar]
- 15.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 9514863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellery, P. J., E. Tippett, Y. L. Chiu, G. Paukovics, P. U. Cameron, A. Solomon, S. R. Lewin, P. R. Gorry, A. Jaworowski, W. C. Greene, S. Sonza, and S. M. Crowe. 2007. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J. Immunol. 1786581-6589. [DOI] [PubMed] [Google Scholar]
- 17.Endo, T. A., M. Masuhara, M. Yokouchi, R. Suzuki, H. Sakamoto, K. Mitsui, A. Matsumoto, S. Tanimura, M. Ohtsubo, H. Misawa, T. Miyazaki, N. Leonor, T. Taniguchi, T. Fujita, Y. Kanakura, S. Komiya, and A. Yoshimura. 1997. A new protein containing an SH2 domain that inhibits JAK kinases. Nature 387921-924. [DOI] [PubMed] [Google Scholar]
- 18.He, X. S., X. Ji, M. B. Hale, R. Cheung, A. Ahmed, Y. Guo, G. P. Nolan, L. M. Pfeffer, T. L. Wright, N. Risch, R. Tibshirani, and H. B. Greenberg. 2006. Global transcriptional response to interferon is a determinant of HCV treatment outcome and is modified by race. Hepatology 44352-359. [DOI] [PubMed] [Google Scholar]
- 19.Irish, J. M., N. Anensen, R. Hovland, J. Skavland, A. L. Borresen-Dale, O. Bruserud, G. P. Nolan, and B. T. Gjertsen. 2007. Flt3 Y591 duplication and Bcl-2 overexpression are detected in acute myeloid leukemia cells with high levels of phosphorylated wild-type p53. Blood 1092589-2596. [DOI] [PubMed] [Google Scholar]
- 20.Irish, J. M., D. K. Czerwinski, G. P. Nolan, and R. Levy. 2006. Altered B-cell receptor signaling kinetics distinguish human follicular lymphoma B cells from tumor-infiltrating nonmalignant B cells. Blood 1083135-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irish, J. M., R. Hovland, P. O. Krutzik, O. D. Perez, O. Bruserud, B. T. Gjertsen, and G. P. Nolan. 2004. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell 118217-228. [DOI] [PubMed] [Google Scholar]
- 22.Irish, J. M., N. Kotecha, and G. P. Nolan. 2006. Mapping normal and cancer cell signalling networks: toward single-cell proteomics. Nat. Rev. Cancer 6146-155. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson, J. M., M. M. Lederman, J. Spritzler, H. Valdez, P. Tebas, G. Skowron, R. Wang, J. B. Jackson, L. Fox, A. Landay, M. J. Gilbert, D. O'Neil, L. Bancroft, L. Al-Harthi, M. A. Jacobson, T. C. Merigan, Jr., and M. J. Glesby. 2003. Granulocyte-macrophage colony-stimulating factor induces modest increases in plasma human immunodeficiency virus (HIV) type 1 RNA levels and CD4+ lymphocyte counts in patients with uncontrolled HIV infection. J. Infect. Dis. 1881804-1814. [DOI] [PubMed] [Google Scholar]
- 24.Kohler, J. J., D. L. Tuttle, C. R. Coberley, J. W. Sleasman, and M. M. Goodenow. 2003. Human immunodeficiency virus type 1 (HIV-1) induces activation of multiple STATs in CD4+ cells of lymphocyte or monocyte/macrophage lineages. J. Leukoc. Biol. 73407-416. [DOI] [PubMed] [Google Scholar]
- 25.Krutzik, P. O., J. M. Irish, G. P. Nolan, and O. D. Perez. 2004. Analysis of protein phosphorylation and cellular signaling events by flow cytometry: techniques and clinical applications. Clin. Immunol. 110206-221. [DOI] [PubMed] [Google Scholar]
- 26.Kryworuchko, M., V. Pasquier, H. Keller, D. David, C. Goujard, J. Gilquin, J. P. Viard, M. Joussemet, J. F. Delfraissy, and J. Theze. 2004. Defective interleukin-2-dependent STAT5 signalling in CD8 T lymphocytes from HIV-positive patients: restoration by antiretroviral therapy. AIDS 18421-426. [DOI] [PubMed] [Google Scholar]
- 27.Lee, C., B. Tomkowicz, B. D. Freedman, and R. G. Collman. 2005. HIV-1 gp120-induced TNF-α production by primary human macrophages is mediated by phosphatidylinositol-3 (PI-3) kinase and mitogen-activated protein (MAP) kinase pathways. J. Leukoc. Biol. 781016-1023. [DOI] [PubMed] [Google Scholar]
- 28.Meier, A., G. Alter, N. Frahm, H. Sidhu, B. Li, A. Bagchi, N. Teigen, H. Streeck, H. J. Stellbrink, J. Hellman, J. van Lunzen, and M. Altfeld. 2007. MyD88-dependent immune activation mediated by HIV-1-encoded TLR ligands. J. Virol. 818180-8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naka, T., M. Narazaki, M. Hirata, T. Matsumoto, S. Minamoto, A. Aono, N. Nishimoto, T. Kajita, T. Taga, K. Yoshizaki, S. Akira, and T. Kishimoto. 1997. Structure and function of a new STAT-induced STAT inhibitor. Nature 387924-929. [DOI] [PubMed] [Google Scholar]
- 30.Ott, M., S. Emiliani, C. Van Lint, G. Herbein, J. Lovett, N. Chirmule, T. McCloskey, S. Pahwa, and E. Verdin. 1997. Immune hyperactivation of HIV-1-infected T cells mediated by Tat and the CD28 pathway. Science 2751481-1485. [DOI] [PubMed] [Google Scholar]
- 31.Paukku, K., and O. Silvennoinen. 2004. STATs as critical mediators of signal transduction and transcription: lessons learned from STAT5. Cytokine Growth Factor Rev. 15435-455. [DOI] [PubMed] [Google Scholar]
- 32.Qiao, X., B. He, A. Chiu, D. M. Knowles, A. Chadburn, and A. Cerutti. 2006. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat. Immunol. 7302-310. [DOI] [PubMed] [Google Scholar]
- 33.Schrager, J. A., V. Der Minassian, and J. W. Marsh. 2002. HIV Nef increases T-cell ERK MAP kinase activity. J. Biol. Chem. 2776137-6142. [DOI] [PubMed] [Google Scholar]
- 34.Selliah, N., M. Zhang, D. DeSimone, H. Kim, M. Brunner, R. F. Ittenbach, H. Rui, R. Q. Cron, and T. H. Finkel. 2006. The gammac-cytokine regulated transcription factor, STAT5, increases HIV-1 production in primary CD4 T cells. Virology 344283-291. [DOI] [PubMed] [Google Scholar]
- 35.Sharp, E. R., J. D. Barbour, R. K. Karlsson, K. A. Jordan, J. K. Sandberg, A. Wiznia, M. G. Rosenberg, and D. F. Nixon. 2005. Higher frequency of HIV-1-specific T-cell immune responses in African American children vertically infected with HIV-1. J. Infect. Dis. 1921772-1780. [DOI] [PubMed] [Google Scholar]
- 36.Starr, R., T. A. Willson, E. M. Viney, L. J. Murray, J. R. Rayner, B. J. Jenkins, T. J. Gonda, W. S. Alexander, D. Metcalf, N. A. Nicola, and D. J. Hilton. 1997. A family of cytokine-inducible inhibitors of signalling. Nature 387917-921. [DOI] [PubMed] [Google Scholar]
- 37.Tomkowicz, B., C. Lee, V. Ravyn, R. Cheung, A. Ptasznik, and R. G. Collman. 2006. The Src kinase Lyn is required for CCR5 signaling in response to MIP-1β and R5 HIV-1 gp120 in human macrophages. Blood 1081145-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toschi, E., I. Bacigalupo, R. Strippoli, C. Chiozzini, A. Cereseto, M. Falchi, F. Nappi, C. Sgadari, G. Barillari, F. Mainiero, and B. Ensoli. 2006. HIV-1 Tat regulates endothelial cell cycle progression via activation of the Ras/ERK MAPK signaling pathway. Mol. Biol. Cell 171985-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trial, J., H. H. Birdsall, J. A. Hallum, M. L. Crane, M. C. Rodriguez-Barradas, A. L. de Jong, B. Krishnan, C. E. Lacke, C. G. Figdor, and R. D. Rossen. 1995. Phenotypic and functional changes in peripheral blood monocytes during progression of human immunodeficiency virus infection: effects of soluble immune complexes, cytokines, subcellular particulates from apoptotic cells, and HIV-1-encoded proteins on monocytes phagocytic function, oxidative burst, transendothelial migration, and cell surface phenotype. J. Clin. Investig. 951690-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trinchieri, G., and A. Sher. 2007. Cooperation of Toll-like receptor signals in innate immune defense. Nat. Rev. Immunol. 7179-190. [DOI] [PubMed] [Google Scholar]
- 41.Trushin, S. A., A. Algeciras-Schimnich, S. R. Vlahakis, G. D. Bren, S. Warren, D. J. Schnepple, and A. D. Badley. 2007. Glycoprotein 120 binding to CXCR4 causes p38-dependent primary T-cell death that is facilitated by, but does not require cell-associated CD4. J. Immunol. 1784846-4853. [DOI] [PubMed] [Google Scholar]
- 42.Wang, J., G. Roderiquez, T. Oravecz, and M. A. Norcross. 1998. Cytokine regulation of human immunodeficiency virus type 1 entry and replication in human monocytes/macrophages through modulation of CCR5 expression. J. Virol. 727642-7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warby, T. J., S. M. Crowe, and A. Jaworowski. 2003. Human immunodeficiency virus type 1 infection inhibits granulocyte-macrophage colony-stimulating factor-induced activation of STAT5A in human monocyte-derived macrophages. J. Virol. 7712630-12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, X., and D. Gabuzda. 1999. Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. J. Virol. 733460-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]