Abstract
Human T lymphotropic virus type 1 (HTLV-1) -associated myelopathy/tropic spastic paraparesis is a demyelinating inflammatory neurologic disease associated with HTLV-1 infection. HTLV-1 Tax11–19-specific cytotoxic T cells have been isolated from HLA-A2-positive patients. We have used a peptide-loaded soluble HLA-A2–Ig complex to directly visualize HTLV-1 Tax11–19-specific T cells from peripheral blood and cerebrospinal fluid without in vitro stimulation. Five of six HTLV-1-associated myelopathy/tropic spastic paraparesis patients carried a significant number (up to 13.87%) of CD8+ lymphocytes specific for the HTLV-1 Tax11–19 peptide in their peripheral blood, which were not found in healthy controls. Simultaneous comparison of peripheral blood and cerebrospinal fluid from one patient revealed 2.5-fold more Tax11–19-specific T cells in the cerebrospinal fluid (23.7% vs. 9.4% in peripheral blood lymphocyte). Tax11–19-specific T cells were seen consistently over a 9-yr time course in one patient as far as 19 yrs after the onset of clinical symptoms. Further analysis of HTLV-1 Tax11–19-specific CD8+ T lymphocytes in HAM/TSP patients showed different expression patterns of activation markers, intracellular TNF-α and γ-interferon depending on the severity of the disease. Thus, visualization of antigen-specific T cells demonstrates that HTLV-1 Tax11–19-specific CD8+ T cells are activated, persist during the chronic phase of the disease, and accumulate in cerebrospinal fluid, showing their pivotal role in the pathogenesis of this neurologic disease.
Human T lymphotropic virus type 1 (HTLV-1) is a human retrovirus. It can cause human adult T cell leukemia/lymphoma (1) and a slowly progressive demyelinating neurologic disease, HTLV-1-associated myelopathy/tropic spastic paraparesis (HAM/TSP) (2, 3). The clinical symptoms of HAM/TSP are characterized by upper motor neuron signs and mild sensory and sphincter dysfunction (3). Histopathologically, one finds thoracic spinal cord atrophy involving perivascular demyelination and axonal degeneration (4, 5). The pathophysiology of HAM/TSP is still not completely understood (6). Serum reactivity to HTLV-1 was first associated with HAM/TSP by Osame et al. in 1986 (3). The immunologic hallmark of patients with HAM/TSP is the spontaneous proliferation of peripheral blood lymphocytes (PBLs) in vitro (7–9).
It has been previously demonstrated that circulating CD8+ cytotoxic T lymphocytes (CTLs) in patients with HAM/TSP react against HTLV-1 protein products (10), and an immunodominant HLA-A2-restricted epitope (HTLV-1 Tax11–19) has been well characterized (11). HTLV-1 Tax11–19-specific CTL precursor frequency was estimated by limiting dilution analysis in the range of 1:75 to 1:320 CD8+ lymphocytes (12) in peripheral blood. Tax-specific CTLs were also found in cerebrospinal fluid (CSF) (12, 13) and HTLV-1-specific clones could be generated in vitro from a spinal cord lesion that was obtained from a HAM/TSP patient (14). Immunohistochemical analysis of affected spinal cord lesions in the early stage of the disease from patients with HAM/TSP reveals the presence of infiltrating CD4+ and CD8+ lymphocytes, in which the CD8+ population predominates with duration of the disease (15). Moreover, an increase in activated lymphocytes has been shown in PBLs and CSF (16, 17). Recently, we have been able to demonstrate that peripheral CD8+ T lymphocytes produce interleukin (IL) 2, γ-interferon (IFN-γ), and tumor necrosis factor α (TNF-α) in HAM/TSP patients (18). Collectively, this evidence has supported the view that virus-specific CD8+ T lymphocytes may play a critical role in the immunopathogenesis of HAM/TSP.
One major limitation in these studies has been the inability to identify HTLV-1-specific CD8+ T cells directly from biological samples not only to quantitate the actual number of antigen-specific T cells in vivo but also to further characterize the antigen-specific population of T lymphocytes without in vitro amplification. Recently, tetrameric major histocompatibility complex (MHC) –peptide complex crosslinked by streptavidin were shown to bind stably to antigen-specific T cells based on the increased avidity afforded by polyvalency (19). Based on this avidity principal, we have developed divalent MHC class I constructs using Ig as a scaffold (20) and used these to elucidate the role of antigen-specific CD8+ T cells in different human immunologic diseases such as HAM/TSP.
Here, we have analyzed HTLV-1 Tax11–19-specific, HLA-A2+-restricted CD8+ T lymphocytes directly from peripheral blood and CSF using peptide-loaded divalent HLA-A2/Ig chimeras. Flow cytometric analysis showed that peptide-loaded HLA-A2/Ig specifically stained antigen-specific T cells. A surprisingly high frequency of HTLV-1 Tax11–19-specific CD8+ T cells were seen in the peripheral blood of patients with HAM/TSP and a selective enrichment of these cells were seen in the CSF from a HAM/TSP patient. Tax11–19-specific CD8+ T cells are activated in vivo in HAM/TSP and express proinflammatory cytokines. Finally, HTLV-1 Tax11–19 specific CD8+ T cells persist at high numbers over a period of 9 yrs during the chronic phase of the disease even 19 yrs after the first symptoms of the disease.
MATERIALS AND METHODS
Study Subjects and Specimens.
Clinical characteristics of patients with HAM/TSP and asymptomatic HTLV-1-infected individuals as well as HLA type were described previously (12). HTLV-1 infection was confirmed by Western blot in serum of HAM/TSP patients and asymptomatic carriers. The diagnosis of HAM/TSP was determined according to W.H.O. criteria using their neurological symptoms and serological testing of HTLV-1. PBLs were prepared by Ficoll–Hypaque centrifugation and stored in liquid nitrogen until use. Histocompatibility typing was performed on PBLs or Epstein–Barr virus-transformed lymphoblastoid cell lines by the Tissue Typing Laboratory at the National Institutes of Health Clinical Center. Some of the healthy HLA-A2-uninfected controls were tested for HLA-A2 expression by fluorescence-activated cell-sorting analysis using a panel of HLA-A2-specific mAbs: MA2.1, BB7.2, and PA2.1 (American Type Culture Collection).
Peptides.
Peptides HTLV-1 Tax11–19 (LLFGYPVYV), influenza virus A M158–66 (GILGFVFTL), and p17 Gag77–85 (SLYNTVATL) were synthesized and HPLC purified at the Colorado State University Macromolecular Resources (Fort Collins, CO). Identity of the peptides was confirmed by mass spectrometry measurement.
Cloning the Construct and Protein Expression.
Using oligonucleotide-directed PCR, we introduced a 5′ MluI and a 3′ NotI site (sites underlined) into the extracellular domain (α1–α3) of HLA-A2 cDNA (kindly provided by P. F. Robbins, National Cancer Institute, National Institutes of Health, Bethesda, MD) using the following primer pair: 5′-GATACGCGTTGGGCTCTCACTCCATGAG-3′ and 5′-CAGTCGATGCGGCCGCCCATCTCAGGGTGAGGGGCT-3′. After PCR amplification the α1–α3 region of HLA-A2 was sequenced and directional cloned in exchange for H-2Kb into the previously described pX/Ig vector (20). Subsequently, the construct was cotransfected with DNA encoding the chimeric HLA-A2/Ig protein and the human β2-microglobulin by electroporation into J558L cells. Transfectants were screened for secretion of the chimeric protein by ELISA as described previously (20). ELISA plates were coated with BB7.2, an antibody specific for peptide-loaded conformations of HLA-A2 or a goat anti-mouse IgG–Fc antibody (10 μg/ml; Cappel). High secretors were picked and grown in Hybridoma-SFM (GIBCO/BRL). HLA-A2/Ig secretion from the transfected J558L was confirmed by SDS/PAGE. The chimeric protein was harvested from cell supernatant and was concentrated with Centriflo-membrane cones (Amicon). HLA-A2/Ig was loaded with 660-fold molar excess of peptide for 10–14 days before fluorescence-activated cell-sorting analysis. Three micrograms of protein was used for staining of 1 × 106 cells.
Flow Cytometric Analysis.
Murine monoclonal anti-human CD8–fluorescein isothiocyanate (FITC; Sigma), anti-CD4-phycoerythrin (PE; Becton Dickinson), anti-CD8-Tri (Caltag Laboratories, Burlingame, CA), anti-HLA-DR (PharMingen) were used to detect cell surface molecules of lymphocytes. PK1.36–FITC was used as an isotype-matched control for anti-HLA-DR–FITC. HLA-A2/Ig bound onto the cell surface was detected using goat anti-mouse IgG1–PE or a goat anti-mouse IgG1–Tri (Caltag). Monoclonal anti-TNF-α–FITC, anti-IFN-γ–FITC, or isotype-matched IgG1–FITC antibodies (PharMingen) were used for intracellular cytokine staining. PBLs (1 × 106) were incubated with peptide-loaded HLA-A2/Ig on ice followed by PE or Tri-conjugated goat anti-mouse IgG1–PE. Intracellular staining was performed according to the manufacturer’s instructions, with slight modifications (21), after a 4-h incubation at 37°C followed by a 10-h incubation in the presence of 10 μg/ml brefeldin A (Sigma). Three-color fluorometric analysis was carried out on a FACScan (Becton Dickinson). Lymphocytes were gated on forward and side scatter. One × 105 (2 × 105 for three-color analysis) gated events were analyzed using CELLQuest software (Becton Dickinson).
PBLs from all HLA-A2-positive patients have been stained at least twice with similar results.
RESULTS
Peptide-Loaded HLA-A2/Ig Binds Specifically HLA-A2-Restricted CTLs.
We have engineered a divalent HLA-A2/Ig molecule using Ig as a scaffold that can be loaded with various peptides. Peptide-loaded HLA-A2/Ig was specific in its interaction with HLA-A2-restricted CTL clones analyzed by flow cytometry. Thus, Tax-A2/Ig was specifically reactive with a HLA-A2-restricted, HTLV-1 Tax11–19-specific CTL clone, A6 (22). Mean channel fluorescence of A6 cells stained with HTLV-1 Tax11–19-loaded HLA-A2/Ig (Tax-A2/Ig) was 426, whereas mean channel fluorescence of A6 cells stained with the control Gag-A2/Ig complex was only 13 and almost identical to staining controls using no chimeric protein, unloaded HLA-A2/Ig or M1-loaded HLA-A2/Ig (Fig. 1A). In complementary experiments, Gag-A2/Ig specifically stained the HIV p17 Gag77–85-specific clone SL09 (23) (Fig. 1B), whereas staining with Tax-A2/Ig was virtually identical to unloaded HLA-A2/Ig, M1-loaded HLA-A2/Ig, or no HLA-A2/Ig.
Figure 1.
Peptide-loaded HLA-A2/Ig specifically stains HTLV-1 Tax11–19 or HIV p17 Gag77–85-specific T cell clones. (A) Fluorescence-activated cell-sorting analysis was carried out using A6 cells (22), specific for the immunodominant HLA-A2-restricted Tax epitope Tax11–19. HTLV-1 Tax11–19-loaded HLA-A2/IgG stably bound on the cell surface and was detected using PE-labeled goat anti-mouse Ig (heavy line). Gag-A2/Ig was used as an irrelevant control (thin line) as well as no HLA-A2/Ig, unloaded HLA-A2/Ig, and M1-loaded HLA-A2/Ig (dotted and stripped lines overlapping). (B) T cells specific for the HLA-A2-restricted HIV p17 Gag77–85 epitope were stained to demonstrate the peptide specificity of peptide-loaded HLA-A2/Ig. Gag77–85-loaded HLA-A2/IgG was stably bound on the cell surface and detected using PE-labeled goat anti-mouse Ig (heavy line). HTLV-1 Tax11–19-loaded HLA-A2/Ig as well as no peptide loaded, M1-loaded HLA-A2/Ig, and no HLA-A2/Ig were used as an irrelevant control and stained virtually identical (overlapping thin, dotted, and stripped lines).
Visualization of Antigen-Specific T Lymphocytes in Peripheral Blood of Patients with HAM/TSP.
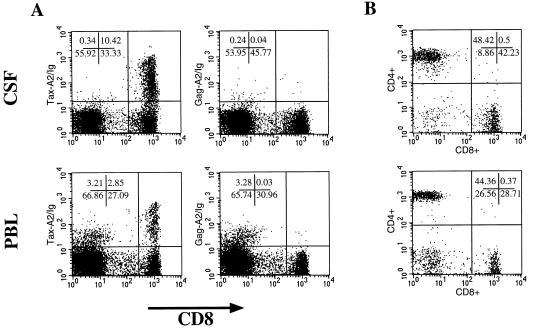
HTLV-1 Tax11–19-specific CTL activity has been demonstrated directly in PBLs from HAM/TSP patients (10). In HLA-A2+ patients, HTLV-1-specific reactivity is predominately directed against the HTLV-1 Tax11–19 epitope (11). Previous estimates of precursor frequencies of HTLV-1 Tax11–19-specific CTLs using limiting dilution analysis (LDA) gave values in the range of 1:75–1:320 CD8+ T cells. To directly quantitate the HTLV-1 Tax11–19-specific CD8+ population in peripheral blood of HAM/TSP patients, we performed two-dimensional flow cytometric analysis of cells using Tax-A2/Ig and anti-CD8+ (Fig. 2). PBLs from patients with HAM/TSP showed a significant high number of HTLV-1 Tax11–19-specific CD8+ T lymphocytes. In one patient the frequency of Tax11–19-specific cells was as high as 13.8% (Figs. 2 and 3). The percentage of Tax-A2/Ig-specific cells ranged between 0.64 and 13.87% of CD8+ T cells with values >2% for four of the six HAM/TSP patients (Fig. 3). In one of the six HLA-A2-positive HAM/TSP patients, no Tax-A2/Ig-reactive cells were detected. However, this same patient’s PBLs also did not lyse Tax protein-expressing, autologous, Epstein–Barr virus-transformed B cells (our unpublished data). Moreover, we were able to detect Tax-specific T cells with Tax-A2/Ig in every patient sample that lysed Tax-expressing, HLA-A2+ target cells.
Figure 2.
Peptide-loaded HLA-A2/Ig can be used to visualize antigen-specific CD8+ T cells in peripheral blood. Two-color flow cytometry was performed using Tax-A2/Ig or Gag-A2/Ig and mouse anti-human CD8-FITC. PE-labeled goat anti-mouse IgG1 mAb was used to detect the peptide-A2/Ig complex. Frozen PBLs from a HAM/TSP patient (donor H1) were examined. Four and three-tenths percent of the PBLs from the HAM/TSP patient stained positive with anti-human CD8-FITC and Tax-A2/Ig. This represents 10.49% of all CD8+ cells. No significant signal was seen using Gag-A2/Ig (<0.1%). PBLs from a HLA-A2+ HIV-1-infected individual showed 3% Gag-A2/Ig-positive CD8+ T lymphocytes.
Figure 3.
HAM/TSP patients carry high numbers of HTLV-1 Tax11–19-specific CD8+ cells in their PBLs. Presented is the percentage of HTLV-1 Tax11–19-specific, CD8+ T cells from HLA-A2+ HAM/TSP patients, HTLV-1-infected HLA-A2+ carriers, HLA-A2− HAM/TSP patients, and healthy individuals. Between 0.64 and 10.25% of the CD8+ cells from HAM/TSP patients are specific for the HTLV-1 Tax11–19 peptide. One of the analyzed HTLV-1-infected donors had 0.33% Tax-specific CD8+ cells, whereas no significant number of Tax-specific CD8+ cells were found in non-HLA-A2 HAM/TSP donors or HLA-A2+ healthy individuals. Positive samples were stained at least twice.
We also analyzed peripheral blood from two HLA-A2+ HTLV-1 carriers who did not have HAM/TSP (Fig. 3). One of the two HTLV-1 carriers had no detectable HTLV-1 Tax11–19-specific T cells, whereas the other contained a low but reproducibly detectable number of CD8+ T cells that stained with Tax-A2/Ig (0.33%). To confirm the specificity of staining of peripheral blood samples with these reagents and their relationship to the disease, we analyzed PBLs from HAM/TSP patients who do not carry the HLA-A2 allele (Fig. 3), uninfected healthy HLA-A2+ donors (Fig. 3), and a HIV-1-infected HLA-A2+ individual (Fig. 2). Less then 0.1% of CD8+ T cells from all of these samples stained positively with the Tax-A2/Ig. However, a significant number (3.03%) of the CD8+ T lymphocytes from the HIV-infected individual was stained using Gag-A2/Ig. Collectively, these results demonstrate that the presence of high numbers of Tax-A2/Ig-staining CD8+ cells is specific for HTLV-1+ HLA-A2+ individuals with HAM/TSP.
Visualization of Antigen-Specific T Lymphocytes in CSF of Patients with HAM/TSP.
To corroborate the significance of HTLV-I-specific CTLs for the pathogenesis of this demyelinating disease, we analyzed lymphocytes from CSF of a patient with HAM/TSP (H1) and compared them with the T cells in his peripheral blood from the same day. Of the CD8+ lymphocyte population in CSF, 23.7% was Tax-A2/Ig specific. In contrast, we detected 9.4% Tax-A2/Ig-specific CD8+ T cells in the peripheral blood (Fig. 4A). Interestingly, we found the same number of CD4+ T cells in peripheral blood and CSF (44% in the PBLs and 48% in the CSF) as well as an increased number in total CD8+ T cells in the CSF (42%) compared with the peripheral blood (29%) (Fig. 4B).
Figure 4.
HTLV-1 Tax11–19-specific CD8+ T cells accumulate in CSF. CSF and peripheral blood were obtained from a HAM/TSP patient (H1) the same day and analyzed using Tax-A2/Ig. Cells (9 × 104) were obtained from CSF and stained using Tax/A2-Ig and Gag/A2-Ig in combination with mouse anti-human CD8-FITC as described in Fig. 3. Freshly obtained, Ficoll-purified PBLs were analyzed in parallel. Of the CD8+ T cells in CSF, 23.7% were Tax specific and only 9.4% of the CD8+ T cells from peripheral blood. No significant signal was seen using Gag-A2/Ig (<0.1%) (A). CD4/CD8 analysis showed that the ratio of CD4:CD8 was 1.1 in the CSF and 1.5 in the PBLs due to a higher number of CD8+ T cells in CSF (B).
HTLV-1 Tax11–19-Specific T Cells in Patients with HAM/TSP Are Activated.
The ability to directly stain HTLV-1 Tax11–19-specific T lymphocytes provides the opportunity to analyze their state of activation. If HTLV-1-specific CD8+ T cells indeed mediate the immunopathogenesis of HAM/TSP, a significant proportion of them might be expected to be activated. Since activated human T cells express elevated levels of MHC class II molecules, we further characterized the HTLV-1 Tax11–19-specific T cells from HAM/TSP patients for HLA-DR expression by multicolor flow cytometry.
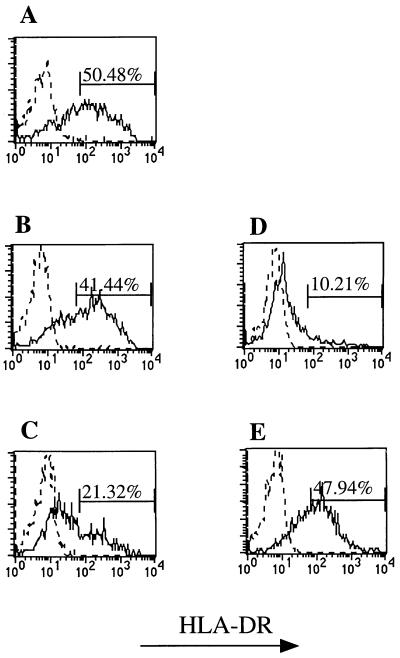
Tax-A2/Ig-reactive CD8+ T cells from the three HAM/TSP patients had significantly increased expression of DR (Fig. 5). In two of three patients, the level of DR expression was virtually identical to that seen in control phytohemagglutinin-stimulated PBLs (Fig. 5A and B, Tax-A2/Ig- reactive-specific CD8+ T cells from patients, to E, activated human PBLs). Similar results were obtained for IL-2 receptor expression (data not shown). Of particular interest, one patient, H6, demonstrated a significantly lower population of DR+, Tax-specific CD8+ cells (Fig. 5C). This individual, the spouse of a HAM/TSP patient, was originally thought to be an asymptomatic carrier, since no neurologic symptoms were reported. However, on a physical examination a hyperreflexia in the lower extremities and extensor plantar responses were observed, indicative of corticospinal tract lesion(s).
Figure 5.
Tax-specific CD8+ cells from HAM/TSP patients express cell surface activation marker HLA-DR. Using three-color flow cytometry, Tax-specific CD8+ cells were gated and analyzed for HLA-DR expression using mouse anti-human HLA-DR-FITC (solid line) and an isotype-matched irrelevant mouse IgG2a-FITC control (dotted line). Forty-one to 50% of the Tax-specific CD8+ T cells from two different symptomatic HAM/TSP patients stain positive for HLA-DR expression (A and B) in contrast to only 21% from patient H6 (C), who has a very mild form of the disease. Nonstimulated (D) and phytohemagglutinin-stimulated (E) CD8+ PBLs from a healthy donor served as staining controls.
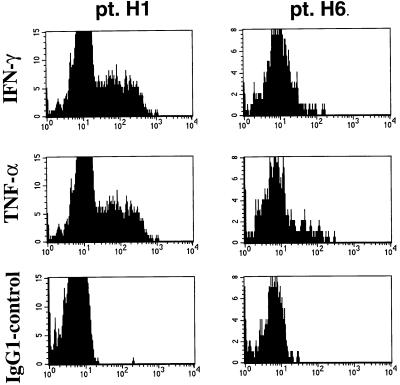
Inflammatory cytokines are considered critical mediators of the central nervous system immunopathology in many autoimmune models. Elevated levels of several proinflammatory cytokines have been detected in the serum or CSF of HAM/TSP patients (16, 17). Therefore, it is of particular interest to analyze Tax-specific CD8+ cells from HAM/TSP patients for the presence of relevant intracellular cytokines. Recently, we have found that bulk CD8+ T cells from HAM/TSP patients demonstrated significant expression of cytokines such as TNF-α, IFN-γ, and IL-2 but no IL-4 (18). Tax-specific CD8+ T cells from patients H1 (Fig. 6), H5 (data not shown), and H6 (Fig. 6) demonstrated distinct patterns of intracellular TNF-α and IFN-γ expression. Although the HAM/TSP patients H1 and H5 displayed a high proportion of Tax-A2/Ig-specific CD8+ cells with intracellular TNF-α and IFN-γ (roughly 30%), patient H6 had a very low proportion of Tax-specific CD8+ cells expressing intercellular TNF-α and IFN-γ (2%). These results, along with HLA-DR analysis, demonstrate a disassociation between expansion of antigen-specific T cells and their activation state. They further demonstrate how the ability to use multiparameter flow cytometry to analyze the activation state of antigen-specific T cell populations may provide further insight into the relationship between state of T cell activation and disease pathogenesis.
Figure 6.
Tax-specific CD8+ cells from HAM/TSP patients express intracellular IFN-γ and TNF-α. Using three-color flow cytometry, Tax-specific CD8+ cells were gated and analyzed for intracellular cytokine expression as described in Materials and Methods. Using mouse anti-human IFN-γ–PE, mouse anti-human TNF-α–PE, and an isotype-matched irrelevant mouse IgG1–PE control, 28% of the Tax-specific CD8+ cells from H1 (left panel) expressed intracellular IFN-γ and 29% expressed TNF-α; however, only 8% of the Tax-specific CD8+ cells from H6 (right panel) expressed intracellular IFN-γ and TNF-α.
Tax-Specific CD8+ T Cells Persist in Peripheral Blood from HAM/TSP Patients.
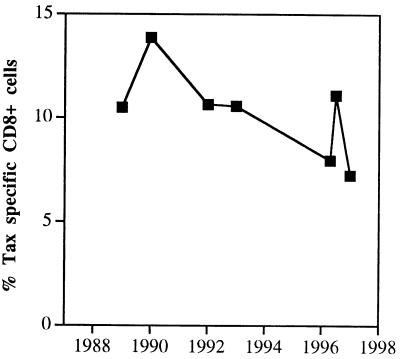
An important aspect in understanding the pathogenesis of autoimmune diseases, such as HAM/TSP, is to understand the dynamics of antigen-specific T cell responses. We therefore compared the numbers of Tax-specific, CD8+ T cells from a HAM/TSP patient (H1) who had serial PBLs drawn between 1989 and 1998. This patient was initially diagnosed in 1979. Interestingly, even 10 yrs after diagnosis, this patient had about 10% Tax-specific CD8+ T cells which did not change significantly over the next 8 yr (Fig. 7). Thus, in this patient pathogenic Tax-specific CD8+ T cells are sustained at high levels even after 19 yrs of active disease.
Figure 7.
High levels of HTLV-1 Tax-specific CD8+ T lymphocytes persist during HAM/TSP. PBL samples obtained between 1989 and 1998 from patient H1 were analyzed for Tax-specific CD8+ cells. Between 7.24 and 13.87% specific CD8+ T cells were detected in the frozen PBL samples.
DISCUSSION
In the present study, we demonstrate that divalent peptide loaded HLA-A2/Ig can be used to directly stain antigen-specific, HLA-A2-restricted T cells in peripheral blood. Initially, we chose the most common HLA class I allele (24) because many HLA-A2-restricted antigens have been identified for human viral infections, including HIV (25), autoimmune diseases, and cancer (26). However, we have already successfully used this approach for other MHC class I molecules (data not shown). This class of reagents represents a new tool for understanding a variety of immunologic diseases. The observation that a divalent MHC/Ig chimeric protein stably binds to its cognate T cell receptor is surprising since the monovalent peptide–MHC complex dissociates rapidly from complexes with TCR with a half-life of <1 min (27), and the affinity of the T cell receptor for peptide–MHC complexes is relatively low (≈10−5 μM) (28–30). Stable binding of the dimeric HLA-A2/Ig complex to cognate T cells is based on two features related to the Ig scaffold. First, the divalent nature of the complex provides an increase in avidity. An increase in avidity afforded by polyvalence has also been demonstrated using tetrameric peptide–MHC complexes crosslinked by streptavidin (19). Second, it is likely that the unique flexibility of the Ig hinge region promotes maximal avidity enhancement with our divalent construct.
Using the Tax-A2/Ig to detect antigen-specific T cells, we have been able to identify surprisingly large numbers of Tax-specific CD8+ T cells in the peripheral blood of the majority of HAM/TSP patients without in vitro amplification. These high numbers apparently do not represent acute spikes of T cell expansion since consistently high numbers of cells were observed in at least one patient (Fig. 7) on eight different analyses over a 9-yr period. Finally, similar numbers of Tax-specific CD8+ T cells over time were also found in blood samples from other patients that were taken at different time points (data not shown).
The ability to simultaneously quantitate Tax-specific CD8+ T cells in both PBLs and CSF has enabled us to directly analyze the role of these cells at the site of pathology. Since HAM/TSP is a demyelinating neurological disease in which CD4+ and CD8+ T cells infiltrate the spinal cord (14), analysis of T lymphocytes from CSF is pivotal. We found a higher number of Tax-specific CD8+ T cells in the CSF than in peripheral blood, suggesting that these antigen-specific T cells accumulate in the central nervous system. Accumulation of Tax11–19-specific CD8+ T cells at the pathological site, the central nervous system, could be due to either migration and selective accumulation of antigen-specific T cells in the central nervous system or selective expansion of these cells in the central nervous system. In either event, selective enrichment of these cells strongly suggests that these cells are directly involved in the pathogenesis of HAM/TSP.
Quantitation of HTLV-1 Tax11–19-specific T cells in peripheral blood with peptide-loaded HLA-A2/Ig demonstrates that previous precursor frequency analysis of Tax-specific CD8+ CTLs by LDA may have significantly underestimated the actual number of Tax-specific T cells (12). We were able to show that the number of Tax-specific CD8+ T cells assessed by Tax-A2/Ig staining was roughly 10- to 30-fold higher than previously estimated by LDA (12). The accuracy of LDA is probably compromised by the fact that it depends on the ability to expand individual cells in vitro to numbers large enough to be detected by functional tests such as chromium release (31–34). The frequencies determined by direct staining with Tax-A2/Ig are not due to nonspecific binding. Tax-A2/Ig positively staining CD8+ cells were virtually undetectable in HTLV-1− individuals, in HLA-A2− patients with HAM/TSP, and in control HLA-A2 patients infected with a different retrovirus, HIV, where a significant number of cells were specific for p17 Gag77–85 when stained with p17 Gag77–85-loaded A2/Ig.
It is still unknown why some HTLV-1-infected individuals develop an inflammatory neurologic disease and what pathophysiologic mechanisms lead to the development of HAM/TSP. In many models HTLV-1-specific CD8+ lymphocytes are considered to play a crucial role, although it remains unknown which cells in the central nervous system are preferential targets for these cells (35–40). The ability to directly visualize Tax-specific T cells in peripheral blood and the characterization of this cell population has provided new insights into the pathogenesis of HAM/TSP that could also be of relevance to other autoimmune diseases. We were able to clearly demonstrate for the first time that circulating Tax-specific, CD8+ T lymphocytes are activated as measured by expression of HLA-DR staining in HAM/TSP patients. We also further characterized these antigen-specific CD8+ T lymphocytes for cytokine production since we recently found that bulk CD8+ T lymphocytes from HAM/TSP patients release cytokines dependent on the presence of either HTLV-1-infected CD4+ T lymphocytes or peptide-pulsed allogeneic HLA-A2+ B lymphocytes (18). Three-color analysis (CD8+ vs. Tax-A2/Ig vs. TNF-α or IFN-γ) of PBLs from patients H1 and H5 revealed a number of interesting features. Although a very large proportion of CD8+ Tax-A2/Ig+ cells expressed intracellular IFN-γ and TNF-α (28 and 29%, respectively), the majority were negative for these cytokines, suggesting that circulating Tax-specific CD8+ cells are not uniformly activated. The cytokine-negative cells may represent a dormant cell population not actively participating in disease pathogenesis.
Although the number of analyzed individuals is clearly too small to draw any definite conclusions, the observation that Tax-specific T cells from H6 showed lower expression of intracellular IFN-γ and TNF-α as well as less MHC class II expression than other HAM/TSP patients may be significant. This individual has a very mild form of the disease (no clinical symptoms and neurologic deficits limited to hyperreflexia in the lower extremities and extensor plantar responses indicative of a corticospinal tract lesion). One might therefore speculate that this individual has not developed any additional symptoms and still might be at an very early stage of the disease due to the lack of activated HTLV-1 Tax11–19-specific CD8+ T lymphocytes. Characterization of HTLV-1-specific lymphocytes from a larger cohort of patients to determine which parameters of T cell activation and cytokine expression correlate the best with disease severity and progression will further elucidate the role of activated HTLV-1 Tax11–19-specific CD8+ T lymphocytes in the pathogenesis of HAM/TSP.
In addition to using peptide-loaded HLA-A2/Ig to analyze antigen-specific T cell populations, the Ig scaffold of the HLA-A2 Ig chimera could provide for a variety of other applications, including targeting of antigen-specific T cells in vivo. The diversity of in vivo biological effects mediated by the different Fc regions which can be inserted onto this molecule allow for its application in targeting antigen-specific T cells in vivo, either for amplification of antigen-specific T cells as a vaccine or for elimination of pathogenic T cells in diseases such as HAM/TSP as well as classical autoimmune diseases. The high number of HTLV-1 Tax11–19-specific CD8+ T cells in HLA-A2+ HAM/TSP patients suggests the HTLV-1 Tax11–19 may represent the immunodominant epitope in this pathogenic immune response. Thus, soluble divalent MHC/Ig could also have therapeutic application in targeting and eliminating HTLV-1-specific CD8+ T lymphocytes in HAM/TSP.
Acknowledgments
We thank Paul F. Robbins for providing the human HLA-A2 cDNA, Spyros A. Kalams for providing the HIV p17 Gag77–85-specific T cell clone, William E. Biddison for the HTLV-1 Tax11–19-specific clone A6, and Lucy Carruth for staining PBLs from HIV-1-infected individuals. T.F.G. was supported by a grant from the Deutsche Forschungsgemeinschaft (Gr 1511/1–1) and the National Multiple Sclerosis Society (RG2637A2/1). J.P.S. was supported by grants from the National Institutes of Health and National Multiple Sclerosis Society (RG 2637A2/1).
ABBREVIATIONS
- HTLV-1
human T lymphotropic virus 1
- HAM/TSP
HTLV-1-associated myelopathy/tropic spastic paraparesis
- PBL
peripheral blood lymphocyte
- CTL
cytotoxic T lymphocyte
- CSF cerebrospinal fluid
IL, interleukin
- IFN-γ
γ-interferon
- TNF-α
tumor necrosis factor α
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- LDA
limiting dilution analysis
- MHC
major histocompatibility complex
References
- 1.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Blood. 1977;50:481–492. [PubMed] [Google Scholar]
- 2.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de Thé G. Lancet. 1985;2:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 3.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. Lancet. 1986;1:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 4.Akizuki S, Nakazato O, Higuchi Y, Tanabe K, Setoguchi M, Yoshida S, Miyazaki Y, Yamamoto S, Sudou S, Sannomiya K, et al. Lancet. 1987;1:156–157. doi: 10.1016/s0140-6736(87)91984-2. [DOI] [PubMed] [Google Scholar]
- 5.Izumo S, Usuku K, Osame M, Machigashira K, Johnsonso M, Nakagawa M. In: The Neuropathology of HTLV-I Associated Myelopathy in Japan: Report of an Autopsy Case and Review of the Literature. Roman G C, Vernant J C, Osame M, editors. New York: Alan R. Liss Inc.; 1988. pp. 261–267. [Google Scholar]
- 6.Hollsberger P, Hafler D A. N Engl J Med. 1993;328:1173–1182. doi: 10.1056/NEJM199304223281608. [DOI] [PubMed] [Google Scholar]
- 7.Itoyama Y, Minato S, Kira J, Goto I, Sato H, Okochi K, Yamamoto N. Neurology. 1988;38:816–818. doi: 10.1212/wnl.38.5.816. [DOI] [PubMed] [Google Scholar]
- 8.Usuku K, Sonoda S, Osame M, Yashiki S, Takahashi K, Matsumoto M, Sawada T, Tsuji K, Tara M, Igata A. Ann Neurol. 1988;23:S143–S150. doi: 10.1002/ana.410230733. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson S, Gupta A, Mattson D, Mingioli E, McFarlin D E. Ann Neurol. 1990;27:149–156. doi: 10.1002/ana.410270209. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson S, Shida H, McFarlin D E, Fauci A S, Koenig S. Nature (London) 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 11.Koenig S, Woods R M, Brewah Y A, Newell A J, Jones G M, Boone E, Adelsberger J W, Baseler M W, Robinson S M, Jacobson S. J Immunol. 1993;151:3874–3883. [PubMed] [Google Scholar]
- 12.Elovaara I, Koenig S, Brewah A Y, Woods R M, Lehky T, Jacobson S. J Exp Med. 1993;177:1567–1573. doi: 10.1084/jem.177.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson S, McFarlin D E, Robinson S, Voskuhl R, Martin R, Brewah A, Newell A J, Koenig S. Ann Neurol. 1992;32:651–657. doi: 10.1002/ana.410320508. [DOI] [PubMed] [Google Scholar]
- 14.Levin M C, Lehky T J, Flerlage A N, Katz D, Kingma D W, Jaffe E S, Heiss J D, Patronas N, McFarland H F, Jacobson S. N Engl J Med. 1997;336:839–845. doi: 10.1056/NEJM199703203361205. [DOI] [PubMed] [Google Scholar]
- 15.Umehara F, Izumo S, Nakagawa M, Ronquillo A T, Takahashi K, Matsumuro K, Sato E, Osame M. J Neuropathol Exp Neurol. 1993;52:424–430. doi: 10.1097/00005072-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Ijichi S, Eiraku N, Osame M, Izumo S, Kubota R, Maruyama I, Matsumoto M, Niimura T, Sonoda S. J Neuroimmunol. 1989;25:251–254. doi: 10.1016/0165-5728(89)90143-4. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson S, Zaninovic V, Mora C, Rodgers J P, Sheremata W A, Gibbs C J, Gajdusek C, McFarlin D E. Ann Neurol. 1988;23:S196–S200. doi: 10.1002/ana.410230744. [DOI] [PubMed] [Google Scholar]
- 18.Kubota, R., Kawanishi, T., Matsubara, H., Manns, A. & Jacobson, S. (1998) J. Immunol., in press. [PubMed]
- 19.Altman J D, Moss P, Goulder P, Barouch D H, McHeyzer W M, Bell J I, McMichael A J, Davis M M. Science (Washington DC) 1996;274:94–96. [Google Scholar]
- 20.Dal Porto J. Proc Natl Acad Sci USA. 1993;90:6671–6675. doi: 10.1073/pnas.90.14.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biddison W E, Kubota R, Kawanishi T, Taub D D, Cruikshank W W, Center D M, Connor E W, Utz U, Jacobson S. J Immunol. 1997;159:2018–2025. [PubMed] [Google Scholar]
- 22.Utz U, Banks D, Jacobson S, Biddison W E. J Virol. 1996;70:843–851. doi: 10.1128/jvi.70.2.843-851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sipsas N V, Kalams S A, Trocha A, He S, Blattner W A, Walker B D, Johnson R P. J Clin Invest. 1997;99:752–762. doi: 10.1172/JCI119221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imanishi T, Akaza T, Kimura A, Tokunaga K, Gojobori T. In: Allele Haplotype Frequencies for HLA- and Complement Loci in Various Ethnic Groups. Tsuji K, Aizawa M, Sasazuki T, editors. Oxford, U.K.: Oxford Univ. Press; 1992. pp. 1065–1220. [Google Scholar]
- 25.Goulder P J, Sewell A K, Lalloo D G, Price D A, Whelan J A, Evans J, Taylor G P, Luzzi G, Giangrande P, Phillips R E, McMichael A J. J Exp Med. 1997;185:1423–1433. doi: 10.1084/jem.185.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van den Eynde B, van der Bruggen P & B. Curr Opin Immunol. 1997;9:684–693. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 27.Margulies D H. Curr Opin Immunol. 1997;9:390–395. doi: 10.1016/s0952-7915(97)80086-6. [DOI] [PubMed] [Google Scholar]
- 28.Corr M, Slanetz A E, Boyd L F, Jelonek M T, Khilko S, al R B, Kim Y S, Maher S E, Bothwell A L, Margulies D H. Science (Washington DC) 1994;265:946–949. doi: 10.1126/science.8052850. [DOI] [PubMed] [Google Scholar]
- 29.Matsui K, Boniface J J, Steffner P, Reay P A, Davis M M. Proc Natl Acad Sci USA. 1994;91:12862–12866. doi: 10.1073/pnas.91.26.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sykulev Y, Brunmark A, Jackson M, Cohen R J, Peterson P A, Eisen H N. Immunity. 1994;1:15–22. doi: 10.1016/1074-7613(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 31.Doherty P C, Topham D J, Tripp R A. Immunol Rev. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 32.Moss P A, Rowland J S, Frodsham P M, McAdam S, Giangrande P, McMichael A J, Bell J I. Proc Natl Acad Sci USA. 1995;92:5773–5777. doi: 10.1073/pnas.92.13.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A, McMichael A J. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pantaleo G, Koenig S, Baseler M, Lane H C, Fauci A S. J Immunol. 1990;144:1696–1704. [PubMed] [Google Scholar]
- 35.Kira J, Itoyama Y, Koyanagi Y, Tateishi J, Kishikawa M, Akizuki S, Kobayashi I, Toki N, Sueishi K, Sato H, et al. Ann Neurol. 1992;31:39–45. doi: 10.1002/ana.410310108. [DOI] [PubMed] [Google Scholar]
- 36.Kubota R, Umehara F, Izumo S, Ijichi S, Matsumuro K, Yashiki S, Fujiyoshi T, Sonoda S, Osame M. J Neuroimmunol. 1994;53:23–29. doi: 10.1016/0165-5728(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 37.Hara H, Morita M, Iwaki T, Hatae T, Itoyama Y, Kitamoto T, Akizuki S, Goto I, Watanabe T. J Exp Med. 1994;180:831–839. doi: 10.1084/jem.180.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehky T J, Fox C H, Koenig S, Levin M C, Flerlage N, Izumo S, Sato E, Raine C S, Osame M, Jacobson S. Ann Neurol. 1995;37:167–175. doi: 10.1002/ana.410370206. [DOI] [PubMed] [Google Scholar]
- 39.Gessain A, Saal F, Giron M L, Lasneret J, Lagaye S, Gout O, De T G, Sigaux F, Peries J. J Gen Virol. 1990;71:333–341. doi: 10.1099/0022-1317-71-2-333. [DOI] [PubMed] [Google Scholar]
- 40.Moritoyo T, Reinhart T A, Moritoyo H, Sato E, Izumo S, Osame M, Haase A T. Ann Neurol. 1996;40:84–90. doi: 10.1002/ana.410400114. [DOI] [PubMed] [Google Scholar]