Abstract
Herpes simplex virus (HSV) entry into cells is a multistep process that engages the host cell machinery. The proteasome is a large, ATP-dependent, multisubunit protease that plays a critical role in the maintenance of cell homeostasis. A battery of assays were used to demonstrate that proteasome inhibitors blocked an early step in HSV entry that occurred after capsid penetration into the cytosol but prior to capsid arrival at the nuclear periphery. Proteasome-dependent viral entry was not reliant on host or viral protein synthesis. MG132, a peptide aldehyde that competitively inhibits the degradative activity of the proteasome, had a reversible inhibitory effect on HSV entry. HSV can use endocytic or nonendocytic pathways to enter cells. These distinct entry routes were both dependent on proteasome-mediated proteolysis. In addition, HSV successfully entered cells in the absence of a functional host ubiquitin-activating enzyme, suggesting that viral entry is ubiquitin independent. We propose that proteasomal degradation of virion and/or host proteins is required for efficient delivery of incoming HSV capsids to the nucleus.
In the earliest stage of infection of the host cell, viruses utilize and manipulate preexisting cellular machineries before initiating their genetic program. Host cell surface receptors, signaling molecules, and cytoskeletal elements are examples of host factors that are commandeered during virus entry. The cellular proteasome has recently been shown to play a role in the entry of several viruses (36, 58, 59, 69). In addition, studies have documented a role for the proteasome in postentry stages of herpes simplex virus (HSV) infection (4, 11, 19, 20, 28). However, a role for the proteasome in HSV entry has not been described.
The proteasome system is the major pathway of intracellular protein degradation in eukaryotes. The 26S proteasome is an approximately 2.5 MDa, ATP-dependent protease complex that is critical for the maintenance of cell homeostasis (38, 66). Six proteolytic active sites are housed in a hollow, barrel-shaped structure called the 20S catalytic core particle. Substrates enter this channel and are processively cleaved into smaller peptides. The addition of two 19S regulatory caps to the ends of the 20S particle results in the 26S proteasome. The proteasome executes both the regulated hydrolysis of functionally active proteins and the degradation of aberrantly folded polypeptides.
The degradative activity of the 20S subunit regulates processes such as cell cycle progression, apoptosis, and antigen presentation (27). Many viruses depend on proteasome-mediated proteolysis for postentry events, including successful gene expression, replication, egress, and immune evasion (1). The majority of proteins destined for proteasomal degradation are tagged with chains of the 76-amino-acid protein ubiquitin (29). The polyubiquitin chain serves as the recognition motif for the proteasome. There are also examples of ubiquitin-independent proteolysis by the proteasome (14, 26, 32, 34, 40, 51).
HSV entry is a multistep process that engages the host cell machinery in a coordinated fashion (42, 64). Viral entry can be broadly defined as all events, leading to the deposition of the uncoated virus genome into the nucleus (49). Several cellular receptors can function to mediate HSV entry (5, 63), including nectin-1 (10, 24), a cell-cell adhesion molecule that is a component of cadherin-based adherens junctions (60). HSV can utilize either the endocytic machinery or a nonendocytic mechanism for productive entry into host cells (53). Host kinases have been shown to be involved in HSV entry (7, 9, 25, 30, 52, 54, 55). For example, cellular phosphatidylinositol 3-kinase activity facilitates endocytic trafficking of HSV and notably is not required for HSV entry via the direct penetration at the cell surface (54). After membrane penetration, the HSV capsid utilizes the minus-end-directed motor complex dynein/dynactin to travel on microtubules to the nuclear periphery (16, 41, 62, 65).
Using several experimental approaches, we have identified a role for the host proteasome in HSV entry. Interestingly, viral entry does not appear to require an active intracellular polyubiquitination machinery. Proteasome-mediated degradation facilitates the transport of incoming capsids to the nucleus. The completion of HSV entry by endocytosis or by direct penetration at the plasma membrane requires a proteasome-dependent step(s).
MATERIALS AND METHODS
Cells and viruses.
Vero cells (American Type Culture Collection, Rockville, MD) were propagated in Dulbecco modified Eagle medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (Gemini Bio-Products, West Sacramento, CA). CHO-nectin-1 cells (24) are CHO-K1 cells stably transformed with the human nectin-1 gene and the Escherichia coli lacZ gene under the control of the HSV ICP4 promoter (M3A cells provided by G. Cohen and R. Eisenberg, University of Pennsylvania). The cells were propagated in Ham F-12 nutrient mixture (Invitrogen) supplemented with 10% fetal bovine serum, 150 μg of puromycin (Sigma, St. Louis, MO)/ml, and 250 μg of G418 sulfate (Fisher Scientific, Fair Lawn, NJ)/ml. Cells were subcultured in nonselective medium prior to use in experiments. Mouse ts20 cells (70) (obtained from Harvey S. Ozer, University of Medicine and Dentistry of New Jersey) are derived from BALB/c 3T3 fibroblasts and contain a temperature-sensitive E1 enzyme (8), which activates free ubiquitin in an ATP-dependent manner. BALB/c 3T3 cells (American Type Culture Collection) and ts20 cells were maintained at the permissive temperature of 35°C. The nonpermissive temperature was 39°C.
HSV-1 strain KOS was provided by Priscilla Schaffer (Harvard University). HSV-1 KOS-tk12 (provided by Patricia Spear, Northwestern University) contains the lacZ gene under control of the ICP4 promoter (67). HSV-1 KOS K26GFP contains green fluorescent protein (GFP) fused to the N terminus of the VP26 capsid protein (13) (provided by Prashant Desai, Johns Hopkins University). HSV-1 strain ANG path (33) was obtained from Thomas Holland, Wayne State University. All viruses were propagated and titers were determined on Vero cells.
Chemicals.
Stocks of 0.5 M cycloheximide (Sigma), 1 mM epoxomicin (Peptides International, Louisville, KY), and 4 mM MG132 (Sigma) were prepared in ethanol. Lactacystin (1 mM; Peptides International) was prepared in dimethyl sulfoxide (Sigma), and 0.5 mg of heparin (Sigma)/ml was prepared in water. All stocks were stored at −20°C.
Citrate inactivation of infectious HSV.
Vero cells were grown in 24-well dishes overnight. Cells were mock treated or treated with MG132 for 30 min at 37°C. HSV-1 KOS was added (100 PFU/well) in the presence or absence of MG132 for various times at 37°C. Sodium citrate buffer (135 mM NaCl, 40 mM C6H5Na3O7, 10 mM KCl [pH 3.0]) or phosphate-buffered saline (PBS) warmed to 37°C was added for 1 min at 37°C. Culture medium was added, or cells were subjected to four 5-min washes with warmed medium. Infection proceeded for 18 to 24 h, and plaque formation was quantified.
β-Galactosidase reporter assay for HSV entry.
Confluent cell monolayers grown in 96-well dishes were treated with medium containing proteasome inhibitors or vehicle controls for 15 to 30 min at 37°C. HSV-1 KOS or KOS-tk12 (multiplicity of infection [MOI] of 4, unless otherwise indicated) was added. Cells were incubated in the constant presence of agent for 6 to 7.5 h. 0.5% Nonidet P-40 (Sigma) cell lysates were prepared, chlorophenol red-β-d-galactopyranoside (Roche Diagnostic, Indianapolis, IN) was added, and the β-galactosidase activity was read at 595 nm with an ELx808 microtiter plate reader (BioTek Instruments, Winooski, VT). β-Galactosidase activity indicated successful entry. Similar results were obtained at an MOI of 1. Mean results and standard errors were calculated for four replicate samples.
Cell viability.
Cultures in 96-well dishes were treated with culture medium plus inhibitor or mock-treated for 7.5 h. Cell monolayers were treated with trypsin and resuspended in a 0.07% solution of trypan blue (Sigma). Cells were counted on a hemacytometer by using light microscopy. The 100% viability was equal to the number of cells that exclude trypan blue/total number of cells in mock-treated samples. Mean results and the standard error were calculated for four replicate samples.
Plaque assay.
At 18 to 24 h postinfection (p.i.), culture medium was removed, and cells were fixed with ice-cold methanol-acetone solution (2:1 ratio) for 20 min at −20°C and air dried. Virus titers were determined by immunoperoxidase staining with anti-HSV polyclonal antibody HR50 (Fitzgerald Industries, Concord, MA).
Binding of GFP-tagged HSV to the cell surface.
Vero cells were grown overnight on glass coverslips in 24-well dishes. Cells were treated with the indicated inhibitor for 15 min at 37°C. Cultures were then chilled on ice, and HSV-1 K26GFP was added at an MOI of 30. Dishes were centrifuged at 200 × g for 1 h at 4°C to enhance virus binding. Cells were washed with ice-cold PBS (Invitrogen) and then fixed with 3% paraformaldehyde (Thomas Scientific, Swedesboro, NJ) in PBS prior to confocal microscopy.
Laser scanning confocal microscopy.
Paraformaldehyde-fixed cells on coverslips were permeabilized with 0.2% Triton X-100 (Fisher Scientific) in PBS for 8 min. Cells were then blocked with PBS containing 1% bovine serum albumin (Sigma) for 20 min. Nuclei were counterstained with DAPI (4′,6′-diamidino-2-phenylindole; Roche). Coverslips were washed with PBS, mounted with Fluoromount G (Electron Microscopy Sciences), and viewed with a Zeiss LSM 510 Meta microscope equipped with a 63× oil immersion objective lens. Digital images were processed with Adobe Photoshop CS2 version 9.
Subcellular localization of GFP-tagged HSV.
Vero cells were grown overnight on glass coverslips in 24-well dishes. Cells were treated with proteasome inhibitor or vehicle control in the presence of 0.5 mM cycloheximide for 15 min at 37°C. Cultures were rapidly chilled on ice, and then a supernatant preparation of HSV-1 KOS K26GFP was added (MOI of 10) in the continued presence of agent. Virus was spinoculated onto the cell surface by centrifugation at 200 × g for 1 h at 4°C. Entry was initiated by shifting dishes to 37°C for 2.5 h in the continued presence of agent. Cells were washed three times with PBS and fixed in 3% paraformaldehyde in PBS prior to confocal microscopy.
Fluorescence protease protection assay.
Modified from (45). HSV entry into Vero cells proceeded in the presence of 25 μΜ MG132 for 2.5 h as described above for subcellular localization of GFP-tagged HSV. Cells were then chilled and washed twice with ice-cold KHM buffer (110 mM KC2H3O2, 20 mM HEPES, 2 mM MgCl2 [pH 7.2]). For protease treatment, 100 μg of ice-cold proteinase K (Invitrogen)/ml in KHM buffer was added to live cells for 2.5 min on ice. Proteolysis was halted with 4 mM phenylmethylsulfonyl fluoride (Sigma) and 1% bovine serum albumin in KHM buffer. For permeabilized samples, 25 μΜ digitonin (EMD Chemicals, Inc., Darmstadt, Germany) in KHM buffer was added to cells for 3 min at 37°C prior to proteinase K treatment. Cells were washed twice with ice-cold PBS, fixed with 3% paraformaldehyde, and then visualized by confocal microscopy.
Fusion-from-without assay.
As described previously (12), Vero cells were pretreated with growth medium containing 0.5 mM cycloheximide with or without 50 μΜ MG132 for 15 min. Cell-free supernatant preparations of HSV-1 ANG path were added to cells (MOI of 50) for 3 h at 37°C in the constant presence of 0.5 mM cycloheximide. Cells were rinsed with PBS and then fixed in 100% methanol. Monolayers were air dried, and then nuclei were stained with Giemsa (Sigma).
Micrographs were taken with a Zeiss Axiovert 40C microscope equipped with a Canon PowerShot G6 digital camera. Digital images were processed with Adobe Photoshop CS2 version 9.0. To quantitate fusion, photomicrographs of random fields from triplicate wells (>500 cells/well) were scored. The number of nuclei present in clusters of four or more divided by the total number of nuclei yielded the percent fusion.
Stability of p53 in the presence or absence of E1, the ubiquitin-activating enzyme.
BALB/c 3T3 or ts20 cells were maintained at 35°C or transferred to 39°C for 18 h. Lysates were prepared in radioimmunoprecipitation assay buffer (100 mM NaCl, 25 mM Tris [pH 7.5], 2% Nonidet P-40, 0.5% sodium deoxycholate, 0.2% sodium dodecyl sulfate [SDS]) containing protease inhibitor cocktail (Roche). Samples in Laemmli buffer were separated by SDS-10% polyacrylamide gel electrophoresis, blotted onto nitrocellulose, and probed with 20 ng of mouse monoclonal antibody/ml, either pab421 (EMD Chemicals) to p53 or DM1A (Sigma) to alpha-tubulin. Nitrocellulose membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (Pierce, Rockford, IL), developed with enhanced chemiluminescence detection reagents (Pierce), and exposed to X-ray film (Kodak).
RESULTS
Proteasome inhibitors block HSV infection.
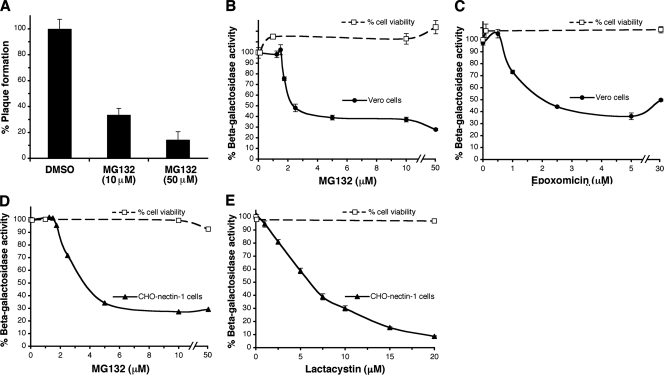
MG132 is a peptide aldehyde that is commonly used as an inhibitor of cellular proteasome activity (3). The presence of MG132 during the first 3 h of infection inhibited HSV plaque formation by >60% (Fig. 1A). HSV-induced expression of β-galactosidase is frequently used as a measure of successful viral entry. HSV-1 KOS-tk12 entry into Vero cells as measured by a beta-galactosidase reporter assay was inhibited by MG132 in a concentration-dependent manner (Fig. 1B). Epoxomicin is a specific and potent inhibitor of proteasomal degradation (50), and it too blocked HSV-induced β-galactosidase activity (Fig. 1C). Since reporter gene activity is measured at 6 to 7.5 h p.i., it is important to note that steps up to and including immediate-early gene expression might be affected in this assay. The inhibitors were effective at concentrations that were not toxic to the cells, as measured by a cell viability assay (Fig. 1). Together, the data suggested a role for the proteasome in HSV entry and infection.
FIG. 1.
Effect of proteasome inhibitors on HSV infection. (A) Effect of MG132 on HSV plaque formation. HSV-1 KOS (100 PFU/well) was added to Vero cells in the presence of MG132. At 3 h p.i., medium was removed, extracellular virus was acid inactivated, and plates were incubated for 24 h. Plaques were detected by immunoperoxidase staining and quantified. The data are means of quadruplicate determinations with the standard error. Vero cells (B and C) or CHO cells expressing nectin-1 (D and E) were treated with the indicated concentrations of inhibitor for 15 min. HSV-1 KOS-tk12 was added for 7.5 h in the continued presence of inhibitor. The percent β-galactosidase activity relative to that obtained in the absence of agent is indicated. The data are means of quadruplicate determinations with the standard errors. The effect of inhibitor on cell viability was measured by trypan blue exclusion. Viability in the absence of inhibitor was set to 100%. The mean viabilities and the standard errors of quadruplicate samples is shown.
HSV infection by endocytic or nonendocytic routes depends on the proteasome.
Vero cells support nonendocytic entry via direct fusion at the plasma membrane (23, 39, 53, 62). CHO-nectin-1 cells are a prototype line that supports HSV entry via fusion with an internal membrane after endocytosis (53). Both MG132 and lactacystin, a bacterial metabolite that inhibits the 20S proteasome, impaired HSV KOS-tk12 infection of CHO-nectin-1 cells, as measured by HSV-induced gene expression (Fig. 1D and E). Lactacystin appears to have a greater effect than MG132 or epoxomicin. This may be because the efficacy of a proteasome inhibitor can differ depending on the substrate being degraded (37). Similar results were obtained with HSV-1 KOS and HSV-2 G (data not shown). This suggests that HSV infection is dependent on proteasomal degradation, regardless of whether endocytic or nonendocytic entry routes are utilized.
The cellular proteasome, not a proteasome-like function of HSV, is blocked by lactacystin.
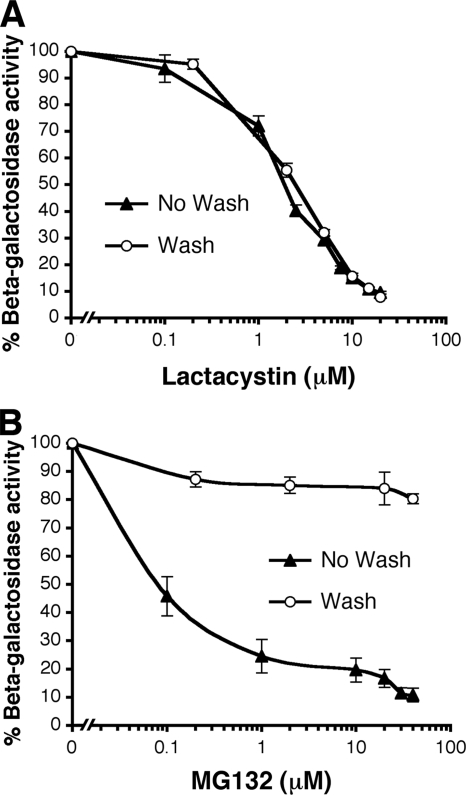
Herpesviruses mimic a myriad of cellular functions (57). It was possible that in the previous experiments the proteasome inhibitors were interfering with a virus-encoded function of HSV that is needed for entry. To determine whether this was the case, we took advantage of the known irreversible effect of lactacystin on the proteasome. Lactacystin binds covalently to the beta subunits of the 20S catalytic core (3). Once bound, its inhibitory effect on the proteasome cannot be reversed by washing the cells. CHO-nectin-1 cells were pretreated with medium containing lactacystin. Medium was removed, and cells were washed prior to addition of HSV. Lactacystin inhibited HSV-induced β-galactosidase activity in an equivalent manner whether the cells were washed prior to infection or whether lactacystin was present for the duration of the assay (Fig. 2A). This result is consistent with the inhibitor acting directly on the proteasome itself and not on a function of HSV. If lactacystin were affecting HSV directly, then infection would have resumed after the removal and washout of lactacystin. This was not the case (Fig. 2A). In contrast to lactacystin, MG132 is a reversible inhibitor (56). Cells that were treated with MG132 and then washed prior to addition of virus were much more susceptible to HSV than the cells that were not washed (Fig. 2B). The effect of MG132 was ∼85% reversible in this cell system.
FIG. 2.
Lactacystin acts directly on the cell proteasome and not on an HSV-specific function. CHO-nectin-1 cells were treated with lactacystin (A) or MG132 (B) for 30 min at 37°C. The medium was removed. Either the cells were subjected to four 5-min washes with culture medium, and then HSV-1 KOS-tk12 (MOI of 1) was added in medium without inhibitor (Wash), or else virus was added in the continued presence of agent (No Wash). The β-galactosidase activity was measured at 6 h p.i. (as in Fig. 1).
Proteasome-mediated proteolysis is needed for an early step in infection.
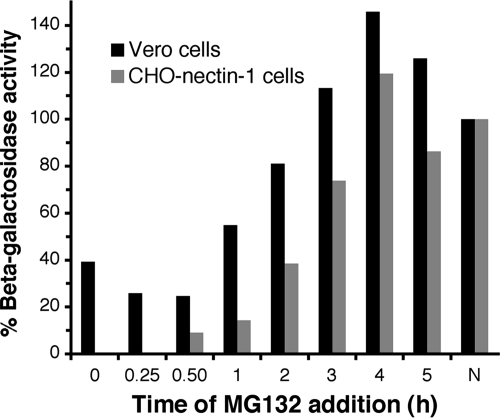
Early steps in HSV entry by either endocytic or nonendocytic routes are rapid (12, 31, 54, 62). Surface-bound virions are internalized by endocytosis (in CHO-nectin-1 cells) (12, 54) or penetrate directly at the plasma membrane (in Vero cells) (23, 39, 54, 62), with a t1/2 of <10 min. However, penetration of the capsid from an endocytic compartment in CHO-nectin-1 cells is necessarily delayed (t1/2 of ∼30 min) (54). Regardless of the entry pathway taken, incoming capsids dock at the nuclear envelope in <1 h (2, 48, 54, 62). To begin to identify the viral process that involves proteasomal degradation, cells were infected with HSV, and then MG132 was added at different times postinfection. The later the MG132 was added, the less of an inhibitory effect there was on HSV infection of either Vero or CHO-nectin-1 cells (Fig. 3). MG132 was most effective at blocking virus-induced β-galactosidase activity when added to Vero cells during the first hour postinfection or when added to CHO-nectin-1 cells during the first 2 h p.i. (Fig. 3). The results suggest that an early event in HSV infection, such as viral entry, requires the proteasome.
FIG. 3.
MG132 is most effective when added early in infection. HSV-1 KOS-tk12 or KOS (MOI of 1) was bound to Vero or CHO-nectin-1 cells, respectively, for 1 h at 4°C. Cells were rapidly warmed to 37°C to initiate infection. At the indicated times, 25 μΜ MG132 was added to the cells. The β-galactosidase activity was measured at 6 h p.i. as an indication of viral entry (as in Fig. 1). N, no MG132 added.
Binding of HSV to the cell surface is independent of proteasome activity.
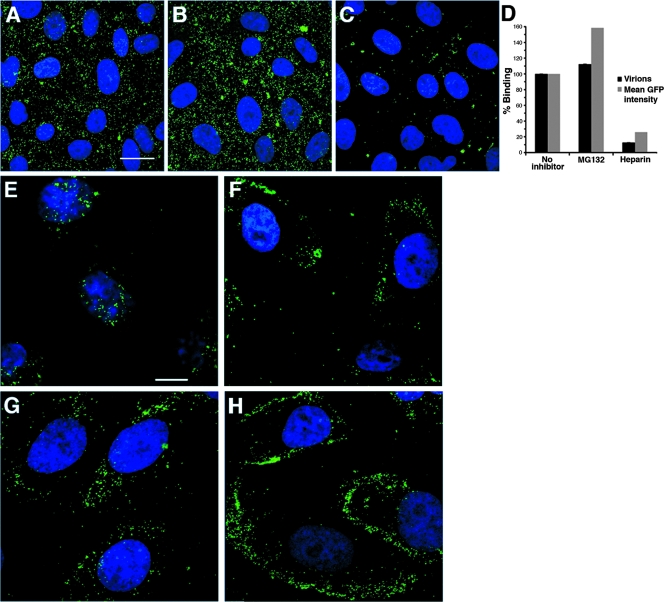
To define more precisely the step in viral entry that is influenced by proteasome activity, the effect of MG132 on steps prior to gene expression was analyzed by confocal microscopy. First, we determined whether proteasome inhibitors disrupted HSV binding to Vero cells. Vero cells were treated with MG132, and then HSV-1 K26GFP was added for 1 h at 4°C. MG132 treatment had no discernible inhibitory effect on HSV binding to cells (Fig. 4A and B). In contrast, control treatment with 1 μg of heparin/ml (68) blocked virus binding to cells by more than 80% (Fig. 4C and D). This suggested that the proteasome is not needed for virus binding to the cell surface.
FIG. 4.
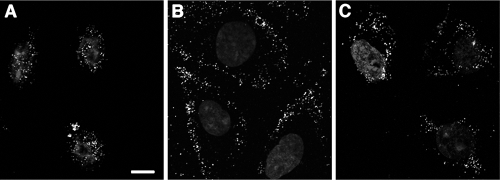
MG132 affects an entry event that occurs after surface binding, but prior to incoming capsid arrival at the nucleus. (A to D) Effect of MG132 on virus binding to cells. HSV-1 KOS K26GFP (MOI of 30) was added to prechilled Vero cells for 1 h at 4°C in the presence of either no inhibitor (A), 25 μΜ MG132 (B), or 1 μg of heparin/ml (C). Cells were fixed, and confocal images were obtained. Bar, 100 μm. (D) The number of bound GFP-tagged virions was quantified by three observers blinded to experimental conditions (Virions). The data are means with the standard errors. In parallel, mean GFP intensity was measured by the histogram function of Adobe Photoshop. Binding in the absence of inhibitor was set to 100%. (E to H) Effect of proteasome inhibitors on incoming capsid transport to the nuclear periphery. Vero cells were mock treated (E) or treated with 25 μΜ MG132 (F), 10 μΜ lactacystin (G), or 10 μΜ epoxomicin (H) for 15 min at 37°C. HSV-1 KOS K26GFP (MOI of 10) was added for 2.5 h in the constant presence of agent and 0.5 mM cycloheximide. Cells were fixed, and confocal images were obtained. Images are representative of cell population. Bar, 10 μm.
Reduced capsid transport to the nucleus in the absence of proteasome activity.
We investigated the role of the proteasome in delivery of incoming HSV-1 K26GFP capsids to the nucleus during entry. In HSV-infected Vero cells, the bulk of the GFP signal is detected at or near the nucleus by 2.5 h p.i. (Fig. 4E). Each punctate fluorescent signal is an individual virus particle. In the continued presence of MG132, lactacystin, or epoxomicin, GFP-tagged capsids were not effectively transported to the nuclear periphery (Fig. 4F, G, and H). Instead, the bulk of GFP-tagged viral particles appeared to be trapped at the cell periphery. These results were obtained in the presence of the protein synthesis inhibitor cycloheximide to ensure that GFP signal was from input virions. This also suggests that newly synthesized viral factors do not affect the proteasome dependence of entry. Together, the results suggest that proteasome activity is needed for a step in HSV entry that occurs after cell binding but prior to capsid arrival at the nucleus.
The inhibitory effect of MG132 on HSV entry is reversible.
MG132 can be washed out of cells prior to addition of virus, resulting in a much-reduced inhibitory effect (Fig. 2B). This is consistent with the reversible effect of MG132 on the proteasome. We next determined whether the block to entry imposed by MG132 could be reversed by washout. Vero cells were infected with HSV-1 K26GFP for 2 h in the presence of MG132 (Fig. 5B). After washout of MG132 and incubation for an additional hour, GFP-tagged capsids appeared to migrate toward the nucleus (Fig. 5C). Punctate GFP signals were visualized at an intermediate site between the cell periphery and the nucleus and in the perinuclear region (Fig. 5C). This is in line with the known reversible effect of MG132 on the 20S proteasome.
FIG. 5.
The block to entry imposed by proteasome inhibitors is partially reversible. Vero cells were mock treated (A) or treated with 25 μΜ MG132 (B and C) for 15 min at 37°C. HSV-1 KOS K26GFP was allowed to infect cells for 2 h in the absence (A) or presence of MG132 (B and C). Cells were fixed (B) or were washed four times for 5 min each time with culture medium and returned to 37°C for an additional hour in normal culture medium prior to fixation (A and C). Bar, 10 μm.
The proteasome is needed at a postpenetration step in HSV entry.
From the fluorescence microscopy experiments (Fig. 4F and 5B), the location of MG132-arrested virions in Vero cells cannot be ascertained unambiguously. The GFP signal may represent either enveloped virions that have bound but not yet fused or capsids that are halted in the cytosol. We utilized multiple approaches to determine the location of these virions.
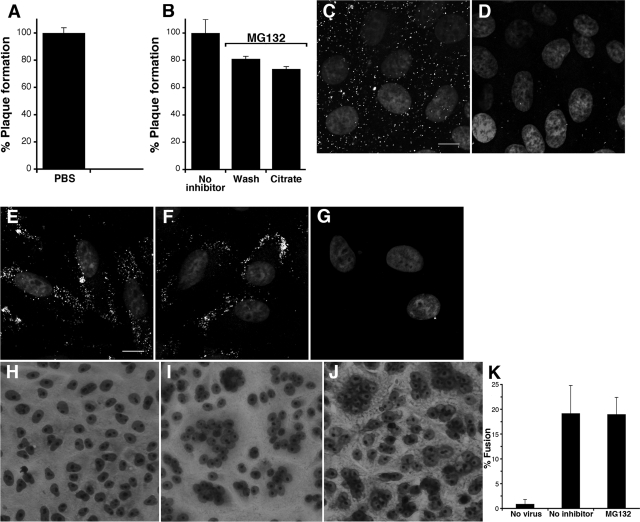
First, we used a citrate inactivation assay to determine whether MG132-arrested virions were bound to the cell surface. Sodium citrate buffer (pH 3.0) inactivates cell-bound HSV that has not penetrated into the cytosol (31). For example, the infectivity of HSV that is bound to Vero cells at 4°C is completely inactivated by citrate treatment (Fig. 6A). When citrate buffer was added to the surface of infected cells treated with MG132, HSV infectivity was not significantly affected (Fig. 6B). Similar infectivity was observed in the control cells that received PBS treatment (Fig. 6B). In these experiments, MG132 was washed out after citrate or PBS treatment to allow infection. The resistance to citrate inactivation supports the notion that HSV penetrates into the cytosol of Vero cells in the presence of MG132. Thus, proteasome activity may be needed for a step that occurs after fusion with a cell membrane.
FIG. 6.
Incoming HSV relies on proteasome activity at a postpenetration step. (A and B) Citrate treatment of the cell surface has no effect on the infectivity of MG132-arrested virions. (A) Vero cells were chilled on ice, and HSV-1 KOS (100 PFU/well) was added for 1 h at 4°C. Cells were treated with warmed PBS or citrate buffer (pH 3.0). Plates were shifted to 37°C, and plaques were quantified at 24 h p.i. (B) Vero cells were mock treated (no inhibitor) or treated with 25 μM MG132 for 30 min at 37°C. Cells were chilled, and then HSV-1 KOS (100 PFU/well) was added for 1 h at 4°C to synchronize the infection. Cultures were shifted to 37°C, and MG132 concentrations were maintained for 2.5 h at 37°C. Cells were then treated with PBS or citrate buffer (Citrate) and then extensively washed with culture medium to reverse the effect of MG132. Plaques were quantified at 18 h p.i. The data are the means of triplicate samples with the standard deviations. (C to G) Permeabilization of the plasma membrane followed by treatment with protease allows the removal of MG132-arrested virions. HSV-1 KOS K26GFP (MOI of 10) was bound to prechilled Vero cells for 1 h at 4°C (C), and then 100 μg of proteinase K/ml was added for 2.5 min (D) prior to fixation and confocal microscopy. Bar, 10 μm. (E to G) HSV-1 KOS K26GFP (MOI of 10) was added to cells in the presence of 25 μM MG132 for 2.5 h p.i. at 37°C. MG132-treated cells received no additional treatment (E), were treated with proteinase K (F), or were permeabilized with digitonin followed by proteinase K treatment (G). Cells were fixed and analyzed by confocal microscopy. Bar, 10 μm. (H to K) MG132 has no effect on virion-induced fusion in a fusion-from-without assay. Vero cells were mock treated (H and I) or treated with 50 μM MG132 (J) for 30 min at 37°C. Cells were left uninfected (H), or HSV-1 ANG path (MOI of 50) was added (I and J) for 3 h in the presence of cycloheximide. Cells were fixed and stained with Giemsa. (K) Fusion was quantitated from photomicrographs of random fields as described in Materials and Methods. The data are means of triplicate determinations with the standard errors.
Second, we used a fluorescence protease protection assay to determine the accessibility of MG132-arrested virions to proteinase K. HSV that is bound to the cell surface can be removed by protease treatment (54, 62). HSV-1 K26GFP was bound to Vero cells at 4°C (Fig. 6C). Proteinase K added to the cell surface effectively removed the GFP signal (Fig. 6D). In contrast, proteinase K did not effectively remove GFP-tagged HSV from MG132-treated cells (Fig. 6F), suggesting that arrested virions are not present on the cell surface. This is consistent with the inefficacy of citrate treatment (Fig. 6B). When cells were first permeabilized with digitonin and then treated with protease, the GFP signal was removed more effectively (Fig. 6G). In this case, proteinase K may enter the permeabilized cell and act on cytosolic capsids such that they can be washed away or their GFP degraded. Digitonin treatment alone did not have an effect on retention of GFP signal (not shown). These results support the notions that capsids are halted in the cytosol of MG132-treated cells and that the proteasome is needed for a step in entry that follows penetration.
Third, we addressed more directly the possibility that proteasome activity was needed for membrane fusion itself. Virus-cell fusion (or penetration) during HSV entry is refractory to direct study. We used a fusion-from-without (FFWO) assay as a surrogate means to measure virion-induced fusion (12). FFWO is the induction of target cell fusion by addition of virions to the monolayer surface in the absence of viral protein expression (22). A subset of syncytial strains of HSV-1, including the ANG path strain (33) has FFWO activity. Relative to uninfected cells (Fig. 6H), the addition of HSV-1 ANG path to Vero cell monolayers resulted in the clustering of nuclei, which is indicative of FFWO. We next assessed the effect of MG132 on FFWO. Cells treated with MG132 were susceptible to virion-induced FFWO (Fig. 6J) in a manner similar to untreated cells (Fig. 6K), suggesting that the proteasome is not needed for the fusion activity of the virion.
Proteasome-dependent entry of HSV does not require cellular activation of ubiquitin.
We next determined whether the host ubiquitination machinery is required for HSV entry. Modification with polyubiquitin chains serves to target many cellular proteins to the proteasome (29), but it is not always required. The initial step in ubiquitination is the ATP-dependent activation of a single ubiquitin molecule by the host enzyme E1 (27). In the absence of E1 activity, substrates are not polyubiquitinated and in turn are not degraded by the proteasome. To assess HSV entry under conditions where ubiquitin monomers are not activated, we used the mouse ts20 cell line (8), which is derived from BALB/c 3T3 fibroblasts. These cells have a temperature-sensitive E1 enzyme, which is nonfunctional at 39°C (8).
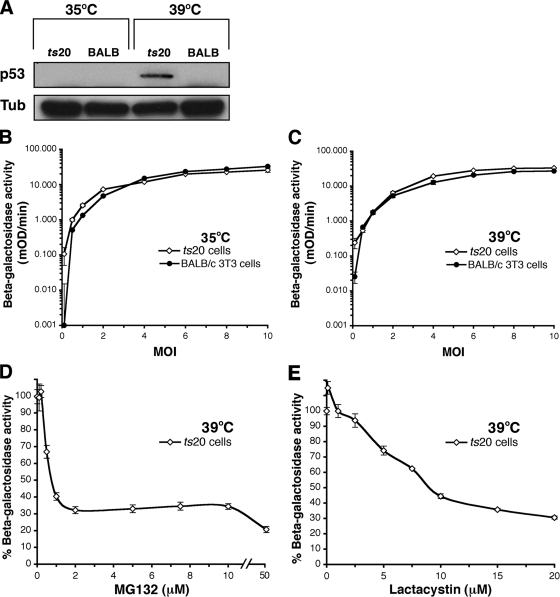
We first verified that E1 is nonfunctional in ts20 cells at the nonpermissive temperature by using a p53 stability assay. The transcription factor p53 is a regulator of cell proliferation, differentiation, and apoptosis (43). Wild-type p53 has a short half-life and is degraded by the ubiquitin-proteasome system (8). p53 was stabilized in ts20 cells at the restrictive temperature (Fig. 7A), confirming inactivation of E1.
FIG. 7.
HSV entry is independent of the ubiquitin-activating enzyme, E1. ts20 or BALB/c 3T3 cells were cultured at 35 or 39°C for 18 h. (A) p53 is not degraded in ts20 cells at the nonpermissive temperature. Lysates were prepared and analyzed by SDS-polyacrylamide gel electrophoresis and immunoblotted for p53 or α-tubulin (Tub). (B and C) HSV infects cells with an inactive polyubiquitination machinery. HSV-1 KOS-tk12 was added to cells cultured at 35°C (B) or 39°C (C). The β-galactosidase activity was determined at 6 h p.i. (D to E) Ubiquitin-independent entry of HSV requires proteasome activity. ts20 cells maintained at 39°C were treated with MG132 (D) or lactacystin (E) for 15 min. HSV-1 KOS-tk12 was added in the presence of inhibitor. The β-galactosidase activity was measured at 6 h p.i. (as in Fig. 1). The data are means of quadruplicate determinations with the standard errors.
Notably, HSV-1 KOS-tk12 expressed β-galactosidase in ts20 cells at 39°C in a manner similar to that seen at 35°C (Fig. 7B and C). Thus, viral entry proceeded under conditions that inhibited the polyubiquitination of proteins (Fig. 7A and C). HSV also infected control BALB/c 3T3 cells at both temperatures (Fig. 7B and C). Virus-induced gene expression in ts20 cells at the nonpermissive temperature was inhibited by MG132 and lactacystin (Fig. 7D and E), indicating that entry is still reliant on active proteasomes. Together, the results suggest that HSV enters cells in a proteasome-dependent, ubiquitin-independent manner.
DISCUSSION
Several virus-cell interactions have been identified during the initial infection of the host cell by HSV. Here we show that functional proteasomes are needed for efficient entry of HSV into cells. Interestingly, viral entry is not dependent on the ubiquitination activity of the cell. Proteasome inhibitors block HSV entry into cells that mediate either endocytic or nonendocytic entry pathways. Together, our studies suggest that a common step in both pathways, namely, the delivery of newly penetrated capsids to the nuclear periphery, is proteasome dependent.
To our knowledge HSV entry into Vero cells is the first example of a nonendocytic viral entry process to require cellular proteasome activity. The ubiquitin-proteasome system is important for the entry of several other viruses (36, 58, 59, 69), all of which utilize endocytic pathways. MG132 traps influenza virus in what are thought to be sorting endosomes (36). Murine hepatitis virus is blocked in an endocytic compartment, prior to penetration (69). In contrast, MG132-arrested virions of the nonenveloped minute virus of mice appear in the nuclear periphery, after they have been released from endosomes (58, 59). Canine parvovirus is similarly sensitive to MG132 but, interestingly, bovine parvovirus is not (59). Thus, the subcellular distribution of MG132-arrested HSV reported here appears to be distinct. In total, proteasome-mediated proteolysis may play multiple roles in the intracellular trafficking of viruses.
Our results are consistent with the idea that the proteasome is also needed at an early, postpenetration step in CHO cell entry. (Fig. 3). However, this needs to be determined conclusively. After endocytosis in CHO-nectin-1 cells, the HSV capsid penetrates from a low pH endocytic compartment (9, 25, 53, 54), but the precise site has not been established. Hence, Vero cells were more amenable to use in several aspects of our study because the penetration of capsid at the plasma membrane is synchronized easily and can be analyzed more directly.
The two major target cells for HSV in the human host are mucosal epithelial cells and sensory neurons of the peripheral nervous system. We have proposed that HSV utilizes a pH-dependent, endocytic pathway for initial entry into the epithelia, and a pH-independent entry route into neurons (52). The distance that the capsid must travel in order to reach the nucleus in epithelial cells, and in the cultured cells used in the present study, is much shorter than the distance to be traveled down the axon of an innervating neuron (18). While proteasome inhibitors block 60 to 90% of HSV entry into Vero, CHO-nectin-1, BALB/c 3T3 (the present study), B78-nectin-1, HeLa, and HaCaT cells (data not shown), the proteasome may play an even more significant role in neuronal entry.
There have been several studies on the role of the proteasome in HSV infection of cultured cells (4, 6, 11, 19-21, 28, 44). These studies were carried out under postentry conditions and at times in which newly expressed viral gene products are active. For example, in the infected cell, HSV ICP0 is associated with proteasomal degradation of ND10 components, which may serve to stimulate lytic infection (20, 28). Our results suggest that the proteasome dependence of HSV entry is not reliant on active host or viral protein synthesis. Whether our findings bear on downstream, proteasome-mediated events in HSV infection remains to be seen.
Three distinct proteolytic activities that line the channel of the 20S proteasome are responsible for degradation (27). Based on the nature of the active sites, the activities are designated chymotrypsin-like, trypsin-like, and caspase-like. The relative importance of an active site depends on the substrate (37). MG132 and lactacystin target the chymotrypsin-like activity primarily but do inhibit the remaining catalytic activities to a lesser extent (38). Epoxomicin is highly specific for the chymotrypsin-like active site and binds irreversibly (50). MG132, lactacystin, epoxomicin, and the chymotrypsin inhibitor TPCK (data not shown) all inhibit HSV entry. Agents that primarily affect the trypsin-like or caspase-like catalytic sites had no effect on viral entry (data not shown). Thus, the chymotrypsin-like active site, which is located on the beta-5 subunit of the 20S particle, is likely important for substrate proteolysis during HSV entry.
The nature of the degradation signal involved in proteasome-dependent entry of HSV is currently not known. Entry proceeds unimpeded in cells that lack a functional form of the ubiquitin-activating enzyme, E1 (Fig. 7). In the absence of E1 function, activated ubiquitin is not transferred to the carrier enzyme E2, and consequently both monoubiquitination and polyubiquitination are blocked, as is proteolysis by the ubiquitin-proteasome system (27). Although the majority of proteins degraded by the proteasome are first modified with polyubiquitin chains, there are examples of substrates that are targeted for proteasomal degradation independently of ubiquitin (26, 32, 34, 40, 51). The cyclin-dependent kinase p21 and the retinoblastoma protein p130 are substrates for ubiquitination, but they can also be degraded by the proteasome in a ubiquitin-independent manner (34, 61). Also, a number of ubiquitin-like modifications have been identified, such as sumoylation (35). It remains to be determined whether such ubiquitin-related moieties play a role in viral entry.
Efforts are under way to identify the polypeptide(s) that is degraded during HSV entry. Retrograde movement of herpesvirus capsids toward the nucleus likely involves the coordinated action of virus and cell components (15-17, 46, 47). HSV capsid and capsid-associated tegument proteins are candidate substrates for the 20S proteasome. Also, cytoskeletal elements and their associated motor proteins and regulatory molecules are host factors that may be targeted for degradation. Discovery of the substrate(s) will be critical for understanding the mechanism of proteasome-dependent viral entry.
Acknowledgments
This study was supported by Public Health Service grant AI-60702 from the National Institute of Allergy and Infectious Diseases and by a grant from the Jeffress Memorial Trust.
We are grateful to Gary Cohen, Prashant Desai, Roselyn Eisenberg, Thomas Holland, Harvey Ozer, Priscilla Schaffer, Patricia Spear, and Stephen Straus for kindly providing reagents. We thank Farzaneh Rostami for technical assistance and Michael McVoy for critical reading of the manuscript.
Footnotes
Published ahead of print on 30 January 2008.
Dedicated to the memory of Stephen E. Straus.
REFERENCES
- 1.Banks, L., D. Pim, and M. Thomas. 2003. Viruses and the 26S proteasome: hacking into destruction. Trends Biochem. Sci. 28452-459. [DOI] [PubMed] [Google Scholar]
- 2.Batterson, W., D. Furlong, and B. Roizman. 1983. Molecular genetics of herpes simplex virus. VIII. Further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J. Virol. 45397-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogyo, M., M. Gaczynska, and H. L. Ploegh. 1997. Proteasome inhibitors and antigen presentation. Biopolymers 43269-280. [DOI] [PubMed] [Google Scholar]
- 4.Burch, A. D., and S. K. Weller. 2004. Nuclear sequestration of cellular chaperone and proteasomal machinery during herpes simplex virus type 1 infection. J. Virol. 787175-7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10305-319. [DOI] [PubMed] [Google Scholar]
- 6.Chelbi-Alix, M. K., and H. de The. 1999. Herpesvirus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18935-941. [DOI] [PubMed] [Google Scholar]
- 7.Cheshenko, N., W. Liu, L. M. Satlin, and B. C. Herold. 2005. Focal adhesion kinase plays a pivotal role in herpes simplex virus entry. J. Biol. Chem. 28031116-31125. [DOI] [PubMed] [Google Scholar]
- 8.Chowdary, D. R., J. J. Dermody, K. K. Jha, and H. L. Ozer. 1994. Accumulation of p53 in a mutant cell line defective in the ubiquitin pathway. Mol. Cell. Biol. 141997-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clement, C., V. Tiwari, P. M. Scanlan, T. Valyi-Nagy, B. Y. Yue, and D. Shukla. 2006. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J. Cell Biol. 1741009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 729992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai-Ju, J. Q., L. Li, L. A. Johnson, and R. M. Sandri-Goldin. 2006. ICP27 interacts with the C-terminal domain of RNA polymerase II and facilitates its recruitment to herpes simplex virus 1 transcription sites, where it undergoes proteasomal degradation during infection. J. Virol. 803567-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delboy, M. G., J. L. Patterson, A. M. Hollander, and A. V. Nicola. 2006. Nectin-2-mediated entry of a syncytial strain of herpes simplex virus via pH-independent fusion with the plasma membrane of Chinese hamster ovary cells. Virol. J. 3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai, P., and S. Person. 1998. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 727563-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dick, L. R., C. Aldrich, S. C. Jameson, C. R. Moomaw, B. C. Pramanik, C. K. Doyle, G. N. DeMartino, M. J. Bevan, J. M. Forman, and C. A. Slaughter. 1994. Proteolytic processing of ovalbumin and β-galactosidase by the proteasome to a yield antigenic peptides. J. Immunol. 1523884-3894. [PMC free article] [PubMed] [Google Scholar]
- 15.Dohner, K., K. Radtke, S. Schmidt, and B. Sodeik. 2006. Eclipse phase of herpes simplex virus type 1 infection: efficient dynein-mediated capsid transport without the small capsid protein VP26. J. Virol. 808211-8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dohner, K., A. Wolfstein, U. Prank, C. Echeverri, D. Dujardin, R. Vallee, and B. Sodeik. 2002. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol. Biol. Cell 132795-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas, M. W., R. J. Diefenbach, F. L. Homa, M. Miranda-Saksena, F. J. Rixon, V. Vittone, K. Byth, and A. L. Cunningham. 2004. Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport. J. Biol. Chem. 27928522-28530. [DOI] [PubMed] [Google Scholar]
- 18.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51237-347. [DOI] [PubMed] [Google Scholar]
- 19.Eom, C. Y., and I. R. Lehman. 2003. Replication-initiator protein (UL9) of the herpes simplex virus 1 binds NFB42 and is degraded via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA 1009803-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22761-770. [DOI] [PubMed] [Google Scholar]
- 21.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 726581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falke, D., A. Knoblich, and S. Muller. 1985. Fusion from without induced by herpes simplex virus type 1. Intervirology 24211-219. [DOI] [PubMed] [Google Scholar]
- 23.Fuller, A. O., and P. G. Spear. 1987. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc. Natl. Acad. Sci. USA 845454-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 2801618-1620. [DOI] [PubMed] [Google Scholar]
- 25.Gianni, T., G. Campadelli-Fiume, and L. Menotti. 2004. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J. Virol. 7812268-12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glass, J. R., and E. W. Gerner. 1987. Spermidine mediates degradation of ornithine decarboxylase by a non-lysosomal, ubiquitin-independent mechanism. J. Cell Physiol. 130133-141. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg, A. L. 2007. Functions of the proteasome: from protein degradation and immune surveillance to cancer therapy. Biochem. Soc. Trans. 3512-17. [DOI] [PubMed] [Google Scholar]
- 28.Hagglund, R., and B. Roizman. 2004. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J. Virol. 782169-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67425-479. [DOI] [PubMed] [Google Scholar]
- 30.Hoppe, S., M. Schelhaas, V. Jaeger, T. Liebig, P. Petermann, and D. Knebel-Morsdorf. 2006. Early herpes simplex virus type 1 infection is dependent on regulated Rac1/Cdc42 signalling in epithelial MDCKII cells. J. Gen. Virol. 873483-3494. [DOI] [PubMed] [Google Scholar]
- 31.Huang, A. S., and R. R. Wagner. 1964. Penetration of herpes simplex virus into human epidermoid cells. Proc. Soc. Exp. Biol. Med. 116863-869. [DOI] [PubMed] [Google Scholar]
- 32.Hwang, J., and R. F. Kalejta. 2007. Proteasome-dependent, ubiquitin-independent degradation of Daxx by the viral pp71 protein in human cytomegalovirus-infected cells. Virology 367334-338. [DOI] [PubMed] [Google Scholar]
- 33.Kaerner, H. C., C. H. Schroder, A. Ott-Hartmann, G. Kumel, and H. Kirchner. 1983. Genetic variability of herpes simplex virus: development of a pathogenic variant during passaging of a nonpathogenic herpes simplex virus type 1 virus strain in mouse brain. J. Virol. 4683-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalejta, R. F., and T. Shenk. 2003. Proteasome-dependent, ubiquitin-independent degradation of the Rb family of tumor suppressors by the human cytomegalovirus pp71 protein. Proc. Natl. Acad. Sci. USA 1003263-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerscher, O., R. Felberbaum, and M. Hochstrasser. 2006. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 22159-180. [DOI] [PubMed] [Google Scholar]
- 36.Khor, R., L. J. McElroy, and G. R. Whittaker. 2003. The ubiquitin-vacuolar protein sorting system is selectively required during entry of influenza virus into host cells. Traffic 4857-868. [DOI] [PubMed] [Google Scholar]
- 37.Kisselev, A. F., A. Callard, and A. L. Goldberg. 2006. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J. Biol. Chem. 2818582-8590. [DOI] [PubMed] [Google Scholar]
- 38.Kisselev, A. F., and A. L. Goldberg. 2001. Proteasome inhibitors: from research tools to drug candidates. Chem. Biol. 8739-758. [DOI] [PubMed] [Google Scholar]
- 39.Koyama, A. H., and T. Uchida. 1987. The mode of entry of herpes simplex virus type 1 into Vero cells. Microbiol. Immunol. 31123-130. [DOI] [PubMed] [Google Scholar]
- 40.Krappmann, D., F. G. Wulczyn, and C. Scheidereit. 1996. Different mechanisms control signal-induced degradation and basal turnover of the NF-κB inhibitor IκB alpha in vivo. EMBO J. 156716-6726. [PMC free article] [PubMed] [Google Scholar]
- 41.Kristensson, K., E. Lycke, M. Roytta, B. Svennerholm, and A. Vahlne. 1986. Neuritic transport of herpes simplex virus in rat sensory neurons in vitro. Effects of substances interacting with microtubular function and axonal flow [nocodazole, taxol and erythro-9-3-(2-hydroxynonyl)adenine]. J. Gen. Virol. 672023-2028. [DOI] [PubMed] [Google Scholar]
- 42.Krummenacher, C., A. Carfi, R. J. Eisenberg, and G. H. Cohen. 2007. Herpesvirus entry into cells: the enigma variations, p. 1-17. In S. Poehlmann and G. Simmons (ed.), Viral entry into host cells. Landes Bioscience, Austin, TX.
- 43.Lavin, M. F., and N. Gueven. 2006. The complexity of p53 stabilization and activation. Cell Death Differ. 13941-950. [DOI] [PubMed] [Google Scholar]
- 44.Lopez, P., C. Van Sant, and B. Roizman. 2001. Requirements for the nuclear-cytoplasmic translocation of infected-cell protein 0 of herpes simplex virus 1. J. Virol. 753832-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lorenz, H., D. W. Hailey, C. Wunder, and J. Lippincott-Schwartz. 2006. The fluorescence protease protection (FPP) assay to determine protein localization and membrane topology. Nat. Protoc. 1276-279. [DOI] [PubMed] [Google Scholar]
- 46.Luxton, G. W., S. Haverlock, K. E. Coller, S. E. Antinone, A. Pincetic, and G. A. Smith. 2005. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. USA 1025832-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luxton, G. W., J. I. Lee, S. Haverlock-Moyns, J. M. Schober, and G. A. Smith. 2006. The pseudorabies virus VP1/2 tegument protein is required for intracellular capsid transport. J. Virol. 80201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lycke, E., B. Hamark, M. Johansson, A. Krotochwil, J. Lycke, and B. Svennerholm. 1988. Herpes simplex virus infection of the human sensory neuron: an electron microscopy study. Arch. Virol. 10187-104. [DOI] [PubMed] [Google Scholar]
- 49.Marsh, M., and A. Helenius. 2006. Virus entry: open sesame. Cell 124729-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng, L., R. Mohan, B. H. Kwok, M. Elofsson, N. Sin, and C. M. Crews. 1999. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo anti-inflammatory activity. Proc. Natl. Acad. Sci. USA 9610403-10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller, C. L., and D. J. Pintel. 2001. The NS2 protein generated by the parvovirus minute virus of mice is degraded by the proteasome in a manner independent of ubiquitin chain elongation or activation. Virology 285346-355. [DOI] [PubMed] [Google Scholar]
- 52.Nicola, A. V., J. Hou, E. O. Major, and S. E. Straus. 2005. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J. Virol. 797609-7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 775324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicola, A. V., and S. E. Straus. 2004. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J. Virol. 787508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qie, L., D. Marcellino, and B. C. Herold. 1999. Herpes simplex virus entry is associated with tyrosine phosphorylation of cellular proteins. Virology 256220-227. [DOI] [PubMed] [Google Scholar]
- 56.Rock, K. L., C. Gramm, L. Rothstein, K. Clark, R. Stein, L. Dick, D. Hwang, and A. L. Goldberg. 1994. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78761-771. [DOI] [PubMed] [Google Scholar]
- 57.Roizman, B., D. M. Knipe, and R. J. Whitley. 2007. Herpes simplex viruses, p. 2501-2602. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott/The Williams & Wilkins Co., Philadelphia, PA. [Google Scholar]
- 58.Ros, C., C. J. Burckhardt, and C. Kempf. 2002. Cytoplasmic trafficking of minute virus of mice: low-pH requirement, routing to late endosomes, and proteasome interaction. J. Virol. 7612634-12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ros, C., and C. Kempf. 2004. The ubiquitin-proteasome machinery is essential for nuclear translocation of incoming minute virus of mice. Virology 324350-360. [DOI] [PubMed] [Google Scholar]
- 60.Sakisaka, T., and Y. Takai. 2004. Biology and pathology of nectins and nectin-like molecules. Curr. Opin. Cell Biol. 16513-521. [DOI] [PubMed] [Google Scholar]
- 61.Sheaff, R. J., J. D. Singer, J. Swanger, M. Smitherman, J. M. Roberts, and B. E. Clurman. 2000. Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol. Cell 5403-410. [DOI] [PubMed] [Google Scholar]
- 62.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 1361007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 2751-8. [DOI] [PubMed] [Google Scholar]
- 64.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 7710179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Topp, K. S., K. Bisla, N. D. Saks, and J. H. Lavail. 1996. Centripetal transport of herpes simplex virus in human retinal pigment epithelial cells in vitro. Neuroscience 711133-1144. [DOI] [PubMed] [Google Scholar]
- 66.Voges, D., P. Zwickl, and W. Baumeister. 1999. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 681015-1068. [DOI] [PubMed] [Google Scholar]
- 67.Warner, M. S., R. J. Geraghty, W. M. Martinez, R. I. Montgomery, J. C. Whitbeck, R. Xu, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246179-189. [DOI] [PubMed] [Google Scholar]
- 68.WuDunn, D., and P. G. Spear. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 6352-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu, G. Y., and M. M. Lai. 2005. The ubiquitin-proteasome system facilitates the transfer of murine coronavirus from endosome to cytoplasm during virus entry. J. Virol. 79644-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng, G. C., J. Donegan, H. L. Ozer, and R. Hand. 1984. Characterization of a ts mutant of BALB/3T3 cells and correction of the defect by in vitro addition of extracts from wild-type cells. Mol. Cell. Biol. 41815-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]