Abstract
Acanthamoeba infections are difficult to treat due to often late diagnosis and the lack of effective and specific therapeutic agents. The most important reason for unsuccessful therapy seems to be the existence of a double-wall cyst stage that is highly resistant to the available treatments, causing reinfections. The major components of the Acanthamoeba cyst wall are acid-resistant proteins and cellulose. The latter has been reported to be the major component of the inner cyst wall. It has been demonstrated previously that glycogen is the main source of free glucose for the synthesis of cellulose in Acanthamoeba, partly as glycogen levels fall during the encystment process. In other lower eukaryotes (e.g., Dictyostelium discoideum), glycogen phosphorylase has been reported to be the main tool used for glycogen breakdown in order to maintain the free glucose levels during the encystment process. Therefore, it was hypothesized that the regulation of the key processes involved in the Acanthamoeba encystment may be similar to the previously reported regulation mechanisms in other lower eukaryotes. The catalytic domain of the glycogen phosphorylase was silenced using RNA interference methods, and the effect of this phenomenon was assessed by light and electron microscopy analyses, calcofluor staining, expression zymogram assays, and Northern and Western blot analyses of both small interfering RNA-treated and control cells. The present report establishes the role of glycogen phosphorylase during the encystment process of Acanthamoeba. Moreover, the obtained results demonstrate that the enzyme is required for cyst wall assembly, mainly for the formation of the cell wall inner layer.
Free-living amoebae of the genus Acanthamoeba represent one of the most prevalent protists found in the environment. They are also causative agents of rare but serious human diseases: a fatal encephalitis termed granulomatous amoebic encephalitis; disseminated, mostly cutaneous and nasopharyngeal infections in immunocompromised patients; and a sight-threatening ulceration of the cornea called amoebic keratitis, which affects mostly immunocompetent contact lens wearers (15, 26, 42). Acanthamoeba infections are difficult to treat due to the often late diagnosis and the lack of effective and specific therapeutic agents. The most important reason for unsuccessful therapy appears to be the existence of a cyst stage that tends to resist the available treatments, causing reinfections (19, 43, 52, 53).
The cyst is one of two distinct stages formed by acanthamoebae during their life cycle and presents two wall layers, which are usually readily recognizable by their morphologies, the outer one termed the exocyst and the inner one termed the endocyst (36). Under favorable environmental conditions, motile vegetative amoeboid trophozoites feeding on bacteria crawl in the soil and on the ground and divide by fission. Under unfavorable conditions such as starvation, desiccation, and changes in temperature and pH, etc., the trophozoites stop dividing and undergo differentiation to form nonmotile cysts. The process of encystment leads to profound morphogenetic and metabolic changes involving the de novo synthesis of a highly resistant double-layered cyst wall, which serves as a shelter under stressful external conditions (26, 53).
The major components of the Acanthamoeba cyst wall are acid-resistant proteins (of unknown composition, except for cyst-specific protein 21 [CSP21] [17]) and cellulose (4, 51). Cellulose has been reported to be the major constituent of the endocyst in acanthamoebae, constituting more than 30% of the total components of this layer in Acanthamoeba castellanii (2, 51). On the other hand, the exocyst has been reported to be composed mainly of proteins (17, 55).
Cellulose is the major polysaccharidic component of the cell walls in vascular plants, algae, and many bacteria (11, 21, 34, 38, 39, 40, 41, 44) and consists of linear chains of glucose units joined by β-1,4 linkages. Actively growing acanthamoebae store glucose in the form of glycogen, and earlier biochemical studies suggested that glycogen serves as a source of glucose for the synthesis of cellulose during cyst wall formation (33, 46, 56). Moreover, it has been demonstrated previously that glycogen is the most rapidly degraded macromolecule during the initial phase of Acanthamoeba encystment (8, 56). However, the mechanisms by which glycogen levels decrease during the early hours of encystment are still unclear (55). In general, glycogen breakdown into units of glucose occurs due to hydrolytic cleavages by lysosomal hydrolases (amylases) and phosphorylitic cleavages by glycogen phosphorylase. Both routes have been suggested as possible ways of glycogen breakdown during the encystment of Acanthamoeba (55).
In mammals, glycogen degradation is regulated posttranslationally by the activation and inactivation of the glycogen phosphorylase which is continuously expressed in the cell (29). Two different glycogen phosphorylases in lower eukaryotes, such as the slime mold Dictyostelium discoideum, have been described previously (3, 48, 49). In the vegetative stages, there is glycogen phosphorylase I, which is functional until the moment when Dictyostelium cells undergo differentiation into environmentally resistant spores with cellulose-containing walls (58). At this phase, another type of glycogen phosphorylase undetectable in the vegetative stage is expressed (16, 36, 48, 49). The expression of this second type of glycogen phosphorylase is regulated at the level of transcription.
Therefore, it was hypothesized that the regulation of the key processes involved in the cell wall assembly in Acanthamoeba may be similar to the previously described regulation in other lower eukaryotes during cyst formation (3, 22, 54, 57). The present report describes the role of glycogen phosphorylase during the aforementioned encystment process in Acanthamoeba, as assessed using RNA interference (RNAi) methods.
MATERIALS AND METHODS
Cultures of Acanthamoeba.
Two Acanthamoeba strains from the American Type Culture Collection, A. castellanii (Neff strain) ATCC 30010, genotype T4, and A. astronyxis ATCC 30137, genotype T7 (5, 47), and two highly pathogenic isolates of A. polyphaga, MN-7 and SWT-22, both belonging to genotype T4 (23), were included in this study. The MN-7 strain is a clinical isolate from a human mesenteric node (31), and SWT-22 is an environmental isolate obtained from seawater in Tenerife, Canary Islands, Spain (23, 24). All strains were axenically grown without shaking in PYG 712 medium (American Type Culture Collection) at 25°C.
Encystation conditions.
For the encystment studies, cells from the late exponential phase of growth (a nearly confluent monolayer) were used. The spent PYG medium was quantitatively poured off, and the attached trophozoites were immediately overlaid with nonnutrient Neff's encystment medium (NEM; 0.1 M KCl-8 mM MgSO4 · 7H2O-0.4 mM CaCl2 · 2H2O-1 mM NaHCO3-20 mM ammediol [2-amino-2-methyl-1,3-propanediol; Sigma], pH 8.8, at 25°C) (30), with or without the addition of 1 mM glucose (the concentration of glucose corresponded to that used in the PYG medium).
Glycogen phosphorylase PCR.
A specific primer pair (AGP forward, 5′-AAGCTGGAGGACCTCTACGA-3′, and AGP reverse, 5′-TGCTTCATCGACAGCTGCGCGTA-3′) was designed using the Primer3 software program (35; http://fokker.wi.mit.edu/primer3/) and was based on a previously described sequence from the glycogen phosphorylase gene of the A. castellanii Neff strain (EMBL database accession no. EC109277).
PCR amplifications with the four Acanthamoeba strains included in this study were carried out in a MyCycler thermal cycler (Bio-Rad, Hercules, CA) using each primer at a concentration of 5 pmol/ml in a total volume of 30 μl containing 200 mM deoxynucleoside triphosphate, 10 ng of template DNA, and 0.4 U of Taq DNA polymerase (Applied Biosystems, Branchburg, NJ). Conditions for all PCRs were as follows: an initial denaturing phase of 95°C for 4 min and 35 repetitions of denaturation at 95°C for 30 s, annealing at 52°C for 30 s, and extension at 72°C for 15 s. An additional extension phase at 72°C for 7 min was included. Amplified products were electrophoretically resolved on 2% agarose gels and stained with ethidium bromide (0.5 μg/ml) for visual analysis under UV light. The obtained fragments were purified using a PCR purification kit (Qiagen, Hilden, Germany) and were sequenced using an ABI automatic sequencer (Sistemas Genómicos, Valencia, Spain). The obtained sequences were aligned using the Mega 3.0 software program (20).
Gene silencing technique.
Small interfering RNAs (siRNAs) targeting the catalytic domain of the glycogen phosphorylase of the A. castellanii Neff strain were designed based on the previously described sequence mentioned above (EMBL database accession no. EC109277) by using the BLOCK-iT RNAi designer (Invitrogen Corp., Carlsbad, CA) and synthesized by Invitrogen Ltd. (Carlsbad, CA). The siRNA duplex with the following sense and antisense sequences was used: 5′-CCGGCUACCGCACCAACAA and UUGUUGGUGCGGUAGCCGG-5′.
The soaking method, which was successfully applied to Acanthamoeba in a previous study, was used for the delivery of the siRNAs (25). Briefly, siRNAs were added directly to NEM to a final concentration of 20 μg/ml by using the siPORT NeoFX transfection agent (Ambion, Madrid, Spain). Cultures were grown for 96 h without shaking.
As a control, a scrambled sequence absent from the A. castellanii genome was used. The scrambled siRNA duplex sequence (5′-CAAGCUGACCCUGAAGUUC and GUUCGACUGGGACUUCAAG-5′ for the sense and the antisense strands, respectively) was based on the gene encoding the green fluorescent protein and was used, at the same concentration as the glycogen phosphorylase siRNAs, to treat the A. castellanii Neff strain cultured in PYG medium. Controls with the same strains treated with siRNA were also developed in PYG medium.
During the silencing procedure, living cells were monitored using a DMIL inverted microscope (Leica, Wetzlar, Germany) and harvested at different time points, 0, 6, 8, 12, 24, 48, 72, and 96 h after the induction of encystation, for Northern blot analyses, Western blot analyses, zymogram assays, electron microscopy, and calcofluor staining.
Northern blot analyses.
To determine the phase of the Acanthamoeba life cycle at which glycogen phosphorylase is expressed, Northern analyses of actively growing cells and cells stimulated by starvation in NEM to encyst were performed. Briefly, Acanthamoeba strain poly(A) mRNAs were isolated using the poly(A) Purist kit (Ambion Inc., Austin, TX). The electrophoretically separated nucleic acids were transferred onto nylon membranes (Roche Diagnostics). A glycogen phosphorylase cDNA from the A. castellanii Neff strain (ATCC 30010), prepared as previously described (25), was used as a control probe.
Northern blot analyses were performed as previously described (37); briefly, the hybridization was developed for 16 h at 68°C, and the materials were washed twice in a mixture of 2% standard saline citrate (SSC; 0.15 M NaCl-0.015 M sodium citrate, pH 7.0), 0.1% N-laurylsarcosine, and 0.1% (wt/vol) sodium dodecyl sulfate (SDS) at room temperature for 15 min each time, twice in 0.1% SSC-0.1% (wt/vol) SDS at 68°C, and once in 0.1% SSC-0.1% (wt/vol) Tween 20 at 68°C. The membranes were developed with antidigoxigenin antibody using the digoxigenin-RNA labeling kit (Roche). Assay results were normalized by using 18S mRNA from Acanthamoeba as described previously (25).
Preparation of protein extracts.
Acanthamoebae collected from cultures at different time points during encystation (0, 6, 8, 12, 24, 48, and 72 h after the induction of encystation) were harvested by centrifugation (5 min at 2,000 × g for samples from 0, 6, 8, and 12 h and 10 min at 4,000 × g for samples from 24, 48, and 72 h), and the obtained pellets were resuspended in 2 ml of phosphate-buffered saline, pH 7.2, containing 0.5 mM phenylmethylsulfonyl fluoride (Sigma). All suspensions underwent 10 rounds of freeze-thaw procedures (in a liquid nitrogen bath at 37°C). Suspensions from 0, 6, 8, and 12 h were sonicated (three times for 1 min each, with 30-s pauses) using an ultrasonic homogenizer model 47105 (Cole Parmer Instrument Corporation). Cysts (in samples from 24, 48, and 72 h) were disrupted by homogenization with a mini Beadbeater (Biospec Products, OK) using 0.5-mm glass beads until 90% of the cysts were destroyed, as determined by examination under the inverted microscope (DMIL; Leica, Wetzlar, Germany). The unbroken cells were removed by centrifugation at 20,000 × g for 20 min at 4°C. The supernatant was stored at −20°C until analysis. The protein concentration was measured according to the method of Bradford by using bovine serum albumin as the standard.
Zymogram assays of glycogen phosphorylase.
The activity of glycogen phosphorylase in the direction of glycogen synthesis was determined using a method of Steup (45). Proteins (18 μg per line) were electrophoretically separated on nondenaturing polyacrylamide gel electrophoresis (PAGE) slab gels containing 0.1% (wt/vol) glycogen (type II, from oyster; Sigma), and zymograms of phosphorylase glucan-synthesizing activity were prepared as described previously (45). Briefly, the gel with the separated proteins was equilibrated in 0.1 M sodium citrate (pH 6.5)-14 mM 2-mercaptoethanol buffer for 30 min and then incubated in 0.75% (wt/vol) glucose-1-phosphate in the same buffer at 37°C overnight.
After being washed twice in bidistilled water, the gel was flooded with 0.13% iodine-0.3% potassium iodine in bidistilled water and incubated until staining was observed (approximately 10 min at room temperature). Glycogen phosphorylase b from rabbit muscle (Sigma) was used as a control. The gels were photographed with a Kodak EDAS 290 digital documentation system.
Western blot analysis.
Total protein extracts collected and prepared as described above were electrophoretically separated on 10% SDS-PAGE gels and transferred onto nylon membranes using a Mini-Protean III system (Bio-Rad, Hercules, CA). Membranes were incubated with an anti-human polyclonal glycogen phosphorylase BB rabbit antibody (Spectral Diagnostic, Canada), and the results were developed with an anti-rabbit immunoglobulin G antibody labeled with alkaline phosphatase (Sigma, Tres Cantos, Madrid) by using the antidigoxigenin method as previously described (37).
Staining procedures.
Aliquots collected at each time point were fixed and stained for the presence of cellulose with 0.1% calcofluor fluorescent dye (Sigma) in phosphate-buffered saline, pH 7.2, for 10 min (12). The slides were examined with a Nikon Eclipse 90i fluorescent microscope, and digital images were further processed using Adobe Photoshop. A total of approximately 500 cells per time point were observed.
Scanning electron microscopy.
Amoeba aliquots were fixed in 2.5% glutaraldehyde in cacodylate buffer at 22°C for 12 h. The samples were then postfixed with 1% (wt/vol) osmium tetroxide, and dehydration was carried out by using a graded series of ethanol solutions. Fixed cells were sedimented onto electron microscopy stubs previously covered with poly-l-lysine. The critical point was reached by drying with liquid carbon dioxide. The dried samples were gold coated, and the electron microscopy pictures were obtained with a LEO (Zeiss) GEMINI-1530 scanning electron microscope.
Sensitivity and viability assays.
To test the resistance of Acanthamoeba cysts, a 5-min treatment with a nonionic detergent (SDS) was used as previously described (12). SDS (0.5% [wt/vol] final concentration) was added to the 96-h cultures in NEM. After exposure, 30-μl aliquots were inoculated onto nonnutrient agar plates covered with a heat-inactivated suspension of Enterobacter aerogenes. The plates were incubated at 27°C for the next 7 days. The viability of the cysts was monitored by their ability to excyst, i.e., to allow trophozoites to emerge from the cysts and to multiply. The presence of the trophozoites was detected microscopically using inverted microscopy.
Nucleotide sequence accession numbers.
The sequences obtained in this study were deposited in the GenBank database under the following accession numbers: EU273887 to EU273889.
RESULTS
Glycogen phosphorylase in Acanthamoeba.
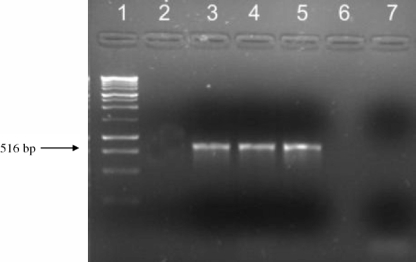
PCRs with the AGP primer pair amplified a 516-bp fragment from each of three of the four Acanthamoeba isolates used in this study, with the exception being the A. astronyxis isolate (Fig. 1). After sequencing of the amplified products and BLAST analysis, the obtained sequences showed levels of homology to the available A. castellanii Neff glycogen phosphorylase sequence of 98% (MN-7 and SWT-22) and 99% (A. castellanii Neff strain ATCC 30010).
FIG. 1.
Results from agarose gel electrophoresis showing PCR amplifications with the AGP primer pair. Lanes: 1, 100-bp DNA ladder; 2, A. astronyxis; 3, SWT-22; 4, MN-7; 5, A. castellanii Neff; 6, empty; 7, negative control.
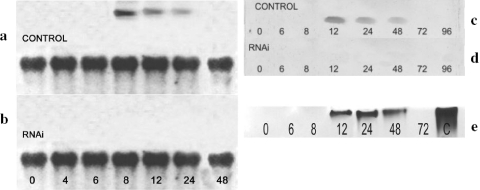
Northern blot analyses revealed that the expression of glycogen phosphorylase mRNA was limited to the cells in the early phase of encystment, between 8 and 24 h poststimulation, while no expression was detected either in the dividing and nondividing trophozoites, propagated in PYG and in NEM at the beginning of starvation, respectively, or in the cells entering the late phase of encystment (Fig. 2a). The levels of the expressed mRNAs decreased during encystation and became undetectable after 24 h (Fig. 2a).
FIG. 2.
Analyses of glycogen phosphorylase expression during encystation. (a and b) Northern blot analysis of glycogen phosphorylase expression during encystation in siRNA-treated (b) and control (a) cultures of Acanthamoeba. Total RNA extracted at different time points during encystation was electrophoresed at 80 V, blotted onto a nylon membrane, and probed with cDNA for glycogen phosphorylase. Top lanes, total RNA hybridized with cDNA corresponding to the catalytic domain of Acanthamoeba glycogen phosphorylase. Bottom lanes, hybridization with 18S mRNA as a control. Numbers indicate the hours after the induction of encystation. (c and d) Western blot analysis of glycogen phosphorylase during encystation in siRNA-treated (d) and control (c) cultures of Acanthamoeba. Protein extracts from different time points during encystation were incubated with polyclonal anti-human glycogen phosphorylase (BB) rabbit antibody, and results were developed with digoxigenin. Numbers indicate the hours after the induction of encystation. (e) Analysis of glycogen phosphorylase activity during encystation of Acanthamoeba. Proteins were isolated in time course experiments and separated by nondenaturing PAGE on a gel containing glycogen. Zymograms were developed by iodine staining of polyglucan synthesized from glucose-1-phosphate. Numbers indicate the hours after the induction of encystation. Lane C, positive control with glycogen phosphorylase b from rabbit muscle.
Glycogen phosphorylase was detected by Western blotting in the aliquots taken at 12, 24, and 48 h after the induction of encystment (Fig. 2c). Moreover, zymogram assays of glycogen phosphorylase detected enzymatic activity (Fig. 2e) at 12 h after the stimulation of encystation, and this activity persisted up to 48 h, while no activity was detected 72 h poststimulation.
Silencing of the glycogen phosphorylase gene by siRNA treatment.
The data above suggested glycogen phosphorylase to be significant for Acanthamoeba differentiation. In order to examine this idea more directly, the encystation process was initiated in the presence of the designed siRNAs in order to knock down the glycogen phosphorylase gene.
Regarding possible cytotoxicity of siRNAs for acanthamoebae, trophozoites (grown in the PYG medium) exposed to the same concentration of the siRNA as the encysting cells in the NEM did not show affected morphologies, and no mature cysts (which would reflect a cellular stress response) were detected after 96 h. Scrambled-siRNA-treated cultures did not show affected morphologies and cyst formation either (data not shown). Glucose added to NEM at the concentration corresponding to that in PYG medium (1 mM) had no effect on the differentiation of Acanthamoeba either in the absence or in the presence of siRNA.
Northern blot analyses demonstrated high efficiency of the designed siRNAs in the downregulation of the glycogen phosphorylase gene during the encystation assays. Moreover, transfection with these siRNAs resulted in complete silencing of the glycogen phosphorylase gene (Fig. 2b). As a result of the silencing procedure, no glycogen phosphorylase was detected by Western blot analysis (Fig. 2b).
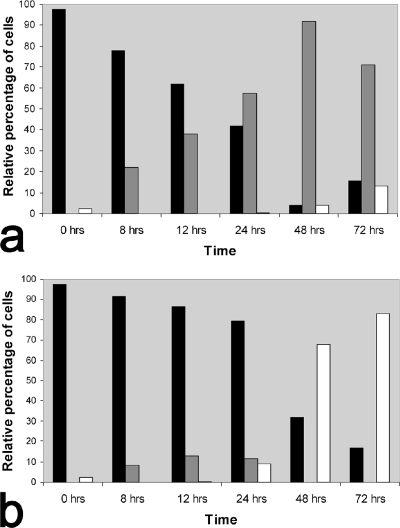
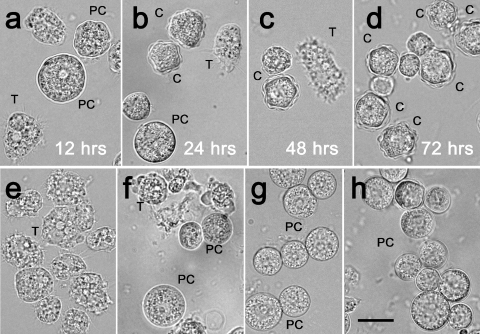
To explore what effect the silencing had on the phenotypes of the encysting cells, a comparative microscopic analysis of the living acanthamoebae differentiating in the presence or in the absence of siRNA (with glycogen phosphorylase knockdown or expression, respectively) was performed. In living cultures, both siRNA treated and untreated, three cell phenotypes were seen: amoeboid trophozoites, rounded immature precysts, and mature cysts (Fig. 3; also see Fig. 5). As shown in Fig. 3, there were considerable differences in the proportions of the cellular phenotypes between the populations of encysting cells with and without the silencing of the glycogen phosphorylase gene. In comparison to the normal course of the encystment process demonstrated in Fig. 3b and Fig. 4a to d, the glycogen phosphorylase knockdown resulted in a dramatic decrease in the number of mature cysts and an increase in the number of immature precysts (Fig. 3a and 4e to h). In both treated and untreated cultures, trophozoites predominated until 12 h poststimulation, which was the stage when the cells started to round up (Fig. 4a and e). In the siRNA-treated cultures, approximately 38% of cells (n = 130) detached and became rounded to form the precysts after approximately 24 h of encystment (Fig. 3a). The number of precysts further increased, so that after 72 h, 70% of the cells with the glycogen phosphorylase knockdown presented the precyst phenotype (Fig. 3a and 4h). As shown in Fig. 3b and 4d, at the corresponding stage of the normal encystment process, the precysts were absent and the mature cysts constituted 83% of the population. Some mature cysts were detected in siRNA-treated cultures (Fig. 3a and 4h), but their levels did not exceed 4.5%.
FIG. 3.
Distribution of trophozoites, immature precysts, and mature cysts during the course of encystment in the presence (a) or absence (b) of glycogen phosphorylase siRNA. The cell distribution (percentages of different cell types at different time points after the induction of encystation) was determined by counting cells viewed under an inverse microscope. The minimum number of cells counted per time point was 130; the maximum number of cells counted per time point was 600. All experiments were repeated five times. Black columns, trophozoites; gray columns, immature precysts; white columns, mature cysts.
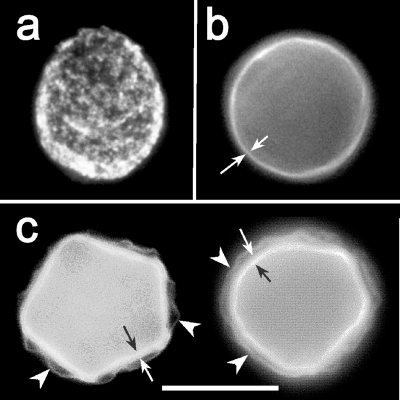
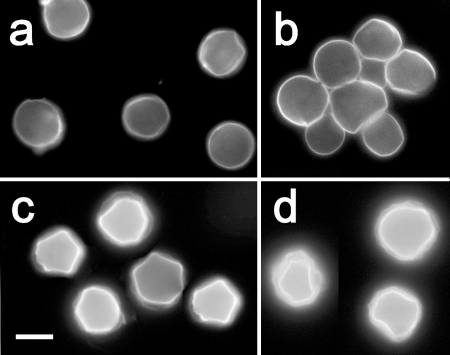
FIG. 5.
Phases of cyst wall formation as detected by calcofluor staining. (a) The rounded trophozoite with cellulose patches on the cell surface appears approximately 12 h after the induction of encystation. (b) Immature precyst with a continuous cell wall containing cellulose (approximately 24 h after the induction of encystation). A single layer of the cell wall is indicated by arrows. (c) Two mature cysts with both layers of the cell wall: a wrinkled exocyst (arrows) and an endocyst (arrowheads) containing a larger amount of cellulose than the exocyst.
FIG. 4.
Morphology of the living acanthamoebae at different time points after the induction of encystation. (a to d) Control culture. (a) Twelve hours after the induction of encystation, the trophozoites are detaching from the flask and starting to round up. (b) Twenty-four hours after the induction of encystation, immature cysts with a single-layered cyst wall are present; the first mature cysts with both layers of the cyst wall are also shown. (c and d) From 48 to 72 h after the induction of encystation, the number of mature cysts with both walls gradually increases, and at 72 h, the population of acanthamoebae consists mainly of mature cysts. (e to h) siRNA-treated cultures. (e) Twelve hours after the induction of encystation, trophozoites are starting to round up, similar to those in the control culture. (f) Twenty-four hours after the induction of encystation, single-layered “pseudocysts” are observed as a main cell type. (g) No mature cysts are observed 48 h after the induction of encystation. The main cell type present is the single-layered immature cyst. (h) Immature cysts represent approximately 70% of the cell population; the first mature cysts are also observed. T, trophozoite; PC, immature precyst; C, mature cyst. Scale bar, 10 μm.
Cellulose visualization by calcofluor staining of acanthamoebae resulted in the observation of three different patterns of cellulose-containing structures on the cell surfaces: (i) irregular discontinuous patches (Fig. 5a); (ii) a single confluent circular layer (Fig. 5b); and (iii) two parallel confluent layers partially separated from each other, the inner one thick and smooth, the outer one thin and irregularly wrinkled (Fig. 5c). Whereas the cellulose patches representing an early phase of cyst wall development occurred in both siRNA-treated and untreated cells approximately 12 h after the induction of encystment, significant differences in late phases of the process (between 48 and 72 h) were identified (Fig. 6). In the siRNA-treated cultures, the majority of rounded cells representing the precysts presented a single layer of calcofluor-positive material on their surfaces (Fig. 6a and b), in contrast to double-layered walls, which surrounded the mature cysts predominating in the untreated cultures (Fig. 6c and d).
FIG. 6.
Cyst types characteristic of stages of Acanthamoeba encystment in the presence or absence of siRNA for glycogen phosphorylase. (a and b) siRNA-treated culture after 24 h (a) or 48 h (b) of encystment. (c and d) Untreated control culture after 24 h (c) or 48 h (d) of encystment. The single-layered immature precysts represented the main cell type in the siRNA-treated cultures at both time points, whereas in the untreated cultures, the mature cysts with both cyst wall layers were already present after 24 h and prevailed after 48 h of encystment. Scale bar, 10 μm.
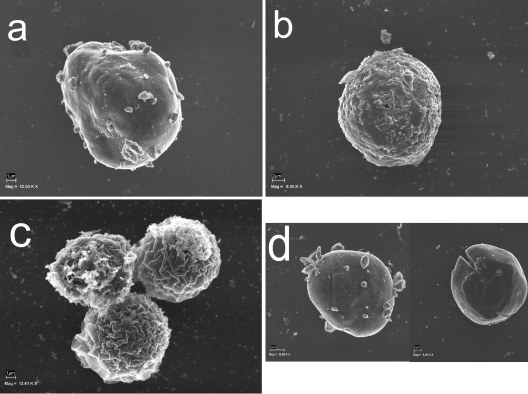
The addition of glucose to encysting cultures (both siRNA treated and control) had no effect on the cell phenotypes or proportions or the patterns of cellulose on the cell surfaces (data not shown). Scanning electron microscopy data obtained at the same stages further confirmed the observations described above (Fig. 7).
FIG. 7.
Phases of cyst wall formation as detected by scanning electron microscopy. (a) The rounded trophozoite with cellulose patches on the cell surface appears approximately 12 h after the induction of encystation. (b) Immature precyst with a continuous cell wall (approximately 24 h after the induction of encystation). A single layer of the cell wall is indicated by arrows. (c) Three mature cysts. The wrinkled exocyst is clearly recognizable. (d) RNAi-treated acanthamoebae were not able to form a mature cyst. Mag, magnification; 12.00 K X, ×12,000.
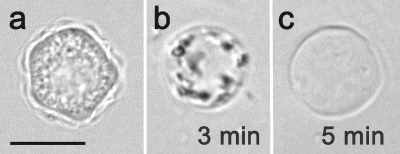
Mature cysts (presenting both layers of the cyst wall after 72 h of encystment) were fully resistant to the treatment with 0.5% SDS, as demonstrated by their morphology (Fig. 8a) and ability to excyst on agar plates within 24 h at 27°C (data not shown), whereas single-walled precysts (observed mainly in the siRNA-treated cultures after 72 h) did not survive the exposure and underwent cell lysis (Fig. 8b and c). Furthermore, no trophozoites were detected on agar plates until the stage 7 days postinoculation, when the viability tests were finished.
FIG. 8.
Sensitivity to 0.5% SDS of 72-h-old cyst stages formed in siRNA-treated and untreated cultures. (a) The mature cyst from the control cultures shows no affected morphology after a 5-min exposure to SDS. Scale bar, 10 μm. (b) The immature precyst from the siRNA-treated culture is undergoing lysis after a 3-min treatment with SDS. (c) The empty single-layered cyst wall shelter is a residuum of the SDS-treated precyst.
In contrast to the other isolates included in this study, A. astronyxis yielded no amplification products when PCR was performed with the AGP primer pair (Fig. 1). When the course of encystation of this particular strain was monitored, there were nearly no differences found between siRNA-treated and untreated cultures. By 96 h, the same percentages of mature cysts presenting both layers of the cyst wall and exhibiting full resistance to SDS treatment were observed in treated and untreated populations (data not shown).
DISCUSSION
In this study, the possible role of glycogen phosphorylase during the encystation process of Acanthamoeba spp. was surveyed using RNAi methods in order to knock down glycogen phosphorylase expression. When the expression of the glycogen phosphorylase gene was silenced using siRNAs, the vast majority of acanthamoebae were unable to form a resistant double-layered cyst wall, as detected by cellulose staining and electron microscopy. Moreover, siRNA-treated amoebae were able to complete only the first part of the encystation process, e.g., the synthesis of the outer layer of the cyst wall (exocyst), which was shown not to be resistant enough in order to provide protection against detergent lysis. Furthermore, the obtained results provide evidence of the interrelationship between glycogen phosphorylase and the biosynthesis of cellulose in the cyst wall. It is also important to mention that these results further point to possible differences in the mechanisms that provide glucose for the synthesis of polysaccharides in the inner and outer cyst wall layers.
Beside the CSP21 gene (17), the glycogen phosphorylase gene is the second gene that has been proven to be specifically expressed during the differentiation of Acanthamoeba cells into cysts. Indeed, there are more candidate genes, including the cellulose synthase gene(s) recently announced in the A. castellanii genome project (1). Analogous to the CSP21 gene, the glycogen phosphorylase gene of the A. castellanii and A. polyphaga strains (all belonging to genotype T4) included in this study can be regarded as inducible in that it seems to be active only during the encystment process of these strains. Northern blot analysis showed that the expression of glycogen phosphorylase mRNA was restricted to the period between 8 and 24 h after the induction of encystment. No expression was detectable in actively growing and stationary trophozoites, as well as in the mature cysts. In this respect, the glycogen phosphorylase gene expression pattern strongly resembles that of the CSP21 gene (17). Although a mechanism that underlies the activation of the glycogen phosphorylase gene during encystment remains to be elucidated, we can hypothesize that, similar to the CSP21 gene (10), the glycogen phosphorylase gene may be repressed in growing cells and induced by the removal of a specific repressor early in encystment. It seems likely that cyst-specific genes, including those of the cellulose biosynthetic pathway, may be coordinately regulated by the same mechanisms. Antirepression has recently been suggested as a major mechanism regulating Acanthamoeba differentiation (10).
During Acanthamoeba encystment, genes coding for both structural and functional proteins involved in the cyst wall assembly are expressed. Whereas the function of the CSP21 gene is yet to be proven, the incapacity to synthesize a double-layered cyst wall resulting from interference with glycogen phosphorylase gene expression clearly demonstrates that the glycogen phosphorylase enzyme is required for normal cyst wall assembly, most likely for the formation of the cell wall inner layer. Although any specific marker for a particular layer of the cyst wall is not available so far, we believe that it is the endocyst assembly that is affected as a consequence of the glycogen phosphorylase silencing. This assumption is based on a comparative analysis of the normal course of encystation and encystation after siRNA treatment. It has already been demonstrated by ultrastructural and autoradiography studies that the outer layer of the cyst wall is formed first (8, 9, 46). Our study has further confirmed this finding. Moreover, it has shown that there are no differences in the early phase of encystment between siRNA-treated and control cells. In both cell populations, the calcofluor-stained material forms a fine single layer uniformly covering the entire surface of a rounded cell. This layer corresponds to the early exocyst, as previously described (36). However, the completion of the exocyst, including the typical separation from the plasma membrane, and particularly, the assembly of the endocyst are completely blocked by glycogen phosphorylase RNAi. Thus, the posttranscriptional silencing of the glycogen phosphorylase gene yields immature precysts covered by the exocyst, with apparently no ability to form the endocyst, which strongly indicates the involvement of the glycogen phosphorylase in the assembly of this particular layer. Participation in cellulose biosynthesis through the degradation of glycogen into phosphorylated glucose is the most likely function of this enzyme.
Furthermore, the obtained results are in accordance with those in previous reports addressing biochemical changes during the encystment process (33, 46, 56). Stewart and Weisman (46) referred to the incorporation of 3H-labeled glucose into glycogen during the growth phase and subsequently, after the induction of encystation, showing that the radioactivity was found in the cellulose fraction of the cells.
Incomplete inhibition of the synthesis of polysaccharide (cellulose; stained by calcofluor white in the siRNA-treated cultures) raises the possibility of the involvement of other metabolic pathways providing glucose for the synthesis of the outer wall. Our observations are in agreement with those in a previous study by Chávez-Munguía et al. (9), in which it was reported that calcofluor-stained material, first observed on the encysting cells, was present in both layers of the mature cyst wall and, consequently, also in a single layer of the immature precyst wall. However, the specific wall component which is stained by calcofluor has not been resolved yet, although it clearly binds to cell walls that are composed of chitin, cellulose, and other β-1,4-linked carbohydrates (18). Calcofluor has been used to detect other exopolysaccharides with different oligosaccharide repeat units in Rhizobium sp. (14). Therefore, the stained material which is present in the outer layer of the cyst wall may not correspond to cellulose. Moreover, according to Potter and Weisman (33), who analyzed alkali-soluble and -insoluble fractions of A. castellanii cyst wall homogenates, a portion of β-1,4-glucan with close similarity to but distinct from cellulose may be synthesized beside cellulose during the encystment process of these amoebae.
Additionally, the assembly of the outer cyst wall layer in the RNAi-treated cells suggests that glycogen phosphorylase may not be involved in this process. The activity of glycogen phosphorylase was shown to be the highest after 24 h of encystation but moderate 12 h before, at the stage when the calcofluor-staining patches were already present on the surfaces of the encysting cells. No enzyme activity was observed between 0 and 12 h of encystment, as was previously reported (55). This finding may indicate that there may be another mechanism providing glucose for the synthesis of polysaccharides in the exocyst. In earlier studies, lysosomal hydrolases were suggested as an alternative to the phosphorylitic breakdown of the glycogen storage (55, 56). A functional glyoxylate pathway in encysting acanthamoebae may provide some levels of glucose via acetyl coenzyme A from lipid sources also (27, 50). The involvement of a glyoxylate pathway in the synthesis of cellulose should be considered, even though Stewart and Weisman (46) excluded this possibility; it was previously shown that the inhibition of the isocitrate lyase, a key enzyme of the pathway, leads to partial inhibition of the encystation process (27). However, further studies are needed in order to clarify the contribution of different metabolic pathways in the synthesis of polysaccharides involved in the formation of a resistant mature cyst wall.
Precysts surrounded by the single-layered wall were not protected against stressful conditions, as was shown by SDS treatment. As SDS denaturizes proteins, this result may further confirm that those acanthamoebae in which the expression of glycogen phosphorylase was disabled by siRNAs were able to finish only the first part of the cyst wall formation—the assembly of the exocyst that consists mainly of proteins (17).
Negative PCR results and a very weak response of the isolate of A. astronyxis to glycogen phosphorylase RNAi further support the view that this species is genetically very distinct from other Acanthamoeba species (5, 6, 47). Similar differences were described previously when the CSP21 protein was analyzed by Western blotting (17), as well as in a study on surface carbohydrates in acanthamoebae (7). The fact that the rest of the strains included in the present study belonged to genotype T4, a group that includes pathogenic Acanthamoeba strains, may also be related to the observations reported in this study. Nevertheless, further studies using the glycogen phosphorylase siRNAs and including other strains from the A. astronyxis group (genotype T7) should be carried out.
In conclusion, as the encystation process leads to higher-level resistance of acanthamoebae to the available treatments (52), understanding it is crucial for the future development of specific therapeutic targets. The development of new agents which could block the encystment may be a powerful tool in order to decrease the resistance of these amoebae, as was previously shown (28, 32). Moreover, in a recent study (13), the cellulose biosynthesis pathway was proposed as a possible target for the treatment of Acanthamoeba infections.
Finally, the results obtained in this study highlight the in vivo potential of RNAi technology as an attractive candidate to be included as an active component of future amoebicidal agents for the treatment of Acanthamoeba infections.
Acknowledgments
This work was supported by the project RICET (project no. RD06/0021/0005 of the program of Redes Temáticas de Investigación Cooperativa, FIS), Spanish Ministry of Health, Madrid, Spain; the Dirección General de Universidades e Investigación del Gobierno de Canarias project PI042005/049; and the project BIOPOLIS Interreg IIIB (Canarias, Açores, Madeira). Jacob Lorenzo-Morales was funded by a Dirección General de Fomento Industrial e Innovación Tecnológica postdoctoral contract (no. IDT-TF-06/055) from the Consejería de Industria y Nuevas Tecnologías of the Canary Islands government. This work was also partly supported by research project MSM 0021620806 from the Ministry of Education of the Czech Republic (E.N. and B.P.) and grants GACR 310/05/H533 and GAUK 119907 from Grant Agencies of the Czech Republic and Charles University in Prague, respectively (E.N. and J.K.). We also thank Hlavkova nadace for financial support through an internship in Spain (J.K.).
Footnotes
Published ahead of print on 25 January 2008.
REFERENCES
- 1.Anderson, I. J., R. F. Watkins, J. Samuelson, D. F. Spencer, W. H. Majoros, M. W. Gray, and B. J. Loftus. 2005. Gene discovery in the Acanthamoeba castellanii genome. Protist 156203-214. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, R. A., and M. Alexander. 1977. Resistance of cysts of amoebae to microbial decomposition. Appl. Environ. Microbiol. 33670-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop, J. D., B. C. Moon, F. Harrow, D. Ratner, R. H. Gomer, R. P. Dottin, and D. T. Brazill. 2002. A second UDP-glucose pyrophosphorylase is required for differentiation and development in Dictyostelium discoideum. J. Biol. Chem. 3632430-32437. [DOI] [PubMed] [Google Scholar]
- 4.Blanton, W. E., and C. L. Villemez. 1978. Molecular-size and chain-length distribution in Acanthamoeba cellulose. J. Protozool. 25264-267. [Google Scholar]
- 5.Booton, G. C., D. J. Kelly, Y. W. Chu, D. V. Seal, E. Houang, D. S. Lam, T. J. Byers, and P. A. Fuerst. 2002. 18S ribosomal DNA typing and tracking of Acanthamoeba species isolates from corneal scrape specimens, contact lenses, lens cases, and home water supplies of Acanthamoeba keratitis patients in Hong Kong. J. Clin. Microbiol. 401621-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booton, G. C., G. S. Visvesvara, T. J. Byers, D. J. Kelly, and P. A. Fuerst. 2005. Identification and distribution of Acanthamoeba species genotypes associated with nonkeratitis infections. J. Clin. Microbiol. 431689-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bose, K., D. K. Ghosh, and A. Bhattacharya. 1989. Membrane carbohydrate characterization of Acanthamoeba astronyxis, A. castellanii and Naegleria fowleri by fluorescein-conjugated lectins. Int. J. Parasitol. 19737-741. [DOI] [PubMed] [Google Scholar]
- 8.Bowers, B., and E. D. Korn. 1969. The fine structure of Acanthamoeba castellanii (Neff strain). J. Cell Biol. 41786-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chávez-Munguía, B., M. Omana-Molina, M. Gonzáles-Lázaro, A. Gonzáles-Robles, P. Bonilla, and A. Martínez-Palomo. 2005. Ultrastructural study of encystation and excystation in Acanthamoeba castellanii. J. Eukaryot. Microbiol. 52153-158. [DOI] [PubMed] [Google Scholar]
- 10.Chen, L., T. Orfeo, G. Gilmartin, and E. Bateman. 2004. Mechanism of cyst specific protein 21 mRNA induction during Acanthamoeba differentiation. Biochim. Biophys. Acta 169123-31. [DOI] [PubMed] [Google Scholar]
- 11.Dauvillée, D., V. Chochois, M. Steup, S. Haebel, N. Eckermann, G. Ritte, J. P. Ral, C. Colleoni, G. Hicks, F. Wattebled, P. Deschamps, C. d′Hulst, L. Liénard, L. Cournac, J. C. Putaux, D. Dupeyre, and S. G. Ball. 2006. Plastidial phosphorylase is required for normal starch synthesis in Chlamydomonas reinhardtii. Plant J. 48274-285. [DOI] [PubMed] [Google Scholar]
- 12.Dudley, R., A. Matin, S. Alsam, J. Sissons, A. H. Maghsood, and N. A. Khan. 2005. Acanthamoeba isolates belonging to T1, T2, T3, T4 but not T7 encyst in response to increased osmolarity and cysts do not bind to human corneal epithelial cells. Acta Trop. 95100-108. [DOI] [PubMed] [Google Scholar]
- 13.Dudley, R., S. Alsam, and N. A. Khan. 2007. Cellulose biosynthesis pathway is a potential target in the improved treatment of Acanthamoeba keratitis. Appl. Microbiol. Biotechnol. 75133-140. [DOI] [PubMed] [Google Scholar]
- 14.Gray, J. X., H. J. Zhan, S. B. Levery, L. Battisti, B. G. Rolfe, and J. A. Leigh. 1991. Heterologous exopolysaccharide production in Rhizobium sp. strain NGR234 and consequences for nodule development. J. Bacteriol. 1733066-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammersmith, K. M. 2006. Diagnosis and management of Acanthamoeba keratitis. Curr. Opin. Ophthalmol. 17327-331. [DOI] [PubMed] [Google Scholar]
- 16.Higgins, R. C., and M. E. Dahmus. 1982. Glycogen phosphorylase synthesis in Dictyostelium discoideum. J. Biol. Chem. 2575068-5076. [PubMed] [Google Scholar]
- 17.Hirukawa, Y., H. Nakato, S. Izumi, T. Tsuruhara, and S. Tomino. 1998. Structure and expression of a cyst specific protein of Acanthamoeba castellanii. Biochim. Biophys. Acta 139847-56. [DOI] [PubMed] [Google Scholar]
- 18.Hughes, J., and M. E. McCully. 1975. The use of an optical brightener in the study of plant structure. Stain Technol. 50319-329. [DOI] [PubMed] [Google Scholar]
- 19.Khan, N. A. 2006. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol. Rev. 30564-595. [DOI] [PubMed] [Google Scholar]
- 20.Kumar, S., K. Tamura, and K. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5150-163. [DOI] [PubMed] [Google Scholar]
- 21.Lerouxel, O., D. M. Cavalier, A. H. Liepman, and K. Keegstra. 2006. Biosynthesis of plant cell wall polysaccharides—a complex process. Curr. Opin. Plant Biol. 9621-630. [DOI] [PubMed] [Google Scholar]
- 22.Linder, M., J. Wieniecka-Krusnell, and E. Linder. 2002. Use of recombinant cellulose-binding domains of Trichoderma reesei cellulase as a selective immunocytochemical marker for cellulose in protozoa. Appl. Environ. Microbiol. 682503-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenzo-Morales, J. 2006. Estudios de amebas de vida libre del género Acanthamoeba en las Islas Canarias. Ph.D. thesis. Universidad de La Laguna, La Laguna, Tenerife, Spain.
- 24.Lorenzo-Morales, J., A. Ortega-Rivas, P. Foronda, E. Martínez, and B. Valladares. 2005. Isolation and identification of pathogenic Acanthamoeba strains in Tenerife, Canary Islands, Spain from water sources. Parasitol. Res. 95273-277. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo-Morales, J., A. Ortega-Rivas, P. Foronda, N. Abreu-Acosta, D. Ballart, E. Martínez, and B. Valladares. 2005. RNA interference (RNAi) for the silencing of extracellular serine proteases genes in Acanthamoeba: molecular analysis and effect on pathogenicity. Mol. Biochem. Parasitol. 14410-15. [DOI] [PubMed] [Google Scholar]
- 26.Marciano-Cabral, F., and G. Cabral. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16273-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehdi, H., and N. K. Garg. 1987. Changes in the lipid composition and activities of isocitrate dehydrogenase and isocitrate lyase during encystation of Acanthamoeba culbertsoni strain A-1. Trans. R. Soc. Trop. Med. Hyg. 81633-636. [DOI] [PubMed] [Google Scholar]
- 28.Murdoch, D., T. B. Gray, R. Cursons, and D. Parr. 1998. Acanthamoeba keratitis in New Zealand, including two cases with in vivo resistance to polyhexamethylene biguanide. Aust. N. Z. J. Ophthalmol. 3231-236. [DOI] [PubMed] [Google Scholar]
- 29.Murray, R. K., D. K. Granner, P. A. Mayes, and V. W. Rodwell. 1993. Harper's biochemistry, 23rd ed. Appleton and Lange, East Norwalk, CT.
- 30.Neff, R. J., and R. H. Neff. 1969. The biochemistry of amoeba encystment. Symp. Soc. Exp. Biol. 2351-81. [PubMed] [Google Scholar]
- 31.Ortega-Rivas, A., J. Lorenzo-Morales, E. Martinez-Carretero, M. Villa Lloberas, B. Valladares Hernandez, and A. del Castillo-Remiro. 2004. Design and evaluation of a specific primer pair for the diagnosis and identification of Acanthamoeba polyphaga. Curr. Microbiol. 48360-363. [DOI] [PubMed] [Google Scholar]
- 32.Pérez-Santonja, J. J., S. Kilvington, R. Hughes, A. Tufail, M. Matheson, and J. K. Dart. 2003. Persistently culture positive Acanthamoeba keratitis: in vivo resistance and in vitro sensitivity. Ophthalmology 81593-1600. [DOI] [PubMed] [Google Scholar]
- 33.Potter, J. L., and R. A. Weisman. 1971. Differentiation in Acanthamoeba: beta-glucan synthesis during encystment. Biochim. Biophys. Acta 23765-74. [DOI] [PubMed] [Google Scholar]
- 34.Romling, U. 2002. Molecular biology of cellulose production in bacteria. Res. Microbiol. 153205-212. [DOI] [PubMed] [Google Scholar]
- 35.Rozen, S., and H. J. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers, p. 365-386. In S. Krawetz and S. Misener (ed.), Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 36.Rutherford, L. C., O. Selmin, and S. Peters-Weigel. 1997. Temporal regulation of the Dictyostelium glycogen phosphorylase 2 gene. Biochim. Biophys. Acta 1351111-125. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 38.Saxena, I. M., and R. M. Brown, Jr. 2000. Cellulose synthases and related enzymes. Curr. Opin. Plant Biol. 3523-531. [DOI] [PubMed] [Google Scholar]
- 39.Saxena, I. M., and R. M. Brown, Jr. 2005. Cellulose biosynthesis: current views and evolving concepts. Ann. Bot. (London) 969-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheible, W. R., and M. Pauly. 2004. Glycosyltransferases and cell wall biosynthesis: novel players and insights. Curr. Opin. Plant Biol. 7285-295. [DOI] [PubMed] [Google Scholar]
- 41.Schupp, N., and P. Ziegler. 2004. The relation of starch phosphorylases to starch metabolism in wheat. Plant Cell Physiol. 451471-1484. [DOI] [PubMed] [Google Scholar]
- 42.Schuster, F. L., and G. S. Visvesvara. 2004a. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 341001-1027. [DOI] [PubMed] [Google Scholar]
- 43.Schuster, F. L., and G. S. Visvesvara. 2004b. Opportunistic amoebae: challenges in prophylaxis and treatment. Drug Resist. Updat. 741-51. [DOI] [PubMed] [Google Scholar]
- 44.Somerville, C., S. Bauer, G. Brininstool, M. Facette, T. Hamann, J. Milne, E. Osborne, A. Paredez, S. Persson, T. Raab, S. Vorwerk, and H. Youngs. 2004. Toward a systems approach to understanding plant-cell walls. Science 3062206-2211. [DOI] [PubMed] [Google Scholar]
- 45.Steup, M. 1990. Starch degrading enzymes, p. 103-128. In P. J. Lea (ed.), Methods in plant biochemistry, vol. 3. Academic Press, London, United Kingdom. [Google Scholar]
- 46.Stewart, J. S., and R. A. Weisman. 1974. A chemical and autoradiographic study of cellulose synthesis during the encystment of Acanthamoeba castellanii. Arch. Biochem. Biophys. 161488-498. [DOI] [PubMed] [Google Scholar]
- 47.Stothard, D. R., J. M. Schroeder-Diedrich, M. H. Awwad, R. J. Gast, D. R. Ledee, S. Rodriguez-Zaragoza, C. L. Dean, P. A. Fuerst, and T. J. Byers. 1998. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J. Eukaryot. Microbiol. 4545-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas, D. A., and B. E. Wright. 1976. Glycogen phosphorylase in Dictyostelium discoideum. I. Purification and properties of the enzyme. J. Biol. Chem. 2511253-1257. [PubMed] [Google Scholar]
- 49.Thomas, D. A., and B. E. Wright. 1976. Glycogen phosphorylase in Dictyostelium discoideum. II. Synthesis and degradation during differentiation. J. Biol. Chem. 2511258-1263. [PubMed] [Google Scholar]
- 50.Tomlinson, G. 1967. The glyoxylate pathway in Acanthamoeba sp. J. Protozool. 14114-116. [Google Scholar]
- 51.Tomlinson, G., and E. A. Jones. 1962. Isolation of cellulose from the cyst wall of a soil amoeba. Biochim. Biophys. Acta 63194-200. [DOI] [PubMed] [Google Scholar]
- 52.Turner, N. A., A. D. Russell, J. R. Furr, and D. Lloyd. 2000. Emergence of resistance to biocides during differentiation of Acanthamoeba castellanii. J. Antimicrob. Chemother. 4627-34. [DOI] [PubMed] [Google Scholar]
- 53.Turner, N. A., A. D. Russell, J. R. Furr, and D. Lloyd. 2004. Resistance, biguanide sorption and biguanide-induced pentose leakage during encystment of Acanthamoeba castellanii. J. Appl. Microbiol. 961287-1295. [DOI] [PubMed] [Google Scholar]
- 54.Upadhyay, J. M., S. Crow, and A. Cox. 1984. The cyst wall composition of Hartmannella glebae. Proc. Soc. Exp. Biol. Med. 4424-428. [DOI] [PubMed] [Google Scholar]
- 55.Weisman, R. A. 1976. Differentiation in Acanthamoeba castellanii. Annu. Rev. Microbiol. 30189-219. [DOI] [PubMed] [Google Scholar]
- 56.Weisman, R. A., R. S. Spiegel, and J. G. McCauley. 1970. Differentiation in Acanthamoeba: glycogen levels and glycogen synthase activity during encystment. Biochim. Biophys. Acta 20145-53. [DOI] [PubMed] [Google Scholar]
- 57.Werth, J. M., and A. J. Kahn. 1967. Isolation and preliminary chemical analysis of the cyst wall of the amoeba-flagellate Naegleria gruberi. J. Bacteriol. 941272-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.West, C. M., and G. W. Erdos. 1990. Formation of the Dictyostelium spore coat. Dev. Genet. 11492-506. [DOI] [PubMed] [Google Scholar]