Abstract
Blastocystis is a ubiquitous enteric protozoan found in the intestinal tracts of humans and a wide range of animals. Evidence accumulated over the last decade suggests association of Blastocystis with gastrointestinal disorders involving diarrhea, abdominal pain, constipation, nausea, and fatigue. Clinical and experimental studies have associated Blastocystis with intestinal inflammation, and it has been shown that Blastocystis has potential to modulate the host immune response. Blastocystis is also reported to be an opportunistic pathogen in immunosuppressed patients, especially those suffering from AIDS. However, nothing is known about the parasitic virulence factors and early events following host-parasite interactions. In the present study, we investigated the molecular mechanism by which Blastocystis activates interleukin-8 (IL-8) gene expression in human colonic epithelial T84 cells. We demonstrate for the first time that cysteine proteases of Blastocystis ratti WR1, a zoonotic isolate, can activate IL-8 gene expression in human colonic epithelial cells. Furthermore, we show that NF-κB activation is involved in the production of IL-8. In addition, our findings show that treatment with the antiprotozoal drug metronidazole can avert IL-8 production induced by B. ratti WR1. We also show for the first time that the central vacuole of Blastocystis may function as a reservoir for cysteine proteases. Our findings will contribute to an understanding of the pathobiology of a poorly studied parasite whose public health importance is increasingly recognized.
Blastocystis is a ubiquitous protozoan parasite found in the intestinal tracts of humans and a wide range of animals (46, 49). Blastocystis infections have a worldwide distribution, and incidences of up to 60% have been reported in tropical, subtropical, and developing countries (49). Blastocystis is also of special clinical relevance to developed countries, since millions of travelers going to developing countries are at risk of infection (44). The clinical symptoms of Blastocystis infections are mainly diarrhea and abdominal pain, as well as nonspecific gastrointestinal symptoms, such as nausea, vomiting, bloating, and anorexia (12, 31, 44). Intriguingly, even though it was first described almost a century ago (1, 7), the pathogenic mechanisms of this enteric protozoan remain elusive. In recent years, many reports have shown that Blastocystis is associated with intestinal disorders (2, 16, 23). Patients with human immunodeficiency virus infections undergoing immunosuppressive therapy were reported to be more susceptible to Blastocystis-associated diarrhea (18, 20, 38). Similar to giardiasis, most of the people infected with Blastocystis are asymptomatic, and infection frequently resolves spontaneously (46).
We recently reported that Blastocystis ratti WR1, an isolate with zoonotic potential (32, 51), can induce contact-independent apoptosis and disrupt barrier function in IEC-6 cells (35), and it possesses proteases (43) that can degrade human secretory immunoglobulin A (36). Reports have suggested that this parasite can cause acute and chronic gastroenteritis (31), and inflammation of the intestinal mucosa has been associated with Blastocystis infections (19, 21, 39, 54). Intense infiltration of inflammatory cells in the colon was shown after Blastocystis infection in mice (26), and it was reported that Blastocystis modulates interleukin-8 (IL-8) response in intestinal epithelial cells (24); however, nothing is known about the parasitic virulence factors and the early events occurring in host cells following Blastocystis-host interactions. Proteases from protozoan parasites are known to play significant roles in pathogenesis (25, 40), and proteases from some pathogens have been reported to induce the production of proinflammatory cytokines from host cells (6).
IL-8 (CXCL8) is a CXC chemokine that attracts polymorphonuclear leukocytes to the site of inflammation, activates monocytes, and is considered to play an important role in the pathogenesis of inflammatory diseases (8, 34). Expression of the IL-8 gene is regulated by a number of pathways, and its promoter region has binding sequences for a range of transcription factors, including NF-κB, NF-IL-6, and AP-1 (28). In most cell types, activation of NF-κB is the most important step for IL-8 gene transcription (27). In unstimulated cells, NF-κB exists in an inactive form in the cytoplasm, bound to inhibitory proteins called IκBs. Stimulation by various inducers may activate a signaling cascade that culminates in the phosphorylation of IκBs, resulting in the degradation of IκB proteins (11), and the released NF-κB translocates to the nucleus to activate target genes. Numerous pathogens, such as Helicobactor pylori (4), Escherichia coli (10), and Bacteroides fragilis (22), have been shown to induce IL-8 production from host cells via NF-κB activation.
In the present study, we demonstrate for the first time that B. ratti WR1 cysteine proteases can activate IL-8 gene expression in human colonic epithelial cells. Furthermore we show that NF-κB activation is involved in the production of IL-8. In addition, our findings show that treatment with the antiprotozoal drug metronidazole can decrease IL-8 production induced by B. ratti WR1. We also show for the first time that the central vacuole of Blastocystis contains cysteine proteases.
MATERIALS AND METHODS
Parasite culture and preparation of lysate.
B. ratti WR1, an axenized zoonotic isolate, was used in this study and cultured as described previously (35). In brief, parasites were cultured in prereduced Iscove's modified Dulbecco's medium supplemented with 10% inactivated horse serum (Gibco) and incubated anaerobically at 37°C in an Anaerojar (Oxoid, United Kingdom). Four- to 5-day-old parasites at log phase were washed two times in ice-cold T84 cell complete medium at 500 × g for 10 min at 4°C. The pellet was resuspended in T84 cell medium supplemented with only 0.5% fetal bovine serum, the parasites were counted with a hemocytometer, and the concentration was adjusted to 1 × 107 parasites/ml. The parasites were examined microscopically for their viability in this T84 cell medium and were found to be viable for >48 h under T84 growth conditions (data not shown). Parasite lysates were prepared by three freeze-thaw cycles in liquid nitrogen and a 37°C water bath, respectively.
Measurement of the protease activity of Blastocystis by azocasein assay.
The protease activity of B. ratti WR1 was determined by the azocasein assay as previously described by us (43). In brief, 4 × 106 parasites were washed two times in phosphate-buffered saline (PBS) (pH 7.4), and lysates were prepared by three freeze-thaw cycles in liquid nitrogen and a 37°C water bath, respectively. Parasite lysates were preincubated with 2 mM dithriothreitol (Sigma) at 37°C for 10 min to activate protease activity. One hundred microliters of 5-mg/ml azocasein (Sigma) solution was prepared in PBS (pH 7.4) and incubated with 100 μl of parasite lysate for 1 h at 37°C. The reaction was stopped by adding 300 μl of 10% trichloroacetic acid, and samples were incubated on ice for 30 min. Undigested azocasein was removed by centrifugation (5,000 × g; 5 min), and the resulting supernatant was transferred to a clean tube containing 500 μl of NaOH (525 mM). Absorbance was measured at 442 nm on a spectrophotometer (Tecan Magellan). For controls, lysates were boiled at 90°C for 15 min to inactivate the proteases, and 100 μl trypsin (2.5 mg/ml) was used as a positive control. To determine the types of proteases that are present in B. ratti WR1, the effects of protease inhibitors on the protease activity of B. ratti WR1 were studied by adding one of the following inhibitors during a 1-h incubation of parasite lysate with azocasein: E-64, iodoacetamide, EDTA, phenylmethylsulfonyl fluoride (PMSF) (all 1 mM), and pepstatin A (100 μg/ml). All inhibitors were purchased from Sigma except pepstatin A (Chemicon), iodoacetamide (MP Biomedicals, LLC), and PMSF (BDH).
Cellular localization of cysteine proteases.
The cellular localization of cysteine proteases in living Blastocystis parasites was determined as described previously (41). Briefly, cells were incubated at room temperature in PBS (pH 7.0) containing 5 mM of the substrate Arg-Arg-4-methoxy-2-naphtylamide and 2.5 mM 5-nitro-2-salicylaldehyde. After 2 h, the cells were spun down and washed four times in PBS at 2,500 rpm in a benchtop centrifuge. Smears were made on a glass slide and viewed under a fluorescence microscope using 360- to 430-nm excitation and 550- to 600-nm emission. For inhibition of cysteine proteases, parasites were preincubated for 1 h in PBS containing iodoacetamide (50 μM).
Colonic-cell culture, inoculation protocol, and experimental planning.
T84 human colonic carcinoma epithelial cells were obtained from the American Type Culture Collection. T84 cell stock was maintained in T-75 flasks in a humidified 37°C incubator with 5% CO2, and passages 20 to 25 were used for all experiments. The complete growth medium consisted of a 1:1 mixture of Dulbecco modified Eagle medium and Ham's F-12 (Sigma) supplemented with 5% heat-inactivated fetal bovine serum (Gibco). Cell viability was analyzed by trypan blue assay, and cell cultures with >95% viability were used for the experiments. The cells were trypsinized with 0.25% trypsin-EDTA (Gibco), seeded in six-well plates (Costar), and grown to 75 to 80% confluence for all experiments. In some assays, cells were grown to confluence on collagen (BD)-coated 12-mm glass coverslips in 12-well tissue culture plates (Costar). As described previously (35), a concentration of 1 × 107 parasites or equal parasite lysate per 1 ml medium was used for all experiments. Either live parasites or parasite lysate was added to T84 cells grown in 6-well or 12-well plates and coincubated for the indicated times in a humidified 37°C incubator with 5% CO2. The culture medium was changed 1 day before experiments, and fresh complete medium supplemented with only 0.5% heat-inactivated fetal bovine serum was used during incubation with parasites or parasite lysate.
In protease inhibition experiments, parasite lysates were preincubated for 1 h in T84 complete medium containing one of the following inhibitors: EDTA, E-64, pepstatin A (all 10 μM), PMSF (1 μM), or iodoacetamide (50 μM). For antiprotozoal drug treatment, live parasites were pretreated in complete medium with 10 μg/ml metronidazole (Sigma) prior to the experiments. In some experiments, for NF-κB inhibition, T84 cells were pretreated for 3 h in complete medium containing 30 μM BAY11-7082 (Sigma). Cholera toxin (5 μg/ml) was added to the medium in some experiments as a positive control for the production of IL-8.
ELISA and real-time reverse transcription (RT)-PCR for IL-8.
An IL-8 enzyme-linked immunosorbent assay (ELISA) kit (R&D) was used to measure IL-8 in the supernatants of T84 cell cultures coincubated with live B. ratti WR1 parasites or parasite lysates for various times. For real-time PCR, total cellular RNA was extracted by a single-step method using Trizol reagent (Invitrogen). First-strand cDNA was synthesized with 1 μg RNA using SuperScript II reverse transcriptase (Invitrogen) following the manufacturer's instructions. The quantitative real-time PCR was performed with an Applied Biosystems 7500 instrument (Applied Biosystems) using Sybr green PCR core reagents (Applied Biosystems). The reaction conditions were designed as follows: initial denaturation at 95°C for 10 min, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C, and finally 15 s at 95°C, 1 min at 60°C, and 15 s at 95°C. Relative quantitation of IL-8 mRNA was calculated by the ΔΔCT method, and the amount of the target relative to the β-actin mRNA was expressed as 2−ΔΔCT. The primers used were IL-8 sense (5′ ATGACTTCCAAGCTGGCCGTGGCT 3′) and antisense (5′ TCTCAGCCCTCTTCAAAAACTTCTC 3′) and β-actin sense (TGACGGGGTCACCCACACTGTGCCCATCTA 3′) and antisense (5′ CTAGAAGCATTGCGGTGGACGATGGAGGG 3′).
Western blotting for IκB-α.
T84 cells grown on six-well plates were first lysed in cell lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM EDTA, 1% Triton X-100, 0.5% sodium dodecyl sulfate, and protease inhibitor cocktail) and then exposed to live B. ratti WR1 parasites and parasite lysate for 6 h. Equal amounts of proteins were loaded on 10% polyacrylamide gels for sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a Mini-Protean II system (Bio-Rad) and blotted onto nitrocellulose membranes (Amersham). After being blocked with 5% nonfat milk in 10 mM Tris-HCl, pH 7.5, 100 mM NaCl, and 0.1% Tween 20, the membrane was probed first with rabbit anti-IκB-α antibody (Santa Cruz) and then with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Santa Cruz). Rabbit anti-actin antibody (Sigma) was used as a loading control. The blots were developed on Hyperfilm ECL (Amersham) using chemiluminescent ECL Plus Detection Reagent (Amersham). The densities of bands were quantified with a gel documentation and analysis system (Gel Doc XR; Bio-Rad).
EMSA and measurement of NF-κB activation by ELISA.
Nuclear extracts were prepared using an NE-PER nuclear and cytoplasmic extraction reagent kit (Pierce), and electrophoretic mobility shift assays (EMSA) were performed according to the manufacturer's protocol using a LightShift chemiluminescent EMSA kit (Pierce). Target DNA sequences were used as described previously (47). Oligonucleotide duplexes containing either a wild-type NF-κB binding site (sense strand, 5′ GATCCGTGGAATTTCCTCTG 3′) present in the IL-8 promoter or a mutant NF-κB binding site (sense strand, 5′ GATCCGTTAACTTTCCTCTG 3′) were biotinylated at the 3′ end (synthesized by 1st Base, Singapore).
In a separate experiment, the NF-κB activity was measured with a commercial ELISA kit (TransAM NF-κB p65 Assay Kit; Active Motif) as described by the manufacturer. In brief, this assay uses a 96-well plate coated with an oligonucleotide containing the NF-κB consensus binding site (5′-GGGACTTTCC-3′). The active form of NF-κB in the nuclear extracts binds to the consensus site and is detected by a primary antibody specific for the activated p65 of NF-κB. A horseradish peroxidase-conjugated secondary antibody is then used for colorimetric quantification by spectrophotometry at 450 nm. The results are expressed as the increase over the control group.
Immunostaining for NF-κB nuclear translocation.
T84 cells grown on collagen-coated glass coverslips were exposed to B. ratti WR1 parasite lysate for 6 h and fixed in 2% paraformaldehyde for 30 min. All procedures were carried out at room temperature. Monolayers were permeabilized with 0.25% Triton X-100 for 15 min and washed three times with PBS (1 min each). The monolayers were blocked with blocking solution (Protein Block; Dako) for 10 min, and the solution was decanted. The monolayers were incubated for 1 h with 1:100 dilutions of rabbit anti-NF-κB p50 antibody (Santa Cruz) diluted in 0.2% bovine serum albumin and were subsequently washed three times with PBS (5 min each). Cells were incubated for 30 min in the dark with 1:200 dilutions of a Cy3-conjugated sheep anti-rabbit secondary antibody (Sigma) diluted in 0.2% bovine serum albumin. The monolayers were washed four times with PBS (5 min each) and mounted with mounting medium (Vectashield) with 4′,6-diamidino-2-phenylindole (DAPI) to counterstain the nucleus. The monolayers were visualized with a fluorescence microscope (Olympus), and images were captured using Image Pro software.
Statistical analysis.
The means of experimental groups were compared by one-way analysis of variance with Tukey's posttest using GraphPad Prism software. Differences were considered significant at the level of a P value of <0.05.
RESULTS
B. ratti WR1 contains mainly cysteine proteases.
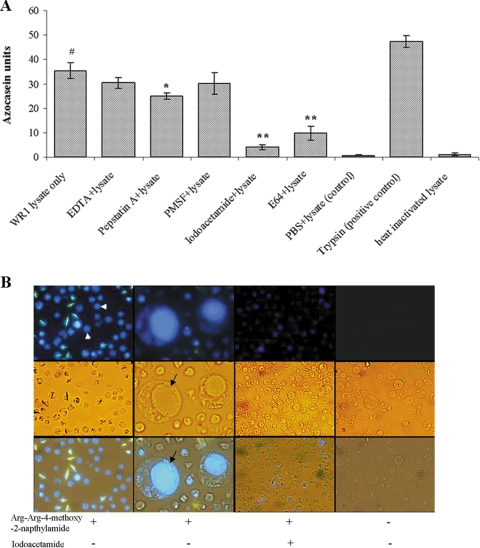
We employed an azocasein assay for the determination of parasite protease activity. Azocasein consists of casein conjugated to azo dye and serves as a substrate for proteolytic enzymes. Degradation of casein liberates free azo dye into the supernatant, which can be quantitatively analyzed. Figure 1A shows the protease activity of WR1 represented in azocasein units. One azocasein unit was defined as the amount of enzyme producing an increase of 0.01 optical density units per hour. Lysates of B. ratti WR1 showed significant protease activity (35.3 ± 3.2; P < 0.01) in comparison to the control (0.8 ± 0.1). A significant inhibition of the protease activity of WR1 lysate was observed with the use of the cysteine protease inhibitors iodoacetamide (4.1 ± 0.8; P < 0.01) and E-64 (9.8 ± 2.9; P < 0.01) (Fig. 1A). Partial inhibition was noticed with the aspartic protease inhibitor pepstatin A (25.1 ± 1.3; P = 0.058), and insignificant inhibition was seen with the metalloprotease inhibitor EDTA and the serine inhibitor PMSF. These findings suggested that the protease activity of B. ratti WR1 is mainly due to cysteine proteases of the parasite.
FIG. 1.
Protease activity of B. ratti WR1 and cellular localization of cysteine proteases. (A) Protease activity was determined with azocasein as a substrate. Lysates from 4 × 106 parasites were used for each sample. The assay was performed with and without protease inhibitors as described in Materials and Methods. Significant inhibition of protease activity could be noticed with cysteine protease inhibitors (iodoacetamide and E-64), and less significant inhibition was seen with the aspartic inhibitor pepstatin A. The metalloprotease inhibitor EDTA and the serine inhibitor PMSF showed no inhibition. One azocasein unit was defined as the amount of enzyme producing an increase of 0.01 optical density units/h. The values are means ± standard deviations (n = 3). #, P < 0.01 versus the negative control; *, P = 0.058 versus lysate; **, P < 0.01 versus lysate. (B) Representative fluorescence, light, and merged micrographs show the activities and localization of cysteine proteases in live parasites. Parasites were incubated in a cysteine protease substrate, Arg-Arg-4-methoxy-2-naphtylamide, as described in Materials and Methods. All parasites showed fluorescence (arrowhead, first column on the left), and the higher-magnification (×400) images in the second column show that fluorescence was limited to the central vacuole (arrow) of the parasite. Treatment of parasites with the cysteine inhibitor iodoacetamide resulted in diminished fluorescence (third column). Control parasites without cysteine protease substrate showed no fluorescence (fourth column). (Magnification in first, third, and fourth columns, ×100).
Cysteine proteases are confined to the central vacuole.
To determine the cellular localization of cysteine proteases, we incubated live parasites in PBS containing the substrates Arg-Arg-4-methoxy-2-naphtylamide and 5-nitro-2-salicylaldehyde. 5-Nitro-2-salicylaldehyde forms an insoluble adduct with the proteolytically released naphtylamine derivative. Our fluorescence microscopy results (Fig. 1B) showed that fluorescence was clearly localized within the central vacuole of the parasite. This suggests that cysteine proteases are mainly confined to this organelle. Almost no fluorescence was observed when parasites were pretreated with the cysteine protease inhibitor iodoacetamide.
Cysteine proteases of B. ratti WR1 induce IL-8 production.
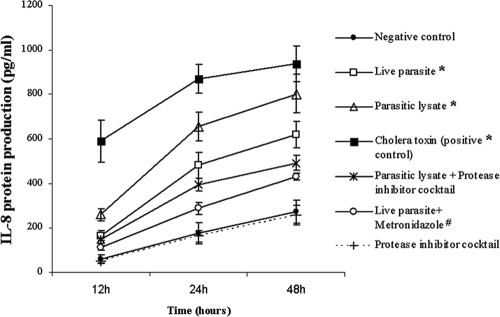
To assay for IL-8 protein in culture supernatants, T84 cells were incubated with B. ratti WR1 live cells or lysates for various times. As shown in Fig. 2, significant production of IL-8 protein occurred 12, 24, and 48 h after stimulation with B. ratti WR1 live parasites (166 ± 22, 482 ± 58, and 620 ± 60 pg/ml, respectively; P < 0.05) and with parasite lysates (260 ± 25, 654 ± 64, and 802 ± 87 pg/ml, respectively). B. ratti WR1-induced increase in IL-8 was significantly inhibited with the use of protease inhibitor cocktail for 12, 24, and 48 h (149 ± 16, 395 ± 27, and 492 ± 34 pg/ml, respectively; P < 0.05 in comparison to lysate). These observations suggest that B. ratti WR1 proteases are involved in the induction of IL-8 from T84 cells. Pretreatment of live B. ratti WR1 parasites with the antiprotozoal drug metronidazole significantly retarded the ability of the parasites to induce an increase in IL-8 production at 12, 24, and 48 h (111 ± 8, 289 ± 26, and 429 ± 15 pg/ml, respectively; P < 0.05).
FIG. 2.
Induction of IL-8 production in human intestinal epithelial T84 cells by B. ratti WR1. T84 cells were coincubated with B. ratti WR1 for 12, 24, and 48 h. The IL-8 concentrations in the supernatants were measured by ELISA. Live WR1 parasites and lysate induced significant increases in IL-8 production in a time-dependent manner (*, P < 0.05 versus the negative control for each time point). Use of a protease inhibitor cocktail reduced the WR1 lysate-induced IL-8 production significantly (**, P < 0.05 versus WR1 lysate). Pretreatment of live parasites with the antiprotozoal drug metronidazole significantly reduced the IL-8 production induced by live WR1 parasites (#, P < 0.05 versus live WR1 parasites). Treatment of cells with only protease inhibitor cocktail did not show any significant effect on the baseline production of IL-8. To confirm that IL-8 can be induced in T84 cells, purified cholera toxin was used as a positive control. The values are means ± standard deviations (n = 3).
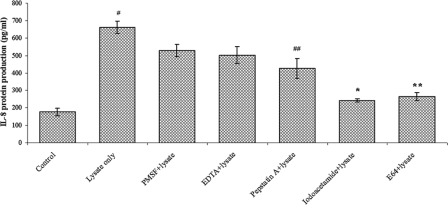
To study the particular type of protease involved, different protease inhibitors were used, and it was found that the cysteine protease inhibitors iodoacetamide (243 ± 9 pg/ml; P = 0.002) and E-64 (265 ± 23 pg/ml; P = 0.007) significantly inhibited IL-8 increase induced by parasitic lysates at 24 h (Fig. 3). A less significant reduction in IL-8 production was also seen with the aspartic protease inhibitor pepstatin A (426 ± 57; P = 0.048). Insignificant inhibition was noticed with serine and metalloprotease inhibitors, PMSF and EDTA, respectively. These findings suggest that B. ratti WR1 cysteine proteases are mainly responsible for the induction of increased IL-8 from T84 cells and that aspartic proteases also contribute to some extent to IL-8 production.
FIG. 3.
Effects of protease inhibitors on IL-8 production from T84 cells induced by B. ratti WR1. T84 cells were coincubated for 24 h with WR1 lysate pretreated with one of several protease inhibitors. Cysteine protease inhibitors, iodoacetamide and E-64, most significantly reduced WR1-induced IL-8 production from T84 cells (*, P = 0.002, and **, P = 0.007, versus WR1 lysate). Considerable reduction in IL-8 production was seen with the aspartic inhibitor pepstatin A (##, P = 0.04 versus WR1 lysate). Minimal inhibition was observed with serine and metalloprotease inhibitors, PMSF and EDTA, respectively. The values are means ± standard deviations (n = 3). #, P < 0.02 versus the control.
B. ratti WR1 cysteine proteases increase IL-8 mRNA levels in human colonic cells.
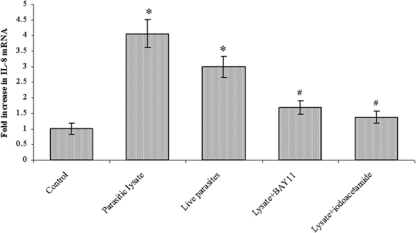
To determine whether B. ratti WR1 or its cysteine proteases could increase IL-8 mRNA expression, T84 cells were cocultured with B. ratti WR1 live parasites or lysate for 12 h. Total RNA extracted from the cells was used for real-time RT-PCR, and β-actin was used as a housekeeping gene. As shown in Fig. 4, there were marked increases in the expression of IL-8 mRNA in T84 cells after coincubation with B. ratti WR1 lysate (∼4-fold) and live parasites (∼3-fold). WR1 lysate-induced IL-8 mRNA expression levels were significantly inhibited when a cysteine protease inhibitor, iodoacetamide, was used (∼1.4-fold). To find out if the NF-κB pathway was activated upon exposure to B. ratti WR1, T84 cells were pretreated with an NF-κB inhibitor, BAY11-7082, and coincubated with parasite lysate. BAY11-7082 markedly decreased the B. ratti WR1-induced increase in mRNA levels (∼1.7-fold). Altogether, these findings indicated that B. ratti WR1 cysteine proteases are capable of increasing IL-8 mRNA in human colonic T84 cells with involvement of the NF-κB pathway.
FIG. 4.
B. ratti WR1 induces up-regulation of IL-8 mRNA levels in T84 cells. T84 cells were coincubated with live WR1 parasites or parasite lysate for 12 h. Total cellular RNA was isolated, and IL-8 and β-actin mRNA levels were measured by quantitative real-time RT-PCR as described in Materials and Methods. WR1 lysate caused a significant increase in IL-8 mRNA levels. A significant decrease in IL-8 mRNA levels was noticed after treatment with the NF-κB inhibitor BAY11-7082 and the cysteine protease inhibitor iodoacetamide. The increase in IL-8 mRNA relative to untreated T84 cells was calculated for each experiment based on internal β-actin controls. The values are means ± standard deviations (n = 3). *, P < 0.05 versus the control; #, P < 0.05 versus WR1 lysate.
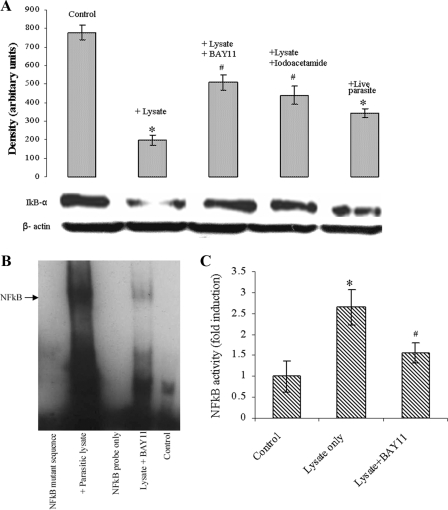
B. ratti WR1 degrades IκB-α and activates NF-κB.
One of the key pathways for NF-κB activation involves the phosphorylation of IκBs, which is followed by degradation of IκBs and subsequent translocation of NF-κB dimers from the cytoplasm to the nucleus. Therefore, we examined the kinetics of IκB-α degradation by Western blot analysis in T84 cells cocultured with live B. ratti WR1 parasites or lysate for 5 h. As shown in Fig. 5A, cells incubated with parasite lysate and live parasites showed significant degradation of IκB-α (74.7% and 55.8% degradation, respectively). Use of the cysteine protease inhibitor iodoacetamide decreased the B. ratti WR1 lysate-induced degradation of IκB-α (43.5%). Similarly, pretreatment of T84 cells with the NF-κB inhibitor BAY11-7082 also decreased the B. ratti WR1 lysate induced-degradation of IκB-α (34.7%).
FIG. 5.
B. ratti WR1 exposure to intestinal epithelial cells causes IκB-α degradation and induces NF-κB activation. (A) Live WR1 parasite or lysate coincubation with T84 cells resulted in IκB-α degradation at 5 h, as shown in a representative result with a β-actin loading conrol. The cell lysates were analyzed by Western blotting using an anti-IκB-α antibody. Pretreatment of the cells with the NF-κB inhibitor BAY11-7082 resulted in decreased IκB-α degradation. Iodoacetamide, a cysteine protease inhibitor, decreased the WR1 lysate-induced degradation of IκB-α. The histogram represents densitometry values expressed as arbitrary units ± standard deviations (SD). (B) A representative EMSA shows NF-κB/IL-8 promoter binding activity in nuclear extracts of T84 cells coincubated with WR1 lysate for 6 h. No NF-κB/DNA binding was observed in a mutant NF-κB oligoduplex incubated with nuclear extract, a wild-type NF-κB oligoduplex without nuclear extract, and the control. Pretreatment of T84 cells with the NF-κB inhibitor BAY11-7082 resulted in decreased WR1-induced NF-κB/DNA binding. (C) Histogram showing the increase in NF-κB activity in nuclear extracts of T84 cells. The cells were coincubated with WR1 lysate for 6 h, and NF-κB activity was measured by specific ELISA. The values are means ± SD (n = 3). *, P < 0.05 versus the control; #, P < 0.05 versus WR1 lysate.
To determine the NF-κB binding activity with the IL-8 promoter, oligonucleotide duplexes containing a wild-type NF-κB binding site in the IL-8 promoter were used in EMSA. Stimulation of T84 cells with B. ratti WR1 lysate for 6 h caused NF-κB/DNA binding activity in nuclear extracts of T84 cells (Fig. 5B). EMSA with a mutant NF-κB oligoduplex did not show any NF-κB/DNA binding. Decreased NF-κB/DNA binding activity was noticed when T84 cells were pretreated with the NF-κB inhibitor BAY11-7082. To measure the NF-κB p65 activity, nuclear extracts were subjected to a specific ELISA. As shown in Fig. 5C, coincubation of T84 cells with B. ratti WR1 lysate caused a significant increase in NF-κB p65 activity (P < 0.05). Pretreatment of T84 cells with the NF-κB inhibitor BAY11-7082 significantly inhibited the B. ratti WR1 lysate-induced increase in NF-κB p65 activity.
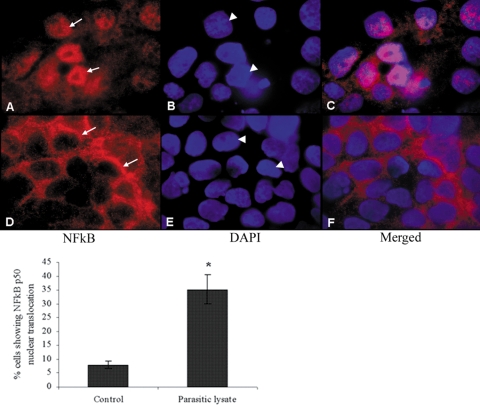
Nuclear translocation of NF-κB.
The most commonly found form of NF-κB is a heterodimer composed of the p50 and p65 subunits. In unstimulated cells, NF-κB resides in the cytosol in the inactive form bound to inhibitory IκB proteins. Various stimuli initiate a series of signaling events that culminate in the phosphorylation and degradation of IκBs. Activated free NF-κB translocates to the nucleus and stimulates transcription by binding to correlated κB sites in the promoter regions of various target genes. To visualize the nuclear translocation of NF-κB, we performed immunohistochemistry of T84 cell monolayers stimulated for 6 h with B. ratti WR1 lysates (Fig. 6). Monolayers were probed with rabbit anti-NF-κB p50 antibody, and a Cy3-conjugated sheep anti-rabbit secondary antibody was used for visualization. Nuclei were counterstained with DAPI to determine the cellular localization of p50. T84 cells exposed to B. ratti WR1 lysate showed nuclear translocation of the p50 subunit (Fig. 6C). Control cells showed a normal cytosolic localization of p50 (Fig. 6F). A significant increase (P < 0.05) in the percentage of cells showing nuclear translocation of NF-κB p50 was observed after exposure to B. ratti WR1 lysate (Fig. 6, histogram).
FIG. 6.
Representative micrographs showing nuclear translocation of NF-κB in intestinal epithelial T84 cells following exposure to B. ratti WR1. T84 cell monolayers were grown on collagen-coated glass coverslips and coincubated with WR1 lysate for 6 h. The monolayers were fixed and incubated first with rabbit anti-NF-κB p50 antibody and then with Cy3-conjugated sheep anti-rabbit secondary antibody. The monolayers were mounted using mounting medium containing DAPI to counterstain the nuclei and visualized by fluorescence microscopy. (A to C) T84 cells exposed to WR1 lysate showed nuclear translocation of the p50 subunit. (D to F) Control cells exhibited normal perinuclear localization of p50. All of the micrographs were obtained at a magnification of ×400. Arrows, p50 localization; arrowheads, nuclei. As shown in the histogram, a significant increase in the percentage of cells showing nuclear translocation of NF-κB p50 could be seen in cells exposed to WR1 lysate. The percentage of cells with NF-κB p50 nuclear translocation was calculated from observations of 200 cells in three independent experiments. The values are means ± standard deviations. *, P < 0.05 versus the control.
DISCUSSION
Evidence accumulated over the last decade suggests association of Blastocystis with gastrointestinal disorders (44, 49). Mostly inconclusive clinical and epidemiological studies implicated or exonerated Blastocystis in the etiology of disease. Unlike most other enteric pathogens, Blastocystis is essentially considered noninvasive and does not cause any consistent inflammatory response. However, it was observed that Blastocystis infections may cause gastrointestinal symptoms similar to irritable bowel syndrome involving diarrhea, abdominal pain, constipation, nausea, and fatigue (5, 45, 49, 50). Over the past few decades, major advances in the epidemiology, phylogeny, and cell biology of Blastocystis were made, but only a few studies addressed the parasite's virulence factors and host responses against Blastocystis.
We have recently reported the protease activity of Blastocystis hominis B (43) and also showed that B. hominis B and B. ratti WR1 proteases can degrade human secretory immunoglobulin A (36). We also reported that B. ratti WR1 induces contact-independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells (35). These studies suggested that B. ratti WR1 secretory products exert detrimental effects on host cells and warrant further investigations. Phylogenetic data suggest that Blastocystis is a zoonotic parasite, and animal-to-human transmission is common (32, 51). Intestinal inflammation and edema were reported in patients infected with Blastocystis (19, 21, 39, 54). In a murine model, intense infiltration of inflammatory cells into the colon and inflammation with edematous lamina propria in the cecum and colon were reported following Blastocystis infection (26). One in vitro study showed that Blastocystis may modulate the IL-8 response in intestinal epithelial cells (24). Taken together, these observations suggest that Blastocystis-induced intestinal inflammation is mediated by IL-8 recruitment of inflammatory cells and that some secretory parasite factors are responsible for this.
Our results from the protease profile study showed that B. ratti WR1 mainly contains cysteine proteases. The active-site thiol group of cysteine proteases can function only under reduced conditions (25), and because Blastocystis is an anaerobic enteric protozoan, these cysteine proteases may have important roles in its life cycle. Most other pathogenic protozoans, such as Entamoeba, Giardia, Trichomonas, Leishmania, Trypanosoma, and Plasmodium, are known to possess cysteine proteases (25, 33) that have been shown to be important for the development, differentiation, and pathogenicity of the parasites. These cysteine proteases can be promising chemotherapeutic or vaccine targets for many parasites (40). Based on protease activity, we observed that B. ratti isolate WR1 showed less cysteine protease activity than that reported for B. hominis isolate B (43). High protease activity has been reported in amebic clones of high virulence (17), and it would be interesting to study if high protease activity is associated with virulence in Blastocystis. Furthermore, cysteine proteases were suggested to play a role in the survival of Blastocystis in the gut (36).
Blastocystis is a polymorphic organism, and its recognized forms are vacuolar, granular, amoeboid, and cyst forms. The vacuolar form is the most common form observed in axenized cultures. It has a characteristic large central vacuole and a thin rim of peripheral cytoplasm (49). The exact role of the central vacuole in Blastocystis is not clear, but it has been suggested that it may act as a storage organelle for the deposition of apoptotic bodies during programmed cell death of the parasite (30). Other reports suggest that the central vacuole may have a role in schizogony-like reproduction (13, 48). Results from our study suggest for the first time that the central vacuole may also function as a reservoir for cysteine proteases.
It has been reported that Entamoeba histolytica cysteine proteases are involved in the production of IL-8 from intestinal epithelial cells and that these proteases also induce gut inflammation (53). Our results demonstrate that B. ratti WR1 induces IL-8 production from colonic epithelial T84 cells in a time-dependent manner. The use of protease inhibitors showed that cysteine proteases of B. ratti WR1 were mainly responsible for the induction of IL-8 production. Moreover, increased cytokine production was paralleled by increased gene transcription. Numerous pathogenic gastrointestinal pathogens, like E. histolytica (15), Cryptosporidium parvum (42), Salmonella, E. coli (14), and Vibrio cholerae (37), are known to induce IL-8 production from the intestinal epithelium. Intestinal epithelial cell production of IL-8 causes an influx of inflammatory cells into the intestinal mucosa with resultant tissue damage and gastrointestinal disturbances. It has been reported that invasion of the intestinal epithelium by pathogens is not necessary for the induction of inflammation (3), and since Blastocystis is a noninvasive parasite, secreted products from the parasite might initiate the inflammatory process by activating cell surface receptors. Although, E. histolytica is an invasive protozoan, it can also induce IL-8 secretion from human colonic epithelial cells without parasite-enterocyte contact (52). Similarly, Blastocystis proteases may initiate IL-8-mediated inflammatory processes that ultimately lead to gastrointestinal symptoms.
We observed that metronidazole treatment of live B. ratti WR1 parasites markedly reduced the induction of IL-8 production from T84 cells. Metronidazole induces programmed cell death in Blastocystis, and the plasma membrane integrity of the parasite is preserved (29); therefore, there is minimal leakage of intracellular parasite proteases and other products that could induce a high IL-8 response. However, a pronounced IL-8 response was observed when T84 cells were exposed to parasite lysates, as they contain all soluble or insoluble parasite products. We have previously shown that metronidazole can avert the adverse effects of B. ratti WR1 on the intestinal epithelial barrier function (35). Altogether, these findings suggest the therapeutic potential of metronidazole in Blastocystis infections.
In this study, we investigated the molecular mechanisms by which IL-8 gene expression is regulated in colonic epithelial cells after exposure to B. ratti WR1. First, we demonstrated that B. ratti WR1 can degrade IκB-α and that the use of a cysteine protease inhibitor markedly inhibited the degradation of IκB-α, suggesting the involvement of a parasite-derived cysteine protease. Secondly, our results from EMSA demonstrated that NF-κB is involved in the induction of IL-8 promoter activity in colonic epithelial cells. NF-κB plays a pivotal role in intestinal inflammation, and both invasive and noninvasive enteric pathogens can trigger the inflammatory cascade through activation of NF-κB (3). In most cells, including intestinal epithelial cells, activation of NF-κB is critical for inducible expression of the proinflammatory response genes. NF-κB-mediated induction of IL-8 has been reported in numerous enteric pathogens, like H. pylori (4), E. coli (10), and B. fragilis (22). Protozoan parasites, like E. histolytica (53) and C. parvum (9), are also capable of activating NF-κB in host cells. Results from our immunohistochemistry also support these findings and show nuclear translocation of NF-κB. The finding that B. ratti WR1 has the ability to activate NF-κB has important implications, since other NF-κB-responsive genes, like those for IL-1 and IL-6, may also be affected in Blastocystis infections, which may lead to altered intestinal homeostasis.
In summary, the present study demonstrates for the first time that B. ratti WR1 cysteine proteases induce IL-8 production in colonic epithelial T84 cells and that an NF-κB-dependent transcriptional process is involved. In addition, our findings show that metronidazole treatment can markedly inhibit the ability of B. ratti WR1 to induce IL-8 production. Furthermore, we demonstrated that the central vacuole of B. ratti WR1 may act as a reservoir for cysteine proteases. The findings of this study will certainly help us to understand the pathobiology of a poorly studied parasite whose public health importance is increasingly recognized.
Acknowledgments
This work was supported by a generous grant from the National Medical Research Council (NMRC/1071/2006).
We are grateful to Jing Zhou, Department of Community, Occupational and Family Medicine, for technical help and helpful discussions. We thank Tan Mui Hong and Tan Li Li, Defense Medical and Environmental Research Institute, DSO, for their contributions. We also thank Ng Geok Choo, Yin Jing, and N. P. Ramachandran for technical support.
Footnotes
Published ahead of print on 21 December 2007.
REFERENCES
- 1.Alexeieff, A. 1911. Sur la nature des formations dites kystes de Trichomonas intestinalis. C. R. Soc. Biol. 71296-298. [Google Scholar]
- 2.Barahona Rondon, L., C. Maguina Vargas, C. Naquira Velarde, I. A. Terashima, and R. Tello. 2003. Human blastocystosis: prospective study symptomatology and associated epidemiological factors. Rev. Gastroenterol. Peru. 2329-35. [PubMed] [Google Scholar]
- 3.Berkes, J., V. K. Viswanathan, S. D. Savkovic, and G. Hecht. 2003. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52439-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beswick, E. J., I. V. Pinchuk, K. Minch, G. Suarez, J. C. Sierra, Y. Yamaoka, and V. E. Reyes. 2006. The Helicobacter pylori urease B subunit binds to CD74 on gastric epithelial cells and induces NF-κB activation and interleukin-8 production. Infect. Immun. 741148-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boorom, K. F. 2007. Is this recently characterized gastrointestinal pathogen responsible for rising rates of inflammatory bowel disease (IBD) and IBD associated autism in Europe and the United States in the 1990s? Med. Hypotheses. doi: 10.1016/j.mehy. 2007.01.027. [DOI] [PubMed]
- 6.Borger, P., G. H. Koeter, J. A. Timmerman, E. Vellenga, J. F. Tomee, and H. F. Kauffman. 1999. Proteases from Aspergillus fumigatus induce interleukin (IL)-6 and IL-8 production in airway epithelial cell lines by transcriptional mechanisms. J. Infect. Dis. 1801267-1274. [DOI] [PubMed] [Google Scholar]
- 7.Brumpt, E. 1912. Blastocystis hominis n. sp. et formes voisines. Bull. Soc. Pathol. Exotique Filiates (Paris) 5725-730. [Google Scholar]
- 8.Charo, I. F., and R. M. Ransohoff. 2006. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354610-621. [DOI] [PubMed] [Google Scholar]
- 9.Chen, X. M., S. A. Levine, P. L. Splinter, P. S. Tietz, A. L. Ganong, C. Jobin, G. J. Gores, C. V. Paya, and N. F. LaRusso. 2001. Cryptosporidium parvum activates nuclear factor κB in biliary epithelia preventing epithelial cell apoptosis. Gastroenterology 1201774-1783. [DOI] [PubMed] [Google Scholar]
- 10.Dahan, S., V. Busuttil, V. Imbert, J. F. Peyron, P. Rampal, and D. Czerucka. 2002. Enterohemorrhagic Escherichia coli infection induces interleukin-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-κB and AP-1 in T84 cells. Infect. Immun. 702304-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388548-554. [DOI] [PubMed] [Google Scholar]
- 12.Doyle, P. W., M. M. Helgason, R. G. Mathias, and E. M. Proctor. 1990. Epidemiology and pathogenicity of Blastocystis hominis. J. Clin. Microbiol. 28116-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn, L. A., P. F. Boreham, and D. J. Stenzel. 1989. Ultrastructural variation of Blastocystis hominis stocks in culture. Int. J. Parasitol. 1943-56. [DOI] [PubMed] [Google Scholar]
- 14.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1993. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 614569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckmann, L., S. L. Reed, J. R. Smith, and M. F. Kagnoff. 1995. Entamoeba histolytica trophozoites induce an inflammatory cytokine response by cultured human cells through the paracrine action of cytolytically released interleukin-1 alpha. J. Clin. Investig. 961269-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Shazly, A. M., A. A. Abdel-Magied, S. N. El-Beshbishi, H. A. El-Nahas, M. A. Fouad, and M. S. Monib. 2005. Blastocystis hominis among symptomatic and asymptomatic individuals in Talkha Center, Dakahlia Governorate, Egypt. J. Egypt. Soc. Parasitol. 35653-666. [PubMed] [Google Scholar]
- 17.Espinosa-Cantellano, M., and A. Martinez-Palomo. 2000. Pathogenesis of intestinal amebiasis: from molecules to disease. Clin. Microbiol. Rev. 13318-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Florez, A. C., D. A. Garcia, L. Moncada, and M. Beltran. 2003. Prevalence of microsporidia and other intestinal parasites in patients with HIV infection, Bogota, 2001. Biomedica 23274-282. [PubMed] [Google Scholar]
- 19.Garavelli, P. L., L. Scaglione, A. Merighi, and M. Libanore. 1992. Endoscopy of blastocystosis (Zierdt-Garavelli disease). Ital. J. Gastroenterol. 24206. [PubMed] [Google Scholar]
- 20.Hailemariam, G., A. Kassu, G. Abebe, E. Abate, D. Damte, E. Mekonnen, and F. Ota. 2004. Intestinal parasitic infections in HIV/AIDS and HIV seronegative individuals in a teaching hospital, Ethiopia. Jpn. J. Infect. Dis. 5741-43. [PubMed] [Google Scholar]
- 21.Kain, K. C., M. A. Noble, H. J. Freeman, and R. L. Barteluk. 1987. Epidemiology and clinical features associated with Blastocystis hominis infection. Diagn. Microbiol. Infect. Dis. 8235-244. [DOI] [PubMed] [Google Scholar]
- 22.Kim, J. M., S. J. Cho, Y. K. Oh, H. Y. Jung, Y. J. Kim, and N. Kim. 2002. Nuclear factor-kappa B activation pathway in intestinal epithelial cells is a major regulator of chemokine gene expression and neutrophil migration induced by Bacteroides fragilis enterotoxin. Clin. Exp. Immunol. 13059-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leelayoova, S., R. Rangsin, P. Taamasri, T. Naaglor, U. Thathaisong, and M. Mungthin. 2004. Evidence of waterborne transmission of Blastocystis hominis. Am. J. Trop. Med. Hyg. 70658-662. [PubMed] [Google Scholar]
- 24.Long, H. Y., A. Handschack, W. Konig, and A. Ambrosch. 2001. Blastocystis hominis modulates immune responses and cytokine release in colonic epithelial cells. Parasitol. Res. 871029-1030. [DOI] [PubMed] [Google Scholar]
- 25.McKerrow, J. H., E. Sun, P. J. Rosenthal, and J. Bouvier. 1993. The proteases and pathogenicity of parasitic protozoa. Annu. Rev. Microbiol. 47821-853. [DOI] [PubMed] [Google Scholar]
- 26.Moe, K. T., M. Singh, J. Howe, L. C. Ho, S. W. Tan, X. Q. Chen, G. C. Ng, and E. H. Yap. 1997. Experimental Blastocystis hominis infection in laboratory mice. Parasitol. Res. 83319-325. [DOI] [PubMed] [Google Scholar]
- 27.Mori, N., K. Oishi, B. Sar, N. Mukaida, T. Nagatake, K. Matsushima, and N. Yamamoto. 1999. Essential role of transcription factor nuclear factor-κB in regulation of interleukin-8 gene expression by nitrite reductase from Pseudomonas aeruginosa in respiratory epithelial cells. Infect. Immun. 673872-3878. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Mukaida, N., S. Okamoto, Y. Ishikawa, and K. Matsushima. 1994. Molecular mechanism of interleukin-8 gene expression. J. Leukoc. Biol. 56554-558. [PubMed] [Google Scholar]
- 29.Nasirudeen, A. M., Y. E. Hian, M. Singh, and K. S. Tan. 2004. Metronidazole induces programmed cell death in the protozoan parasite Blastocystis hominis. Microbiology 15033-43. [DOI] [PubMed] [Google Scholar]
- 30.Nasirudeen, A. M., K. S. Tan, M. Singh, and E. H. Yap. 2001. Programmed cell death in a human intestinal parasite, Blastocystis hominis. Parasitology 123235-246. [DOI] [PubMed] [Google Scholar]
- 31.Nimri, L., and R. Batchoun. 1994. Intestinal colonization of symptomatic and asymptomatic schoolchildren with Blastocystis hominis. J. Clin. Microbiol. 322865-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noel, C., F. Dufernez, D. Gerbod, V. P. Edgcomb, P. Delgado-Viscogliosi, L. C. Ho, M. Singh, R. Wintjens, M. L. Sogin, M. Capron, R. Pierce, L. Zenner, and E. Viscogliosi. 2005. Molecular phylogenies of Blastocystis isolates from different hosts: implications for genetic diversity, identification of species, and zoonosis. J. Clin. Microbiol. 43348-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.North, M. J. 1982. Comparative biochemistry of the proteinases of eucaryotic microorganisms. Microbiol. Rev. 46308-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oppenheim, J. J., C. O. Zachariae, N. Mukaida, and K. Matsushima. 1991. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu. Rev. Immunol. 9617-648. [DOI] [PubMed] [Google Scholar]
- 35.Puthia, M. K., S. W. Sio, J. Lu, and K. S. Tan. 2006. Blastocystis ratti induces contact-independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells. Infect. Immun. 744114-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puthia, M. K., A. Vaithilingam, J. Lu, and K. S. Tan. 2005. Degradation of human secretory immunoglobulin A by Blastocystis. Parasitol. Res. 97386-389. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez, B. L., A. Rojas, J. Campos, T. Ledon, E. Valle, W. Toledo, and R. Fando. 2001. Differential interleukin-8 response of intestinal epithelial cell line to reactogenic and nonreactogenic candidate vaccine strains of Vibrio cholerae. Infect. Immun. 69613-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao, K., U. Sekar, K. T. Iraivan, G. Abraham, and P. Soundararajan. 2003. Blastocystis hominis—an emerging cause of diarrhoea in renal transplant recipients. J. Assoc. Physicians India 51719-721. [PubMed] [Google Scholar]
- 39.Russo, A. R., S. L. Stone, M. E. Taplin, H. J. Snapper, and G. V. Doern. 1988. Presumptive evidence for Blastocystis hominis as a cause of colitis. Arch. Intern. Med. 1481064. [PubMed] [Google Scholar]
- 40.Sajid, M., and J. H. McKerrow. 2002. Cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 1201-21. [DOI] [PubMed] [Google Scholar]
- 41.Scholze, H., and E. Tannich. 1994. Cysteine endopeptidases of Entamoeba histolytica. Methods Enzymol. 244512-523. [DOI] [PubMed] [Google Scholar]
- 42.Seydel, K. B., T. Zhang, G. A. Champion, C. Fichtenbaum, P. E. Swanson, S. Tzipori, J. K. Griffiths, and S. L. Stanley, Jr. 1998. Cryptosporidium parvum infection of human intestinal xenografts in SCID mice induces production of human tumor necrosis factor alpha and interleukin-8. Infect. Immun. 662379-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sio, S. W., M. K. Puthia, A. S. Lee, J. Lu, and K. S. Tan. 2006. Protease activity of Blastocystis hominis. Parasitol. Res. 99126-130. [DOI] [PubMed] [Google Scholar]
- 44.Sohail, M. R., and P. R. Fischer. 2005. Blastocystis hominis and travelers. Travel Med. Infect. Dis. 333-38. [DOI] [PubMed] [Google Scholar]
- 45.Stensvold, C. R., G. K. Suresh, K. S. Tan, R. C. Thompson, R. J. Traub, E. Viscogliosi, H. Yoshikawa, and C. G. Clark. 2007. Terminology for Blastocystis subtypes—a consensus. Trends Parasitol. 2393-96. [DOI] [PubMed] [Google Scholar]
- 46.Stenzel, D. J., and P. F. Boreham. 1996. Blastocystis hominis revisited. Clin. Microbiol. Rev. 9563-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun, Q., H. Matta, G. Lu, and P. M. Chaudhary. 2006. Induction of IL-8 expression by human herpesvirus 8 encoded vFLIP K13 via NF-κB activation. Oncogene 252717-2726. [DOI] [PubMed] [Google Scholar]
- 48.Suresh, K., J. Howe, G. C. Ng, L. C. Ho, N. P. Ramachandran, A. K. Loh, E. H. Yap, and M. Singh. 1994. A multiple fission-like mode of asexual reproduction in Blastocystis hominis. Parasitol. Res. 80523-527. [DOI] [PubMed] [Google Scholar]
- 49.Tan, K. S. 2004. Blastocystis in humans and animals: new insights using modern methodologies. Vet. Parasitol. 126121-144. [DOI] [PubMed] [Google Scholar]
- 50.Tungtrongchitr, A., S. Manatsathit, C. Kositchaiwat, J. Ongrotchanakun, N. Munkong, P. Chinabutr, S. Leelakusolvong, and W. Chaicumpa. 2004. Blastocystis hominis infection in irritable bowel syndrome patients. Southeast Asian J. Trop. Med. Public Health 35705-710. [PubMed] [Google Scholar]
- 51.Yoshikawa, H., K. Morimoto, Z. Wu, M. Singh, and T. Hashimoto. 2004. Problems in speciation in the genus Blastocystis. Trends Parasitol. 20251-255. [DOI] [PubMed] [Google Scholar]
- 52.Yu, Y., and K. Chadee. 1997. Entamoeba histolytica stimulates interleukin 8 from human colonic epithelial cells without parasite-enterocyte contact. Gastroenterology 1121536-1547. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, Z., L. Wang, K. B. Seydel, E. Li, S. Ankri, D. Mirelman, and S. L. Stanley, Jr. 2000. Entamoeba histolytica cysteine proteinases with interleukin-1 beta converting enzyme (ICE) activity cause intestinal inflammation and tissue damage in amoebiasis. Mol. Microbiol. 37542-548. [DOI] [PubMed] [Google Scholar]
- 54.Zuckerman, M. J., M. T. Watts, H. Ho, and F. V. Meriano. 1994. Blastocystis hominis infection and intestinal injury. Am. J. Med. Sci. 30896-101. [DOI] [PubMed] [Google Scholar]