Abstract
The role of Ca2+ signaling in triggering hypertrophy was investigated in neonatal rat cardiomyocytes in vitro. We show that an increase in cell size and sarcomere reorganization were elicited by receptor agonists such as Angiotensin II, aldosterone, and norepinephrine and by a small rise in medium KCl concentration, a treatment devoid of direct effects on receptor functions. All these treatments increased the frequency of spontaneous [Ca2+] transients, caused nuclear translocation of transfected NFAT(GFP), and increased the expression of a NFAT-sensitive reporter gene. There was no increase in Ca2+ spark frequency in the whole cell or in the perinuclear region under these conditions. Hypertrophy and NFAT translocation but not the increased frequency of [Ca2+] transients were inhibited by the calcineurin inhibitor cyclosporine A. Hypertrophy by the different stimuli was insensitive to inhibition of myofilament contraction. We concluded that calcineurin–NFAT can act as integrators of the contractile Ca2+ signal, and that they can decode alterations in the frequency even of rapid Ca2+ oscillations.
Cardiac hypertrophy accompanies many forms of heart pathologies, such as genetic or congenital defects, ischemia, and hypertension. The molecular signaling pathways through which the different hypertrophic stimuli modulate cardiac cell size include mitogen-activated protein kinase, Gp130/Stat3, Calmodulin (CaM)-dependent kinases, and the calcineurin-regulated pathway (1). The latter has recently received much attention, in part because it can be the target of therapeutic intervention with well known drugs. For example, in transgenic mice, it has been demonstrated that overexpression of the Ca2+-dependent phosphatase calcineurin causes a dramatic increase in the size of the heart, inhibited by cyclosporine A (CsA), a calcineurin blocker (2). Along the same line, overexpression of Cain/Cabin (molecules that associate with the calcineurin and inhibit its activity) attenuate cardiac hypertrophy caused not only by calcineurin overexpression but also by pressure overload or β-adrenergic receptor stimulation (3). The effect of CsA on cardiac hypertrophy, however, has led to contradictory results in different model systems (4, 5).
At the cellular level, cardiomyocyte hypertrophy is characterized by an increase in cell size, enhanced protein synthesis, activation of fetal genes, and cytoskeleton reorganization (4, 5). A number of treatments are known to induce cardiac cell hypertrophy in vitro, including angiotensin II (Ang II) (6), catecholamines (7), endothelin (8), and aldosterone (9). Many, but not all, of these hormones are known to be coupled to alterations in Ca2+ homeostasis. In particular, (i) Ang II (6) and endothelin (10) are coupled to IP3 generation and Ca2+ mobilization from stores; (ii) catecholamines, in particular through β1 receptors, are known to induce an increase in the frequency and amplitude of Ca2+ spiking in cardiomyocytes (7); and (iii) the mechanism of aldosterone-induced hypertrophy in vitro has not been clarified yet, but indirect evidence suggests that it may affect Ca2+ homeostasis (11, 12). Even admitting that many, if not all, hypertrophic agents modulate cardiac cell size via a modification of cellular Ca2+ handling, a question that remains open is how a hypertrophic Ca2+ signal can be discriminated from the repetitive Ca2+ rises underlying contractions. Differences in the amplitude, frequency, duration, or subcellular localization of the Ca2+ signals elicited by the hypertrophic agents may represent the biological code for such an action (13–15).
Here, we have addressed the problem of the molecular signaling pathway through which a variety of treatments induce cardiac cell hypertrophy in vitro and, in particular whether they exert their action through a modification of Ca2+ homeostasis and calcineurin activation. The capability of calcineurin to decode oscillatory Ca2+ signals is still a debated question. It has been shown that Ca2+ oscillations of tens of seconds are sufficient to activate calcineurin-dependent gene expression in cell lines (16–18). Regarding faster oscillatory patterns, such as those occurring in striated muscle, the situation is more complex: in skeletal muscle calcineurin-dependent NFAT activation/translocation depends on the frequency of the stimulation pattern (19); similarly, the high-frequency pacing of atrial cardiac preparations can induce calcineurin-dependent gene regulation (20). However, it is unclear whether calcineurin can indeed decode the frequency of Ca2+ oscillations or rather is activated by the prolonged increases of intracellular [Ca2+], [Ca2+]i, after high-frequency stimulation. Our data demonstrate that a variety of hypertrophic agents modulate the frequency of spontaneous Ca2+ oscillations. By monitoring [Ca2+]i, NFAT translocation and/or activation of a calcineurin gene reporter, we here demonstrate that calcineurin can act as an integrator of the single rapid Ca2+ transients occurring in spontaneously beating cardiomyocytes in vitro, eventually resulting in the translocation of NFAT into the nucleus and the downstream activation of the hypertrophic program.
Results
Confirming previous results, we found that incubation of neonatal cardiomyocytes in serum-free medium with Ang II caused a time-dependent increase in cell area and a reorganization of the cytoskeleton [supporting information (SI) Fig. 6]. The unsolved and most important question, however, is the signaling pathway through which this and other hypertrophic stimuli exert their effect. A large body of data indicates that with a variety of agents, including Ang II, changes in Ca2+ homeostasis are pivotal in the hypertrophy of cardiac cells both in vitro and in vivo (21).
To measure the dynamics of intracellular Ca2+ concentration in the cytosol, we used the classical fluorescent Ca2+ indicators fura-2 and fluo-3. As shown in SI Fig. 7, we found that, under standard conditions, both indicators caused clear phototoxic effects in cardiomyocytes. Conditions can be found, however, where the light-induced damage can be reduced to a minimum (see SI Text), and these conditions were used for all of the after experiments.
In unstimulated cells, two types of [Ca2+] behavior were observed: in >90% of the cells there were spontaneous [Ca2+] transients, accompanied by contractions; in the remaining cells, [Ca2+] was stable and no contractions were visible (Fig. 1A). In cells undergoing spontaneous contractions, the Ca2+ increase caused by Ang II was small and superimposed on the Ca2+ oscillations, whereas in nonoscillating cells the Ca2+ peaks were much larger and more prolonged (Fig. 1A). The question is whether the nonbeating cells represent contaminating nonmyocytes or cardiomyocytes that exhibit no spontaneous action potentials. The cultures were thus on purpose enriched in fibroblasts and other noncardiomyocytes, loaded with fluo-3 and (i) the cells were challenged with high K+ (40 mM) to depolarize the plasma membrane and thus fully activate voltage-operated Ca2+ channels, if present; (ii) after stimulation with Ang II the monolayer was fixed and stained with antibodies against cardiac α-actinin; the staining pattern (Fig. 1C) was compared with the Ca2+ signaling behavior of the individual cells (Fig. 1B). The results of these experiments demonstrate that the cells that did not show spontaneous Ca2+ oscillations did not show any significant Ca2+ increase upon K+ challenge (unlike beating cells) (data not shown); cells that did not oscillate were also negative for the cardiac isoform of α-actinin (10/10) (Fig. 1 B and C). In both cell types, the effect of Ang II on [Ca2+] was prevented by ZD7155 (data not shown).
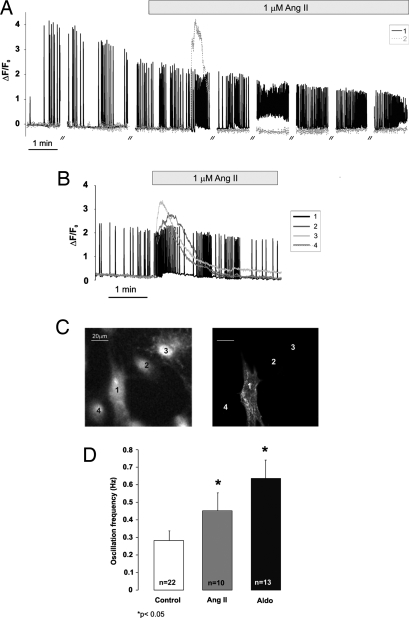
Fig. 1.
Acute effects of Ang II and aldosterone on [Ca2+]i. (A) The fluorescence changes of two cells, typical of the behavior of many other cells analyzed, loaded with fluo-3 are plotted as ΔF/F0, where F0 is the value of fluorescence at time 0, and ΔF is the change in fluorescence intensity at any given time. // indicates an interruption of the illumination for 2 min. (B) These four cells were obtained from a culture on purpose enriched in noncardiomyocytes. Where indicated, Ang II (1 μM) was added. Other conditions were as in A. (C) At the end of the imaging experiment, the cells whose Ca2+ changes are shown in B were fixed and permeabilized on the microscope stage and then immunostained with an anti-α actinin antibody. (D) The mean frequency of spontaneous Ca2+ oscillations was monitored in cardiac cells after 48-h incubation in medium supplemented with either 1 μM Ang II or 1 μM aldosterone. Data are means ± SEM of different independent experiments (the number of experiments is indicated in each column); for each experiment, at least 20 randomly chosen single cells were measured. *, P < 005 vs. control.
Two types of spontaneous Ca2+ oscillation patterns were observed: one regular in terms of frequency (in 79% of the cells analyzed) and the other characterized by bursts of activity followed by periods of rest of variable duration, from 10 s to 1 min (SI Fig. 7 B and C, respectively). On average, the Ca2+ oscillation frequency of control cells was ≈0.3 Hz (0.28 ± 0.06; n = 22). Addition of Ang II induced the small transient increase of basal [Ca2+] in 55.15% of the spontaneously oscillating cells (91/165, 19 experiments). A closer look at the effect of Ang II on [Ca2+] dynamics in bona fide cardiomyocytes indicated that the peptide also caused an increase in the frequency of Ca2+ oscillations that initially superimposed on the transient increase in diastolic Ca2+ (when present). The increase in Ca2+ oscillation frequency in some cells continued for the period of observation (>5 min; see for example Fig. 1A). In other cells, the Ca2+ oscillation frequency returned to the original value after 3–5 min (see for example Fig. 1B), and then oscillations stopped. This latter behavior is attributable to photoxicity, because closely located nonilluminated cells continued to beat. The effect of Ang II was prolonged, because an increase in the mean oscillation frequency was observed in cells analyzed after 48 h of continuous treatment with Ang II (Fig. 1D). Of note, in oscillating cells that did not show the small transient rise in diastolic [Ca2+], the increase in spike frequency was almost always observed (>90%).
The effect on Ca2+ oscillation frequency of two other in vitro hypertrophic agents was tested, aldosterone and norepinephrine (NE). NE caused, as expected, a rapid increase in both the frequency and amplitude of Ca2+ oscillations, whereas aldosterone had no acute effect on Ca2+ dynamics (data not shown). However, prolonged incubation with 1 μM aldosterone (Fig. 1D) resulted in a clear increase in oscillation frequency, from 0.28 ± 0.06 (n = 22) in controls to 0.62 ± 0.12 (n = 13) in cells treated with the hormone (P < 0.05). This effect was inhibited not only by a classical mineralcorticoid receptor antagonist (spironolactone), but also by ZD7155, suggesting that somehow aldosterone affects Ca2+ homeostasis via the type 1 Ang II receptor, AT1R (data not shown and see Fig. 3).
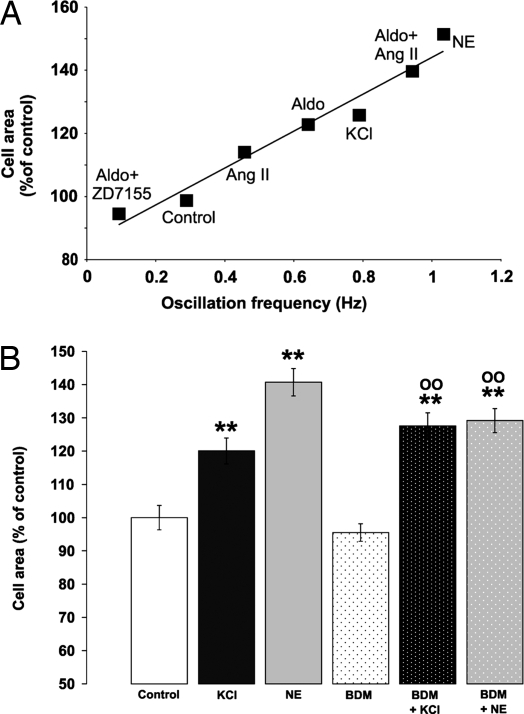
Fig. 3.
Correlation between Ca2+ oscillation frequency, cell area, and effects of BDM. (A) Cells were incubated for 48 h with different stimuli: Ang II (1 μM), aldosterone (1 μM), alone or preincubated with 10 μM ZD7155, KCl (10 mM), and NE (1 μM). For each condition, oscillation frequency and cell area were measured in the same samples. Represented is the mean of at least three independent experiments, with at least 20 cells per experiment. (B) Neonatal cardiomyocytes were incubated in serum-free medium supplemented with 5 mM KCl (final concentration, 10 mM) in the presence or absence of 7.5 mM BDM for 48 h. Cells were fixed, permeabilized, stained with TRITC phalloidin, and examined by fluorescence microscopy. The data presented are expressed as percentage of control and are the means ± SEM of three independent experiments (ncells = 250 for each treatment). **, P < 0.005 vs. control; 00, P < 0.005 vs. BDM.
The question thus arises whether the key event in the hypertrophy induced by Ang II is the transient increase in diastolic [Ca2+], or whether it is due to the prolonged increase in Ca2+ oscillation frequency caused by this agent. To address this question, cells were treated with Ang II for 5 min, washed and incubated for 48 h with an AT1R antagonist, ZD7155. The area of the cells treated in this way was slightly smaller than that of controls (87.08 ± 2.27%; nexp = 5, ncell = 279) and the oscillation frequency, measured 10 min or 48 h after Ang II, was indistinguishable from controls.
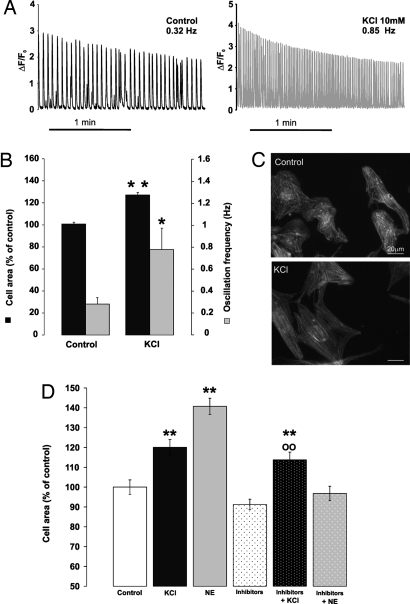
Hypertrophic agents such as Ang II and NE control complex signal transduction pathways, i.e., G proteins, kinases, and phosphatases or, in the case of aldosterone, directly activate nuclear gene expression. It is possible that downstream effects, other than changes in Ca2+ oscillation frequency, may be common among these stimuli and thus responsible for inducing cell hypertrophy. Therefore, we used a protocol that should affect only the frequency of Ca2+ oscillations, without perturbing membrane or intracellular receptors. Cells were thus incubated in media where KCl concentration was doubled (to 10 mM), causing a partial depolarization of the plasma membrane and an increase in the frequency of spontaneous Ca2+ oscillations. Measurement of the membrane potential by patch pipette (in the current clamp mode) revealed that the membrane potential of the cells in normal medium (5 mM KCl) was −70 ± 5 mV and decreased to −55 ± 5 mV in a medium containing 10 mM KCl. The incubation of cardiomyocytes in medium containing 10 mM KCl caused a rapid increase in the Ca2+ oscillation frequency (>2-fold; Fig. 2 A and B), and this increase persisted during the whole incubation time. Myocytes treated for 48 h with 10 mM KCl appeared conical-shaped, and their cell areas increased by ≈25% (126.19 ± 1.73%; ncell = 294, nexp = 14; P < 0.005) compared with controls; the myofilaments presented a more regular organization (Fig. 2C). The hypertrophic effect of KCl was not modified by a mixture of inhibitors, such as the AT1R inhibitor ZD7155, β and α adrenergic antagonists (Fig. 2D), but was completely prevented by the inhibitor of voltage-gated Ca2+ channel verapamil (see below).
Fig. 2.
A rise in extracellular KCl increases Ca2+ oscillation frequency and cell size and causes sarcomere reorganization. The incubation medium was supplemented with 5 mM KCl (final concentration 10 mM). (A) Other conditions were as in Fig. 1. On the left is the fluorescent trace of a typical control cell; on the right is that of a cell incubated in medium containing 10 mM KCl. (B) Mean ± SEM of the oscillation frequency (gray columns; control, nexp = 22; KCl, nexp = 4, >5 cells analyzed per experiment) and cell area (black columns; control: nexp = 16, ncell = 367; KCl: nexp = 14, ncell = 294). *, P < 0.05; **, P < 0.005 vs. control. (C) Representative images of sarcomere organization (labeling with TRITC phalloidin) in controls and cells treated with 10 mM KCl. (Scale bars, 20 μm.) (D) Mean ± SEM of cell area of cardiomyocytes incubated for 48 h with KCl (10 mM) or NE (1 μM), alone or preincubated with a mixture of receptor inhibitors: α,β adrenoreceptor inhibitors and AT1 receptors antagonist (10 μM Prazosin, 100 nM ICI118,51, 300 nM CGP20712A, and 10 μM 10 μM ZD7155, respectively) (nexp = 3, ncells = 250 for each treatment). **, P < 0.005 vs. control; 00, P < 0.005 vs. inhibitors.
The hypertrophic effect of NE and its inhibition by β adrenoreceptor antagonists are presented for comparison in Fig. 2D. An increase of 5 mM in NaCl concentration had no effect on either Ca2+ oscillation frequency or cell size (data not shown). In the graph presented in Fig. 3A, we compared the hypertrophic and chronotropic effects of Ang II, NE, aldosterone (with and without ZD7155), aldosterone +Ang II, and KCl. It should be stressed that, despite the very different signaling pathways modulated by these stimuli, a striking linear relationship (r2 = 0.9629) between these two parameters is observed.
It is known that cardiac myocytes show spontaneous local increases in cytoplasmic Ca2+, named Ca2+ sparks (22). Wu et al. (23) recently suggested that local Ca2+ events, in particular those occurring in the perinuclear region, have a primary effect on gene expression in adult cardiomyocytes. However, they did not measure Ca2+ sparks directly. We thus used line-scan confocal microscopy to investigate whether hypertrophic stimuli had any effect on the frequency and localization of Ca2+ sparks in the neonatal cardiomyocyte preparation. Table 1 shows that the overall frequency of sparks was not significantly affected by Ang II and was actually depressed slightly in the presence of 10 mM KCl. Moreover, there were no significant increases in spark frequency in any of the subcellular regions examined, and the nuclear and perinuclear regions exhibited the lowest spark frequencies under all conditions (Table 1). The intracellular distribution of Ca2+ sparks measured from 2D confocal image time series also showed no increase in spark density after Ang II or KCl treatment (SI Fig. 8). These Ca2+ spark maps are also consistent with the data of Table 1 in showing higher spark frequency at the cell periphery than in the nuclear and perinuclear regions. Finally, we examined the spatial and temporal properties of the Ca2+ sparks and again found no evidence for enhanced signal generation by Ca2+ sparks in the presence of the hypertrophic stimuli (SI Table 2).
Table 1.
Effect of Ang II and elevated extracellular KCl on Ca2+ spark frequency
| Spark frequency (no. of sparks per 100 μm/s) |
|||||
|---|---|---|---|---|---|
| Whole cell | Cell periphery | Cytoplasmic | Perinuclear | Nuclear | |
| Control | 2.43 ± 0.14 | 3.87 ± 0.37 | 2.68 ± 0.25 | 1.96 ± 0.34 | 0.59 ± 0.18 |
| Ang II, 1 μM | 2.36 ± 0.18 | 4.13 ± 0.55 | 2.58 ± 0.27 | 1.37 ± 0.31 | 0.22 ± 0.13 |
| KCl, 10 mM | 1.78 ± 0.22* | 3.25 ± 0.60 | 2.33 ± 0.57 | 1.07 ± 0.32 | 0.07 ± 0.05 |
Neonatal cardiac myocytes were treated with the indicated agonists for 10 min prior to recording Ca2+ sparks from line-scan images. Spark frequency is expressed as the number of sparks normalized to 100-μm scan-line length and 1-s duration. Data are mean ± SEM. Whole cell refers to all sparks recorded in each cell for the following number of cells analyzed: Control, 83; Ang II, 39; KCl, 41. Spark data for subcellular regions were obtained from the same cells with at least 20 cells for each region (scan line did not pass through all regions in all cells).
*, P < 0.05 vs. control.
All of the agents tested, in addition to causing an increase in Ca2+ oscillation frequency, also increase either the force and/or frequency of contraction. The possibility that the hypertrophic effect is somehow mediated by a mechanochemical coupling was investigated by treating the cells with 2,3-butanedione monoxime (BDM), an excitation–contraction uncoupler (24). Incubation with 7.5 mM BDM, a dose that inhibits myofilament contraction, while leaving Ca2+ oscillations unaffected had no effect on the hypertrophic effects of KCl or NE (Fig. 3B).
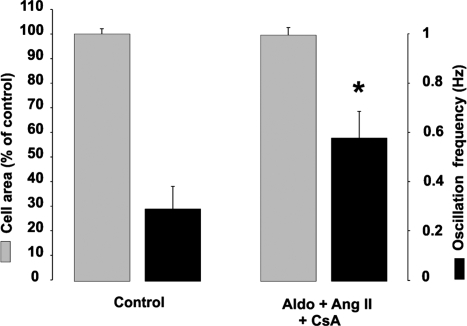
The Ca2+-dependent phosphatase calcineurin has been at the center of major interest as a key transducer in the Ca2+-activated signaling pathway, particularly in the field of heart hypertrophy (5, 25, 26). A major, although not unique, pathway regulated by calcineurin is that involving the transcription factor NFAT, which, upon dephosphorylation, translocates to the nucleus and eventually results in the transcription of a number of genes. To determine whether calcineurin is involved in the hypertrophic effect of the different treatments described above, cells stimulated with Ang II were treated with CsA, the classical inhibitor of calcineurin. The increase in cell area caused by the stimulus was completely blocked, whereas the frequency of Ca2+ oscillations remained comparable to that of cells incubated with Ang II only (Fig. 4). CsA was also able to prevent the hypertrophic effect induced by KCl, NE and aldosterone, again without affecting the changes in Ca2+ oscillation frequency induced by these agents (data not shown).
Fig. 4.
Translocation of NFATc4-GFP into the nucleus and effects of CsA. Conditions were as in SI Fig. 7. When present, CsA was 1 μM. Other conditions were as in SI Fig. 6. The results are mean ± SEM of independent experiments. Cell area: control, nexp = 3, ncell = 192; aldosterone + Ang II + CsA: nexp = 3, ncell = 178. Oscillation frequency: control, nexp = 5, ncell = 5; aldosterone + Ang II + CsA: nexp = 9, ncell = 9. *, P < 0.05 vs. aldosterone + Ang II.
To more directly address the involvement of calcineurin–NFAT, cells were transfected with NFAT-GFP, and its subcellular localization in control and treated cells was compared (SI Fig. 9). Quantitation of such results indicated that the percentage of unstimulated cardiomyocytes with NFAT-positive nuclei was 12% (14/140 cells in six different experiments); these data are in agreement with previous studies (27). In cells treated with aldosterone for 20 h, the number of cells containing NFAT-GFP in the nucleus increased to 59% (142 of 245 cells, in 11 different experiments). Similarly, the number of cells with NFAT-GFP-positive nuclei increased to 47%, 45%, and 58% in cells treated for 4 h with Ang II (1 μM), NE (1 μM), and KCl (10 mM), respectively (average of three to five experiments for each condition). In cells artificially paced for 3 h at a frequency of 3.5 Hz, 72% of the nuclei were positive for NFAT-GFP. Translocation of NFAT-GFP was inhibited by pretreatment with 1 μM CsA (data not shown). The time course of KCl-induced NFAT-GFP translocation was followed in more detail. SI Fig. 10 shows that a significant translocation of NFAT is observed after 1 h of treatment with KCl and increased by almost 3-fold after 5-h treatment.
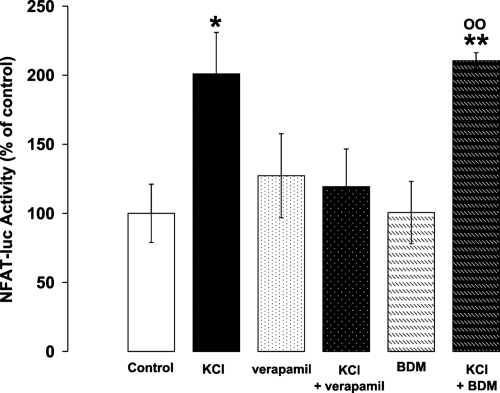
The final question is whether endogenous NFAT translocation to the nucleus is sufficient to activate the transcription of downstream gene targets. To this end, cells were transfected with the reporter gene luciferase engineered to include an NFAT-sensitive element at the 5′ end (27). Fig. 5 shows that the expression of luciferase was increased on average 2-fold by 10 mM KCl, and this increase was not affected by inhibition of contraction with BDM, whereas it was completely suppressed by an inhibitor of voltage-gated Ca2+ channels such as verapamil. The increase in luciferase expression by KCl was unaffected by a mixture of different receptor inhibitors (ZD71055 to inhibit AT1RR; and prazosin, ICI, and CGP to block α, β1, and β2 receptors, respectively). Similar results were obtained with the other hypertrophic stimuli (data not shown).
Fig. 5.
NFAT transcriptional responses upon treatment with 10 mM KCl. 9xNFAT-TATA luciferase transfected neonatal cardiomyocytes (27) were incubated in serum-free medium for 16 h. Where indicated, the medium was supplemented with 5 mM KCl (final concentration, 10 mM), 10 μM verapamil, or 7.5 mM BDM. NFAT-luciferase expression was measured as described in Materials and Methods. The data presented are means ± SEM of at least three independent experiments, each condition tested in triplicate (*, P < 0.05; **, P < 0.001 vs. control; 00, P < 0.001 vs. BDM).
Discussion
Cardiac hypertrophy is a highly complex phenomenon that can be activated by physiological and pathological conditions. The molecular mechanisms underlying the transformation of physiological into pathological cardiac hypertrophy are the subject of very intense investigation, given that hypertrophy, followed by fibrosis, is the major cause of heart failure in the world.
Results obtained in cardiomyocyte cell cultures from neonatal animals are obviously difficult to extrapolate to the in vivo adult situation, because the number, repertoire, and coupling mechanism of different receptors may vary between adult and neonatal cells. The latter model system, however, allows an easier molecular dissection of the mechanism of action of different agents, without interference from effects at the organism level.
Regarding the molecular mechanisms linking Ang II to hypertrophy and cytoskeletal rearrangements, strong evidence supports the idea that changes in cytoplasmic [Ca2+] play an essential role in hypertrophic signaling in the heart (26, 28, 29). Indeed, a fundamental role of extra- and intracellular Ca2+ in acute cytoskeleton reorganization by Ang II has been uncovered (30).
Surprisingly, there are few and often contradictory studies on the effects of Ang II on cardiomyocyte Ca2+ homeostasis. In particular, some authors have provided evidence that Ang II decreases [Ca2+] transients in cultured neonatal cardiomyocytes (31), whereas others showed intracellular [Ca2+] increases after Ang II stimulation (32). In light of the data presented in Fig. 1 and SI Fig. 7A, these contradictory results could be explained by the occurrence of technical problems, such as toxicity of the fluorophore or contaminant nonmyocytes. Differences in culture conditions and/or in the method of Ca2+ measurements may explain some discrepancies. We here show unambiguously that, in bona fide neonatal cardiomyocytes in culture, the primary effect of Ang II is a modest rise of the diastolic Ca2+ level lasting 1–2 min.
One thus wonders how such a minor effect on [Ca2+], particularly in a cell that continuously undergoes spontaneous large changes in cellular Ca2+ levels tens of times per minute, could have such dramatic and long-term effects on cell size (and cytoskeletal organization). Indeed, addition of ZD7155 5–10 min after Ang II resulted in the complete blocking of hypertrophy, indicating that a prolonged occupancy of the receptors is necessary to fully activate the hypertrophic program. However, we found that treatment with Ang II had an unpredicted long-term effect on cardiomyocyte Ca2+ handling, i.e., it increased the frequency of their spontaneous Ca2+ oscillations. The molecular mechanism of this positive chronotropic effect is presently not yet clear, but it is long-lasting, because it is observable also in cells treated with Ang II for 24/48 h. Other hypertrophic agents, such as NE and aldosterone, also cause an increase in oscillation frequency. Although the positive chronotropic effect of NE has been extensively investigated, the effect of aldosterone was somewhat unexpected. The hormone effect on Ca2+ oscillation frequency requires several hours to be appreciated and, based on the inhibitory effect not only of spironolactone but also of ZD7155, it requires the stimulation of both the mineralcorticoid receptor and of AT1R (M.C. and T.P., unpublished results).
The final but key question is the identification of the signaling pathway downstream Ca2+ oscillation frequency that leads to activation of the hypertrophy program. In the field of cardiac hypertrophy, much attention has been devoted in the last few years to kinases and phosphatases controlled by CaM. Indeed, overexpression of CaM has been reported to induce hypertrophic growth of cardiomyocytes in transgenic mice (33). The Ca2+/CaM complex exerts its functions through downstream effectors, such as Ca2+/CaM-dependent protein kinases (CaMKs), and calcineurin, a Ca2+/CaM-activated serine-threonine phosphatase. Calcineurin activation by Ca2+ results in dephosphorylation of the transcription factor NFAT in the cytosol, followed by its translocation into the nucleus and subsequent transcription of a number of cardiac genes (2, 5, 29). The idea that calcineurin (and NFAT) is primarily involved in cardiac hypertrophy in humans, however, is not shared by all investigators in the field, and this remains a hot topic of debate.
We here addressed the hypothesis that the increased frequency of Ca2+ oscillations, if prolonged for hours, is sufficient to substantially activate the calcineurin/NFAT pathway and eventually turns it into a hypertrophic signal. Indeed, not only were all of the hypertrophic effects of Ang II, NE, and KCl inhibited by CsA, but, more importantly, all these stimuli resulted in a substantial, CsA-sensitive, translocation of NFAT into the nucleus. Even simple and direct ways of changing the beating frequency (i.e., pacing of the cells with an extracellular electrode or increases of the extracellular KCl concentration) resulted in an efficient translocation of NFAT. Is it noteworthy that these stimuli activated the expression of a transfected reporter gene under the control of endogenous NFAT, and such an activation was insensitive to the mechanical activity.
The question thus arises of the molecular mechanism of such NFAT activation, because of the very brief duration of each single Ca2+ spike in cardiac cells. In previous reports, translocation of NFAT into the nucleus has been obtained by either sustained increases in cellular Ca2+ levels (34) or oscillatory Ca2+ increases, yet of several tens of seconds duration (16, 17), i.e., 10–100 times more prolonged than that of a single Ca2+ oscillation in cardiac cells. More recently, however, it was shown that pacing of atria at high frequency (>4 Hz) results in a substantial activation of NFAT-dependent gene expression (20). Under such conditions, however, the high-frequency pacing resulted in a large increase of diastolic Ca2+. Under our experimental conditions, up to a frequency of 2 Hz, the increase in diastolic Ca2+ level is negligible, yet substantial NFAT translocation is observed after a few hours. It can be argued that in the 100/200-ms duration of a single Ca2+ oscillation, there is no time for a significant dephosphorylation of NFAT and its translocation into the nucleus. We here propose that calcineurin/NFAT act as integrators of the Ca2+ signal. During a single Ca2+ oscillation of ≈200-ms duration, one may expect a very minor fraction of NFAT to be dephosphorylated by calcineurin. If the interval between two Ca2+ spikes is sufficiently long, this minute dephosphorylated fraction of NFAT will be rephosphorylated during diastole, and no net transfer of NFAT into the nucleus will occur. If the interval between two successive Ca2+ spikes is shortened, the balance between phosphorylation/dephosphorylation will be shifted toward the second situation, and dephosphorylated NFAT will tend to slowly accumulate. If such even marginal imbalance is repeated tens of times every minute, eventually, after hours, a significant fraction of NFAT will end up in the nucleus, as observed. This simple kinetic scheme not only has the advantage of explaining NFAT translocation as a function of Ca2+ oscillation frequency, but it can also be applied to stimuli that may affect the amplitude rather than the frequency of Ca2+ spikes. In this case, a larger Ca2+ increase will activate more calcineurin molecules and, even if minute, the shift toward a more dephosphorylated NFAT will tend to accumulate if the episodes occur thousands of times in the same cells.
A similar model, named “integrative tracking,” has been proposed by Berridge et al. (13–15). The authors proposed that each Ca2+ transient has a small effect on itself, but individual transients can be integrated over time to provide a significant change in some cellular process. They identified the NFAT shuttle as a typical example of “integrative tracking.” The present data provide experimental support to the model.
An alternative possibility would be that local Ca2+ increases, in particular those occurring close to the nucleus, might be part of this integrative tracking system. Wu et al. (23) showed that endothelin-1 enhances hypertrophic gene transcription in ventricular myocytes via CaM Kinase II phosphorylation of HDAC. The authors suggested that this effect depends on IP3-mediated mobilization of Ca2+ from the nuclear envelope, although they were unable to detect local nuclear Ca2+ increases (23). In our studies, Ca2+ sparks were observed throughout the cell during periods between Ca2+ transients, including in the perinuclear region, although they were rare inside the nuclear matrix. More importantly, the translocation of NFAT into the nucleus in response to either Ang II or KCl was not associated with any increase in nuclear or perinuclear Ca2+ spark frequency, and these agents did not affect the spatial or temporal spreading of Ca2+ sparks. Thus, it appears that cytosolic Ca2+ transients, rather than localized perinuclear or nuclear Ca2+ signals, are responsible for the regulation of NFAT phosphorylation and hence translocation into the nucleus.
It can be argued that the spontaneous activity of the cultures used in this study (0.3–0.5 Hz) is much lower than that of the physiological beating rate of rat heart (3–4 Hz). However, in the kinetic scheme proposed above, it is not difficult to hypothesize that, by adapting the activity and/or level of the NFAT kinases to the activity of calcineurin, cells can adapt to a higher frequency of beating, maintaining NFAT in a largely phosphorylated state under nonstimulated conditions. In addition, the kinetic scheme predicts that short-duration increases in the beating frequency will not result in a substantial translocation of NFAT into the nucleus. The key aspect of the model is that the calcineurin/NFAT pathway has intrinsically the ability to perceive and transduce into NFAT activation the frequency (or amplitude) of Ca2+ oscillations, independently of the duration of the single episode of Ca2+ increase. A kinetic scheme of this type could, in theory, be hypothesized for other Ca2+-dependent phosphorylation/dephosphorylation processes that involve translocation across the nuclear membrane of transcriptonal regulators, e.g., HDAC and CaM kinases (35, 36), i.e., other signaling pathways that can lead to cardiac cell hypertrophy. Finally, a similar mechanism may apply to the activation of the slow fiber-type program in skeletal muscle (that depends on NFAT translocation to the nucleus) (19), where the single episode of Ca2+ rise can be repeated several hundreds/thousands of times, depending on the frequency and pattern of the motor nerve impulse.
Materials and Methods
Cardiomyocyte Culture.
Cultures of cardiomyocytes were prepared from ventricles of neonatal Wistar rats (0–2 d after birth), as described (37). On the first day of culture, cells were washed and thereafter maintained in serum-free medium until used.
cDNA Constructs and Transfection.
The NFATc4-GFP plasmid, a gift from Chi-Wing Chow (Department of Molecular Pharmacology, Albert Einstein College of Medicine, Bronx, NY) and 9xNFAT-TATA luciferase plasmid, a kind gift from J. D. Molkentin (27), were transfected on the second day of culture by using the FuGENE 6 Transfection Reagent (Roche), as described in ref. 37. For a 24-mm diameter coverslip, 3 μg of DNA was mixed with 6 μl of FuGENE (37). All experiments were performed 1 d after transfection.
Ca2+ Measurements.
See SI Text.
Statistical Analysis.
Data are reported as mean ± SEM of independent experiments or, in cell-area determination, of different cells. Statistical differences were evaluated by one-tailed Student's t test, a P value <0.05 being considered statistically significant.
Materials.
All chemicals were of analytical or highest available grade and, unless otherwise stated, were obtained from Sigma. ZD7155 was from Tocris; fura-2 a.m. and fluo-3 a.m. were from Molecular Probes. When dimethyl sulfoxide or ethanol was used as solvents, the final solvent concentration never exceeded 0.01% or 0.1%, respectively.
Supplementary Material
ACKNOWLEDGMENTS.
This work was supported by grants from the Italian Telethon, the Italian Association for Cancer Research, and the Veneto Region (Biotech 2) (T.P.), and by National Institutes of Health Grant AA014980 (to A.P.T.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812316105/DC1.
References
- 1.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 2.Molkentin JD, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Windt LJ, et al. Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc Natl Acad Sci USA. 2001;98:3322–3327. doi: 10.1073/pnas.031371998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 5.Molkentin JD. Calcineurin and beyond: cardiac hypertrophic signaling. Circ Res. 2000;87:731–738. doi: 10.1161/01.res.87.9.731. [DOI] [PubMed] [Google Scholar]
- 6.Sadoshima J, Qiu Z, Morgan JP, Izumo S. Angiotensin II, other hypertrophic stimuli mediated by G protein-coupled receptors activate tyrosine kinase, mitogen-activated protein kinase, and 90-kD S6 kinase in cardiac myocytes. The critical role of Ca2+-dependent signaling. Circ Res. 1995;76:1–15. doi: 10.1161/01.res.76.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 8.Sugden PH. An overview of endothelin signaling in the cardiac myocyte. J Mol Cell Cardiol. 2003;35:871–886. doi: 10.1016/s0022-2828(03)00153-6. [DOI] [PubMed] [Google Scholar]
- 9.Young M, Funder JW. Aldosterone and the heart. Trends Endocrinol Metab. 2000;11:224–226. doi: 10.1016/s1043-2760(00)00270-8. [DOI] [PubMed] [Google Scholar]
- 10.Mackenzie L, et al. The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J Physiol. 2002;541:395–409. doi: 10.1113/jphysiol.2001.013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mano A, et al. Aldosterone directly induces myocyte apoptosis through calcineurin-dependent pathways. Circulation. 2004;110:317–323. doi: 10.1161/01.CIR.0000135599.33787.CA. [DOI] [PubMed] [Google Scholar]
- 12.Lalevee N, et al. Aldosterone increases T-type calcium channel expression and in vitro beating frequency in neonatal rat cardiomyocytes. Cardiovasc Res. 2005;67:216–224. doi: 10.1016/j.cardiores.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 14.Berridge MJ. Remodelling Ca2+ signalling systems and cardiac hypertrophy. Biochem Soc Trans. 2006;34:228–231. doi: 10.1042/BST20060228. [DOI] [PubMed] [Google Scholar]
- 15.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 17.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 18.Tomida T, Hirose K, Takizawa A, Shibasaki F, Iino M. NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO J. 2003;22:3825–3832. doi: 10.1093/emboj/cdg381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Cseresnyes Z, Randall WR, Schneider MF. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J Cell Biol. 2001;155:27–39. doi: 10.1083/jcb.200103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavi P, et al. Pacing-induced calcineurin activation controls cardiac Ca2+ signalling and gene expression. J Physiol. 2004;554:309–320. doi: 10.1113/jphysiol.2003.053579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vega RB, Bassel-Duby R, Olson EN. Control of cardiac growth and function by calcineurin signaling. J Biol Chem. 2003;278:36981–36984. doi: 10.1074/jbc.R300023200. [DOI] [PubMed] [Google Scholar]
- 22.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, et al. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlasblom R, et al. Contractile arrest reveals calcium-dependent stimulation of SERCA2a mRNA expression in cultured ventricular cardiomyocytes. Cardiovasc Res. 2004;63:537–544. doi: 10.1016/j.cardiores.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Frey N, McKinsey TA, Olson EN. Decoding calcium signals involved in cardiac growth and function. Nat Med. 2000;6:1221–1227. doi: 10.1038/81321. [DOI] [PubMed] [Google Scholar]
- 26.Olson EN. A decade of discoveries in cardiac biology. Nat Med. 2004;10:467–474. doi: 10.1038/nm0504-467. [DOI] [PubMed] [Google Scholar]
- 27.Braz JC, et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through up-regulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 29.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Aoki H, Sadoshima J, Izumo S. Myosin light chain kinase mediates sarcomere organization during cardiac hypertrophy in vitro. Nat Med. 2000;6:183–188. doi: 10.1038/72287. [DOI] [PubMed] [Google Scholar]
- 31.Kinugawa K, Takahashi T, Kohmoto O, Yao A, Ikenouchi H, Serizawa T. Ca2+-growth coupling in angiotensin II-induced hypertrophy in cultured rat cardiac cells. Cardiovasc Res. 1995;30:419–431. [PubMed] [Google Scholar]
- 32.Miyata S, Haneda T. Hypertrophic growth of cultured neonatal rat heart cells mediated by type 1 angiotensin II receptor. Am J Physiol. 1994;266:H2443–H2451. doi: 10.1152/ajpheart.1994.266.6.H2443. [DOI] [PubMed] [Google Scholar]
- 33.Colomer JM, Terasawa M, Means AR. Targeted expression of calmodulin increases ventricular cardiomyocyte proliferation and deoxyribonucleic acid synthesis during mouse development. Endocrinology. 2004;145:1356–1366. doi: 10.1210/en.2003-1119. [DOI] [PubMed] [Google Scholar]
- 34.Timmerman LA, Clipstone NA, Ho SN, Northrop JP, Crabtree GR. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–840. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Randall WR, Schneider MF. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J Cell Biol. 2005;168:887–897. doi: 10.1083/jcb.200408128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang S, McKinsey TA, Zhang CL, Richardson JA, Hill JA, Olson EN. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol Cell Biol. 2004;24:8467–8476. doi: 10.1128/MCB.24.19.8467-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robert V, et al. Beat-to-beat oscillations of mitochondrial [Ca2+] in cardiac cells. EMBO J. 2001;20:4998–5007. doi: 10.1093/emboj/20.17.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.