Abstract
TNFα levels are elevated in the marrows of patients with myelodysplastic syndrome (MDS) and are associated with high rates of apoptosis, which contributes to hematopoietic failure. We observed that exposure of human marrow stroma cell lines HS5 and HS27a to TNFα increases levels of IL-32 mRNA. IL-32, in turn, induces TNFα. Marrow stroma from patients with MDS expressed 14- to 17-fold higher levels of IL-32 mRNA than healthy controls. In contrast, cells from patients with chronic myelomonocytic leukemia (CMML) expressed only one tenth the level of IL-32 measured in healthy controls. Human KG1a leukemia cells underwent apoptosis when cocultured with HS5 stromal cells, but knockdown of IL-32 in the stromal cells by using siRNA abrogated apoptosis in the leukemia cells. IL-32 knockdown cells also showed dysregulation of VEGF and other cytokines. Furthermore, CD56+ natural killer cells from patients with MDS and CMML expressed IL-32 at lower levels than controls and exhibited reduced cytotoxic activity, which was unaffected by IL-2 treatment. We propose that IL-32 is a marrow stromal marker that distinguishes patients with MDS and CMML. Furthermore, IL-32 appears to contribute to the pathophysiology of MDS and may be a therapeutic target.
Keywords: marrow stroma, TNFα
Myelodysplastic syndrome (MDS) and myeloproliferative disorder (MPD) are clonal diseases of hematopoietic stem/precursor cells. Most patients are not candidates for hematopoietic cell transplantation, which is the only current treatment with curative potential (1, 2). Whereas patients with MPD show proliferative characteristics in the bone marrow, apoptosis is prominent in patients with MDS. Cytokines such as TNFα, and the proapoptotic Fas-ligand and TNF-related apoptosis-inducing ligand (TRAIL), are up-regulated in patients with MDS (3), and the neutralization of TNFα (4–6), for example with the soluble TNF receptor etanercept (7), enhances hematopoiesis in vitro and in vivo (6, 8, 9). TNFα is of particular interest because, in addition to proinflammatory activity, it provides both proapoptotic and proliferative signals (9). In early stage MDS, apoptosis occurs in both residual normal and clonal hematopoietic precursors (8). The rate of apoptosis in clonal precursors declines as the disease progresses. Concurrently, NFκB, a transcription factor involved in the regulation of cytokine pathways, shows increasing levels of activation (9).
Molldrem et al. (10) showed that a proportion of patients with MDS harbored autoreactive T lymphocytes, which provided a source of TNFα. Macrophages from MDS marrow also generate increased amounts of TNFα spontaneously and after stimulation (11). However, whereas TNFα is a recognized factor in the dysregulation of hematopoiesis, the proapoptotic property of TNFα alone cannot satisfactorily explain the entire spectrum of MDS.
Here, we observed that the leukemia-derived cell line KG1a, which is resistant to TNFα-mediated apoptosis, became sensitive to TNFα-induced apoptosis when cocultured with the human marrow-derived stroma cell lines HS5 or HS27a. Apoptosis was contact-dependent, consistent with a model that TNFα-modified signals in the microenvironment affected the leukemia (or MDS) clone. Microarray gene expression analysis of HS5 and HS27a cells revealed prominent induction of IL-32 by TNFα (12). Because IL-32 appears to contribute to inflammatory and autoimmune diseases (13–16) and induces TNFα and apoptosis (17), we hypothesized that IL-32 and TNFα participate in an autoamplification loop in MDS that promotes apoptosis. Therefore, the levels of TNFα, IL-32, or both should be lower in patients in whom proliferation, rather than apoptosis, was the dominant feature. One such disorder is chronic myelomonocytic leukemia (CMML), originally categorized as MDS (18), but recently reclassified by the World Health Organization as a distinct entity (19). Indeed, ex vivo studies showed low TNFα levels in patients with CMML (20–22).
Similar to other cytokines, IL-32 may exert its functions even in a membrane-bound form. Also, IL-32 is released from cells upon apoptosis, thus allowing for the induction of TNFα (and apoptosis) in other target cells (23). Although we realize that cytokine levels in mononuclear cells may not reflect levels in marrow stroma, we determined IL-32 expression in marrow and peripheral blood cells from patients with MDS and CMML, in comparison with cells from healthy control subjects, and we began to characterize its functional relevance.
Results
IL-32 Expression in Human Marrow Stroma.
Human marrow stroma cell lines.
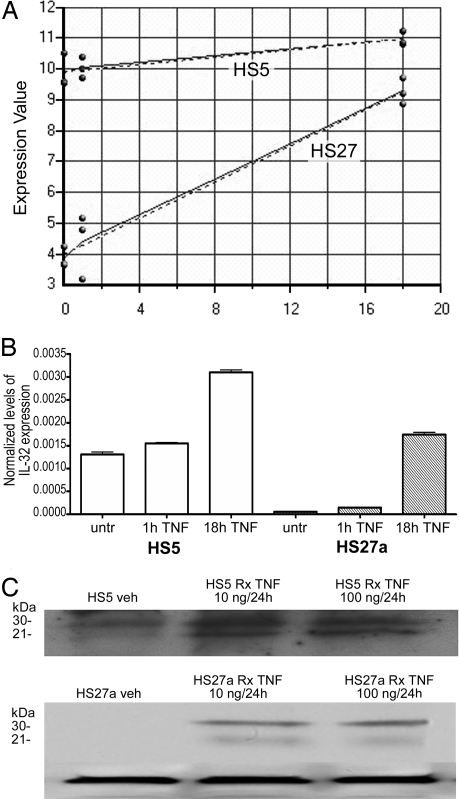
Preliminary studies indicated that exposure of the human marrow stroma cell lines HS5 and HS27a to TNFα increased the expression of several genes, including IL-32 (Fig. 1A). We confirmed those findings by using quantitative RT-PCR for RNA (Fig. 1B) and Western blot analysis for the IL-32 protein (Fig. 1C). Because others had shown that IL-32 induces the expression of TNFα (17), the present observations suggested the possibility of an autoamplification loop of TNFα and IL-32.
Fig. 1.
Expression of IL-32 in HS5 and HS27a cells in response to TNFα. (A) Log2 expression (y axis) of IL32 before (0) and 1 and 18 h after TNFα exposure (50 ng/ml) in HS5 and HS27a stroma cell lines (dotted lines, predicted expression; solid lines, measured expression). (B) IL-32 expression by quantitative RT-PCR before (untr) and 1 and 18 h after TNFα exposure (shown are results at 20 ng/ml TNF). (C) Western Blot of IL-32 protein in HS5 (Upper) and HS27a (Lower) cells. (Lower) β-actin served as control for both cell lines. Shown are IL-32 levels before treatment (Veh) and after treatment (Rx), with 10 ng/ml and 100 ng/ml of TNFα, respectively, for 24 h. The lower weight band likely represents IL-32α, and the higher likely represents IL-32γ.
Primary marrow stroma cells.
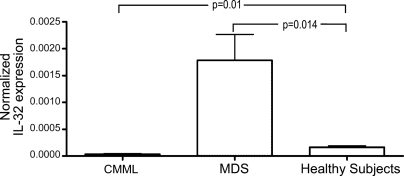
Primary adherent stroma cells were obtained from human marrows by depleting CD45+ cells (including macrophages) (see Materials and Methods). The purity was ≥95% as determined by the absence of staining for CD14, CD34, CD11b, and CD45. Adherent cells derived from the marrows of 13 patients with untreated MDS expressed significantly higher levels of steady-state IL-32 mRNA, compared with cells from six healthy subjects (P = 0.014) (Fig. 2). In contrast, adherent cells derived from six patients with CMML exhibited a significantly lower constitutive expression of IL-32 (P = 0.01), and patients with CMML also were shown to express low levels of TNFα (21, 24).
Fig. 2.
Expression of IL-32 in primary marrow-derived stroma cells from untreated patients with CMML (n = 6) or MDS (n = 13) and healthy control subjects (n = 6). mRNA levels were determined by quantitative RT-PCR. P values are in comparison with healthy subjects. IL-32 levels were significantly lower in patients with CMML (P = 0.01) and higher in patients with MDS (P = 0.014).
Coculture with Stroma Cells Renders KG1a Cells Sensitive to TNFα-Induced Apoptosis.
The leukemia cell line KG1a is nearly completely resistant to TNFα-induced apoptosis in both the absence and presence of stroma (25). However, when TNFα was added to contact cocultures of KG1a and stroma, apoptosis of KG1a cells increased significantly, and KG1a cells detached from the stroma. KG1a cells that remained adherent to stroma showed little or no apoptosis.
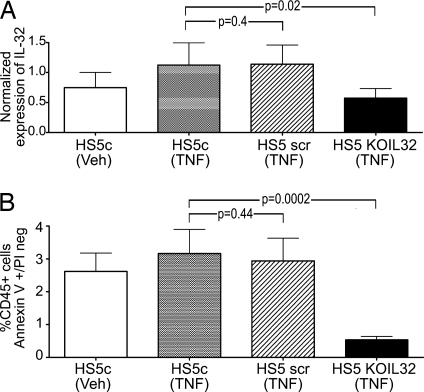
We speculated that the induction of apoptosis was related to the up-regulation of IL-32 (or other proapoptotic factors) in HS5 cells after TNFα exposure. To determine the role of IL-32, we reduced the constitutive level of IL-32 expression in HS5 cells by using siRNA and performed the same coculture experiments. As shown in Fig. 3A (HS5KOIL32), a lower expression of IL-32 was accomplished. As illustrated in Fig. 3B, KG1a cells cocultured with HS5KOIL32 cells failed to undergo apoptosis even in the presence of exogenous TNFα. Identical results were obtained with HS27a [supporting information (SI) Fig. 7]. We interpreted these data as due to a biologically active form of IL-32, which is likely membrane-bound on stroma cells (15), and the induction of apoptosis in KG1a cells.
Fig. 3.
IL-32 expression in HS5 stroma and effect on apoptosis in cocultured KG1a cells. (A) Expression of IL-32 in unmodified (HS5c) and modified (HS5 scr or HS5 KOIL32) stroma cells under control conditions (Veh) or after exposure to 100 ng/ml TNFα. IL-32 mRNA levels were determined by quantitative RT-PCR (n = 7; mean ± 1 SD). (B) Early stage apoptosis in KG1a cells in control cultures containing unmodified HS5 cells (HS5c) and in cocultures with HS5 cells transfected with either a scrambled siRNS sequence (HS5scr) or siRNA specific for IL-32 (HS5KOIL32). Cells were cultured in normal medium (Veh) or in the presence of 100 ng/ml TNFα. Apoptosis was determined by flow cytometry. Only CD45+ (KG1a) cells were considered.
Interactions of TNFα, IL-32, and VEGF.
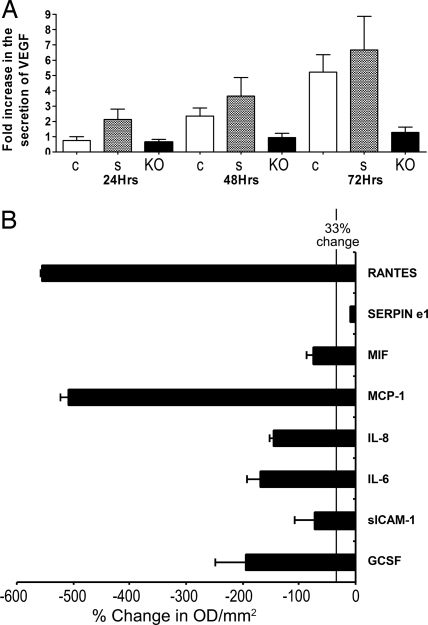
Others have suggested that the inhibitory effect of VEGF on hematopoiesis was mediated by the local generation of TNFα (26) and that neutralization of VEGF reduced the transcription of TNFα (6). We observed that, after overnight culture of HS5 and HS5KOIL32 cells, concentrations of VEGF were significantly lower in the supernatant of HS5KOIL32 cells (Fig. 4A). Concentrations of TNFα were below the level of assay sensitivity. In addition, a broad array of 36 cytokines, using protein dot blots, showed significant reduction of RANTES, MCP-1, IL-6, IL-8, G-CSF, sICAM-1, MIF, and Serpine 1 in HS5KOIL32 supernatant (VEGF was not represented) (Fig. 4B).
Fig. 4.
Secretion of cytokines from HS5 stroma cells. Unmodified HS5 cells (c) or HS5 cells transfected with scrambled siRNA (s) or with IL-32-specific siRNA (KO) were used. (A) VEGF levels at 24, 48, and 72 h in the stroma cell supernatants by using ELISA. Changes in cytokine concentration are expressed as fold increase over control supernatants mean ± SD. (B) Expression of 36 cytokines in HS5KOIL32 cells in comparison with unmodified HS5 cells was determined by proteome profiler cytokine array (R&D Systems). Shown are changes (mean ± SD) in eight cytokines, which decreased significantly in HS5KOIL32 cells. The vertical line indicates a decrease of 33%. Levels were determined after 72 h of culture. The membrane contained probes for C5a, ICAM-1, IL-4, IL-13, IL-32α, MIP-1β, CD40 ligand, IFN-γ, IL-5, IL-16, IP-10, RANTES, G-CSF, IL-1α, IL-6, IL-17, I-TAC, SDF-1, GM-CSF, IL-1β, IL-8, IL-17E, MCP-1, Serpin-E1, GROα, IL-1ra, IL-10, IL-23, MIF, TNFα, I-309, IL-2, IL-12p70, IL-27 MIP-1α, and TREM-1.
IL-32 Expression in Natural Killer (NK) Cells.
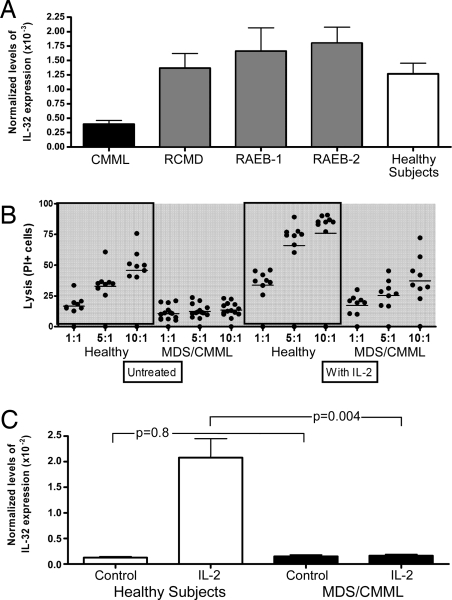
The cDNA of IL-32 was originally cloned from NK cells (27). NK cells were of interest to us because abnormal NK function has been reported in patients with MDS (27). Thus, in a final set of experiments, we determined steady-state levels of IL-32 mRNA in CD56+ NK cells from patients with MDS or CMML (Fig. 5A). The baseline expression of IL-32 in CD56+ cells from peripheral blood of patients with CMML was lower than in CD56+ cells from healthy donors (P = 0.001). Conversely, in patients with MDS, the baseline expression of IL-32 tended to be higher than in healthy controls, although the difference did not reach statistical significance (P = 0.48).
Fig. 5.
IL-32 expression and NK activity. (A) IL-32 expression in peripheral blood CD56+ cells (n = 60). Shown are steady-state relative levels of expression of mRNA determined by quantitative RT-PCR. CMML, chronic myelomonocytic leukemia. MDS patients are shown by disease category [RCMD, refractory cytopenia with multilineage dysplasia; RAEB-1, refractory anemia with excess blasts type 1 (5–9% blasts); RAEB-2, refractory anemia with excess blasts, type 2 (10–19% blasts)]. Baseline expression of IL-32 in CD56+ cells from peripheral blood of patients with CMML was significantly lower than in CD56+ cells from healthy subjects (P = 0.001). Conversely, in patients with MDS, baseline expression of IL-32 tended to be higher than in healthy controls, although the difference did not reach statistical significance (P = 0.48). (B) Cytolytic function of NK cells. CD56+ cells were obtained from the peripheral blood of healthy subjects (n = 7) and patients with CMML or MDS (n = 13) and assayed at three different effector/target ratios (1:1; 5:1, and 10:1) against K562 cells. CD56+ cells were untreated or cultured for 24 h with 500 units/ml IL-2. Lytic activity was measured after 4 h of incubation of the effector cells (CD56+ cells) with target cells (K562). Lytic units were calculated from triplicate. Black dots show individual results, horizontal bars the mean percentage of lysis. (C) Expression of IL-32 in incubated peripheral blood CD56+ cells from healthy subjects (n = 7) and patients with MDS or CMML (because no difference was noted between MDS and CMML cells, results are lumped; n = 26). IL-32 levels were determined in unmodified cells (control) and cells that had been cultured with 500 units/ml IL-2 for 18 h. P values are for MDS/CMML patients in comparison with healthy subjects.
In other cell populations such as CD34+ cells and more differentiated granulocytes, the patterns of expression were less consistent (data not shown).
IL-32 Expression and NK Cell Function.
Fig. 5B summarizes data on cytotoxicity of CD56+ NK cells against K562 target cells. CD56+ cells from healthy donors exhibited the expected cytotoxicity, which increased with higher effector/target ratios. In contrast, no increase was seen with CD56+ cells from MDS or CMML patients (P = 0.001). A slight increase in NK cytotoxicity in MDS-derived CD56+ cells did occur at higher effector/target ratios after activation with IL-2. However, at identical ratios, cytotoxicity consistently lagged behind that of cells from healthy donors. Despite differences in baseline IL-32 expression, CD56+ cells from the peripheral blood of patients with CMML showed cytotoxicity comparable to that of CD56+ cells from patients with MDS, suggesting that factors in addition to IL-32 were involved.
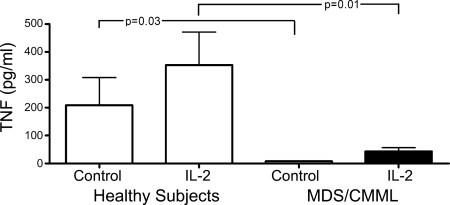
Fig. 5C shows IL-32 expression in peripheral blood CD56+ cells at baseline (control) and 18 h after IL-2 addition (IL-2) in healthy donors and patients with MDS or CMML. Because we combined MDS (higher IL-32 at baseline) and CMML (lower IL-32 at baseline) patients, the average level of expression was similar to that in healthy donors in unstimulated samples. However, after IL-2 stimulation, in contrast to healthy donors, no up-regulation of IL-32 occurred in either MDS or CMML cells. Further, TNFα secretion by CD56+ cells from patients with MDS or CMML was consistently lower than in cells from healthy controls both without (P = 0.03) and with (P = 0.01) IL-2 stimulation (Fig. 6). Again, because baseline IL-32 levels differed between MDS and CMML cells, factors in addition to IL-32 are likely involved in NK dysfunction in these patients.
Fig. 6.
Secretion of TNFα by CD56+ NK cells from healthy subjects (n = 8) and patients with MDS or CMML (MDS; n = 16). TNFα was measured by ELISA at baseline (control) and 24 h after 500 units/ml IL-2. Shown is the mean ± SD (pg/ml). Although there was considerable variation in cells from healthy donors, little or no TNFα was secreted from MDS-derived cells even after activation by IL-2.
Discussion
The present study revealed significant dysregulation of IL-32 expression in marrow and peripheral blood cells from patients with MDS and CMML.
In comparison with healthy controls, the constitutive expression of IL-32 mRNA was higher in primary stroma cells from patients with MDS and significantly reduced in patients with CMML. A similar pattern was observed in peripheral blood CD56+ NK cells (i.e., the cells from which the IL-32 cDNA was cloned originally) (27). Because recent data indicate that the marrow microenvironment contributes to the pathophysiology of MDS and because NK function is abnormal in MDS (6, 12, 28), we were interested in determining IL-32's possible role in these disorders.
We observed previously that TNFα protein levels were higher in the marrow of patients with MDS, and we showed that neutralization of TNFα activity enhanced hematopoiesis (4). The gene expression profiling of the human stroma lines HS5 and HS27a revealed a striking up-regulation of IL-32 in response to TNFα (12). Because IL-32 had been shown to induce TNFα (17), we hypothesized that an autoamplification loop of TNFα and IL-32 contributed to the dysregulation of apoptosis in MDS marrow. If so, then the marrow of patients with proliferative disorders, such as CMML, with low rates of apoptosis, should, as observed here, show low levels of TNFα, IL-32, or both.
The role of TNFα in the pathophysiology of MDS has been characterized (4, 29), whereas the role of IL-32 is unknown. KG1a cells, used as a model (no stable MDS cell lines are available), were nearly completely resistant to TNFα-induced apoptosis (25). However, when exposed to TNFα in cocultures with HS5 stroma, KG1a cells underwent apoptosis. When the same experiment was carried out with HS5KOIL32 cells, apoptosis in KG1a cells was significantly reduced. Thus, IL-32 in and on HS5 cells appears to deliver proapoptotic signals to KG1a cells.
These results are not in conflict with the concept that stroma contact protects leukemic cells (30, 31). KG1a cells became apoptotic only after contact with stroma in the presence of TNFα. Our data are in agreement with a report by Goda et al.(23), who showed that up-regulation of IL-32 induced apoptosis, whereas down-regulation resulted in proliferation, a pattern similar to that observed in stroma cells from patients with MDS (high levels of IL-32 and apoptosis) and CMML (low levels of IL-32 and no apoptosis), respectively. The present data indicate that the final outcome of cell death or survival depended on the milieu in which stroma interactions occurred.
To further define the mechanism that resulted in lower rates of apoptosis in KG1a cells cocultured with HS5KOIL32 cells, we determined patterns of other cytokines that are dysregulated in MDS marrow (3, 22). For example, Bellami et al. (6) suggested that the neutralization of VEGF led to the suppression of TNFα transcription. Such a model is supported by our data, which show that silencing of IL-32 in stroma resulted in a significant decrease in the secretion of VEGF.
In primary stroma (and hematopoietic) cells from patients with CMML, lower steady-state levels of IL-32 mRNA, as expected, were associated with lower levels of TNFα and VEGF production. Molnar et al. (21) also showed lower levels of TNFα and VEGF in the marrow of CMML patients, compared with healthy controls and patients with MDS, consistent with the hypothesis of an autoamplification loop of TNFα and IL-32.
IL-32 was initially reported as a cDNA without known function(s) in IL-2-activated NK and T cells (27), both cell types involved in the pathophysiology of MDS (10, 28). Immunosuppressive therapy (e.g., with antithymocyte globulin) or immunomodulation (e.g., with thalidomide, which interferes with T and NK cell function) have shown therapeutic benefits in some patients with MDS, but not with CMML (21, 24). Kiladjian et al. (28) reported recently that CD56+ NK cells from patients with MDS were quantitatively normal, but functionally abnormal. The present data support those findings and indicate that NK dysfunction was associated with the dysregulation of IL-32 and impaired activation in response to IL-2. However, whereas constitutive expression of IL-32 was low in CMML, it was increased in patients with MDS, compared with healthy controls. Thus, additional factors are likely to be involved in NK dysfunction in these patients. These data offer insights, but also add to the complex picture of the pathophysiology of MDS. The in vivo relevance of IL-32 dysregulation for MDS remains to be proven. It may provide an important link between the immune system [both innate (NK) and adaptive (T cells)] and dysregulated hematopoiesis (10, 28), as well as between hematopoietic cells and the microenvironment. Intriguing in this context are observations by Roth et al. (32), who showed that phenotypic and functional maturation of NK cells required intimate interactions with stroma cells.
In summary, the current study provides evidence for a role of IL-32 in the pathophysiology of clonal myeloid diseases and provides objective data for a distinction between CMML and MDS, supporting the reclassification of CMML as a distinct disorder (19). Conceivably, IL-32 (or components in the signaling pathway) could serve as therapeutic targets for patients with MDS and MPD.
Materials and Methods
Cell Lines, Cultures, and Reagents.
HS5 and HS27a are human stroma cell lines derived from the marrow of a healthy volunteer (33). The human myeloid leukemia cell line, KG1a, was obtained from D. Banker (Fred Hutchinson Cancer Research Center) (34). TNFα was purchased from PeproTech. IL-2 was obtained from Invitrogen.
Cell lines were maintained and processed for use in experiments as described (25). Primary cells from marrow aspirates and peripheral blood from healthy volunteers and patients with MDS or CMML were obtained, and CD34+ cells were selected as described (9, 35). All subjects had given informed consent.
CD56+CD3− cells were isolated from peripheral blood by EasySep Negative Selection Human NK Cell Enrichment Mixture (CD3, CD4, CD14, CD19, CD20, CD36, CD66b, CD123, HLA-DR, and glycophorin A) obtained from StemCell Technologies.
Isolation and Purification of Marrow Stroma Cells.
Marrow cells, 25–30 × 106 cells per 75-ml cell flasks were cultured in nonhematopoietic expansion medium (Miltenyi Biotec) at 37°C in a humidified atmosphere containing 5% CO2, with weekly medium replacement until adherent cells reached 70% confluence. Adherent cells were washed with PBS, detached with trypsin 0.25%, washed again, and analyzed by flow cytometry. Antibody specificities to identify myeloid/lymphoid cells included CD45, CD11b, CD14, and CD34. To identify stroma cells, we used antibodies for CD54, CD73, CD90, CD133, and CD177 (BD Biosciences/R&D Systems). cDNA from isolated cells was synthesized by using the one-step micromacs mRNA/cDNA isolation kit (Miltenyi Biotec).
Western Blot Analysis.
Total cell lysates were obtained by using CHAPS cell extract buffer (Cell Signaling Technology), protein concentrations were determined by Bradford assay (Bio-Rad), and equal amounts of protein (30 μg) were separated by SDS/4–20% PAGE and transferred to PVDF (Bio-Rad). After blocking with 5% BSA for 1.5 h, membranes were incubated overnight at 4°C with the primary polyclonal anti-human IL-32 antibody (produced in the laboratory of C. Dinarello), binding all isoforms of IL-32. Blots were developed by using the ECL system.
Quantitative RT-PCR Analysis of IL-32.
Quantitative RT-PCR was carried out by using the ABI PRISM 7900HT sequence-detection system (Applied Biosystems) according to the manufacturer's instructions (plates were warmed to 50°C for 2 min, then 96°C for 2 min, then 40 cycles, 15 s at 96°C, 30 s at 59°C, and 1 min at 72°C). Data were collected and analyzed according to manufacturer's instructions. The cDNA content was normalized to the number of copies of endogenous β2microglobulin (β2m).
The PCR primers used to detect all IL-32 isoforms were: 5′-GGG CCT TCA GCT TCT TCA TGT-3′ and 3′-GAA CTT TTG GCC GCC ATG T-5; for β2m, 5′-TGC TTA CAT GTC TCG ATC CCA CTT-3′ and 3′-TGC TCG CGC TAC TCT CTC TT-5.
IL-32 cDNA was cloned into a pcDNA3.1 plasmid as described (11, 17). β2m cloned into pcr2.1 TOPOVector (Invitrogen) was used to construct a standard reference.
RNAi and Transient Transfection.
HS5 stroma cells plated in 12-well plates at 0.2–0.5 × 105 cells per well in 1,000 μl of growth medium without antibiotics until 90–95% confluence were transfected with siRNA oligonucleotides and Lipofectamine 2000 (Invitrogen). siRNAs were incubated in growth media for 5–10 min. Lipofectamine diluted in DMEM was added, and complexation continued for 30 min. The Lipofectamine/RNA ratio was 3 μl to 1 ng. After 6 h, DMEM plus 10% FBS was added to minimize toxicity. The scrambled siRNA sequences were: 5′-CGAAUCCUAAUGCUGCUCCCUACUU-3′ and 3′-AAGUAGGGAGGAGCAUUACCAUUCG-5′. The specific IL-32 sequences were: 5′-CUUUGGUGCCAACUCUGCCUCUCUU-3′ and 3′-AAGAGAGGCAGAGUUGGCACCAAAG-5′ (16).
Assessment of Apoptosis.
KG1a and stroma cells were cocultured in contact in the presence or absence of 10–100 ng/ml TNFα for 18 h, and apoptosis was determined as described (5, 9). KG1a cells remaining attached to HS5 stroma cells were removed with the stroma after trypsin treatment labeled with FITC/CD45 [to distinguish stroma (HS5) and hematopoietic cells (KG1a)] and analyzed by flow cytometry for apoptosis by using Annexin V-PE and propidium iodide (PI).
Enzyme-Linked Immunosorbent Assays.
Selected cytokines in marrow plasma and culture supernatants were determined (in triplicate) by using commercially available ELISA kits (R&D Systems) for human TNFα (detection limit 0.5 pg/ml) and VEGF (detection limit 31.2 pg/ml). The color intensity of the chromogenic reaction was determined at 490 nm for TNFα and at 450 nm for VEGF by plate reader (Biotech Laboratories). The ELISA for VEGF recognizes two secreted isoforms (121 and 165 kDa), but lacks reactivity with the membrane-bound forms (189 and 206 kDa).
Cytokine Protein Array.
To determine additional cytokines, we used the proteome profiler array membrane kit (R&D Systems) according to the manufacturer's instructions. Equal volumes (1 ml) of supernatant from HS5KOIL32 cells propagated for 72 h were added to the precoated membrane, and the dot blots (standardized for loading control) were analyzed by using ImageQuant software (Molecular Dynamics).
NK Cytotoxicity Assays and Cytokine Release.
NK cytotoxicity was determined with the flow-cytometric system, ACT-1 (Cell Technology). Peripheral blood CD56+CD3− lymphocytes were added to K562 target cells (labeled with a CFSE analog; Cell Technology) at effector/target ratios of 10:1, 5:1, and 1:1 for 4 h at 37°C. Cells were stained with PI (Molecular Probes) and analyzed by flow cytometry. Experiments were performed in triplicate. Standard errors were within 5% of the mean. Analogous experiments were carried out with CD56+CD3− cells precultured with IL-2 at 500 units/ml for 24 h.
In addition, peripheral blood CD56+CD3− cells were incubated overnight with or without IL-2 (500 units per 0.6 × 106 cells per milliliter), and TNFα in the supernatant was determined by ELISA.
Statistical Analysis.
The relationship between gene expression levels in different disease categories was assessed by two-sample t test. Mean and standard deviations are shown.
Supplementary Material
ACKNOWLEDGMENTS.
We thank S. Bair, E. Spaulding, S. Seal, and B. Torok-Storb for technical advice, and H. Crawford and B. Larson for help with manuscript preparation. This work was supported in part by National Institutes of Health Grants HL082941, HL36444 (to H.J.D.), AI-15614, CA-046934, and HL-68743 (to C.A.D.) and K23 CA92405 (to D.L.S.) and a J. P. McCarthy Fund grant (to A.M.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712391105/DC1.
References
- 1.Deeg HJ. In: Thomas' Hematopoietic Cell Transplantation. 4th Ed. Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Oxford: Blackwell; 2007. [Google Scholar]
- 2.de Lima M, Giralt S. Allogeneic transplantation for the elderly patient with acute myelogenous leukemia or myelodysplastic syndrome. Semin Hematol. 2006;43:107–117. doi: 10.1053/j.seminhematol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Mundle SD, et al. Indication of an involvement of interleukin-1 beta converting enzyme-like protease in intramedullary apoptotic cell death in the bone marrow of patients with myelodysplastic syndromes. Blood. 1996;88:2640–2647. [PubMed] [Google Scholar]
- 4.Gersuk GM, et al. A role for tumor necrosis factor-α, Fas and Fas-Ligand in marrow failure associated with myelodysplastic syndrome. Br J Haematol. 1998;103:176–188. doi: 10.1046/j.1365-2141.1998.00933.x. [DOI] [PubMed] [Google Scholar]
- 5.Zang DY, Goodwin RG, Loken MR, Bryant E, Deeg HJ. Expression of tumor necrosis factor-related apoptosis-inducing ligand, Apo2L, and its receptors in myelodysplastic syndrome: effects on in vitro hemopoiesis. Blood. 2001;98:3058–3065. doi: 10.1182/blood.v98.10.3058. [DOI] [PubMed] [Google Scholar]
- 6.Bellamy WT, et al. Vascular endothelial cell growth factor is an autocrine promoter of abnormal localized immature myeloid precursors and leukemia progenitor formation in myelodysplastic syndromes. Blood. 2001;97:1427–1434. doi: 10.1182/blood.v97.5.1427. [DOI] [PubMed] [Google Scholar]
- 7.Deeg HJ, et al. Hematologic responses of patients with MDS to antithymocyte globulin plus etanercept correlate with improved flow scores of marrow cells. Leuk Res. 2004;28:1177–1180. doi: 10.1016/j.leukres.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Bryant E, Deeg HJ. Simultaneous demonstration of clonal chromosome abnormalities and apoptosis in individual marrow cells in myelodysplastic syndrome. Int J Hematol. 2004;80:140–145. doi: 10.1532/ijh97.na0402. [DOI] [PubMed] [Google Scholar]
- 9.Kerbauy DMB, et al. NF-κB and FLIP in arsenic trioxide (ATO)-induced apoptosis in myelodysplastic syndromes (MDSs). Blood. 2005;106:3917–3925. doi: 10.1182/blood-2005-04-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molldrem JJ, et al. Haematological response of patients with myelodysplastic syndrome to antithymocyte globulin is associated with a loss of lymphocyte-mediated inhibition of CFU-GM and alterations in T-cell receptor Vbeta profiles. Br J Haematol. 1998;102:1314–1322. doi: 10.1046/j.1365-2141.1998.00920.x. [DOI] [PubMed] [Google Scholar]
- 11.Deeg HJ, et al. Negative regulators of hemopoiesis and stroma function in patients with myelodysplastic syndrome. Leuk Lymphoma. 2000;37:405–414. doi: 10.3109/10428190009089441. [DOI] [PubMed] [Google Scholar]
- 12.Stirewalt DL, et al. TNF induced gene expression in human marrow stroma: Clues to the pathophysiology of MDS. Br J Haematol. 2008;140:444–453. doi: 10.1111/j.1365-2141.2007.06923.x. [DOI] [PubMed] [Google Scholar]
- 13.Shioya M, et al. Epithelial overexpression of interleukin-32alpha in inflammatory bowel disease. Clin Exp Immunol. 2007;149:480–486. doi: 10.1111/j.1365-2249.2007.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinarello CA, Kim SH. IL-32, a novel cytokine with a possible role in disease. Ann Rheum Dis. 2006;65(Suppl 3):iii61–iii64. doi: 10.1136/ard.2006.058511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joosten LA, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA. 2006;103:3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Netea MG, et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci USA. 2005;102:16309–16314. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SH, et al. Interleukin-32: A cytokine and inducer of TNFalpha. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Bennett JM, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199. [PubMed] [Google Scholar]
- 19.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasm. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 20.Allampallam K, et al. Biological significance of proliferation, apoptosis, cytokines, and monocyte/macrophage cells in bone marrow biopsies of 145 patients with myelodysplastic syndrome. Int J Hematol. 2002;75:289–297. doi: 10.1007/BF02982044. [DOI] [PubMed] [Google Scholar]
- 21.Molnar L, Berki T, Hussain A, Nemeth P, Losonczy H. Detection of TNFalpha expression in the bone marrow and determination of TNFalpha production of peripheral blood mononuclear cells in myelodysplastic syndrome. Pathol Oncol Res. 2000;6:18–23. doi: 10.1007/BF03032653. [DOI] [PubMed] [Google Scholar]
- 22.Raza A, et al. Novel insights into the biology of myelodysplastic syndromes: Excessive apoptosis and the role of cytokines. Int J Hematol. 1996;63:265–278. doi: 10.1016/0925-5710(96)00455-0. [DOI] [PubMed] [Google Scholar]
- 23.Goda C, et al. Involvement of IL-32 in activation-induced cell death in T cells. Int Immunol. 2006;18:233–240. doi: 10.1093/intimm/dxh339. [DOI] [PubMed] [Google Scholar]
- 24.Kitagawa M, et al. Overexpression of tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma by bone marrow cells from patients with myelodysplastic syndromes. Leukemia. 1997;11:2049–2054. doi: 10.1038/sj.leu.2400844. [DOI] [PubMed] [Google Scholar]
- 25.Ying SX, et al. Differential effects of bexatrone on intrinsic and extrinsic pathways in TRAIL-induced apoptosis in two myeloid leukemia cell lines. Leuk Lymphoma. 2007;48:1003–1014. doi: 10.1080/10428190701242358. [DOI] [PubMed] [Google Scholar]
- 26.Broxmeyer HE, et al. Myeloid progenitor cell regulatory effects of vascular endothelial cell growth factor. Int J Hematol. 1995;62:203–215. doi: 10.1016/0925-5710(95)00412-2. [DOI] [PubMed] [Google Scholar]
- 27.Dahl CA, Schall RP, He HL, Cairns JS. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol. 1992;148:597–603. [PubMed] [Google Scholar]
- 28.Kiladjian JJ, et al. Cytolytic function and survival of natural killer cells are severely altered in myelodysplastic syndromes. Leukemia. 2006;20:463–470. doi: 10.1038/sj.leu.2404080. [DOI] [PubMed] [Google Scholar]
- 29.Reza S, Shetty V, Dar S, Qawi H, Raza A. Tumor necrosis factor-alpha levels decrease with anticytokine therapy in patients with myelodysplastic syndromes. J Interferon Cytokine Res. 1998;18:871–877. doi: 10.1089/jir.1998.18.871. [DOI] [PubMed] [Google Scholar]
- 30.Konopleva M, et al. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 31.Garrido SM, Appelbaum FR, Willman CL, Banker DE. Acute myeloid leukemia cells are protected from spontaneous and drug-induced apoptosis by direct contact with a human bone marrow stromal cell line (HS-5). Exp Hematol. 2001;29:448–457. doi: 10.1016/s0301-472x(01)00612-9. [DOI] [PubMed] [Google Scholar]
- 32.Roth C, Rothlin C, Riou S, Raulet DH, Lemke G. Stromal-cell regulation of natural killer cell differentiation. J Mol Med. 2007 doi: 10.1007/s00109-007-0195-0. [DOI] [PubMed] [Google Scholar]
- 33.Roecklein BA, Torok-Storb B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood. 1995;85:997–1005. [PubMed] [Google Scholar]
- 34.Koeffler HP, Billing R, Lusis AJ, Sparkes R, Golde DW. An undifferentiated variant derived from the human acute myelogenous leukemia cell line (KG-1). Blood. 1980;56:265–273. [PubMed] [Google Scholar]
- 35.Graf L, Heimfeld S, Torok-Storb B. Comparison of gene expression in CD34+ cells from bone marrow and G-CSF mobilized peripheral blood by high-density oligonucleotide array analysis. Biol Blood Marrow Transplant. 2001;7:486–494. doi: 10.1053/bbmt.2001.v7.pm11669215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.