Abstract
Receptor-operated Ca2+ entry (ROCE) and store-operated Ca2+ entry (SOCE) into cells are functions performed by all higher eukaryotic cells, and their impairment is life-threatening. The main molecular components of this pathway appear to be known. However, the molecular make-up of channels mediating ROCE and SOCE is largely unknown. One hypothesis proposes SOCE channels to be formed solely by Orai proteins. Another proposes SOCE channels to be composed of both Orai and C-type transient receptor potential (TRPC) proteins. Both hypotheses propose that the channels are activated by STIM1, a sensor of the filling state of the Ca2+ stores that activates Ca2+ entry when stores are depleted. The role of Orai in SOCE has been proven. Here we show the TRPC-dependent reconstitution of Icrac, the electrophysiological correlate to SOCE, by expression of Orai1; we also show that R91W-Orai1 can inhibit SOCE and ROCE and that Orai1 and STIM1 expression leads to functional expression of Gd-resistant ROCE. Because channels that mediate ROCE are accepted to be formed with the participation of TRPCs, our data show functional interaction between ROCE and SOCE components. We propose that SOCE/Icrac channels are composed of heteromeric complexes that include TRPCs and Orai proteins.
Keywords: capacitative calcium entry, signal transduction, store depletion
Reported independently by three laboratories at the beginning of 2006, Orai (also known as CRACM) proteins, especially Orai1, have emerged as the molecular candidates for the underlying structure of store-operated Ca2+ entry (SOCE) channels responsible for the store-depletion activated Ca2+ current, Icrac, without apparent role(s) for transient receptor potential ion channels (TRPCs) (reviewed in refs. 1–3). Before the discovery of Orai genes and their gene products, the only candidates presumed to underlie SOCE had been the members of the TRPC class of ion channels, of which there are seven. This proposition was based on primary research reports from many laboratories showing that expression of the cloned TRPCs enhances store-depletion activated Ca2+ entry (cf. table 1.3 in ref. 4) and/or that injection of specific anti-TRPC antibodies or genetic ablation of TRPC genes reduces SOCE and/or Icrac. Moreover, Zagranichnaya et al. (5) showed partial reduction of SOCE in response to siRNAs that targeted TRPC1, TRPC3, or TRPC7, which, when combined, were partially additive. The idea promoted in the reviews listed above that channels responsible for SOCE and Icrac form without involvement of TRPC proteins is especially difficult to reconcile with the total loss of Icrac in vascular endothelial cells seen in TRPC4 knock-out mice (6) and the 80% loss of SOCE found in submaxillary acinar cells of TRPC1 knockout mice (7).
Based on the foregoing data, and recognizing that Orai/CRACMs are part of SOCE/Icrac channels, we proposed that SOCE/Icrac channels may be heteromeric complexes of TRPC and Orai proteins (8). If this were the case, we reasoned, expression of Orai1 in cells expressing excess TRPCs might lead to increases in SOCE, as seen in Fura2 imaging experiments.¶ We indeed found that Orai1 increased SOCE in cells stably expressing TRPC3 or TRPC6 but not in untransformed HEK293 cells or HEK cells that were expressing unrelated molecules, such as the V2 or the V1a vasopressin receptors (8). In these studies we also showed that expression of SOCE-enhancing levels of Orai1 tends to “silence”‖ spontaneous activity of TRPC3 in cells that stably overexpress these channels. It is worth noting that the parental HEK293 cells do not present with spontaneously active nonselective cation currents and are devoid of Gd3+-resistant receptor-operated Ca2+ entry (ROCE), despite being positive by RT-PCR analysis for the presence of mRNA coding for TRPC3 and TRPC6 (5). Gd3+ is commonly used to assess TRPC3-, TRPC6- or TRPC7-mediated ROCE.
In contrast to SOCE and Icrac, which are molecularly defined phenomena mediated by the SOCE/Icrac channels activated by mere store depletion without involvement of plasma membrane receptors or their signaling pathways, ROCE is an operational definition describing Ca2+ entry occurring through TRPC as well as SOCE/Icrac channels activated secondarily to the stimulation of a receptor–Gq/11–phospholipase C signaling pathway. TRPC activities contributing to ROCE are stimulated as a consequence of one or more of the following events: (i) Gq-coupled receptor signaling independent of store depletion, such as the generation of diacylglycerol, which activates TRPC3, -6, and -7 but not TRPC1, -4, or -5 (ref. 9 and unpublished observations); (ii) lysophospholipids, such as lysophosphatidylcholine, shown for TRPC5 in ref. 13; (iii) conformational coupling by which the IP3–IP3 receptor complex activates TRPCs through direct protein–protein interaction (10–12); (iv) translocation of membrane vesicles by which TRPCs located in intracellular vesicles are shuttled to the plasma membrane (shown for TRPC5 in ref. 14); and (v) other as yet undefined mechanisms. SOCE/Icrac channels contributing to ROCE are activated by the store-depleting action of IP3 generated by receptor activation. Operationally, ROCE is triggered with agonists, hormones, neurotransmitters, and/or growth factors, whereas SOCE/Icrac is triggered without involvement of elements acting upstream of store depletion. The “cleanest” assessment of ROCE without the contribution of SOCE is obtained when ROCE is mediated by Gd3+-resistant channels. Under this condition SOCE/Icrac channels are blocked by 0.5–1 μM Gd3+. Because only the C3, C6, and C7 TRPCs are resistant to inhibition by low concentrations of Gd3+, presumably by forming homomeric multimers (15), it is believed that Gd-resistant ROCE represents Ca2+ entry mediated by molecules that include one or more of these three TRPCs. Mediation by these channels does not exclude participation of additional components, including Orai/CRACM and/or other TRPC proteins, but it does identify participation of the C3, C6, and C7 TRPCs. Absence of measurable Gd-resistant ROCE in native HEK293 cells is therefore likely to be a reflection of the heteromultimerization of Gd-resistant TRPCs with Gd-sensitive TRPCs, such as that described in ref. 14.
The experiments, reported in Results, were performed to address the question whether expression of Orai1 at SOCE-enhancing levels in TRPC-expressing cells is associated with reconstitution of Icrac. In the course of these experiments, we also tested the effects of expressing R91W-Orai1, a mutant found in humans to be responsible for a familial form of severe combined immunodeficiency (SCID). Unexpectedly, this mutant displayed dominant negative properties affecting not only SOCE but also ROCE.
Results
TRPC-Dependent Reconstitution of Icrac by Expression of Orai1.
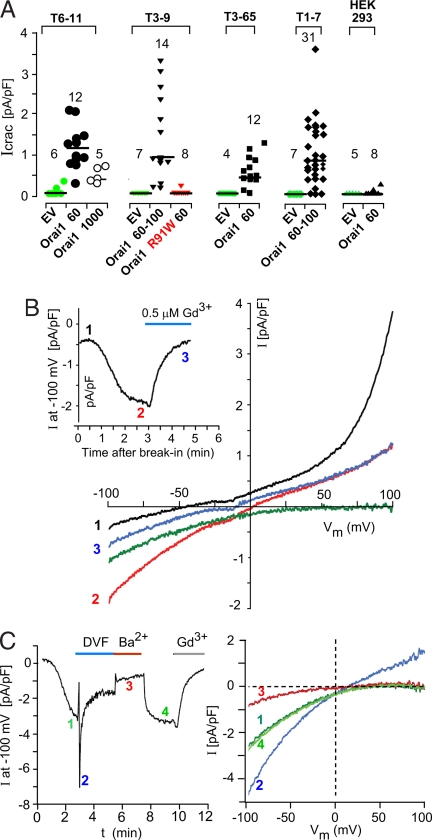
The T1, T3, T6, and T7 cell lines used in this study are clonal HEK293 cell lines that stably express TRPC1, TRPC3, TRPC6, and TRPC7, respectively. When ROCE is elicited in TRPC3- and TRPC6-expressing cells, they develop agonist-induced whole-cell currents with reversal potentials at or very near 0 mV, which show no inward rectification (cf. refs. 16 and 17). This is indicative of the activation of nonselective cation channels. On the other hand, protocols that elicit Icrac in mast cells (e.g., ref. 18) fail, in our hands, to elicit Icrac in either HEK293 cells or in cells stably expressing TRPCs (Fig. 1A). Thus, in the present experiments, infusing the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA) at 10 mM into cells through a patch pipette to promote passive depletion of internal Ca2+ stores led either to no current development (HEK293 cells, n = 6 of 8; untransfected TRPC-expressing cells, n = 22 of 24) or, when developed over a 5-min time span, to <0.25 pA/pF at −100 mV (n = 2 of 24). In contrast, transient expression of SOCE-enhancing levels of Orai1 (60–100 ng of expression plasmid per 60-mm dish under our transfection conditions) elicited measurable Icrac ranging from a low of 0.3 pA/pF to a high of 3.25 pA/pF at −100 mV in 61 of 69 cells (Fig. 1A). Fig. 1B shows typical I–V relationships recorded from an Orai1- and TRPC6-expressing cell before Icrac development, i.e., at the time of break-in (black trace); 3 min later, after Icrac had developed (red trace); and after the addition of 0.5 μM Gd3+ (blue trace at 5 min). Icrac, defined by the difference (green trace) between the I–V relationships before and after Gd3+ addition, shows the typical strong inward rectification and a reversal potential of >50 mV, which are the hallmarks of Icrac. Fig. 1B Inset shows the time course of the development of the inwardly rectifying Icrac and its inhibition by 0.5 μM Gd3+. Icrac reconstituted by expression of Orai1 in TRPC-expressing cells develops with a typical slow time course, reflecting the time course with which store depletion develops its signal to activate Icrac channels, i.e., to activate STIM (Fig. 1C Left), and exhibits a characteristic pattern of ion selectivity. Thus, shifting from Ca2+- and Mg2+-containing external solution to a divalent-free solution, the current, now carried by monovalent ions, increased instantaneously, followed by rapid inactivation. Shifting to standard external solution with 10 mM Ba2+ instead of 10 mM Ca2+, established a new steady-state current level at approximately one-third of that obtained with Ca2+. Ca2+-carried Icrac was reestablished upon return to standard solution and was inhibited by Gd3+. Fig. 1C Right depicts I–V relationships at different stages of the protocol shown in Fig. 1C Left. As exemplified for a TRPC3-expressing cell, these maneuvers indicate that expression of Orai1 in cells stably expressing excess TRPCs reconstitutes typical Icrac currents. Taken together, our results show TRPC-dependent reconstitution of Icrac currents upon expression of low levels of Orai and are an indication of a functional interaction between Orai1 and TRPCs. The results further suggest that Orai1 expression in HEK293 cells and its TRPC-expressing derivatives is likely to be rate-limiting.
Fig. 1.
Expression of Orai in TRPC-expressing cells reconstitutes Icrac. (A) Summary of Gd3+-sensitive Icrac at −100 mV developed 3–5 min after break-in in the various cell types transfected as shown. EV, empty vector. Shown below each group are the nanograms of WT or mutant or Orai1-carrying expression vector used in the standard transfection to which the analyzed cells had been subjected. (B) I–V relationships recorded from a TRPC6-expressing cell transfected with Orai1 after break-in (trace 1, black), at 180 sec before addition of 0.5 μM GdCl3 (trace 2, red) and at 500 sec after Gd3+ block had set in (trace 3, blue). The green trace indicates net Icrac (trace 2 minus blue trace 3). (Inset) Time course of current at −100 mV during the course of the experiment. (C) (Left) Changes in Icrac recorded from a TRPC3-expressing cell transfected with Orai1 as seen by changing the ionic composition of the external solution from Ca2+ to divalent-free and Ba2+. (Right) I–V relations of the leak-subtracted currents at the times indicated in Left.
Positive and Negative Regulation of SOCE in TRPC-Expressing Cells by WT and Mutant Orai1.
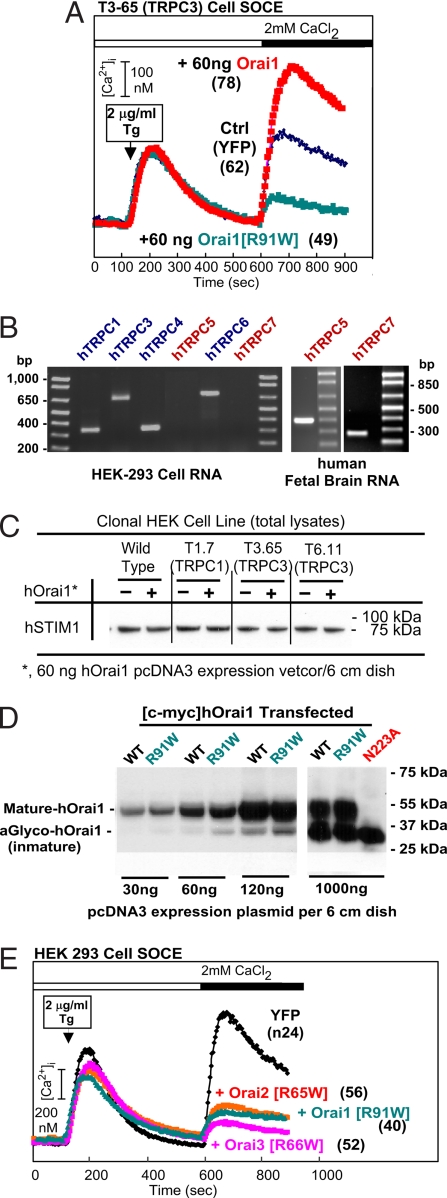
Expression of low levels of Orai (typically between 50 and 90 ng of Orai1-carrying pcDNA3 plasmid to transfect the cells grown to near confluence in a 60-mm dish) leads to an enhancement of SOCE between 50% and 150% for cells stably expressing either TRPC3 or TRPC6 (8). Orai1 also enhances SOCE in TRPC1- and TRPC7-expressing cells (data not shown). TRPC2, C4, and C5 have not yet been tested for their responsiveness to Orai. When the SCID-causing mutant R91W-Orai1 (19) was transfected into TRPC-expressing or HEK293 (see below) cells, we saw no enhancement of SOCE. Instead, and to our surprise, SOCE was inhibited (Fig. 2A). This finding was true also in HEK293 cells (Fig. 2E). This result was unexpected because the mutation was reported to be recessive (19), and it prompted us to seek a better characterization as to which of the relevant genes is expressed in HEK cells. As seen by RT-PCR analysis, HEK293 cells express all three Orai genes, both STIM genes, and TRPC3 and TRPC6 (8). Upon expanding the screen for TRPCs, we found that HEK293 cells also express TRPC1 and TRPC4 but do not express TRPC5 or TRPC7 (Fig. 2B). Given that coexpression of excess STIM1 and Orai1 leads to massively enhanced SOCE and Icrac, we tested whether Orai1 expression alters the levels of STIM1 in TRPC3-expressing cells, which we found does not occur (Fig. 2C). Because overexpression of high levels of Orai1, instead of being stimulatory or without effect, are inhibitory (Fig. 3), we tested whether the inhibitory effect of R91W-Orai1 might come about by a more efficient translation of its cDNA as compared with that of WT Orai1. More efficient translation or greater stability of the protein could lead to excessive accumulation and an attendant inhibitory action. However, this also is not the case, because the accumulation of the transfected myc-tagged WT and R91W-Orai1 proteins were found to be about the same (Fig. 2D). Fig. 2E shows that the same mutation introduced into Orai2 and Orai3 also creates effective inhibitors of SOCE and that inhibition by R91W-Orai1 does not require stable coexpression of a TRPC because it was elicited in HEK293 cells, in contrast to the stimulatory effect of WT Orai1, which requires a stably overexpressed TRPC.
Fig. 2.
Inhibition of SOCE by mutant Orai. (A) Representative SOCE elicited in Fura2-loaded, TRPC3-expressing cells transfected with YFP and empty vector alone (YFP), with YFP plus Orai1, or YFP plus R91W-Orai1. In this and all other figures, SOCE was triggered by the addition of 2 μg/ml Tg after previous removal of Ca2+ from the medium. SOCE developed after 450 sec is seen by the rate of rise in [Ca2+]i and the extent of increase in [Ca2+]i. The time of addition of Ca2+ is indicted by the black bar. Numbers in parentheses denote numbers of cells analyzed. (B) Survey of TRPC expression in HEK293 cells by RT-PCR. Primer sequences are available upon request. (C) Expression of STIM1 protein in control HEK293 and TRPC-expressing cells is unaffected by expression of SOCE augmenting levels of Orai1 as seen by Western blot analysis of total cell lysates. (D) Western blot analysis shows that the exogenous myc-tagged WT and R91W-Orai1 are expressed at similar levels in transfected cells. N223A, WT myc-tagged Orai1 in which the site for posttranslational glycosylation has been changed from Asn to Ala. (E) Mutations cognate to R91W-Orai1 in Orai2 and Orai3 also inhibit SOCE. HEK293 cells were used.
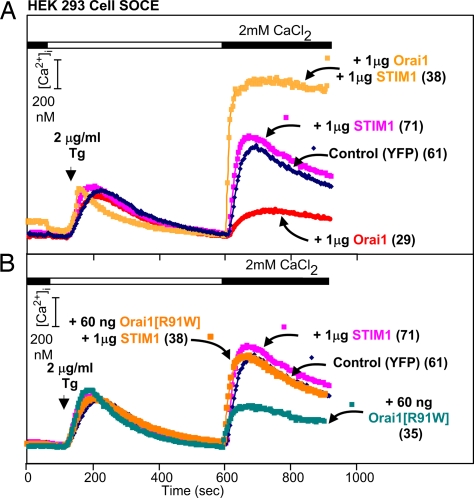
Fig. 3.
Block of inhibition of SOCE and effects of WT and mutant Orai coexpressed with STIM1 as seen in HEK293 cells. The results shown are from a single representative experiment. (A) Block of the inhibitory effect of excess WT Orai1. (B) Block of the effect of low levels of R91W-Orai1.
The results reported so far suggested that Orai may be limiting. Confirming data are presented in the experiment of Fig. 3, in which HEK293 cells were transfected with high levels of Orai1 or STIM1, i.e., levels that, when combined, generate very high SOCE and very large Icrac. We found that transfection of 1,000 ng of STIM1 alone had no effect on SOCE (Fig. 3A) but led to a much increased SOCE when combined with 1,000 ng of Orai1 (Fig. 3A), indicating that there is no excess of Orai1 to complement the overexpressed STIM1 and cause an enhancement of SOCE. The inverse is also true, i.e., expression of low levels of Orai1 would be expected to augment SOCE if there were unpaired STIM1 molecules available, which it did not. The experiment further shows that STIM1 and the SCID mutant of Orai1 are able to interact (Fig. 3B), given that STIM1 rescued SOCE from inhibition by R91W-Orai1 but was not able to assemble or contribute to the formation of functional SOCE channels, as evidenced by the fact that cells expressing R91W-Orai1 and excess STIM1 exhibited SOCE indistinguishable from that in control cells.
Not Only SOCE but Also ROCE Is Affected by Orai.
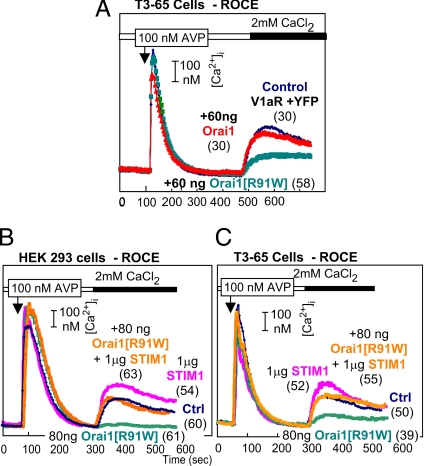
A survey of the literature shows that functions of Orai (CRACMs) have apparently only been studied in the context of SOCE or Icrac and not for potential role(s) in ROCE. However, under physiologic conditions, SOCE is a subset of ROCE. In view of our finding that R91W-Orai1 inhibits SOCE in HEK293 cells, we tested for a possible effect of this mutation on ROCE in HEK293 cells. As shown in Fig. 4A, low levels of the SCID-causing Orai1 mutant potently inhibit ROCE. WT Orai1 does not affect ROCE in HEK293.
Fig. 4.
Block of V1a receptor-triggered ROCE in TRPC3-expressing cells (A and C) and in control HEK cells (B). (B and C) Block of the inhibition by STIM1.
As is the case with SOCE, coexpression of STIM1 prevents the inhibitory effect of the mutant Orai1 in both native HEK293 cells and in HEK cells expressing a recombinant TRPC (Fig. 4 B and C). In contrast to SOCE, expression of STIM1 alone augmented ROCE in HEK cells by 20–50% (Fig. 4B). In TRPC-expressing cells, this enhancement also is seen but is transient (shown for T3–65 cells in Fig. 4C).
Coexpression of Orai1 and STIM1 Affects Gd-Block of ROCE.
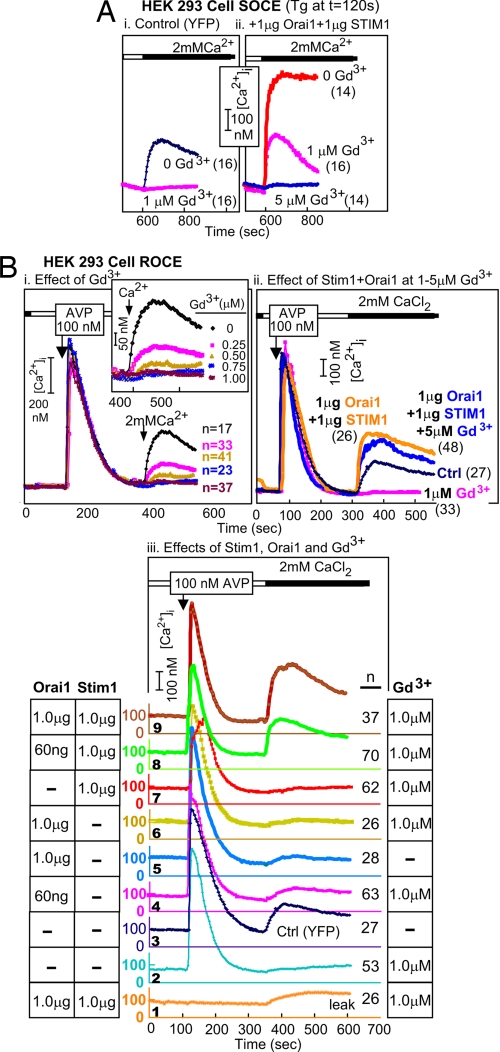
In HEK293 cells, SOCE and ROCE are inhibited by low concentrations of Gd3+ (Fig. 5 Ai and Bi). Upon coexpression of Orai1 and STIM1, SOCE remains sensitive to Gd3+ inhibition, albeit requiring a somewhat higher concentration for full block (Fig. 5 Ai and Aii). Upon testing whether ROCE in the presence of an inhibiting level of WT Orai1 plus a “rescuing” level of STIM1 is still inhibited by 1 μM Gd3+, we unexpectedly found that it is largely Gd3+-resistant. Gd3+-resistant ROCE appeared in HEK293 cells only upon coexpression of STIM1 with WT Orai1 (Fig. 5Bii), and was at about half the level at 60 ng Orai1 as when 1,000 ng of Orai1 was used (Fig. 5Biii). Thus, conditions that elicit very high SOCE and Icrac activities in HEK293 cells also elicit an up to 2-fold increase in ROCE, which, instead of being fully blocked by 5 μM Gd3+, is inhibited only by 15–20% (Fig. 5Bii).
Fig. 5.
Development of resistance of ROCE, but not SOCE, to inhibition by Gd3+ upon coexpression of Orai1 and STIM1. (A) Gd3+ block of SOCE. (Ai) SOCE in HEK cells is blocked by 1 μM Gd3+. (Aii) SOCE induced by coexpression of Orai1 and STIM1 also is blocked by Gd3+. (B) Gd3+ block of ROCE and appearance of Gd3+-resistant ROCE in cells cotransfected with Orai1 plus STIM1. (Bi) ROCE is highly sensitive to inhibition by Gd3+. (Bii) Coexpression of Orai1 and STIM1 leads to the appearance of Gd3+-resistant ROCE (Biii) Gd3+-resistant ROCE requires both STIM1 and inhibitory levels of WT Orai1. The results are from a single experiment; each trace represents the averaged changes in the indicated number of cells.
We asked whether the development of Gd3+-resistant ROCE required Orai1 and STIM1 or only one of them. As shown in Fig. 5Biii, generation of Gd3+-resistant ROCE in HEK293 cells requires both Orai1 and STIM1 (Fig. 5Biii, traces 2, 6, and 7 vs. traces 8 and 9). Furthermore, the results in Fig. 5Biii illustrate that high SOCE-inhibiting levels of WT Orai1 (Fig. 3B) also inhibit ROCE (Fig. 5Biii, trace 3 vs. trace 5). Gd3+-resistant ROCE, produced by coexpression of Orai1 and STIM1, was not a leak artifact (Fig. 5Biii, trace 1 vs. trace 9).
Discussion
The data presented in this report argue strongly for functional interaction between Orai1 and TRPCs and are difficult to interpret with models in which Orai and TRPCs coexist in plasma membranes independently from each other, each subserving an independent regulatory role. Thus, we showed TRPC-dependent reconstitution of Icrac upon expression of Orai1; we showed that low levels of a mutant Orai (e.g., R91W-Orai1) are able to almost totally inhibit endogenous ROCE, a phenomenon commonly ascribed to TRPC-based channels; we further showed that coexpression of Orai1 and the store's calcium sensor STIM1 leads to the assembly and/or formation of Gd-resistant ROCE channels, a phenomenon also commonly accepted as evidence for participation of one of the TRPC3-family channels (TRPC3, -6, and/or -7). These findings need to be integrated with the finding that Orai1 enhances SOCE only if TRPCs are coexpressed [so far, found for TRPC1, -3, -6, and -7 and not yet tested for TRPC4 and 5 (human TRPC2 is a pseudogene], and that spontaneous single-channel activities seen in TRPC3-expressing cells are silenced by low, SOCE-enhancing levels of Orai1. We take these findings also to point toward a TRPC–Orai interaction and possibly to be at the root of the fact that HEK cells do not present with spontaneously active single-channel activities resembling those of TRPC3 or TRPC6, despite being positive for presence of their mRNAs.** Moreover, WT untransfected HEK293 cells, when tested for classical Icrac, show little or no activity of this type, despite having mRNAs coding for all of the known players thought to contribute to ROCE and SOCE, i.e., four TRPCs, three Orai, and two STIMs (Fig. 2B) (8). It would appear that Orai levels are limiting in HEK cells, because expression of STIM led to no increase in SOCE. Expression of Orai1 plus STIM1, on the other hand, leads to robust SOCE and Icrac. Likewise, the steady-state levels of STIM1 also appear to be limiting or balanced, because expression of Orai1 at levels that in TRPC-expressing cells augment SOCE did not increase SOCE in HEK293 cells. High levels of Orai1 also failed to increase SOCE and, instead, inhibited it. Availability of free STIM1 would have been predicted to lead to an increase in SOCE upon increased expression of Orai1 if one were to argue for existence of Orai-only channels activated by STIM1.
Perhaps the most striking discovery was the fact that STIM and Orai functionally interact with the ROCE process: inhibition of ROCE by R91W-Orai1 and the appearance of Gd3+-resistant ROCE. ROCE is a realm ascribed to TRPCs as a result of expression studies in mammalian cells and of genetic ablation studies in the Drosophila visual signal transduction system, which led to the discovery of mammalian TRPCs. The role of TRPCs in ROCE is predicted to operate both independently of store depletion and in concert with the STIM signaling system. Given that SOCE and its likely electrophysiological correlate, Icrac, are inhibited by submicromolar Gd3+ concentrations, the appearance of Gd-resistant ROCE upon coexpression of Orai and STIM argues for TRPCs being regulated by these proteins. Appearance of Gd-resistant ROCE (and also to some extent SOCE) argues further for a STIM–Orai-directed change in the heteromultimerization status of TRPCs, because Gd3+-resistance is otherwise only seen upon overexpression (transient or stable) of C3, C6, or C7 TRPCs. These are conditions conducive to the formation of homomeric channels.
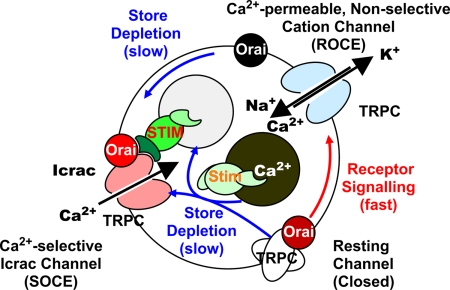
Although it may be premature to propose a subunit structure for the TRPC- and Orai-containing complexes regulated by STIM, it is nevertheless tempting to lay out the scenario depicted in Fig. 6. Based on the finding that spontaneous activity of stably overexpressed TRPC3 channels is suppressed by Orai1 (8), we propose that nonactivated TRPCs in unstimulated cells are stabilized in their inactive conformation through association with Orai molecules. Stimulation by the receptor–Gq–phospholipase C–IP3–IP3 receptor pathway is proposed to cause the rapid release of Orai from TRPCs with concomitant activation of TRPCs operating in a Ca2+-permeable, nonselective cation channel mode. As Ca2+ stores are depleted, the store's Ca2+ sensor STIM1 changes conformation with activation of a scaffolding function in its cytosolic C terminus. This promotes (i) the gradual reorganization and migration of Orai molecules into microdomains visible by confocal and total internal reflection fluorescence microscopy as puncta [described by Lewis and coworkers (20, 21)], in which STIM and Orai colocalize; and (ii) the gradual migration of TRPCs to the same microdomains as a consequence of interacting with STIM activated by the Ca2+ depletion process [described by Muallem, Worley, and coworkers (22–24)]. TRPCs associated with STIM–Orai complexes are proposed to be channels that operate in the SOCE/Icrac mode. In the absence of cytosolic Ca2+, as is the case in protocols in which Icrac is activated with BAPTA, the model views TRPCs transitioning directly from being kept in the resting state by Orai to operating in the SOCE/Icrac mode induced by the STIM–Orai complex. STIM is shown to interact with both Orai and TRPC, as could be expected from a molecule with scaffolding properties. The model does not rule out, however, that, even in the absence of cytosolic Ca2+, TRPCs may transition to the SOCE/Icrac- gating mode via an intermediary mode in which they are associated with neither Orai nor STIM–Orai and gate in the nonselective ion channel mode. R→W mutants would reduce SOCE and ROCE by associating with and stabilizing the resting state and interfering with the activation process. Excess STIM would revert this inhibition by sequestering the mutant Orai molecules, allowing the endogenous WT Orai to work as if no R→W mutants were present. Our experiments show that the quantities of STIM and Orai are balanced (Figs. 3 and 5). In contrast, the model assumes that cells express TRPC molecules in excess of Orai and STIM. Overexpression of both STIM1 and Orai1 would then be able to engage all of the TRPCs, which under physiologic conditions does not happen because of substoichiometric amounts of Orai and STIM compared with TRPCs. Overexpression of either STIM or Orai has no effect because Orai and STIM are limiting.
Fig. 6.
Model of the interplay between TRPCs, Orai, and STIM. TRPCs are depicted as being stabilized in an inactive state by interacting with Orai. Receptor signaling is proposed to cause fast dissociation of Orai from TRPC, promoting its activation. Activated TRPC gating in the nonselective cation channel mode is then trapped by STIM–Orai complexes induced by depletion of Ca2+ from the store and dissociation of Ca2+ from STIM's luminal, Ca2+-sensing N terminus. Clustered aggregates of STIM–Orai associate with activated TRPCs and stabilize their conformation in a state in which it operates as an Icrac channel. Normal cells have excess of neither Orai nor STIM but do have more TRPC molecules than Orai molecules. For further discussion of the model, see the text.
The proposal that TRPCs operating in the nonselective gating mode have a transient lifetime is in agreement with experimental findings (cf. figure 1A in ref. 14), given that, upon addition of the muscarinic receptor agonist carbachol to T3-9 cells, the nonselective current it elicited was transient.
Taken together, our results show the existence of functional interactions between Orai1 and TRPCs under the influence of STIM1 and do not support views of ROCE and/or SOCE being mediated by TRPCs and Orai operating as noninteracting pathways.
Materials and Methods
Cell and Molecular Biology.
For transfections, cDNAs coding for enhanced yellow fluorescent protein (eYFP; Clontech), V1a vasopressin receptor (V1aR), myc-tagged Orai and mutants, and STIM1 were placed into pcDNA3 (Invitrogen). For expression of proteins in HEK and HEK-derived cells, cells were grown in 60-mm dishes to ≈80% confluence and were transfected (Lipofectamine method; Invitrogen) with a mixture of plasmids that included 0.1–0.5 μg of plasmids coding for eYFP; the indicated plasmids coding for WT or mutant Orai, STIM1, and a combination thereof; and various amounts of pcDNA3 without insert (empty vector) to give 5.0 μg of total plasmid DNA. For ROCE measurements, 1.5 μg of V1aR plasmid was included in the transfection mixture. For measurement of [Ca2+]i, the cells were replated 24 h after the transfection onto polylysine-coated coverslips and allowed to recover for another 24 h before preparing them for SOCE or ROCE measurements. Loading with Fura2 was done by incubation with 2 μM Fura2AM in Hepes-buffered saline for 30 min at 37°C. Changes in [Ca2+]i were monitored by dual excitation fluorescence ratiometric video microscopy as described in Zhu et al. (25) and Liao et al. (8). The materials and all other methods were as described previously (8).
Electrophysiology.
Icrac currents were elicited by passive store depletion with BAPTA in whole-cell recordings using an intracellular solution containing 120 mM Cs-glutamate, 8 mM NaCl, 10 mM Cs-BAPTA, 3 mM MgCl2, and 10 mM Hepes (pH-adjusted to 7.2 with CsOH). The external solution consisted of 120 mM NaCl, 10 mM CsCl, 2.8 mM KCl, 2 mM MgCl2, 10 mM CaCl2 or BaCl2, 10 mM Hepes, and 10 mM glucose [pH-adjusted to 7.2 with tetraethylammonium hydroxide (TEA-OH)]. The divalent-free solution contained 135 mM NaCl, 10 mM CsCl, 2.8 mM KCl, 10 mM Hepes, 0.1 mM EDTA, 10 mM glucose (pH-adjusted to 7.2 with TEA-OH). Patch electrodes were of between 2- and 3-MΩ resistance and were coated with Sylgard 184 elastomer (Dow Corning) to minimize capacitive and noise components, and currents were recorded with an EPC-10 amplifier in combination with a PC running Pulse software (HEKA Instruments). Icrac vs. t plots show the time course of inward current at −100 mV obtained during voltage ramps (1 V/s) from −100 mV to +100 mV from a holding potential of 0 mV at a rate of 0.5 Hz. A 10-mV junction potential correction was applied to all values of voltage across the membrane. I–V curves for Icrac were determined as the difference between averaged ramps (four to eight) during maximally activated current and after block of Icrac with 0.5 μM Gd3+. In some cases, averaged ramps before Icrac activation were used as leak currents. Current amplitudes are normalized to cell size and are presented as pA/pF.
ACKNOWLEDGMENTS.
We thank Dr. Brian Kawasaki for performing the experiment whose results are shown in Fig. 5A and Dr. Zhenshan Wang and Ms. Diana Walstad for technical assistance. This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
The authors declare no conflict of interest.
In these types of experiments, SOCE is quantified by analyzing the Ca2+ influx into cells in which the stores are depleted by inactivation with thapsigargin (Tg) of the sarcoplasmic–endoplasmic reticulum Ca2+-activated ATPases. ROCE is quantified by triggering store depletion secondarily to activation of the Gq–phospholipase Cβ pathway, which stimulates cells through an endogenous or a transfected receptor that activates the Gq/11 pathway.
“Silencing” is used as an operational term denoting inability to record, without connotation of physical removal from the membrane.
References
- 1.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 2.Hogan PG, Rao A. Dissecting ICRAC, a store-operated calcium current. Trends Biochem Sci. 2007;32:235–245. doi: 10.1016/j.tibs.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Vig M, Kinet JP. The long and arduous road to CRAC. Cell Calcium. 2007;42:157–162. doi: 10.1016/j.ceca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abramowitz J, Yildirim E, Birnbaumer L. The TRPC Family of Ion channels: Relation to the TRP Superfamily and Role in Receptor- and Store-Operated Calcium Entry, In: Liedke W, editor. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton, FL: CRC Press; 2006. pp. 1–30. [PubMed] [Google Scholar]
- 5.Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem. 2005;280:29559–29569. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- 6.Freichel M, et al. Lack of an endothelial store-operated Ca2+ current impairs agonist dependent vasorelaxation in Trp4−/− mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci USA. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao YH, et al. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci USA. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann T, et al. Direct activation of human TRPC6 and TRPC3 channels by diacyglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 10.Boulay G, et al. Modulation of Ca2+ entry by polypeptides of the inositol 1,4,5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): Evidence for roles of TRP, IP3R in store depletion-activated Ca2+ entry. Proc Natl Acad Sci USA. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiselyov K, et al. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 12.Rosado JA, Brownlow SL, Sage SO. Endogenously expressed Trp1 is involved in store-mediated Ca2+ entry by conformational coupling in human platelets. J Biol Chem. 2002;277:42157–42163. doi: 10.1074/jbc.M207320200. [DOI] [PubMed] [Google Scholar]
- 13.Flemming PK, et al. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem. 2006;281:4977–4982. doi: 10.1074/jbc.M510301200. [DOI] [PubMed] [Google Scholar]
- 14.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation, insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurst RS, Zhu X, Boulay G, Birnbaumer L, Stefani E. Ionic currents underlying hTrp3 mediated agonist-dependent Ca2+ influx in stably transfected HEK293 cells. FEBS Lett. 1998;422:333–338. doi: 10.1016/s0014-5793(98)00035-0. [DOI] [PubMed] [Google Scholar]
- 17.Boulay G, et al. Cloning and expression of mTrp6, a novel mammalian homolog of Drosophilia transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G proteins. J Biol Chem. 1997;272:29672–29680. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- 18.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast mells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 19.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 20.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: Local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang GN, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 23.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worley PF, et al. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu X, et al. TRP, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]