Abstract
The pituitary gland adapts the proportion of each of its endocrine cell types to meet differing hormonal demands throughout life. There is circumstantial evidence that multipotent adult progenitor cells contribute to this plasticity, but these cells have not been identified. Here, we describe a small (<0.05%) population of progenitor cells in the adult pituitary gland. We show that these cells express SOX2, a marker of several early embryonic progenitor and stem cell types, and form “pituispheres” in culture, which can grow, form secondary spheres, and differentiate to all of the pituitary endocrine cell types, as well as folliculostellate cells. Differentiation of cells in the pituispheres was associated with the expression of nestin, SOX9, and S100. Cells expressing SOX2 and E-cadherin are found throughout Rathke's pouch (RP) in embryos but persist in the adult gland, mostly in a narrow zone lining the pituitary cleft, but also are scattered throughout the pituitary. However, unlike in embryonic RP, most of these SOX2+ cells in the adult gland also express SOX9 and S100. We suggest that this SOX2+/SOX9+ population represents transit-amplifying cells, whereas the SOX2+/SOX9− cells we identify are multipotent progenitor/stem cells persisting in the adult pituitary.
Keywords: stem cell, folliculostellate cells
Tissue-specific stem cells have been identified in several adult organs on the basis of their expression of key marker genes of undifferentiated cells, their capacity to proliferate and self-renew, their differentiation potential, and their ability to regenerate tissue after cell loss. They are most readily demonstrated in organs with high cell turnover and/or regenerative capacity, such as the skin, intestine, or bone marrow (1), but they also are present in specialized niches in tissues long considered to be postmitotic, or with little potential for repair, such as the brain (2) and the heart (3).
The anterior pituitary is an organ with a low steady-state cell turnover (4), with its secretory activity and cell proliferation under neuroendocrine control from the hypothalamus, regulated by peripheral endocrine feedback signals. However, the adult pituitary has the ability to adapt the absolute and relative number of each of its cell types in response to changing physiological demands, and this ability also is thought to be mediated via the hypothalamus. For example, the number of growth hormone (GH)-producing cells (somatotrophs) doubles during puberty, whereas the number of prolactin (PRL)-secreting cells (lactotrophs) expands and contracts several fold during pregnancy, lactation, and weaning (5). The mechanisms underlying these changes are poorly understood. Fluctuations in the rate of cell division and cell death in the differentiated endocrine cell populations could explain some of the changes, but direct studies of the pituitary mitotic responses after stimuli that increase the number of ACTH and LH cells show that these changes do not arise by cell division from mature endocrine cells but rather by differentiation from uncommitted progenitors (6). Furthermore, it is difficult to explain the remarkable ability of the adult pituitary gland to repopulate after extreme loss of specific cell populations by genetic (7) or immune (8) lesions without the involvement of a multipotent stem or progenitor population in the adult gland (reviewed in refs. 9 and 10).
Several minor cell populations in the pituitary are candidates for such progenitors, including marginal cells (MCs), which line the pituitary cleft [the adult remnants of the embryonic Rathke's pouch (RP)], and folliculostellate (FS) cells, which are found throughout the pituitary parenchyma (11). FS cells are enriched in pituitary fractions with colony-forming abilities, from which sporadic somatotroph differentiation has been reported both in vitro (12) and in vivo (13). However, FS cells are heterogeneous, representing several types of cells with diverse morphology and gene expression, and convincing evidence for their role as multipotent progenitors is lacking (14–16). Recently, Chen et al. (17, 18) used FACS to isolate a side population from dispersed pituitary cells, enriched for markers reminiscent of stem cell and embryonic progenitors, such as Sca-1 and CD133. Although this fraction could give rise to nonadherent spheres in culture, this population contains a large mixture of different cell types and markers, and their differentiation potential was not established. Thus, the identity and location of the elusive adult pituitary stem or progenitor cell remains to be established.
SOX2, a member of the SOXB1 subfamily of HMG box transcription factors, is required for the maintenance of several stem cell populations (19), for the pluripotent cells of the preimplantation mouse embryo, and for their in vitro counterpart, ES cells (20). We recently reported that the heterozygous loss of Sox2 in both mice and humans also is associated with hypopituitarism (21) and endocrine disruption, with variable abnormalities in pituitary morphology (distorted or additional clefts) in embryonic and adult Sox2+/− mice (21). An increasing body of evidence supports the importance of SOX2 in stem cells of the developing and adult CNS and within sensory organs (22). For example, humans heterozygous for SOX2 mutations and mice carrying a regulatory mutation in Sox2 show hippocampal atresia, which likely reflects the loss of neural stem cells (23). Here, we provide evidence that SOX2+ cells found throughout the embryonic RP remain as a small population of multipotent stem and/or progenitor cells in the adult pituitary gland, capable of differentiating into all of the pituitary cell types.
SOX2 Is Expressed in Embryonic Pituitary Progenitors and Nonendocrine Cells of the Adult Pituitary.
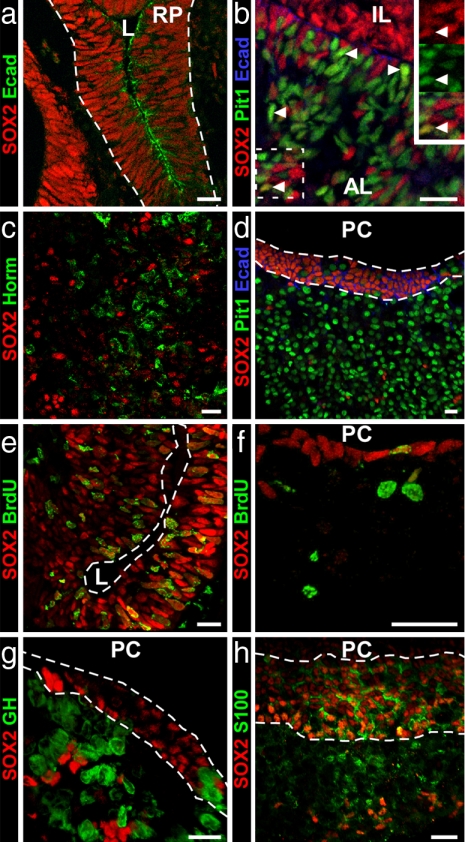
The pituitary primordium of RP is initially organized as an epithelial layer of progenitors surrounding a lumen. At 11.5 days postcoitum (dpc), SOX2 is expressed ubiquitously throughout RP (Fig. 1a) (21) in cells that also express E-cadherin, a marker of adult MCs and FS cells (see Fig. 1d). At this stage, E-cadherin is enriched in membranes facing the lumen of the pouch (E-cad) (Fig. 1a), and the cells are highly proliferative as shown by BrdU incorporation at 12.5 dpc (Fig. 1e). By 16.5 dpc, cells are differentiating ventrally. SOX2 expression is still widespread (Fig. 1b), but only a few SOX2+ cells express the intermediate lineage marker Pit-1 (Fig. 1b), and none expresses SF1, which is present in gonadotroph precursors (data not shown). By 18.5 dpc (Fig. 1c), SOX2 is mostly expressed in the dorsal proliferative zone of the developing pituitary and in scattered cells of the anterior lobe. There is no colocalization of SOX2 with endocrine cell markers (Fig. 1c).
Fig. 1.
SOX2 expression in embryonic and adult pituitary. (a) At 11.5 dpc, SOX2 is ubiquitous in RP, and cell membranes facing the pouch's lumen (L) show E-cad immunoreactivity. (b and c) At 16.5 dpc (b), a few ventral cells coexpress SOX2 and Pit1 (arrowheads), but SOX2 is excluded from the endocrine cell population in the anterior lobe at 18.5 dpc (c). (d) In the adult pituitary, no colocalization is found with Pit1, and SOX2+ cells in the cleft still express E-cad. (e) One and one-half hour BrdU labeling shows high mitotic activity in SOX2+ embryonic pituitary progenitors at 12.5 dpc. (f) In the adult, a few cells lining the pituitary cleft (PC) are still mitotically active. (g and h) SOX2 is not expressed in somatotrophs (g) but is expressed in marginal cells (between dashed lines) and FS cells as shown by its coexpression with S100 protein (h). AL, anterior lobe; IL, intermediate lobe; Horm, pituitary hormones. (Scale bars: 15 μm.)
In adult pituitary glands, SOX2+ cells (representing 3–5% of the cells in the anterior lobe) are present in a concentrated layer lining the pituitary cleft and are scattered throughout the parenchyma (Fig. 1d). This SOX2+ population remains mitotically active, although the proportion of SOX2+ cells labeled after a 90-min pulse of BrdU was much lower than at 12.5 dpc (1% vs. 80%) (Fig. 1 e and f). No SOX2+ cells in the adult gland coexpressed Pit 1 (Fig. 1d), GH [Fig. 1g and supporting information (SI) Fig. 5], or other hormones (data not shown). Based on their localization, the SOX2+ cells in adults most likely correspond to MCs and FS cells. One marker for these cells is S100 protein (24), which is present from postnatal day 10 (P10) in MCs and P15 in FS cells (25). We found that all S100-expressing cells were SOX2+, but not all SOX2+ cells expressed S100 (Fig. 1h and SI Fig. 6). E-cad also is a marker of MCs and FS cells in the rat pituitary (26). In the mouse pituitary, E-cad and SOX2 colocalize in both the embryo (Fig. 1a) and the adult gland (Fig. 1d). The lack of coexpression of SOX2 with CD-31, F4/80, or ER-TR7 (SI Fig. 7) suggests that the SOX2+ cells are not endothelial, macrophage, or fibroblast cells.
SOX9 Expression in the Pituitary Marks Differentiating Progenitors in the Embryo and Most SOX2+ Cells in the Adult.
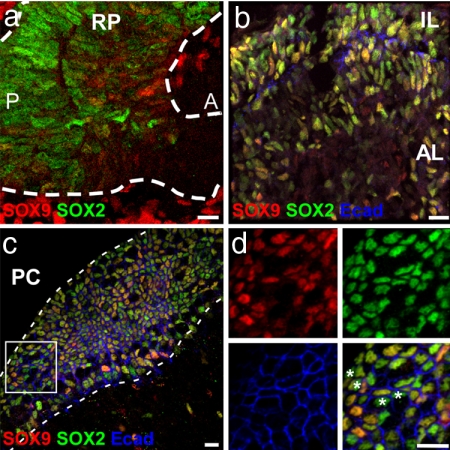
In the developing nervous system, SOX2 marks all cells of the early neuroepithelium. Although SOX2 is generally lost as cells differentiate, it is retained all the way through to the adult in neural stem cell populations wherever they have been described. SOX9, a member of the SoxE subfamily, also is expressed by multipotent progenitor cell types with stem cell properties in some areas of the CNS, for example, during the generation of the neural crest and glia (27, 28) (C. Scott, J. Briscoe, and R.L.-B., unpublished data). We therefore investigated the expression of SOX9 in the pituitary in relation to SOX2+ cells. At 12.5 dpc, SOX9 is expressed in RP in a few SOX2+ cells at low levels (Fig. 2a). By 18.5 dpc, more cells express SOX9 (Fig. 2b), and in the adult pituitary, SOX9 expression colocalizes with SOX2 in both MC and FS cells (Fig. 2c and SI Fig. 6). Note, however, that ≈1% of SOX2+ E-cad+ cells do not express SOX9 (Fig. 2d). Five hours after a single BrdU injection, the few SOX2+ cells incorporating BrdU also were positive for SOX9. However, with a BrdU-labeling protocol designed to look for label-retaining cells, the BrdU+ SOX2+ cells were all negative for SOX9 (SI Fig. 8). These data are consistent with the latter corresponding to slowly dividing cells, a feature displayed by some stem cells, and the former, containing SOX9, to a more rapidly dividing progenitor that could represent a transit-amplifying cell type.
Fig. 2.
SOX9 is expressed in the embryonic pituitary and defines two populations of SOX2+ cells in the adult gland. (a and b) At 12.5 dpc (a), SOX9 is only sparsely expressed within the RP but shows a strong colocalization with SOX2 at 18.5 dpc (b). At the earlier stage, there are mesenchymal cells (Mes) strongly positive for SOX9 outside the pouch. (c and d) In the adult cleft (c), SOX2 and SOX9 are coexpressed in marginal cells (between dotted lines) and FS cells, although a few SOX2+ cells, indicated by asterisks, are negative for SOX9 (d). L, Rathke's pouch lumen; A, anterior; P, posterior; PC, pituitary cleft. (Scale bars: 15 μm.)
A Subpopulation of SOX2+ Cells Generate Pituispheres.
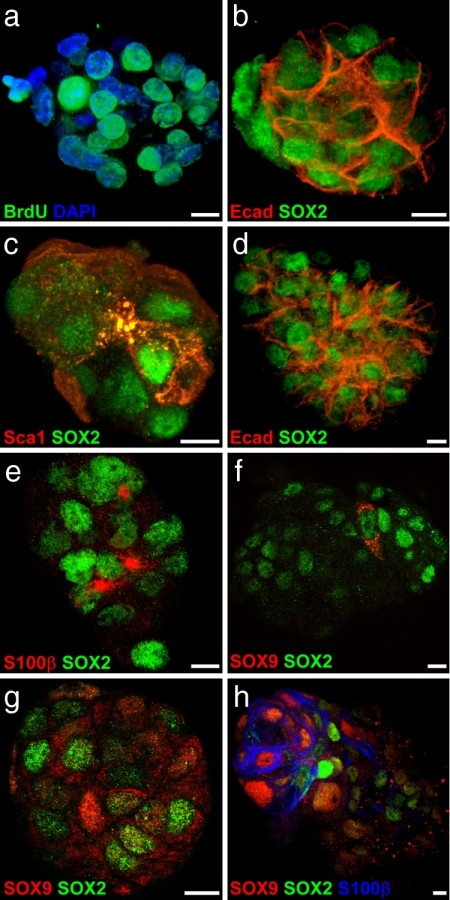
To test the progenitor potential of adult pituitary SOX2+ cell populations, nonadherent pituispheres were grown in culture from dispersed adult anterior pituitaries (17). After 4 days, pituispheres were found at a density of 1 per 5,000 to 1 per 10,000 cells seeded. The number of cells per pituisphere was 22.7 ± 0.9 (mean ± SEM of 20 spheres; range, 16–30). When cultured in the presence of BrdU for 24 h after the first day of culture, virtually all cells were labeled by day 4, suggesting growth by cell division rather than reaggregation (Fig. 3a). Notably, SOX2 and E-cad were coexpressed in the vast majority of cells in 4-day-old pituispheres (20 spheres double-stained for SOX2 and Ecad, representing 583 cells, 98% of which express SOX2 and 97% Ecad) (Fig. 3b) as well as Stem cell antigen-1 (Sca-1) (Fig. 3c). However, no S100 (whole protein or β subunit) could be detected, nor were any hormones detectable. Secondary pituispheres could be obtained from disaggregated primary pituispheres, although infrequently (1 per 150 primary pituispheres or ≈1 per 3,500 cells).
Fig. 3.
The SOX2+ subpopulation forms pituispheres in culture. (a) BrdU labeling shows that pituispheres grow by cell division, not aggregation. (b and c) SOX2, E-cad (b), and Sca-1 (c) are highly expressed in 4-day-old pituispheres. (d–f) SOX2 and E-cad are still highly expressed after 7 days in culture (d), and an FS cell-like population appears as shown by S100β immunostaining (e), which coincides with the onset of SOX9 expression (f). (g) As SOX9 expression increases, the protein shows more nuclear localization. (h) When pituispheres are grown in differentiating conditions, SOX9 quickly becomes nuclear, and regions of higher SOX9 nuclear localization correlate with regions of S100β expression. (Scale bars: 5 μm.)
After 7 days in culture, the number of cells per pituisphere had doubled (43 ± 1.6, mean ± SEM of 20 spheres; range, 29–57), and the percentage of SOX2+ E-cad+ (Fig. 2d) cells was slightly decreased (93%, 20 spheres, 837 cells). Secondary spheres also could be obtained from these larger primary pituispheres but at a similar infrequent rate. In 7-day-old pituispheres, Sca-1 was no longer detectable, whereas S100β was now expressed (Fig. 3e), as was nestin (data not shown). SOX9 expression also became detectable after 6–7 days, initially with cytoplasmic immunostaining (Fig. 3f), but with nuclear locaslization as expression increased (Fig. 3g). Interestingly, if growth factors were removed from the medium after 4 days in culture, SOX9 expression appeared within 36 h, and stronger S100β immunostaining appeared 24 h later, where SOX9 nuclear staining was most intense (Fig. 3h). These results suggest that pituispheres can form from the SOX2+ E-cad+ S100− cells present in vivo in adult pituitary cleft-lining cells and initially contain a self-renewing cell type. As these pituispheres enlarge, they decrease their expression of early progenitor markers, with the emergence of markers characteristic of FS cell-like phenotypes.
SOX2+ Progenitors Are Multipotent, and Their Differentiation Is Influenced by Cell Culture Conditions.
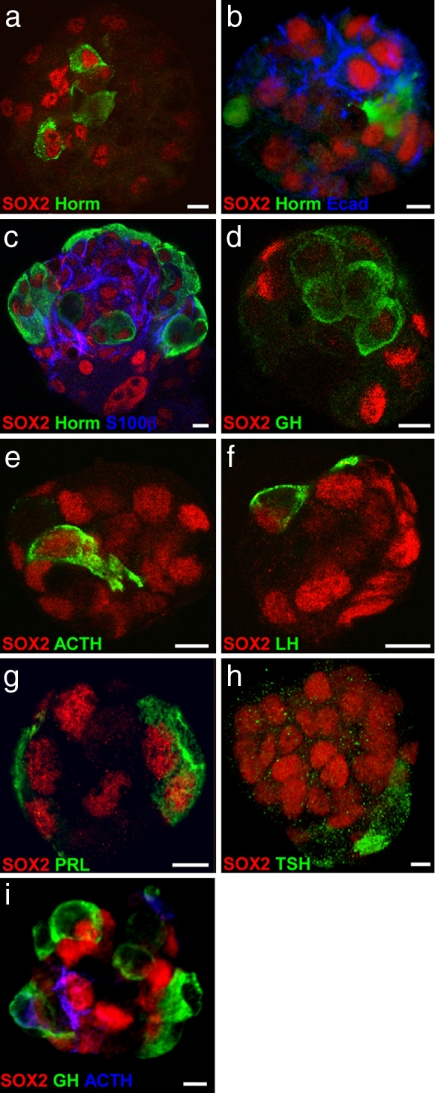
A true test of multipotent pituitary progenitors is their ability to generate all of the endocrine cell types. After 7 days in culture in the continued presence of growth factors, only a small proportion of pituispheres contained one or two isolated hormone-producing cells, always located in the center of the sphere (7.8%, 19 of 242 spheres) (Fig. 4a and SI Movie 1). Some of these cells showed hormone and SOX2 staining, a feature never observed in vivo. It is unlikely that these cells were trapped by aggregation during pituisphere formation because they were not present in 4-day-old cultures. Nevertheless, the differentiation from SOX2+ progenitors was clearly a rare occurrence under these conditions. Therefore, we sought to identify culture conditions more conducive to differentiation. After 5 days in culture [when only 4.4% of the spheres (8 of 182) contained any differentiated endocrine cell types], pituispheres were picked at random and cultured in medium without bFGF and EGF or serum on coverslips coated unevenly with concentrated (1/10) Matrigel (which helped to preserve the 3D structure of the sphere). Under these conditions, virtually all of the pituispheres (69 of 71) contained differentiated cells within 3–4 days (Fig. 4b), with increasing numbers after 7 days (Fig. 4c). The differentiating cells were mostly at the surface, often on one side, although some were present in the center of the pituisphere (Fig. 4c).
Fig. 4.
Pituispheres contain multipotent progenitors. (a) In the presence of growth factors, a few endocrine cells differentiate inside pituispheres, and some still express SOX2. (b) When 5-day-old pituispheres are cultured on Matrigel-coated coverslips in the absence of growth factors, endocrine cells differentiate within 4 days, although SOX2- and E-cad-expressing cells remain evident. (c) After 8 days, more hormone-producing cells are found mostly at the surface of the sphere, whereas S100β is found in regions within the structure. (d–h) GH (d), ACTH (e), LH (f), PRL (g), and TSH (h) immunostainings after 9 days in differentiating conditions. (i) Pituisphere cells can give rise to differentiated cells from separate lineages as shown by the presence of both GH- and ACTH-producing cells in the same pituisphere. (Scale bars: 5 μm.)
All of the anterior pituitary hormone cell types were found in such spheres (Fig. 4 d–h). Furthermore, different endocrine cell types could be found within single spheres. For example, staining for GH and ACTH cells, which represent unrelated lineages, showed that 65% of spheres (n = 34) expressing GH or ACTH contained both endocrine cell types (e.g., Fig. 4i). Moreover, for each hormone cell type, we saw at least one cell costaining for SOX2, which is consistent with their differentiation from SOX2+ progenitors. Conversely, SOX2 immunostaining decreased in cell number and intensity in spheres differentiating under these conditions, with a strong concomitant decrease in E-cad (Fig. 4b) and a complete loss of Sca-1 (data not shown). S100β (Fig. 4c) was highly expressed, but not ubiquitously, often in cells positioned between differentiated endocrine cells and SOX2+ S100β− cells. We did not observe the expression of S100β in hormone-positive cells.
In contrast, adding serum to the differentiation medium and coating the coverslips evenly with diluted Matrigel resulted in the pituispheres flattening into a monolayer of very large cells. Virtually all of these cells expressed SOX2 and nestin, but not Sca-1, and >80% of the cells stained for S100β (see SI Fig. 9), similar to the FS cell-like population in pituispheres after 7 days in nondifferentiating conditions. Because a few endocrine cells could be detected in these monolayers after 7 days (see SI Fig. 9), the remaining S100β− cells in this monolayer may retain some progenitor capabilities. However, no pituispheres were obtained when the cells in this monolayer were dissociated and cultured in medium containing growth factors. Together these data show that, within the pituispheres, the SOX2+ progenitors can differentiate into all of the endocrine cell types present in the anterior pituitary probably through a transitory FS cell-like phenotype.
Discussion
In this study, we show that the adult anterior pituitary gland contains a small subpopulation of SOX2+ cells that, when cultured in vitro, are able to form pituispheres that can proliferate, self-renew into secondary spheres, and differentiate into all pituitary cell types. In both the spheres and the gland, this subpopulation of SOX2+ cells expresses E-cad, but not SOX9 or S100, and corresponds to BrdU+ label-retaining cells. We estimate that these progenitors represent only 0.03–0.05% of the total number of cells in the gland, a percentage similar to the frequency of pituisphere generation. They share other markers with early embryonic progenitors, and their location is consistent with data from other studies proposing the existence of pituitary stem cells (9, 10). The pituispheres that develop by proliferation from such cells give rise in vitro to differentiated GH-, ACTH-, PRL-, LH-, and TSH-producing cells, as well as S100+ FS cells. Moreover, a single sphere often contains more than one endocrine cell type, which is consistent with their progenitors being multipotent. Under these in vitro conditions, a few endocrine cells still stained for low levels of SOX2 protein. We believe this staining represents residual protein, rather than continued transcription, and supports our findings that the hormone-producing cells arise from SOX2+ progenitors, rather than rare SOX2− cells within the pituispheres. SOX2+ endocrine cells are not seen in vivo, but this finding may be explained by the more rapid appearance of hormone markers after an abrupt switch to differentiating conditions in our in vitro model.
Early pituispheres adopted a morphology that could be analogous to a folded epithelium, very similar to RP. In “nondifferentiating” conditions, a few hormone-producing cells appeared at the center of the sphere, whereas one might expect them on the outside (if “outside” corresponded to “away from” the MCs). However, a previous study with quail RPs in culture also showed differentiation in the center, with cells lining the lumen of the pouch migrating toward the outside of the explant (29). It was notable that when growth factors that prevent differentiation were absent from the pituisphere medium, the extensive differentiation that occurred was now mostly at the surface.
Under differentiating conditions, pituisphere cells progressed through a transitional phase of SOX9, nestin, and S100β expression. During pituitary development in vivo, SOX9 expression is detected from ≈12.5 dpc, when most pituitary progenitors begin their commitment to specific lineages and is maintained in the majority of the SOX2+ population throughout life. SOX9 coincided with nestin and S100β expression in later pituispheres, and we were unable to generate secondary pituispheres from monolayers of these cells. Because S100β expression in SVZ astrocytes coincides with the loss of their neural stem cell potential (30), the appearance of S100 in pituitary cells may mark a transition from uncommitted to committed progenitor cells (9, 10).
Some nestin+ S100β+ cells may correspond to the small subpopulation of double-positive FS cells in vivo, whereas most adult pituitary FS cells express S100 only (18). These cells are enriched in our pituispheres when cultured in the presence of serum, but do not express hormone markers. It is possible that S100β expression must be down-regulated before the SOX9+ cells differentiate because SOX9 has been shown to block terminal differentiation of chondrocytes by activating S100 protein expression (31). MCs and FS cells are the most proliferative cell type in the pituitary (32), whereas the SOX2+ SOX9− population we describe has a slow division rate. Taken together, the data suggest that the SOX9+ cells in vitro correspond to the MCs and FS cells expressing SOX9 and S100 in vivo and represent actively dividing progenitors at a later stage committed toward differentiation. If our model is correct, the progenitor and differentiating regions, spatially separated as cells migrate in the developing pituitary (33), are compressed and intermingled in the residual SOX2+ population in the adult pituitary as either SOX9− cells (multipotent progenitor/stem cells) or SOX9+ cells (transit-amplifying cells). Thus, it is possible that their locations in the cleft and throughout the parenchyma represent different residual stem or progenitor “niches” in the adult gland, responsive to different requirements for minor or major remodeling according to physiological demand.
The FS cell population makes a sizeable contribution to the pituitary gland and remains remarkably resistant to insults that virtually wipe out endocrine cell lineages (K.R. and I.C.A.F.R., unpublished data). The terms MCs and FS cells clearly cover a heterogeneous group of SOX2+ cells, and other markers may be needed to identify a subpopulation of FS cells that could contribute in vivo to postnatal pituitary remodeling. Indeed, FS cells (or, more likely, a subpopulation of such cells) are ideal candidates for such a role. Although the network organization of endocrine cells has recently become recognized (35), the first such network to be identified anatomically (36), and then functionally (37, 38), was that of FS cells, which pervade the gland. Moreover, they are in close contact with blood vessels and can integrate signals originating from the pituitary, the brain, peripheral organs, and the immune system (14). Finally, FS cells secrete bFGF (39) and LIF (40), two factors crucial for the maintenance of other stem cell populations and that could play an important role in supporting the SOX2+ E-cad+ SOX9− population in their niche(s).
In the embryo, the orderly emergence of the different cell lineages is thought to be controlled by diffusible factors emanating dorsally from the ventral diencephalon (BMP4 and FGFs), ventrally from the oral ectoderm and surrounding mesenchyme (Shh, BMP2), and then within the early pituitary to establish the proportion and spatial organization of each cell type at birth (34). Because S100 expression starts during the second week after birth, it is tempting to propose that the adult transit-amplifying cells appear when pituitary trophic activity is needed to respond to altered physiological demands for particular endocrine cell populations, rather than overall organ size, and differentiate only when they receive the appropriate signals. In the adult, these cues most likely arise as neuroendocrine signals from hypothalamic hypophysiotropic neurons and/or peripheral endocrine feedback delivered via a complex vasculature. Now that the pituitary progenitor population can be identified in vivo and manipulated in vitro, it will be interesting to study how these external factors regulate this progenitor population to achieve the changing numbers and types of differentiated endocrine cells required in the adult gland throughout life.
Materials and Methods
Isolation of Pituitary Cells and Pituisphere Cultures (Modified from ref. 17).
After removing the neurointermediate lobe, anterior pituitary lobes from 10- to 12-week-old MF1 males were dissociated into single cells by using successive incubations in collagenase (0.5% in HBSS) with decreasing calcium concentrations. The yield was ≈60,000 cells per pituitary, with cell viability >85%. Freshly dissociated cells were seeded at a density of 75,000–100,000 cells per ml in 6-cm dishes (3 ml per dish) in DMEM/F12 containing 0.5% BSA, B27, and N2 supplements plus 20 ng/ml bFGF and EGF. A 10X growth factors solution was added daily, and the medium was changed after 3 days.
Differentiation Assays.
Pituispheres were harvested after 5 days in culture, and floating cells were collected and submitted to a series of short low-speed centrifugations (1′, 12 × g) to separate pituispheres from the few remaining isolated cells. Pituispheres were handpicked individually unless they showed obvious aggregation of endocrine cells at their surface. Another series of brief centrifugations was then applied, and one-third of the pituispheres were fixed, and their purity was assessed by immunostaining. If >5% of the spheres already showed endocrine cells, such experiments usually were not taken further. The rest of the pituispheres were diluted to approximately five spheres per well and seeded on glass coverslips coated with Matrigel (1/10). Matrigel was dissolved at 8°C to create an uneven coating with small lumps of polymerized proteins. Growth factors were omitted from the medium.
For differentiation in serum, FCS (10%) and horse serum (10%) were added to the medium, and Matrigel was used at a 1/30 concentration (dissolved at 2°C to obtain a homogeneous solution).
Immunofluorescence.
At 11.5 and 12.5 dpc, embryo heads were fixed in 4% paraformaldehyde (PFA) on ice for 2 h. From 14.5 dpc onward, pituitaries were dissected and fixed. Tissues were cryoprotected in sucrose (20% in PBS) overnight at 4°C and embedded in OCT (BDH), and immunofluorescence was performed on 12-μm cryosections. For most immunostaining of adult pituitaries, mice were anesthetized and perfused with 4% PFA, pituitaries were postfixed for 2 h in 4% PFA on ice, and 25-μm sections were obtained by using a vibratome. BrdU immunostaining was performed on 12-μm cryosections of pituitaries taken at different times after a single BrdU injection or a prolonged labeling protocol (13 daily BrdU injections, followed by a 4-week washout before analysis). Pituispheres were fixed (4% PFA for 15–30 min) and processed under microscopic control. Cells were permeabilized with Triton X-100 (0.1% in PBS), blocked with normal donkey serum (10% in PBS), and incubated with primary antibody: goat anti-SOX2 (1:500; ISL), rabbit anti-SOX2 (1:750; Chemicon), rabbit anti-SOX9 (1:750; a gift from F. Poulat, Institut de Génétique Humaine, Montpellier, France), mouse anti-nestin (1:1,000; Chemicon), rabbit anti-Pit1 (1:750; a gift from S. Rhodes, Indiana University School of Medicine, Indianapolis, IN), mouse anti-S100β and rat anti-E-cad (1:1,000; Sigma–Aldrich), rabbit anti-S100 (1:250; Dako), rat anti-ScaI (1:100; BD Pharmingen), and hormone antisera (National Institute of Diabetes and Digestive and Kidney, courtesy of A. F. Parlow), mouse anti-ACTH (1:1,000; Fitzgerald), rat anti-CD31 (1:50; Pharmingen), rat anti- F4/80 (1:1,000; BMA Biomedical), and rat anti-ER-TR7 (1:100; Abcam). All secondary antibodies were raised in donkey (The Jackson Laboratory) and used at a 1:300 dilution. Sections or pituispheres were mounted in Fluorsave (Calbiochem).
Confocal Imaging.
Samples were imaged by using a Leica TCS SP1 confocal microscope with 40X (1 NA), 63X (1,32 NA), and 100X (1,4 NA) objective lenses. Each fluorescent label was imaged independently. Sequential laser excitation at 351, 488, 568, and 647 nm and emission collection between 460 and 480, 495 and 525, 590 and 620, and 675 and 730 nm was used for DAPI, Alexa488, Rhodamine Red, and Cy5 fluorescence, respectively, and bleed-through was checked. Confocal images were captured in a Z-series with an interslice gap between 0.2 and 1 μm. Images were prepared by using Leica LCS Lite, Volocity 4.2 (Improvision), ImageJ, and Adobe Photoshop 7.0.
Supplementary Material
ACKNOWLEDGMENTS.
We thank M. Hannah, P. LeTissier, and other members of the National Institute for Medical Research for their comments and encouragement, B. Boizet-Bonhoure and F. Poulat for SOX9 antibody and helpful discussions, and A. Gould for critical reading of the manuscript. This work was supported by the Medical Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707886105/DC1.
References
- 1.Moore KA, Lemischka IR. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 2.Gage FH. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 3.Leri A, Kajstura J, Anversa P. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 4.Nolan LA, Kavanagh E, Lightman SL, Levy A. J Neuroendocrinol. 1998;10:207–215. doi: 10.1046/j.1365-2826.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- 5.Levy A. J Endocrinol. 2002;174:147–155. doi: 10.1677/joe.0.1740147. [DOI] [PubMed] [Google Scholar]
- 6.Nolan LA, Levy A. J Neuroendocrinol. 2006;18:655–661. doi: 10.1111/j.1365-2826.2006.01459.x. [DOI] [PubMed] [Google Scholar]
- 7.Borrelli E, Heyman RA, Arias C, Sawchenko PE, Evans RM. Nature. 1989;339:538–541. doi: 10.1038/339538a0. [DOI] [PubMed] [Google Scholar]
- 8.De Jersey J, Carmignac D, Le Tissier P, Barthlott T, Robinson I, Stockinger B. Immunology. 2004;111:254–261. doi: 10.1111/j.1365-2567.2004.01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vankelecom H. Neuroendocrinology. 2007;85:110–130. doi: 10.1159/000100278. [DOI] [PubMed] [Google Scholar]
- 10.Vankelecom H. Semin Cell Dev Biol. 2007;18:559–570. doi: 10.1016/j.semcdb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Inoue K, Couch EF, Takano K, Ogawa S. Arch Histol Cytol. 1999;62:205–218. doi: 10.1679/aohc.62.205. [DOI] [PubMed] [Google Scholar]
- 12.Lepore DA, Roeszler K, Wagner J, Ross SA, Bauer K, Thomas PQ. Exp Cell Res. 2005;308:166–176. doi: 10.1016/j.yexcr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Lepore DA, Thomas GP, Knight KR, Hussey AJ, Callahan T, Wagner J, Morrison WA, Thomas PQ. Stem Cells. 2007;25:1730–1736. doi: 10.1634/stemcells.2007-0012. [DOI] [PubMed] [Google Scholar]
- 14.Allaerts W, Vankelecom H. Eur J Endocrinol. 2005;153:1–12. doi: 10.1530/eje.1.01949. [DOI] [PubMed] [Google Scholar]
- 15.Horvath E, Kovacs K. Ultrastruct Pathol. 2002;26:219–228. doi: 10.1080/01913120290104476. [DOI] [PubMed] [Google Scholar]
- 16.Inoue K, Mogi C, Ogawa S, Tomida M, Miyai S. Arch Physiol Biochem. 2002;110:50–53. doi: 10.1076/apab.110.1.50.911. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. Endocrinology. 2005;146:3985–3998. doi: 10.1210/en.2005-0185. [DOI] [PubMed] [Google Scholar]
- 18.Krylyshkina O, Chen J, Mebis L, Denef C, Vankelecom H. Endocrinology. 2005;146:2376–2387. doi: 10.1210/en.2004-1209. [DOI] [PubMed] [Google Scholar]
- 19.Boiani M, Scholer HR. Nat Rev Mol Cell Biol. 2005;6:872–884. doi: 10.1038/nrm1744. [DOI] [PubMed] [Google Scholar]
- 20.Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelberman D, Rizzoti K, Avilion A, Bitner-Glindzicz M, Cianfarani S, Collins J, Chong WK, Kirk JM, Achermann JC, Ross R, et al. J Clin Invest. 2006;116:2442–2455. doi: 10.1172/JCI28658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham V, Khudyakov J, Ellis P, Pevny L. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 23.Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, et al. Development (Cambridge, UK) 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Yamaguchi H, Takahashi K. Brain Res. 1980;191:523–531. doi: 10.1016/0006-8993(80)91300-1. [DOI] [PubMed] [Google Scholar]
- 25.Shirasawa N, Kihara H, Yamaguchi S, Yoshimura F. Cell Tissue Res. 1983;231:235–249. doi: 10.1007/BF00222177. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi M, Yatabe M, Fujiwara K, Takigami S, Sakamoto A, Soji T, Yashiro T. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1183–1189. doi: 10.1002/ar.a.20384. [DOI] [PubMed] [Google Scholar]
- 27.Wegner M, Stolt CC. Trends Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J. Dev Cell. 2005;8:179–192. doi: 10.1016/j.devcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Fremont PH, Ferrand R. Anat Embryol (Berl) 1980;160:275–284. doi: 10.1007/BF00305108. [DOI] [PubMed] [Google Scholar]
- 30.Raponi E, Agenes F, Delphin C, Assard N, Baudier J, Legraverend C, Deloulme JC. Glia. 2007;55:165–177. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito T, Ikeda T, Nakamura K, Chung UI, Kawaguchi H. EMBO Rep. 2007;8:504–509. doi: 10.1038/sj.embor.7400934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson DB. Acta Anat (Basel) 1986;126:121–126. doi: 10.1159/000146199. [DOI] [PubMed] [Google Scholar]
- 33.Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Mol Endocrinol. 2005;19:698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
- 34.Zhu X, Gleiberman AS, Rosenfeld MG. Physiol Rev. 2007;87:933–963. doi: 10.1152/physrev.00006.2006. [DOI] [PubMed] [Google Scholar]
- 35.Bonnefont X, Lacampagne A, Sanchez-Hormigo A, Fino E, Creff A, Mathieu MN, Smallwood S, Carmignac D, Fontanaud P, Travo P, et al. Proc Natl Acad Sci USA. 2005;102:16880–16885. doi: 10.1073/pnas.0508202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vila-Porcile E. Z Zellforsch Mikrosk Anat. 1972;129:328–369. [PubMed] [Google Scholar]
- 37.Fauquier T, Guerineau NC, McKinney RA, Bauer K, Mollard P. Proc Natl Acad Sci USA. 2001;98:8891–8896. doi: 10.1073/pnas.151339598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fauquier T, Lacampagne A, Travo P, Bauer K, Mollard P. Trends Endocrinol Metab. 2002;13:304–309. doi: 10.1016/s1043-2760(02)00616-1. [DOI] [PubMed] [Google Scholar]
- 39.Ferrara N, Schweigerer L, Neufeld G, Mitchell R, Gospodarowicz D. Proc Natl Acad Sci USA. 1987;84:5773–5777. doi: 10.1073/pnas.84.16.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrara N, Winer J, Henzel WJ. Proc Natl Acad Sci USA. 1992;89:698–702. doi: 10.1073/pnas.89.2.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.