Abstract
The possibility for fishery-induced evolution of life history traits is an important but unresolved issue for exploited fish populations. Because fisheries tend to select and remove the largest individuals, there is the evolutionary potential for lasting effects on fish production and productivity. Size selection represents an indirect mechanism of selection against rapid growth rate, because individual fish may be large because of rapid growth or because of slow growth but old age. The possibility for direct selection on growth rate, whereby fast-growing genotypes are more vulnerable to fishing irrespective of their size, is unexplored. In this scenario, faster-growing genotypes may be more vulnerable to fishing because of greater appetite and correspondingly greater feeding-related activity rates and boldness that could increase encounter with fishing gear and vulnerability to it. In a realistic whole-lake experiment, we show that fast-growing fish genotypes are harvested at three times the rate of the slow-growing genotypes within two replicate lake populations. Overall, 50% of fast-growing individuals were harvested compared with 30% of slow-growing individuals, independent of body size. Greater harvest of fast-growing genotypes was attributable to their greater behavioral vulnerability, being more active and bold. Given that growth is heritable in fishes, we speculate that evolution of slower growth rates attributable to behavioral vulnerability may be widespread in harvested fish populations. Our results indicate that commonly used minimum size-limits will not prevent overexploitation of fast-growing genotypes and individuals because of size-independent growth-rate selection by fishing.
Keywords: behavior, fisheries, selection, temperament
It is well known that fisheries tend to select for larger and older fish individuals because of preference and/or regulations imposing minimum size limits for harvest. The result of sustained and heavy size-selective harvesting over time has been the removal of larger and/or later-maturing individuals from populations, leaving behind populations consisting of small, early-maturing individuals, with low fecundity (1–3). Because growth rate affects fish size at age and size at maturity, a size-selective fishery may indirectly remove faster-growing individuals from a population. Studies suggest that this effect may represent contemporary evolution, leaving behind genotypes that are slower-growing and early-maturing; this then can lead to reductions in harvestable biomass and population fecundity that in turn hinders population recovery from harvest (2–5). The possibility for evolutionary responses should not be surprising given high heritability of growth rate and other life history parameters in fish and the intensity of size-selective fish harvest reviewed in refs. 2, 6, and 7. However, we are aware of only two studies providing strong evidence of fisheries-induced evolution of growth and/or other life history traits (4, 5).
A fishery may select upon growth rate through both indirect and direct mechanisms. Indirect selection occurs through removal of larger individuals from a population, whereby faster-growing individuals attain harvestable size at a younger age, thus increasing time spent vulnerable to the fishery. However, size selection alone may not be a strong selective pressure on growth rate if fish are large predominantly because they are old but slow-growing. By contrast, direct selection would occur if faster-growing individuals are more vulnerable to fishing gear because of their behavior, independent of their body size. Direct selection on growth rate is likely because faster growth usually is achieved by individuals and genotypes that expend more effort to secure food resources, even at the expense of risk of predation (8–11). Fast-growing individuals/genotypes typically are more active, more bold in the face of risk, and more aggressive than slow-growing individuals/genotypes (5, 8–10, 12). Given these positive correlations between activity and boldness traits and growth rate, we expect that fast-growing individuals will encounter fishing gear more frequently because of greater activity rates, be less likely to detect and avoid them because of decreased vigilance (i.e., greater boldness), and aggressively pursue lures and baits. Therefore, the probability of harvest of individual fish should be proportional to their behavioral vulnerability, even for fish of equal size. Although size selection is known to result in evolution of lower growth rate and age at maturity in the laboratory (5) and in wild populations (4), the possibility that a fishery may directly select on growth rate because of behavior has not been tested. If so, behavioral vulnerability would represent a unique mechanism for selection on growth rate not previously considered in the context of fisheries harvest management. If this unique mechanism of selection exists, it may suggest that evolutionary changes in harvested fish populations are more likely and occur more rapidly than previously thought when based solely on predictions from indirect size-selection effects.
Here, we present data from whole-lake experiments showing that genotypes of rainbow trout with high intrinsic growth rate and bold behavioral traits (fast/bold) are more vulnerable to a simulated commercial fishery than slow-growing and shy genotypes (slow/shy), even when the fishing gear does not target specific sizes of fish and size variation in the catch is controlled for statistically. That is, exploitation removes faster-growing individuals from a population by direct selection on behavioral genotype, independent of body size. For the experiments, we use two genotypes of trout known to differ in intrinsic growth rate when fed ad libitum, activity rates, and degree of boldness (see Methods for details). Briefly, we stocked equal densities of each genotype into two small experimental lakes, simulated an intensive commercial gillnet fishery for 5 consecutive days, and quantified the relative rates of harvest of each genotype and the resultant shift in genotypic distribution.
Results
At the whole-population level, the cumulative impact of intensive gillnet fishing over 5 consecutive days in two small lakes was to remove a significantly larger proportion of the fast/bold genotypes from within each population than the slow/shy type (Fig. 1). This difference was not simply the result of differences in fish size among genotypes that affect vulnerability to the nets. The mean length of harvested fish did not differ between populations (lakes), genotypes, or between genotypes within a population (i.e., population × genotype interaction; all P > 0.3; mean length of overall harvest = 26 cm). In addition, the mean length of fish within each size class (each genotype was split into two size classes at stocking) did not differ between populations, genotypes, or their interaction (all P > 0.25). Although these genotypes are known to differ in intrinsic growth rate when fed ad libitum (9), the fast/bold genotype apparently was unable to realize its growth potential because of a combination of feeding on small food items (plankton; P.A.B., unpublished data), high metabolic rates, and depleted plankton abundance resulting from high stocking densities (13) (see also Methods).
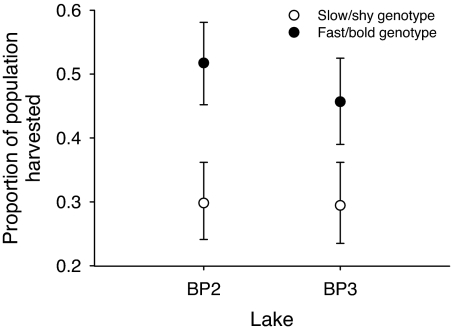
Fig. 1.
Harvest of two genotypes of trout from a gillnet fishery in experimental populations in two small lakes. Shown are the observed proportions harvested over 5 consecutive days of netting and their associated 95% confidence interval (CI) as estimated from likelihood profiles based on a binomial distribution. Nonoverlapping CIs indicate significant differences.
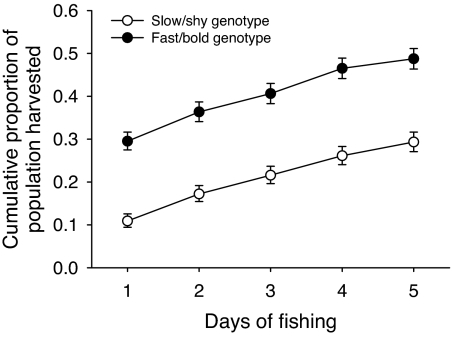
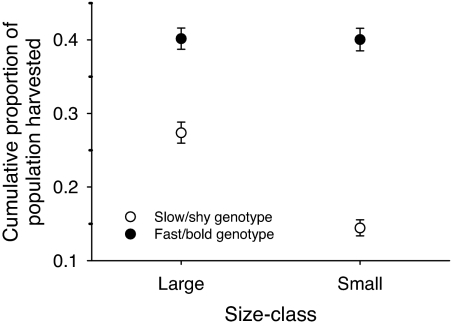
The proportion of fast/bold genotypes removed by fishing was initially three times that observed for the slow/shy genotype (0.3 versus 0.1), followed by similar (but slightly higher rate for fast/bold types) rates of harvest over time that maintained the initial difference (genotype × net day interaction, χ82 = 100, P < 0.0001; Fig. 2). In addition, the proportion of fast/bold genotypes harvested was not size-dependent, indicating that large and small fast/bold fish were equally vulnerable within a population; in contrast, the larger slow/shy individuals were more vulnerable to the fishery than the smaller ones (genotype × size class interaction, χ12 = 32, P < 0.0001; Fig. 3). Finally, the effect of the fishery on relative depletion of the genotypes did not vary between lakes, indicating that the results are robust across populations (genotype × lake interaction, χ12 = 3.7, P > 0.05; see also Fig. 1).
Fig. 2.
Cumulative proportion of each genotype of trout harvested by an intensive gillnet fishery over time, averaged over the two populations in two small lakes (genotype × net day interaction). Shown are the back-transformed least-squares estimates and standard errors.
Fig. 3.
Cumulative proportion of each genotype of trout harvested in an intensive gillnet fishery, in relation to fish size (genotype × size class interaction). Shown are the back-transformed least-squares estimates and standard errors.
Discussion
By simulating an intensive commercial gillnet fishery on trout populations in two small lakes, we were able to show that fast-growing/bold genotypes were harvested to a greater extent, and more rapidly, than slower-growing/shyer genotypes. Given that the fast-growing trout genotype is highly active and bold (9, 12, 14), we predicted that they would encounter nets frequently and tend not to avoid such threats, leading to greater harvest. The greater observed harvest rate of the fast/bold genotype supports this mechanistic hypothesis and demonstrates that there is direct selection against fast-growing individuals in fish populations. Previously, studies have suggested that early maturity and small size in harvested fish populations represents contemporary evolution, resulting from indirect selection on growth rate and size at maturity via size-selective harvest (1, 2, 4, 5, 15). In contrast, our study provides direct evidence of a fishery selecting against a life history trait (growth) within fish populations in nature. Additionally, our study shows that fish behavior has significant effects on fish catchability and harvest leading to direct selection against genotypes with rapid growth rate. Given positive correlations between activity/boldness and growth rate, we show that fishing selects directly on growth rate that is in addition to the well known indirect selection that results from targeting large fish. Our results also have important implications for fisheries management. Given these behavioral effects on vulnerability to harvest, the common practice of regulating the size of fish harvested (minimum size limits) will do little to mitigate the loss of fast-growing genotypes and individuals from fish populations. And, because fast-growing fish often attain larger size at age and are more fecund, the loss of these genotypes may potentially hinder the recovery of exploited fish populations and lower the fishable biomass and yield (1–3, 5, 16).
Our results should apply broadly to many fish populations given that genetic variation in growth rate and corresponding genetic variation in behavior appear common. Fish growth is genetically determined (6), and recent studies show that faster-growing fish tend to be more active and bold (reviewed in refs. 8, 9, 11, 14, 17, and 18). Indeed, the fast/bold genotype used in this experiment was developed by selection on growth rate, resulting in elevated thyroid and growth hormone levels, greater appetite and consumption, and greater expression of behaviors that help secure food resources (i.e., activity, boldness, and aggression; refs. 9, 14, 19, and 20). Additionally, the bold genotype displays all of the normal foraging and antipredator behaviors one would expect of a fish, although antipredator behavior is reduced relative to the shy genotype to gain access to food (9, 21) (see also Methods). Although our study uses a trout genotype possessing maximum mean growth rates not observed in nature, similar rates of selection against fast/bold genotypes should occur within natural populations because the fast/bold type originated from a wild population and there is still considerable overlap in individual growth rates between the two genotypes (P.A.B., unpublished data). In other words, our results are not outside the realm of possible selection in natural populations. Nevertheless, our aim is to demonstrate the potential for selection on fish growth and not to suggest that the actual rate of selection (in an evolutionary sense) should be inferred from our data.
Our results, although obtained from a single type of fishing gear, also should apply to fishing gear other than gillnets. For instance, trout in small lakes appear to be composed of highly vulnerable individuals that are rapidly caught by angling and individuals that more difficult to catch (22). Indeed, a recent study shows that vulnerability of fish to angling is a heritable trait in fish and is related to metabolic rate and some parental behaviors (23). In fact, greater activity rates and boldness by fast-growing individuals are likely to increase encounter rates with any fishing type of fishing gear, although the relative harvest of fast/bold versus slow/shy genotypes is likely to be diminished for gear such as trawling that sweeps the water column. Further, if faster-growing individuals tend to congregate to a larger extent in food-rich but risky habitats (9, 14), then knowledgeable fishers with high-tech fish-locating devices may well target those areas, harvesting fast-growing fish to a greater extent than our data would suggest (we specifically did not target high-quality habitats; see Methods). Finally, our study highlights the importance of adopting behavioral and evolutionary ecology perspectives to our understanding of the short- and long-term effects of fish harvest and the need to foster collaboration between fishery scientists and evolutionary ecologists.
Methods
We used two genotypes of rainbow trout known to differ in intrinsic growth rate and behavior, where the fast-growing genotype also is highly active, bold, and more aggressive than the slow-growing genotype. A wild genotype and a “domestic” genotype that had been selected for rapid growth were obtained from and raised at the Fraser Valley Trout Hatchery (FVTH), Abbotsford, BC, Canada. Wild genotypes were reared from eggs and milt collected from a nearby wild trout population (Tunkwa Lake). The domestic strain resided entirely within the hatchery and originated from a stock in California that was widely distributed across North America (K. Scheer, FVTH, personal communication). Despite having no experience with predators for many generations, the domestic genotype nonetheless displays appropriate antipredator behavior by avoiding risky habitats when predators are present but using them when predators are absent (9, 14).
The domestic strain of trout has a higher intrinsic growth rate than the wild strain when reared under identical conditions and fed ad libitum, achieving greater growth rates attributable to greater appetite and consumption (K. Scheer, FVTH, personal communication; also refs. 9 and 19). Correlated with faster growth rates is a tendency to be generally more active and more prone to take risks while foraging (i.e., more bold; refs. 9, 12, and 21). Furthermore, the fast/bold genotype used in this experiment was developed by selection on growth rate, with correlated responses to selection including elevated thyroid and growth hormone levels, greater appetite and consumption, and greater expression of behaviors that help secure food resources in nature (i.e., activity, boldness and aggression; refs. 9, 14, 19, and 20). In addition, there is additive genetic variation in growth rate evident when these wild and domestic trout are crossed and then back-crossed (19, 20), and wild-domestic hybrids are more bold than wild types (12). For these reasons, we refer to the wild and domestic trout as slow/shy and fast/bold genotypes, respectively. Because both genotypes were reared identically, variation in behaviors affecting catchability should be driven primarily by genetic variation between these strains (12, 19). In fact, we know that the fast/bold genotype of the same size used in this study is generally more active and tends to use risky but food-rich habitats to a greater extent than the slow/shy genotype when stocked into our experimental lakes (21). Both genotypes were raised to a common average length (mean length = 15 cm); we then split the size frequency of each into equal halves to create two nonoverlapping size classes. The adipose fin (a vestigial fin) was clipped to identify the fast/bold genotype and opposite ventral fins were clipped to identify size class in both types. Each genotype and size class was stocked June 1 at identical rates to create a density of 160 trout per ha of each type into each lake, yielding a total fish density of 640 trout per ha. No other fish were present in the lakes because of winterkill the previous winter. The experimental gillnet fishery took place September 25–30.
We stocked the trout into two small lakes, B2 and B3, located within 100 m of one another in southwestern British Columbia, Canada. The lakes are very similar in trophic status, invertebrate abundance, and morphometry and are closed to fishing (details in ref. 10). Although small, these lakes have all of the features of much larger lakes, and fish display normal feeding and antipredator behaviors when stocked into them (13, 24). These two lakes we used are known to be free of the major avian predator on large trout, the common loon (Gavia immer). Many years of experiments and observations confirm that loons never feed on these lakes (which are too small for the large bird to take off from), and that loons are the only significant source of mortality on trout of the size used in this experiment (25). In fact, estimates of mortality do not differ from zero for a dataset comprising 16 lake-years of data in these lakes (25). If there was any significant mortality attributable to predation by other bird species, then it should reduce the relative abundance of the fast/bold genotype, making our assessment of the effect of fishery on selection conservative. Other potential sources of mortality such as disease also seem unlikely because we observed no indications of it when dissecting subsamples of our catch, and given no significant mortality in these lakes (above) suggests that disease in general does not affect trout significantly in our lakes. For those reasons, therefore, we assumed that mortality was zero in both lakes for the purposes of analyses. Because of the absence of a source of mortality in these lakes, fish were not affected by behavioral tradeoffs that influence growth and survival and therefore were free to feed and grow at rates limited only by food abundance, their intrinsic appetite, digestion, and metabolism. However, as outlined above, equal mean mass achieved by autumn between the genotypes suggests that the fast/bold genotype became food-limited at some point relative to the wild type that has lower basal metabolic requirements (see Results).
We simulated an intensive commercial gillnet fishery by using established experimental gillnet protocols (26, 27). Gillnet density was standardized among lakes based on lake area (each lake = 1.4 ha), set at 490 m2·ha−1·night−1. In each lake, we set two sinking gillnet gangs (2.3-m tall) with graded, stretched mesh ranging in size from 24 to 89 mm (1 to 3.5 inches), where each gang of gillnet consisted of seven panels, alternating between small and large mesh sizes (additional details in ref. 26). Nets were set during the day and retrieved 24 h later, for 5 consecutive nights. Nets were moved each day to sample all habitats and areas of the lake over the 5 nights of netting. All captured fish not already dead were killed.
To estimate the population impact of fishing on underlying genotypes, we first calculated the overall proportion of each genotype harvested by fishing in each population. Next, to estimate the rate at which each genotype was removed from the population, we calculated the proportion of each genotype harvested over time during the 5-day fishery while accounting for those harvested the previous day. We used Proc Genmod (SAS Institute) and the raw binomial data to test for the significance of factors affecting fish harvest, evaluating each effect using type III contrasts. We tested for the effects of lake, size class, genotype, and any interactions of genotype with lake, size class, and net day. After removal of the nonsignificant three-way interaction (χ82 = 8.3, P > 0.4), we reran the analyses to test for remaining two-way interactions and main effects.
ACKNOWLEDGMENTS.
We thank the Trends in Environmental Research group for helpful feedback on an earlier version of this paper. P.A.B. was supported by a University of Technology Sydney Chancellor's Postdoctoral Fellowship. Field research was supported by an National Science and Engineering Research Council discovery grant to J.R.P.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.W. is a guest editor invited by the Editorial Board.
References
- 1.Hutchings JA, Baum JK. Measuring marine fish biodiversity: temporal changes in abundance, life history and demography. Philos Trans R Soc London Ser B Biol Sci. 2005;360:315–338. doi: 10.1098/rstb.2004.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Law R. Fishing, selection, and phenotypic evolution. ICES J Mar Sci. 2000;57:659–668. [Google Scholar]
- 3.Jørgensen C, et al. Managing evolving fish stocks. Science. 2007;318:1247–1248. doi: 10.1126/science.1148089. [DOI] [PubMed] [Google Scholar]
- 4.Olsen EM, et al. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004;428:932–935. doi: 10.1038/nature02430. [DOI] [PubMed] [Google Scholar]
- 5.Walsh MR, Munch SB, Chiba S, Conover DO. Maladaptive changes in multiple traits caused by fishing: Impediments to population recovery. Ecol Lett. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- 6.Gjedrem T. Genetic improvement of cold-water fish species. Aquaculture Res. 2000;31:25–33. [Google Scholar]
- 7.Reznick DN, Ghalambor CK. Can commercial fishing cause evolution? Answers from guppies (Poecilia reticulata). Can J Fish Aquatic Sci. 2005;62:791–801. [Google Scholar]
- 8.Stamps JA. Growth-mortality tradeoffs and ‘personality’ traits in animals. Ecol Lett. 2007;10:355–363. doi: 10.1111/j.1461-0248.2007.01034.x. [DOI] [PubMed] [Google Scholar]
- 9.Biro PA, Abrahams MV, Post JR, Parkinson EA. Behavioural trade-offs between growth and mortality explain evolution of submaximal growth rates. J Anim Ecol. 2006;75:1165–1171. doi: 10.1111/j.1365-2656.2006.01137.x. [DOI] [PubMed] [Google Scholar]
- 10.Biro PA, Post JR, Parkinson EA. From individuals to populations: Risk-taking by prey fish mediates mortality in whole-system experiments. Ecology. 2003;84:2419–2431. [Google Scholar]
- 11.Mangel M, Stamps J. Trade-offs between growth and mortality and the maintenance of individual variation in growth. Evol Ecol Res. 2001;3:583–593. [Google Scholar]
- 12.Johnsson JI, Abrahams MV. Domestication increases foraging under threat of predation in juvenile steelhead trout (Oncorhynchus mykiss): An experimental study. Can J Fish Aquatic Sci. 1991;48:243–247. [Google Scholar]
- 13.Post JR, Parkinson EA, Johnston NT. Density-dependent processes in structured fish populations: Assessment of interaction strengths in whole-lake experiments. Ecol Monogr. 1999;69:155–175. [Google Scholar]
- 14.Biro PA, Abrahams MV, Post JR, Parkinson EA. Predators select against high growth rates and risk-taking behaviour in domestic trout populations. Proc R Soc London B. 2004;271:2233–2237. doi: 10.1098/rspb.2004.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conover DO, Arnott SA, Walsh MR, Munch SB. Darwinian fishery science: Lessons from the Atlantic silverside (Menidia menidia). Can J Fish Aquatic Sci. 2005;62:730–737. [Google Scholar]
- 16.Hutchings JA. Life history consequences of overexploitation to population recovery in Northwest Atlantic cod (Gadus morhua). Can J Fish Aquatic Sci. 2005;62:824–832. [Google Scholar]
- 17.Ward AJW, Thomas P, Hart PJB, Krause J. Correlates of boldness in three-spined sticklebacks (Gasterosteus aculeatus). Behav Ecol Sociobiol. 2004;55:561–568. [Google Scholar]
- 18.Reale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biol Rev. 2007;82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x. [DOI] [PubMed] [Google Scholar]
- 19.Tymchuk WE, Devlin RH. Growth differences among first and second generation hybrids of domesticated and wild rainbow trout (Oncorhynchus mykiss). Aquaculture. 2005;245:295–300. [Google Scholar]
- 20.Tymchuk WE. Vancouver: Univ of British Columbia; 2006. Vancouver Physiological, behavioural, and fitness consequences of introgression of domestic genotypes into wild salmonid populations. PhD thesis. [Google Scholar]
- 21.Biro PA, Abrahams MV, Post JR. Direct manipulation of behaviour reveals a mechanism for variation in growth and mortality among prey populations. Anim Behav. 2007;73:891–896. [Google Scholar]
- 22.Askey PJ, Richards SA, Post JR, Parkinson EA. Linking angling catch rates and fish learning under catch-and-release regulations. N Am J Fish Manag. 2006;26:1020–1029. [Google Scholar]
- 23.Cooke SJ, Suski CD, Ostrand KG, Wahl DH, Philipp DP. Physiological and behavioural consequences of long-term artificial selection for vulnerability to recreational angling in a teleost fish. Physiol Biochem Zool. 2007;80:480–490. doi: 10.1086/520618. [DOI] [PubMed] [Google Scholar]
- 24.Post JR, Parkinson EA, Johnston NT. Spatial and temporal variation in risk to piscivory of age-0 rainbow trout: Patterns and population consequences. Trans Am Fish Soc. 1998;127:932–942. [Google Scholar]
- 25.Beckmann C, Biro PA, Post JR. Asymmetric effect of bird predation on fish population size-structure in whole-lake experiments. Can J Zool. 2006;84:1584–1593. [Google Scholar]
- 26.Askey PJ, et al. Estimation of gillnet efficiency and selectivity across multiple sampling units: A hierarchical Bayesian analysis using mark-recapture data. Fish Res. 2007;83:162–174. [Google Scholar]
- 27.Biro PA, Post JR, Parkinson EA. Population consequences of a predator-induced habitat shift by trout in whole-lake experiments. Ecology. 2003;84:691–700. [Google Scholar]