Abstract
Natal dispersal, the process through which immature individuals permanently depart their natal area in search of new sites, is integral to the ecology and evolution of animals. Insights about the underlying causes of natal dispersal arise mainly from research on species whose short dispersal distances or restricted distributions make them relatively easy to track. However, for small migratory animals, the causes of natal dispersal remain poorly understood because individuals are nearly impossible to track by using conventional mark–recapture approaches. Using stable-hydrogen isotope ratios in feathers of American redstarts (Setophaga ruticilla) captured as immature birds and again as adults, we show that habitat use during the first tropical nonbreeding season appears to interact with latitudinal gradients in spring phenology on the temperate breeding grounds to influence the distance traveled on the initial spring migration and the direction of natal dispersal. In contrast, adult redstarts showed considerable site fidelity between breeding seasons, indicating that environmental conditions did not affect dispersal patterns after the first breeding attempt. Our findings suggest that habitat occupancy during the first nonbreeding season helps determine the latitude at which this species of Neotropical–Nearctic migratory bird breeds throughout its life and emphasize the need to understand how events throughout the annual cycle interact to shape fundamental biological processes.
Keywords: seasonal interactions, American redstart (Setophaga ruticilla), carryover effects, nonbreeding season, phenology
Dispersal shapes the ecology and evolution of animals by regulating gene flow, linking subdivided populations, and influencing the distribution of species. In most animals, adult dispersal distances between breeding seasons are relatively short (1–3), suggesting that these critical biological processes are driven mainly by natal dispersal, the process through which immature individuals permanently depart their natal area in search of their first breeding site. Accurately measuring natal dispersal has been notoriously difficult because juvenile survival rates are low compared with those of adults, and dispersal distances often exceed study area boundaries (4, 5). Research on natal dispersal has therefore focused on species that are relatively easy to follow because of their short dispersal distances or restricted distributions (6). Studies of natal dispersal in small, migratory animals are rare in comparison because individuals are nearly impossible to track by using conventional mark–recapture approaches. For all but a handful of these species, we are presently unable to measure natal dispersal, study its underlying causes, and develop appropriate conservation plans (7–10).
Natal dispersal strategies are thought to arise from a complex interplay between genetic and environmental forces. In many organisms, the traits that determine natal dispersal potential have a genetic basis (10–12). However, expression of these traits and their ultimate role in directing natal dispersal patterns can depend on environmental conditions experienced by juveniles within the natal area or soon after they leave (10, 11). In birds, evidence is accumulating that juveniles gather public information (PI) during the postfledging period to assess the suitability of future breeding sites (13–15). Exploiting PI to enhance reproductive success requires that juveniles arrive on the breeding grounds sufficiently early to acquire high-quality territories. Temperate zone residents and short-distance migrants may solve this problem by wintering as close as possible to future breeding areas, thereby enabling early arrival at preferred breeding sites (16). In contrast, long-distance migratory birds spend the nonbreeding period in tropical habitats thousands of miles away from temperate breeding areas. The quality of the nonbreeding habitat is known to influence overwinter body condition, the timing of departure on spring migration, and the date of arrival on temperate breeding grounds (17–19). This inability to regulate breeding ground arrival time may prevent reliance on PI from the previous summer and force immature birds making their first breeding attempt to use other settlement cues. We tested the hypothesis that habitat-specific differences in overwinter performance combine with latitudinal gradients in spring phenology on the temperate breeding grounds to influence natal dispersal patterns in one species of Neotropical–Nearctic migratory bird, the American redstart (Setophaga ruticilla).
From 2002 to 2006, we monitored the overwinter performance of redstarts in two habitats at a nonbreeding site in Jamaica: a wet, mangrove forest where food is abundant through the winter; and a dry, second-growth scrub habitat where food becomes scarce in late winter. We then estimated patterns of natal dispersal by using stable-hydrogen isotope ratios (δD) in redstart tail feathers collected across multiple years. In North America, δD in growing season precipitation varies with latitude (20), and birds incorporate these signatures into their feathers via the supporting food web (21). Because immature redstarts grow their feathers in the nest and adults molt their wing and tail feathers in late summer on or close to the breeding site (22, 23), feathers collected on tropical nonbreeding areas allow inferences about natal or molting sites from the previous summer. We collected one tail feather from immature redstarts during their first nonbreeding season to estimate the latitude of the natal area and a second feather 1 year later from the same individuals, if they returned as adults, to estimate the latitude of the first breeding attempt. We also sampled feathers from the same adult redstarts in each of two consecutive years to understand whether winter habitat occupancy influenced patterns of dispersal between breeding seasons. These multiyear δD profiles for the same individuals enabled us to examine the geography of dispersal without tracking banded birds throughout their annual cycle.
Results
Regardless of sex or body size, immature redstarts wintering in mangrove forest maintained body mass over winter, whereas those in second-growth scrub lost mass (repeated measures mixed model for sex × season, F1,37 = 2.45, P = 0.13; for body size, F1,37 = 1.05, P = 0.31; for habitat × season, F1,37 = 5.52, P = 0.02; 0.01 ± 0.08 g for mangrove; −0.18 ± 0.07 g for scrub; mean ± SE). Individuals that maintained mass departed earlier on spring migration than those that lost mass (r = 0.48, P = 0.01, n = 27), allowing redstarts in mangrove to leave an average of 7 days before those in scrub (Kaplan–Meier log-rank test for habitat: χ2 = 22.26, P < 0.0001, n = 78; 31 ± 1 days since April 1 for mangrove; 38 ± 1 days since April 1 for scrub; mean ± SE).
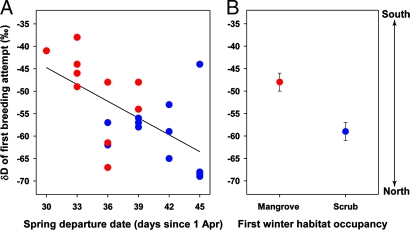
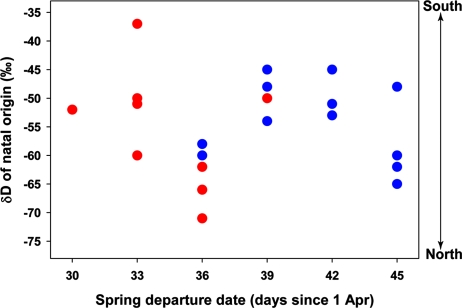
Of the 41 redstarts first captured during their first winter, 22 were recaptured the next year when they returned as adults. Feathers of recaptured birds indicated that the date of departure on the first spring migration was positively correlated with the δD of the first breeding attempt (r = 0.60, P = 0.004, n = 22) (Fig. 1A), but not with the δD of natal origin (r = 0.09, P = 0.69, n = 22) (Fig. 2). Immature redstarts departing early from mangrove forest migrated comparatively short distances and made their first breeding attempt in the southern part of the breeding range, whereas later-departing individuals from scrub migrated and made their first breeding attempt further north (t20 = −2.88, P = 0.009) (Fig. 1B). Natal feathers of immature redstarts overwintering in mangrove forest and second-growth scrub had a similar range of δD values (t20 = −0.34, P = 0.73) (Fig. 2), suggesting that the natal origin of each bird had little effect on the location of the first breeding attempt.
Fig. 1.
Spring departure dates of immature American redstarts and the δD of their first breeding attempt. (A) Immature redstarts wintering in mangrove forest (red circles) tended to depart early on spring migration and migrate comparatively short distances, whereas redstarts in second-growth scrub (blue circles) were more likely to depart later and migrate longer distances. (B) As a consequence, immature birds from mangrove made their first breeding attempt (mean ± SE) in southerly areas, whereas those birds from scrub bred at more northerly sites.
Fig. 2.
Spring departure dates of American redstarts and the δD of their natal origin. Immature redstarts overwintering in mangrove forest (red circles) and second-growth scrub (blue circles) did not differ in the δD of their natal origin, and there was no conclusive relationship between the timing of departure from the nonbreeding grounds and natal δD.
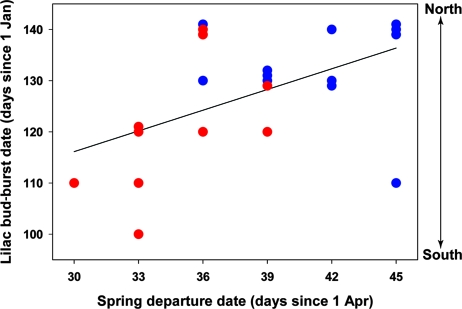
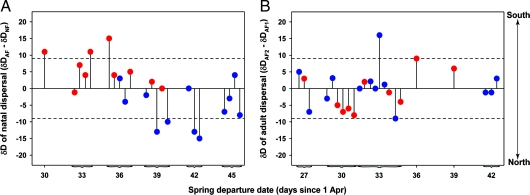
Breeding ground settlement patterns of immature redstarts were consistent with latitudinal differences in the start of the growing season, as indexed by lilac bud-burst dates modeled from daily temperature extremes (24). Early departing redstarts settled at latitudes with comparatively early lilac bud-burst dates, whereas later-departing birds settled at latitudes with later bud-burst dates (r = 0.52, P = 0.01, n = 22) (Fig. 3). It is unlikely that birds use lilac phenology as a cue for selecting breeding territories, but lilac budburst occurs during redstart migration and can be considered a surrogate for the onset of photosynthesis in other plant species (25). The apparent interaction between habitat-specific differences in spring departure schedules and breeding ground phenology resulted in marked differences in natal dispersal direction. Regardless of sex and body size, individuals wintering in a scrub dispersed north of their natal area, whereas birds wintering in mangrove dispersed south of their natal area (repeated measures mixed model for sex, F1,19 = 0.21, P = 0.66; for body size, F1,19 = 0.36, P = 0.55; for year × habitat, F1,19 = 5.07, P = 0.02) (Fig. 4A).
Fig. 3.
Spring departure dates of immature American redstarts and lilac bud-burst dates at the δD of their first breeding attempt. Lilac bud-burst dates index the start of the growing season at different latitudes and are indicators of phenology in other plant species (25). The correlation between spring departure schedules of immature redstarts and lilac bud-burst dates at the latitude of their first breeding attempt suggests that early departing birds from mangrove (red circles) settled in southerly breeding areas to exploit early plant phenology, whereas later-departing birds from second-growth scrub (blue circles) migrated further north to find suitable conditions.
Fig. 4.
Dispersal patterns of American redstarts inferred from δD in tail feathers molted on or close to temperate breeding sites and sampled from the same individuals in consecutive nonbreeding seasons. (A) δD differences between adult and natal feathers (δDAF − δDNF) indicated that immature redstarts overwintering in mangrove and scrub habitats differed in their natal dispersal direction. Birds wintering in mangrove forest (red circles) dispersed south of their natal area, whereas redstarts in second-growth scrub (blue circles) dispersed north of their natal area. (B) δD differences between adult feathers in the second and first year of capture (δDAF2 − δDAF1) showed that adult redstarts exhibited considerable breeding site fidelity and that nonbreeding habitat occupancy had no apparent effect on adult dispersal. Horizontal dashed lines depict the 95% C.I. (±9‰) of δD in feathers (23).
Consistent with our results from immature redstarts, adults wintering in mangrove maintained body mass, whereas those in scrub habitat lost mass (repeated measures mixed model for habitat × season, F1,41 = 8.60, P = 0.0008; 0.00 ± 0.08 for mangrove; −0.24 ± 0.07 g for scrub; mean ± SE). Adult redstarts in mangrove also departed earlier on spring migration, compared with those in scrub (Kaplan–Meier log-rank test for habitat: χ2 = 16.82, P < 0.0001, n = 137; 30 days since April 1 ±1 for mangrove; 34 days since April 1 ±1 for scrub; mean ± SE). Despite the comparable overwinter performance of adults and immature birds, adult dispersal between breeding seasons was not influenced by winter habitat occupancy (repeated measures mixed model for year × habitat, F1,21 = 0.05, P = 0.83) (Fig. 4B).
δD can vary significantly among feathers grown at the same latitude, leading to potential bias in the interpretation of dispersal patterns (23, 26). We therefore used the 95% C.I. of δD in redstart feathers molted at a known location (±9‰, n = 42) to judge whether birds truly dispersed from their latitude of origin (23). Based on this cutoff, the rate of natal dispersal was greater than the rate of adult dispersal between breeding seasons (χ2 = 5.81, P = 0.02; 7 of 22 natal dispersers; 1 of 23 adult dispersers).
Discussion
Our results indicate that environmental conditions in locations thousands of miles apart can interact across periods of the annual cycle to influence the distance and direction of natal dispersal in American redstarts. Immature redstarts securing territories in mangrove forest maintained body mass over winter, allowing them to depart earlier from Jamaica than individuals wintering in scrub and to undertake a shorter spring migration. Because migratory birds appear to use phenological cues to select breeding habitat and synchronize reproduction with food availability for nestlings (27), early migrants probably benefit by settling in southern parts of the breeding range, where the early vegetation flush supports an early food supply. Conversely, immature birds holding territories in second-growth scrub lost mass, forcing them to depart later and complete a longer migration. Birds departing later would need to migrate further north to locate necessary resources for breeding or risk costly mismatches between food availability and nestling nutritional demands (27, 28). Immature birds arriving late also could find southerly breeding areas saturated with early arrivals, creating additional stimulus to migrate further north in search of breeding territories. Because redstarts from both nonbreeding habitats fledged from a similar range of natal latitudes, birds spending their first winter in mangrove ultimately dispersed south of their natal area, whereas those birds in scrub dispersed north of their natal area.
It could be argued that variation in individual quality determined the overwinter performance and dispersal direction of immature redstarts not habitat occupancy. In autumn, immature redstarts arrive in Jamaica before adults and establish winter territories randomly with respect to habitat (29). Dominance status in redstarts is a function of both sex and body size, allowing adult males and large-bodied adult females to displace many immature birds from mangrove forest (29, 30). These data raise the possibility that variation in sex- and size-based competitive ability among immature redstarts ultimately controls overwinter performance and, thus, natal dispersal patterns. However, such effects were not apparent in the present study because neither sex nor body size mediated the effect of habitat occupancy on overwinter performance and dispersal. Additionally, previous research in this system has demonstrated that, regardless of sex and body size, immature males and females experimentally upgraded from second-growth scrub to mangrove forest maintained mass and departed earlier on migration, compared with control redstarts that remained in scrub for the duration of the nonbreeding period (18). Together these data support the idea that winter habitat occupancy is the most important driver of overwinter performance in redstarts and suggest it was the underlying factor responsible for the natal dispersal patterns observed in this study.
In many species, immature birds identify future breeding sites through postfledging exploration or by gathering PI near their natal site (14, 15). These behaviors can be advantageous because they enable birds undertaking their first breeding attempt to settle in familiar areas where they have some knowledge of expected reproductive success (13). Our results do not preclude the possibility that immature redstarts prospected for and identified future breeding territories near their natal site before leaving on fall migration. Indeed, the similarity between the δD of the natal and first breeding attempt for many redstarts is consistent with natal site fidelity, particularly for birds leaving during the middle of the spring departure period, when departure schedules from each habitat overlapped (days 36 and 39) (Fig. 4A). It is interesting to note that during this 6-day time frame, immature redstarts departing from mangrove forest tended to disperse south of their natal area, whereas the majority of those from scrub habitat dispersed north even when they left their nonbreeding territories on the same day. One explanation for this pattern is that, before migratory departure, immature redstarts holding mangrove territories were in superior body condition, compared with those in scrub. Initiating migration in good condition could facilitate a more rapid migration and earlier arrival at southern breeding areas by reducing refueling time at stopover sites, an idea supported by both orientation experiments and recapture rates of birds during spring passage (31, 32). Thus, the interaction between winter habitat occupancy and breeding ground phenology may have influenced natal dispersal patterns for redstarts we classified as “site-faithful,” as well as those we labeled “dispersers.”
Once redstarts gain breeding season experience, dispersal appears to become decoupled from environmental conditions experienced during the nonbreeding period. The overwinter performance of adults was similar to that of immature birds; however, unlike immature redstarts, the habitat-specific spring departure schedules of adults did not conclusively influence patterns of breeding dispersal. After accounting for the potential environmental variation in feather δD, adults showed considerable breeding site fidelity, with only 4% (1 of 23) of birds dispersing away from the latitude occupied in the previous breeding season. Adult redstarts may not have returned to the exact location of their first breeding attempt; it is possible that they dispersed short distances to new breeding sites. Unfortunately, δD does not provide the resolution needed to distinguish between these possibilities. Nonetheless, this result suggests that the environmental conditions during the first nonbreeding season that drive natal dispersal patterns also appear to influence the location at which birds breed in future years. Because adult redstarts show high fidelity to nonbreeding territories (30), repeated use of the same nonbreeding sites could help synchronize spring departure schedules with phenology on breeding areas. Importantly, mismatches between breeding ground arrival time and resource phenology could still occur if environmental conditions on nonbreeding areas vary among years (33).
Unfortunately, environmental change on nonbreeding areas appears certain. Multiple independent models of climate change predict significant long-term drying trends in the Caribbean region (34), the primary nonbreeding range of many species of migratory songbirds (35). Because moisture directly affects nonbreeding season arthropod populations, declining rainfall in future years could severely limit food availability for birds, resulting in progressively delayed migration schedules (17, 32). In North America, climate change scenarios predict further advances in spring resource phenology (36), which means that suitable resources for both early and late-arriving birds may lie progressively further north than in the past. Therefore, climate change within the Caribbean could promote longer natal dispersal distances, resulting in northerly range shifts and the eventual disappearance of more southerly populations.
Finally, our results underscore the need to understand how events throughout the annual cycle of migratory species interact to shape fundamental biological processes. Although caution should be exercised in estimating linear distances from δD values, dispersal movements greater than our chosen 95% C.I. of ±9‰ likely correspond to distances of >150 miles. Our evidence for broad-scale demographic exchange in redstarts is consistent with low levels of phylogeographic structure demonstrated for other species of Neotropical–Nearctic migratory birds (37). Therefore, nonbreeding season events appear to help structure breeding populations of redstarts at regional rather than local scales, making it unlikely that birds are adapted to local conditions on North American breeding areas.
Methods
Fieldwork was conducted in southwestern Jamaica at the Font Hill Nature Preserve (18°02′N, 77°57′W). In winter (January 15–February 20) of 2002–2005, we captured redstarts in mist nets, color-banded and processed them, and plucked a single tail feather for δD analysis. From March 20 to April 15 of each year, we recaptured birds to assess overwinter change in body mass. We estimated the date of departure on spring migration by searching territories of color-banded redstarts every 3 days from April 1 to May 15. When we failed to resight a bird, we rechecked the territory twice more during the 3-day period and once more in the next 3-day period. On this final visit, we broadcast a recording of redstart songs and chips for five bouts of 20 s interspersed with 30 s of silence. We considered birds to have left on migration when the playback drew no response. In each following winter, we captured color-banded redstarts returning from the previous winter to sample a second feather for δD analysis.
Isotope analyses were performed at the Queen's University Facility for Isotope Research (QFIR). Feathers were washed of surface oils and debris in a 2:1 chlorform:methanol solution and air-dried under a fume hood for 48 h. After transport to the QFIR, feathers were allowed to equilibrate with the local atmosphere for 72 h. A small sample of each feather (0.10–0.15 mg) was clipped, loaded into a silver capsule, and placed in a drying oven at 100°C for 24 h to remove potential surface water. The capsules were crushed, combusted at 1,450°C in an elemental analyzer (TC/EA; Finnegan), and introduced online to an isotope ratio mass spectrometer (MAT Delta Plus XL; Finnegan). One in-house standard was run for every five unknowns. We reported isotope ratios in δ notation relative to Vienna Standard Mean Ocean Water (VSMOW), where δD = (2H/1Hsample/2H/1Hstandard)−1) × 1,000. Analytical error (±1 SD) was 2‰ based on replicate analyses of the same feather (n = 18) and analyses of standards (kaolinite, n = 11; brucite, n = 12). We adjusted the isotope ratio of each feather by 19‰ to account for isotopic fractionation among precipitation, redstart prey, and feathers (38). The δD values reported here included both exchangeable and nonexchangeable hydrogen. To minimize any potential systematic error caused by nonexchangeable hydrogen, we analyzed all feather samples during a period of 6 days and included an approximately equal number of feathers from each habitat and age-class in each run of the mass spectrometer.
We examined the relationship between spring departure schedules of immature redstarts and date of lilac bud-burst at the latitude of their first breeding attempt by using data from World Data Center of Paleoclimatology (24) and a previously published δD base map (39). We first assigned the δD ratio of each feather sampled after the first breeding attempt to one 10‰ division on the δD base map. We then determined the average lilac bud-burst date within each 10‰ division of the δD base map. By assuming that the rate of migration was the same for individuals, we were able to assess whether immature redstarts used plant phenology as a cue for settling their first breeding territory.
We judged whether redstarts dispersed from or were faithful to their latitude of origin during the previous summer by calculating the 95% C.I. of δD in feathers sampled from separate population of redstarts (±9‰, n = 42) known to have bred at the Queens University Biological Station (44°43′N, 76°19′W) (23). In the present study, only individuals whose feathers from successive years had differences in δD in excess of ±9‰ were considered to be dispersers. This cutoff likely caused us to label as site-faithful some birds that actually dispersed short distances from their origin the previous summer. Despite the potential for such error, we believed this approach to defining dispersal events was warranted given published estimates of variation in δD in feathers molted at the same latitude (23, 26, 40).
Data on overwinter body mass change were examined by using a repeated measures mixed model with year, sex, and habitat as main effects and season (winter and spring) as the repeated measure. Data on natal and breeding dispersal were examined by using a repeated measures mixed model with year, sex, and habitat as main effects and bird age (immature and adult) as the repeated measure. Tarsus length was included in each model as a covariate to adjust for body size differences. Individual bird nested within habitat was considered a random effect in each of the above analyses. Departure schedules were analyzed with a Kaplan–Meier log-rank test. The relationship between overwinter body mass change and migratory departure date, between migratory departure date and δD, and between migratory departure date and lilac bud-burst date were tested by using Pearson's correlation. Differences between the latitude of the first breeding attempt for birds wintering in mangrove and scrub were examined with Student's t test. Comparisons between rates of natal and breeding dispersal were made with Pearson's χ2 test. All data met test assumptions, so no transformations were used. Analyses were done with SAS version 8.2 (41).
ACKNOWLEDGMENTS.
We thank T. Biasiolli, D. Brown, K. Cramer, H. Davis, M. Evans, P. Goulet, J. Greenwood, Q. Hays, E. Klein, M. Reudink, and K. Strum for assistance in the field; M. Schwartz for discussions about phenology; R. Aviram, R. Greenberg, D. Inouye, S. LaDeau, K. Langin, M. Reudink, S. Sillett, and three anonymous reviewers for comments on the manuscript; the Petroleum Corporation of Jamaica for permission to conduct this research at the Font Hill Nature Preserve; and Yvette Strong and Andrea Donaldson of the Jamaica National Environment Planning Agency for their cooperation. This work was supported by National Science Foundation Grants DEB-085965 and DEB-640195 (to P.P.M.), the Cosmos Club Foundation, the Wilson Ornithological Society, the Smithsonian Predoctoral Fellowship Program (C.E.S.), the Natural Sciences and Engineering Research Council, the Canadian Foundation for Innovation, and the Ontario Innovation Trust (T.K.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Gaines MS, McClenaghan LR., Jr Dispersal in mammals. Annu Rev Ecol Syst. 1980;11:163–196. [Google Scholar]
- 2.Berven KA, Grudzien TA. Dispersal in the wood frog (Rana sylvatica): Implications for genetic population structure. Evolution (Lawrence, Kans) 1990;8:2047–2056. doi: 10.1111/j.1558-5646.1990.tb04310.x. [DOI] [PubMed] [Google Scholar]
- 3.Paradis E, Baillie SR, Sutherland WJ, Gregory RD. Patterns of natal and breeding dispersal in birds. J Anim Ecol. 1998;67:518–536. [Google Scholar]
- 4.Anders AD, Dearborn DC, Faaborg J, Thompson FR. Juvenile survival rates in population of Neotropical migrant birds. Conserv Biol. 1997;11:698–707. [Google Scholar]
- 5.Koenig WD, Van Vuren D, Hooge PN. Detectability, philatropy, and the distribution of dispersal distances in vertebrates. Trends Ecol Evol. 1996;11:514–517. doi: 10.1016/s0169-5347(96)20074-6. [DOI] [PubMed] [Google Scholar]
- 6.Clobert J, Danchin E, Dhondt AA, Nichols JD. Dispersal. Oxford, UK: Oxford Univ Press; 2001. [Google Scholar]
- 7.Tittler R, Fahrig L, Villard MA. Evidence of large-scale source-sink dynamics and long-distance dispersal among wood thrush populations. Ecology. 2007;87:3029–3036. doi: 10.1890/0012-9658(2006)87[3029:eolsda]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Winkler DW, et al. The natal dispersal of tree swallows in a continuous mainland environment. J Anim Ecol. 2005;74:1080–1090. [Google Scholar]
- 9.Møller AP, Flensted-Jensen E, Mardal W. Dispersal and climate change: A case study of the Arctic Tern Sterna paradisaea. Global Change Biol. 2006;12:2005–2013. [Google Scholar]
- 10.Hansson B, Bensch S, Hasselquist D. Heritability of dispersal in the great reed warbler. Ecol Lett. 2003;6:290–294. [Google Scholar]
- 11.Pasinelli G, Schiegg K, Walters JR. Genetic and environmental influences on natal dispersal distances in a resident bird species. Am Nat. 2004;264:660–669. doi: 10.1086/424765. [DOI] [PubMed] [Google Scholar]
- 12.Roff DA, Fairbairn DJ. The genetic basis of dispersal and migration, and its consequences for the evolution of correlated traits. In: Clobert J, Danchin E, Dhondt AA, Nichols JD, editors. Dispersal. Oxford, UK: Oxford Univ Press; 2001. pp. 191–202. [Google Scholar]
- 13.Doligez B, Danchin E, Clobert J. Public information and breeding habitat selection in a wild bird population. Science. 2002;297:1168–1170. doi: 10.1126/science.1072838. [DOI] [PubMed] [Google Scholar]
- 14.Nocera JJ, Forbes GJ, Giraldeau LA. Inadvertent social information in breeding site selection of natal dispersing birds. Proc R Soc London Ser B. 2006;273:349–355. doi: 10.1098/rspb.2005.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hénaux V, Bregnballe T, Lebreton JD. Dispersal and recruitment during population growth in a colonial bird, the great cormorant Phalacrocorax carbo sinensis. J Avian Biol. 2007;38:44–57. [Google Scholar]
- 16.Cristol DA, Baker MB, Carbone C. Differential migration revisited: Latitudinal segregation by age and sex class. Curr Ornith. 1999;15:33–88. [Google Scholar]
- 17.Marra PP, Hobson KA, Holmes RT. Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science. 1998;282:1884–1886. doi: 10.1126/science.282.5395.1884. [DOI] [PubMed] [Google Scholar]
- 18.Studds CE, Marra PP. Nonbreeding habitat occupancy and population processes: An upgrade experiment with a migratory bird. Ecology. 2005;86:2380–2385. [Google Scholar]
- 19.Saino N, et al. Ecological conditions during winter predict arrival at the breeding quarters in a trans-Saharan migratory bird. Ecol Lett. 2004;7:21–25. [Google Scholar]
- 20.International Atomic Energy Agency. Environmental Isotope Data No. 10: World Survey of Isotope Concentration in Precipitation (1988–1991) Tech Rep Ser. Vol. 371. Vienna, Austria: IAEA; 1994. [Google Scholar]
- 21.Hobson KA, Wassenaar LI. Linking breeding and wintering grounds of Neotropical migrant songbirds using stable-hydrogen isotopic analysis of feathers. Oecologia. 1997;109:142–148. doi: 10.1007/s004420050068. [DOI] [PubMed] [Google Scholar]
- 22.Pyle P. Identification Guide to North American Birds. Part I, Columbidae to Ploceidae. Bolinas, CA: Slate Creek; 1997. pp. 496–497. [Google Scholar]
- 23.Langin KM, et al. Hydrogen isotopic variation in migratory bird tissues of known origin: Implications for geographic assignment. Oecologia. 2007;152:449–457. doi: 10.1007/s00442-007-0669-3. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz MD, Caprio JM. North American First Leaf and First Bloom Lilac Phenology Data. Boulder, CO: NOAA/NGDC Paleoclimatology Program; 2003. ftp://ftp.ncdc.noaa.gov/pub/data/paleo/phenology/north_america_lilac.txt. [Google Scholar]
- 25.Schwartz MD. Green-wave phenology. Nature. 1998;394:839–840. [Google Scholar]
- 26.Wunder MB, Kester CL, Knopf FL, Rye RO. A test of geographic assignment using isotope tracers in feathers of known origin. Oecologia. 2005;144:607–617. doi: 10.1007/s00442-005-0071-y. [DOI] [PubMed] [Google Scholar]
- 27.Thomas DW, Blondel J, Perret P, Lambrechts LM, Speakman JR. Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science. 2001;291:2598–2600. doi: 10.1126/science.1057487. [DOI] [PubMed] [Google Scholar]
- 28.Both C, Bouwhuis S, Lessells CM, Visser ME. Climate change and population declines in a long-distance migratory bird. Nature. 2006;441:81–83. doi: 10.1038/nature04539. [DOI] [PubMed] [Google Scholar]
- 29.Marra PP. The role of behavioral dominance in structuring patterns of habitat occupancy in a migrant bird during the nonbreeding season. Behav Ecol. 2000;11:299–308. [Google Scholar]
- 30.Marra PP, Holmes RT. Consequences of dominance-mediated habitat segregation in American redstarts during the non-breeding season. Auk. 2001;118:92–104. [Google Scholar]
- 31.Parejo D, White J, Clobert J, Dreiss A, Danchin E. Blue tits use fledgling quantity and quality as public information in breeding site choice. Ecology. 2007;89:2373–2382. doi: 10.1890/06-2000.1. [DOI] [PubMed] [Google Scholar]
- 32.Parejo D, White J, Danchin E. Settlement decisions in blue tits: Difference in the use of social information according to age and individual success. Naturwissenschaften. 2007;94:749–757. doi: 10.1007/s00114-007-0253-z. [DOI] [PubMed] [Google Scholar]
- 33.Studds CE, Marra PP. Linking fluctuations in rainfall to nonbreeding season performance in a long-distance migratory bird Setophaga ruticilla. Clim Res. 2007;35:115–122. [Google Scholar]
- 34.Neelin JD, Münnich M, Su H, Meyerson JE, Holloway CE. Tropical drying trends in global warming models and observations. Proc Natl Acad Sci USA. 2006;103:6110–6115. doi: 10.1073/pnas.0601798103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wunderle JM, Waide RB. Distribution of overwintering Nearctic migrants in the Bahamas and Greater Antilles. Condor. 1993;95:904–933. [Google Scholar]
- 36.Zhang XY, Friedl MA, Schaaf CB, Strahler AH. Climate controls on vegetation phenological patterns in northern mid- and high latitudes inferred from MODIS data. Global Change Biol. 2004;10:1133–1145. [Google Scholar]
- 37.Lovette IJ, Clegg SM, Smith TB. Limited utility of mtDNA markers for determining connectivity among breeding and overwintering locations in three Neotropical migrant birds. Conserv Biol. 2004;18:159–166. [Google Scholar]
- 38.Hobson KA, Wassenaar LI, Bayne E. Using isotopic variance to detect long-distance dispersal and philatropy in birds. Condor. 2004;106:732–743. [Google Scholar]
- 39.Dunn EH, Hobson KA, Wassenaar LI, Hussell DJT, Allen ML. Identification of summer origins of songbirds migrating through southern Canada in autumn. Avian Con Ecol. 2006;1:4. [Google Scholar]
- 40.Rocque DA, Ben-David M, Barry RP, Winker K. Assigning birds to wintering and breeding grounds using stable isotopes: lessons from two feather generations among three intercontinental migrants. J Ornith. 2006;147:395–404. [Google Scholar]
- 41.SAS Institute. SAS/STAT Users Guide, Version 8.2. Cary, NC: SAS Institute; 1999. [Google Scholar]