Abstract
The role of chromosomal inversions in speciation has long been of interest to evolutionists. Recent quantitative modeling has stimulated reconsideration of previous conceptual models for chromosomal speciation. Anopheles gambiae, the most important vector of human malaria, carries abundant chromosomal inversion polymorphism nonrandomly associated with ecotypes that mate assortatively. Here, we consider the potential role of paracentric inversions in promoting speciation in A. gambiae via “ecotypification,” a term that refers to differentiation arising from local adaptation. In particular, we focus on the Bamako form, an ecotype characterized by low inversion polymorphism and fixation of an inversion, 2Rj, that is very rare or absent in all other forms of A. gambiae. The Bamako form has a restricted distribution by the upper Niger River and its tributaries that is associated with a distinctive type of larval habitat, laterite rock pools, hypothesized to be its optimal breeding site. We first present computer simulations to investigate whether the population dynamics of A. gambiae are consistent with chromosomal speciation by ecotypification. The models are parameterized using field observations on the various forms of A. gambiae that exist in Mali, West Africa. We then report on the distribution of larvae of this species collected from rock pools and more characteristic breeding sites nearby. Both the simulations and field observations support the thesis that speciation by ecotypification is occurring, or has occurred, prompting consideration of Bamako as an independent species.
Keywords: chromosomal inversion, ecological speciation, Bamako chromosomal form, inversion polymorphism, selection
Differences in chromosome number or structure are often found between species, even those that are very closely related. This pattern has stimulated interest in the contribution of chromosomal changes to speciation itself. It has been argued that chromosome mutations are causal by directly inducing some degree of reproductive incompatibility [e.g., sterility (1)]. However, this argument does not hold for paracentric chromosomal inversions (i.e., those not involving the centromere). Such inversions are commonly polymorphic and not associated with reduced fertility in dipteran species like fruitflies and anopheline mosquitoes. Indeed, clinal, microspatial, and seasonal shifts in inversion frequencies associated with environmental conditions testify to the adaptive significance of chromosomal inversions (2). In this light, more recent considerations have hypothesized an indirect causal relationship to speciation, whereby chromosomal rearrangements protect adaptive divergence that may lead to the evolution of reproductive isolation (3). This effect is achieved through suppressed recombination between alternative arrangements bearing sets of genes differentially adapted to environmental heterogeneities. Kirkpatrick and Barton (4) formalized these arguments. They presented a general model showing that chromosomal inversions can become established because they capture sets of genes that confer local adaptations, protecting these gene combinations from recombination with genes from migrants. This may occur even in the absence of epistasis, the presumed basis of the coadaptation theory of maintenance of inversions (2, 5).
Twenty-five years earlier, Coluzzi (6) (see also ref. 7) presented verbal arguments for a chromosomal speciation process much like that modeled by Kirkpatrick and Barton (4). At its core, it is a conceptual model of ecological speciation in which ecological and adaptive divergence among populations leads to reproductive isolation that promotes speciation (ref. 8 and refs. therein). However, Coluzzi's model was proposed with reference to anophelines, which are particularly good candidates due to ubiquitous inversion polymorphisms within and fixed inversion differences between species. The chromosome number (n = 3) and genome recombination length (9) are small, so the probability of an inversion capturing locally adapted alleles is relatively high. In addition, anopheline populations may undergo extreme fluctuations in size and distribution due to seasonal, climatic, or ecological oscillations. Coluzzi (6) hypothesized that during times of high growth rates, populations expand to colonize adaptively marginal habitats at the geographic or ecological periphery of the normal range. During population contractions, such peripheral populations could be stabilized by alleles adaptive to the marginal conditions. Renewed population expansion would reunite peripheral and central populations. Resulting interbreeding would swamp locally adapted alleles, unless they were protected from recombination by chance inclusion in a chromosomal inversion newly arisen in the peripheral population. The process has three possible outcomes: (i) extinction of the peripheral population, (ii) incorporation of inversion polymorphism by the central population, and (iii) speciation of the peripheral population via “ecotypification,” a term that refers to differentiation arising from local adaptation in anopheline mosquitoes (M. Coluzzi, personal communication). New alleles adapted to the recently occupied habitat are expected to accumulate within the inversion. Alleles promoting reproductive isolation can also arise (within or outside the inversion) due to pleiotropy, epistasis, or simply chance and increase through Batson/Dobzhansky/Muller or other processes (10, 11), leading eventually to speciation.
Here, we consider the potential role of paracentric inversions in promoting evolution of reproductive isolation among ecotypes of Anopheles gambiae, the most important vector of human malaria. In particular, we focus on the Bamako form, a chromosomally defined ecotype, which best fits the definition of an ecologically peripheral isolate (12, 13). Unlike other forms of A. gambiae whose range can extend across tropical Africa, the Bamako form has an extremely restricted range in southern Mali and northern Guinea, primarily around the upper Niger River. The Bamako form is characterized by low-inversion polymorphism overall (only 2Rb/+b) and fixation of an inversion (2Rj) that is very rare or absent in all other forms of A. gambiae. It is likely that inversion 2Rj is associated with breeding sites peculiar to its range. Characteristic breeding sites for A. gambiae (i.e., where they lay their eggs, and where these mature to adults) are temporary rain-dependent pools not possible such as hoof prints (typical of the Savanna form) or semipermanent swamps or irrigated sites (typical of Mopti) (13, 14). In the upstream portion of the Niger River and its associated tributaries where the Bamako form occurs, an additional breeding site is available, laterite rock pools. Laterite is an iron-rich surface formation locally common in a few parts of Africa (15). It forms a thick crust [“duricrust” (16)] that hardens upon repeated inundation and exposure to the air. After erosion by water and rocks, holes as deep as ≥1 m can develop and may interconnect beneath the duricrust. These “rock pools” (Fig. 1B) offer protected sites where mosquito larvae can develop during the rainy season without being swept away by the currents on top of them, if the mosquitoes are suitably adapted. The rock pools offer a unique breeding site that has chemical and physical features distinct from more characteristic breeding sites for A. gambiae [Fig. 1B (17, 18)]. It also appears that, by being more permanent, rock pools host a richer biota than do more temporary sites of the same size. These laterite rock pools are effectively marginal parapatric habitats where locally adapted constellations of genes might become established.
Fig. 1.
Characteristic breeding sites of A. gambiae around the study site in Banambani Village, Mali. (A) Puddles and (B) laterite rock pools. Note rapidly flowing water at the upper part of the rock pool area, which could sweep away unprotected mosquito immatures. Photos were taken at time of sampling in 2006.
With this possibility in view, we first present computer simulations to formally investigate whether the population dynamics of A. gambiae are compatible with the hypothesis of speciation by ecotypification. The model was parameterized using field observations on the various forms of A. gambiae that exist in Mali, West Africa. We then present empirical evidence based on larval collections in Mali that the 2Rj inversion frequency is highest in rock pools, only a short distance from alternative puddle and swamp-edge larval breeding habitats. This frequency difference is repeatable and stable over several years. Carriers of the 2Rj inversion are almost completely reproductively isolated from the 2R+j karyotype, because virtually no j/+ heterozygotes were observed. This provides support for both the simulated models and the status of Bamako as a distinct reproductively isolated genetic unit that merits recognition as a new species.
Results
Simulations.
Response of the simulation model to several single parameters (Table 1) was tested and compared with expectations from Kirkpatrick and Barton (4) to validate its behavior. It conformed to expectation in all cases, indicating that the simulation captures the essential elements of the ecotypification hypothesis [see supporting information (SI) Text].
Table 1.
Parameters and their ranges explored by simulation
| Parameter | Abreviation | Range | Refs. |
|---|---|---|---|
| Core population (P0) min. size | S0 | 200–600 (Ne=1,648–4,945) | 32, 35–37 |
| Peripheral population (P1) min. size | S1 | 5–200 (Ne=41–1,648) | 32, 35–37 |
| Migration rate (P1 to P0) | m0 | 0.001–0.01 | 37, 38–43 |
| Migration rate (P0 to P1) | m1 | 0.001–0.5 | 32, 44–46 |
| Relative fitness of maladapted allele | s | 0.1–1.0 | 32, 44–46* |
| Time/population size when inversion occurs | ti | 290–299 | N/A |
| Number of loci captured by inversion | ni | 1–15 | 26 |
| Recombination rate | r | 0.006–0.06 | 26, 47 |
*These sources gave qualitative comparisons, so a large range was adopted.
The model was used to determine the likelihood of a new inversion becoming established in A. gambiae given realistic parameter ranges. To assess the importance of stochastic factors, experiments were conducted with 1,000, 2,500, and 5,000 simulations distributed over the same parameter space. These had, respectively, 7.20%, 7.44%, and 7.70% of simulations end with inversion frequencies (pf) greater than zero. Statistical analyses were conducted on the 5,000-simulation dataset to maximize power, although results were qualitatively the same as for the 2,500-simulation experiment. The inverted karyotype never reached fixation under the conditions explored, so the outcomes reflect establishment of inversion polymorphism (0< pf < 1) or loss of the inversion (pf = 0) at the end of the simulations.
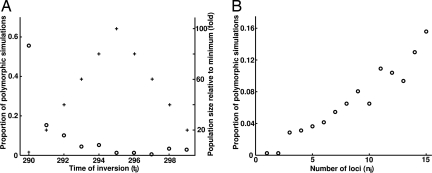
Binomial regression indicated that several factors affected whether chromosomal inversions persisted. Minimum size of the peripheral population (S1), migration rate to the peripheral habitat (m1), time of the year when the inversion occurred (ti), number of loci captured by the inversion (ni), and recombination rate (r) all had a statistically significant effect on inversion persistence; core population size (S0), migration rate from the peripheral habitat (m0), and relative fitness of the maladapted (migrant) alleles (s) had no such effect (Table 2). An analysis of deviance on the binomial regression model was used to rank the importance of the variables using the R programming language (www.r-project.org). It revealed two classes of significant predictors (Table 2): ti and ni explained much more of the deviance, whereas r and m1 had relatively minor roles. All other factors were not significant at P = 0.05. Examination of ti and ni individually shows that inversions persisted most often when introduced to a small and growing population (Fig. 2A) and when the inversion encompassed more loci conferring adaptive properties (Fig. 2B).
Table 2.
Factors that lead to inversion polymorphism based on binomial regression and analysis of deviance of model parameters
| Coefficient | Binomial regression |
Analysis of deviance |
||||
|---|---|---|---|---|---|---|
| Estimate | SE | z | P | RD | Percent | |
| Intercept | 192.2 | 10.8 | 17.88 | <0.001 | 2713.80 | |
| S0 | −5.4 × 10−4 | 5.5 × 10−4 | −0.98 | 0.33 | 2713.05 | 0.03 |
| S1 | −2.7 × 10−3 | 1.1 × 10−3 | −2.39 | 0.02 | 2710.13 | 0.11 |
| m0 | 361.0 | 243.2 | −1.48 | 0.14 | 2709.60 | 0.02 |
| m1 | 4.4 | 0.5 | 9.54 | <0.001 | 2630.48 | 2.82* |
| r | 38.4 | 3.9 | 9.82 | <0.001 | 2536.10 | 0.04* |
| s | 7.0 × 10−4 | 2.4 × 10−2 | −0.29 | 0.77 | 2535.00 | 0.04 |
| ti | −0.6 | 3.7 × 10−2 | −18.34 | <0.001 | 1945.47 | 21.72* |
| ni | 0.2 | 1.7 × 10−2 | 13.67 | <0.001 | 1714.76 | 8.50* |
Binomial regression: null deviance, 2,714 on 4,999 degrees of freedom (df); residual deviance (RD), 1,715 on 4,991 df. For analysis of deviance, terms were added sequentially in the order presented above. *, P(χ2) < 0.05.
Fig. 2.
Main factors affecting inversion polymorphism. Proportion of simulations ending in inversion polymorphism as a function of (A) time of inversion introduction (ti) and (B) number of loci captured in the inversion (ni). In A, ti is given in time steps of the simulation, where each time step is a single generation between step 290 and 299 (1 year). Population size (crosses) is indicated on the right vertical axis as fold-increase relative to the minimum S1 at step 290. Proportion of polymorphic simulations (circles) is indicated on the left vertical axis.
In a separate analysis restricted to those simulations which ended in inversion polymorphism, we used a conditional model to test which factors were significant determinants of final inversion frequency. These were m1, s, and ni. For detailed results, see SI Table 4.
Field Results.
Distribution of the 2Rj inversion was compared in the core (puddle and swamp) and peripheral (rock pool) breeding sites. A total of 459 larvae from collections in September of 2000 and 221 larvae from September 2006 were successfully karyotyped for 2Rj using molecular diagnostics (19). Collections in 2000 were identified by day and puddle/pool; the numbers from each category were too small to permit detailed analysis, so they were pooled into rock pool vs. puddles/swamp. Collections in 2006 were made on the same day from each source; the numbers collected from each puddle/pool were small and not recorded, thus they were also pooled. Table 3 gives the numbers and frequencies of each 2Rj karyotype (i.e., +j/+j, +j/j, and j/j) found in alternative habitats, rock pools or puddles/swamp, for both years. The distribution of larval genotypes in both years was statistically different between samples from rock pools vs. puddles/swamp, with P < 0.001 [Fisher's exact test (20)]. Further, in both years, there was an excess of the 2Rj chromosome arrangements in the rock pools relative to the puddles/swamp, just as predicted by the ecotypification hypothesis for origin of the Bamako form. Of 680 larvae analyzed, only four 2Rj/+j heterozygotes were found.
Table 3.
Distribution of the 2Rj inversion among larval breeding sites in two years in Banambani, Mali
| Year | 2Rj karyotypes |
Total | ||
|---|---|---|---|---|
| +/+ | +/j | j/j | ||
| 2000 | ||||
| Rock pools | 191 | 2 | 52 | 245 |
| Puddles/swamp | 193 | 2 | 19 | 214 |
| Total | 384 | 4 | 71 | 459 |
| 2006 | ||||
| Rock pools | 50 | 0 | 29 | 79 |
| Puddles/swamp | 126 | 0 | 16 | 49 |
| Total | 176 | 0 | 45 | 221 |
Only larvae of the S molecular form of An. gambiae s.s. are included.
Discussion
We note three particularly important aspects of these results. First, the simulations based on models parameterized specifically to coincide with the population dynamics of A. gambiae indicate that establishment of a new chromosomal inversion through local adaptation and recombination suppression in a peripheral habitat is feasible. Approximately 7.5% of the parameter space explored allowed for such establishment. The parameters most influential in determining whether a newly arisen inversion in a peripheral population will be retained are time of introduction (ti); number of adaptive loci captured (ni); migration rate from the core to the peripheral population (m1); and, to a lesser extent, overall size of the peripheral population (S1) and recombination rate (r) (Table 2, Fig. 2). Assuming that migrants are maladapted to the peripheral environment, higher migration and larger numbers of locally adapted loci captured by the inversion increased the probability of its establishment and were important factors affecting the final inversion frequency (SI Fig. 3). The time parameter (ti) captures both population size and change in population size, because the simulations were performed on populations cycling between S0 and 100·S0 each 10 generations (≈1 year). Introduction of the new inversion into the population when it is small and growing increased the probability of establishment of the inversion as a polymorphism compared with introduction to a large or shrinking population (Fig. 2A). The importance of this last parameter was not explored by Kirkpatrick and Barton (4) and would seem especially relevant to species with large fluctuations in population sizes, a characteristic of many multivoltine insects and most important vectors of diseases.
Second, the field observations on the distribution of the 2Rj inversion among A. gambiae larval breeding sites at a location in Mali, West Africa, are consistent with the simulations and support Coluzzi's (6) model for the establishment of an inversion in marginal habitats. In West Africa, A. gambiae larvae are found in pools with soil substrates such as hoof prints near water, tire ruts in dirt roads, edges of seasonal swamps, and irrigated rice fields (13). Laterite rock pools with stone substrate are unusual as a larval breeding site for A. gambiae and must be considered ecologically marginal. In addition, such pools are found in this part of Africa only during and immediately after the rainy season, which coincides with the population maximum of the presumed ancestral A. gambiae Savanna form (13), the stage when they are predicted to expand into ecologically marginal areas. These are the dynamics envisioned by Coluzzi as important in establishing inversions as a prelude to speciation (6) (see also ref. 7).
The computer simulations and field observations presented here refer strictly to the dynamics and distribution of chromosomal inversions. What is the evidence they are relevant to speciation in this group of mosquitoes? It is clear that considerable reproductive isolation exists between Bamako and the other sympatric chromosomal forms as indicated by lack of conformity to Hardy–Weinberg expectations of a panmictic unit (Table 3; and see also refs. 13 and 19). Coulibaly et al. (19) reported that, in a karyotyped sample of nearly 1,000 adult A. gambiae collected from seven villages (including Banambani) in southern Mali in 2004, only 6 2Rj/+j heterokaryotypes were found. This was not due to a Wahlund effect, because the sample of 607 karyotyped mosquitoes from a single village (Kela) contained only four 2Rj/+j heterokaryotypes (the rest comprised 243 +j/+j and 360 j/j), representing an extreme deviation from random mating. In our data from Banambani, only four heterokaryotypes were observed in a sample of 680 larvae. That both 2Rj/j and 2R+j/+j homozygotes are found in the same larval pools is strong evidence these forms are in sympatry in the strictest sense in this region of Mali. Given the lack of evidence for postzygotic isolation between the forms (13), this implies considerable nonrandom mating between Bamako and Savanna (as defined by the 2Rj; see ref. 19). That 2Rj is found almost exclusively in the Bamako form, that the Bamako form is found almost exclusively in areas that contain rock pools, and that it is disproportionately present in rock pool breeding sites, all implicate inversion 2Rj in conferring adaptation to this unique niche. These results are consistent with the longstanding hypothesis that ecological adaptation promotes reproductive isolation and speciation, a generalization of Coluzzi's concept of “ecotypification” in anopheline mosquitoes. This hypothesis has been supported not only by modeling but also by a recent metaanalysis of published data for >500 species pairs, suggesting that this causal relationship may be a general evolutionary rule (8).
For such adaptation to lead to reproductive isolation requires that genes involved in reproductive behavior are in linkage disequilibrium with the ecologically adapted alleles or, perhaps less likely, that the ecologically adapted alleles themselves affect reproductive isolation. Linkage disequilibrium is most likely when there is low or no recombination. This is exactly the major effect of paracentric inversions: reduction of recombination within and especially near the breakpoints between alternative arrangements. In Drosophila, another dipteran, where detailed studies are possible, genes involved in reproductive isolation have been shown to be concentrated in inversions fixed for alternative gene arrangements in very closely related species (21). Similarly, in the apple maggot fly Rhagoletis pomonella, genetic mapping revealed evidence that chromosomal inversions were pivotal to formation of the sympatric apple and haw host races (22). The importance of suppressed recombination in speciation of A. gambiae is supported by the large concentration of fixed nucleotide differences between the M and S forms in low recombination regions near the centromeres of chromosomes X and 2L (23–26). Indeed, there is good evidence of “selective introgression” among full species in the A. gambiae sibling species complex, all except one of which differ, not coincidentally, by at least one fixed inversion difference. Many regions of the genome are shared, and only regions of low recombination remain differentiated between species (27). In regions of reduced (but nonzero) recombination, a selection-migration balance can preserve adaptive combinations of alleles from homogenization in the face of gene flow through elimination of hybrids; alleles that limit production of hybrids and that are associated with adaptive loci in low-recombination regions would be favored to the extent they eliminate wasteful reproductive effort (28).
Because there is good support for both the genetic and now ecological cohesion (in the sense of ref. 29) of the Bamako form, it seems reasonable to consider it as a separate species and to assign a new species name. Although this recommendation does not include a consideration of geographic variation, the narrow distribution of Bamako makes it likely these observations near Banambani hold generally for the taxon.
Finally, this study emphasizes the importance of being able to identify the taxon to which larvae of the A. gambiae complex belong, especially those taxa based solely on chromosome structure, i.e., the chromosomal forms of A. gambiae s.s. Until now, such identification has been possible only for adult females based on polytene chromosomes in ovarian tissue. This has complicated detailed study of ecological and other aspects of immature life stages and males of these taxa. The development of a DNA-based diagnostic test for inversions by Coulibaly et al. (19) now allows much more detailed understanding of these forms. Application of this tool allowed us to combine empirical and simulation approaches to address an issue of extreme importance in understanding the population biology, from a medical perspective, of arguably the most important species of insects in the world.
Materials and Methods
Model Simulations.
We created a “minimal model” (30) that includes the essentials of the ecotypification hypothesis with regard to chromosomal inversions in A. gambiae s.s. All parameters used are summarized in Table 1, along with the ranges simulated based on empirical studies referenced in that table. We simulated two populations of mosquitoes connected by migration. The first population represented the core portion of the range and supported a large number of individuals. The second population represented a marginal habitat supporting fewer individuals. Each mosquito had a diploid genome of 15 equally spaced (recombination distance) biallelic loci under selection. Ten generational steps (≈1 year) were simulated with population sizes changing from a low in the dry season (S0 for core, S1 for peripheral) to a maximum in the wet season (100·S0, 100·S1), then back to S0 and S1 in generations 10, 20, 30, etc. The evolutionary variables were selection (s), recombination (r), and migration between populations (m).
Each simulation started with randomly chosen alleles for all individuals and was run for 289 generations to attain near-equilibrium migration-selection balance. During the next year (generations 290–299), an inversion was introduced into the peripheral population at a single time step, ti,; note that ti also determines the population size into which an inversion was introduced. The inversion was assumed to have “captured” ni alleles at the 15 biallelic loci that have adaptive advantage in the peripheral habitat. The simulation was then run to generation 600, and the frequencies of the inversion in the core and peripheral populations were recorded. The outcome was either polymorphism of the inversion [(0< pf < 1) or loss (pf = 0)]. The range of the parameter space explored was defined by the empirical data or reasonable estimates based on the biology of the mosquitoes as indicated in Table 1. Approximately 5,000 simulations were run exploring this parameter space [for additional details, see SI Text (31)].
Field Observations.
The sampling locations were near the village of Banambani, Mali. Banambani is in a largely agricultural area of the Sudan-Savanna vegetation belt, ≈20 km from Bamako, at 12°48′ N and 8°03′ W. The climate is of the Northern Sudan type, with a rainy season extending from May to October and a dry season during the rest of the year. Three sources of development for anopheline larvae have been identified in the area, as shown in the map of Edillo et al. (17): a field of laterite rock with holes that hold water (“rock pools”) to the south east of the village, puddles primarily on the near west side of the village and a swamp/pond farther west of the village beyond the puddles. Photographs of these are shown in Fig. 1. The distance between them was <2 km, well within the flight range of individual female A. gambiae. For information about dispersal by A. gambiae around Banambani and more comprehensive description of the area, see ref. 32.
Our collections were made during the peak of the rainy season in 2000 and 2006. The puddles in subarea 1 (17) had few or no larvae, so we sampled predominantly from subareas 2 and 3. The rock pool collections came from all of the rock pool subareas, predominantly from the lower rock pools, subarea 3. The sampling procedures were slightly different for 2000 and 2006, because the objectives of the original studies differed slightly. In 2000, samples of mosquito immatures were collected every alternate day from 28 July to 25 August, whenever weather permitted. Using a standard aquatic larval dipper, 60 dips (350 ml) were taken from edges of sampling sites within each type of habitat. The larvae were transported to the laboratory in Bamako, where DNA was extracted and sent to the University of Texas Medical Branch, Galveston, for further identification to species (33) and molecular form (34). See Edillo et al. (17) for further details. After storage at 4°C in individual vials, extracts of S form larval DNA were shipped to the University of Notre Dame (UND) for karyotype analysis. The 2006 samples were made by placing a tray into the pool or puddle, then removing individual A. gambiae s.l. larvae with a dropper (Fig. 1A). Approximately 10 larvae per bag were stored in small plastic bags with water from that source for transport to the laboratory in Bamako. After drying on filter paper, larvae were placed individually in small vials with 80% ethanol and shipped to UND for molecular characterization. Approximately 90–95% of the larvae were A. gambiae (the others were Anopheles arabiensis); among the A. gambiae s.s., ≈95% corresponded to the S molecular form. The data presented here are confined to the S molecular form of A. gambiae, which in Mali may be either the Savanna or Bamako chromosomal forms.
The 2Rj inversion that largely distinguishes Savanna from Bamako was molecularly karyotyped using a recently developed PCR diagnostic assay that has been extensively validated in Mali (19).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Mario Coluzzi (University of Rome, Rome) for from his help and encouragement; this paper grew from the seeds of inspiration that he planted in each of us. We thank G. Lanzaro (University of California, Davis) for generous sharing of DNA, T. Collier (University of California, Los Angeles) for help with simulations, and M. Kern and four Notre Dame undergraduate researchers (D. Thaner, M. Belton, J. Liu, and V. Au) for excellent technical assistance. The suggestions of two anonymous reviewers are gratefully acknowledged. Funding was provided by National Institutes of Health Grants 1 R01 AI51633 (to C.E.T.), R01 AI046018 (to J.R.P.), and R01 AI63508 (to N.J.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709806105/DC1.
References
- 1.White MJD. Modes of Speciation. San Francisco: Freeman; 1978. [Google Scholar]
- 2.Powell JR. Progress and Prospects in Evolutionary Biology: The Drosophila Model. Oxford, UK: Oxford Univ Press; 1997. [Google Scholar]
- 3.Butlin RK. Mol Ecol. 2005;14:2621–2635. doi: 10.1111/j.1365-294X.2005.02617.x. [DOI] [PubMed] [Google Scholar]
- 4.Kirkpatrick M, Barton N. Genetics. 2006;173:419–434. doi: 10.1534/genetics.105.047985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobzhansky T. Genetics and the Origin of Species. New York: Columbia Univ Press; 1951. [Google Scholar]
- 6.Coluzzi M. In: Mechanisms of Speciation. Barigozzi C, editor. New York: Liss; 1982. pp. 143–153. [Google Scholar]
- 7.Ayala FJ, Coluzzi M. Proc Natl Acad Sci USA. 2005;102(Suppl 1):6535–6542. doi: 10.1073/pnas.0501847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funk DJ, Nosil P, Etges WJ. Proc Natl Acad Sci USA. 2006;103:3209–3213. doi: 10.1073/pnas.0508653103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, et al. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- 10.Gavrilets S. Evol Int J Org Evol. 2003;57:2197–2215. doi: 10.1111/j.0014-3820.2003.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 11.Navarro A, Barton NH. Science. 2003;300:321–324. doi: 10.1126/science.1080600. [DOI] [PubMed] [Google Scholar]
- 12.Coluzzi M, Petrarca V, DiDeco MA. Bull Zool. 1985;52:45–63. [Google Scholar]
- 13.Toure YT, Petrarca V, Traore SF, Coulibaly A, Maiga HM, Sankare O, Sow M, DiDeco MA, Coluzzi M. Parassitologia. 1998;40:477–511. [PubMed] [Google Scholar]
- 14.Gillies MT, De Meillon B. The Anophelinae of Africa South of the Sahara. Johannesburg, South Africa: South African Institute for Medical Research; 1968. [Google Scholar]
- 15.Goudie AS. In: The Physical Geography of Africa. Adams WM, Goudie AS, Orme AR, editors. Oxford, UK: Oxford Univ Press; 1996. pp. 148–160. [Google Scholar]
- 16.Areola A. In: The Physical Geography of Africa. Adams WM, Goudie AS, Orme AR, editors. Oxford, UK: Oxford Univ Press; 1996. pp. 134–147. [Google Scholar]
- 17.Edillo FE, Tripet F, Toure YT, Lanzaro GC, Dolo G, Taylor CE. Malar J. 2006;5:35. doi: 10.1186/1475-2875-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Touré YT. Marseille, France: Université de Droit, d'Economie et des Sciences Aix-Marseille III; 1985. PhD thesis. [Google Scholar]
- 19.Coulibaly MB, Pombi M, Caputo B, Nwakanma D, Jawara M, Konate L, Dia I, Fofana A, Kern M, Simard F, et al. Malar J. 2007;6:133. doi: 10.1186/1475-2875-6-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.StataCorp. Stata Statistical Software: Release 9. College Station, TX: StataCorp LP; 2005. [Google Scholar]
- 21.Noor MA, Grams KL, Bertucci LA, Reiland J. Proc Natl Acad Sci USA. 2001;98:12084–12088. doi: 10.1073/pnas.221274498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feder JL, Roethele JB, Filchak K, Niedbalski J, Romero-Severson J. Genetics. 2003;163:939–953. doi: 10.1093/genetics/163.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stump AD, Fitzpatrick MC, Lobo NF, Traore S, Sagnon N, Costantini C, Collins FH, Besansky NJ. Proc Natl Acad Sci USA. 2005;102:15930–15935. doi: 10.1073/pnas.0508161102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slotman MA, Reimer LJ, Thiemann T, Dolo G, Fondjo E, Lanzaro GC. Genetics. 2006;174:2081–2093. doi: 10.1534/genetics.106.059949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner TL, Hahn MW. Mol Biol Evol. 2007;24:2132–2138. doi: 10.1093/molbev/msm143. [DOI] [PubMed] [Google Scholar]
- 26.Turner TL, Hahn MW, Nuzhdin SV. PLoS Biol. 2005;3:e285. doi: 10.1371/journal.pbio.0030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Besansky NJ, Krzywinski J, Lehmann T, Simard F, Kern M, Mukabayire O, Fontenille D, Toure YT, Sagnon NF. Proc Natl Acad Sci USA. 2003;100:10818–10823. doi: 10.1073/pnas.1434337100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felsenstein J. Evolution. 1981;35:124–138. doi: 10.1111/j.1558-5646.1981.tb04864.x. [DOI] [PubMed] [Google Scholar]
- 29.Templeton AR. In: Speciation and Its Consequences. Otte D, Endler JA, editors. Sunderland, MA: Sinauer; 1989. pp. 3–27. [Google Scholar]
- 30.Roughgarden JSB, Shafir S, Taylor CE. In: Adaptive Individuals in Evolving Populations: Models and Algorithms. Belew RK, Mitchell M, editors. Redwood City, CA: Addison Wesley; 1996. pp. 25–32. [Google Scholar]
- 31.Manoukis NC. Los Angeles: University of California; 2006. PhD thesis. [Google Scholar]
- 32.Toure YT, Dolo G, Petrarca V, Traore SF, Bouare M, Dao A, Carnahan J, Taylor CE. Med Vet Entomol. 1998;12:74–83. doi: 10.1046/j.1365-2915.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- 33.Scott JA, Brogdon WG, Collins FH. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 34.Favia G, Lanfrancotti A, Spanos L, Siden-Kiamos I, Louis C. Insect Mol Biol. 2001;10:19–23. doi: 10.1046/j.1365-2583.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 35.Charlwood JD, Vij R, Billingsley PF. Am J Trop Med Hyg. 2000;62:726–732. doi: 10.4269/ajtmh.2000.62.726. [DOI] [PubMed] [Google Scholar]
- 36.Pinto J, Donnelly MJ, Sousa CA, Malta-Vacas J, Gil V, Ferreira C, Petrarca V, do Rosario VE, Charlwood JD. Heredity. 2003;91:407–414. doi: 10.1038/sj.hdy.6800348. [DOI] [PubMed] [Google Scholar]
- 37.Taylor C, Toure YT, Carnahan J, Norris DE, Dolo G, Traore SF, Edillo FE, Lanzaro GC. Genetics. 2001;157:743–750. doi: 10.1093/genetics/157.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Besansky NJ, Lehmann T, Fahey GT, Fontenille D, Braack LE, Hawley WA, Collins FH. Genetics. 1997;147:1817–1828. doi: 10.1093/genetics/147.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carnahan J, Zheng L, Taylor CE, Toure YT, Norris DE, Dolo G, Diuk-Wasser M, Lanzaro GC. J Hered. 2002;93:249–253. doi: 10.1093/jhered/93.4.249. [DOI] [PubMed] [Google Scholar]
- 40.Costantini C, Li SG, Della Torre A, Sagnon N, Coluzzi M, Taylor CE. Med Vet Entomol. 1996;10:203–219. doi: 10.1111/j.1365-2915.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- 41.Kayondo JK, Mukwaya LG, Stump A, Michel AP, Coulibaly MB, Besansky NJ, Collins FH. Malar J. 2005;4:59. doi: 10.1186/1475-2875-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tripet F, Dolo G, Lanzaro GC. Genetics. 2005;169:313–324. doi: 10.1534/genetics.104.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onyabe DY, Vajime CG, Nock IH, Ndams IS, Akpa AU, Alaribe AA, Conn JE. Trans R Soc Trop Med Hyg. 2003;97:605–608. doi: 10.1016/s0035-9203(03)80045-7. [DOI] [PubMed] [Google Scholar]
- 44.Bayoh MN, Thomas CJ, Lindsay SW. Med Vet Entomol. 2001;15:267–274. doi: 10.1046/j.0269-283x.2001.00298.x. [DOI] [PubMed] [Google Scholar]
- 45.Onyabe DY, Conn JE. Heredity. 2001;87:647–658. doi: 10.1046/j.1365-2540.2001.00957.x. [DOI] [PubMed] [Google Scholar]
- 46.Toure YT, Petrarca V, Traore SF, Coulibaly A, Maiga HM, Sankare O, Sow M, Di Deco MA, Coluzzi M. Genetica. 1994;94:213–223. doi: 10.1007/BF01443435. [DOI] [PubMed] [Google Scholar]
- 47.Coluzzi M, Sabatini A, Della Torre A, Di Deco MA, Petrarca V. Science. 2002;298:1415–1418. doi: 10.1126/science.1077769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.