Abstract
Interaction of the activating receptor NKG2D with its ligands is a major stimulatory pathway for cytotoxicity of natural killer (NK) cells. Here, the signaling pathway involved after NKG2D ligation is examined. Either incubation of the NKG2D-bearing human NKL tumor cell line with K562 target cells or cross-linking with NKG2D mAb induced strong activation of the mitogen-activated protein (MAP) kinases. Selective inhibition of JNK MAP kinase with four different means of inhibition greatly reduced NKG2D-mediated cytotoxicity toward target cells and furthermore, blocked the movement of the microtubule organizing center (MTOC), granzyme B (a component of cytotoxic granules), and paxillin (a scaffold protein) to the immune synapse. NKG2D-induced activation of JNK kinase was also blocked by inhibitors of Src protein tyrosine kinases and phospholipase PLCγ, upstream of JNK. Similarly, a second MAP kinase pathway through ERK was previously shown to be required for NK cell cytotoxicity. Thus, activation of two MAP kinase pathways is required for cytotoxic granule and MTOC polarization and for cytotoxicity of human NK cells when NKG2D is ligated.
Keywords: D-JNK-1, ERK, paxillin, siRNA, synapse
Natural killer (NK) cells are a population of lymphocytes that lack surface Ig and CD3, express CD16 and CD56, and mediate the killing of some virus-infected and tumor cells (1–3). The function of NK cells is controlled by a coordinated signal generated from the ligation of inhibitory and activating receptors. The ligation of activating receptors leads to activation of a cascade of intracellular downstream signaling molecules, ultimately resulting in polarization and exocytosis of granules to lyse the target cells (4–7).
NKG2D is a C-type lectin-like receptor whose activation can trigger NK-cell mediated killing of virus-infected and tumor cells (8, 9). Its ligands on targets cells are cell surface major histocompatibility complex (MHC) I-related chain A and B (MICA/B) and UL16-binding proteins (ULBP) 1, 2, 3, and 4 in humans (8, 10–12), and retinoic acid early transcript-1, minor histocompatibility antigen H-60, and murine ULBP-like transcript-1 in mice (13–16). Stimulation of NK cells with soluble NKG2D ligands causes activation of phosphatidylinositol 3-kinase (PI3K) and Akt signaling pathways and also JAK1 and STAT5 (17). Furthermore, cross-linking of NK cells with anti-NKG2D mAb activates Vav1, Rho GTPases, and PLC-γ (18–25).
Mitogen-activated protein kinases (MAPKs) are a family of serine/threonine protein kinases consisting of three subgroups: extracellular signal-regulated protein kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 (26–32). They have been shown to play important roles in a variety of cellular functions, including cell proliferation, apoptosis, oncogenesis, differentiation, inflammation, cell migration, and cytoskeleton remodeling (33–36). In NK cells, ERK is in the pathway PI3K–Rac1–PAK–MEK–ERK that regulates granule polarization mediated by reorientation of the microtubule organizing center (MTOC) to the synapse (6, 37, 38). Furthermore, ERK is activated when NK cells are stimulated with soluble NKG2D ligands (17) or when pNK cells or the NK tumor cell line YTS are stimulated through CD28 by CD80 or CD86 on targets (38). JNK as well as ERK has been briefly reported to be required for NKG2D-mediated lysis in cytotoxic T lymphocytes (39). However, the high concentration of the JNK inhibitor SP600125 used (25 μM) is far above that which is selective for JNK (40), and the concentration used decreases cell viability and induces apoptosis (41). No other supporting data were presented.
In this report, two specific inhibitors of JNK at low concentration, a dominant-negative mutant and siRNA, were used to determine whether inhibiting the activation of JNK in addition to ERK interferes with the signaling of NKG2D-mediated cytotoxicity and elements of synapse formation that may be involved.
Results
Expression of NKG2D and Its Functional Activity in NK Cells.
Examination of the expression of the NKG2D activating receptor in three NK tumor cell lines revealed that both NK92 and NKL express NKG2D, whereas no significant expression of NKG2D was detected on YTS cells [supporting information (SI) Fig. 6]. More than 90% of CD56+ peripheral blood NK cells (pNK) cells expressed NKG2D on their cell surface (SI Fig. 6A, Right). To examine whether NKG2D plays a functional role in the killing activity of these NK cells, K562 was used as a target. This target cell line expressed two NKG2D ligands, ULBP2 and MICA/B, on its surface, but neither ULBP1 nor ULBP3 (SI Fig. 6B). At an E:T ratio of 20:1, NKL, NK92, YTS, and pNK all showed 30–50% lysis of K562 cells. The addition of blocking NKG2D mAb significantly attenuated cytotoxicity of NKL, NK92, and pNK, but not of YTS (SI Fig. 6C). Thus, NKG2D is a functional activating receptor. NKL and primary pNK cells were blocked more efficiently and were, therefore, chosen for further study.
Effect of Two Specific Inhibitors on Activation of JNK MAPK by NKG2D Stimulation and Cytotoxicity.
NKL cells were cross-linked with anti-NKG2D mAb for different times. All three major MAPKs, JNK, ERK, and p38, were phosphorylated (Fig. 1A, Left). Similarly, JNK was activated in NKL cells after incubation with K562 target cells (Fig. 1A, Right).
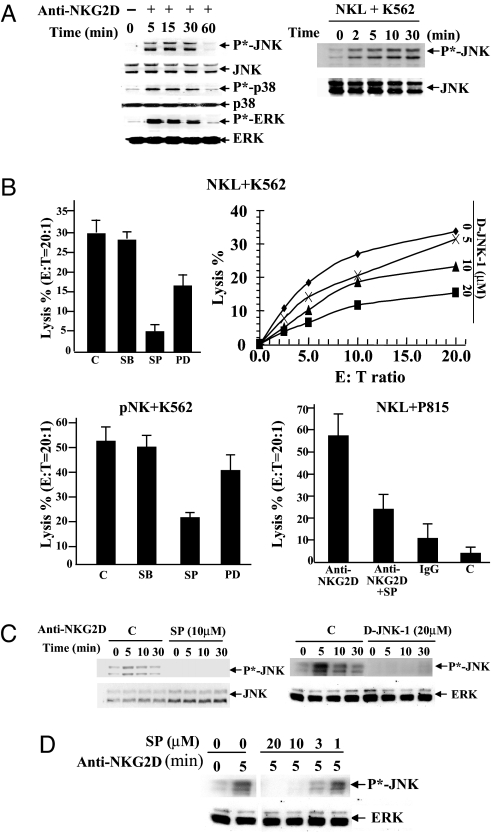
Fig. 1.
Activation of JNK is required for NKG2D-mediated cytotoxicity. (A) NKL cells were cross-linked with anti-NKG2D mAb for the indicated times at 37°C. (Left) Activation of MAPKs was detected with anti-phospho-JNK, p38, or ERK, and total proteins were measured by blotting with anti-JNK, p38, and ERK. (Right) NKL cells were cultured with K562 cells at a 1:1 ratio for 0–30 min. Activation of JNK was detected by anti-phospho-JNK. Anti-JNK was used as a loading control. (B) Percentage of lysis of target cells by NK cells. NKL (Upper) or pNK (Lower Left) cells, pretreated for 30 min at 37°C with either DMSO, 20 μM p38 MAPK inhibitor SB, 10 μM MEK1/2 inhibitor PD, 10 μM JNK inhibitor SP600125 (SP), or potent peptide inhibitor D-JNK-1 were tested for lysis of 51Cr-labeled K562 cells. (Lower Right) Murine mastocytoma P815 cells labeled with 51Cr were coated with anti-NKG2D and then incubated with NKL cells pretreated with JNK inhibitor SP at E:T ratio of 20:1. (C) NKL cells pretreated with 10 μM SP or 20 μM D-JNK-1 were cross-linked with anti-NKG2D mAb for the indicated times. Phosphorylation of JNK was then detected by anti-phospho-JNK antibody. Total JNK or ERK was measured as a loading control. (D) NKL cells pretreated with JNK inhibitor SP at different concentrations were cross-linked with anti-NKG2D mAb for 5 min. Phosphorylation of JNK was then detected by anti-phospho-JNK antibody. Total ERK was measured as a loading control. These bands were run in the same gel as C, Right, and the controls for the two experiments are the same. Data shown in A–D are representative of three or more individual experiments.
To examine which of those MAPK signaling pathways is involved in the NKG2D-mediated cytolytic activity of NK cells, specific inhibitors of MAPKs were used. Specific inhibition of JNK activation by 10 μM SP600125 or by the potent peptide inhibitor D-JNK-1 (42) significantly reduced cytotoxicity of NKL on K562 targets (Fig. 1B, Upper Left and Right). Specific inhibition of ERK by the upstream MAPK kinase MEK1/2 inhibitor PD98059 (PD) also partially decreased cytolytic activity, but the p38 inhibitor SB203580 (SB) had no significant effect in either NKL or pNK cells (Fig. 1B, Upper and Lower Left). A redirected cytolytic assay was also performed by using NKL cells pretreated with anti-NKG2D as effector cells and P815 cells expressing Fc receptor as target cells. SP600125 blocked the NKG2D-redirected cytotoxicity of NKL cells against P815 cells (Fig. 1B, Lower Right).
Treatment of NKL cells with 10 μM SP600125 or 20 μM D-JNK-1 also efficiently blocked the phosphorylation of JNK (Fig. 1C). Substantial inhibition of JNK phosphorylation was observed even at a very low concentration (3 μM) (Fig. 1D).
Effect of a Dominant-Negative JNK Mutant and JNK siRNA on NKG2D-Mediated Killing Activity.
To examine further whether JNK activity is specifically required for killing of NK cells, GFP-JNKdn (S183A,Y185F), a dominant-negative (dn) mutant of JNK (43), was transfected into NKL cells. The killing of NKL cells was greatly reduced by the expression of the GFP-JNKdn mutant but not by similarly transfected GFP-p38dn (Fig. 2 A and B).
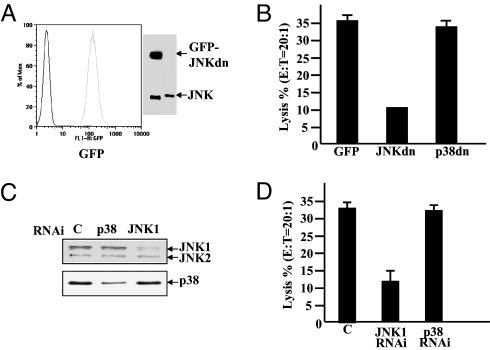
Fig. 2.
Inhibition of JNK activity by a JNK dominant-negative mutant or depletion of JNK by RNAi reduces NK killing activity. (A) NKL cells were transiently transfected with the GFP-JNKAF (JNKdn) mutant by using the Amaxa nucleofection system. Expression of GFP-JNKdn was confirmed by FACS analysis (Left) and Western blot analysis with anti-JNK antibody (Right). The lower band is endogenous JNK, and the upper band is the fusion protein GFP-JNKdn. (B) NKL cells expressing JNKdn together with NKL cells expressing GFP or GFP-p38AF (p38dn) as control were tested for lysis of 51Cr-labeled K562 cells (E:T = 20:1). (C) NKL cells were transfected with siRNAs specific for JNK-1, p38, or negative control siRNA. Depletion of JNK-1 expression was confirmed by Western blotting with anti-JNK antibody (Upper) or with anti-p38 as a control (Lower). (D) NKL cells treated with JNK-1, p38, or negative control siRNAs were tested for lysis of 51Cr-labeled K562 cells (E:T = 20:1, Right). Data are representative of three or more experiments.
Additionally, NKL cells were transfected with siRNAs specific for JNK-1. Transfection of JNK-1 siRNA markedly reduced the expression of JNK-1 protein (Fig. 2C). NKL cells transfected with siRNA targeting JNK-1 showed markedly reduced killing of K562 cells, whereas depletion of p38 had no significant effect (Fig. 2D).
Effects of Specific Inhibition of JNK Activation on Conjugation and Synapse Formation.
Cytolytic activity of NK cells is mediated through effector cell–target cell conjugation, synapse formation including actin ring formation, polarization of the MTOC and cytolytic granules, and finally exocytosis of cytolytic proteins including perforin and granzymes (6, 44). Inhibition of JNK activation by SP600125 had no effect on conjugation between NK cells and target cells (SI Fig. 7).
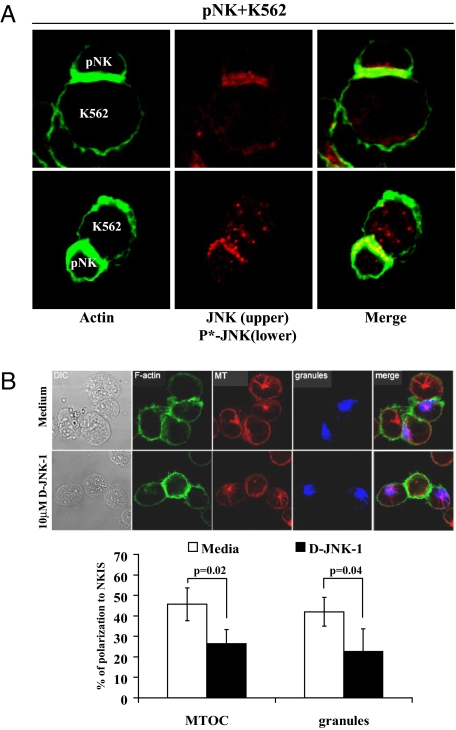
Both JNK (Upper) and phospho-JNK (Lower) accumulated at the synapse of pNK-K562 conjugates (Fig. 3A). Importantly, inhibition of JNK activity by D-JNK-1, SP600125, or JNK-1-specific siRNA blocked polarization of granzyme B, a component of cytolytic granules (blue circle), and the MTOC (γ-tubulin, red dot) to the synapse, although it had no effect on the localization of actin to the synapse (Fig. 3B and SI Fig. 8). JNK is localized to the centrosome (an alternative name for MTOC) during the cell cycle (45).
Fig. 3.
Effect of specific inhibition of JNK on synapse formation between NK cells and K562 cells. (A) Fixed pNK-K562 conjugates were stained with rabbit polyclonal anti-JNK that also reacts with phospho-JNK or anti-phospho-JNK, followed by the staining with Alexa Fluor 568-conjugated goat anti-rabbit IgG. (B) NKL cells pretreated with 10 μM D-JNK-1 for 30–45 min at 37°C were incubated with K562 cells for 30 min, allowing formation of cell–cell conjugates. After cell fixation and permeabilization, microtubules (MT) were stained with anti-α-tubulin mAb, followed by Alexa Fluor 568-conjugated anti-mouse IgG (red). F-actin (green) was stained with phalloidin-Alexa Fluor 488, and granules (blue) were stained with Alexa Fluor 647-conjugated anti-granzyme B mAb. Representative images are shown. Average percentages of NKL conjugates with MTOC or granule polarization are shown with SD and P values after counting >50 conjugates. Data are representative of three or more independent experiments. See Materials and Methods and SI Fig. 8B for details of determination of polarization.
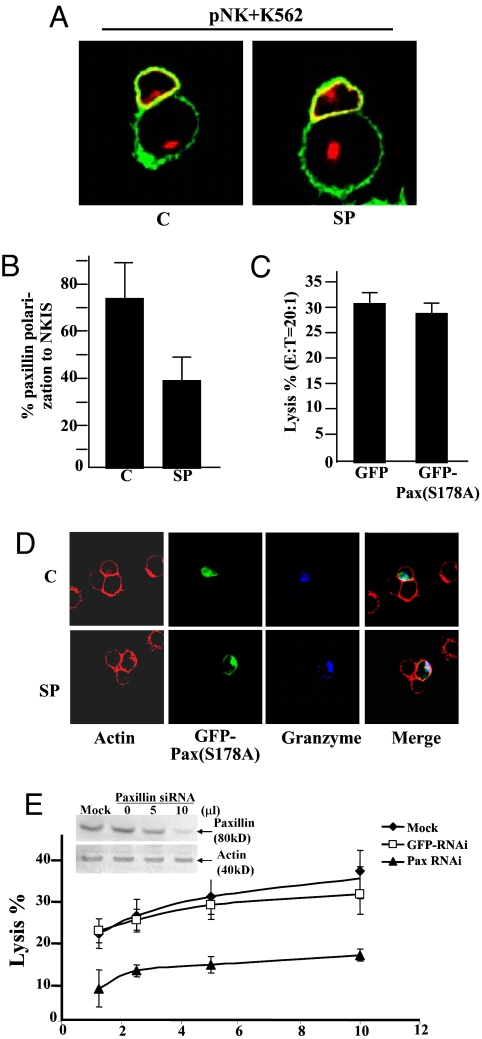
Paxillin, a scaffold protein, also moves to the MTOC of T cells, where it colocalizes with α- and γ-tubulin (46–48). Paxillin moved to the synapse in association with the MTOC of NK cells also, and this localization was inhibited by SP600125 (Fig. 4 A and B). Cytotoxicity of NKL cells on K562 targets was decreased after NKL cells were transfected with specific siRNA for human paxillin (Fig. 4E). JNK phosphorylates Ser-178 on paxillin, and this phosphorylation regulates cell migration (44, 47). However, overexpression of PaxS178A (GFP-PaxS178A) had no effect on killing by NKL cells of K562 cells (Fig. 4C). Moreover, GFP-PaxS178A still moved to the synapse and was still excluded in the presence of SP600125 (Fig. 4D).
Fig. 4.
JNK and paxillin. (A) pNK cells treated with 10 μM SP600125 (SP) or without (C, control) JNK inhibitor were incubated with K562 cells and then stained with mouse anti-actin (green) and monoclonal anti-paxillin (red) in both NK cells (upper cell) and target cell (lower cell). (B) Percentage of paxillin polarization to the synapse of pNK cells treated or not treated with JNK inhibitor. For each effector–target cell, 40 conjugates were examined. (C) NKL cells were transfected with pBABE-GFP-PaxS178A mutant and then tested for the lysis of 51Cr-labeled K562 cells (E:T = 20:1). (D) GFP-PaxS178A-transfected NKL cells were treated or not treated with JNK inhibitor and then incubated with K562 cells. The conjugates were stained with Alexa Fluor 488-conjugated phalloidin (green) and Alexa Fluor 647-conjugated anti-granzyme B (blue). (E) NKL cells were transfected with paxillin siRNA, GFP siRNA, or control siRNA (mock). (Inset) Depletion of JNK expression was confirmed by Western blotting with anti-paxillin antibody. NKL cells treated with these siRNAs were tested for lysis of 51Cr-labeled K562 cells. Data are representative of three or more experiments.
Multiple Signaling Pathways Are Required for NKG2D-Mediated Cytotoxicity of NK Cells.
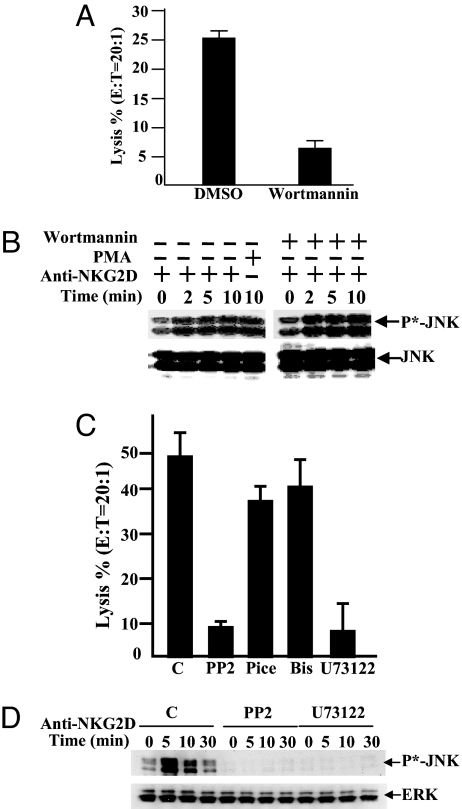
Binding of the p85 regulatory subunit of PI3K to the tyrosine-phosphorylated YNIM motif of DAP10 couples NKG2D-initiated signaling downstream to PI3K (18, 20, 25). Although inhibition of PI3K in the ERK pathway by wortmannin greatly reduced the killing of K562 by NKL cells (Fig. 5A), it had no effect on NKG2D-induced JNK activation (Fig. 5B).
Fig. 5.
Upstream regulators for the NKG2D-induced JNK activation. (A) NKL cells were preincubated with 200 nM wortmannin and then incubated with 51Cr-labeled K562 cells at E:T = 10:1 for 4 h at 37°C. Percentage of lysis was measured as 51Cr release. (B) NKL cells were pretreated with 200 nM wortmannin and then stimulated by cross-linking with anti-NKG2D monoclonal antibody at 37°C for 0–30 min. NKL were also treated with phorbol 12-myristate 13-acetate (PMA) and NaCl as positive controls. Activation of JNK was detected by anti-phospho-JNK (Upper), and the total protein was measured by anti-JNK (Lower). (C) NKL cells, treated with DMSO, 20 μM Src PTK inhibitor PP2, 100 μM Syk inhibitor piceatannol (Pice), 2 μM PKC inhibitor bisindolylmaleimide (Bis), or 10 μM PLC inhibitor U73122 were incubated with 51Cr-labeled K562 cells (ratio 20:1) to measure percentage of lysis. (D) NKL cells pretreated with DMSO, PP2, and U73122 were cross-linked with anti-NKG2D mAb for the indicated times. Activation of JNK was detected with anti-phospho-JNK antibody, and total JNK protein was examined by using anti-JNK antibody. Results are representative of three experiments.
Because Src, Syk, PLC-γ, and PKC have been implicated in some modes of lymphocyte activation, their involvement in NKG2D-induced JNK activation was examined. Pretreament of NKL cells with the Src inhibitor PP2 or the PLC-γ inhibitor U73122 [but neither with the inactive analog U73343 (data not shown), the Syk inhibitor piceatannol, nor the PKC inhibitor bisindolylmaleimide] markedly reduced their killing activity against K562 cells (Fig. 5C). Moreover, pretreatment of NKL cells with PP2 or U73122 followed by stimulation of the cells with NKG2D mAb attenuated NKG2D-induced JNK activation (Fig. 5D), suggesting PLC-γ is upstream of JNK in the pathway of NKG2D-mediated cytotoxicity.
Discussion
NK cells express diverse families of activating receptors that can trigger lysis of the target cells upon recognition of specific ligands. NKG2D is an activating receptor that plays a significant role in NK cell-mediated tumor cell recognition and cytolysis. Stimulation of NKG2D results in binding of DAP10 and recruitment of PI3K through its PI3K-binding motif YNIM (20) and then triggers a Syk-independent pathway involving PI3K, PLC-γ Grb2, Vav1, and Rho family GTPase (18, 25). The present studies have extended this signaling pathway to include the MAPK JNK in the general picture. Selective inhibition of JNK activation by a JNK inhibitor significantly attenuated NKG2D-mediated cytotoxicity of NK cells. Inhibition of ERK by a selective inhibitor of its MAPK kinase also blocked cytotoxicity, although to a less extent at the concentration used. Inhibition of p38 had no significant effect on NKG2D-mediated cytolysis of NK cells. Because use of high concentrations of MAPK inhibitors may decrease their specificity (40), low concentrations of inhibitors were used in the present experiments to avoid the nonspecific inhibition of other kinases. Furthermore, a dominant-negative JNK-1 mutant or depletion of JNK expression by siRNA significantly impaired NKG2D-mediated cytotoxicity, but importantly, neither treatment of p38 had any effect. Thus, JNK is a key downstream player in the NKG2D signaling pathway. In addition, inhibition of JNK also reduced the cytolytic activity of YTS cells, which do not express NKG2D but depend on CD28 for killing activity (38). Thus, the role of JNK in the regulation of cytolytic activity is general in NK cells. However, the reports from Chini et al. (49) and Trotta et al. (50) provided evidence for p38 involvement in development of NK cytotoxicity. In particular, Trotta et al. (50) excluded a major role for JNK-1 in this process. Such a discrepancy could be caused by the use of different analysis methods or cell lines. The data shown in Figs. 1 and 2 suggest that p38 may not be involved but should not be regarded as definitive proof of lack of involvement of this kinase in NK cytotoxicity without further study.
Phosphorylation of paxillin, a focal adhesion-associated protein, at Ser-178 by JNK has been shown to be essential for cell migration (43). Paxillin has also been shown to localize to the MTOC of T cells, where it colocalizes with α- and γ-tubulin (47). In NK cells, both JNK and activated phospho-JNK localized to the synapse of NK cells together with the MTOC after specific recognition of target cells. The MTOC, granzyme B (cytolytic granules), and paxillin all moved to the synapse, and inhibition of JNK activation blocked these movements. Moreover, siRNA knockdown of paxillin greatly impaired cytotoxicity of NK cells. However, expression of a S178A mutant of paxillin (PaxS178A) did not inhibit the cytotoxicity. Thus, the role of paxillin in synapse formation of NK cells is not mediated through the phosphorylation of the Ser-178 residue. JNK might phosphorylate other serine, threonine, or tyrosine sites of paxillin to regulate the movement of paxillin directly, or mediate its effect through another downstream target protein to affect paxillin localization indirectly. Detailed analysis of the six Ser/Thr and four Tyr phosphorylation sites in paxillin (46) is required in the future.
PI3K has been demonstrated to be a pivotal player in the regulation of NK cytotoxicity by controlling the activation of ERK (18, 37, 38). However, NKG2D-induced JNK activation was independent of PI3K, but was partly dependent on Src kinase and PLC. Caraux et al. (51) have recently shown that PLC-γ2 is pivotal for the cytotoxicity of NK cells, but MTOC polarization was shown to be normal in PLC-γ2-deficient NK cells. JNK activation might depend on other PLC isoforms. Taken together, two signaling pathways, Src–PI3K–ERK and Src–PLC–JNK, acting in an additive rather than synergistic manner, contribute to NKG2D-mediated cytotoxicity. JNK and ERK are both phosphorylated during granule and MTOC polarization using a variety of different NK cells and target cells, but only after activation of some NK surface receptors, thus providing a definition of a NK-activating receptor and generalizing the results described here (52).
Materials and Methods
Antibodies and Reagents.
NKG2D mAb was purchased from R&D Systems. Anti-CD56 mAb, phycoerythrin-conjugated goat anti-mouse IgG1, and anti-paxillin mAb were from BD PharMingen. Anti-ERK, JNK, and anti-phospho-ERK, phospho-JNK, and phospho-p38 were from Cell Signaling. Rabbit anti-p38 was a gift from Jiahuai Han (Scripps, La Jolla, CA). Anti-γ-tubulin mAb was from Sigma. The JNK inhibitor SP600125 was from BioSource. The potent JNK peptide inhibitor D-JNK-1 was kindly provided by Xigen S.A. The MEK1/2 inhibitor PD (upstream of ERK), p38 inhibitor SB, the PI3K inhibitor wortmannin, the Src inhibitor PP2, the Syk inhibitor piceatannol, the PKC inhibitor bisindolylmaleimide, and the PLC inhibitor U73122 were from Calbiochem. Plasmid pBABE-GFP-Paxillin(S178A) was constructed by PCR from pEGFP-Paxillin(S178A), kindly provided by K. Jacobson (University of North Carolina, Chapel Hill, NC) (43).
Cells and Tranfectants.
pNK cells were isolated from peripheral blood from anonymous healthy donors by using Ficoll either directly or after enrichment for NK cells with Rosettesep according to the manufacturer's instructions (StemCell Technologies). Enriched NK cell populations were >85% CD56+/CD3− with <0.5% CD3+ cells. NKL and NK92 tumor cells were grown in RPMI medium containing 100 units/ml IL-2 (PetroTech, Inc.), 10% FBS, 1% l-glutamine, 1% sodium pyruvate, and streptomycin/penicillin. K562 erythroleukemia cells were used as target cells. Transfection of NKL cells was performed by using the nucleofection technology (Amaxa).
siRNA and siRNA Transfection.
siRNAs for human p38 (sc-29433), JNK-1 (sc-29380), paxillin (sc-29439), and control (sc-37007) were purchased from Santa Cruz Biotechnology. Transfection of siRNA into NKL cells was performed according to the manufacturer's instructions.
Laser Scanning Confocal Microscopy and Analysis of Polarization and Conjugation.
The imaging work was done as described in ref. 44. To evaluate granule polarization, cell–cell conjugates showing F-actin polymerization were examined by visually dividing the cell into four radial sectors (SI Fig. 8); the granules and MTOC were deemed polarized when they were oriented toward the conjugated cell within the sector in contact with the target cell. This method has a blank value of ≈25% because of random distribution within this sector and is therefore semiquantitative. This blank has not been subtracted from the data shown. At least three independent experiments were performed to give a minimum of 50 conjugates counted for each cell–drug combination. A two-tailed t test was used to compare the differences between groups. A P value < 0.05 was considered statistically significant.
Cell Activation and Immunoblot Analysis.
In experiments involving NKG2D cross-linking, NK cells were incubated for 30 min in ice with 10 μg/ml anti-NKG2D before adding goat anti-mouse F(ab′)2 at 37°C for the indicated period of times. For experiments in which NK cells were activated by target cells, NK cells were incubated with paraformaldehyde-fixed target cells at 37°C for the indicated period of times. Activation of MAPK was detected by immunoblotting with anti-phospho-MAPK rabbit antibody, and total MAPK protein was blotted with anti-MAPK rabbit antibody.
Cytotoxicity Assays.
The 51Cr-release assays were done as described in ref. 53. In redirected cytotoxicity assays, effector cells and target cells were mixed in the presence of soluble anti-NKG2D or anti-IgG for 30 min. For experiments with inhibitors, effector cells were incubated with inhibitors for 30 min at 37°C, 5% CO2 before the cytotoxic assay. Assays were typically performed three times, unless otherwise indicated.
Antibodies.
Anti-MICA/B mAb, anti-ULBP-1, 2, 3 mAb, and phycoerythrin (PE)-conjugated anti-NKG2D antibody were purchased from R&D Systems.
Flow Cytometry.
For NKG2D, cells were stained with PE-conjugated anti-NKG2D antibody. To detect surface expression of NKG2D ligands in K562 cells, the cells were incubated with unconjugated mAbs for 20 min and washed with PBS containing 2% FBS. PE-conjugated goat anti-mouse IgG was then used for further staining. Stained cells were analyzed with FACSCalibur (Becton Dickinson).
FACS-Based Conjugation Assay.
Cell conjugate formation was determined by flow cytometry as described (54, 55). NK cells at 5 × 106 cells per ml were labeled with PKH67 (green; Sigma) for 2–5 min at 25°C; they were mixed with K562-RFP cells transfected with RFP (red). After incubation at 37°C for 10–15 min, the cells were resuspended and then fixed by the addition of 0.5 ml of ice-cold PBS/0.5% paraformaldehyde. The relative proportion of conjugation (red/green) events was promptly determined by two-color flow cytometric analysis.
Supplementary Material
ACKNOWLEDGMENTS.
We thank B. Rybalov for technical assistance with FACS analysis and other laboratory members for help at all stages. This work was supported by National Institutes of Health Research Grant AI50207.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712310105/DC1.
References
- 1.Long EO, Wagtmann N. Natural killer cell receptors. Curr Opin Immunol. 1997;9:344–350. doi: 10.1016/s0952-7915(97)80080-5. [DOI] [PubMed] [Google Scholar]
- 2.Biassoni R, et al. Human natural killer cell receptors and coreceptors. Immunol Rev. 2001;181:203–214. doi: 10.1034/j.1600-065x.2001.1810117.x. [DOI] [PubMed] [Google Scholar]
- 3.Orange JS, Fassett MS, Koopman LA, Boyson JE, Strominger JL. Viral evasion of natural killer cells. Nat Immunol. 2002;3:1006–1012. doi: 10.1038/ni1102-1006. [DOI] [PubMed] [Google Scholar]
- 4.Moretta A, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 5.Colucci F, Di Santo JP, Leibson PJ. Natural killer cell activation in mice and men: Different triggers for similar weapons? Nat Immunol. 2002;3:807–813. doi: 10.1038/ni0902-807. [DOI] [PubMed] [Google Scholar]
- 6.Vyas YM, Maniar H, Dupont B. Visualization of signaling pathways and cortical cytoskeleton in cytolytic and noncytolytic natural killer cell immune synapses. Immunol Rev. 2002;189:161–178. doi: 10.1034/j.1600-065x.2002.18914.x. [DOI] [PubMed] [Google Scholar]
- 7.Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 8.Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–730. [PubMed] [Google Scholar]
- 9.Jamieson AM, et al. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 10.Cosman D, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 11.Chalupny N, Sutherland CL, Lawrence WA, Rein-Weston A, Cosman D. ULBP4 is a novel ligand for human NKG2D. Biochem Biophys Res Commun. 2003;305:129–135. doi: 10.1016/s0006-291x(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 12.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 13.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerwenka A, et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 15.Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM. Cutting edge. Murine UL16-binding protein-like transcript 1: A newly described transcript encoding a high-affinity ligand for murine NKG2D. J Immunol. 2002;169:4079–4083. doi: 10.4049/jimmunol.169.8.4079. [DOI] [PubMed] [Google Scholar]
- 16.Diefenbach A, Hsia JK, Hsiung MY, Raulet DH. A novel ligand for the NKG2D receptor activates NK cells and macrophages and induces tumor immunity. Eur J Immunol. 2003;33:381–391. doi: 10.1002/immu.200310012. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland CL, et al. UL16-binding proteins, novel MHC class I-related proteins, bind to NKG2D and activate multiple signaling pathways in primary NK cells. J Immunol. 2002;168:671–679. doi: 10.4049/jimmunol.168.2.671. [DOI] [PubMed] [Google Scholar]
- 18.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol. 2003;4:557–564. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 19.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 21.Diefenbach A, et al. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol. 2002;3:1142–1149. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 22.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3:1150–1155. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 23.Zompi S, et al. NKG2D triggers cytotoxicity in mouse NK cells lacking DAP12 or Syk family kinases. Nat Immunol. 2003;4:565–572. doi: 10.1038/ni930. [DOI] [PubMed] [Google Scholar]
- 24.Rosen DB, et al. A Structural basis for the association of DAP12 with mouse, but not human, NKG2D. J Immunol. 2004;173:2470–2478. doi: 10.4049/jimmunol.173.4.2470. [DOI] [PubMed] [Google Scholar]
- 25.Upshaw JL, et al. NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol 3-kinase in human natural killer cells. Nat Immunol. 2006;7:524–532. doi: 10.1038/ni1325. [DOI] [PubMed] [Google Scholar]
- 26.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 27.Cobb MH, Boulton TG, Robbins DJ. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991;2:965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derijard B, et al. JNK-1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 29.Kyriakis JM, et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 30.Lee JC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 31.Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: Specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 33.Ono K, Han J. The p38 signal transduction pathway: Activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 34.Pearson G, et al. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 35.Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- 36.Xia Y, Karin M. The control of cell motility and epithelial morphogenesis by Jun kinases. Trends Cell Biol. 2004;14:94–101. doi: 10.1016/j.tcb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Jiang K, et al. Pivotal role of phosphoinositide 3-kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1:419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, et al. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc Natl Acad Sci USA. 2006;103:10346–10351. doi: 10.1073/pnas.0604236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meresse B, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 40.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: An update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masamune A, et al. A c-Jun NH2-terminal kinase inhibitor SP600125 (anthra[1,9-cd]pyrazole-6(2H)-one) blocks activation of pancreatic stellate cells. J Pharmacol Exp Ther. 2004;310:520–527. doi: 10.1124/jpet.104.067280. [DOI] [PubMed] [Google Scholar]
- 42.Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: Novel blockers of beta cell death. Diabetes. 2001;50:77–82. doi: 10.2337/diabetes.50.1.77. [DOI] [PubMed] [Google Scholar]
- 43.Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–223. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- 44.Orange JS, et al. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci USA. 2003;100:14151–14156. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacCorkle-Chosnek RA, VanHooser A, Goodrich DW, Brinkley BR, Tan TH. Cell cycle regulation of c-Jun N-terminal kinase activity at the centrosomes. Biochem Biophys Res Commun. 2001;289:173–180. doi: 10.1006/bbrc.2001.5948. [DOI] [PubMed] [Google Scholar]
- 46.Brown MC, Turner CE. Paxillin: Adapting to change. Physiol Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- 47.Herreros L, et al. Paxillin localizes to the lymphocyte microtubule organizing center and associates with the microtubule cytoskeleton. J Biol Chem. 2000;275:26436–26440. doi: 10.1074/jbc.M003970200. [DOI] [PubMed] [Google Scholar]
- 48.Robertson LK, Mireau LR, Ostergaard HK. A role for phosphatidylinositol 3-kinase in TCR-stimulated ERK activation leading to paxillin phosphorylation and CTL degranulation. J Immunol. 2005;175:8138–8145. doi: 10.4049/jimmunol.175.12.8138. [DOI] [PubMed] [Google Scholar]
- 49.Chini CC, Boos MD, Dick CJ, Schoon RA, Leibson PJ. Regulation of p38 mitogen-activated protein kinase during NK cell activation. Eur J Immunol. 2000;30:2791–2798. doi: 10.1002/1521-4141(200010)30:10<2791::AID-IMMU2791>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 50.Trotta R, et al. Differential role of p38 and c-Jun N-terminal kinase 1 mitogen-activated protein kinases in NK cell cytotoxicity. J Immunol. 2000;165:1782–1789. doi: 10.4049/jimmunol.165.4.1782. [DOI] [PubMed] [Google Scholar]
- 51.Caraux A, et al. Phospholipase C-γ2 is essential for NK cell cytotoxicity and innate immunity to malignant and virally infected cells. Blood. 2006;107:994–1002. doi: 10.1182/blood-2005-06-2428. [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Trivedi PP, Ge B, Krzewski K, Strominger JL. Many NK cell receptors activate ERK2 and JNK-1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc Natl Acad Sci USA. 2007;104:6329–6334. doi: 10.1073/pnas.0611655104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mandelboim O, Wilson SB, Valez-Gomez M, Reyburn HT, Strominger JL. Natural killer activating receptors trigger interferon gamma secretion from T cells and natural killer cells. Proc Natl Acad Sci USA. 1997;94:4604–4609. [Google Scholar]
- 54.Burshtyn DN, Shin J, Stebbins C, Long EO. Adhesion to target cells is disrupted by the killer cell inhibitory receptor. Curr Biol. 2000;10:777–780. doi: 10.1016/s0960-9822(00)00568-6. [DOI] [PubMed] [Google Scholar]
- 55.Morgan MM, et al. Superantigen-induced T cell:B cell conjugation is mediated by LFA-1 and requires signaling through Lck, but not ZAP-70. J Immunol. 2001;167:5708–5718. doi: 10.4049/jimmunol.167.10.5708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.