Abstract
A critical component of nervous system development is synapse elimination during early postnatal life, a process known to depend on neuronal activity. Changes in synaptic strength in the form of long-term potentiation (LTP) and long-term depression (LTD) correlate with dendritic spine enlargement or shrinkage, respectively, but whether LTD can lead to an actual separation of the synaptic structures when the spine shrinks or is lost remains unknown. Here, we addressed this issue by using concurrent imaging and electrophysiological recording of live synapses. Slices of rat hippocampus were cultured on multielectrode arrays, and the neurons were labeled with genes encoding red or green fluorescent proteins to visualize presynaptic and postsynaptic neuronal processes, respectively. LTD-inducing stimulation led to a reduction in the synaptic green and red colocalization, and, in many cases, it induced a complete separation of the presynaptic bouton from the dendritic spine. This type of synapse loss was associated with smaller initial spine size and greater synaptic depression but not spine shrinkage during LTD. All cases of synapse separation were observed without an accompanying loss of the spine during this period. We suggest that repeated low-frequency stimulation simultaneous with LTD induction is capable of restructuring synaptic contacts. Future work with this model will be able to provide critical insight into the molecular mechanisms of activity- and experience-dependent refinement of brain circuitry during development.
Keywords: cortex, critical period, development, long-term depression, schizophrenia
Overproduction of synapses and their subsequent elimination through activity- and experience-dependent processes are critical for refinement of neuronal circuits during development (1). One useful model of synapse elimination has been the mammalian visual cortex, where pruning of geniculocortical synapses takes place in response to monocular visual deprivation (MD) during postnatal critical periods (2). Neuronal activity is essential for this type of synapse elimination because blockade of action potentials in the cortex with tetrodotoxin (TTX) completely prevents synaptic loss and restructuring after MD (3, 4). Moreover, the loss of synapses in several models requires NMDA receptors, suggesting that postsynaptic processes also may be important (5–7). These findings indicate that synapse elimination is an active process, not merely a function of nonuse (8).
Structural plasticity such as that described after MD has been associated with retraction of entire axonal branches (9, 10), but it is likely this process begins at the synaptic level. How might synapses be eliminated in such an activity-dependent manner? As predicted by the Beinenstock, Cooper, and Munroe (BCM) theory (11, 12), low-frequency synaptic stimulation weakens glutamatergic synapses in hippocampal slices [long-term depression (LTD)] (13) and such synaptic depression might provide the first step in the experience-dependent refinement of neural circuits (11, 13). Current biochemical and physiological evidence supports a link between the mechanisms of deprivation-induced plasticity and those that mediate LTD; synaptic depression induced with MD was accompanied by AMPA receptor dephosphorylation and internalization, which subsequently occluded LTD induction (14).
Changes in dendritic spines resulting from the loss of visual stimuli have been reported (15), but only recently have the advances in confocal and multiphoton microscopy in vitro and in vivo allowed live observation of such changes (16, 17). For example, glutamate uncaging that produces long-term potentiation (LTP) leads to a rapid and selective enlargement of spines, providing direct live evidence of activity-dependent spine remodeling (18). In addition, LTP-inducing stimulation has been found to stimulate formation of new spines (16, 19). Because LTD is similar to depotentiation in its dependence on low-frequency stimulation, NMDA receptors, and phosphatases (13, 20–24), one might predict that the opposite structural change in spines would occur in response to LTD-inducing stimulation. Indeed, LTD not only results in spine shrinkage (25), but it also facilitates spine loss (19). Methods used in those studies, however, did not allow for observation of presynaptic structures, and so virtually nothing is known about what happens to the synapse itself as result of LTD.
Using two-color labeling of presynaptic and postsynaptic neurons that permits direct examination of synapses, we tested the hypothesis that LTD-inducing stimulation leads to synapse elimination in a cortical model. We also investigated whether spine size has any bearing on the likelihood that synapses are eliminated. Here, we demonstrate that LTD-inducing stimulation leads to elimination of synapses and that initial spine size can be a determining factor. We also demonstrate that synapses can be lost with little or no change in spine volume, indicating that spine shrinkage after LTD can be independent of synapse elimination.
Results
Low-Frequency Stimulation Induces Synapse Loss.
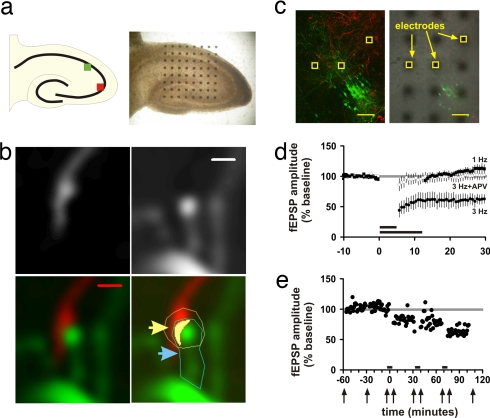
To allow for concurrent observation of putative synapses and electrophysiological recordings, neurons in hippocampal slice cultures were labeled with virally expressed fluorescent proteins and cultured on multielectrode arrays (Fig. 1A, see also Materials and Methods for details). This preparation allowed for extended stable stimulating and recording from any of 64 electrode sites and identification of presynaptic boutons (red) and postsynaptic dendritic spines (green). Because cultures of brain slices may not behave identically to their acute counterparts, we tested whether 900 pulses delivered at a low-frequency stimulation (1 Hz) to the stratum radiatum would induce LTD as is observed in acute slice preparations (13); they did not (Fig. 1D). Nine hundred pulses delivered at 3 Hz, however, were very effective at inducing LTD, and this depression was blocked by an antagonist of the NMDA receptor, as described for acute slices (13) (Fig. 1D). Responses to the test stimuli from unstimulated (control) electrodes were not depressed after low-frequency stimulation, suggesting that the observed LTD was input-specific [supporting information (SI) Fig. 6]. Therefore, even with the slight shift in optimal frequency, the synaptic depression we observe in this preparation can be considered “classical” input-specific NMDA receptor-dependent hippocampal LTD.
Fig. 1.
Imaging synapses in hippocampal slice culture. (a–d) To allow for concurrent observation of putative synapses and electrophysiological recordings, neurons in hippocampal slice cultures were labeled with virally expressed fluorescent proteins (a Left) and cultured on multielectrode arrays (a Right). Putative en passant synapses were identified as the presence of a thickening in the presynaptic (red) axon (b, raw image, Upper Left) that apposed a postsynaptic (green) spine (b, raw image, Upper Right, and colored, Lower Left). (Scale bars, 1 μm.) (b Lower Right) Regions of interest (ROIs) were drawn around the presynaptic bouton (yellow outline and arrow) and the postsynaptic spine (blue outline and arrow) after examining all images in the Z stacks for possible interfering neighboring structures. Areas (pixels) of red/green colocalization were detected by the MetaMorph software and colored here in yellow (b Lower Right). Pixels of colocalization and spine area from each Z stack then were summed to give volume pixels (voxels). When the presynaptic axon could be traced back to an electrode site, as visualized in bright-field illumination (c), the axon was considered to be a candidate for stimulation. (Scale bar, 100 μm.) Cases where axons did not traverse near an electrode and those slices that had separated from the electrode array were designated as control (unstimulated) cases. Field potential recordings were made in the general area of the imaged synapses (c). To determine the frequency that best induced LTD in slices cultured on electrode arrays, 900 pulses were delivered at differing stimulation frequencies. Three-hertz afferent stimulation (short bar) was effective at inducing LTD, but 1-Hz stimulation (long bar) was not effective (d). The depression induced with 3 Hz was blocked with a 50 μM concentration of the NMDA receptor antagonist 2-amino-5-phosphonopentanoic acid (APV). (e) Schematic of a typical experimental design with a recording from one representative experiment. Baseline fEPSPs and images were taken for 1 h before LTD-inducing stimulation (900 pulses, 5 min of 3 Hz, indicated by the bars). The times that images were acquired are indicated by arrows. The stimulation was repeated up to four times (three in this case). Experiments were performed at 32°C.
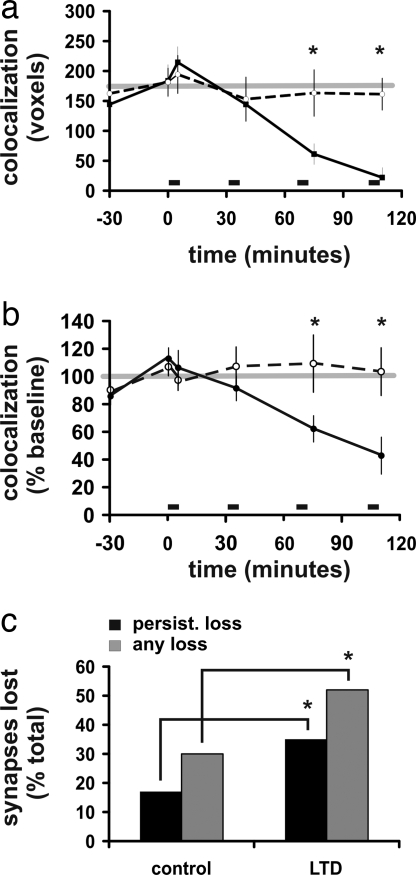
To determine whether LTD induces synapse loss, colocalization of green and red at sites of putative synapses were observed for 60 min before (baseline), immediately after, and 30 min after each of at least two episodes of low-frequency stimulation (Fig. 1E). Putative synapses were identified as colocalization of green and red fluorescence from a postsynaptic (green) spine and a presynaptic (red) en passant bouton (Fig. 1 B and C) (26, 27). This colocalization of red and green fluorescence is representative of the two objects that are closer than the optical resolution; colocalization correlates inversely with the distance between structures, and this distance in turn correlates inversely with existence of a synapse. The volume of presynaptic/postsynaptic colocalization at unstimulated control synapses, measured in voxels, did not change over this time period on average (Fig. 2A and SI Fig. 7). In contrast, the colocalization decreased significantly in the cases where LTD had been induced (Fig. 2 A and B). In many of these instances synapses appeared to separate completely (colocalization dropping to <10 voxels), only to reassociate at a later point in time. We refer to synapses that reassociated during the imaging period as having a “transient loss” and those that did not as having a “persistent loss.” When individual synapses were examined for complete loss of colocalization over the course of the imaging period, synapses had significantly more of both transient losses and persistent losses in cases in which LTD had been induced than in the control, unstimulated slices (55% vs. 38%, P < 0.05). This finding was true for synapses that were stable over the course of the 1-h baseline period (control: 19 of 64 vs. LTD: 25 of 48; P < 0.03, Fig. 2C) but not for cases that had transient loss during the baseline period (control: 14 of 22 vs. LTD: 26 of 29; P = 0.056). When we restricted our analysis to those synapses with both stable baselines and with a persistent loss of presynaptic/postsynaptic colocalization, we continued to find a significant difference between LTD and control cases (control: 11 of 64 vs. LTD: 17 of 48, P < 0.05). The number of persistent losses in stimulated cases in which LTD failed to be induced did not differ from the control cases (P > 0.05). Hence, LTD-inducing stimulation increases synapse separation.
Fig. 2.
Colocalization of presynaptic and postsynaptic structures decreases after LTD. (a) Average red/green (presynaptic/postsynaptic) colocalization (voxels) over time is shown for both unstimulated control cases (n = 85, 85, 78, 74, 37, and 37 synapses from 14, 14, 13, 12, 4, and 4 slices for time points, respectively) and for cases in which LTD exceeded 15% (n = 77, 77, 70, 63, 27, and 15 synapses from 10, 10, 9, 8, 4, and 2 slices for time points, respectively). One pixel volume (voxel) is equivalent to 0.0007 μm3. Low-frequency stimulation (900 pulses at 3 Hz) is indicated by the bars. (b) Data from a expressed as a percentage of baseline values are shown. *, P < 0.01. (c) LTD-inducing stimulation enhanced both persistent and temporary loss of putative synapses. Only cases without loss during the baseline period are included. n = 64 unstimulated controls; n = 48 LTD; *, P < 0.05.
Synapse Loss Is Associated with Smaller Initial Spine Size but Not Spine Shrinkage During LTD.
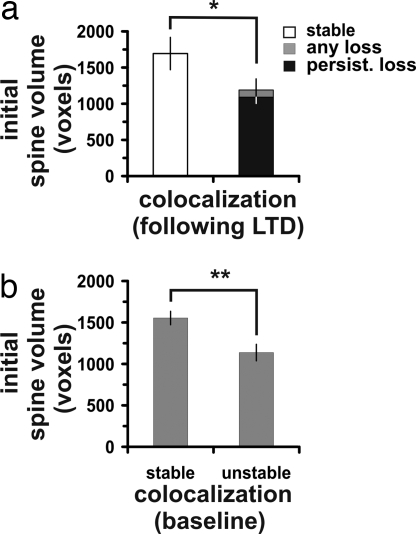
Dendritic spine size generally reflects the functional ability of postsynaptic cells to respond to presynaptic signals; larger spines tend to contain greater numbers of AMPA receptors (28) and the number of AMPA receptors in the postsynaptic membrane is proportional to the size of the synaptic junction (29). Therefore, we first sought to determine whether initially smaller, presumably less effective, synapses were more likely to be eliminated after LTD induction. We found that the baseline spine volumes at synapses that were later observed to separate were smaller than the spine volumes at synapses that were maintained after LTD (P < 0.05, Fig. 3A). We observed no difference in initial spine volumes when synapses with persistent separations were compared with those that had transient separations after LTD-inducing stimulation. In our analysis of initial spine volume and loss of colocalization, we noted that not all synapses were maintained throughout the initial 1-h baseline period in that some synaptic structures appeared to separate from each other during that time and then reassociate. We found that among the spines associated with these spontaneous transient losses, the average volumes were significantly smaller than those spines associated with stable synapses (stable synapse spine volume: 1,553 ± 81.3 voxels; unstable synapse spine volume: 1,138 ± 100.6 voxels, P < 0.005) (Fig. 3B). These results indicate that synapses on smaller spines are more likely to be lost, either spontaneously or as a result of LTD-inducing stimulation. LTD, however, increases the likelihood that small synapses will be eliminated.
Fig. 3.
Small initial spine volume is associated with greater likelihood of synapse loss. (a) In stimulated cases in which LTD was induced, spines that were associated with synapses that were eventually lost had smaller initial volumes than spines that remained stable throughout the imaging period (*, P < 0.05). No significant difference was observed between spine volumes associated with either persistent loss of synapses or all types of synapse loss (persistent plus transient). (b) Some synapses separated during the baseline period and later reassociated (called “unstable” synapses). Compared with the spines with “stable” synapses, the spines with unstable synapses were smaller. **, P < 0.01.
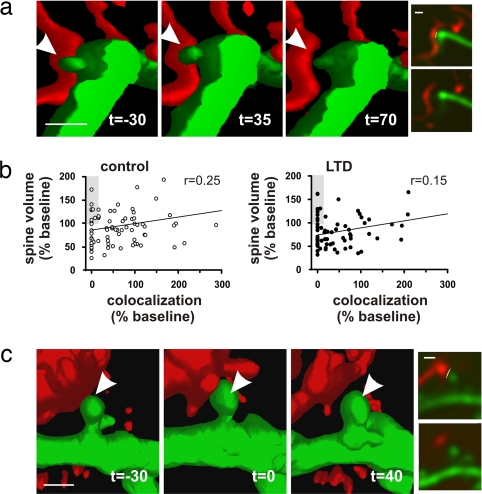
Because LTD has been associated with spine shrinkage and loss (19, 25), we next sought to determine whether there is any relationship between the amount of loss of red/green colocalization and changes in spine size. We found that changes in spine volume after LTD were not predictive of the loss of colocalization (Fig. 4 and raw images in SI Fig. 8) (r = 0.25 and 0.15 for control and for LTD cases, respectively). Note that although we did observe spine shrinkage after LTD (SI Fig. 9), we observed no cases of synapses that were associated with a complete loss of spines, at least over the time frame imaged here. These results indicate that spine shrinkage or loss after LTD was not necessary for a loss of colocalization, and spines with axons initially associated with them appeared to be resistant to complete loss over the observation period.
Fig. 4.
Synapse loss is not associated with decreases in spine volume. (a) Example of a persistent synapse loss after LTD with a reduction in spine volume. (b) Even though decreases in spine volumes were commonly observed coincident with synapse elimination (colocalization decreased to zero), the association was not significant. As represented by the symbols in the gray regions, many examples of synapse loss without decreases in spine volume were observed. Note that no complete spine losses were observed. In the LTD case, only synapses from slices in which LTD >15% was induced were included in this analysis (n = 76 synapses from 10 slices). For clarity of the graphs, x axes were truncated at 300% of the baseline colocalization. Arrowheads indicate the synaptic region of colocalization (a) or the postsynaptic spine (b). (c) An example of synapse elimination with no decrease in spine volume is shown. (Insets) Raw images from single Z stacks are shown with the pixels of colocalization pseudo-colored yellow before (Upper) and after (Lower) loss of colocalization (a Right and c Right). (Scale bars, 1 μm.)
Consistent with the lack of any significant correlation between spine shrinkage and synapse separation, we found that a substantial fraction of the synapses that had separated was associated with stable spine volumes (represented in the gray regions of the graphs in Fig. 4B). In these cases, the loss of colocalization cannot be caused by reductions in postsynaptic spine size, and in this example (Fig. 4C), it is accompanied by a loss or shrinkage of presynaptic structure. Therefore, we conclude that postsynaptic spine shrinkage is not a requirement for synapse disassembly; presynaptic involvement is likely.
Synapse Loss Is Associated with Greater Synaptic Depression.
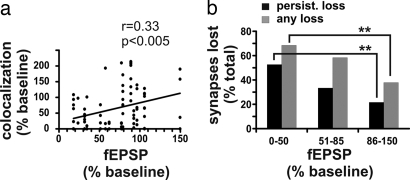
NMDA receptor-dependent LTD caused by trafficking of glutamate receptors recently has been shown to diverge mechanistically from decreases in spine size, indicating that low-frequency stimulation and not expression of LTD itself results in spine shrinkage (30). As a first step in establishing whether LTD leads to synapse elimination, we sought to determine whether the magnitude of the depression measured in field potentials correlated with amount of loss of red/green colocalization. We found that cases that had greater LTD expressed in the field potentials had a larger percentage of their synapses lost than those cases where LTD was not induced (Fig. 5 A and B, and SI Fig. 10). These data indicate that there is a relationship between the conditions leading to LTD and those leading to synapse loss. Further work will be required to establish causality for the role of LTD in synapse elimination.
Fig. 5.
Synapse elimination correlates with LTD expression. (a) The degree of synaptic depression, indicated as a percentage of the baseline field EPSP, is correlated with the degree of colocalization reduction (percentage of baseline value). Only synapses with stable baselines were included. (b) When the final level of LTD was used to classify cases, the percentages of synapses lost is significantly different between cases with large LTD and those with minimal LTD. Percentage baseline fEPSP of 86–150% equals no synaptic depression; 0–50% baseline equals maximal depression. **, P < 0.01, χ2 test. See also SI Fig. 10.
Discussion
Loss of synapses during development requires neuronal activity and postsynaptic receptors in many species and nervous system structures (8, 31). Encouraged by the experimental evidence linking LTD to ocular dominance plasticity (14), we tested the hypothesis that LTD could lead to synapse elimination in a cortical model. Here, we demonstrate that repeated LTD-inducing stimulation is capable of driving the separation of presynaptic and postsynaptic structures in hippocampal slice cultures. These findings suggest that rapid activity-dependent refinement of neuronal circuitry during development likely occurs on a large scale throughout cortical tissue. The result should not be surprising considering one study that estimated that 5,000 synapses are lost per second in primate visual cortex during one stage of postnatal development (32).
Since Ramón y Cajal's first description of dendritic spines (33), debates about the role of spines in neuronal communication have continued (34–36). Many classical and recent studies indicate that spine loss correlates with many forms of mental retardation, schizophrenia, and other developmental disorders (37, 38). In most cases, however, the distinction between failure of synapses and spines to develop and an actual loss has been difficult to prove. Nevertheless, our finding that small initial dendritic spine size predicts a higher likelihood of synapse loss could help explain why problems with spine maturation during development could lead to a profound impact on adult brain circuitry.
Although the majority of excitatory synapses in cortical structures exist on spines and not dendritic shafts (39), loss of spines does not necessarily indicate loss of synapses. Because shrinking spines often continue to remain connected at synapses (40), direct observation of a physical separation between presynaptic and postsynaptic neurons is critical before it can be stated that synapses are lost after LTD. In contrast to the notion that spine shrinkage drives synapse loss, we find that even though spine shrinkage occurs after LTD (SI Fig. 9), it is not a requirement for synapse separation, indicating that the two structural changes have independent induction or downstream mechanisms. Although we do observe a correlation between the amount of LTD and synapse loss (Fig. 5 and SI Fig. 10), it remains to be tested whether NMDA receptors and subsequent AMPA receptor internalization are required to firmly establish that LTD is necessary for synapse separation. Because loss of colocalization does not correlate with spine shrinkage, and a substantial number of presynaptic structures shrink, we do not rule out a substantial role for presynaptic changes in these separation events. It is also possible that neither presynaptic nor postsynaptic shrinkage is directly related to synapse separation, because dissolution of the extracellular matrix or glial actions may be the primary events leading to loss of synapses.
LTD previously has been associated with examples of spine loss (19, 25), but we observed no complete loss of spines associated with synapses, even in cases where the red/green colocalization drops to zero. Without knowledge of the presynaptic status of each spine in previous studies, based on our findings here, we speculate that an initial presence of presynaptic structures protects a spine from complete loss until much later after the presynaptic structure detaches, beyond the period of our imaging. In neocortex, for example, <4% of spines lack synapses. The spines that lack synapses were smaller and resembled dendritic filopodia (41). We found no indication that the stable spines fit this profile, nor did the area of colocalization differ in these cases from the average. This finding leads us to conclude that presynaptic separation from the postsynaptic structure does not require spine shrinkage but merely co-occurs with shrinkage in some cases. Future mechanistic studies should be able to disentangle the processes of spine shrinkage from synapse separation.
Clearly, LTD in the field potentials does not result in synapse loss in all cases and vice versa. In fact, a certain number of synapses are lost in the control, unstimulated cases. We are unable to determine at this time, however, whether the individual cases that are lost are in fact associated with LTD resulting from spontaneous activity that is not detected in the field excitatory postsynaptic potentials (fEPSPs), or whether LTD is not the only route by which synapses are eliminated. Although our observations of synapse loss are not surprising when one considers how many synapses are lost during postnatal development (32), our findings were unexpected in light of the fact that LTD is reversible and does not appear to lower the “ceiling” for subsequent LTP (21). The most likely explanation for this apparent paradox is that, in the earlier study, the LTD was not saturated before its reversal with LTP-inducing stimulation. The results of our current study allow us to make the specific prediction that saturating LTD will lead to a decrease in the LTP ceiling because of loss of synapses after 1 h or more.
The hippocampus, although a cortical structure, is obviously not the same as the visual cortex in that the synapses studied here contain axons other hippocampal pyramidal cells, whereas most of the structural studies in the visual cortex have used the geniculocortical synapses onto layer IV neurons (9). Even with the differences between hippocampal and geniculocortical synapses, some features of layer IV neurons make them more similar to hippocampal CA1 than to other cortical layers, namely, that both areas have similar molecular mechanisms for LTD expression (42). Therefore, the mechanisms involved in hippocampal and visual cortical synapse elimination may turn out to be similar. Because of the relative simplicity of the hippocampal structure, we expect rapid progress on the molecular mechanisms of synapse elimination; lessons learned in the hippocampus may then be tested for their applicability in other brain regions.
Materials and Methods
Labeling CA1 Synapses.
On day 6–7 in vitro, the organotypic slices were infected by applying 100 nl of media with modified Sindbis viruses containing genes for either tdTomato (hippocampal area CA3) (43) or for enhanced green fluorescent protein (eGFP) (area CA1) to visualize presynaptic and postsynaptic neurons, respectively. Vectors were constructed as described in ref. 44. Successfully infected slices with sufficient dual-color expression were transferred 1 day later to the MED64 probes (Panasonic, MED-P5155, interpolar distance 150 μm).
Image Analysis.
One hundred and thirteen stimulated synapses with corresponding spines from 16 organotypic slices (from 16 animals) and 84 unstimulated synapses with corresponding spines from 14 organotypic slices (14 animals) were analyzed. Of the stimulated cases, 76 synapses (10 slices) were from slices where low-frequency stimulation resulted in LTD. Putative synapses were defined as the volume, in voxels, of colocalization between spines (green fluorescence) and axonal boutons (red fluorescence) (27). Spine volumes and colocalization volumes corresponding to each putative synapse were calculated by using the automatic MetaMorph function after manually constructing the outline of regions of interest (ROIs) around each spine or colocalization area. Briefly, to assess the total volumes of the spines or synapses, the program measures the fluorescent areas from individual spines or fluorescent colocalization areas of individual synapses occupied in each slice of the Z-stack voxels and adds them together (1 voxel corresponds to 0.0007 μm3). Investigators analyzing colocalizations and spine volumes based on these thresholds were blind to the experimental condition. We anticipate that, although we may have overestimated synapse colocalization size and thus the number of synaptic contacts, we have likely underestimated the degree and number of separation events. Synapses lost and reassociated in the baseline period were considered unstable. Baseline data from colocalization size were analyzed by using regression and correlation analyses against time (SI Fig. 7). The correlation coefficient between synapse size and time (r = 0.0045) demonstrates that there is no relationship between colocalization and time, thus excluding time as an independent variable that influences a change in colocalization after stimulation.
SI.
Further information can be found in SI Materials and Methods.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Daniel Chun and Dr. Frank Schottler for their assistance with the MED64, Dr. Marc Sommer for advice on the statistics, Drs. Ben Philpot and Richard Weinberg for their careful reading of the manuscript, and our reviewers for their suggestions. We also thank Dr. Roger Tsien (University of California, San Diego) for providing the tdTomato cDNA. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800027105/DC1.
References
- 1.Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- 2.Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc London B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 3.Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiter HO, Waitzman DM, Stryker MP. Cortical activity blockade prevents ocular dominance plasticity in the kitten visual cortex. Exp Brain Res. 1986;65:182–188. doi: 10.1007/BF00243841. [DOI] [PubMed] [Google Scholar]
- 5.Kleinschmidt A, Bear MF, Singer W. Blockade of “NMDA” receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987;238:355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- 6.Bear MF, Kleinschmidt A, Gu QA, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colonnese MT, Constantine-Paton M. Developmental period for N-methyl-d-aspartate (NMDA) receptor-dependent synapse elimination correlated with visuotopic map refinement. J Comp Neurol. 2006;494:738–751. doi: 10.1002/cne.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 9.Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260:1819–1821. doi: 10.1126/science.8511592. [DOI] [PubMed] [Google Scholar]
- 10.Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- 11.Bear MF, Cooper LN, Ebner FF. A physiological basis for a theory of synapse modification. Science. 1987;237:42–48. doi: 10.1126/science.3037696. [DOI] [PubMed] [Google Scholar]
- 12.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-d-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heynen AJ, et al. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat Neurosci. 2003;6:854–864. doi: 10.1038/nn1100. [DOI] [PubMed] [Google Scholar]
- 15.Globus A, Scheibel AB. The effect of visual deprivation on cortical neurons: a Golgi study. Exp Neurol. 1967;19:331–345. doi: 10.1016/0014-4886(67)90029-5. [DOI] [PubMed] [Google Scholar]
- 16.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 17.Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagerl UV, Eberhorn N, Chambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Fujii S, Saito K, Miyakawa H, Ito K, Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res. 1991;555:112–122. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- 21.Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- 23.Barrionuevo G, Schottler F, Lynch G. The effects of repetitive low frequency stimulation on control and “potentiated” synaptic responses in the hippocampus. Life Sci. 1980;27:2385–2391. doi: 10.1016/0024-3205(80)90509-3. [DOI] [PubMed] [Google Scholar]
- 24.Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Umeda T, Ebihara T, Okabe S. Simultaneous observation of stably associated presynaptic varicosities and postsynaptic spines: Morphological alterations of CA3–CA1 synapses in hippocampal slice cultures. Mol Cell Neurosci. 2005;28:264–274. doi: 10.1016/j.mcn.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 27.De Simoni A, Edwards FA. Pathway specificity of dendritic spine morphology in identified synapses onto rat hippocampal CA1 neurons in organotypic slices. Hippocampus. 2006;16:1111–1124. doi: 10.1002/hipo.20236. [DOI] [PubMed] [Google Scholar]
- 28.Baude A, Nusser Z, Molnar E, McIlhinney RA, Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neurosci. 1995;69:1031–1055. doi: 10.1016/0306-4522(95)00350-r. [DOI] [PubMed] [Google Scholar]
- 29.Nusser Z, et al. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- 30.Wang XB, Yang Y, Zhou Q. Independent expression of synaptic and morphological plasticity associated with long-term depression. J Neurosci. 2007;27:12419–12429. doi: 10.1523/JNEUROSCI.2015-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Fields RD, Fitzgerald S, Festoff BW, Nelson PG. Proteolytic activity, synapse elimination, and the Hebb synapse. J Neurobiol. 1994;25:325–335. doi: 10.1002/neu.480250312. [DOI] [PubMed] [Google Scholar]
- 32.Bougeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cajal SR. Neue Darstellung vom histologischen bau des centralnervensystems. Anatomische Abtheilung des Arch fur Anat und Physiol. 1893:319–428. [Google Scholar]
- 34.Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: Implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araya R, Jiang J, Eisenthal KB, Yuste R. The spine neck filters membrane potentials. Proc Natl Acad Sci USA. 2006;103:17961–17966. doi: 10.1073/pnas.0608755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloodgood BL, Sabatini BL. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–869. doi: 10.1126/science.1114816. [DOI] [PubMed] [Google Scholar]
- 37.Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- 38.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 39.Gray EG. Axosomatic and axodendritic synapses of the cerebral cortex: An electron microscopic study. J Anat. 1959;83:420–433. [PMC free article] [PubMed] [Google Scholar]
- 40.Konur S, Yuste R. Imaging the motility of dendritic protrusions and axon terminals: Roles in axon sampling and synaptic competition. Mol Cell Neurosci. 2004;27:427–440. doi: 10.1016/j.mcn.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Arellano JI, Espinosa A, Fairen A, Yuste R, DeFelipe J. Non-synaptic dendritic spines in neocortex. Neuroscience. 2007;145:464–469. doi: 10.1016/j.neuroscience.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 42.Crozier RA, Wang Y, Liu CH, Bear MF. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc Natl Acad Sci USA. 2007;104:1383–1388. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaner NC, et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 44.Jeromin A, Yuan LL, Frick A, Pfaffinger P, Johnston D. A modified Sindbis vector for prolonged gene expression in neurons. J Neurophysiol. 2003;90:2741–2745. doi: 10.1152/jn.00464.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.