Abstract
The goal of this study was to define the impact of colonization of gnotobiotic (Gn) pigs with lactic acid bacteria (LAB) on development of intestinal and systemic B cell responses to human rotavirus (HRV). The LAB-specific and total B cell responses were also assessed. Gn pigs were inoculated with LAB (Lactobacillus acidophilus and L. reuteri) and virulent Wa strain HRV (LAB+HRV+), HRVonly (LAB−HRV+), LAB only (LAB+HRV−) or mock (LAB−HRV−). The HRV infection induced similar HRV-specific intestinal and systemic antibody and B cell responses in pigs with or without LAB, whereas LAB significantly enhanced total intestinal IgA secreting cell responses and total serum IgM and intestinal IgM and IgG titers. The LAB colonization did not reduce HRV shedding or diarrhea, this may be partly due to the short time interval between the first LAB feeding and HRV inoculation. Further studies are needed with longer time for LAB to establish before HRV inoculation. However, our studies demonstrate that Gn pigs infected with HRV develop a similar magnitude of virus-specific B cell responses as those of HRV-infected and LAB colonized pigs. LAB colonization alone is not as efficient in promoting intestinal B cell responses, as is HRV infection.
Keywords: Rotavirus, Probiotic, Lactobacillus, Gnotobiotic pigs, B cell responses
1. Introduction
Earlier studies comparing germfree animals with conventional animals showed that germfree animals have less developed lymphoid tissues, with fewer immunoglobulin (Ig) A antibody-secreting cells (ASC) and intraepithelial lymphocytes in the intestinal mucosa and a lower level of systemic Ig when compared with animals that have a conventional indigenous microflora (Crebbe et al., 1968; Golden and Pesti, 1971). However, these observations were from germfree animals that were not exposed to pathogens. After germfree animals are inoculated with a defined microbe (e.g. a virus), they become gnotobiotic (Gn). Our hypothesis is that the lack of gut flora in Gn animals may cause a reduced magnitude of intestinal immune responses to enteric viral infection. Lactic acid bacteria (LAB) (e.g. Lactobacillus) colonization may promote development of the intestinal immune system and may have stimulating or regulatory effects on the B cell immune responses to rotavirus infection. In addition, although the beneficial effects of Lactobacilli as probiotics have been evaluated widely and shown to be effective for partial prevention and treatment of acute rotavirus gastroenteritis in children (Kaila et al., 1992; Rosenfeldt et al., 2002; Shornikova et al., 1997a,b; Szajewska and Mrukowicz, 2005), the mechanism of action remains largely undefined. Thus, the goal of this study was to define the influence of colonization by two strains of LAB (Lactobacillus acidophilus and L. reuteri) on the development of virus-specific, LAB-specific, and total B cell responses using the neonatal Gn pig model of human rotavirus (HRV) infection and disease. The study was also designed to evaluate the effects of prophylactic use of LAB in infants and the effects of competition between commensal colonization and enteric virus infection. In a study of humans, prophylactic use of LGG significantly reduced the risk of nosocomial rotavirus gastroenteritis in infants (Szajewska et al., 2001).
2. Materials and methods
2.1. Virus
The virulent Wa strain HRV (VirWaHRV) (pooled intestinal contents) was used for inoculation at a dose of 1 × 105 fluorescent-forming units (FFU). The 50% infectious dose (ID50) of VirWaHRV in pigs was determined as ~1 FFU (Ward et al., 1996a). Cell culture adapted attenuated Wa strain HRV (AttWaHRV) was used as antigen for isotype-specific ELISA, and ELI-SPOT assays and was propagated in MA104 cells (Yuan et al., 1996).
2.2. Bacterial strains
The Lactobacilli L. acidophilus strain NCFM™ and L. reuteri strain ATCC 23272 (ATCC, Manassas, VA, USA) were used in this study. Both strains were propagated in Lactobacilli MRS broth (Weber, Hamil-ton, NJ) overnight at 37 °C anaerobically (85% nitrogen, 10% hydrogen, 5% carbon dioxide). Cultures were subcultured once and inoculated into 10 ml of MRS broth. After 24 h, serial dilutions were made in sterile 0.1% peptone water (Becton Dickinson [BD] Biosciences, Sparks, MD) and 0.1 ml of the dilution was spread onto MRS agar (BD) for determining the colony-forming units (CFU)/ml. The remaining bacteria suspensions were aliquoted into 1 ml volumes, stored at −80 °C and thawed and washed with 0.1% peptone water and titrated on MRS agar 1 day prior to feeding of animals.
2.3. Experimental design
Gnotobiotic pigs from three sows were derived near term and maintained in sterile isolation units as described previously (Meyer et al., 1964). Pigs were assigned randomly to four groups as follows: HRV-infected LAB-fed (LAB+HRV+) (n = 8), HRV-infected non-LAB-fed (LAB−HRV+) (n = 10), non-infected LAB-fed (LAB+HRV−) (n = 4), and non-infected non-LAB-fed (LAB−HRV−) (n = 4). Pigs were orally dosed with 103, 104, 105, 106 and 106 CFU at 3, 5, 7, 9, 11 days of age, respectively with 1:1 mixture of L. acidophilus and L. reuteri in 2 ml of 0.1% peptone water. Non-LAB-fed pigs were orally dosed with an equal volume of peptone water. At 5 days of age, pigs were orally inoculated with 105 FFU VirWaHRV or diluent (non-infected) as described previously (Ward et al., 1996b).
Post-HRV-inoculation, pigs were examined daily for clinical signs, including % with diarrhea, duration of diarrhea and diarrhea scores as described (Yuan et al., 1996). Rectal swabs were collected daily for HRV [from post-inoculation day (PID) 0 to 7] and Lactobacilli shedding (PID 5, 10, 21 and 28). Rotavirus shedding was determined by antigen capture enzyme-linked-immunosorbent-assay (ELISA) and cell culture immunofluorescence (CCIF) as described (Ward et al., 1996a). Serum samples were collected at PID 0, 10, 21 and 28 and viremia was assessed by the antigen capture ELISA (Azevedo et al., 2005). Pigs were euthanized at PID 28 to isolate mononuclear cells (MNC) from intestinal and systemic lymphoid tissues for enumeration of antibody-secreting cells (ASC) and total immunoglobulin-secreting cells (IgSC) by enzyme-linked-immunospot (ELISPOT) assays. The small intestinal contents (SIC) and large intestinal contents (LIC) were collected at euthanasia for detection of intestinal antibodies.
2.4. Enumeration of LAB
Each rectal swab was diluted in 4 ml of 0.1% peptone water (~1:10) and a 100 μl aliquot was diluted in 900 μl of peptone water and plated onto MRS agar. The plates were incubated in sealed BBL Gaspak jars (Fisher, Hanover Park, IL) containing Anaerogen packs (BD) for 24 h at 37 °C. The number of CFU on plates with 20–200 colonies were enumerated and recorded. LAB shedding was expressed as CFU/ml. Bacteremia was assessed by plating pig sera onto MRS agar plates and incubated in the same way as for LAB enumeration.
2.5. Isolation of MNC and ELISPOT assay
The ileum, spleen, and peripheral blood lymphocytes (PBL) were collected from pigs at euthanasia and the MNC were isolated and subjected to ELISPOTassays as described (Yuan et al., 1996) for enumeration of HRV-specific IgM, IgA and IgG ASC on fixed HRV-infected MA104 cell monolayers in 96-well plates (Corning Costar, Corning, NY) and total IgSC on plates (Nunc-Immuno, Rochester, NY) coated with goat anti-porcine IgM, IgA or IgG antibodies (KPL, Gaithersburg, MD and Bethyl, Montgomery, TX).
2.6. ELISA for HRV-specific antibody, LAB-specific antibody and total Ig
To determine antibody titers to Wa HRV in the sera, LIC and SIC of the pigs, an indirect isotype-specific antibody ELISA was used as previously described (Parreno et al., 1999). The LAB-specific antibody titers were determined by using an ELISA modified from that described previously (Verbrugh et al., 1981). Antibody titer to peptidoglycan (PGN), which is a common cell-wall component of all Gram + bacteria (90% dry weight), was measured as a proxy of antibody responses to LAB in Gn pigs. Briefly, 96-well plates (Nunc-Immuno) were coated with 4 μg/ml staphylococcal peptidoglycan (Fluka, St. Louis, MO) and washed with PBS–Tween (0.1%) between addition of samples and reagents. All samples/reagents were added in the following sequence: serial fourfold dilutions of each test sample; horseradish peroxidase-conjugated goat anti-porcine IgM (KPL), IgA (Bethyl) and IgG (KPL) (dilutions of 1:3000, 1:3000 and 1:500, respectively); and ABTS (2,2′-azinobis[3-ethylbenzthiazoline-6-sulfonate]) substrate (KPL). The antibody titers measured on PGN-coated plates were compared to the titers measured by using whole LAB cell coated plates (105 CFU/ml fixed with 80% acetone) for the same set of serum samples and they did not differ significantly. Consequently, the PGN-specific antibody titers were presented as LAB-specific antibody titers.
Total Ig titers were determined by using an ELISA as described previously. Briefly, 96-well plates were coated with goat anti-porcine IgM (15 μg/ml) (KPL), IgA (15 μg/ml) (Bethyl) or IgG (3 μg/ml KPL) polyclonal antibodies. Incubation with serially diluted samples on the plates was followed by addition of horseradish peroxidase-conjugated goat anti-porcine IgM (KPL), IgA (Bethyl) and IgG (KPL) antibodies (dilutions of 1:5000, 1:5000 and 1:500, respectively) and ABTS substrate (KPL). The Ig titer was calculated as the reciprocal of the highest sample dilution that produced a mean Absorbance (A414) greater than the cut-off value [mean of negative controls A414 + three times standard deviation (S.D.)], after subtracting the mean mock coated well A414 from the antigen coated well A414 of each sample. The initial dilution of the tested samples was 1:4 (for virus-specific and LAB-specific antibody) and 1:64 (for total Ig). Titers of <4 were assigned a value of 2 (for HRV-specific and LAB-specific antibodies) or <64 were assigned a value of 16 (for total Ig) for calculation of geometric mean titers (GMT).
2.7. Statistical analyses
Fisher’s exact test was used to compare percent of pigs with diarrhea and virus shedding among groups. One-way analysis of variance (ANOVA-general linear model), followed by Duncan’s multiple range test, were used to compare mean duration of virus shedding and diarrhea, mean diarrhea scores, mean peak titers of virus shed, mean titers of LAB shed and mean isotype-specific ELISA antibody titers. The numbers of virus-specific ASC and total IgSC were compared among groups using the Kruskal–Wallis rank sum (non-parametric) test. When differences among the groups were detected, the same test was used in a pairwise fashion to clarify the nature of the differences. Statistical significance was assessed at p < 0.05 for all comparisons. All the analyses were performed using the SAS program (SAS Institute, NC).
3. Results and discussion
3.1. Clinical signs, virus shedding, viremia and bacteremia
The clinical signs (i.e. % with diarrhea, mean duration and diarrhea scores) and virus shedding (i.e. % with shedding, mean duration and peak titers) in LAB+HRV+ and LAB−HRV+ groups are statistically similar (data not shown). Thus, L. acidophilus and L. reuteri feeding of the Gn pigs did not have a significant impact on alleviation of rotavirus infection or diarrhea at an early age. The pigs were fed LAB starting at 3 days of age and feeding continued for 9 days, and they were inoculated with virulent HRVat 5 days of age. The short time interval between the first feeding and inoculation with HRV may not have been enough for the gut microflora to promote increased non-specific defense against the virulent rotavirus challenge. All the LAB+HRV+ and LAB−HRV+ pigs developed viremia but none of the LAB+HRV+ or LAB+HRV− pigs developed bacteremia (data not shown), indicating that dual infection did not significantly alter the infectivity/pathogenicity in terms of distribution of the HRV or LAB.
3.2. LAB colonization
The numbers of LAB (CFU/ml) in feces of LAB+HRV+ pigs and LAB+HRV− pigs were compared in Fig. 1. From PID 5 to 28, the average daily fecal LAB counts in both groups ranged between 1.5 × 106 and 7.4 × 107 CFU/ml which was greater than the amount (106 CFU/ml) in the last LAB feeding. High LAB counts in the feces at PID 28, 3 weeks after the last LAB feeding (at PID 7) indicate that LAB effectively colonized the gastrointestinal tract of pigs. Both Lactobacilli strains were previously evaluated for their colonization pattern in Gn pigs (Glass, 2003) and similar results were reported. We used a mixture of L. acidophilus and L. reuteri in hoping to achieve better immune stimulating effects than monoassociation.
Fig. 1.
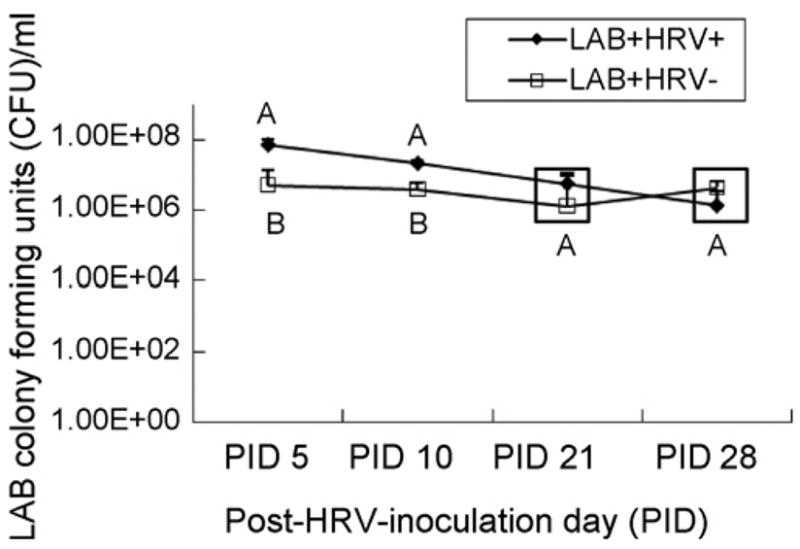
Mean number of LAB colony-forming units (CFU)/ml of feces from LAB-fed Gn pigs inoculated with HRV (LAB+HRV+, n = 4) or diluent (LAB+HRV−, n = 4). Pigs were orally dosed with 103 to 106 CFU of LAB every other day from 3 to 11 days of age with increasing doses (10-fold incremental increases from each previous dose fed and 106 for the last two feedings). The total LAB received by each pig from the five doses was 2.11 × 106 CFU. Pigs were inoculated orally at 5 days of age with the virulent Wa HRV. Different capital letters indicate significant difference in CFU/ml (GLM on log transformed titer, p < 0.05) for the same time. The values that are statistically similar are in the same box.
The LAB+HRV+ pigs had significantly higher fecal LAB counts than LAB+HRV− pigs at PID 5 and 10. The mechanism for the increased LAB growth in HRV-infected pigs is unclear. One explanation might be that HRV infection induces malabsorption, resulting in the transit of undigested mono- and disaccharides, carbohydrates, fats and proteins into the colon (Ramig, 2004), and these undigested components were favorable for LAB growth.
3.3. HRV-specific ASC and total IgSC responses
The numbers of HRV-specific IgM, IgA and IgG ASC did not differ significantly between the LAB+HRV+ and LAB−HRV+ groups in intestinal (ileum) or systemic lymphoid tissues (spleen and PBL). These results differed from a study showing that children with rotavirus diarrhea who received LGG had significantly higher rotavirus-specific IgA ASC responses in PBL (Majamaa et al., 1995). On the other hand, the results suggest that the lack of gut flora in Gn animals did not cause impairment of intestinal immune responses to HRV infection. However, LAB colonization plus HRV infection significantly enhanced the magnitude of total intestinal IgA SC responses compared to HRV infection alone or LAB colonization alone in neonatal Gn pigs (Table 1), thus LAB and HRV synergistically enhanced the development of B cell compartment in the gut at PID 28, but did not increase the mucosal resistance against HRV infections at the time of virus inoculation (5 days of age). Further studies with a longer time for LAB to establish before HRV inoculation are needed.
Table 1.
ASC and total IgSC in the ileum, spleen and PBL of Gn pigs infected with HRV and/or colonized with LAB or mock at PID 28
| Treatment | n | Ileum
|
Spleen
|
PBL
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASCa |
IgSCa |
ASC
|
IgSC
|
ASC
|
IgSC
|
||||||||||||||
| IgM | IgA | IgG | IgM | IgA | IgG | IgM | IgA | IgG | IgM | IgA | IgG | IgM | IgA | IgG | IgM | IgA | IgG | ||
| LAB+HRV+ | 4 | 0 A (0)b | 37 A (15) | 24 A (4) | 1141 A (60) | 5198 A (272) | 247 A (50) | 0 A (0) | 4 A (1) | 6 A (3) | 129 A (31) | 101 A (18) | 18 A (6) | 0 A (0) | 2 AB (2) | 2 A (1) | 38 A (11) | 42 A (18) | 4 B (1) |
| LAB−HRV+ | 3 | 0 A (0) | 57 A (20) | 44 A (13) | 1024 A (156) | 2562 B (648) | 488 A (158) | 0 A (0) | 8 A (1) | 11 A (0) | 148 A (44) | 92 A (4) | 47 A (13) | 0 A (0) | 4 A (2) | 2 A (0) | 13 A (3) | 70 A (37) | 9 AB (1) |
| LAB+HRV− | 4 | 0 A (0) | 0 B (0) | 0 B (0) | 1058 A (508) | 1773 B (728) | 137 A (44) | 0 A (0) | 0 B (0) | 0 B (0) | 186 A (105) | 98 A (12) | 87 A (57) | 0 A (0) | 0 B (0) | 0 A (0) | 30 A (24) | 78 A (15) | 20 A (12) |
| LAB−HRV− | 4 | 0 A (0) | 0 B (0) | 0 B (0) | 5 B (3) | 21 C (12) | 0 B (0) | 0 A (0) | 0 B (0) | 0 B (0) | 4 B (2) | 3 B (2) | 0 B (0) | 0 A (0) | 0 B (0) | 0 A (0) | 3 B (3) | 1 B (1) | 0 C (0) |
Numbers of Wa rotavirus-specific ASC and total IgSC per 5 × 105 MNC were assessed by ELISPOT assays at PID 28 in the ileum, spleen and PBL.
Standard error of the mean.
3.4. HRV-specific, LAB-specific and total Ig responses
Figs. 2 and 3 depict antibody responses in serum and intestinal contents, respectively including HRV-specific, LAB-specific and total Ig. The LAB colonization did not significantly alter HRV-specific serum or intestinal antibody responses as indicated by similar titers of HRV-specific IgM, IgA and IgG antibodies between LAB+HRV+ and LAB−HRV+ groups. Our observations agreed with the results of Shornikova et al.’s studies (Shornikova et al., 1997a,b) that children with rotavirus diarrhea with or without treatment with L. reuteri had similar rotavirus-specific serum IgA and IgG antibodies. Our results also agreed with Kaila et al.’s findings (Kaila et al., 1992) that serum antibodies to rotavirus were comparable in children who received LGG or placebo. However, these results differed from the study showing that children with rotavirus diarrhea who received LGG had significantly higher rotavirus-specific serum IgA antibody titers at convalescent stage (Majamaa et al., 1995). Conventional piglets (3-week old) with rotavirus infection that received Bifidobacterium lactis had significantly higher fecal rotavirus-specific IgM, IgA and IgG antibodies (Shu et al., 2001). As reviewed by Szajewska (Szajewska and Mrukowicz, 2005), the beneficial effects of probiotics in rotavirus infection in children seem to be bacterial strain-dependent and dose-dependent. Different doses, administration regimens (e.g. once vs. twice or more frequent administration per day) and duration of treatment may be the reasons for the conflicting results.
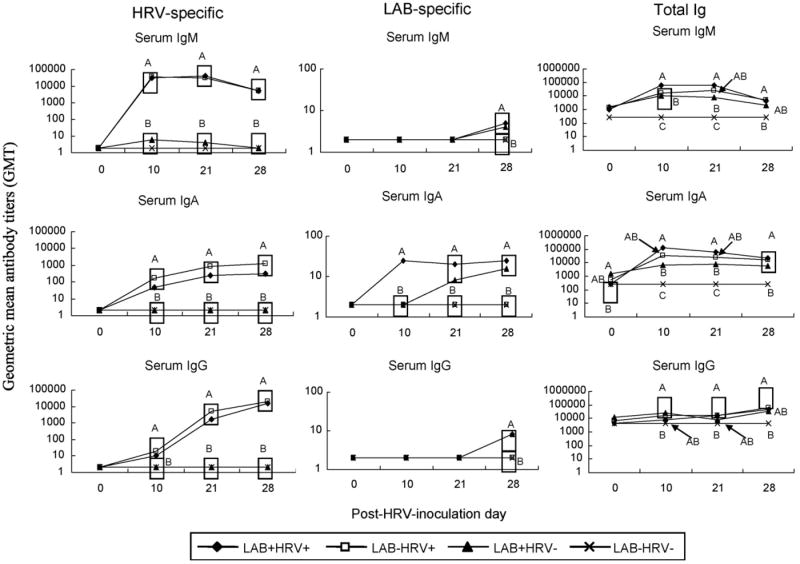
Fig. 2.
Isotype-specific geometric mean antibody titers (GMT) to Wa human rotavirus (HRV), bacterial peptidoglycan (PGN) and total Ig in serum of gnotobiotic pigs. Serum samples were collected on PID 0, 10, 21 and 28. Data represent the GMTs for four to eight pigs at each time point. Different capital letters indicate significant difference. The GMT that are statistically similar are in the same box.
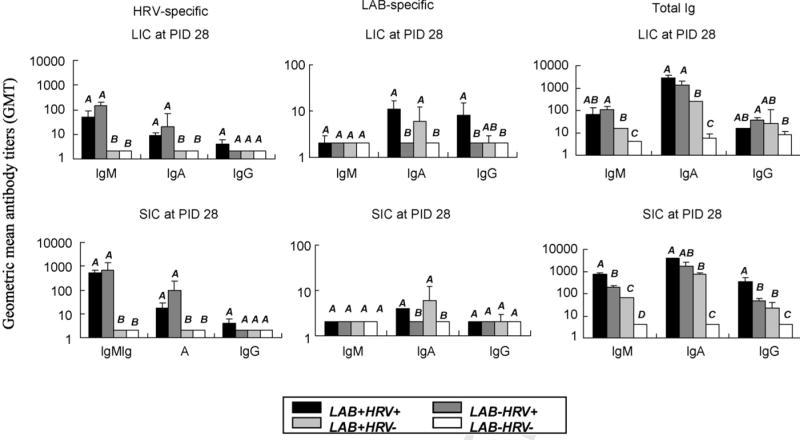
Fig. 3.
Isotype-specific GMT to HRV, PGN and total Ig in intestinal contents of gnotobiotic pigs. The large and small intestinal content samples were collected at PID 28. Data represent the GMTs for four to eight pigs at PID 28. Different capital letters indicate significant difference. The GMT that are statistically similar are in the same box.
It has been recognized that either microbial colonization or viral infection of the gut could profoundly influence the status of cellular and humoral elements of the gut mucosal immune system (Cebra, 1999). HRV alone (LAB−HRV+) induced significantly higher titers of total IgM in LIC and SIC and total IgA in SIC compared to LAB alone (LAB+HRV−) (Fig. 3). Therefore, LAB colonization alone is not as efficient in promoting intestinal B cell responses, as is HRVinfection of neonatal pigs. Consistent with the increased LAB counts, the LAB-specific serum IgA antibody titers of LAB+HRV+ pigs were significantly higher (eightfold) than those of LAB+HRV− pigs at PID 10 (Fig. 2). However, compared to the kinetics and magnitude of HRV-specific antibody responses, the antibody responses to LAB increased much more slowly in the serum (IgM and IgG were not detectable until PID 28) and the overall LAB-specific antibody titers were substantially lower (50–2000-fold) than the HRV-specific antibody titers in serum and intestine (Figs. 2 and 3). These results suggest that LAB are not as immunogenic as HRVand the lack of LAB-specific IgM and IgG antibodies in serum at PID 10 and 21 may reflect the development of systemic tolerance to commensal bacteria colonization.
In conclusion, our findings demonstrate that Gn pigs develop a similar magnitude of virus-specific B cell responses to HRV infection as those of the HRV-infected pigs colonized with the mixture of L. acidophilus and L. reuteri. Also LAB colonization alone is not as efficient in promoting development of the B cell compartment as HRV infection of neonatal Gn pigs. Because the two LAB strains did not protect the naïve pigs from HRV infection or diarrhea, further studies are needed to evaluate a different strain of LAB or different regimen of LAB feeding and time of HRV inoculation that provides protection against HRV diarrhea or acts as an oral HRV vaccine adjuvant.
Acknowledgments
This work was supported by grants from the National Institutes of Health (1R21AT002524 to LY and R01AI033561 to LJS) and the Ohio Agricultural Research and Development Center, The Ohio State University (OHOA1208 to LY). We thank Dr. Juliette Hanson, Ms. Peggy Lewis and Mr. Rich McCormick for technical assistance. Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center (OARDC), The Ohio State University.
References
- Azevedo MS, Yuan L, Jeong KI, Gonzalez A, Nguyen TV, Pouly S, Gochnauer M, Zhang W, Azevedo A, Saif LJ. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J Virol. 2005;79:5428–5436. doi: 10.1128/JVI.79.9.5428-5436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999;69:1046S–1051S. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- Crebbe PA, Bazin H, Eyssen H, Hermans JF. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int Arch Allergy Immunol. 1968;34:365–375. doi: 10.1159/000230130. [DOI] [PubMed] [Google Scholar]
- Glass MD. Master Thesis. The Ohio State University; Wooster: 2003. Effects of Lacobacillus acidophilus and Lactobacillus reuteri on bovine Cryptosporidium parvum and C. hominis in vitro and in vivo. (Advisors: P.D. Courtney and L.A. Ward) [Google Scholar]
- Golden HA, Pesti L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol Rev. 1971;35:390–429. doi: 10.1128/br.35.4.390-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res. 1992;32:141–144. doi: 10.1203/00006450-199208000-00002. [DOI] [PubMed] [Google Scholar]
- Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1995;20:333–338. doi: 10.1097/00005176-199504000-00012. [DOI] [PubMed] [Google Scholar]
- Meyer RC, Bohl EH, Kohler EM. Procurement and maintenance of germ-free Seine for microbiological investigations. Appl Environ Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreno V, Hodgins DC, de Arriba L, Kang SY, Yuan L, Ward LA, To TL, Saif LJ. Serum and intestinal isotype antibody responses to Wa human rotavirus in gnotobiotic pigs are modulated by maternal antibodies. J Gen Virol. 1999;80(Pt 6):1417–1428. doi: 10.1099/0022-1317-80-6-1417. [DOI] [PubMed] [Google Scholar]
- Ramig RF. Pathogenesis of intestinal and systemic rotavirus infection. J Virol. 2004;78:10213–10220. doi: 10.1128/JVI.78.19.10213-10220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Moller PL, Pedersen P, Tvede M, Weyrehter H, Valerius NH, Paerregaard A. Effect of probiotic Lactobacillus strains in young children hospitalized with acute diarrhea. Pediatr Infect Dis J. 2002;21:411–416. doi: 10.1097/00006454-200205000-00012. [DOI] [PubMed] [Google Scholar]
- Shornikova AV, Casas IA, Isolauri E, Mykkanen H, Vesikari T. Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. J Pediatr Gastroenterol Nutr. 1997a;24:399–404. doi: 10.1097/00005176-199704000-00008. [DOI] [PubMed] [Google Scholar]
- Shornikova AV, Casas IA, Mykkanen H, Salo E, Vesikari T. Bacteriotherapy with Lactobacillus reuteri in rotavirus gastroenteritis. Pediatr Infect Dis J. 1997b;16:1103–1107. doi: 10.1097/00006454-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Shu Q, Qu F, Gill HS. Probiotic treatment using Bifidobacterium lactis HN019 reduces weanling diarrhea associated with rotavirus and Escherichia coli infection in a piglet model. J Pediatr Gastroenterol Nutr. 2001;33:171–177. doi: 10.1097/00005176-200108000-00014. [DOI] [PubMed] [Google Scholar]
- Szajewska H, Mrukowicz JZ. Use of probiotics in children with acute diarrhea. Paediatr Drugs. 2005;7:111–122. doi: 10.2165/00148581-200507020-00004. [DOI] [PubMed] [Google Scholar]
- Szajewska H, Kotowska M, Mrukowicz JZ, Armanska M, Mikolajczyk W. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J Pediatr. 2001;138:361–365. doi: 10.1067/mpd.2001.111321. [DOI] [PubMed] [Google Scholar]
- Verbrugh HA, Peters R, Rozenberg-Arska M, Peterson PK, Verhoef J. Antibodies to cell wall peptidoglycan of Staphylococcus aureus in patients with serious staphylococcal infections. J Infect Dis. 1981;144:1–9. doi: 10.1093/infdis/144.1.1. [DOI] [PubMed] [Google Scholar]
- Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996a;77(Pt 7):1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- Ward LA, Yuan L, Rosen BI, To TL, Saif LJ. Development of mucosal and systemic lymphoproliferative responses and protective immunity to human group A rotaviruses in a gnotobiotic pig model. Clin Diagn Lab Immunol. 1996b;3:342–350. doi: 10.1128/cdli.3.3.342-350.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]