Abstract
Studies with genetically modified insulinoma cells suggest that Group VIA Phospholipase A2 (iPLA2β) participates in amplifying glucose-induced insulin secretion. INS-1 insulinoma cells that overexpress iPLA2β, for example, exhibit amplified insulin secretory responses to glucose and cAMP-elevating agents. To determine whether similar effects occur in whole animals, we prepared transgenic mice in which the Rat Insulin 1 Promoter (RIP) drives iPLA2β overexpression, and two characterized transgenic mouse lines exhibit similar phenotypes. Their pancreatic islet iPLA2β expression is increased several-fold, as reflected by quantitative PCR of iPLA2β mRNA, immunoblotting of iPLA2β protein, and iPLA2β enzymatic activity. Immunofluorescence microscopic studies of pancreatic sections confirm iPLA2β overexpression in RIP-iPLA2β-transgenic (TG) islet β-cells without obviously perturbed islet morphology. Male RIP-iPLA2β-TG mice exhibit lower blood glucose and higher plasma insulin concentrations than wild-type (WT) mice when fasting and develop lower blood glucose levels in glucose tolerance tests, but WT and TG blood glucose levels do not differ in insulin tolerance tests. Islets from male RIP-iPLA2β-TG mice exhibit greater amplification of glucose-induced insulin secretion by a cAMP-elevating agent than WT islets. In contrast, islets from male iPLA2β-null mice exhibit blunted insulin secretion, and those mice have impaired glucose tolerance. Arachidonate incorporation into and the phospholipid composition of RIP-iPLA2β-TG islets are normal, but they exhibit reduced Kv2.1 delayed rectifier current and prolonged glucose-induced action potentials and elevations of cytosolic [Ca2+] that suggest a molecular mechanism for the physiological role of iPLA2β to amplify insulin secretion.
Keywords: transgenic mice, glucose tolerance, insulin tolerance, insulin secretion
Introduction
Glucose homeostasis requires that pancreatic β-cells secrete insulin when blood glucose concentrations exceed 5 mM. Autoimmune β-cell destruction causes Type I diabetes mellitus, and in type II diabetes mellitus, there is 50% loss of β-cell mass and impaired insulin secretion and action (12, 15). Intensive insulin therapy reduces diabetic complications but increases risks of hypoglycemia (16), and β-cell replacement might someday be a superior therapy (59, 62).
Understanding control of β-cell growth, death, and secretion might permit iterative introduction of genes into β-cell lines or precursors to optimize secretion, proliferation, and resistance to injury (24, 69). Combined with empirical selection and encapsulation, such β-cell engineering might someday provide a renewable source of β-cells for replacement therapy (44, 45). Developing such potential future therapies requires increased understanding of the genes and gene products that govern β-cell physiology (46).
Insulin secretion involves glucose transport into β-cells, phosphorylation by glucokinase at a rate proportional to extracellular glucose concentrations, and further metabolism that increases [ATP]/[ADP] (20, 39, 40). This inactivates β-cell ATP-sensitive K-channels (KATP), causing membrane depolarization, voltage-operated Ca2+-channel opening, and a rise in [Ca2+] that induces exocytosis (1, 14, 21). Several other, incompletely understood processes modulate or amplify insulin secretion, e.g., non-selective cation channels activated by Ca2+-store depletion, an electrogenic Na/K-ATPase, and voltage-operated K channels also affect β-cell membrane potential and secretion (17, 23, 29, 48).
Reducing equivalent shuttles to and from mitochondria are also required for secretion (19, 58, 76), and glucose augments secretion by KATP-independent mechanisms (60, 77). Elevating β-cell cAMP also amplifies secretion by protein kinase A-dependent and -independent mechanisms (50, 61), and β-cell signaling involves phospholipid hydrolysis and accumulation of phospholipid-derived mediators (31, 41, 48, 50, 52, 63, 68, 70).
We and others have developed evidence that an islet phospholipase A2 that does not require Ca2+ (iPLA2β) is activated by secretagogues and that its products participate in β-cell signaling (48, 68, 70). Characterizing this activity resulted in our cloning an 84 kDa PLA2 from islet cDNA libraries (34, 37). Recombinant iPLA2β is Ca2+-independent, activated by ATP, and inhibited by a suicide substrate that also attenuates glucose-induced insulin secretion, arachidonate release, and rises in β-cell [Ca2+] (52).
Genetic gain- and loss-of-function studies with insulinoma cells support the possible involvement of iPLA2β in insulin secretion. INS-1 insulinoma cells stably transfected to express siRNA that reduces iPLA2β expression exhibit reduced insulin secretion when stimulated with glucose and markedly impaired amplification of insulin secretion by cAMP-elevating agents (5). In contrast, stably transfected INS-1 cells that overexpress iPLA2β exhibit amplified insulin secretory responses to glucose, particularly in the presence of cAMP-elevating agents, and this is associated with subcellular redistribution and membrane association of iPLA2β (35).
To determine whether these findings with cultured insulinoma cells are relevant to animal physiology, we have prepared transgenic mice in which iPLA2β overexpression is driven by the Rat Insulin 1 Promoter (RIP) and studied fasting concentrations of glucose and insulin, blood glucose excursions upon administration of glucose or exogenous insulin, and insulin secretory and electrophysiologic responses of pancreatic islets isolated from RIP-iPLA2β-transgenic and wild-type mice.
Experimental Procedures
Materials
BEL [(E)-6-(bromo-methylene)tetrahydro-3-(1-naphthalenyl)-2H-pyran-2-one] was obtained from Cayman Chemical (Ann Arbor, MI); enhanced chemiluminescence (ECL) reagents from Amersham Biosciences (Piscataway, NJ); sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) supplies from BioRad (Richmond, CA); ATP, common reagents, and salts from Sigma Chemical Co. (St. Louis, MO); culture media, penicillin, streptomycin, Hanks' balanced salt solution (HBSS), L-glutamine, agarose, molecular mass standards, and RT-PCR reagents from Invitrogen (Carlsbad, CA); fetal bovine serum from Hyclone (Logan UT); Pentex bovine serum albumin (BSA, fatty acid free, fraction V) from ICN Biomedical (Aurora, OH); and forskolin from Calbiochem (La Jolla, CA). Krebs-Ringer bicarbonate buffer (KRB) contained 25 mM HEPES (pH 7.4), 115 mM NaCl, 24 mM NaHCO3, 5 mM KCl, 1 mM MgCl2, and 2.5 mM CaCl2.
Generation and genotyping of RIP-iPLA2β transgenic mice
As described (10), full-length iPLA2β cDNA was inserted in a RIP-I/β-globin expression vector and microinjected into fertilized eggs of C57BL/6J mice. Transgenic founders were mated with wild-type (WT) C57BL/6J mice (Jackson Laboratory), and N2 and N3 generations from 2 lines with similar phenotypes were studied. The genetic background of resultant mice is pure C57BL/6J, and wild-type littermates were used as controls. All protocols were approved by the Washington University Animal Studies Committee.
Genotyping was performed with tail clipping DNA either by Southern blotting analyses or by PCR. For Southern blots, DNA was digested with EcoRI restriction endonuclease. Digests were analyzed by electrophoresis and transferred to nylon membranes, which were incubated with a [32P]-labeled probe that recognizes sequence in the rabbit hemoglobin gene contained in the original construct. For PCR analyses, DNA was used as a template with two pairs of primers. One pair amplifies sequence in the internal control fatty acid binding protein gene (Fabpi) gene, and the primer sequences are: (Fabpi 5′) CCT CCG GAG AGC AGC GAT TAA AAG TGT CAG; (Fabpi 3′) TAG AGC TTT GCC ACA TCA CAG GTC ATT CAG. The expected size of the product is 450 bp. The other primer pair amplifies sequence that spans the junction of iPLA2β and globin cDNA in the transgene construct. The primer sequences are: (TG5′) CTA GGC TCA GAC ATC ATG CTG GAC GAG GT and (TG3′) AAG ATC TCA GTG GTA TTT GTG AGC CAG GG. The expected size of the product is 200 bp.
Generating and genotyping iPLA2β-/--null mice
Preparation of the iPLA2β knockout construct, its introduction into 129/SvJ mouse embryonic stem (ES) cells, their selection with G418, characterization by Southern blotting, injection into C57BL/6 mouse blastocysts, production of chimeras and then heterozygotes, and mating of heterozygotes to yield wild-type, heterozygous, and iPLA2β-null mice in a Mendelian distribution are described elsewhere, as is their genotyping by Southern blotting of tail genomic DNA (7-9). The genetic background of the resultant mice is mixed 129/SvJ × C57BL/6.
Islet Isolation
Islets were isolated from pancreata of male wild-type, RIP-iPLA2β transgenic mice, and iPLA2β-null mice by collagenase digestion after mincing, followed by Ficoll step density gradient separation, and manual selection under stereomicroscopic visualization to exclude contaminating tissues (9, 49). Mouse islets were counted and used for PCR and immunoblotting of iPLA2β mRNA and protein, respectively; for measuring iPLA2β specific enzymatic activity and ex vivo secretion of insulin and electrophysiologic responses; and for extraction of phospholipids.
PCR of iPLA2β mRNA in mouse islets
As described (9, 13), total RNA was extracted with TRIzol reagent (Invitrogen). After treatment with DNase I, 1 μg of total RNA was reverse transcribed with an oligo-dT primer, and quantitative PCR was performed with the ABI Prism 7700 PCR instrument (Applied Biosystems) using the SYBR Advantage qPCR Premix (Clontech). Each assay included a negative control using RNA not subjected to reverse transcription. PCR performed with a pair of primers designed to amplify a fragment of iPLA2β cDNA. The sequence of primer 1 is GCC CTG GCC ATT CTA CAC AGT A and that of primer 2 is CAC CTC ATC CTT CAT ACG GAA GT. Amplification specificity was verified by agarose gel electrophoresis of products and a heat-dissociation protocol. Sequence-specific amplification was detected with increasing fluorescent signal of FAM (reporter dye) during the amplification cycle. The amplification of 18s rRNA served as control.
Western blotting analyses
As described (35), proteins in islet homogenates were analyzed by SDS-PAGE (7.5%), transferred onto a PVDF membrane, and probed with antibody 506 provided by Dr. Richard Gross (Washington University, St. Louis, MO). Protein bands were visualized by ECL. Membranes were also probed with actin antibodies as a loading control.
Ca2+-independent phospholipase A2 activity assay
As described (37), the protein content of islet cytosolic fractions was determined by Bio-Rad assay, and iPLA2 activity was measured in aliquots (ca. 20 μg protein) added to assay buffer (200 mM Tris-HCl, pH 7.0; total assay volume, 200 μl) containing 5 mM EGTA with or without 1 mM ATP. Some aliquots were pretreated (2 min) with BEL (10 μM) before assay. Reactions were initiated by injecting substrate (L-alpha-1-palmitoyl-2-[14C]arachidonoyl-phosphatidylethanolamine; specific activity, 50 Ci/mol; final concentration, 5 μM) in ethanol (5 μl). Assay mixtures were incubated (3 min, 37 °C), and reactions were terminated by adding butanol (0.1 ml) and vortexing. After centrifugation (2000 × g, 4 min), products in the butanol layer were analyzed by silica gel G TLC in hexane/ethyl ether/acetic acid (80:20:1). The TLC region containing free arachidonic acid (Rf, 0.58) was scraped into vials, and its [14C] content was determined. Specific activity was calculated from released [14C]-dpm and protein content.
Extracting islet phospholipids
As described (9), islets were placed in a solution (2 mL) of chloroform/methanol (1/1, v/v), homogenized. and sonicated on ice (20% power, 5 sec bursts for 60 sec, Vibra Cell probe sonicator, Sonics and Materials, Danbury, CT). After centrifugation (2,800 × g, 5 min) to remove tissue debris, supernatants were transferred to silanized 10 mL glass tubes and extracted with methanol (1 mL), chloroform (1 mL), and water (1.8 mL). Samples were vortex-mixed and centrifuged (900 g, 5 min). Supernatants were removed, concentrated, and dissolved in methanol/chloroform (9/1), and lipid phosphorus content was determined.
Immunofluorescence microscopy
As described (10), pancreata were removed from mice, fixed in 4% paraformaldehyde, and embedded in paraffin to prepare sections, which were placed on glass slides and deparaffinized. Immunostaining for insulin was performed with guinea pig anti-human insulin antibody (1:300; BioGenex Laboratories, San Ramon, California, USA) and FITC-conjugated secondary antibody. Immunostaining for glucagon was performed with rabbit anti-glucagon (1:500; Chemicon International, Temecula, California, USA) as the primary antibody. Incubations with primary antibodies were performed overnight in a humidified chamber. Secondary antibodies were donkey anti-guinea pig FITC (for insulin) and donkey anti-rabbit CY3 (for glucagon). Slides were examined with a Nikon TE300 microscope.
Fasting and fed blood glucose and insulin concentrations
As described (10), blood samples were obtained from the lateral saphenous vein in heparinized capillary tubes, and glucose concentrations were measured in whole blood with a Blood Glucose Monitor (Becton-Dickenson) or an Ascensia ELITE XL blood glucose meter. Plasma was prepared from heparinized blood by centrifugation, and insulin levels were determined in aliquots (5 μl) with a rat insulin ELISA kit (Crystal Chem Inc.). Fasting blood samples were obtained after an overnight fast, and fed blood samples were obtained between 9:00 and 10:00 AM.
Glucose and insulin tolerance tests
As described (9), intraperitoneal glucose tolerance tests were performed in mice fasted overnight from which a baseline blood sample was obtained, followed by intraperitoneal injection of D-glucose (2 mg/g) and collection of blood for measurement of glucose after 30, 60, and 120 min. Oral glucose tolerance tests (42) were performed in a similar manner except that glucose (2 mg/kg) was administered enterally by gavage. Insulin tolerance tests were performed in mice with free access to water and chow that received an intraperitoneal injection (0.75 units/kg) of human regular insulin (Lilly, Indianapolis, IN), followed by collection of blood after 30, 60, and 120 min for glucose determinations (9, 49).
Insulin secretion by isolated pancreatic islets
Islets were isolated from pancreata of male mice as described above. Insulin secretion studies were performed with 30 islets per incubation, as described (9, 49). Islets were rinsed with KRB medium containing 3 mM glucose and 0.1% bovine serum albumin and placed in silanized tubes (12 × 75 mm) in the same buffer, through which 95% air/5% CO2 was bubbled before incubations. Tubes were capped and incubated (37 °C, 30 min) in a shaking water bath. Buffer was then replaced with KRB medium containing 3, 8, or 20 mM glucose and 0.1% BSA without or with forskolin (2.5 μM), and samples were incubated for 30 min. Secreted insulin was measured by radioimmunoassay.
Determination of [3H]arachidonic acid incorporation into islet phospholipids
As described (55), islets were washed thrice in KRB medium containing 5.5 mM glucose and 0.1% BSA, resuspended in that medium, and preincubated for 30 min at 37°C. Islets (100 per condition) were then placed in fresh KRB medium that contained 5.5 mM glucose, 0.1% BSA, and 2.5 mM CaCl2, and [3H]arachidonic acid (final concentration 0.5 μCi/ml, 5 nM) was added to the medium. Incubations were performed for 10-60 min at 37°C, and islets were then washed thrice in KRB medium containing 5.5 mM glucose and 0.1% BSA to remove unincorporated [3H]arachidonic acid. Phospholipids were then extracted as described above and analyzed by TLC, and the [3H]arachidonate content of GPC lipids was determined by liquid scintillation spectrometry and normalized to lipid phosphorus content.
Positive ion electrospray ionization mass spectrometric analyses of choline-containing glycerolipids
Diradyl-glycerophosphocholine (GPC) lipids and lysophosphatidylcholine (LPC) species were analyzed as Li+ adducts by positive ion ESI/MS on a Finnigan (San Jose, CA) TSQ-7000 triple stage quadrupole mass spectrometer with an ESI source controlled by Finnigan ICIS software. Phospholipids were dissolved in methanol/chloroform (2/1, v/v) containing LiOH (10 pmol/μl), infused (1 μl/min) with a Harvard syringe pump, and analyzed as described (25, 26). For tandem MS, precursor ions selected in the first quadrupole were accelerated (32-36 eV collision energy) into a chamber containing argon (2.3-2.5 mtorr) to induce collisionally-activated dissociation (CAD), and product ions were analyzed in the final quadrupole. Identities of GPC species were determined from their tandem spectra (25, 26), and their quantities were determined relative to internal standards by interpolation from standard curves (5, 55).
Whole-cell ruptured-patch electrophysiological recording
Voltage-activated currents were recorded using whole-cell ruptured-patch clamps with an Axopatch 200B amplifier and pCLAMP9 software (Molecular Devices), as described (28, 29). Patch electrodes (2 to 4 MΩ) were loaded with intracellular solution containing (in mmol/l) 140 KCl, 1 MgCl2[H2O]6, 10 EGTA, 10 HEPES, 5 MgATP (pH 7.25) with KOH. Islets were perfused with KRB containing (in mmol/l) 119 NaCl, 2 CaCl2[(H2O)6], 4.7 KCl, 10 HEPES, 1.2 MgSO4, 1.2 KH2PO4, adjusted to pH 7.3 with NaOH with the indicated glucose concentration.
Islet perforated-patch electrophysiological recording
Patch electrodes (2 to 4 MΩ) were loaded with intracellular solution containing (in mmol/l) 140 KCl, 1 MgCl2[H2O]6, 10 EGTA, 10 HEPES (pH 7.25) with KOH containing the pore-forming antibiotic amphotericin B (52) (Sigma), as described (28, 29). Islets were perfused with KRB containing (in mmol/l) 119 NaCl, 2 CaCl2[(H2O)6], 4.7 KCl, 10 HEPES, 1.2 MgSO4, 1.2 KH2PO4, and glucose as indicated, adjusted to pH 7.3 with NaOH. Cells on the periphery of islets on glass coverslips were sealed in voltage clamp at −80V while the amphotericin allowed perforation and good access over several minutes. After switching to current clamp, cells that had a resting membrane voltage near −65mV in 2mM glucose and near −75mV in 0mM glucose were assumed to be β cells, the glucose-containing solution (at 37°C) was perfused into a heated chamber (at 37°C).
High-speed intracellular calcium imaging
Mouse islets attached to glass-bottom Matek tissue culture dishes were loaded with 5 μM Fluo-4 AM loaded in KRH2 for 30 min at 37°C, as described (28, 29). Loaded islets were placed in a temperature controller (TC-202, Medical Systems Corp.) mounted on the stage of an inverted microscope (Nikon Eclipse TE2000-U) for imaging. Experiments were performed at 37°C islets were excited with at 488 nm from a monochrometer (Till Photonics) and the emitted light was filtered with a 535/40 filter (Chroma) and recorded with a photomultiplier tube (Photon Technology International). Acquisition was controlled with pClamp9 software (Molecular Devices). In all experiments, to obtain data with sufficiently high time resolution we used 10 KHz acquisition. Data analysis was performed with pClamp9 and Microsoft Excel.
Statistical Methods
Results are presented as mean ± SEM. Data were evaluated by unpaired, two-tailed Student's t test or by analysis of variance with appropriate post-hoc tests. Significance levels are described in figure legends.
Results
Generation of transgenic mice that overexpress iPLA2β in pancreatic islet β-cells
In the construct used to generate the transgenic mice (Figure 1A), rat iPLA2β cDNA was inserted downstream of the rat insulin 1 promoter (RIP) at a site within rabbit globin gene sequence. Transcription of sequence encoding iPLA2β is under control of RIP, and transgenic overexpression of iPLA2ß is expected in cells that express insulin, e.g. pancreatic islet β-cells, but not in other cells. Transgene incorporation was determined by Southern blotting (Figure 1B) and PCR (Figure 1C) analyses. Two founders were identified, and progeny from each were viable and fertile. Mice from transgenic lines TG1 and TG2 exhibited similar phenotypes.
Figure 1. Preparation of transgenic mice that overexpress iPLA2β in pancreatic islet β-cells.
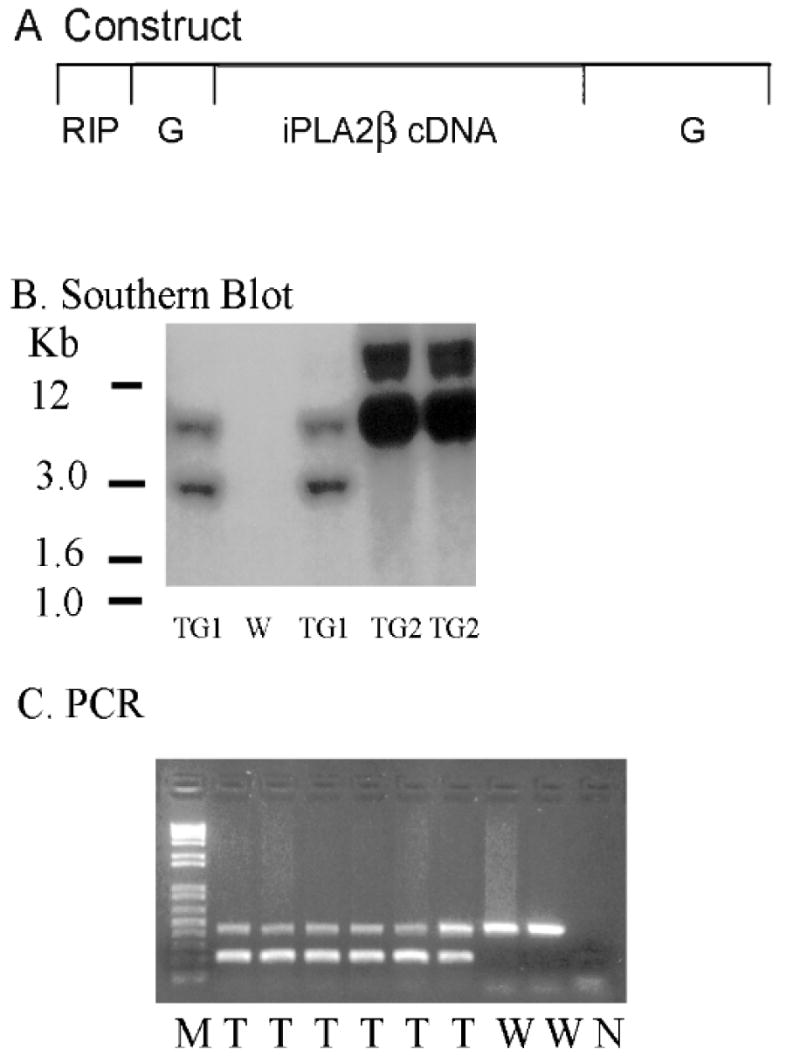
Panel A is a schematic representation of the construct used to prepare the transgenic mice. Full-length iPLA2β cDNA was inserted into the RIP (rat insulin promoter)-I/β-globin (G) expression vector downstream of RIP and within β-globin gene sequence. Panel B is a Southern blot of tail clipping DNA from wild-type (W) or RIP-iPLA2β transgenic mice of line TG1 or TG2 with a [32P]-labeled probe that recognizes transgene sequence. Panel C illustrates PCR analyses using DNA from wild-type (W) or RIP-iPLA2β transgenic (T) mice, and primers that amplify sequence that either spans the junction between iPLA2β and globin cDNA (200 bp product, lower band) or is within the internal control Fabpi (450 bp product, upper band). Lane M represents MW markers and lane N a control reaction without template.
Overexpression of iPLA2β in pancreatic islet β-cells of transgenic mice
Real-time PCR measurements indicated that iPLA2β mRNA was expressed in islets isolated from RIP-iPLA2β transgenic (TG) mice at levels several-fold higher than those from wild-type littermates (Figure 2A). Immunoblotting analyses similarly indicated that iPLA2β protein is expressed at much higher levels in islets from RIP-iPLA2β-TG compared to wild-type mice (Figure 2B). The iPLA2β enzymatic specific activity was 12-fold higher in islets from RIP-iPLA2β-TG mice compared to those from wild-type mice, and activity was stimulated by ATP and inhibited by the suicide substrate BEL (Figure 2C), as is characteristic of iPLA2β (8, 9, 34-37). The size and morphology of pancreatic islets from RIP-iPLA2β transgenic mice were not obviously different from those of wild-type mice, but iPLA2ß expression, as visualized by fluorescence microscopy with antibodies to iPLA2β, was much greater in islets from RIP-iPLA2β-TG mice (Figure 3).
Figure 2. Overexpression of iPLA2β in pancreatic islets of RIP-iPLA2β transgenic mice.
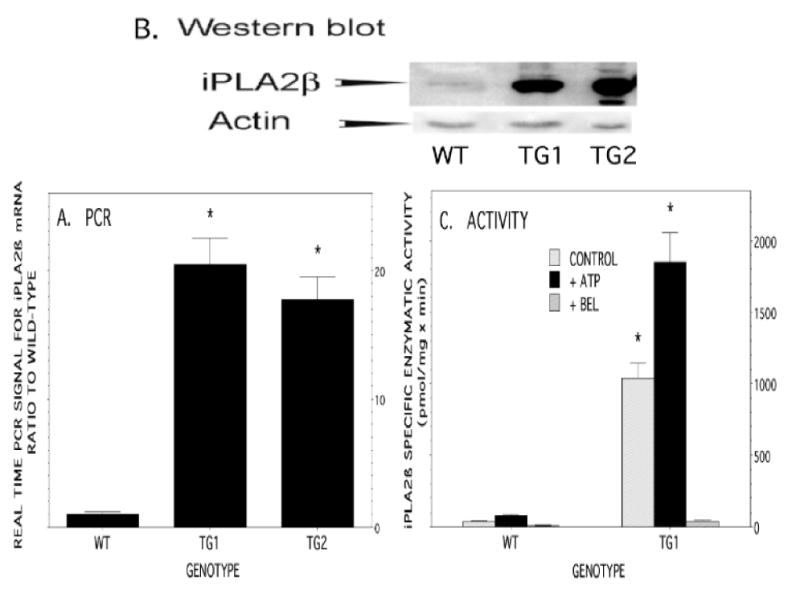
Panel A summarizes Real-Time PCR analyses of iPLA2β mRNA levels in islets from wild-type (WT) or RIP-iPLA2β-transgenic (TG) mice of line TG1 or TG2. Panel B is a Western blot of immunoreactive iPLA2β protein in pancreatic islets isolated from WT or RIP-iPLA2β-TG mice of line TG1 or TG2. The immunoblots were also probed with actin antibodies as a loading control. Panel C summarizes iPLA2β specific enzymatic activity measurements in islets from WT or RIP-iPLA2β-TG mice from line TG1. Gray bars represent basal specific activity, and solid black bars represent activity in the presence of ATP. The third (rightmost) bar in each set represents activity in the presence of the iPLA2β suicide substrate BEL. Mean values ± SEM (n = 6) are displayed in Panels A and C. An asterisk (*) denotes p < 0.05 for the comparison WT vs. RIP-iPLA2β-TG.
Figure 3. Morphology of and expression of iPLA2β by pancreatic islets of RIP-iPLA2β transgenic or wild-type mice as assessed by immunofluorescence microscopy.
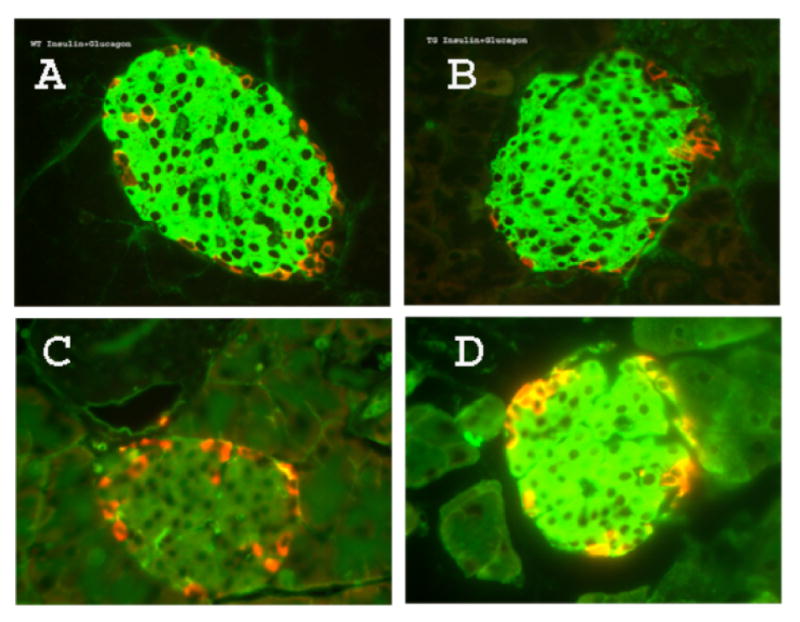
Sections were prepared from pancreata from wild-type (Panels A and C) or RIP-iPLA2β transgenic (Panels B and D) mice. In Panels A and B, sections were probed with antibodies to insulin (green) and glucagon (red) and fluorescent secondary antibodies. In Panels C and D, sections were probed with antibodies to iPLA2β (green) and glucagon (red) and fluorescent secondary antibodies.
Growth of RIP-iPLA2β transgenic male mice
The growth, development, and food intake of RIP-iPLA2β transgenic and wild-type male mice did not differ appreciably, and Figure 4 illustrates that the rates of weight gain of wild-type and RIP-iPLA2β transgenic male mice on an ad libitum regular chow diet were not statistically distinguishable between the ages of 3 and 48 weeks.
Figure 4. Growth of RIP-iPLA2β transgenic and wild-type male mice.
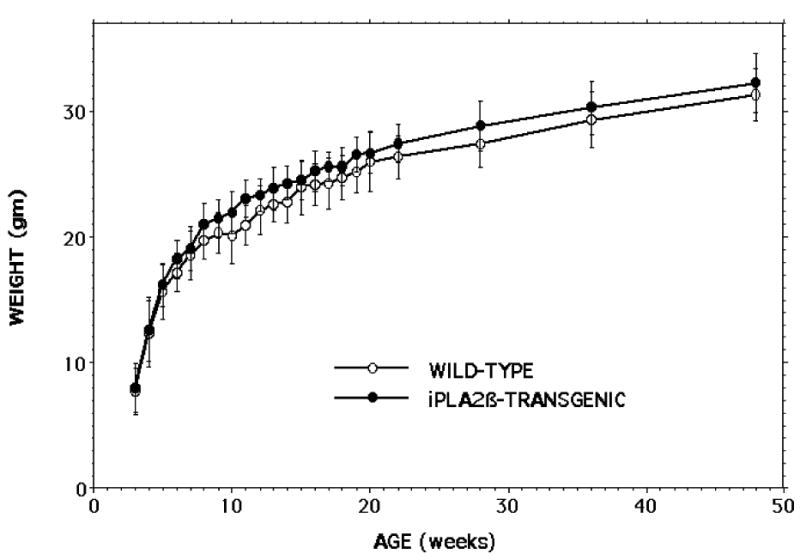
Male RIP-iPLA2β transgenic (closed circles) and wild-type (open circles) of ages 3-48 weeks with free access to water and chow were weighed between 09:00 and 10:00 AM on a top-loading balance. Mean values are displayed, and SD are indicated (n = 44-71). None of the displayed values differed significantly between the genotypes.
Glucose homeostasis in RIP-iPLA2β transgenic male mice
In the fasting state, blood glucose concentrations were significantly lower in RIP-iPLA2β-TG male mice than in wild-type mice (Figure 5A), and fasting insulin levels were significantly higher in RIP-iPLA2β-TG male mice (Figure 5B). After oral glucose administration, blood glucose levels were significantly lower in RIP-iPLA2β-TG male mice compared to wild-type mice at 15 min and remained somewhat but not significantly lower at 30, 60, or 120 min (Figure 6A). Although the fractional increase in peak glucose levels relative to the baseline fasting value was similar for wild-type (2.51 ± 0.11 fold) and RIP-iPLA2β transgenic (2.55 ± 0.13 fold) mice, the area under the concentration versus time curve (AUC) of the GTT was significantly (1.17 ± 0.05 fold) greater for wild-type (434 ± 19 AU) than for RIP-iPLA2β transgenic (370 ± 12 AU) mice. Baseline insulin levels were significantly higher in RIP-iPLA2β-TG male mice than in wild-type mice, and insulin levels in both groups rose somewhat less than two-fold at 15 min after oral glucose administration (Figure 6B).
Figure 5. Fasting blood glucose and plasma insulin concentrations in RIP-iPLA2β transgenic and wild-type male mice.
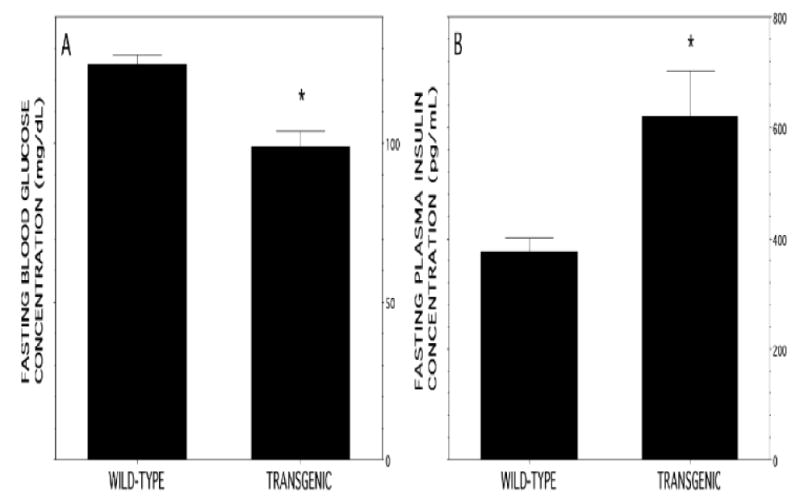
Male mice 20-24 wk of age were fasted overnight and blood obtained from the lateral saphenous vein. Data are expressed as mean ± SEM (n = 30). An asterisk (*) denotes p < 0.01 for the comparison between wild-type and transgenic mice.
Figure 6. Oral glucose-tolerance tests with RIP-iPLA2β transgenic and wild-type male mice.
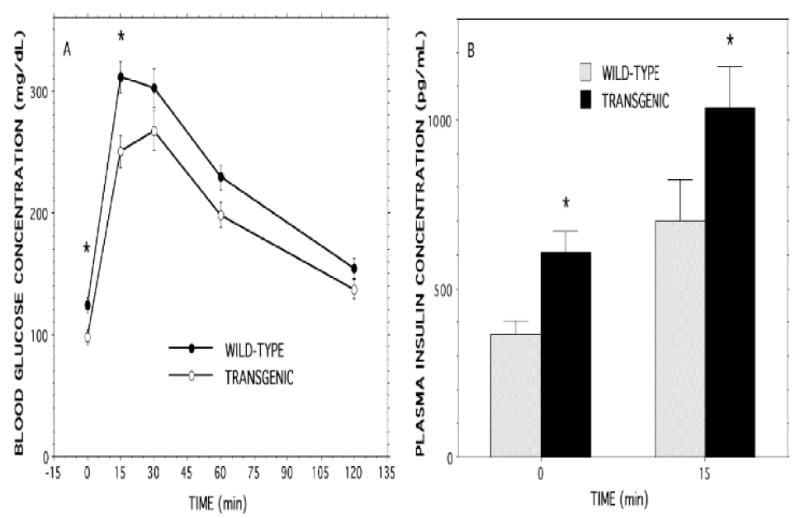
A solution containing 2 mg of D-glucose per g of body weight was administered enterally to wild-type (WT) and RIP-iPLA2β transgenic (TG) male mice 20-24 wk of age by gavage. Panel A illustrates blood glucose concentrations at baseline and at 15, 30, 60, and 120 min after glucose administration to WT (solid circles) or TG (open circles) mice. Panel B illustrates plasma insulin concentrations at baseline and 15 min after glucose administration in WT (gray bars) and TG (solid black bars) mice. Data are expressed as mean ± SEM (n = 19). An asterisk (*) denotes p < 0.01 for the comparison between WT and RIP-iPLA2β-TG mice.
After intraperitoneal administration of glucose, blood glucose concentrations at 30, 60, and 120 min were all significantly lower in RIP-iPLA2β-TG male mice than in wild-type mice (Figure 7A), and the AUC for the GTT was also significantly (1.18 ± 0.05 fold) greater for wild-type (605 ± 25 AU) than for RIP-iPLA2β transgenic (514 ± 20 AU) mice. Blood glucose concentrations were similar in the two groups at all tested time-points after intraperitoneal administration of insulin (Figure 7B). This suggests that sensitivity to insulin is not altered in RIP-iPLA2β-TG male mice, which in turn suggests that the lower glucose levels observed in glucose tolerance tests might reflect enhanced insulin secretion in RIP-iPLA2β-TG male mice. To evaluate this possibility, insulin secretion by pancreatic islets isolated from wild-type or RIP-iPLA2β-TG male mice was compared.
Figure 7. Intraperitoneal glucose and insulin tolerance tests with RIP-iPLA2β transgenic and wild-type male mice.
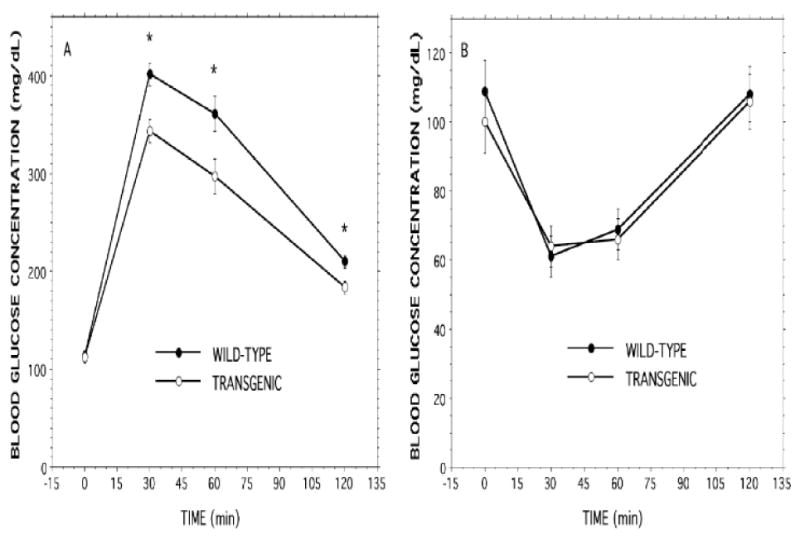
In Panel A, D-glucose (2 mg/g) and in Panel B human regular insulin (0.75 units/kg) was administered by intraperitoneal injection to wild-type (closed circles) or RIP-iPLA2β transgenic (open circlers) male mice 20-24 wk of age, and blood was collected at baseline and at 30, 60, and 120 min after injection to measure glucose concentration. Values are displayed as mean ± SEM (n = 20). An asterisk (*) denotes p < 0.05 for the comparison between wild-type and transgenic.
Insulin secretion by pancreatic islets isolated from RIP-iPLA2β transgenic and iPLA2β-null male mice and their respective wild-type littermates
Compared to insulin secretion at 3 mM glucose, pancreatic islets isolated from either wild-type or RIP-iPLA2β-TG male mice were stimulated by 8 mM and 20 mM glucose to secrete greater amounts of insulin in a concentration-dependent manner, and insulin secretory responses were amplified by the adenylyl cyclase activator forskolin (Figure 8), as expected (9, 50, 61 the Rat Insulin 1 Promoter (RIP)). In the presence of forskolin, insulin secretion stimulated by both 8 mM and 20 mM glucose was significantly greater with islets from RIP-iPLA2β-TG male mice compared to those from wild-type mice (Figure 8). In contrast, islets isolated from iPLA2β-null male mice secreted less insulin in the presence of 20 mM glucose and 2.5 μM forskolin than did islets from wild-type mice (Figure 9), indicating that the amplification of insulin secretion under those conditions correlates with iPLA2β expression level.
Figure 8. Insulin secretion stimulated by D-glucose and forskolin from pancreatic islets isolated from RIP-iPLA2β transgenic and wild-type mice.
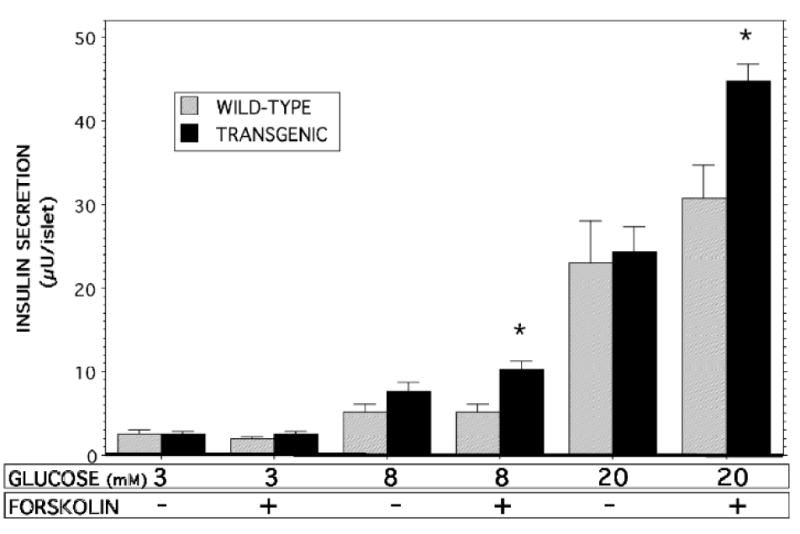
Islets isolated from wild-type (gray bars) or RIP-iPLA2β transgenic (solid black bars) male mice were incubated (30 min, 37 °C) in buffer containing 3, 8, or 28 mM D-glucose without or with 2.5 μM forskolin, and an aliquot of medium was then removed for measurement of insulin. Mean values are displayed (n = 5 experiments in triplicate). An asterisk (*) denotes p < 0.05 for the comparison between wild-type and transgenic.
Figure 9. Insulin secretion stimulated by D-glucose and forskolin from pancreatic islets isolated from iPLA2β-null and wild-type mice.
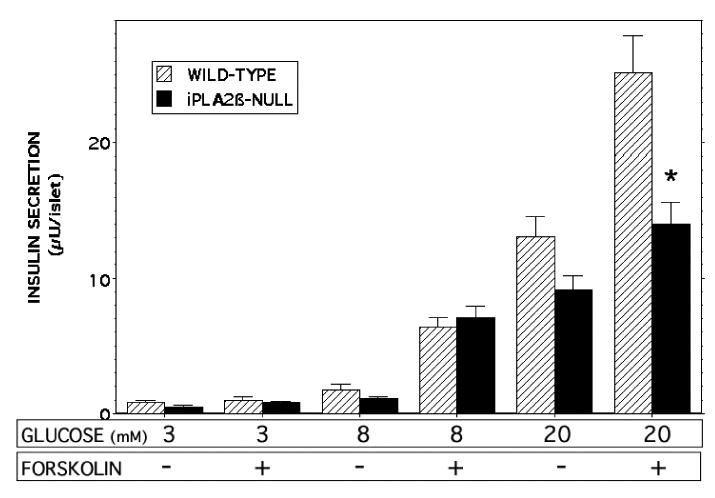
Islets isolated from wild-type (gray bars) or iPLA2β-null (solid black bars) male mice were incubated (30 min, 37 °C) in buffer containing 3, 8, or 28 mM D-glucose without or with 2.5 μM forskolin, and an aliquot of medium was then removed for measurement of insulin. Mean values are displayed (n = 5 experiments in triplicate). An asterisk (*) denotes p < 0.05 for the comparison between wild-type and iPLA2β-null mice.
Glucose and insulin tolerance testing of iPLA2β-null male mice
Although fasting blood glucose levels were not significantly different between the two groups, blood glucose concentrations at 60 and 120 min after intraperitoneal administration of glucose were significantly higher in iPLA2β-null than in wild-type male mice (Figure 10A), indicating that the former are less glucose tolerant. In the fed state, blood glucose levels were significantly higher in iPLA2β-null male mice (134 ± 2.9 mg/dL) compared to wild-type male mice (118 ± 4.5 mg/dL) and remained significantly higher at 30 min (80 ± 5.0 vs. 55 ± 3.9 mg/dL) and 60 min (75 ± 6.4 vs. 51 ± 4.1 mg/dL) and somewhat higher at 120 min (97 ± 7.2 vs. 73 ± 11.6 mg/dL) after intraperitoneal insulin administration. The fractional fall in blood glucose level was also significantly lower in iPLA2β-null male mice at 30 and 60 min (to 59 ± 3% and 56 ± 5%, respectively, of the baseline value) than it was in wild-type male mice (to 47 ± 3% and 38 ± 5%, respectively, of the baseline value), reflecting impaired insulin-sensitivity (Figure 10B).
Figure 10. Intraperitoneal glucose and insulin tolerance tests with iPLA2β-null and wild-type mice.
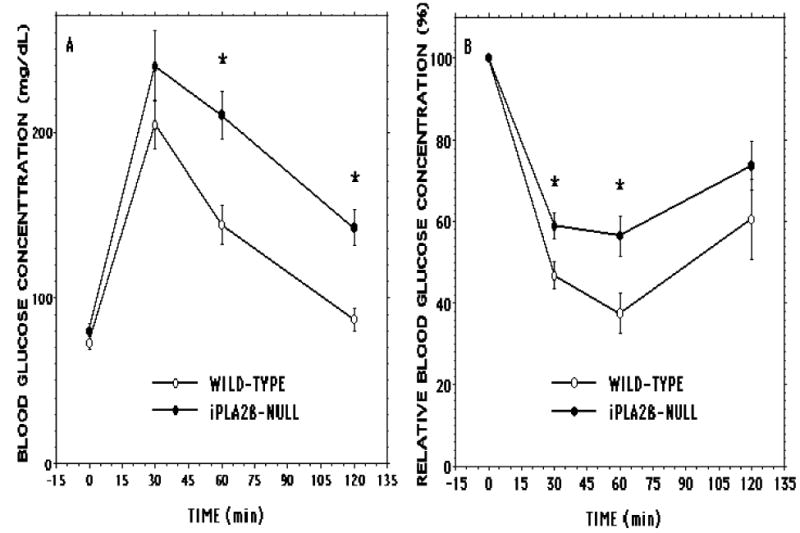
In Panel A D-glucose (2 mg/g) and in Panel B human regular insulin (0.75 units/kg) was administered by intraperitoneal injection to wild-type (open circles) or iPLA2β-null (closed circlers) male mice 20-24 wk of age, and blood was collected at baseline and at 30, 60, and 120 min after injection to measure glucose concentration. Values are displayed as mean ± SEM (n = 20). An asterisk (*) denotes p < 0.05 for the comparison between wild-type and iPLA2β-null mice.
Coupled with defective amplification of insulin secretion (Figure 9), this could account for the impaired glucose tolerance of iPLA2β-null male mice (Figure 10A), which is not observed in female iPLA2β-null mice in the absence of metabolic stressors (9). (Differences in absolute blood glucose concentrations between Figures 7 and 10 and in insulin levels between Figures 8 and 9 reflect different genetic backgrounds of RIP-iPLA2β-TG (pure C57BL6/J) and iPLA2β-null (mixed 129/SvJ × C57BL6) mice (8) and their respective wild-type littermates).
Arachidonic acid incorporation and phospholipid composition of islets from RIP-iPLA2β transgenic male mice and their corresponding wild-type male littermates
Pancreatic islets contain an unusually high fraction of arachidonic acid-containing phospholipids (56), and arachidonic acid released from them is believed to promote insulin secretion (29, 31, 48, 53-55, 74, 75). It has been proposed that iPLA2β plays a critical role in synthesizing arachidonate-containing phospholipids by providing lysophosphatidylcholine (LPC) acceptors for arachidonic acid incorporation into diradyl-glycerophosphocholine (GPC) lipids (4, 64). It was thus considered possible that the enhanced insulin secretory responses of islets from RIP-iPLA2β-TG male mice (Figure 8) might be attributable to increased LPC levels, accelerated incorporation of arachidonic acid, and a higher content of arachidonate-containing GPC lipids to provide substrate for phospholipases activated by insulin secretagogues, and we tested these possibilities.
Incorporation of [3H]arachidonic acid into phospholipids occurs at indistinguishable rates in islets from RIP-iPLA2β-TG and wild-type male mice (Figure 11). ESI/MS/MS analyses indicated that the abundance of the major arachidonate-containing GPC lipids 16:0/20:4-GPC (m/z 788) and 18:0/20:4-GPC (m/z 816) relative to the internal standard (m/z 684) is also virtually identical in islets from RIP-iPLA2β-TG and wild-type male mice (Figure 12), as is their overall content of LPC species (Figure 13). Although there are minor differences in relative abundances of 14:0-LPC (m/z 472) and 16:0-LPC (m/z 502) relative to the internal standard (m/z 544), the relative abundances of 18:1- LPC (m/z 528) and 18:0-LPC (m/z 530) are virtually identical in RIP-iPLA2β-TG and wild-type mouse islets (Figure 13). These findings indicate that overexpression of iPLA2β in islets does not result in any obvious perturbation of their arachidonate incorporation rates or phospholipid composition, and this is unlikely to explain their altered secretory responses.
Figure 11. [3H8]Arachidonic acid incorporation into glycerophosphocholine lipids from pancreatic islets of wild-type and RIP-iPLA2β transgenic mice.
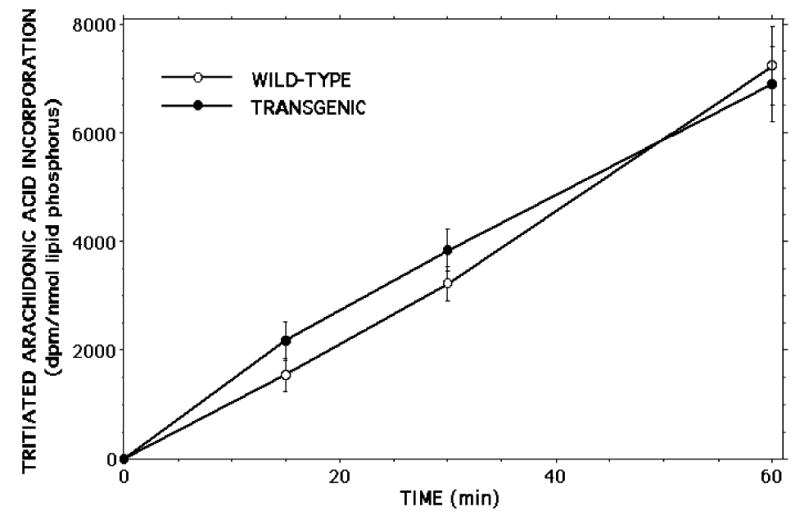
Wild-type (WT) and RIP-iPLA2β transgenic mouse islets were incubated with [3H8]arachidonic acid ([3H]AA) for various intervals and washed with BSA-containing buffer to remove unincorporated label. Phospholipids were extracted after labeling and their phosphorus content measured. GPC lipids were then isolated by TLC and their [3H]AA content determined by liquid scintillation spectrometry. Values are expressed as dpm/nmol lipid phosphorus. Mean values ± SEM are displayed (n = 4).
Figure 12. Electrospray ionization tandem mass spectrometric analyses of diradyl glycerophosphocholine lipids from pancreatic islets of wild-type and RIP-iPLA2β transgenic mice.
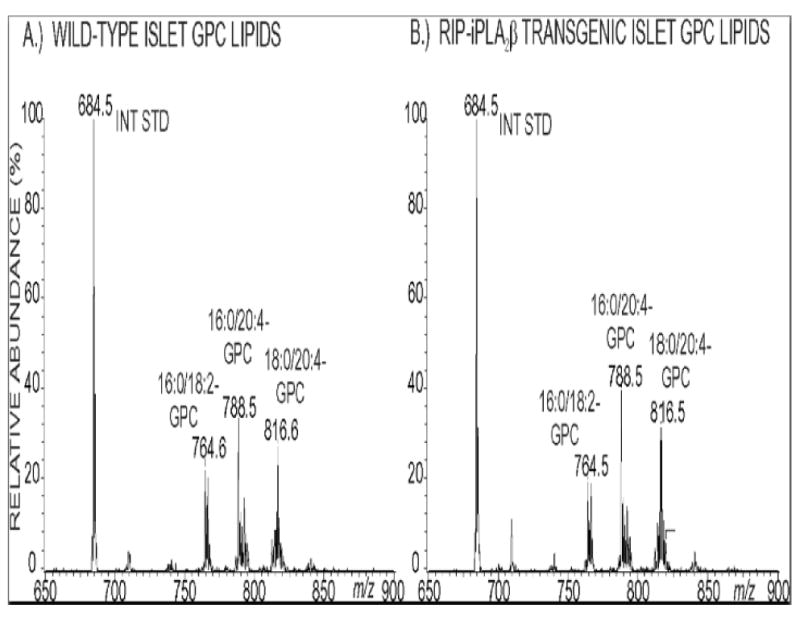
Phospholipids from pancreatic islets of wild-type (Panel A) and RIP-iPLA2β transgenic (Panel B) mice were mixed with internal standard 14:0/14:0-GPC and analyzed as Li+ adducts by positive ion ESI/MS/MS scanning for constant neutral loss of 183 (phosphocholine), and relative abundances of ion currents were plotted vs. m/z value.
Figure 13. Electrospray ionization tandem mass spectrometric analyses of the lysophosphatidylcholine (LPC) content of pancreatic islets of wild-type and RIP-iPLA2β transgenic mice.
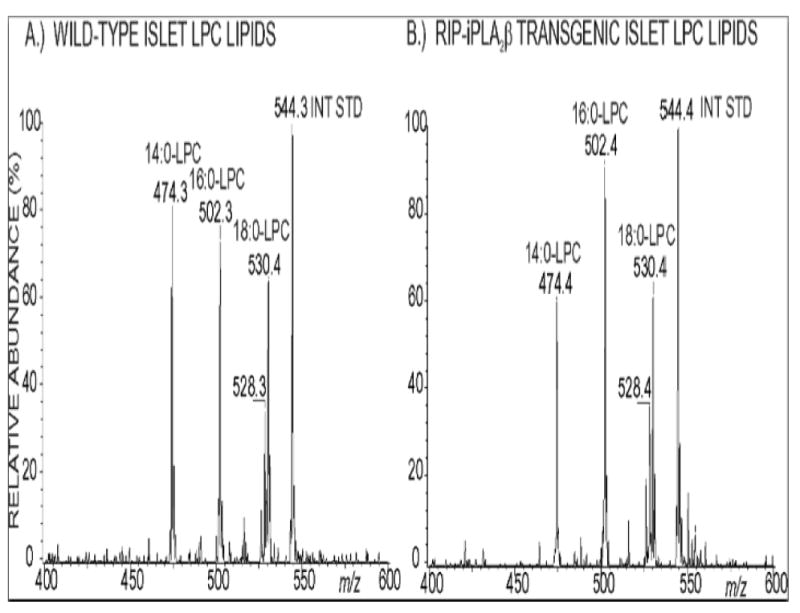
Phospholipids were extracted from pancreatic islets of wild-type (Panel A) and RIP-iPLA2β transgenic (Panel B) mice, mixed with internal standard 19:0-LPC, and analyzed as Li+ adducts by positive ion ESI/MS/MS scanning for constant neutral loss of 59 (trimethylamine) to visualize LPC species.
Kv2.1 currents, membrane potential, and cytosolic [Ca2+] of RIP-iPLA2β transgenic and wild-type male mouse islet β-cells
Another mechanism that was considered as a possible explanation of the enhanced insulin secretory responses of islets from RIP-iPLA2β transgenic mice is attenuation of Kv2.1 currents that ordinarily serve to repolarize the β-cell after secretagogue-induced depolarization. Such repolarization limits the duration of the action potential and the associated rise in cytosolic [Ca2+] that triggers insulin secretion (28, 29). Insulinoma cells that overexpress iPLA2β exhibit reduced amplitude and accelerated inactivation of Kv2.1 channels, and these effects are mimicked by application of the PLA2 reaction product arachidonic acid (29). Mice with a disrupted Kv2.1 gene exhibit a phenotype similar to that of RIP-iPLA2β transgenic mice with mild hypoglycemia and hyperinsulinemia, improved glucose tolerance after intraperitoneal glucose administration, and enhanced insulin secretory responses of isolated islets (28). This is associated with greatly reduced islet Kv currents, action potentials of prolonged duration and decreased frequency, and more sustained periods of elevated cytosolic [Ca2+] (28). We therefore investigated the possibility that islets from RIP-iPLA2β transgenic mice would exhibit similar electrophysiologic properties.
Figure 14 illustrates Kv currents for wild-type control (Panel A) and RIP-iPLA2β-transgenic islet β-cells (Panel B) elicited by 500 mS voltage steps at 10 mV increments from −80mV to 30mV. The RIP-iPLA2β-transgenic β-cells exhibit significantly increased inactivation compared to control β-cells 10 minutes after changing the medium glucose concentration from 0 mM to 20 mM (Panel C), and the amplitude of total Kv current is also reduced in the RIP-iPLA2β-transgenic β-cells compared to control β-cells.
Figure 14. Delayed rectifier currents in pancreatic islet β-cells from wild-type control and RIP-iPLA2β transgenic mice.
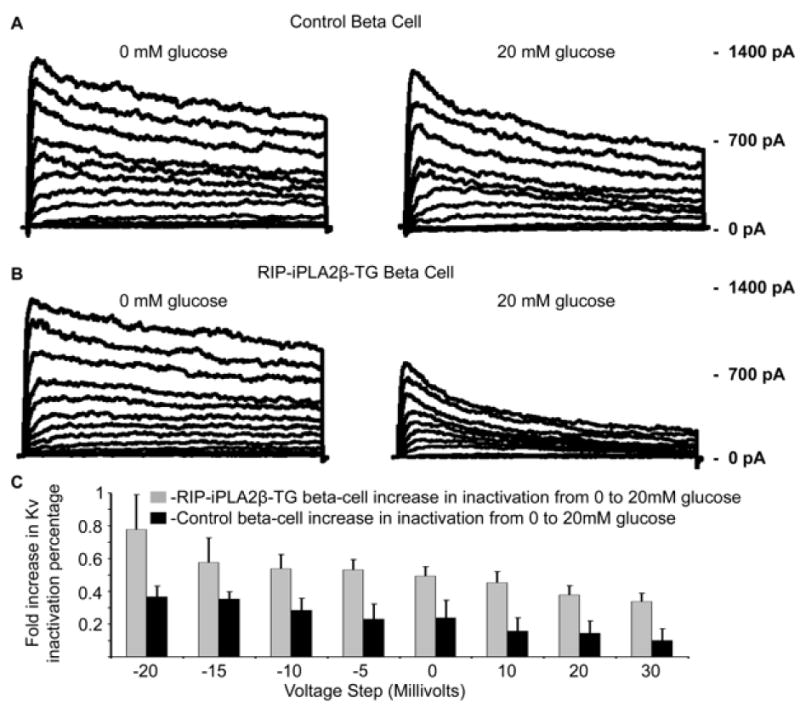
Kv current traces recorded from islet β-cells from wild-type control (Panel A) or RIP-iPLA2β transgenic mice (Panel B) incubated in medium without glucose (leftmost tracings) or with 20 mM glucose (rightmost panels) and subjected to 500-ms depolarization in 10 mV and 5 mV increments from −80 mV to 30 mV. In Panel C, the fold-increase in Kv inactivation percentage is expressed as a function of depolarizing voltage for wild-type control (solid black bars) or RIP-iPLA2β transgenic islet β-cells (gray bars) at 10 minutes after switching the medium glucose concentration from 0 mM to 20 mM. Mean values are displayed and error bars represent SD (n = 7).
Figure 15 illustrates islet action potential frequency during stimulation with 20 mM glucose for wild-type control (Panel A) and RIP-iPLA2β-transgenic islet β-cells (Panel B). The action potential frequency was reduced from 1.7/second for control cells to 0.96/second (p=0.035) for RIP-iPLA2β-transgenic cells 5 minutes post glucose stimulation. This was associated with an increased duration of each action potential for the RIP-iPLA2β-transgenic islet β-cells compared to control cells (middle tracings) and with a more sustained elevation in cytosolic [Ca2+] for the transgenic cells (lower tracings), recorded at a frequency of 10 KHz from the same islet loaded with the Ca2+ indicator Fluo4. The findings with Kv currents (Figure 14) and with membrane potential and cytosolic [Ca2+] (Figure 15) with the RIP-iPLA2β-transgenic islets are quite similar to those observed with islets from Kv2.1-null mice (28), consistent with the phenotype of improved glucose tolerance exhibited by both of these genetically modified mouse lines.
Figure 15. RIP-iPLA2β transgenic mouse islets have increased glucose-induced action potential duration with decreased frequency and corresponding changes in glucose-induced elevation of cytosolic [Ca2+] compared to wild-type control islets.
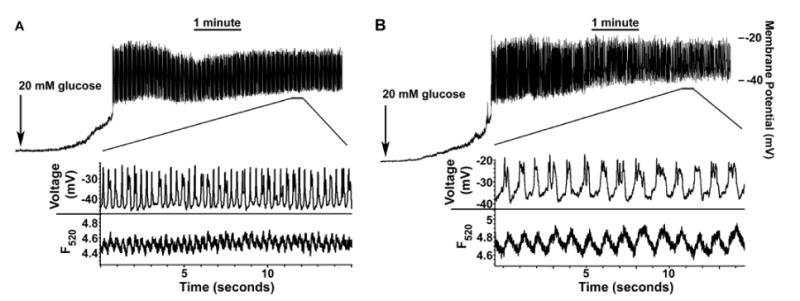
Electrical activity in response to 20 mM glucose is recorded for wild-type control (Panel A) or RIP-iPLA2β transgenic (Panel B) mouse islet attached β-cells. The middle inset in each panel displays the action potentials (APs) from the segment of activity indicated by the horizontal bars. The lowest set of tracings in each panel represents fast-acquisition [Ca2+] traces recorded from the same entire mouse islet during the segment of activity indicated by the horizontal bars, loaded with Fluo-4, and imaged at a frequency of 10 KHz. The decrease in AP frequency of wild-type control vs. RIP-iPLA2β transgenic mouse islets (from 1.7/second to 0.96/second) taken at 5 minutes post glucose was significant (p = 0.035, n = 6 each).
Discussion
These studies represent the first report of genetically modified mice that overexpress iPLA2β in pancreatic islet β-cells. Like other PLA2 enzymes, iPLA2β catalyzes hydrolysis of the sn-2 fatty acid substituent from glycerophospholipid substrates (36, 64) to yield a free fatty acid, e.g., arachidonic acid, and a 2-lysophospholipid, e.g., lysophosphatidylcholine (LPC), that have intrinsic mediator functions (11, 51) and can initiate synthesis of other mediators (43). Other mammalian PLA2s include the PAF-acetylhydrolases; the low molecular weight secretory PLA2 (sPLA2) that require mM [Ca2+] for catalysis (64); and Group IV cytosolic PLA2 (cPLA2) enzymes (22, 47, 64, 71), of which the prototype cPLA2α prefers substrates with sn-2 arachidonoyl residues, undergoes Ca2+-induced membrane association, and is regulated by phosphorylation (22).
The iPLA2β is also designated Group VIA PLA2 (3, 34, 67), and it does not require Ca2+ for catalysis, resides in the cytoplasm of resting cells, and undergoes subcellular redistribution upon cellular stimulation (38). It belongs to a larger class of serine lipases encoded by several genes, and all have a GXSXG lipase consensus sequence (30, 73). Various functions have been proposed for iPLA2β, including roles in phospholipid remodeling (2, 4) and in amplifying insulin secretion from pancreatic islet β-cells (5, 36, 48, 53, 63, 66, 68). The latter hypothesis was first developed with pharmacologic inhibitors and insulinoma cell lines, and its relevance to animal physiology has now been evaluated here with genetically modified mice.
We have generated transgenic mice that overexpress iPLA2β in pancreatic islet β-cells by several-fold compared to wild-type littermates, and, in the fasted state, the male RIP-iPLA2β-TG mice exhibit lower blood glucose and higher plasma insulin levels than wild-type male mice. Upon oral or intraperitoneal glucose administration, the male RIP-iPLA2β-TG mice develop lower blood glucose levels than do wild-type male mice, although the hypoglycemic responses of RIP-iPLA2β-TG and wild-type mice to exogenous insulin are indistinguishable, suggesting that insulin sensitivity is not altered in RIP-iPLA2β-TG mice. Islets from the male RIP-iPLA2β-TG mice exhibit greater amplification of glucose-induced insulin secretion by the adenylyl cyclase activator forskolin than do wild-type islets, and this resembles the effect of overexpressing iPLA2β in INS-1 insulinoma cells, in which the amplifying effects of agents that elevate cAMP on glucose-induced insulin secretion are substantially greater than in parental INS-1 cells (35). Our findings with genetically modified mice thus support the hypothesis that iPLA2β plays a role in amplifying insulin secretion (5, 36, 53) and indicate that this has demonstrable effects on in vivo glucose homeostasis in living animals.
It has been reported that introduction of the RIP-Cre construct, in which transcription of sequence encoding the cre recombinase is driven by RIP, into transgenic mice is sufficient to induce glucose intolerance and impaired insulin secretion even in the absence of genes encoded by loxP sites that are the usual targets of attempts to produce β-cell-specific ablation of specific genes (33). This raises the possibility that artifactual impairment of glucose homeostasis can occur as a result of genetic manipulation that is not related to the targeted genes. In our RIP-iPLA2β transgenic mice, however, improved, rather than impaired, glucose tolerance and insulin secretion were observed, and the transgene construct did not include coding sequence for the cre recombinase. Moreover, elimination of iPLA2β expression in iPLA2β-null mice produced effects in a direction opposite to those produced by overexpression of iPLA2β, and this provides independent but complementary support for the hypothesis that iPLA2β acts to augment insulin secretion and glucose tolerance.
The conclusions from experiments with INS-1 insulinoma cells in which iPLA2β expression is suppressed by stable expression of siRNA are also symmetrical with those involving iPLA2β-null mice. Such cell lines exhibit reduced amplification of glucose-induced insulin secretion by cAMP-elevating agents compared to parental INS-1 cells (5). At the whole animal level, we find here that male iPLA2β-null mice generated by homologous recombination exhibit abnormal glucose tolerance in the absence of metabolic stress. Although unstressed female iPLA2β-null mice have normal glucose tolerance, imposing the metabolic stress of high dietary fat feeding causes greater deterioration in glucose-tolerance in female iPLA2β-null mice than in wild-type littermates (9).
Pancreatic islets isolated from female iPLA2β-null mice subjected to high dietary fat feeding also have markedly reduced ability to amplify glucose-induced insulin secretion in the presence of agents that elevate cAMP (9). Here, we find that islets from male iPLA2β-null mice fed a normal chow diet also have reduced insulin secretory responses to 20 mM glucose and the adenylyl cyclase activator forskolin, which provides further support for a role for iPLA2β in amplification of glucose-induced insulin secretion by cAMP-elevating agents.
The opposing but complementary effects of suppressing (5, 9) or increasing (35) iPLA2β expression level, respectively, with both genetically manipulated cell lines and whole animals also argue against the possibility that the improvements in insulin secretion and glucose tolerance in RIP-iPLA2β transgenic mice reported here are non-specific effects of iPLA2β overexpression, although such effects can occur in overexpression models.
The molecular basis for synergy between iPLA2β expression and cAMP elevation is not yet established, but agents that increase β-cell cAMP levels induce iPLA2β subcellular redistribution and association with perinuclear membranes that include ER and Golgi (6, 36, 38). Membrane association could facilitate access of iPLA2β to its substrates, and subcellular redistribution of iPLA2β is suppressed by Protein Kinase A (PKA) inhibitors (38). Although purified, recombinant iPLA2β is a substrate for the catalytic subunit of PKA in vitro (38 the Rat Insulin 1 Promoter (RIP)), to our knowledge PKA-catalyzed phosphorylation of iPLA2β has not yet been demonstrated in intact β-cells or other cells.
The synergy between iPLA2β expression and cAMP elevation in the amplification of insulin secretion from isolated islets caused us to examine glucose tolerance after both oral and intraperitoneal administration in RIP-iPLA2β transgenic and wild-type mice. We reasoned that the incretin effect of gut peptides released after oral glucose administration might increase islet cAMP and cause greater amplification of insulin secretion from RIP-iPLA2β transgenic islets than that which occurred after intraperitoneal glucose administration. Although the glucose tolerance of RIP-iPLA2β transgenic mice was greater than that of wild-type mice both in oral and intraperitoneal GTT, the difference between the genotypes was not greater in the oral test. Among the possibilities these results could reflect is that the incretin effect is not as strong as expected, that countervailing adrenergic influences supervene because of the stress of oral gavage, because of overriding autonomic effects, or for other reasons operative in vivo that do not affect the behavior of isolated islets ex vivo.
Several events that occur as a consequence of iPLA2β-catalyzed phospholipid hydrolysis and accumulation of nonesterified arachidonate and LPC could contribute to amplification of secretagogue-induced rises in β-cell [Ca2+] and insulin secretion. These include facilitating Ca2+ entry (53, 54, 75) by arachidonate effects on voltage-operated Ca2+ channels (72), effects of LPC and arachidonate on KATP (18), effects of an arachidonate 12-lipoxygenase product on a plasma membrane Na+/K+-ATPase (48), and either direct effects of LPC (65) or indirect effects of arachidonate (74) on plasma membrane store-operated cation channels (17).
A recently demonstrated molecular mechanism through which iPLA2β contributes to regulation of β-cell membrane potential and Ca2+ transients is that arachidonate released by iPLA2β inactivates voltage-operated Kv2.1 channels in the β-cell plasma membrane and thereby increases the duration of depolarization and the associated rise in [Ca2+] induced by secretagogues (29). This demonstration rests in part on findings with INS-1 cells, in which exogenous arachidonate suppresses Kv2.1 currents (29). In addition, INS-1 cells that overexpress iPLA2β exhibit reduced depolarization-induced Kv2.1 currents, and such currents are enhanced in iPLA2β-null β-cells (29). Such effects of iPLA2β prolong secretagogue-induced depolarization and Ca2+ entry into β-cells and amplify the insulin secretory response.
Here we demonstrate that islets from RIP-iPLA2β-transgenic mice also exhibit reduced and more rapidly inactivating Kv2.1 currents than do wild-type control mouse islets. This is associated with reduced frequency and increased duration of glucose-induced action potentials in the transgenic islet β-cells, and there is a corresponding prolongation of the glucose-induced rise in cytosolic [Ca2+]. These findings are quite similar to recently reported electrophysiologic properties of islets from Kv2.1-null mice (28). Those mice also exhibit a phenotype similar to RIP-iPLA2β-transgenic mice with respect to glucose homeostasis, including mild hypoglycemia and hyperinsulinemia, improved glucose tolerance after intraperitoneal glucose administration, and enhanced insulin secretory responses of isolated islets (28). These observations suggest that iPLA2β contributes to physiologically significant regulation of β-cell membrane potential and Ca2+ transients in part by restraining Kv2.1 delayed-rectifier channel activity and that this has a demonstrable impact on glucose homeostasis at the whole animal level.
Pancreatic islet β-cells express PLA2 enzymes in addition to iPLA2β, including the Group IB secretory PLA2 (sPLA2-IB), which resides in part in insulin secretory granules and is co-secreted with insulin (57). Although iPLA2β and sPLA2-IB both hydrolyze the sn-2 fatty acid substituents from glycerolipid substrates to yield a free fatty acid and a 2-lsyosphospholipid, the two enzymes reside in different compartments and have different catalytic requirements. iPLA2β is active at nanomolar to low micromolar Ca2+ concentrations that occur within the cytosol of living cells, resides predominantly in cytosol in resting cells, and undergoes subcellular redistribution to associate with membranous organelles upon stimulation of β-cells with secretagogues (35, 38). In contrast, sPLA2-IB requires millimolar concentrations of Ca2+ for catalytic activity and functions as an extracellular secreted enzyme (64).
sPLA2-IB-null mice have been prepared and found to be resistant to high fat diet-induced obesity and insulin resistance and to exhibit reduced post-prandial hyperglycemia, superior glucose tolerance, and increased insulin sensitivity compared to wild-type littermates (27, 32). These effects appear to be attributable to reduced absorption and blood levels of lysophospholipids, such as lysophosphatidylcholine, and to improved glucose uptake by several peripheral tissues, including liver, heart, and skeletal muscle (32). Plasma insulin levels after intraperitoneal glucose administration are slightly but not significantly lower in sPLA2-IB-null mice than in wild-type mice, and the improved glucose tolerance of the sPLA2-IB-null mice is thought to reflect principally improved insulin sensitivity (32).
A widely cited hypothesis is that iPLA2β is critical for synthesis of arachidonate-containing phospholipids and provides LPC acceptors for incorporating arachidonic acid into diradyl-GPC lipids (4, 64). This could be important because pancreatic islets have the highest arachidonate-containing phospholipid content of any known tissue (56), and arachidonic acid released by secretagogue-activated phospholipases is thought to promote insulin secretion (29, 31, 48, 53, 54, 74, 75). If iPLA2β overexpression increased the content of arachidonate-containing phospholipids, this might contribute to enhanced RIP-iPLA2β-TG islet secretion, but [3H]arachidonate incorporation and content of LPC and arachidonate-containing GPC lipids of wild-type and RIP-iPLA2β-TG islets are indistinguishable. This is consistent with data from INS-1 cells that overexpress (35) or underexpress (5) iPLA2β and from several tissues and cells from iPLA2β-null mice, including islets (9), testes (8), and peritoneal macrophages (7). These findings call into question the relevance of the iPLA2β-arachidonate incorporation hypothesis to the physiology of normal cells because it is based on findings with transformed, cultured, macrophage-like cells (4, 64) but the process does not operate in native macrophages (7).
In conclusion, the findings reported here with transgenic mice that overexpress iPLA2β in pancreatic islet β-cells support the hypothesis that iPLA2β plays a role in amplifying insulin secretion and indicate that this is relevant to the physiology of glucose homeostasis in living animals.
Acknowledgments
The authors thank Sheng Zhang and Min Tan for excellent technical assistance, Dr. Sasanka Ramanadham for a critical reading of the manuscript, and Dr. Richard Gross for iPLA2β antibody 506. The authors also gratefully acknowledge O. Pongs, S.A. Goldstein, L.E. Fridlyand, J.P. Lopez, and N.A. Tamarina for helpful discussions and suggestions and F. Mendez and S. Eames for technical expertise and assistance.
Grants
This work was supported by United States Public Health Service Grants R37-DK34388, P41-RR00954, P60-DK20579, and P30-DK56341 to J.T.; by DK48494 to L.H.P.; and by University of Chicago grant DRTC DK20595. D.A.J. was supported in part by a postdoctoral fellowship in β cell research from Takeda Pharmaceuticals North America.
References
- 1.Arkhammar P, Nilsson T, Rorsman P, Berggren PO. Inhibition of ATP-regulated K+ channels precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration in pancreatic beta cells. J Biol Chem. 1987;262:5448–54. [PubMed] [Google Scholar]
- 2.Baburina I, Jackowski S. Cellular responses to excess phospholipid. J Biol Chem. 1999;274:9400–9408. doi: 10.1074/jbc.274.14.9400. [DOI] [PubMed] [Google Scholar]
- 3.Balboa MA, Balsinde J, Jones SS, Dennis EA. Identity between the Ca2+-independent phospholipase A2 enzymes from P388D1 macrophages and Chinese hamster ovary cells. J Biol Chem. 1997;272:8576–8580. doi: 10.1074/jbc.272.13.8576. [DOI] [PubMed] [Google Scholar]
- 4.Balsinde J, Balboa MA, Dennis EA. Antisense inhibition of group VI Ca2+-independent phospholipase A2 blocks phospholipid fatty acid remodeling in murine P388D1 macrophages. J Biol Chem. 1997;272:29317–29321. doi: 10.1074/jbc.272.46.29317. [DOI] [PubMed] [Google Scholar]
- 5.Bao S, Bohrer A, Ramanadham S, Jin W, Zhang S, Turk J. Effects of stable suppression of Group VIA phospholipase A2 expression on phospholipid content and composition, insulin secretion, and proliferation of INS-1 insulinoma cells. J Biol Chem. 2006;281:187–198. doi: 10.1074/jbc.M509105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao S, Jin C, Zhang S, Turk J, Ma Z, Ramanadham S. The beta cell calcium-independent Group VIA phospholipase A2 (iPLA2β). Tracking iPLA2 iPLA2β movements in response to stimulation with insulin secretagogues in INS-1 cells. Diabetes. 2004;53:S186–S189. doi: 10.2337/diabetes.53.2007.s186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao S, Li Y, Lei X, Wohltmann M, Bohrer A, Jin W, Ramanadham S, Tabas I, Turk J. Attenuated free cholesterol loading-induced apoptosis and preserved phospholipid composition of peritoneal macrophages from mice that do not express Group VIA Phospholipase A2. J Biol Chem. 2007;282 doi: 10.1074/jbc.M701316200. JBC Papers In Press, 07/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, Moley K, Turk J. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J Biol Chem. 2004;279:38194–38200. doi: 10.1074/jbc.M406489200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao S, Song H, Wohltmann M, Bohrer A, Turk J. Insulin secretory responses and phospholipid composition of pancreatic islets from mice that do not express Group VIA Phospholipase A2 and effects of metabolic stress on glucose homeostasis. J Biol Chem. 2006;281:20958–20973. doi: 10.1074/jbc.M600075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernal-Mizrachi E, Fatrai S, Johnson JD, Ohsugi M, Otani K, Han Z, Polonsky KS, Permutt MA. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004;114:928–936. doi: 10.1172/JCI20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–100. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 13.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates PPAR α to maintain glucose, lipid, and cholesterol homeostasis. Cell Metabolism. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic β-cells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- 15.Devendra D, Liu E, Eisenbarth GS. Type I diabetes: recent developments. Brit Med J. 2004;328:750–754. doi: 10.1136/bmj.328.7442.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Intensive treatment and cardiovascular disease in patients with type I diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dukes ID, Roe MW, Worley JF, Philipson LH. Glucose-induced alterations in β-cell cytoplasmic Ca2+ involving coupling of intracellular Ca2+ stores and plasma membrane ion channels. Curr Opin Endocrinol Diabetes. 1997;4:262–271. [Google Scholar]
- 18.Eddlestone GT. ATP-sensitive K channel modulation by products of PLA2 action in the insulin-secreting HIT cell line. Am J Physiol. 1995;268:C181–C190. doi: 10.1152/ajpcell.1995.268.1.C181. [DOI] [PubMed] [Google Scholar]
- 19.Eto K, Tsubamoto Y, Terauchi Y, Sugiyama T, Kishimoto T, Takahashi N, Yamauchi N, Kubota N, Murayama S, Aizawa T, Akanuma Y, Aizawa S, Kasai H, Yazaki Y, Kadowaki T. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283:981–985. doi: 10.1126/science.283.5404.981. [DOI] [PubMed] [Google Scholar]
- 20.Garvey WT. Glucose transport and NIDDM. Diabetes Care. 1992;15:396–417. doi: 10.2337/diacare.15.3.396. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh A, Ronner P, Cheong E, Khalid P, Matschinsky FM. The role of ATP and free ADP in metabolic coupling during fuel-stimulated insulin release from islet β-cells in isolated perfused rat pancreas. J Biol Chem. 1991;266:22887–92. [PubMed] [Google Scholar]
- 22.Gijon MA, Spencer DM, Kaiser AL, Leslie CC. Role of phosphorylation sites and the C2 domain in regulation of cytosolic phospholipase A2. J Cell Biol. 1999;145:1219–1232. doi: 10.1083/jcb.145.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilon P, Arredouani A, Gailly P, Gromada J, Henquin JC. Uptake and release of Ca2+ by the endoplasmic reticulum contribute to oscillations of cytosolic Ca2+ concentration triggered by Ca2+ influx in electrically excitable pancreatic beta cells. J Biol Chem. 1999;274:20197–20205. doi: 10.1074/jbc.274.29.20197. [DOI] [PubMed] [Google Scholar]
- 24.Hohmeier HE, BeltrandelRio H, Clark SA, Henkel-Tieger R, Normington K, Newgard CB. Regulation of insulin secretion from novel engineered insulinoma cell lines. Diabetes. 1997;46:968–977. doi: 10.2337/diab.46.6.968. [DOI] [PubMed] [Google Scholar]
- 25.Hsu FF, Bohrer A, Turk J. Formation of lithiated adducts of glycerophosphocholine lipids facilitates their identification by electrospray ionization tandem mass spectrometry. J Am Soc Mass Spectrom. 1998;9:516–526. doi: 10.1016/S1044-0305(98)00012-9. [DOI] [PubMed] [Google Scholar]
- 26.Hsu FF, Turk J, Thukkani AK, Messner MC, Wildsmith KR, Ford DA. Characterization of alkylacyl-, alk-1-enylacyl, and lyso subclasses of glycerophosphocholine lipids by tandem quadrupole mass spectrometry with electrospray ionization. J Mass Spectrom. 2003;38:752–763. doi: 10.1002/jms.491. [DOI] [PubMed] [Google Scholar]
- 27.Huggins KW, Boileau AC, Hui DY. Protection against diet-induced obesity and obesity- related insulin resistance in Group 1B PLA2-deficient mice. Am J Physiol Endocrinol Metab. 2002;283:E994–E1001. doi: 10.1152/ajpendo.00110.2002. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson DA, Kuznetsov A, James P, Lopez JP, Kash S, Ämmälä CE, Louis H, Philipson LH. Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metabolism. 2007;6:229–235. doi: 10.1016/j.cmet.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobson DA, Webber C, Bao S, Turk J, Philipson LH. Modulation of the pancreatic islet beta cell delayed rectifier potassium channel Kv2.1 by the polyunsaturated fatty acid arachidonate. J Biol Chem. 2007;282:7442–7449. doi: 10.1074/jbc.M607858200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 31.Konrad RJ, Jolly YC, Major C, Wolf BA. Inhibition of phospholipase A2 and insulin secretion in pancreatic islets. Biochim Biophys Acta. 1992;1135:215–220. doi: 10.1016/0167-4889(92)90139-3. [DOI] [PubMed] [Google Scholar]
- 32.Labonte ED, Kirby RJ, Schildmeyer NM, Cannon AM, Huggins KW, Hui DY. Group 1B phospholipase A2-mediated lysophospholipid absorption directly contributes to postprandial hyperglycemia. Diabetes. 2006;55:935–41. doi: 10.2337/diabetes.55.04.06.db05-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L. RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J Biol Chem. 2006;281:2649–53. doi: 10.1074/jbc.M512373200. [DOI] [PubMed] [Google Scholar]
- 34.Ma Z, Ramanadham S, Kempe K, Chi XS, Ladenson J, Turk J. Pancreatic islets express a Ca2+-independent phospholipase A2 enzyme that contains a repeated structural motif homologous to the integral membrane protein binding domain of ankyrin. J Biol Chem. 1997;272:11118–11127. [PubMed] [Google Scholar]
- 35.Ma Z, Ramanadham S, Wohltmann M, Bohrer A, Hsu FF, Turk J. Studies of insulin secretory responses and of arachidonic acid incorporation into phospholipids of stably transfected insulinoma cells that overexpress Group VIA Phospholipase A2 (iPLA2β) indicate a signaling rather than a housekeeping role for iPLA2β. J Biol Chem. 2001;276:13198–13208. doi: 10.1074/jbc.M010423200. [DOI] [PubMed] [Google Scholar]
- 36.Ma Z, Turk J. The molecular biology of the Group VIA Ca2+-independent phospholipase. Prog Nucleic Acid Res Mol Biol. 2001;67:1–33. doi: 10.1016/s0079-6603(01)67023-5. [DOI] [PubMed] [Google Scholar]
- 37.Ma Z, Wang X, Nowatzke W, Ramanadham S, Turk J. Human pancreatic islets express mRNA species encoding two distinct catalytically active isoforms of group VI phospholipase A2 (iPLA2) that arise from an exon-skipping mechanism of alternative splicing of the transcript from the iPLA2 gene on chromosome 22q13.1. J Biol Chem. 1999;274:9607–9616. doi: 10.1074/jbc.274.14.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma Z, Zhang S, Turk J, Ramanadham S. Stimulation of insulin secretion and associated nuclear accumulation of iPLA2β in INS-1 insulinoma cells. Am J Physiol Endocrinol Metab. 2002;282:E820–E833. doi: 10.1152/ajpendo.00165.2001. [DOI] [PubMed] [Google Scholar]
- 39.Matschinsky FM. Glucokinase, glucose homeostasis, and diabetes mellitus. Curr Diab Rep. 2005;5:171–176. doi: 10.1007/s11892-005-0005-4. [DOI] [PubMed] [Google Scholar]
- 40.Meglasson MD, Matschinsky FM. Pancreatic islet glucose metabolism and the regulation of insulin secretion. Diabetes/Metabolism Rev. 1986;2:163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- 41.Metz SA. The pancreatic islet as Rubik's cube. Is phospholipid hydrolysis a piece of the puzzle? Diabetes. 1991;40:1565–1573. doi: 10.2337/diab.40.12.1565. [DOI] [PubMed] [Google Scholar]
- 42.Miles PDG, Barak Y, He W, Evans RM, Olefsky JM. Improved insulin-sensitivity in mice heterozygous for PPAR-γ deficiency. J Clin Invest. 2000;105:287–292. doi: 10.1172/JCI8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy RC, Barkley RM, Berry KZ, Hankin J, Harrison K, Johnson C, Krank J, McAnoy A, Uhlson C, Zarini S. Electrospray ionization and tandem mass spectrometry of eicosanoids. Anal Biochem. 2005;346:1–42. doi: 10.1016/j.ab.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 44.Narushima M, Kobayashi, Okitsu T, Tanaka Y, Li SA, Chen Y, Miki A, Tanaka K, Nakaji S, Tajeu K, Gutierrez AG, Rivas-Carillo JD, Navarro-Alvarez N, Jun HS, Westerman KA, Noguchi H, Lakey JRT, Leboulch P, Tanaka N, Yoon JW. A human beta cell line for transplantation therapy to control type I diabetes. Nature Biotech. 2005;23:1274–1282. doi: 10.1038/nbt1145. [DOI] [PubMed] [Google Scholar]
- 45.Newgard CB. While tinkering with the β-cell. Metabolic regulatory mechanisms and new therapeutic strategies. Diabetes. 2002;51:3141–3150. doi: 10.2337/diabetes.51.11.3141. [DOI] [PubMed] [Google Scholar]
- 46.Newgard CB, Hohmeier HE, Lu D, Jensen MC, Tran VV, Chen G, Gasa R, Burgess S, Sherry AD. Understanding basic mechanisms of β-cell function and survival: Prelude to new diabetes therapies. Cell Biochem Biophys. 2004;40:159–168. doi: 10.1385/cbb:40:3:159. [DOI] [PubMed] [Google Scholar]
- 47.Ohto T, Uozumi N, Hirabayashi T, Shimizu T. Identification of novel cytosolic phospholipase A2s, murine cPLA2δ, ε, and η, which form a gene cluster with cPLA2β. J Biol Chem. 2005;280:24576–24583. doi: 10.1074/jbc.M413711200. [DOI] [PubMed] [Google Scholar]
- 48.Owada S, Larsson O, Arkhammar P, Katz AI, Chibalin AV, Berggren PO, Bertorello AM. Glucose decreases Na+, K+-ATPase activity in pancreatic β-cells. An effect mediated by Ca2+-independent phospholipase A2 and protein kinase C-dependent phosphorylation of the α-subunit. J Biol Chem. 1999;274:2000–2008. doi: 10.1074/jbc.274.4.2000. [DOI] [PubMed] [Google Scholar]
- 49.Pappan KL, Pan Z, Kwon G, Marshall CA, Coleman T, Goldberg IJ, McDaniel ML, Semenkovich CF. Pancreatic beta-cell lipoprotein lipase independently regulates islet glucose metabolism and normal insulin secretion. J Biol Chem. 2005;280:9023–9029. doi: 10.1074/jbc.M409706200. [DOI] [PubMed] [Google Scholar]
- 50.Prentki M, Matschinsky FM. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987;67:1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- 51.Radu CG, Yang LV, Riedinger M, Au M, Witte ON. T cell chemotaxis to lysophosphatidylcholine through the G2A receptor. Proc Natl Acad Sci USA. 2004;101:245–250. doi: 10.1073/pnas.2536801100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- 53.Ramanadham S, Gross RW, Han X, Turk J. Inhibition of arachidonate release by secretagogue-stimulated pancreatic islets suppresses both insulin secretion and the rise in beta cell cytosolic Ca2+ concentration. Biochemistry. 1993;32:337–346. doi: 10.1021/bi00052a042. [DOI] [PubMed] [Google Scholar]
- 54.Ramanadham S, Gross R, Turk J. Arachidonic acid induces an increase in the cytosolic calcium concentration in single pancreatic islet beta cells. Biochem Biophys Res Commun. 1992;184:647–653. doi: 10.1016/0006-291x(92)90638-2. [DOI] [PubMed] [Google Scholar]
- 55.Ramanadham S, Hsu FF, Bohrer A, Ma Z, Turk J. Studies of the role of group VI phospholipase A2 (iPLA2) in fatty acid incorporation, phospholipid remodeling, lysophosphatidylcholine generation, and secretagogue-induced arachidonic acid release in pancreatic islets and insulinoma cells. J Biol Chem. 1999;274:13915–13927. doi: 10.1074/jbc.274.20.13915. [DOI] [PubMed] [Google Scholar]
- 56.Ramanadham S, Hsu FF, Bohrer A, Nowatzke W, Ma Z, Turk J. Electrospray ionization mass spectrometric analyses of phospholipids from rat and human pancreatic islets and subcellular membranes. Comparison to other tissues and implications for membrane fusion in insulin exocytosis. Biochemistry. 1998;37:4533–456. doi: 10.1021/bi9722507. [DOI] [PubMed] [Google Scholar]
- 57.Ramanadham S, Ma Z, Arita H, Zhang S, Turk J. Type IB secretory phospholipase A2 is contained in the insulin secretory granules of pancreatic islet beta cells and is cosecreted with insulin upon stimulation of islets with glucose. Biochim Biophys Acta. 1998;1390:301–312. doi: 10.1016/s0005-2760(97)00189-6. [DOI] [PubMed] [Google Scholar]
- 58.Ravier MA, Eto K, Jonkers FC, Nenquin M, Kadowaki T, Henquin JC. The oscillatory behavior of pancreatic islets from mice with mitochondrial glycerol-3-phosphate dehydrogenase knockout. J Biol Chem. 2000;275:1587–1593. doi: 10.1074/jbc.275.3.1587. [DOI] [PubMed] [Google Scholar]
- 59.Robertson RP. Islet transplantation as a treatment for diabetes. A work in progress. N Engl J Med. 2004;350:694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 60.Sato Y, Henquin JC. The K+-ATP channel-independent pathway of regulation of insulin secretion by glucose. In search of the underlying mechanism. Diabetes. 1998;47:1713–1721. doi: 10.2337/diabetes.47.11.1713. [DOI] [PubMed] [Google Scholar]
- 61.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–42. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 62.Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type I diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 63.Simonsson E, Karlsson S, Ahren B. Ca2+-independent phospholipase A2 contributes to the insulinotropic action of cholecystokinin-8 in rat islets. Dissociation from the mechanism of carbachol. Diabetes. 1998;47:1436–1443. doi: 10.2337/diabetes.47.9.1436. [DOI] [PubMed] [Google Scholar]
- 64.Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 65.Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nature Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- 66.Song K, Zhang X, Zhao C, Ang NT, Ma ZA. Inhibition of Ca2+-independent phospholipase A2 results in insufficient insulin secretion and impaired glucose tolerance. Mol Endocrinol. 2005;19:504–515. doi: 10.1210/me.2004-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang J, Kriz RW, Wolfman N, Shaffer M, Seehra J, Jones SS. A novel cytosolic calcium-independent phospholipase A2 contains eight ankyrin motifs. J Biol Chem. 1997;272:8567–8575. doi: 10.1074/jbc.272.13.8567. [DOI] [PubMed] [Google Scholar]
- 68.Thams P, Capito K. Inhibition of glucose-induced insulin secretion by the diacylglycerol lipase inhibitor RHC80267 and the phospholipase A2 inhibitor ACA through stimulation of K+ permeability without diminution by exogenous arachidonic acid. Biochem Pharm. 1997;53:1077–1086. doi: 10.1016/s0006-2952(96)00813-1. [DOI] [PubMed] [Google Scholar]
- 69.Tran VV, Chen G, Newgard CB, Hohmeier HE. Discrete and complementary mechanisms of protection of β-cells against cytokine-induced and oxidative damage achieved by bcl-2 overexpression and a cytokine selection strategy. Diabetes. 2003;52:14232–1432. doi: 10.2337/diabetes.52.6.1423. [DOI] [PubMed] [Google Scholar]
- 70.Turk J, Gross R, Ramanadham S. Amplification of insulin secretion by lipid messengers. Diabetes. 1993;42:367–374. doi: 10.2337/diab.42.3.367. [DOI] [PubMed] [Google Scholar]
- 71.Underwood KW, Song C, Kriz RW, Chang XJ, Knopf JL, Lin LL. A novel calcium-independent phospholipase A2, cPLA2-gamma, that is prenylated and contains homology to cPLA2. J Biol Chem. 1998;273:21926–21932. doi: 10.1074/jbc.273.34.21926. [DOI] [PubMed] [Google Scholar]
- 72.Vacher P, McKenzie J, Dufy B. Arachidonic acid affects membrane ionic conductances of GH3 pituitary cells. Am J Physiol. 1989;257:E203–E211. doi: 10.1152/ajpendo.1989.257.2.E203. [DOI] [PubMed] [Google Scholar]
- 73.van Tienhoven M, Atkins J, Li Y, Glynn P. Human neuropathy target esterase catalyzes hydrolysis of membrane lipids. J Biol Chem. 2002;277:20942–20948. doi: 10.1074/jbc.M200330200. [DOI] [PubMed] [Google Scholar]
- 74.Wolf BA, Turk J, Sherman WR, McDaniel ML. Intracellular Ca2+ mobilization by arachidonic acid. Comparison with myo-inositol 1,4,5-trisphosphate in isolated pancreatic islets. J Biol Chem. 1986;261:3501–3511. [PubMed] [Google Scholar]
- 75.Wolf BA, Pasquale SM, Turk J. Free fatty acid accumulation in secretagogue-stimulated pancreatic islets and effects of arachidonate on depolarization-induced insulin secretion. Biochemistry. 1991;30:6372–6379. doi: 10.1021/bi00240a004. [DOI] [PubMed] [Google Scholar]
- 76.Wollheim CB. Beta cell mitochondria in the regulation of insulin secretion: A new culprit in type II diabetes. Diabetologia. 2000;43:265–277. doi: 10.1007/s001250050044. [DOI] [PubMed] [Google Scholar]
- 77.Yajima H, Komatsu M, Schermerhorn T, Aizawa T, Kaneko T, Nagai M, Sharp GWG, Hashizume K. cAMP enhances insulin secretion by an action on the ATP-sensitive K+ channel-independent pathway of glucose signaling in rat pancreatic islets. Diabetes. 1999;48:1006–1012. doi: 10.2337/diabetes.48.5.1006. [DOI] [PubMed] [Google Scholar]


