Abstract
In the pulmonary vasculature, cGMP concentrations are regulated in part by a cGMP-dependent phosphodiesterase, PDE5. Infants with persistent pulmonary hypertension of the newborn (PPHN) are often mechanically ventilated with high oxygen concentrations. The effects of hyperoxia on the developing pulmonary vasculature and PDE5 are largely unknown. Here, we demonstrate that exposure of fetal pulmonary artery smooth muscle cells (FPASMC) to high levels of oxygen for 24 hours leads to decreased responsiveness to exogenous nitric oxide (NO), as determined by a decreased intracellular cGMP response, increased PDE5 mRNA and protein expression, as well as increased PDE5 cGMP-hydrolytic activity. We demonstrate that inhibition of PDE5 activity with sildenafil partially rescues cGMP responsiveness to exogenous NO. In FPASMC, hyperoxia leads to increased oxidative stress without increasing cell death. Treatment of normoxic FPASMC with H2O2 is sufficient to induce PDE5 expression and activity, suggesting that reactive oxygen species (ROS) mediate the effects of hyperoxia in FPASMC. In support of this mechanism, a chemical antioxidant, N-acetyl-cysteine, is sufficient to block the hyperoxia-mediated increase in PDE5 expression and activity and rescue cGMP responsiveness to exogenous NO. Finally, ventilation of healthy neonatal sheep with 100% O2 for 24 hours leads to increased PDE5 protein expression in the resistance pulmonary arteries and increased PDE5 activity in whole lung extracts. These data suggest that PDE5 expression and activity play a critical role in modulating neonatal pulmonary vascular tone in response to common clinical treatments for PPHN, such as oxygen and iNO.
Keywords: pulmonary circulation, persistent pulmonary hypertension of the newborn, cyclic GMP, phosphodiesterases
INTRODUCTION
Persistent pulmonary hypertension of the newborn (PPHN) is a clinical syndrome that results from a failure of the pulmonary vasculature to transition to extrauterine life. Infants typically present shortly after birth with respiratory distress and cyanosis, but a structurally normal heart. The estimated incidence of PPHN is 0.2% of live born term infants 1. PPHN is a manifestation of a heterogeneous group of disorders, and the response to therapy frequently depends on the underlying disease. Despite advances in clinical therapy, including high frequency ventilation, inhaled nitric oxide (iNO), and extracorporeal membrane oxygenation, there is still significant morbidity and mortality associated with this disease. Furthermore, iNO does not improve survival, and many infants do not respond or sustain their response to iNO, for reasons that are unclear 2, 3.
One factor that confounds the treatment of these infants is the use of supplemental oxygen. Oxygen has long been considered a vasodilator in the pulmonary circulation, and thus, 100% O2 is considered a first line therapy in infants with PPHN 1, 4. However, data from the adult ARDS literature 5, from the neonatal bronchopulmonary dysplasia (BPD) literature 6, and from the neonatal resuscitation literature 7, 8 suggest that exposure to high levels of oxygen may cause lasting lung injury, oxidative stress, and pulmonary vascular remodeling. Thus, the rationale for high inspired oxygen concentrations has come under question, although the mechanisms by which oxygen affects PPHN therapy are not known.
Phosphodiesterases (PDEs) constitute a superfamily of enzymes comprised of eleven different PDE families. Each family of PDEs has unique enzymatic properties, as well as specific tissue and cellular distributions. The prevalent PDE within the lung is PDE5, although PDE3 and PDE4 are also expressed in pulmonary tissue 9, 10. In the fetal lung, PDE5 activity and expression is highest in pulmonary arteries. In lambs, it decreases shortly after birth, and then rises again 4 to 7 days later, suggesting developmental regulation 11, 12. Moreover, as the primary enzyme responsible for regulating cGMP, PDE5 represents an important regulator of NO-mediated vascular relaxation.
Endogenous NO and cGMP are important in the normal pulmonary vascular transition after birth13, and PDE5 activity may be increased in a lamb model of PPHN.14–17. All of the studies that have examined PDE5 expression or activity in the pulmonary vasculature have been conducted in fetal animals exposed to low oxygen tension in utero 16, 17, or in fetal animals exposed to high levels of inspired oxygen for less than two hours14, 15. There is little data regarding the effects of longer hyperoxia exposures on PDE5 expression and activity in the pulmonary vasculature. Recently published data demonstrate that ventilation of newborn sheep with 100% oxygen increases contractile responses to norepinephrine7. Additionally, in a hyperoxic rat model of BPD, treatment with sildenafil, a PDE5 inhibitor, decreased pulmonary vascular resistance and improved lung angiogenesis, suggesting that PDE5 activity plays a critical role in mediating pulmonary vascular tone in the context of hyperoxia 18. We propose that increased PDE5 expression and activity in pulmonary vascular smooth muscle in response to hyperoxia may explain abnormal baseline vasoreactivity, as well as iNO resistance and rebound pulmonary hypertension after iNO withdrawal. A greater understanding of the mechanisms of PDE5 regulation in the pulmonary vasculature will be of critical importance to identifying improved therapies for infants with PPHN.
MATERIALS AND METHODS
Cell Culture
Primary cultures of FPASMC were prepared from intrapulmonary arteries as described in the expanded materials and methods section. FPASMC were treated in incubators with 21%O2-5%CO2, 50%O2-5%CO2, or 95%O2-5%CO2 ± DETANONOate (100 μM; Cayman Chemical, Ann Arbor, MI), ± sildenafil (100 nM, provided by Dr. Sharron Francis and Dr. Jackie Corbin), ± N-acetylcysteine (NAC, 500 μM, Sigma, St. Louis, MO); or with 21%O2-5%CO2 ± a single dose of H2O2 (50 μM; Sigma). FPASMC were harvested for analysis after 24 hours.
Cyclic GMP enzyme-linked immunoassay
Treated cells were lysed, and cGMP content was measured by EIA in triplicate according to the manufacturer’s protocol and as described in the expanded materials and methods (Cayman Chemical). Results are shown as pmol cGMP per mg total protein.
Real-time PCR
FPASMC were harvested for RNA utilizing the Aurum Total RNA Mini Kit (Biorad, Hercules, CA), and RNA was quantified using the Quant-it RiboGreen assay (Molecular Probes/Invitrogen, Carlsbad, CA). cDNA was prepared from total RNA utilizing the iScript cDNA Synthesis Kit (BioRad). Real-time PCR was performed using the iQ SYBR Green Supermix (BioRad) with the iCycler iQ real-time PCR detection system (BioRad) with 50 cycles of real-time data collection using 95°C for 10 seconds and 46.1°C for 45 seconds, followed by melt curve analysis to verify the presence of a single product. Primers were designed using commercially available software and are described in the expanded materials and methods. Relative PDE5 amounts were normalized to β-actin expression using the ΔΔCT method 19.
Western Blot
After treatment, FPASMC were harvested for total protein (40 μg) which was subjected to Western blot analysis as described in the expanded materials and methods section utilizing commercially available antibodies: PDE5 (BD Transduction, San Jose, CA) and β-actin (Sigma). Bands were visualized via chemiluminescence (Pierce, Rockford, IL), using a Digital Science Image Station (Kodak, Rochester, NY). Expression for PDE5 was normalized to β-actin expression.
Immunocytochemistry
FPASMC were plated onto collagen-treated glass coverslips and treated as above. After treatment, cells were fixed, permeabilized, and stained for immunocytochemistry with anti-PDE5 utilized in conjunction with a fluorescent secondary antibody (Molecular Probes/Invitrogen) as described in the expanded materials and methods section.
PDE5 Activity Assay
After treatment, FPASMC were harvested for total protein (5 μg), which was assayed for cGMP hydrolytic activity using a commercially available colorimetric cyclic nucleotide phosphodiesterase assay kit (Biomol, Plymouth Meeting, PA) as described in the expanded materials and methods section. Assays were performed with and without sildenafil (100 nM) to determine PDE5-specific cGMP-hydrolytic activity. Results are shown as PDE5-specific pmol cGMP hydrolyzed per mg total protein per minute.
Detection of Reactive Oxygen Species (ROS)
FPASMC were infected with 100 PFU/cell of a RoGFP adenoviral construct as described in the expanded materials and methods. RoGFP is a previously characterized ratiometric fluorescent probe sensitive to cellular oxidative stress 20. To create this probe, surface-exposed residues in green fluorescent protein (GFP) were replaced with cysteine residues capable of forming disulfide bonds. Assessment of fluorescence ratios therefore provide a real-time measure of cysteine thiol redox status in live cells 20. Ro-GFP infected FPASMC were exposed to 21%O2-5%CO2 or 95%O2-5%CO2 for 24 hours, and subsequently, their oxidative status was analyzed using multilaser flow cytometry as described in the expanded materials and methods.
Ventilation Protocols for Neonatal Sheep
The Laboratory Animal Care Committees at the State University of New York at Buffalo and at Northwestern University approved this study. Near term gestation pregnant ewes (134 days gestation) were anesthetized with pentothal and halothane, and lambs were delivered by cesarean section (n=5). Lambs were intubated and ventilated with 100% O2 for 24 hours as described in the expanded materials and methods section. Control groups included newborn lambs sacrificed approximately 24 hours after spontaneous delivery (one day nonventilated, n = 4) and fetal lambs delivered and sacrificed at 136 d gestation (fetal control, n=6). For tissue harvest, all lambs were anesthetized with pentothal sodium and killed by rapid exsanguinations through a direct cardiac puncture. The heart and lungs were removed en bloc and fifth-generation pulmonary arteries (inner diameters of 500 μm) were dissected and isolated 7. Total protein was isolated from lung and pulmonary artery (PA) tissue using the commercially available PARIS kit (Ambion, Austin, TX). Total protein was then subjected to Western blot analysis for PDE5 protein expression or to measurement of PDE5-specific cGMP hydrolytic activity as described above.
Immunohistochemistry
Tissue blocs were snap frozen, cut into thin sections (8 microns), and collected on glass slides. Sections were fixed with acetone and stained with anti-PDE5, in conjunction with a fluorescent secondary antibody (Molecular Probes/Invitrogen) as described in the expanded materials and methods section.
Statistics
Data are presented as mean ± SEM. Data were analyzed by paired t-test or one-way repeated measures ANOVA with Bonferroni’s multiple comparison test where appropriate, using Prism (GraphPad Software Inc., San Diego, CA). P<0.05 was considered statistically significant.
RESULTS
Hyperoxia Decreases cGMP Induced by Exogenous NO
As recent reports show that ventilation of sheep with 100% oxygen diminishes PA relaxations to an NO donor 7, we sought to determine the effect of hyperoxia on cGMP levels in FPASMC from resistance PA. FPASMC were exposed to 21%O2-5%CO2, 50%O2-5%CO2, or 95%O2-5%CO2 ± DETANONOate (100 μM), a long-acting NO donor, for 24 hours (Figure 1). Treatment with exogenous NO in normoxia led to a significant increase in cGMP levels (446±64% of control, p<0.001). However, treatment with exogenous NO in the context of hyperoxia blunted the increase in cGMP levels (p<0.001 for 21%O2+NO vs. 50%O2+NO and 21%O2+NO vs. 95%O2+NO). Thus, exposure to hyperoxia for 24 hours makes the FPASMC less responsive to exogenous NO, the primary clinical therapy for PPHN.
Figure 1. Exogenous NO Induces Less cGMP in FPASMC Exposed to Hyperoxia.
FPASMC were exposed to 21%O2-5%CO2, 50%O2-5%CO2, or 95%O2-5%CO2 ± DETANONOate (100μM) for 24 hours. Cells were assayed for cGMP by EIA, and cGMP was normalized for mg total protein. Data are shown as mean±SEM (n=6, read in triplicate; *p<0.05 vs. cells not treated with DETANONOate; #p<0.001 vs. 21%O2+NO).
Hyperoxia Increases PDE5 Expression in FPASMC
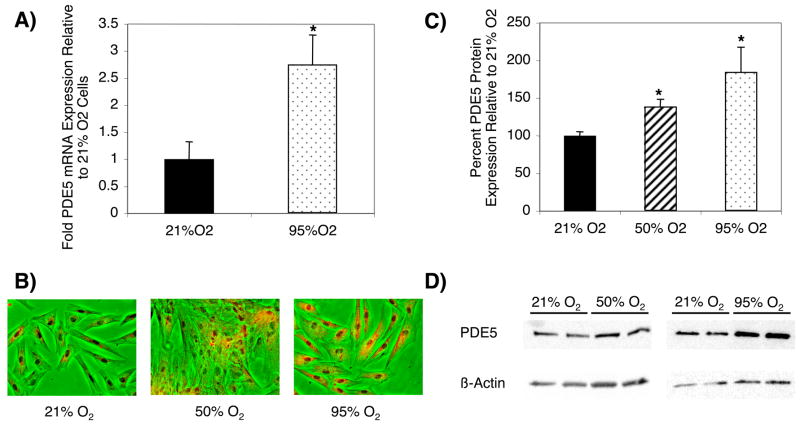
We hypothesized that the blunting of NO-mediated cGMP responsiveness in hyperoxia represents a PDE5-mediated desensitization. FPASMC were exposed to 21%O2-5%CO2, 50%O2-5%CO2, or 95%O2-5%CO2 for 24 hours. FPASMC exposed to hyperoxia showed increased PDE5 mRNA expression (2.7±0.5-fold; p<0.005, Figure 2A) and increased PDE5 protein expression, as determined by immunocytochemistry (Figure 2B) and Western blot (139±9% of control in 50%O2; 185±33% of control in 95%O2; p<0.001, Figures 2C and 2D). Thus, FPASMC exposure to hyperoxia for 24 hours leads to increased PDE5 mRNA and protein expression.
Figure 2. Hyperoxia Induces PDE5 Expression in FPASMC.
FPASMC were exposed to 21%O2-5%CO2, 50%O2-5%CO2, or 95%O2-5%CO2 for 24 hours. A) Cells were harvested for RNA, and RNA was subjected to real-time RT-PCR and normalized to β-actin using the ΔΔCt method. Data are shown as mean±SEM (n=9, read in duplicate, *p<0.005 vs. 21% O2). B) The panels show FPASMC exposed to various oxygen concentrations for 24 hours: left - 21%O2-5%CO2, middle - 50%O2-5%CO2, right - 95%O2-5%CO2. Images are phase contrast pictures overlaid with PDE5 immunofluorescence in red (20X). C) Cells were harvested for total protein and subjected to PDE5 Western Blot analysis, with β-actin normalization. Data are shown as mean±SEM (n=12 for 21% O2, n=9 for 50% O2, n=12 for 95% O2; *p<0.001 vs. 21%O2). D) Representative Western blots are shown for PDE5 and β-actin in FPASMC.
Hyperoxia Increases PDE5 Activity in FPASMC in a Dose-Dependent Fashion
The hypothesis that increased PDE5 expression leads to decreased cGMP responsiveness in FPASMC exposed to hyperoxia is only valid if increased PDE5 expression correlates with increased activity. One of the key events required for PDE5 activity is cGMP binding directly to PDE5, followed by phosphorylation by PKG 21–23. Hyperoxia for 24 hours led to significantly increased PDE5 phosphorylation in FPASMC (Online Data Supplement Figure 1). Similarly, hyperoxia for 24 hours led to significantly increased PDE5 activity in FPASMC (161±26% of control in 50%O2; 309±94% of control in 95%O2, p<0.05, Figure 3). Furthermore, the increase in PDE5 activity in 95%O2-5%CO2 is significantly greater than that seen in 50%O2-5%CO2 (p<0.05, Figure 3). Therefore, the hyperoxia-induced increase in PDE5 protein expression correlates with increased PDE5 phosphorylation and activity, suggesting that increased PDE5 expression and activity contribute to decreased intracellular cGMP responsiveness to exogenous NO in hyperoxia.
Figure 3. Hyperoxia Increases PDE5 Activity in FPASMC.
FPASMC were exposed to 21%O2-5%CO2, 50%O2-5%CO2, or 95%O2-5%CO2 for 24 hours and total protein was harvested. PDE5 specific activity was measured as the sildenafil-inhibitable fraction of total cGMP hydrolysis, normalized for total mg of protein. Data are shown as mean±SEM (n=8, read in duplicate; *p<0.05 vs. 21% O2; #p<0.05 vs. 50% O2).
Sildenafil Partially Rescues cGMP Responsiveness in FPASMC in Hyperoxia
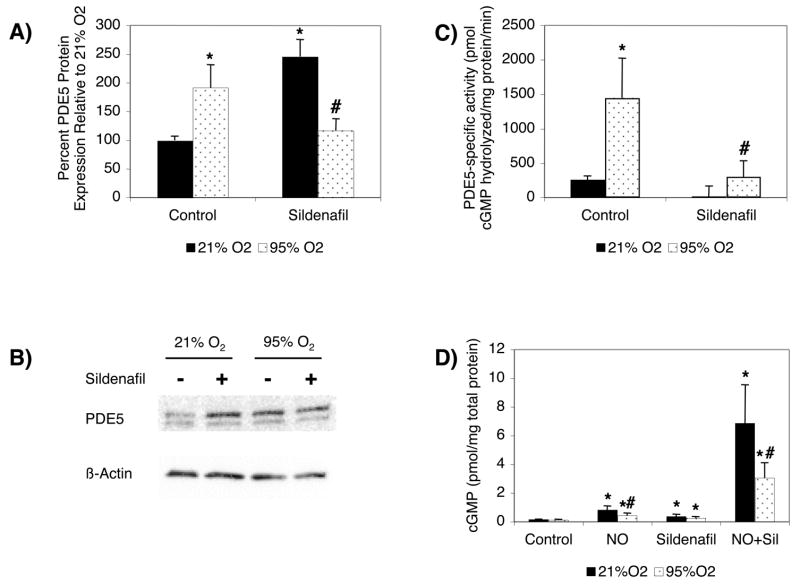
If increased PDE5 activity leads to decreased cGMP responsiveness in FPASMC hyperoxia-exposed, these effects should be blocked by a PDE5 inhibitor, sildenafil. FPASMC were exposed to 21%O2-5%CO2 or 95%O2-5%CO2 for 24 hours ± sildenafil (100 nM). In baseline conditions, addition of sildenafil led to a significant increase in PDE5 protein expression (246±30% of control, p<0.05 vs. 21% O2, Figure 4A and B), likely as a result of increased cGMP levels activating the PDE5 promoter, which has been previously described 24. Hyperoxia for 24 hours led to significantly increased PDE5 protein expression in FPASMC (192±40% of control, p<0.05 vs. 21% O2, Figures 4A and 4B), and in contrast to the effects of sildenafil in 21% O2, this effect is blocked with sildenafil (116±21% of control, p<0.05 vs. 95% O2, Figures 4A and 4B).
Figure 4. Sildenafil Blocks Hyperoxia-Induced PDE5 Expression and Activity and Partially Restores cGMP Responsiveness in FPASMC.
FPASMC were exposed to 21%O2-5%CO2 or 95%O2-5%CO2 for 24 hours ± sildenafil (100 nM). A) Cells were harvested for total protein and subjected to PDE5 Western Blot analysis, with β-actin normalization. Data are shown as mean±SEM (n=14, *p<0.05 vs. 21% O2; #p<0.05 vs. 95% O2). B) Representative Western blots are shown for PDE5 and β-actin in FPASMC. C) PDE5 specific activity was measured as the sildenafil-inhibitable fraction of total cGMP hydrolysis, normalized for total mg of protein. Data are shown as mean±SEM (n=12, read in duplicate; *p<0.05 vs. 21% O2; #p<0.05 vs. 95% O2). D) Cells were assayed for cGMP by EIA, and cGMP was normalized for mg total protein. Data are shown as mean±SEM (n=5, read in triplicate; *p<0.05 vs. untreated cells; +p<0.05 vs. 95% O2; #p<0.05 vs. comparably-treated 21% O2 cells).
As expected, sildenafil significantly blocks PDE5 activity in both 21% O2 and 95% O2 (p<0.05 vs. untreated cells, Figure 4C). As in Figure 1, treatment with exogenous NO in normoxia led to a significant increase in cGMP that was blunted in the context of hyperoxia (p<0.05 for 21%O2+NO vs. 95%O2+NO, Figure 4D). As expected, treatment with sildenafil led to a significant increase in cGMP levels at baseline in both 21% O2 and 95% O2 (p<0.05 vs. untreated cells, Figure 4D). Treatment of cells with exogenous NO and sildenafil led to a marked increase in cGMP in 21% O2 (46.2±18.2-fold relative to 21% O2, p<0.001 vs. 21% O2, Figure 4D) and in 95% O2 (26.2±8.8-fold relative to 95% O2, p<0.001 vs. 95% O2, Figure 4D), but the 95% O2 cGMP levels were still significantly less than those seen in 21% O2 (p<0.01, Figure 4D). Therefore, sildenafil is sufficient to partially rescue the effects of exogenous NO on FPASMC cGMP levels in hyperoxia.
ROS Increase PDE5 Expression and Activity in FPASMC
The effects of hyperoxia are likely mediated via ROS, and others have shown that ROS may play an important role in the underlying pathophysiology of PPHN 25–27. We utilized a ratiometric fluorescent ROS-sensitive probe, Ro-GFP, to measure the oxidative state of the cell, using dual laser flow cytometry 20. Exposure of FPASMC to hyperoxia for 24 hours significantly increased the basal oxidation state of cytosolic proteins within the cell, as measured by the percent maximal oxidation of the Ro-GFP probe (18.8 ± 4.6% in 21%O2-5%CO2 vs. 33.8±7.9% in 95%O2-5%CO2, p<0.05, Figure 5A), without increasing cell death (Online Data Supplement Figure 2). Thus, ROS likely represents a critical mediator for the hyperoxia effects on PDE5 expression and activity in FPASMC.
Figure 5. ROS Increase PDE5 Protein Expression and Activity in FPASMC.
A) FPASMC were infected with adenovirus containing the H2O2-sensitive Ro-GFP probe for 48 hours. Infected cells were exposed to 21%O2-5%CO2 or 95%O2-5%CO2 for 24 hours and then subjected to dual-excitation flow cytometry to quantitate the probe’s oxidative state. Data are expressed as % maximal probe oxidation ± SEM (n=6, *p<0.05 vs. 21% O2). B) FPASMC were treated with a single dose of H2O2 (50 μM) in 21%O2-5%CO2. Total protein was harvested 24 hours later and subjected to PDE5 Western Blot analysis, with β-actin normalization. Data are shown as mean±SEM (n=5, *p<0.05 vs. untreated cells). C) Representative Western blots are shown for PDE5 and β-actin in FPASMC. D) PDE5 specific activity was measured as the sildenafil-inhibitable fraction of total cGMP hydrolysis, normalized for total mg of protein. Data are shown as mean±SEM (n=5, read in duplicate, *p<0.05 vs. untreated cells).
We next sought to determine if treatment of FPASMC with exogenous oxidants in the context of normoxia was sufficient to replicate the hyperoxia effects on PDE5 expression, phosphorylation, and activity. Treatment of FPASMC with a single dose of H2O2 (50 μM) led to a significant increase in PDE5 protein expression (311 ± 87% vs. untreated, p<0.05, Figures 5B and 5C), PDE5 phosphorylation (Online Data Supplement Figure 3), and PDE5 activity (163±32% vs. untreated, p<0.05, Figure 5D). Similarly, pretreatment with sildenafil significantly blocked PDE5 activity in both untreated and H2O2-treated FPASMC (Online Data Supplement Figure 4). These data strongly suggest that ROS, in general, and H2O2 in particular, are sufficient to induce effects similar to hyperoxia on PDE5 expression and activity.
N-Acetyl-Cysteine (NAC) Rescues cGMP Responsiveness in FPASMC in Hyperoxia
The hypothesis that increased ROS leads to decreased cGMP responsiveness in FPASMC exposed to hyperoxia would suggest that these effects can be blocked by an antioxidant, NAC. FPASMC were exposed to 21%O2-5%CO2 or 95%O2-5%CO2 for 24 hours ± NAC (500 μM). Hyperoxia for 24 hours led to significantly increased PDE5 protein expression in FPASMC (304±100% of control, p<0.01 vs. 21% O2, Figures 6A and 6B), and this effect is completely blocked with NAC (106±46% of control, p<0.01 vs. 95% O2, Figures 6A and 6B).
Figure 6. NAC Blocks Hyperoxia-Induced PDE5 Expression and Activity and Restores cGMP Responsiveness in FPASMC.
FPASMC were exposed to 21%O2-5%CO2 or 95%O2-5%CO2 for 24 hours ± NAC (500 μM). A) Cells were harvested for total protein and subjected to PDE5 Western Blot analysis, with β-actin normalization. Data are shown as mean±SEM (n=11, *p<0.01 vs. 21% O2; #p<0.01 vs. 95% O2). B) Representative Western blots are shown for PDE5 and β-actin in FPASMC. C) PDE5 specific activity was measured as the sildenafil-inhibitable fraction of total cGMP hydrolysis, normalized for total mg of protein. Data are shown as mean±SEM (n=11, read in duplicate; *p<0.05 vs. 21% O2; #p<0.05 vs. 95% O2). D) Cells were assayed for cGMP by EIA, and cGMP was normalized for mg total protein. Data are shown as mean±SEM (n=7, read in triplicate; *p<0.05 vs. untreated cells; #p<0.01 vs. 21% O2+NO; + p<0.05 vs. 95% O2+NO).
Furthermore, NAC significantly blocks PDE5 activity in 95% O2 (p<0.05 vs. untreated cells, Figure 6C). Treatment with exogenous NO in normoxia led to a significant increase in cGMP levels that was blunted in the context of hyperoxia (p<0.01 for 21%O2+NO vs. 95%O2+NO, Figure 6D). Treatment of cells with exogenous NO and NAC in 95% O2 restored normal cGMP responsiveness relative to 95% O2 with exogenous NO alone (p<0.05 vs. 95% O2 + NO, Figure 6D). Therefore, NAC, an antioxidant, is sufficient to rescue the effects of exogenous NO on cGMP levels in FPASMC in hyperoxia.
Ventilation of Healthy Neonatal Sheep with 100% O2 For 24 Hours Increases PDE5 Expression and Activity
To verify that the effects of hyperoxia on PDE5 were not confined to isolated FPASMCs, we mechanically ventilated healthy near-term sheep with 100% O2 for 24 hours and then sacrificed the lambs to harvest lung and resistance PA tissue. Ventilation with 100% O2 for 24 hours led to increased PDE5 protein expression in resistance pulmonary arteries as compared to both control nonventilated fetal lambs and one-day non-ventilated lambs (1.8±0.4-fold vs. fetal lambs, 3.2±0.7-fold vs. one-day lambs, p<0.05, Figure 7A). There was a trend toward decreased PDE5 protein expression in one-day lambs versus fetal lambs, but this did not reach statistical significance. Similarly, ventilation with 100% O2 for 24 hours significantly increased lung PDE5 activity relative to fetal and one-day lambs (3.9±0.9-fold vs. fetal lambs, 2.6±0.6-fold vs. one-day lambs, p<0.05, Figure 7B). Immunohistochemistry confirmed that ventilation with 100% O2 for 24 hours leads to increased PDE5 expression, which is primarily seen in the smooth muscle of the resistance PA (Figure 7C). Thus, the increase in PDE5 expression and activity in neonatal sheep ventilated with 100% O2 confirms that our findings are physiologically valid and may explain the impaired vasoreactivity previously reported in sheep after exposure to 100% O2 7.
Figure 7. Ventilation of Healthy Neonatal Sheep with 100% O2 Induces PDE5 Protein Expression and Activity.
Ovine lung parenchyma and resistance pulmonary arteries (PA) from healthy lambs ventilated with 100% O2 were harvested after 24 hours (n=5), and compared to both fetal lambs (Fetus, n=6) and healthy one-day non-ventilated lambs (1DNV, n=4). A) PA PDE5 protein expression was analyzed via Western blot, with β-actin normalization. Data are shown as mean±SEM (*p<0.05 vs. fetus, # p<0.05 vs. 1DNV). B) Lung PDE5 specific activity was measured as the sildenafil-inhibitable fraction of total cGMP hydrolysis, normalized for total mg of protein. Data are shown as mean±SEM (*p<0.05 vs. fetus, #p<0.05 vs. 1DNV). C) PDE5 expression is localized within the PA smooth muscle and nearby airways. The left column shows phase contrast images (10X) of frozen lamb lung sections. The right column shows the corresponding sections stained for PDE5, with immunofluorescence shown as red.
DISCUSSION
Our results demonstrate that in primary cultures of FPASMC exposed to hyperoxia, there is decreased cGMP responsiveness to exogenous NO, which is an important therapeutic vasodilator for infants with pulmonary hypertension. We propose that this is due, in part, to a PDE5-mediated increase in cGMP degradation. In support of that theory, we demonstrate that exposure to hyperoxia for 24 hours increases PDE5 mRNA and protein expression as well as phosphorylation and activity. Inhibition of the hyperoxia-induced PDE5 activity with sildenafil, a PDE5 inhibitor, was sufficient to partially rescue the cGMP response to exogenous NO, indicating that PDE5 is a critical regulator of cGMP in the context of hyperoxia.
As might be expected, exposure to hyperoxia for 24 hours leads to increased oxidative stress with the FPASMC, as detected using a novel ratiometric fluorescent probe sensitive to changes in oxidation. This probe represents a valuable new tool to investigate the oxidation state of cells without relying on fluorescent dyes with their accompanying technical limitations. Utilizing this tool, it is not surprising that 24 hours of hyperoxia increased the oxidation of cellular proteins. However, this oxidative stress was not sufficiently severe to cause an increase in cell death (Online Data Supplement Figure 2). The FPASMC show significant changes in cellular signaling pathways during hyperoxia, as evidenced by the increase in PDE5 expression, phosphorylation, and activity. As such, we hypothesized that ROS may serve as critical mediators in the crosstalk between oxygen and PDE5. In support of that, a single dose of exogenous H2O2 was sufficient to induce long-lasting changes in PDE5 expression, phosphorylation, and activity, which mirror those seen after exposure to hyperoxia. Similarly, the changes in PDE5 expression and activity as well as the decreased cGMP responsiveness in hyperoxia are all reversed with a chemical antioxidant, NAC. This confirms that ROS, in general, and H2O2, in particular, are sufficient to induce significant changes in PDE5 expression and activity, which negatively impact the pulmonary vasculature. Finally, we demonstrate that these results are recapitulated in an in vivo model, where ventilation of healthy neonatal sheep with 100% O2 for 24 hours produced an increase in PDE5 expression and activity.
PDE5 is the key enzyme for cGMP hydrolysis in the pulmonary vasculature. The observation that the response to exogenous NO in FPASMC is diminished in hyperoxia indicates that the increase in PDE5 expression and activity is biologically significant (Figure 1). However, since sildenafil only partially rescues the cGMP response to exogenous NO (Figure 4D), other key signaling mediators may also be impacted by hyperoxia in fetal PASMC. Soluble guanylyl cyclase (sGC) is the primary enzyme responsible for cGMP production. sGC protein expression is decreased in nonventilated fetal lambs with PPHN, suggesting that it may also be an important regulator of neonatal vascular tone 28. Recent studies suggest that sGC protein expression in FPASMC is decreased by ROS, specifically H2O2 29. Little is known regarding the impact of hyperoxia on sGC, although it is possible that changes in sGC further contribute to the decreased cGMP responsiveness to exogenous NO after exposure to hyperoxia. Similarly, ANP and CNP, signal through their respective natriuretic peptide receptors, NPR-A and NPR-B, to produce vasorelaxation. These receptors are coupled to particulate guanylate cyclase (pGC) activity, which can thus impact cGMP concentrations and thereby affect relaxation of the pulmonary arteries. While these molecules play a role in the intact vessels and animals 30, they are not likely to influence the changes seen in the isolated fetal PASMC since there are no adjacent endothelial cells to produce the natriuretic peptides. Thus, while other signaling mediators such as sGC may contribute, the present study demonstrates that PDE5 represents one critical factor for the regulation of intracellular cGMP levels in the context of hyperoxia.
Finally, the mechanism for the interplay between oxygen, ROS, and PDE5 is not fully understood. In corpus cavernosum, the regulation of PDE5 expression is largely cGMP-dependent 31. However, this would seem an unlikely mediator between oxygen and PDE5, given our finding of impaired cGMP signaling in FPASMC under hyperoxic conditions. Promoter analysis shows both an Sp-1 and an AP-1 site in the human PDE5 promoter region31. Both of these transcription factors have previously been shown to be redox-sensitive and may represent potential downstream targets of the ROS-mediated signaling which impacts PDE5 32. In contrast, studies of PDE5 activity in gastric and aortic smooth muscle demonstrate that increased cGMP causes activation of PKG, which in turn phosphorylates and activates PDE5 21, 22. Consistent with these data, we demonstrate that oxygen leads to increased PDE5 phosphorylation, utilizing an antibody raised to a previously characterized PKG site (Online Data Supplement Figure 1) and increased PDE5 activity (Figure 3), suggesting that a likely mechanism for PDE5 activation by oxygen is via PKG-mediated phosphorylation (Figure 8). Unlike PDE5, there is a growing body of literature that PKG is regulated by cellular redox status within aortic smooth muscle as well as the pulmonary vasculature, confirming that it is a likely candidate for the intermediary between ROS and PDE5 33, 34.
Figure 8. Model for the Effects of Hyperoxia on PDE5 in FPASMC.
Exposure to hyperoxia leads to increased ROS production, which increases PDE5 expression and activity, likely in a PKG-mediated fashion. The increased PDE5 activity ultimately decreases the amount of available cGMP, leading to impaired vasorelaxation to exogenous NO.
In conclusion, we have demonstrated the ability of FPASMC to respond to exogenous NO, a critical therapeutic vasodilator, is impaired when FPASMC are exposed to hyperoxia for 24 hours. We have further demonstrated that hyperoxia is sufficient to induce PDE5 expression, phosphorylation, and activity in FPASMC, which is likely one critical mediator of impaired cGMP responsiveness under these conditions. These findings are novel, as they represent the first description of redox-regulation of PDE5. PDE5 appears to be exquisitely sensitive to redox regulation, as even a short burst of oxidative stress such as a single dose of H2O2 is sufficient to produce changes in PDE5. These studies provide key insights into the basic regulation of PDE5 in the pulmonary vasculature and its crosstalk with oxygen, as well as provide a potential mechanism for the impaired vasoreactivity seen in the oxygen-exposed pulmonary vasculature.
Supplementary Material
Acknowledgments
The authors thank Robert D. Guzy for Ro-GFP protocol assistance, Gabriel Loor for cell death assay assistance, and Sharron H. Francis and Jackie D. Corbin for providing the sildenafil utilized here.
SOURCES OF FUNDING:
This work was supported by NIH grants: K12 HD052902 and K08 HL086715 to K.N.F., R01 HL079650 and R01 HL35440 to P.T.S., and R01 HL54705 to R.H.S.
Footnotes
Subject Code: [18] Pulmonary Circulation and Disease, [138] Cell signaling/signal transduction, [91] Oxidant Stress, [156] Pulmonary Biology and Circulation
Publisher's Disclaimer: Disclosure: This is an un-copyedited author manuscript accepted for publication in Circulation Research, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://circres.ahajournals.org/. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
DISCLOSURES: None.
References
- 1.Farrow KN, Fliman P, Steinhorn RH. The diseases treated with ECMO: focus on PPHN. Semin Perinatol. 2005;29:8–14. doi: 10.1053/j.semperi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Group NINOS. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. New Eng J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JD, Fineman JR, Morin FC, 3rd, Shaul PW, Rimar S, Schreiber MD, Polin RA, Zwass MS, Zayek MM, Gross I, Heymann MA, Zapol WM, Thusu KG, Zellers TM, Wylam ME, Zaslavsky A. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. New Eng J Med. 1997;336:605–610. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- 4.Morin FC, 3rd, Egan EA, Ferguson W, Lundgren CE. Development of pulmonary vascular response to oxygen. Am J Physiol. 1988;254:H542–H546. doi: 10.1152/ajpheart.1988.254.3.H542. [DOI] [PubMed] [Google Scholar]
- 5.Sinclair SE, Altemeier WA, Matute-Bello G, Chi EY. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med. 2004;32:2496–2501. doi: 10.1097/01.ccm.0000148231.04642.8d. [DOI] [PubMed] [Google Scholar]
- 6.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 7.Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC, 3rd, Swartz DD, Kumar VH. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res. 2006;59:137–141. doi: 10.1203/01.pdr.0000191136.69142.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vento M, Asensi M, Sastre J, Garcia-Sala F, Pallardo FV, Vina J. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics. 2001;107:642–647. doi: 10.1542/peds.107.4.642. [DOI] [PubMed] [Google Scholar]
- 9.Maclean MR, Johnston ED, McCulloch KM, Pooley L, Houslay MD, Sweeney G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J Pharmacol Exp Ther. 1997;283:619–624. [PubMed] [Google Scholar]
- 10.Pauvert O, Salvail D, Rousseau E, Lugnier C, Marthan R, Savineau JP. Characterisation of cyclic nucleotide phosphodiesterase isoforms in the media layer of the main pulmonary artery. Biochem Pharmacol. 2002;63:1763–1772. doi: 10.1016/s0006-2952(02)00919-x. [DOI] [PubMed] [Google Scholar]
- 11.Hanson KA, Burns F, Rybalkin SD, Miller JW, Beavo J, Clarke WR. Developmental changes in lung cGMP phosphodiesterase-5 activity, protein, and message. Am J Respir Crit Care Med. 1998;158:279–288. doi: 10.1164/ajrccm.158.1.9711042. [DOI] [PubMed] [Google Scholar]
- 12.Okogbule-Wonodi AC, Ibe BO, Yue BW, Hsu S, Raj JU. Phosphodiesterase activity in intrapulmonary arteries and veins of perinatal lambs. Mol Genet Metab. 1998;65:229–237. doi: 10.1006/mgme.1998.2756. [DOI] [PubMed] [Google Scholar]
- 13.Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol. 1990;259:H1921–H1927. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- 14.Dukarm RC, Morin FC, 3rd, Russell JA, Steinhorn RH. Pulmonary and systemic effects of the phosphodiesterase inhibitor dipyridamole in newborn lambs with persistent pulmonary hypertension. Pediatr Res. 1998;44:831–837. doi: 10.1203/00006450-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Dukarm RC, Russell JA, Morin FC, 3rd, Perry BJ, Steinhorn RH. The cGMP-specific phosphodiesterase inhibitor E4021 dilates the pulmonary circulation. Am J Respir Crit Care Med. 1999;160:858–865. doi: 10.1164/ajrccm.160.3.9809120. [DOI] [PubMed] [Google Scholar]
- 16.Hanson KA, Ziegler JW, Rybalkin SD, Miller JW, Abman SH, Clarke WR. Chronic pulmonary hypertension increases fetal lung cGMP phosphodiesterase activity. Am J Physiol. 1998;275:L931–941. doi: 10.1152/ajplung.1998.275.5.L931. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler JW, Ivy DD, Fox JJ, Kinsella JP, Clarke WR, Abman SH. Dipyridamole potentiates pulmonary vasodilation induced by acetylcholine and nitric oxide in the ovine fetus. Am J Respir Crit Care Med. 1998;157:1104–1110. doi: 10.1164/ajrccm.157.4.9701121. [DOI] [PubMed] [Google Scholar]
- 18.Ladha F, Bonnet S, Eaton F, Hashimoto K, Korbutt G, Thebaud B. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med. 2005;172:750–756. doi: 10.1164/rccm.200503-510OC. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem. 2004;279:13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 21.Murthy KS. Activation of phosphodiesterase 5 and inhibition of guanylate cyclase by cGMP-dependent protein kinase in smooth muscle. Biochem J. 2001;360:199–208. doi: 10.1042/0264-6021:3600199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rybalkin SD, Rybalkina IG, Feil R, Hofmann F, Beavo JA. Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J Biol Chem. 2002;277:3310–3317. doi: 10.1074/jbc.M106562200. [DOI] [PubMed] [Google Scholar]
- 23.Turko IV, Francis SH, Corbin JD. Binding of cGMP to both allosteric sites of cGMP-binding cGMP-specific phosphodiesterase (PDE5) is required for its phosphorylation. Biochem J. 1998;329:505–510. doi: 10.1042/bj3290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin G, Xin ZC, Lue TF, Lin CS. Up and down-regulation of phosphodiesterase-5 as related to tachyphylaxis and priapism. J Urol. 2003;170:S15–S19. doi: 10.1097/01.ju.0000075500.11519.e8. [DOI] [PubMed] [Google Scholar]
- 25.Wedgwood S, McMullan M, Bekker JM, Fineman JR, Black SM. Role for endothelin-1-induced superoxide and peroxynitrite production in rebound pulmonary hypertension associated with inhaled nitric oxide therapy. Circ Res. 2001;89:357–364. doi: 10.1161/hh1601.094983. [DOI] [PubMed] [Google Scholar]
- 26.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res. 2003;92:683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 27.Wedgwood S, Black SM. Role of reactive oxygen species in vascular remodeling associated with pulmonary hypertension. Antioxid Redox Signal. 2003;5:759–769. doi: 10.1089/152308603770380061. [DOI] [PubMed] [Google Scholar]
- 28.Tzao C, Nickerson PA, Russell JA, Gugino SF, Steinhorn RH. Pulmonary hypertension alters soluble guanylate cyclase activity and expression in pulmonary arteries isolated from fetal lambs. Pediatr Pulmonol. 2001;31:97–105. doi: 10.1002/1099-0496(200102)31:2<97::aid-ppul1016>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 29.Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, Black SM. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2005;289:L660–666. doi: 10.1152/ajplung.00369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lakshminrusimha S, D’Angelis CA, Russell JA, Nielsen LC, Gugino SF, Nickerson PA, Steinhorn RH. C-type natriuretic peptide system in fetal ovine pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol. 2001;281:L361–368. doi: 10.1152/ajplung.2001.281.2.L361. [DOI] [PubMed] [Google Scholar]
- 31.Lin CS, Chow S, Lau A, Tu R, Lue TF. Identification and regulation of human PDE5A gene promoter. Biochem Biophys Res Comm. 2001;280:684–692. doi: 10.1006/bbrc.2000.4220. [DOI] [PubMed] [Google Scholar]
- 32.Liu MY, Eyries M, Zhang C, Santiago FS, Khachigian LM. Inducible platelet-derived growth factor D-chain expression by angiotensin II and hydrogen peroxide involves transcriptional regulation by Ets-1 and Sp1. Blood. 2006;107:2322–9. doi: 10.1182/blood-2005-06-2377. [DOI] [PubMed] [Google Scholar]
- 33.Sander F, Gao Y, Raj JU. Hypoxia down-regulates cyclic guanidine monophosphate-dependent protein kinase in fetal pulmonary vascular smooth muscle cell through generation of reactive oxygen species and promotes development of pulmonary hypertension. Chest. 2005;128:577S–578S. doi: 10.1378/chest.128.6_suppl.577S-a. [DOI] [PubMed] [Google Scholar]
- 34.Schulz E, Tsilimingas N, Rinze R, Reiter B, Wendt M, Oelze M, Woelken-Weckmuller S, Walter U, Reichenspurner H, Meinertz T, Munzel T. Functional and biochemical analysis of endothelial (dys)function and NO/cGMP signaling in human blood vessels with and without nitroglycerin pretreatment. Circulation. 2002;105:1170–1175. doi: 10.1161/hc1002.105186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.