Abstract
Rationale
3,4-Methylenedioxymethamphetamine (MDMA, ecstasy) is frequently used in hot environments, such as rave parties. Studies in laboratory animals have shown that ambient temperature can alter the behavioral and neurochemical effects of MDMA.
Objective
To examine the influence of ambient temperature on the relative reinforcing strength of MDMA and reinstatement of behavior previously maintained by MDMA.
Methods
The effects of cool (18°C), room (24°C) and warm (31°C) temperatures were examined when MDMA was available under a concurrent fixed-ratio 30 schedule of MDMA (saline, 0.03–0.3 mg/kg/inj) and food choice in rhesus monkeys (n=5). During saline substitutions, the effect of noncontingent MDMA (0.03–0.3 mg/kg) on response allocation was examined at each ambient temperature.
Results
At room temperature, MDMA choice increased as a function of dose, such that food was preferred over a low MDMA dose (0.03 mg/kg/inj) whereas higher doses were preferred over food. Elevating the ambient temperature significantly increased the relative reinforcing strength of 0.03 mg/kg/inj MDMA and lowering the ambient temperature significantly attenuated choice of 0.1 mg/kg/inj MDMA. Noncontingent injections of MDMA administered before a session in which saline was the alternative to food dose-dependently increased injection-lever responding; this effect was not influenced by ambient temperature.
Conclusions
These results suggest that ambient temperature can affect the relative reinforcing strength of MDMA, but not MDMA-induced reinstatement. Furthermore, these results suggest environmental strategies for decreasing the reinforcing strength of MDMA.
Keywords: Ambient Temperature, Drug Choice, MDMA, Reinstatement, Rhesus monkey, Self-Administration
INTRODUCTION
Recent epidemiological data estimate that lifetime use of ecstasy (3,4-methylenedioxymethamphetamine, MDMA) by people ages 19–28 has remained high since 2000 with approximately 15% of respondents reporting use in 2005 (Johnston et al. 2006). One environment where ecstasy use is common is “rave” or dance parties (Irvine et al. 2006) which are characterized by marathon, vigorous group dancing, laser light displays, hypnotic electronic music and use of various illicit drugs (Schwartz and Miller 1997; Weir 2003). These overcrowded, warm environmental conditions of a “rave” may enhance the acute reinforcing and subjective effects of ecstasy, and may increase susceptibility to psychobiological sequelae (Parrott 2004). Thus, the environmental context of ecstasy use may have a profound impact on its behavioral effects.
In subjective reports, MDMA users have noted that “heat was a causal factor in initiating and maintaining its effects” (Bedi and Redman 2006). The impact of ambient temperature on the reinforcing effects of MDMA has been examined in one preclinical study. Cornish et al. (2003) reported that rats responding under a fixed-ratio (FR) schedule of reinforcement earned a greater number of MDMA injections at an elevated ambient temperature. One possible neurochemical mechanism for such an outcome may be enhanced increases in extracellular levels of dopamine (DA) in brain areas that mediate reinforcement such as the nucleus accumbens (Di Chiara and Imperato 1988). Consistent with behavioral data, O’Shea et al. (2005) found that elevating the ambient temperature potentiated nucleus accumbens extracellular DA release induced by MDMA. Others have shown that ambient temperature may impact MDMA-induced neurotoxicity. For example, Malberg and Seiden (1998) found markers of neurotoxicity in rat frontal cortex, hippocampus and striatum when MDMA was given at 26–30°C ambient temperature, but at temperatures of 20–24°C neurotoxic markers were not seen in these brain regions. DA appears to have an important role in this effect (Sprague et al. 1998). Also, the effects of MDMA on body temperature appear to be dependent on the ambient temperature in rats (Gordon et al. 1991; Malberg and Seiden 1998) and monkeys (Banks et al. 2007a; Von Huben et al. 2007). Preclinical studies have not investigated interactions between ambient temperature and MDMA reinforcement in nonhuman primates. Furthermore, the effects of cool ambient temperatures on MDMA reinforcement have not been examined in any species. Such an environmental manipulation might be expected to reduce the reinforcing effects of MDMA. Thus, the goal of the present study was to examine how alterations in ambient temperature influence the abuse-related effects of MDMA in monkeys. To investigate this relationship, we examined the effects of ambient temperature in two paradigms: a concurrent choice procedure (Morgan and Nader 2000; Negus 2003) and reinstatement of MDMA choice (Paronis et al. 2002; Czoty et al. 2005).
Concurrent schedules of reinforcement provide a measure of relative reinforcing strength and possess face validity to human drug use which occurs in the context of alternative reinforcers (cf. Woolverton and Nader 1990; Higgins 1997). We hypothesized that MDMA choice would be increased by elevating the ambient temperature and decreased by lowering the ambient temperature. Few studies have examined MDMA-induced reinstatement (Morley et al. 2004; Daza-Losada et al. 2007) and none have examined the ability of MDMA to reinstate responding previously maintained by MDMA. Reinstatement is often used as a model of relapse or craving (for review, see Shaham et al. 2003); however, recent studies have suggested that the discriminative stimulus effects of drugs have a prominent role in increasing extinguished responding (Odum and Shahan 2004; Cador et al. 2006; Banks et al. 2007b). In contrast to extinguishing responding under a single operant conditioning schedule, substituting saline for MDMA under a concurrent schedule of reinforcement results in shifting response allocation from the MDMA-associated lever to the food-associated lever rather than a cessation of responding. This method may be more analogous to the human condition due to the presence of alternative reinforcers (Gasior et al. 2004; Czoty et al. 2005). We hypothesized that lowering the ambient temperature would attenuate, while elevating the ambient temperature would potentiate the ability of noncontingent MDMA to reinstate MDMA choice.
METHODS
Subjects
Five adult male rhesus monkeys (Macaca mulatta) served as subjects. Each monkey was surgically prepared with an indwelling intravenous catheter into the femoral vein and a subcutaneous vascular access port (Access Technologies, Skokie, IL) as previously described (Martelle et al. 2007). Four monkeys had cocaine self-administration histories (Martelle et al. 2007) whereas one was drug-naïve at the start of the study. Each monkey was fitted with a nylon collar (Primate Products, Redwood City, CA) and trained to sit calmly in a standard primate restraint chair (Primate Products) using a specially designed stainless steel pole (Primate Products) that attached to the collar. Monkeys were weighed weekly and fed enough food daily (LabDiet #5038 Monkey Chow and fresh fruit) to maintain body weights at approximately 90–95% of free-feeding levels. Monkeys were individually housed in stainless steel cages with water available ad libitum and visual and auditory contact with each other. The facilities for housing and care of the animals are accredited by the American Association for the Assessment and Accreditation of Laboratory Animal Care. All procedures were approved by the Animal Care and Use Committee (ACUC) of Wake Forest University and performed in accordance with established practices as described in the National Institutes of Health Guide for Care and Use of Laboratory Animals, except the elevation of ambient temperature that was outside the Guide recommended range. During these experiments, no monkey had a rectal temperature above 41°C that required intervention or termination of the experiment. Rectal temperature was recorded, using a RET-1 thermometer attached to a TH-3 thermocouple (Physitemp, Clifton, NJ), immediately before the monkey was placed in the experimental chamber and at the end of the experimental session. Environmental enrichment was provided as outlined in the ACUC of Wake Forest University Nonhuman Primate Environmental Enrichment Plan.
Procedure
Experimental sessions were conducted in ventilated and sound-attenuating experimental chambers (Med Associates, East Fairfield, VT) designed to accommodate a primate chair. An intelligence panel located on the chamber contained two retractable levers with three stimulus lights (green, amber, and red) located above each lever. The levers were positioned to be within easy reach of the monkey seated in the primate chair. One-gram banana-flavored food pellets (Bio-Serv, Frenchtown, NJ) were delivered into a food receptacle located between the levers on the intelligence panel. The skin over the subcutaneous port was cleaned with Betadine and 95% ethanol and the port was connected to a peristaltic infusion pump (Cole-Parmer Co., Chicago, IL) via a 22-gauge Huber point needle (Access Technologies) for delivering drug injections at a rate of approximately 1.5 ml/10 sec. At the beginning of each session the pump was operated for approximately 3 sec to fill the port and catheter with the concentration of MDMA or saline available for that session.
Subjects were trained initially to respond under an FR 30 schedule of food presentation, with each pellet delivery followed by a 30-sec timeout. During these sessions either the right or left lever was extended and only lights above that lever were illuminated. Sessions lasted until 30 pellets had been obtained or 60 min had elapsed whichever came first. When responding was reliably maintained and maximal food reinforcement was received for at least 5 days, concurrent exposure to MDMA and food began. The lever associated with MDMA was counterbalanced across monkeys, such that for three monkeys, completion of an FR 30 on the left lever activated the infusion pump, producing an injection of 0.1 mg/kg/inj MDMA, whereas responding on the right lever continued to be maintained by food presentation. For the other two monkeys, the reinforcers assigned to each lever were reversed. Initially, levers were presented individually for several sessions. Under the concurrent schedule of reinforcement, sessions began with illumination of the white light above both levers. Completion of 30 consecutive responses resulted in the white light being extinguished and illumination of the red light for 30 sec and delivery of the appropriate reinforcer; responses emitted on the alternate lever before the completion of an FR 30 reset the response requirement. Responding while the red light was illuminated had no schedule consequence. The session ended after 30 total reinforcers had been earned or 60 min had elapsed. Saline and several doses of MDMA (0.03–0.3 mg/kg/inj) were examined as the alternative to food. Each was available for at least five consecutive sessions and remained available until responding was deemed stable (percent injection-lever responding ± 20% of the mean of three consecutive sessions with no trend). Doses were tested in random order in each monkey. (±)MDMA HCl (National Institute on Drug Abuse, Bethesda, MD) was dissolved in sterile saline. Different doses were studied by changing the drug concentration.
In most cases, ambient temperature manipulations were made following determination of drug choice for a dose of MDMA at 24°C (room temperature). Following stable choice behavior, the experimental chamber temperature was either elevated to 31°C using a commercially available space heater placed outside one of the experimental chamber vent holes or lowered to 18°C using a commercially available air conditioner. There was a 10-min timeout at the start of the session to allow for acclimation to the ambient temperature. An indoor/outdoor thermometer was threaded through a hole in the experimental chamber and the digital readout was placed outside of the experimental chamber to monitor the ambient temperature without disrupting the operant behavioral session. The heater or air conditioner settings were adjusted to maintain the temperature within ±1°C according to the digital readout. If changing the ambient temperature altered choice of a dose of MDMA, then that dose was retested at 24°C to see if choice returned to baseline.
Saline substitutions occurred periodically while determining the MDMA dose-effect curves. When injection-lever responding was low (<20%) for three consecutive sessions, a noncontingent MDMA (0.03 – 0.3 mg/kg) injection was administered immediately before the session started. Each MDMA dose was tested once at all three ambient temperatures and at least one saline session occurred between noncontingent MDMA doses.
Data Analysis
Data were analyzed with repeated measures mixed linear regression using SAS Proc Mixed (Version 8.2, SAS, Cary, NC) with ambient temperature and MDMA dose as factors. For the self-administration experiments, the primary dependent variables examined were MDMA choice, defined as percent injection-lever responding (calculated by dividing the total number of MDMA-lever responses by the total responses on both levers), number of MDMA and food reinforcers earned per session and total session MDMA intake (mg/kg) at each ambient temperature. Data from the self-administration experiments are presented as the mean of the last 3 sessions where stability criteria were met. For the reinstatement experiments, the primary dependent variables were percent injection-lever responding, number of saline injections and food reinforcers earned per session. A significant interaction was followed by a post-hoc least squares means difference test for planned comparisons between each MDMA dose and saline within an ambient temperature. In the absence of a significant interaction, the interaction factor was removed from the model and the data reanalyzed with ambient temperature and MDMA dose entered as additive main effects. Differences were considered significant at the 95% level of confidence (p<0.05).
RESULTS
Effects of ambient temperature on MDMA choice under a concurrent schedule of MDMA and food availability
When MDMA injections and food pellets were concurrently available under an FR 30 schedule of reinforcement (Fig. 1A), a significant interaction between MDMA dose and ambient temperature (F5,18=4.40, p<0.05) was detected. At room temperature (24°C), the reinforcing strength of 0.03 mg/kg/inj MDMA relative to food was not significantly different from the relative reinforcing strength of saline compared to food, while higher MDMA doses (0.1 – 0.3 mg/kg/inj) resulted in primarily MDMA choice (p<0.0001). Elevating the ambient temperature (31°C) increased the relative reinforcing strength of MDMA compared to food pellets; injection-lever responding when 0.03 mg/kg/inj MDMA and food were available was significantly greater (t18= −2.58, p<0.05) than when saline was the alternative to food. Similar to room temperature, at 31°C both 0.1 and 0.3 mg/kg/inj MDMA resulted primarily in MDMA choice (p<0.05). At the cool ambient temperature, when compared to injection-lever responding when saline was the alternative to food, only the 0.3 mg/kg/inj dose of MDMA resulted in significantly greater injection-lever responding (t18=−5.61, p<0.0001). Individual differences were observed at 18°C, with 0.1 mg/kg/inj MDMA choice being abolished in three subjects and unaffected in two subjects. In two of the three subjects in which 18°C had an effect, returning to 24°C with 0.1 mg/kg/inj MDMA still available did not restore choice to baseline levels (data not shown).
Figure 1.
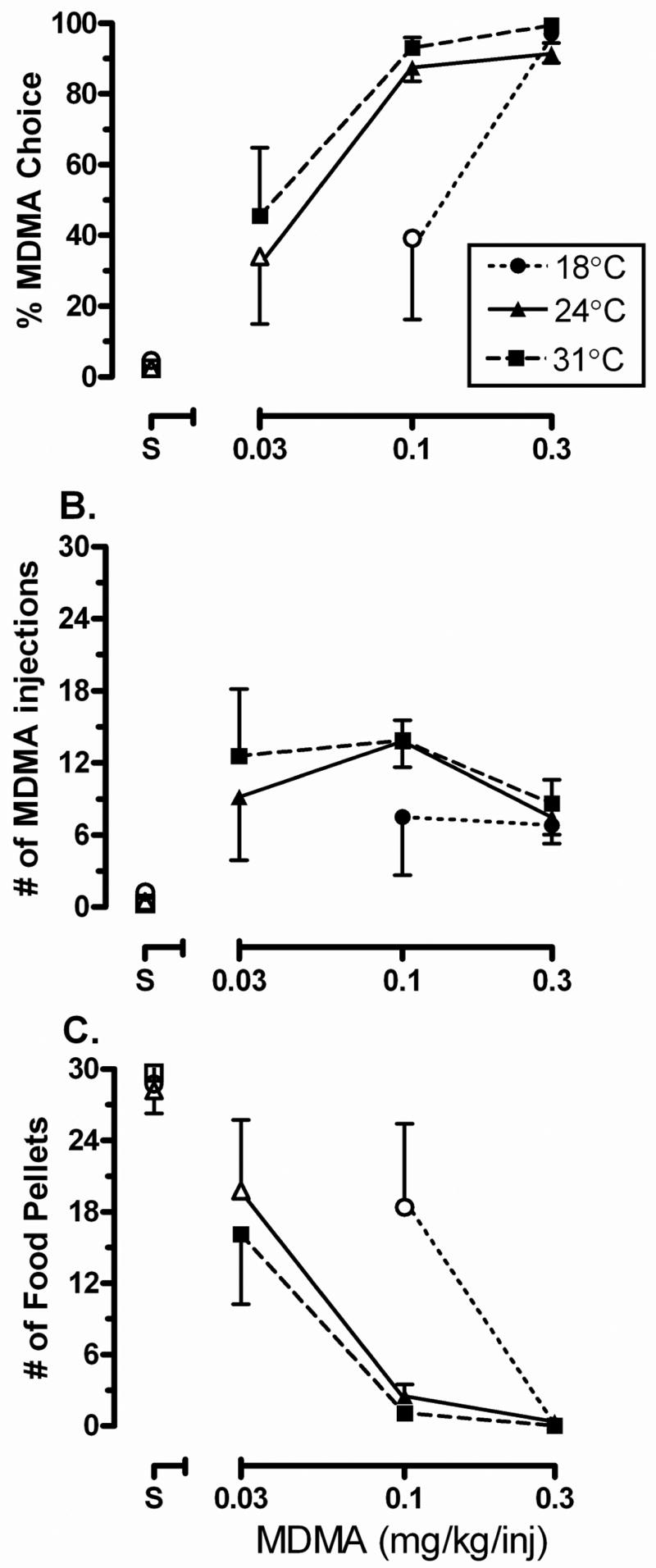
Effects of ambient temperature on MDMA choice under a concurrent schedule of MDMA and food availability. Data are represented as mean ± SEM (n=5). Abscissa represents the MDMA dose (0.03 – 0.3 mg/kg/inj). Values above S represent when saline was the alternative to food. Ordinates represent (A) MDMA choice, (B) MDMA injections earned and (C) food pellets earned. * indicates significantly different from other ambient temperatures at that dose (p<0.001) and filled symbols indicates significantly different from vehicle (saline) at an ambient temperature (p<0.05).
There was no significant interaction between MDMA dose and ambient temperature on number of MDMA injections (Fig. 1B). Upon reanalysis without the interaction factor, there was a significant main effect of MDMA dose (F3,16=4.78, p<0.05), but not ambient temperature. Post-hoc analysis demonstrated a significantly greater number of injections were earned for all MDMA doses compared to saline (p<0.05). Total session intake increased in a dose-dependent manner, but was not affected by changes in ambient temperature (Table 1). There was a significant interaction between MDMA dose and ambient temperature for the number of food pellets earned (F5,18=4.16, p<0.05; Fig. 1C). Similar to the choice data in Fig. 1A, when 0.03 mg/kg/inj MDMA was the alternative to food at room temperature, the number of food pellets earned was not significantly different than when saline was the alternative, but was significantly affected by this MDMA dose at the elevated temperature (t18=2.66, p<0.05). Higher doses of MDMA (0.1 and 0.3 mg/kg/inj) resulted in significant decreases in the number of food pellets earned at both room and warm ambient temperatures. At the cool ambient temperature, compared to saline, only the 0.3 mg/kg/inj dose of MDMA significantly reduced the number of food pellets earned (t18=5.65, p<0.05). There was no significant relationship between changes in rectal temperature and the number of MDMA or food reinforcers earned during the experimental sessions at any ambient temperature (data not shown).
Table 1.
MDMA Session Intake
| MDMA Dose (mg/kg/inj) | Ambient Temperature (°C) | Average Intakea (mg/kg) |
|---|---|---|
| 0.03* | 18 | — |
| 24 | 0.28 ± 0.16 | |
| 31 | 0.38 ± 0.17 | |
| 0.1* | 18 | 0.75 ± 0.48 |
| 24 | 1.38 ± 0.22 | |
| 31 | 1.39 ± 0.17 | |
| 0.3* | 18 | 2.42 ± 0.43 |
| 24 | 2.25 ± 0.43 | |
| 31 | 2.59 ± 0.60 |
Average total session intake (mean ± SEM)
indicates significantly different from all other doses (p < 0.01)
Effects of ambient temperature on MDMA-induced reinstatement
When MDMA was administered noncontingently before an experimental session in which saline was the alternative to food, there was no significant interaction between MDMA dose and ambient temperature for percent injection-lever responding (Fig. 2A). There was also no significant interaction between MDMA dose and ambient temperature for the number of saline injections (Fig. 2B) or food pellets (Fig. 2C) earned per session. Upon reanalysis without the interaction factor, there was a significant main effect of MDMA dose for percent injection-lever responding (F3,16=11.76, p<0.001), number of saline injections (F3,16=7.91, p<0.05) and food pellets (F3,16=16.78, p<0.001) earned. Post-hoc analysis demonstrated that only the 0.3 mg/kg MDMA dose was significantly (p<0.05) different than saline for each dependent measure.
Figure 2.

Effects of ambient temperature on MDMA-induced reinstatement during concurrent saline and food availability. Data are represented as mean ± SEM (n=4). Abscissa represents S (saline) and the pretreatment dose of MDMA (0.03 – 0.3 mg/kg). Ordinates represent (A) % injection-lever responding, (B) saline injections earned and (C) food pellets earned. Filled symbols indicate significantly different from vehicle (saline) for that ambient temperature (p<0.05).
DISCUSSION
The purpose of the present study was to examine the reinforcing strength of MDMA relative to food in monkeys and the effects of ambient temperature manipulations on MDMA choice. At room temperature, MDMA choice increased as a function of dose with higher doses (0.1 – 0.3 mg/kg/inj) resulting in greater choice over food compared to when the alternative to food was saline. Elevating the ambient temperature increased the relative reinforcing strength of 0.03 mg/kg/inj MDMA, while lowering the ambient temperature decreased the relative reinforcing strength of 0.1 mg/kg/inj MDMA. When saline was the alternative to food, noncontingent MDMA injections dose-dependently increased injection-lever responding relative to food. In contrast to the effects on MDMA self-administration, MDMA-induced reinstatement was not significantly affected by ambient temperature.
Cornish et al. (2003) reported that rats responding under an FR schedule of reinforcement self-administered more MDMA at 30°C compared to 21°C. While the present findings in monkeys are consistent with these data, the effects were observed primarily at one MDMA dose, in contrast to more robust effects across doses seen in the earlier study (Cornish et al. 2003). One possible explanation is that in the present study, monkeys were exposed to 31°C for at least five behavioral sessions at each MDMA dose. In contrast, rats in the Cornish et al. (2003) study were exposed to the 30°C ambient temperature during a single “test session” after responding for a particular dose had stabilized. Examination of the data from the present study did not reveal a significant difference between the first day of exposure to 31°C and the three day average graphed in Figure 1. A more likely explanation involves the inherent differences in measuring relative reinforcing strength (concurrent schedules of reinforcement) and reinforcing effects (simple FR schedules of reinforcement). For example, under FR schedules of reinforcement the peak of the MDMA dose-response curve in monkeys was 0.03 mg/kg/inj (Beardsley et al. 1986; Lamb and Griffiths 1987; Fantegrossi et al. 2002). In contrast, this dose of MDMA failed to maintain behavior above vehicle levels under progressive-ratio (Lile et al. 2005; Wang and Woolverton 2007) or concurrent (present study) schedules of reinforcement at 24°C. These results support the notion that measures of reinforcing effects and reinforcing strength do not provide identical information on reinforcement (cf. Woolverton and Nader 1990; Katz 1990).
A particular strength of concurrent schedules of reinforcement that involve food and drug reinforcers is that direct effects of the drug on responding will not affect the primary dependent variable, i.e., percent drug choice. However, it is possible that environmental or pharmacological variables could affect the non-drug reinforcer, which would indirectly impact drug choice, rather than directly affecting drug reinforcement. In the present study, changes in ambient temperature affected MDMA choice, but it appeared that the frequency of food-maintained responding was the more sensitive behavior. Directly comparing MDMA self-administration (present study) with cocaine self-administration (Paronis et al 2002; Czoty et al. 2005) under similar concurrent schedules with food as the alternative shows that there are fundamental differences in behavior maintained by these drugs. For both drugs, percent drug choice increases with dose, drug intake increases in a dose-dependent manner and food reinforcement decreases in a dose-dependent manner. However, the effects of dose on number of injections is different; for cocaine the function is best described as an inverted U-shaped function, while for MDMA it is flat.
Increasing ambient temperature did not affect the number of MDMA injections, but did decrease food reinforcement and consequently increased MDMA choice. It is important to note that this effect of ambient temperature occurred when a dose of MDMA was available that under other circumstances had no effect on frequency of food reinforcement. Thus, it remains possible that the ambient temperature-related increase in relative reinforcing efficacy of MDMA was due to increases in the reinforcing strength of MDMA. Lowering the ambient temperature decreased MDMA choice, but this appears to be due to attenuation of MDMA-induced decreases in the frequency of food reinforcement. Thus, an alternative explanation of the results is that lowering the ambient temperature increased the reinforcing strength of the food pellets. In support for this alternative explanation, Kraly and Blass (1976) reported that rats consumed more food at 4°C compared to 22°C after a 24-hr food deprivation. However, in the present study, the number of food pellets earned when saline was available did not differ significantly between the three ambient temperatures. Because lowering the ambient temperature did not significantly affect number of MDMA injections (or self-administered MDMA dose), it appears that the decreases in ambient temperature directly affected MDMA-induced disruption in food reinforcement, which would be consistent with other findings (e.g., Malberg and Seiden 1998). The observation that the relative reinforcing strength of MDMA is attenuated at lower ambient temperatures because non-drug reinforced behavior is increased should be viewed in a positive light; ultimately, any intervention that decreases adverse effects of the drug of abuse (in this case modeled as decreases in frequency of food reinforcement) is a favorable outcome.
The present study also examined the interaction of ambient temperature on MDMA-induced reinstatement in nonhuman primates using a concurrent schedule of reinforcement. Whereas noncontingent MDMA injections dose-dependently increased responding on the injection lever, there was no significant effect of ambient temperature. Freedman et al. (2005) conducted a clinical study examining the effects of ambient temperature (18°C and 30°C) on the subjective effects of MDMA and also did not find a significant interaction. Raves or dance parties are multi-faceted events and there are a number of stimuli that could serve to provide information about the availability of ecstasy (i.e. discriminative stimuli). For example, these dance parties are characterized by warm ambient temperatures, overcrowding, technotronic music, marathon group dance sessions, drugs, drug paraphernalia and visual stimuli such as glow lights and laser light shows (Schwartz and Miller 1997; Weir 2003). Individually, these stimuli might not serve as a discriminative stimulus for MDMA availability, but the combination of these stimuli may serve as a compound stimulus that has conditioned reinforcing effects. Thus, in the controlled laboratory setting, isolating one of these environmental variables, such as ambient temperature, may not be enough to influence MDMA-induced reinstatement (present study) or the subjective effects in humans (Freedman et al. 2005). Future studies utilizing different cues to reinstate responding, under different ambient temperatures, will be necessary to fully address this hypothesis.
Acknowledgments
We appreciate the insightful comments and statistical assistance of Linda Porrino and Beth Reboussin. This research was supported by National Institute on Drug Abuse grants DA-06634 (MAN) and DA-020281 (MLB).
References
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. Ambient temperature effects on 3,4-methylenedioxymethamphetamine (MDMA)-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Disp. 2007a doi: 10.1124/dmd.107.016261. [DOI] [PubMed] [Google Scholar]
- Banks ML, Czoty PW, Nader MA. The influence of reinforcing effects of cocaine on cocaine-induced increases in extinguished responding in cynomolgus monkeys. Psychopharmacology. 2007b;192:449–56. doi: 10.1007/s00213-007-0732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Harris LS. Self-administration of methylenedioxymethamphetamine (MDMA) by rhesus monkeys. Drug Alcohol Depend. 1986;18:149–57. doi: 10.1016/0376-8716(86)90047-5. [DOI] [PubMed] [Google Scholar]
- Bedi G, Redman J. Recreational ecstasy use: acute effects potentiated by ambient conditions? Neuropsychobiology. 2006;53:113. doi: 10.1159/000092220. [DOI] [PubMed] [Google Scholar]
- Cador M, Isingrini E, Keiflin R. Systemic cocaine can reinstate an instrumental response not directed towards cocaine but previously performed under cocaine. Soc Neurosci Abstract. 2006:189.12. [Google Scholar]
- Cornish JL, Shahnawaz Z, Thompson MR, Wong S, Morley KC, Hunt GE, McGregor IS. Heat increases 3,4-methylenedioxymethamphetamine self-administration and social effects in rats. Eur J Pharmacol. 2003;482:339–41. doi: 10.1016/j.ejphar.2003.09.060. [DOI] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Assessment of the relative reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmcol Exp Ther. 2005;312:96–102. doi: 10.1124/jpet.104.073411. [DOI] [PubMed] [Google Scholar]
- Daza-Losada M, Ribeiro Do Couto B, Manzanedo C, Aguilar MA, Rodriguez-Arias M, Minarro J. Rewarding effects and reinstatement of MDMA-induced CPP in adolescent mice. Neuropsychopharmacology. 2007;32:1750–9. doi: 10.1038/sj.npp.1301309. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abuse by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system in freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G. 3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacology. 2002;161:356–64. doi: 10.1007/s00213-002-1021-6. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Johansen CE, Tancer ME. Thermoregulatory effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2005;183:248–56. doi: 10.1007/s00213-005-0149-6. [DOI] [PubMed] [Google Scholar]
- Gasior M, Paronis CA, Bergman J. Modification by dopaminergic drugs of choice behavior under concurrent schedules of intravenous saline and food delivery in monkeys. J Pharmacol Exper Ther. 2004;308:249–59. doi: 10.1124/jpet.103.052795. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Watkinson WP, O’Callaghan JP, Miller DB. Effect of 3,4-methyleneidoxymethamphetamine on the autonomic thermoregulatory responses of the rat. Pharmacol Biochem Behav. 1991;38:339–44. doi: 10.1016/0091-3057(91)90288-d. [DOI] [PubMed] [Google Scholar]
- Higgins ST. The influence of alternative reinforcers on cocaine use and abuse: a brief review. Pharmacol Biochem Behav. 1997;57:419–27. doi: 10.1016/s0091-3057(96)00446-7. [DOI] [PubMed] [Google Scholar]
- Irvine RJ, Keane M, Felgate P, McCann UD, Callaghan PD, White JM. Plasma Drug Concentrations and Physiological Measures in ‘Dance Party’ Participants. Neuropsychopharmacology. 2006;31:424–30. doi: 10.1038/sj.npp.1300896. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Futurenational survey results on drug use, 1975–2005: Volume II, College students and adults ages 19–45 (NIH Publication No. 06-5884) Bethesda, MD: National Institute on Drug Abuse; 2006. [Google Scholar]
- Katz JL. Models of relative reinforcing efficacy of drugs and their predictive utility. Behav Pharmacol. 1990;1:283–301. doi: 10.1097/00008877-199000140-00003. [DOI] [PubMed] [Google Scholar]
- Kraly FS, Blass EM. Mechanisms for enhanced feeding in the cold in rats. J Comp Physiol Psychol. 1976;90:714–26. doi: 10.1037/h0077225. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Griffiths RR. Self-injection of d, l-3,4-methylenedioxymethamphetamine (MDMA) in the baboon. Psychopharmacology. 1987;91:268–72. doi: 10.1007/BF00518175. [DOI] [PubMed] [Google Scholar]
- Lile JA, Ross JT, Nader MA. A comparison of the reinforcing efficacy of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) with cocaine in rhesus monkeys. Drug Alcohol Depend. 2005;78:135–40. doi: 10.1016/j.drugalcdep.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-Methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–94. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Grundt P, Ross J, Newman AH, Nader MA. Effects of two novel D3-selective compounds, NGB 2904 and CJB 090, on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:573–82. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- Morgan D, Nader MA. Acquisition of intravenous cocaine self-administration with concurrent access to food in cynomolgus monkeys. Exp Clin Psychopharmacol. 2000;8:554–65. doi: 10.1037//1064-1297.8.4.554. [DOI] [PubMed] [Google Scholar]
- Morley KC, Cornish JL, Li KM, McGregor IS. Preexposure to MDMA (“Ecstasy”) delays acquisition but facilitates MDMA-induced reinstatement of amphetamine self-administration behavior in rats. Pharmacol Biochem Behav. 2004;79:331–42. doi: 10.1016/j.pbb.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–31. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Odum AL, Shahan TA. D-amphetamine reinstates behavior previously maintained by food: importance of context. Behav Pharmacol. 2004;15:513–516. doi: 10.1097/00008877-200411000-00007. [DOI] [PubMed] [Google Scholar]
- O’Shea E, Escobedo I, Orio L, Sanchez V, Navarro M, Green AR, Colado MI. Elevation of ambient room temperature has differential effects on MDMA-induced 5-HT and dopamine release in striatum and nucleus accumbens of rats. Neuropsychopharmacology. 2005;30:1312–1323. doi: 10.1038/sj.npp.1300673. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Gasior M, Bergman J. Effects of cocaine under concurrent fixed ratio schedules of food and IV drug availability: a novel choice procedure in monkeys. Psychopharmacology. 2002;163:283–91. doi: 10.1007/s00213-002-1180-5. [DOI] [PubMed] [Google Scholar]
- Parrott AC. MDMA (3,4-Methylenedioxymethamphetamine) or Ecstasy: The Neuropsychobiological Implications of Taking It at Dances and Raves. Neuropsychobiology. 2004;50:329–335. doi: 10.1159/000080961. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Miller NS. MDMA (ecstasy) and the rave: a review. Pediatrics. 1997;100:705–708. doi: 10.1542/peds.100.4.705. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Everman SL, Nichols DE. An integrated hypothesis for the serotonergic axonal loss induced by 3,4-methylenedioxymethamphetamine. Neurotoxicology. 1998;19:427–41. [PubMed] [Google Scholar]
- Von Huben SN, Lay CC, Crean RD, Davis SA, Katner SN, Taffe MA. Impact of Ambient Temperature on Hyperthermia Induced by (±)3,4-Methylenedioxymethamphetamine in Rhesus Macaques. Neuropsychopharmacology. 2007;32:673–81. doi: 10.1038/sj.npp.1301078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Woolverton WL. Estimating the relative reinforcing strength of (±)-3,4-methylenedioxymethamphetamine (MDMA) and its isomers in rhesus monkeys: comparison to (+)-methamphetamine. Psychopharmacology. 2007;189:483–8. doi: 10.1007/s00213-006-0599-5. [DOI] [PubMed] [Google Scholar]
- Weir E. Raves: a review of the culture, the drugs and the prevention of harm. CMAJ. 2000;162:1843–1848. [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Nader MA. Experimental evaluation of the reinforcing effects of drugs, in Modern Methods in Pharmacology. In: Adler MW, Cowan A, editors. Testing and Evaluation of Drugs of Abuse. Vol. 6. Wiles-Liss, Inc.; New York, NY: 1990. pp. 165–192. [Google Scholar]


