Abstract
The place-specific activity of hippocampal cells provides downstream structures with information regarding an animal's position within an environment, and perhaps the location of goals within that environment. In rodents, recent research has suggested that distal cues primarily set the orientation of the spatial representation, whereas the boundaries of the behavioral apparatus determine the locations of place activity. The current study was designed to address possible biases in some previous research that may have minimized the likelihood of observing place activity bound to distal cues. Hippocampal single-unit activity was recorded from 6 freely moving rats as they were trained to perform a tone-initiated place preference task on an open field platform. To investigate whether place activity was bound to the room- or platform-based coordinate frame (or both), the platform was translated within the room at an “early” and at a “late” phase of task acquisition (Shift 1 and Shift 2). At both time points, CA1 and CA3 place cells demonstrated room-associated and/or platform-associated activity, or remapped in response to the platform-shift. Shift 1 revealed place activity that reflected an interaction between a dominant platform-based (proximal) coordinate frame and a weaker room-based (distal) frame, as many CA1 and CA3 place fields shifted to a location intermediate to the two reference frames. Shift 2 resulted in place activity that became more strongly bound to either the platform- or room-based coordinate frame, suggesting the emergence of two independent spatial frames of reference (with many more cells participating in platform-based than room-based representations).
Keywords: spatial cognition, navigation, single-units, rats
The most robust behavioral correlate of rat hippocampal neurons is the location-specific activity observed as an animal moves through space (O'Keefe 1976), with an estimated 30−40% of hippocampal CA1 pyramidal cells displaying “place fields” within a given environment (Wilson and McNaughton 1993; Guzowski et al. 1999). Early investigations suggested that the location of place-specific activity within an environment was determined by distal cues because the rotation of those cues tended to cause equal rotations of place fields, while place fields appeared largely unaffected by rotations of the behavioral apparatus and any associated proximal cues (O'Keefe and Conway 1978; Muller and Kubie 1987). However, further experiments revealed that place cells maintained their firing fields in complete darkness, in the absence of visual input from distal cues, and were disrupted by manipulations of idiothetic inputs. Such findings required the incorporation of path integration mechanisms into models of place-specific activity (Leonard and McNaughton 1990; Quirk et al. 1991; Markus et al. 1994; Samsonovich and McNaughton 1997; Jeffery and O'Keefe 1999; Knierim and McNaughton 2001). Furthermore, experiments in which the proximal cues on an apparatus were made as salient as the distal landmarks along the walls demonstrated that proximal cues could indeed exert some control over the locations of place-specific activity (Young et al. 1994; Shapiro et al. 1997; Tanila et al. 1997), and in some cases were the dominant influence (Knierim 2002; Lee et al. 2004; Renaudineau et al. 2007). Finally, O'Keefe, Burgess, and colleagues (O'Keefe and Burgess 1996; Hartley et al. 2000; Lever et al., 2002) demonstrated that the more proximal boundaries of an enclosed chamber were more important than the boundaries of the room in determining the locations of place activity.
The behavioral performance of rats on spatial learning tasks, such as the Morris water maze, is generally assumed to depend on the rat's perception of its location relative to the configuration of distal landmarks in the room. The assumption is that the rat triangulates its momentary position and the location of its goal relative to these landmarks. However, specific probe tests suggest that, like place-specific activity, the rat's performance can be tied more closely to its perception of location within the reference frame of the behavioral apparatus, rather than the reference frame of the room (Blodgett et al. 1949; Weisend et al. 1995; Horne et al. 2007). In these probes the apparatus was moved to different locations in the room, rather than rotated around its center point. The rats tended to seek the goal location relative to the reference frame of the apparatus, and not relative to the room-associated distal landmarks (although the results of Weisend et al. 1995, were complicated by interactions between the sex of the rat and the amount of training before the probe test.)
The apparent contradiction regarding whether the locations of place-specific activity and goal-seeking behavior are determined by distal or proximal cues appears related to whether the probe tests consisted of cue rotations or the translation of the behavioral apparatus. Most studies that used cue rotations demonstrated a dominance of the distal cue set, whereas studies that translated the behavioral apparatus within the room were more likely to show dominance by proximal cues. It has been suggested that distal cues may determine the orientation of spatial representations, while the exact location of place-specific activity may be determined relative to more proximal cues (such as the boundaries of the behavioral apparatus; O'Keefe and Nadel 1998; O'Keefe and Burgess 1996; Save and Poucet 2000; Lever et al., 2002; Knierim and Rao 2003; Yoganarasimha and Knierim 2005). Head direction cells have been shown in many experiments to be heavily dominated by distal cues (Taube et al. 1991; Zugaro et al. 2001; Yoganarasimha et al. 2006). It is possible that the influence of distal cues on the location of place cell activity is via the influence of distal cues over the orientation of the head direction cell system. Head direction cells, in turn, set the orientation of space-specific activity in entorhinal cortex (grid cells) and the hippocampus (place cells; Sargolini et al. 2006).
Support for the latter hypothesis comes from a set of studies that quantitatively analyzed place cell and head direction cell responses when a behavioral apparatus was moved to different locations relative to distal room cues (Knierim and Rao 2003; Yoganarasimha and Knierim 2005). In these studies, the majority of place cells continued to fire at the same location on a circular or rectangular track when the track was translated to nonoverlapping positions within a circular environment with salient cues at the periphery. Few place cells displayed activity bound to the room-based reference frame. A minority of cells remapped between sessions, by either changing their firing fields completely or developing a new field. New fields then became bound to the track when the track was subsequently moved to a new location. When distal cues were rotated around the track, however, both place fields and head direction cell tuning curves rotated with the landmarks. The results support the idea that distal cues can calibrate the orientation of the head direction system relative to the external world, and thereby control the orientation of the spatial representations downstream in the hippocampal formation. Furthermore, the data suggest that the exact locations of place-specific activity in the x-y plane are largely determined by the boundaries of the local apparatus, rather than by the distal cue set (O'Keefe and Burgess 1996; Lever et al., 2002).
Two confounds in investigating the potential roles of distal cues based on the experiments of Knierim and Rao (2003) and Yoganarasimha and Knierim (2005) are apparent. (1) Because the tracks were shifted to nonoverlapping parts of the room, any room-associated place activity would not have been revealed. Although this possibility does not explain why the activity of a majority of cells showed such strong binding to the track-based reference frame, it is nonetheless a bias in the experimental design that could have influenced the results. (2) The rats were not performing a task that required them to keep track of their location in any reference frame, as they simply moved continuously in a clockwise direction for randomly placed food reward. It is possible that place cells might display a greater degree of room-associated activity if the rats were performing a navigation task that is likely to be hippocampus-dependent (Zinyuk et al. 2000). To address these issues, rats were trained to navigate to an unmarked goal for food reward on an open-field platform upon presentation of a tone (modified from the place preference task of Bures and Fenton 2000). In order to determine whether place-specific activity was bound to the proximal (platform-based) and/or distal (room-based) coordinate frame, the behavioral platform was translated within the room at two time points during task acquisition (once at an ‘early’ phase of learning and again at a ‘late’ phase when task performance had improved), such that the platform's shifted position overlapped with its standard position by 50%. The results revealed a dominant platform-based spatial reference frame under foraging and navigation-associated conditions, as well as the development of a less prevalent room-based reference frame during task conditions as a result of experiencing the platform-room dissociation.
METHODS
Subjects and Behavioral Training
Twelve Long-Evans male rats (approximately 4−5 months of age and weighing 450−600 g) were studied in 2 sets of 6 rats, 12 months apart. The rats were food restricted to 80% of their free-feeding weight and initially trained to forage for chocolate sprinkles on a black circular platform (3−5 sessions). To screen for the best performers, rats received pre-surgical training in a tone-initiated place preference task. Pre-surgical training occurred in the same room but without the curtained environment and the associated cue set used during post-surgical training. Training continued (1 session/day) until rats demonstrated good procedural knowledge of the task but were still inaccurate in locating the goal (see below). Eight rats were chosen for surgical implantation of recording electrodes based on pre-training performance (4 rats from each group). Rats were permitted to free-feed for 3 days prior to surgery. After implantation and surgical recovery (see below), rats were again placed on food restriction until body weight dropped to 80% of free-feeding weight. Training in the place preference task was conducted in a 2.75 m × 1.5 m curtained environment with a number of high contrast cues placed at varying heights and locations around the periphery (e.g., Fig. 1A). The behavioral apparatus consisted of a 1 m × 1 m square platform painted black. Before each training session (and between a standard condition and platform-shift manipulation, see below), the platform was wiped with 70% ethyl alcohol and randomly rotated so that odors and local heterogeneities in the surface of the platform could not be used by rats to locate the unmarked goal across sessions. The platform was never oriented the same way for two consecutive sessions. Rats were given one or two sessions/day separated by at least 4 hours until the point of the first platform-shift manipulation, after which rats received one session/day.
Figure 1.
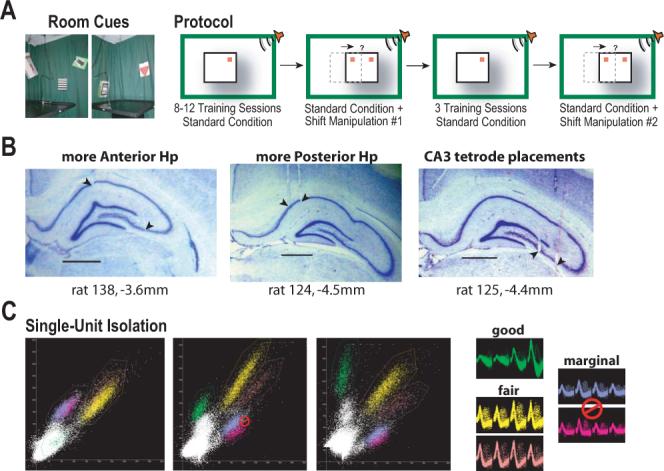
Schematic of behavioral training protocol, example tetrode placements and single-unit isolation. A Pictures showing example room cues (left) and a schematic of the behavioral protocol (right). Rats received training in a tone-initiated place preference task for 8−12 sessions before the first platform-shift manipulation. Rats received 3 more days of training under standard conditions, with a second platform-shift manipulation performed following a standard session on the 4th day. Red squares illustrate the location of the unmarked goal under standard conditions, and possible room- or platform-associated goal locations in the platform-shifted condition. B Representative histology showing the placement of tetrodes across rats. Examples demonstrate the anterior and posterior extent of tetrode placements, with a number of CA3 cells recorded between the upper and lower blades of the dentate gyrus in addition to more lateral placements. No differences among anterior-posterior recording locations were noted in cell responses. (AP coordinates are relative to bregma; scale bar = 1 mm.) C Example scatter plots demonstrating the isolation of single-unit activity for a tetrode and typical ratings regarding the quality of isolation (good = 2 on a scale of 1−4, fair = 3, and marginal = 4; see Methods). The height of triggered waveform peaks were plotted for two wires of a tetrode and displayed as scatter plots. Note that clusters of single-unit activity overlap with each other (yellow with orange and blue with purple) or with background activity (green) on the first projection (left scatter plot), most of which are easily separated when two different wires from the same tetrode are paired (center and right scatter plots). Sampled waveforms are given for isolated single-units and two units that could not be isolated from each other as recorded from the four wires of the tetrode.
Before each training session chocolate sprinkles were dispersed across the platform. A training session began with 2−4 minutes of pre-task foraging before initiating the first trial (∼15 trials/session). A trial was initiated with presentation of a tone (1 kHz for 4 rats and 8 kHz for the other 4), which signalled the availability of food reward. If the rat navigated to an unmarked 16 × 16 cm goal and paused there for more than 1 sec, the tone was turned off and reward was triggered from an automatic overhead dispenser. The rat was permitted ∼90 sec to forage for the reward before initiating the next trial. If a rat was not rewarded within 60 sec of presenting the tone the trial was discontinued without reward, the tone was extinguished, and the next trial was initiated ∼60 sec later. A rat's behavior was shaped by making the task easier in the earliest stages of training (for the first 7 to 10 training sessions). This was accomplished by initially using a larger goal region (20 × 20 cm) and by requiring the rat to stop in the goal for only 250−500 msec. Once the rat persisted in attempting to find the goal location on at least three consecutive trials, the pause requirement was increased to 1 sec and size of the goal reduced (to 16 × 16 cm) for subsequent trials/sessions. The center of the goal was always at the same location for a given rat across all training and standard recording sessions.
Once rats demonstrated good procedural knowledge of the task but were still inaccurate in navigating to the exact location of the goal (i.e., when less than 10% of trials were discontinued due to the 60-sec time limit, ranging between the 8th and 12th sessions depending on the rat), a “shift” manipulation was performed immediately following a standard training session (Fig. 1A). The shift manipulation consisted of first removing the rat from the environment and placing it in its home cage located in the adjacent room with the door closed. The experimenter then shifted the platform 50 cm in one axis within the room-based frame of reference. The rat was brought back into the room without covering or attempting to disorient it, and was placed back onto the platform in the same way as during a standard training session. The rat was permitted 2−4 minutes of pre-task foraging followed by two probe trials. Because the goal location could have been defined by either the platform- or room-based coordinate frame in the shifted condition, a rat was rewarded for choosing either possible goal location during either probe trial. After 3 additional training sessions with the platform in the standard condition (i.e., when rats improved their ability to successfully locate the unmarked goal, see Results), the platform shift protocol was repeated. The location of the goal under standard conditions was always on the half of the platform that overlapped with the platform's new position in the shifted condition, and was counterbalanced among rats within a group (i.e., 2 rats from each of the two groups were trained to an unmarked goal in the upper quadrant, as shown in Fig. 1A, and two rats from each group were trained to a goal in the lower quadrant).
On the day of each shift manipulation, 2 rats did not perform the task under standard conditions, and so data from those rats were not included for those analyses (one rat was omitted from both data sets, a second was omitted only from Shift 1 and a third only from Shift 2). On these occasions, these rats typically displayed poor foraging performance during the pre-task phase. When the task was initiated a rat either remained stationary on the corner of the platform nearest the entrance to the environment or wandered between the platform's edges. We interpreted such behavior as a lack of motivation on the part of the rat, which was usually substantiated by a notable increase in weight relative to the previous day. Therefore, of the 8 implanted rats, 6 are included in the analysis for Shift 1 and 6 are included for Shift 2 (5 rats are included at both time points). On a different day than either shift manipulation, four of the rats were subjected to a standard training session followed by a second session with the platform still in the standard position. These data provided a secondary control regarding expected variability in the location of place-specific activity when a rat is reintroduced to an environment under spatially stable conditions (see Results).
Surgical Procedures
Eight rats were surgically implanted with a microdrive housing 2 bundles of independently movable tetrodes positioned over the CA1 and CA3 regions of the hippocampus. Anesthesia was induced with 4% isoflurane and initially maintained with 60 ml/kg ketamine and 8 ml/kg xylazine until proper placement in the stereotaxic apparatus, at which point rats were maintained on 1−2% isoflurane. A midline incision was made, the skull cleaned and desiccated, and a 3 mm × 4 mm hole was drilled in the skull such that the lateral-most tetrode could be positioned at 4.2 mm posterior to bregma and 3.3 mm lateral to the midline. The dura was reflected and the microdrive positioned with the ends of the bundles just sitting on the surface of the brain. Occasionally, slight adjustments were made in placement to avoid destruction of vasculature, resulting in a range of placements along the longitudinal axis of the hippocampus across animals (e.g., Fig. 1B, see Results). The craniotomy was sealed with Kwiksil (WPI, Sarasota, FL) and the microdrive secured to the skull with 8 skull screws and dental acrylic (one skull screw was electrically connected with a low impedance wire and served as the animal's ground for neurophysiological recordings). A rat was given analgesics (ketoprofen, 5 mg/kg) and permitted at least 5 days to recover before tetrode adjustment and/or food restriction. Rats were maintained on antibiotics until the end of the experiment (Baytril, 0.15 cc/day; tetracycline hydrochloride, 30 mg/kg). All procedures were in accordance with the National Institutes of Health (NIH) and the University of Texas Health Sciences Center at Houston Institutional Animal Care and Use Committee guidelines.
Single-Unit Recordings
Recording tetrodes were constructed from 4 polyimide coated, 12 μm nichrome wires (Kanthal, Palm Coast, FL) and mounted in a “hyperdrive” capable of housing 14 independently moveable recording probes (1 turn of the drive screw = 318 μm). Each wire of a tetrode was electrically connected to an isolated channel on an etched board mounted on top of the hyperdrive (Neuralynx, Tucson AZ) and electrode tips were gold-plated down to impedances of ∼250 kΩ prior to surgical implantation.
After surgical recovery, tetrode adjustments were performed as a rat sat in a towel-lined dish affixed to a pedestal near the recording equipment. Five tetrodes housed in the medial bundle were advanced to the CA1 pyramidal layer over the course of several days (guided by local field recordings according to Buzsáki 1986), with a reference electrode positioned near the corpus callosum. Seven tetrodes housed in the lateral bundle were slowly lowered to the CA3 pyramidal layers, with a reference electrode positioned just above the cell layer. Any tetrode adjustments made after the first platform-shift manipulation (see Behavior) were rarely >40−80 μm, and were followed by a minimum of 6 hours before the next recording session (typically the next session was not run until the following day).
Neurophysiological and behavioral position data were acquired with the Cheetah160 data acquisition system (Neuralynx, Inc., Tucson, AZ). Neural signals from each wire of a tetrode were fed to a multi-channel unity-gain headstage, amplified 1000−5000 times, and bandpass filtered between 600 and 6000 Hz, digitized at 32 kHz, and stored on a personal computer. During behavioral experiments, light-emitting diodes mounted on the headstage amplifier allowed for the tracking and digitization of a rat's momentary position at 30 Hz. In one set of rats (n = 4) the experimenter controlled the presentation of tones and reward (experimenter-initiated automatic release of 3−10 chocolate sprinkles), with the output signals of each fed into two EEG channels of the data acquisition system (sampled at 2 kHz and filtered between 1 and 475 Hz). Event times for tone onsets/offsets and reward trigger were extracted off-line using custom-written software. In the second set of rats (n = 4) a computer program integrated with the neurophysiological acquisition system provided automation of the task (tone control, computer-initiated reward release in the goal zone) and event time-stamping that was synchronized with the neural data.
Analysis
Single-unit activity was isolated off-line using interactive cluster-cutting software on a PC workstation (WinClust, JJ Knierim). In brief, the waveform parameters of digitized neural events recorded from the four closely spaced wires of a tetrode (e.g., spike height) were displayed on scatter plots for one wire versus another. Because many waveform parameters vary as a function of distance from the recording site, the activity of a single neuron can be discriminated from other isolated neurons and from background neural activity by identifying the boundaries of clusters of points on the various scatter plots (Fig. 1C; McNaughton et al. 1983). The timestamps from isolated clusters of neural events were extracted and identified as the activity of a single-unit. The quality of isolation for each identified cell was rated from 1 (very good) to 4 (marginal) prior to examining the associated firing rate maps (see below). Most cells included in analysis were rated as good or fair (values of 2 or 3), with marginally isolated cells excluded from analysis (Fig. 1C).
For each cell, single-unit spike trains and momentary position data were used to create 2-dimensional firing rate maps in order to examine changes in a cell's firing rate as a function of a rat's position in space. Rate maps were created by dividing the camera image into a 64 × 48 array (∼3.2 × 3.2 cm pixels) and computing the average firing rate (total number of spikes/total dwell time) for each pixel of the array. Rate maps initially created for the entire pre-shift standard session and separately for the entire platform-shifted session were used to identify cells that displayed place-specific activity in at least one of the conditions (for consistency with previous place cell identification criteria from our lab, rate maps were first smoothed with the adaptive smoothing technique of Skaggs et al. 1993). Criteria for inclusion were a significant spatial information score (p < 0.01) greater than 0.70 bits/spike (given by I=(∑x px(λx/λ)(log2(λx/λ), where λ is the mean firing rate of the cell, λx is the mean firing rate while the animal is occupying bin x, and px is the probability of occupancy for bin x; Skaggs et al. 1993), when > 50 spikes contributed to the rate map. Statistical significance for the spatial information score was determined using a shuffle procedure, in which the spike train of a given cell was offset from the position record by a random value ≥ 33 sec, a new rate map was created and a corresponding information score calculated, for 100 such iterations. If the spatial information score was greater than all of the scores of the shuffled rate maps, the probability of obtaining the observed score by chance was considered to be less than 0.01. For subsequent analysis, separate rate maps from qualifying cells were created for each epoch of interest (e.g., pre-task standard, task standard, task shifted, etc.) and gently smoothed with a conditional algorithm using a 5 × 5 hybrid box filter (Igor, Wavemetrics, Portland, OR). If >100 spikes contributed to a given rate map the filter was passed 5 times; if <100 spikes were included, the filter was passed 10 times. Passing the box filter additional times for low-firing-rate place fields was a better method for preserving the size and shape of the place fields compared to increasing the size of the smoothing kernel (Siegel et al. 2005; 2006). Within-task behavioral epochs were concatenated to create rate maps for navigating (“tone-on”) and inter-trial foraging (“tone-off”). In order to prevent contamination of inter-trial foraging rate maps with possible goal-associated activity (Kobayashi et al. 1997; Hollup et al. 2001; Hok et al. 2007), the first 5 sec of post-reward random foraging for each trial was not included in order to allow a rat to leave the goal zone (a separate analysis of goal-associated activity is described in the Results). When comparing two different subsessions (e.g., pre-task vs. task, navigating vs. foraging, pre-task standard vs. pre-task shift), cells had to meet an additional inclusion criterion to ensure that at least one of the subsessions displayed a robust place field. In at least one of the two subsessions being compared, the cells had to have a place field defined as 9 or more contiguous pixel-bins with mean firing rates > 1 s.d. above the average for the map.
The basic measure of the similarity of spatial firing patterns between conditions was based on pixel-by-pixel cross-correlations between the rate maps of interest. Only pixels that were occupied during both conditions were included in rate map correlations. Initially, a place cell was considered to have displayed a similar pattern of location-specific activity across two conditions if the rate maps associated with each condition were significantly correlated (p < 0.01). Rate maps that were not significantly correlated were checked for location-specific activity in at least one of the two conditions based on the identification of place fields (> 9 contiguous pixels that were > 1 standard deviation above the mean of the map). If a cell did not display a place field in at least one of the two conditions of interest, the cell was eliminated from that analysis. Cells with rate maps that did not have significantly similar patterns of activity across the two conditions of interest but which displayed location-specific activity in at least one condition were considered to have remapped (i.e., the cell displayed a different pattern of activity between the two conditions).
A shifting correlation analysis was used to test whether place fields were bound more strongly to the platform- or room-based coordinate frames in the platform-shifted condition. For each cell, correlations were calculated between standard and platform-shifted rate maps as the maps were incrementally shifted toward alignment, one pixel at a time. For a given cell, the correlation calculated at the 0-pixel shift corresponded to the room-based coordinate frame and the correlation calculated at either the 15- or 17-pixel shift corresponded to the alignment of the platform's position across conditions. (The video camera was mounted a little higher in the recordings from the second group of rats, and so relative to the camera the platform was shifted 15 pixels in the second group of rats and 17 pixels in the first group of rats. The amount the platform was shifted in the room was the same for both groups.) Cells were considered to have displayed room-associated place-specific activity if a significant correlation was observed for the 0-pixel shift comparison (in addition to at least one significant correlation ± 1 pixel-shift) and not for the platform-based comparison. Similarly, if a significant correlation was observed corresponding to the actual shift of the platform and not for the room-based comparison (at the 0-pixel shift), the cell's activity was considered to be platform-based (again, if at least one significant correlation was also observed ± 1 pixel-shift). Cells that did not display significant room- or platform-associated activity but showed significant place-specific activity in between the two coordinate frames (defined by at least three consecutive significant r-values for the shifting correlation analysis) were categorized as room- or platform-associated depending on the coordinate frame to which the activity was more strongly bound (i.e., whether the number of pixel-shifts necessary to maximize the r-value was closer to the room- or platform-based coordinate frame). A cell's pattern of place-specific activity was considered to be different between the standard and platform-shifted condition if it did not display at least 3 consecutive significant r-values (corresponding to an ∼10 cm spatial overlap), and was categorized as having remapped. Cells with rate maps that were significantly correlated at both the room- and platform-based coordinate frame were considered to be ambiguous.
For platform-shift rate map comparisons, a measure of “binding strength” was used to evaluate the degree to which place-specific activity was associated with either the room- or platform-based coordinate frame. Binding strength was calculated by first determining the number of pixel shifts necessary to maximize rate map similarity, given by the shifting correlation analysis. If the number of pixels shifted to maximize the r-value was closer to the room-based coordinate frame, then binding strength = (num pixels shifted to max r-value). If the number of pixels shifted to maximize the r-value was closer to the platform-based coordinate frame (i.e., 15 or 17 pixels), then binding strength = | (pixels shifted to max r-value) - (actual pixel shift of platform) |.
The alpha level for all rate map correlations was set at 0.01. The alpha level for all group-based statistical comparisons was set at 0.05. Actual p-values are given unless p<0.0001. Descriptive statistics are given as mean ± SEM, except as noted above.
RESULTS
Behavior
Rats learned within 8−12 sessions to respond to the tone by searching for the unmarked goal location in order to trigger reward. At this stage rats were relatively inaccurate in locating the exact position of the unmarked goal on the platform, typically displaying quadrant-specific choices that required persistent corrective behavior before they finally entered and paused within the reward zone (e.g., Fig. 2A and B, top center behavior plots). After 3 additional training sessions rats were able to make more accurate responses that required less corrective behavior (e.g., Fig. 2A and B, bottom center). The improved performance resulted in a significant decrease in average latency to reward between the early and late phases of task acquisition (Wald-Wolfowitz runs test, Z=2.12, p=0.03; Wald and Wolfowitz 1940; Fig. 2C). Note that it was typical even for well-trained rats to fail to respond to the tone on approximately 1 out of every 7−10 trials (e.g., Fig 2A, bottom line graph). One rat did not display this tendency (Fig. 2B, bottom line graph), while another failed to make a response on almost every third trial, performing as efficiently as the other rats when it did respond (note the outlying data point in Fig. 2C and the relatively large error bars associated with it as evidence of good response times interspersed with long latencies). Although the latter rat appeared to be an outlier, data from this rat were included in the analysis because the subject showed improved response latencies compared with the early phase of task acquisition for trials when it did respond and because the rat's performance on those trials suggested that it was able to perform the task at least as well as its cohorts. However, because the “no-response” trials that occurred for most rats substantially increased the observed variability in response latencies at the late phase of learning (by an average of 50%), the 2 longest latencies for each rat/session were excluded at both the early and late phases of learning for statistical comparison.
Figure 2.
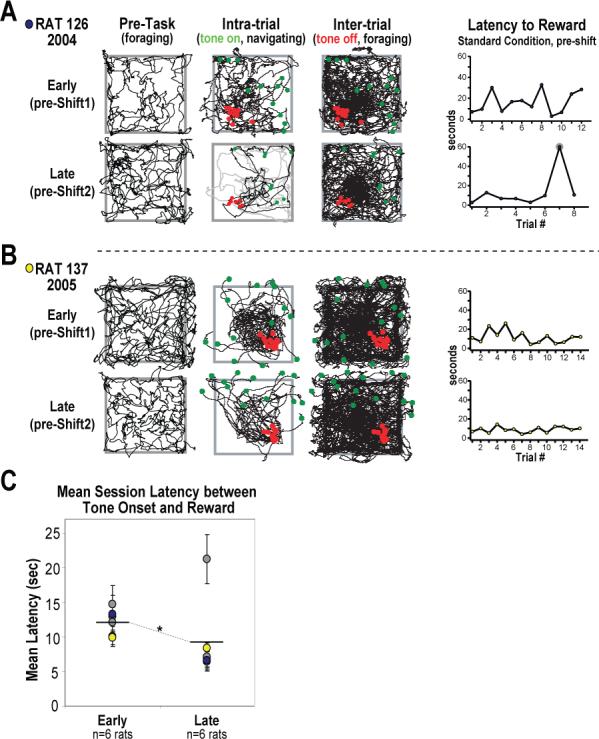
Behavior from two rats at two time points during the acquisition of the place preference task (at “early” and “late” phases of learning), and grouped data showing significant improvement in task performance (as measured by response latency). A,B Behavioral plots and trial response latencies for two rats from each of the groups trained in the task. Behavior is given for both the early (top row) and late (bottom row) phases of acquisition for epochs of pre-task foraging (left), and for concatenated periods of presumed navigation (“tone-on”, center) and inter-trial foraging (“tone-off”, right). Green and red dots represent a rat's location at the onset and offset of the tone, respectively. Note that early in acquisition both rats were inaccurate in locating the goal, often erring toward the center of the platform and having to make persistent corrective behaviors before finding the unmarked goal which triggered reward (top center plots). Late in acquisition rats were able to more accurately find the goal with less corrective behavior (bottom center plots) and shorter response latencies (line graphs, far right). Note that most rats would fail to respond in 1 of every 7−10 trials even late in acquisition, yielding longer response latencies on that trial (A, highlighted in gray on line graph, with behavior on that trial also in gray). The rat shown in B rarely failed to respond to tone-initiated trials. C Grouped data showing significant task improvement as measured by average response latencies. The improvement shown by the rat given in A is a typical example (denoted by blue markers). Note that the rat given in B (yellow dots) displayed the least improved response latencies, yet the improvement in behavior was notable (compare upper and lower navigating behavior plots). *p < .05.
After a standard training session at both the early and late phases of task acquisition, the rat was removed from the environment and the behavioral platform was translated within the room such that the new platform location overlapped with the standard condition by 50% (Fig. 1A). The rat was returned to the environment and permitted 3−4 minutes of foraging followed by two probe trials. Both probe trials were initiated when a rat was approximately equidistant from the two possible goal locations, and were intended to determine whether the rats were primarily using room- or platform-based information to solve the task (in the platform-shifted condition one possible goal was relative to the room-based coordinate frame and the other possible goal was relative to the platform). Rats typically failed to make a clear choice between the two possible goal locations during probe trials. Eventually a rat often made a tentative response that was rewarded, with some rats choosing the platform-based goal and some choosing the room-based goal (apparent goal choices that were not associated with one of the coordinate frames were not observed). Interestingly, the most prevalent response to the platform shift manipulations was remapping (nearly 50% of all cells, see below), which is consistent with the tentative behavior of the rats. When the rats proceeded to make a behavioral response, the goal choice did not predict whether the majority of non-remapping place fields would be more strongly bound to the platform- or room-based coordinate frame within simultaneously recorded ensembles. As described below, many more cells were controlled by the platform-based coordinate frame than the room-based coordinate frame, but no corresponding bias for platform-based goal choices was observed.
Pre-task foraging behavior was examined for evidence that the rats recognized the shifted platform as a change in the environment (Anderson et al. 2006). Rats respond to novelty with increased exploratory activity (Pisula and Siegel 2005). The first time rats experienced the platform-shift condition they explored significantly more of the platform (visited more tracking camera pixels) relative to that of the second shift or during standard conditions (F3 = 4.57, p = 0.005; Fisher's post-hoc LSD, p < 0.02 for all comparisons including Shift 1, all other comparisons were n.s.; the amount of time in the pre-task foraging condition was not different across standard and platform-shifted conditions, F3 = 0.89, p = 0.46, range = 196.6 – 269.8 sec). Additionally, during platform-shift manipulations rats spent the majority of pre-task foraging time on the part of the platform that did not overlap with the platform's position in the standard training condition (F1 = 38.27, p < 0.0001; no significant differences were observed in pre-task exploratory activity during standard conditions at the early and late phases of task acquisition, F1 = 0.66 and 2.43, p = 0.42 and 0.13, respectively). The change in exploratory activity in the shifted condition relative to the preceding standard condition, in addition to the rats' dichotomous behavioral responses during probe trials, suggest that rats perceived the dissociation between the position of the platform within the room-based reference frame.
Single-unit activity
Hippocampal single-unit activity was recorded during standard training sessions (beginning at the early phase of learning) and during the two platform-shift manipulations (Fig. 1A). A total of 84 place cells (57 from CA1 and 27 from CA3) were recorded during the standard and platform-shift conditions at the early phase of learning and 66 place cells (42 from CA1 and 24 from CA3) were recorded at the late phase of learning. Most of the cells that did not meet place cell criteria (see Methods) fired few spikes during behavioral sessions, with a small proportion of cells displaying higher activity levels with little or no spatial selectivity on the platform. All cells included in the analysis were histologically verified to be within the CA1 or CA3 regions of the hippocampus (between 3.5 and 4.8 mm posterior to bregma; ∼45% of putative CA3 cells were recorded from the region between the upper or lower blades of the dentate gyrus; e.g., Fig. 1B). CA1 and CA3 cell responses to the platform-shift manipulations were compared separately for all of the analyses presented below, and included comparisons at the ensemble level when possible. There were no major differences between CA1 and CA3 in the proportions of place cells controlled by the platform- or room-based reference frames (see below), and so cells from both regions were pooled for presentation of the results.
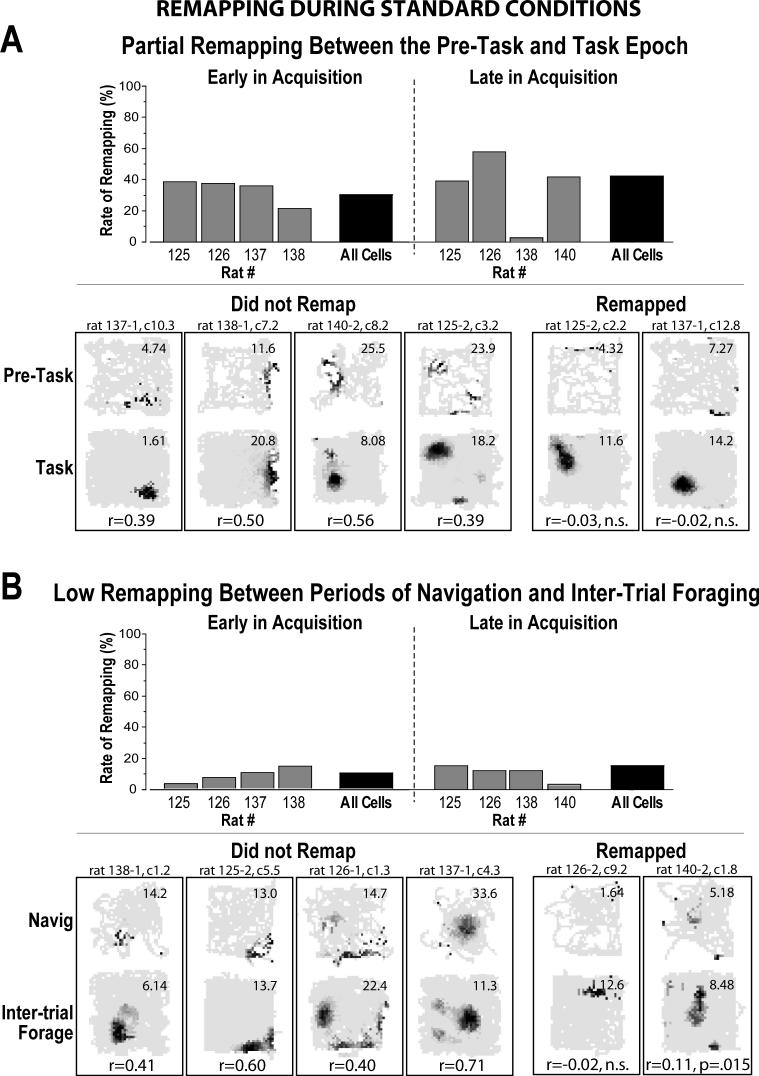
Partial remapping across epochs during standard training conditions
The behavioral task consisted of an initial period of foraging on the platform (pre-task epoch) before the spatial task commenced (task epoch). Similar to previous studies (Markus et al. 1995; Ferbinteanu and Shapiro 2003; Smith and Mizumori 2006), the change in task demands between the pre-task and task epochs induced partial remapping in ensembles of hippocampal place cells during standard conditions (in which a proportion of neurons display a different pattern of place-specific activity, while others maintain the same pattern; Muller and Kubie 1987). It was thus necessary to characterize this partial remapping in order to compare appropriate epochs of the standard and platform-shifted conditions for the main analysis. At both the early and late phases of task acquisition, 30−42% of place cells significantly changed their patterns of place-specific activity between the pre-task foraging epoch and task conditions in standard training conditions (early: 22/73, late: 27/64 active place cells; Fig. 3A). With one exception, the overall rate of remapping between pre-task foraging and task conditions reflected that observed within the ensembles recorded from individual rats (Fig. 3A, top). Given the substantial degree of remapping between the pre-task and task epochs, we also examined whether partial remapping occurred within the task epoch between periods of navigation within trials (i.e., when the tone was present to indicate the availability of reward) and periods of post-trial foraging after successfully locating the goal (after the tone was extinguished and before the next trial). Correlations between rate maps created from “tone on” and “tone-off” periods (excluding the pre-task foraging epoch) were significant for 90% and 85% of all cells at the early and late phases of acquisition, respectively, indicating a low incidence of remapping between task-associated periods of navigation and inter-trial foraging (Fig. 3B). Note that the 5 sec period following the delivery of reward was eliminated from tone-off rate maps to allow a rat time to leave the goal location, so that contamination of inter-trial foraging rate maps with potential goal-associated activity was not possible (Kobayashi et al. 1997; Hollup et al. 2001; Hok et al. 2007). The results suggest partial remapping occurred at both the early and late phases of acquisition between the pre-task foraging epoch and task conditions across rats, but not between periods of navigation and foraging for reward during the task. Therefore, data were analyzed and presented separately for the pre-task and task epochs.
Figure 3.
Partial remapping rates between behavioral epochs under standard training conditions for ensembles of 10 or more cells (gray bars) and for all cells grouped together (black bars). A Partial remapping was observed between pre-task foraging and task-associated conditions at both the early and late phases of acquisition, as defined by the significance of rate map cross-correlations (see Methods). Rate maps with r-values representing the average correlation observed for non-remapping (left) and remapping cells (right) are given. B Relatively low remapping rates were observed between task-associated periods of tone-initiated navigation and inter-trial foraging. Rate maps with r-values representing the average observed correlation for both non-remapping (left) and remapping cells (right) are given. The pair of rate maps and corresponding r-value given at the far right represents cases in which the observed correlation borders on significance. The number at the upper right of each rate map indicates the maximum firing rate (i.e., the darkest pixel). Hyphenated number in cell ID is “1” for early in acquisition and “2” for late in acquisition, followed by the tetrode and cluster number relative to the individual rat.
Dominance of a platform-based representation over a room-based representation in the platform-shifted condition
Based on previous research, the place-specific activity of a given hippocampal cell during the platform-shift manipulation could show stronger binding to local (platform-associated) or distal (room-associated) cues, or both (O'Keefe 1976; O'Keefe and Burgess 1996; Lever et al. 2002; Knierim and Rao 2003; Yoganarasimha and Knierim 2005). Therefore, it was important to utilize an analysis that would be sensitive to platform- and room-based place-specific activity, as well as single-unit responses that reflected an interaction between the room-platform dissociation. To this end, we used a shifting correlation analysis to examine the effect of shifting the behavioral platform within the room-based reference frame. Multiple correlations were calculated between a set of standard and shifted condition rate maps as the maps were shifted toward platform-based alignment in 1-pixel increments. For a given cell, the correlation calculated at the 0-pixel shift corresponded to the room-based coordinate frame and the correlation calculated at either a 15- or 17-pixel shift (depending on which group of rats the cell was recorded from, see Methods) corresponded to the alignment of the platform's position across conditions. Due to partial remapping across task epochs under standard conditions (see above), it was essential to compare rate maps created from the task epoch of the standard and shifted condition separately from pre-task rate map comparisons.
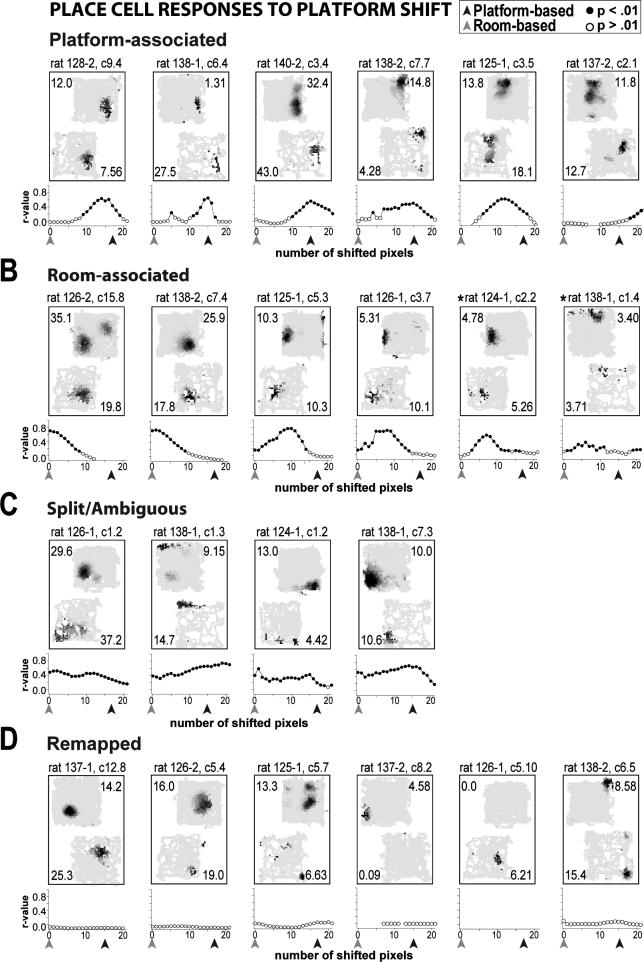
Examples of the shifting correlation results for individual cells are given in Figure 4. If a significant correlation was observed at the room- (0-pixel shift, gray arrow) or platform-based rate map alignment (15- or 17-pixel shift,black arrow) the cell was categorized accordingly (Fig. 4A and B; note that the correlation observed at the pixel-shift before and/or after had to be significant as well). For some cells, particularly during Shift 1, the results of the shifting correlation analysis revealed interactions between the room- and platform-based coordinate frames, such that the number of pixels shifted to maximize rate map similarity fell in between the room- and platform-based alignments (Fig. 4A, 1st and 5th maps; 4B, 3rd and 4th maps). In some cases the interaction was so strong that the location of the place field was in between the room- and platform-based coordinate frame, with insignificant correlations at both the room- and platform-alignments. Cells displaying this pattern of results were considered to have displayed room- or platform-associated depending on whether the number of pixels shifted to maximize the correlation was closest to the room- or platform-based coordinate frame (e.g., Fig. 4B, asterisks; note that < 10% of cells were categorized in this way). Cells with significant correlations at both the room- and platform-based coordinate frames were considered to be ambiguous (Fig. 4C). In some ambiguous cases place fields in the shifted condition appeared stretched between the two coordinate frames (1st map), while in other cases elongated fields were observed in the standard condition along the same axis in which the platform was shifted such that an association with either the room- or platform-based coordinate frame was indiscernible (2nd and 3rd maps). The lack of a significant correlation at any pixel shift suggested that a cell's pattern of place-specific activity was different between the standard and platform-shifted condition, and were considered to have remapped (Fig. 4D). The remapping category also included cells that had fewer than 3 consecutive pixels with significant correlations, as it was deemed likely that these cases arose from chance correlations (i.e., a cell with a relatively small place field [∼10 cm] would be expected to show significant correlations for at least 3 consecutive pixels if the cell did not remap).
Figure. 4.
Representative task-associated rate maps for categories of place cell responses to the platform-shift manipulation. Note that the observed r-values for each pixel-shift correlation (calculated at each step as the maps were aligned in 1 pixel increments) are plotted for each cell (filled black dots: p < .01, open dots: p > .01). A,B Cells with significant correlations observed at pixel-shifts corresponding to either the platform- (black arrows) or room- (gray arrows) based coordinate frame were defined as displaying platform- or room-associated activity, respectively. Additionally, cells displaying activity in between the two coordinate frames (>2 consecutive significant r-values) were categorized according to the reference frame to which the activity was more strongly bound (e.g., asterisks). A majority of cells displayed activity that was more strongly bound to the platform- than to the room-based coordinate frame (see text). C Cells with significant r-values at both coordinate frames were considered ambiguous. D Cells for which fewer than 3 consecutive significant r-values were observed in the shifting correlation analysis were defined as having remapped. Although sometimes a change in the location of place-specific activity between the standard and platform-shifted condition was observed (e.g., first two rate maps), most remapping was the result of a lack of place-specific firing in one condition (∼80%). Rate map scaling and cell identification conventions are described in Figure 3 caption.
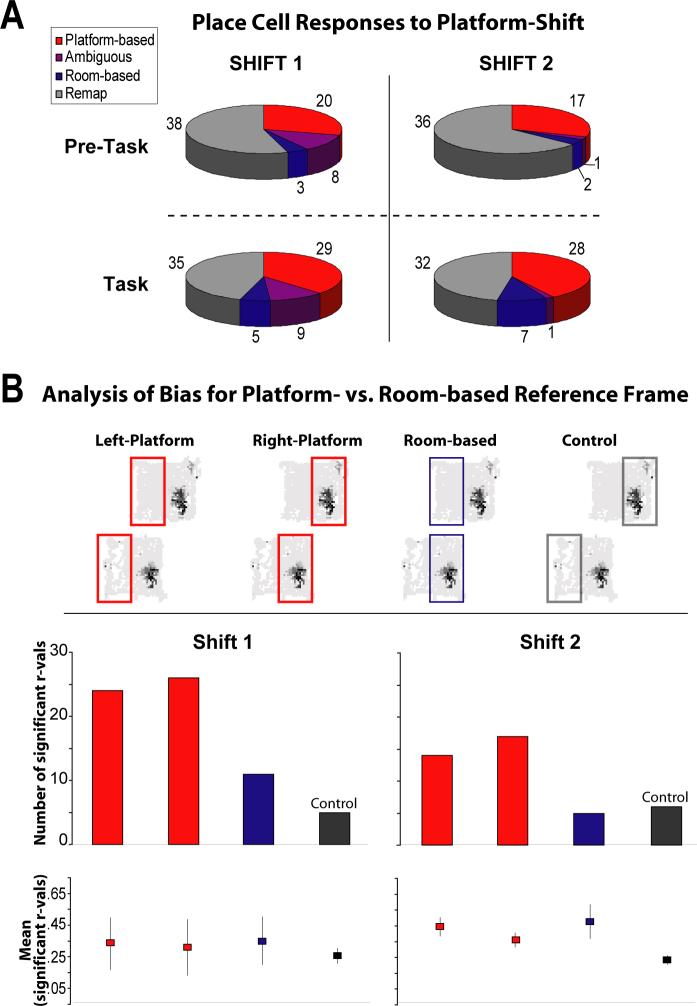
Although a categorization of cell responses cannot fully capture the dynamic place cell responses described above, relying on distinctions that become arbitrary at category boundaries, it is nonetheless useful in providing information about the overall responses of cells to shifting the platform within the room-based coordinate frame. Figure 5A shows the proportions of cells that fell into each response category for the pre-task and task epochs of Shift 1 and Shift 2. A chi-square test comparing the number of observations that fell into each response category for Shift 1 and Shift 2 (collapsed across the pre-task and task epochs) revealed a significant difference that could be directly attributed to the decrease in the number of cells that responded ambiguously (purple regions) to the platform-shift manipulation with a rat's second experience (χ2 = 10.37, df = 3, p = .02, the ambiguous response categories contributed to 91.6% of the statistic). In both Shift 1 and Shift 2 sessions, initiation of the task induced an apparent increase in the number of cells that were room-and platform-based and a decrease in the number of cells that remapped (compare Pre-Task to Task in Fig. 5A). However, a chi-square test comparing the number of observations that fell into each response category for these epochs (collapsed across Shifts 1 and 2) failed to reach significance (χ2 = 5.95, df = 3, p = .11).
Figure 5.
Proportions of place cells observed in each response category for platform-shift manipulations at the early (Shift 1) and late (Shift 2) phases of task acquisition, and the results of a control analysis used to investigate the apparent bias in the prevalence of platform-associated activity relative to room-associated activity. Note that rate maps from pre-task foraging were analyzed separately from task-associated rate maps. A Pie graphs showing the proportions of cells falling into each category during pre-task and task epochs for Shift 1 and Shift 2 (the raw numbers of cells observed in each category are given). Note the decrease in the number of cells displaying ambiguous activity between Shift 1 and Shift 2. The difference between Shift 1 and Shift 2 (collapsed across pre-task and task epochs) was significant (X2 = 10.37, p = .02), but the difference between pre-task and task (collapsed across Shift 1 and Shift 2) failed to reach significance (X2 = 5.95, p = .11). Chi-square comparisons between pairs of individual pie graphs could not be performed due to the low number of cells categorized as room-associated or ambiguous in the pre-task epochs, resulting in expected values that were below 5.0 for tabulated categories. B Rate map comparisons that controlled for the amount of sampled space were made to determine if the apparent bias toward platform-associated activity reflected a real bias in the way hippocampal cells coded for momentary position in the platform-shifted condition. A similar number of significant correlations were observed for left and right half-platform comparisons (compare red bars within histograms), indicating that place fields were homogeneously distributed across the platform. Fewer significant room-based correlations were observed relative to platform-based comparisons, suggesting a bias in the way cells responded to the shifted platform (compare blue bars to the red bars within each histogram). Significant room-based correlations occurred more frequently than predicted from the control comparisons for Shift 1, but were observed at chance levels for Shift 2 (compare blue bar to black bar within histograms). However, room-based comparisons for Shift 2 yielded significantly higher r-values than controls (bottom graph, see text), indicating that room-based activity was significantly more similar to standard conditions than expected based on chance correlations.
The higher proportion of place cells that displayed platform-associated activity relative to the proportion that displayed room-associated activity suggests a bias in favor of a platform-based representation in the hippocampus (O'Keefe and Burgess 1996; Lever et al. 2002; Knierim and Rao 2003; Yoganarasimha and Knierim 2005). However, in the room-based coordinate frame, only half of the space sampled during the standard condition was sampled in the platform-shifted condition. Therefore, even if the platform- and room-based coordinate frames were equally potent in determining the location of place-specific activity, one would expect to see approximately half as many cells displaying room-associated activity as platform-associated activity. Figure 5A shows that the number of cells that displayed platform-associated activity exceeded the number of cells that displayed room-associated activity by greater than the two-fold expected value in all platform-shifted conditions. However, the critical test to reveal biases in cell responses to the platform-shift manipulation was to restrict rate map comparisons to the half of the platform that was re-sampled in both the room- and platform-based coordinate frame in the shifted condition. For example, Figure 5B (top) shows that during the standard session (upper rate map), only the left half of the platform contained place fields that could display either platform-associated (left-platform) or room-associated (room-based) activity when the platform was shifted. Thus, if there was no bias in the binding of place-specific activity to the platform- or room-based coordinate frames, one would predict equal numbers of cells with significant rate map correlations for left-platform and room-based comparisons. (The goal was located on the left half for one group of rats and on the right half for the second group of rats. Note that the results of this analysis are presented as “left” and “right” platform-half comparisons to simplify discussion, but actually refer to the non-goal and goal platform-halves, respectively.)
The null hypothesis stated above was tested by dividing standard and shifted condition rate maps in half along the vertical axis and correlating the left half of the standard rate maps with (a) the left half of the shifted condition rate maps (to test for platform-associated activity), and (b) the right-half of the shifted condition rate maps (to test for room-associated activity). Figure 5B (bottom, histograms) shows that a greater number of cells displayed significant rate map correlations for left-platform comparisons (red, left bar) than room-based comparisons (blue bar) for both shift manipulations (Shift 1: 24 vs. 11, χ2 = 4.83, p = .03; Shift 2: 14 vs. 5, χ2 = 4.26, p = .04), suggesting a bias in the proportion of cells displaying platform-associated activity in the shifted condition. A similar number of cells displayed significant right-platform correlations as was observed for left-platform comparisons (Fig. 5B, compare red bars within graphs; χ2=0.37 and 0.37, p=0.95 and 0.95, for Shift 1 and 2 respectively), suggesting that there were no unexpected inhomogeneities in the distribution of place fields across the platform that could have affected the analysis of platform- or room-associated activity. As a control for the number of significant correlations that could occur by chance in this analysis, rate maps from the right-platform half of the standard session were compared with the left-platform half of the shifted session (which never overlapped in either coordinate frame). All comparisons showed a greater number of significant correlations than expected by chance, with the exception of room-based comparisons for Shift 2. However, the correlations observed for Shift 2 were significantly higher than those observed for the control comparisons (Fig 5B, bottom right plot, compareblue and black markers; Room: r = .46 ± .11, Control: r = .22 ± .03; Mann-Whitney U-test, tied Z = −2.01, tied p = 0.045, ties = 0). The control results suggests that although the number of cells that displayed room-associated activity was low compared to the number that displayed platform-associated activity, the room-associated activity of these individual cells was as robust as that observed for platform-associated activity, and therefore unlikely the result of spurious correlations.
The emergence of independent platform- and room-based spatial reference frames as a result of the platform-shift experience
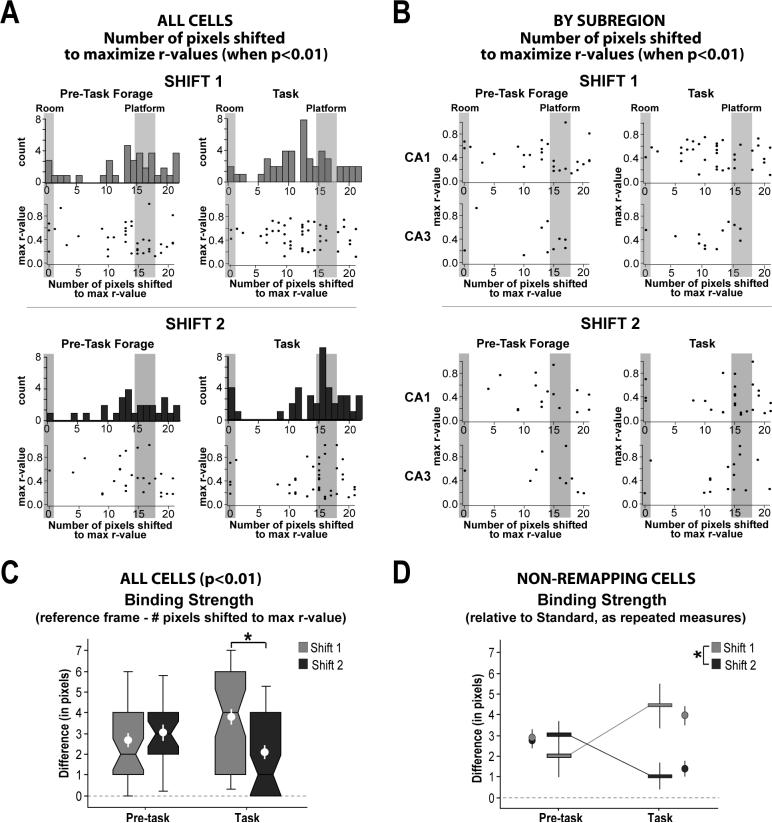
Although the majority of non-remapping cells displayed platform-associated activity, a small proportion of cells displaying room-associated activity was also observed within simultaneously recorded ensembles. The fact that few cells displayed ambiguous activity during the second platform-shift experience suggests that non-remapping cells developed independent room- and platform-based spatial frames of reference as a result of experiencing the room-platform dissociation. Histograms showing the number of pixel-shifts necessary to maximize rate map similarity across conditions for each cell support the predominance of platform-associated activity at both the early and late phases of task acquisition (Figure 6A). Rate map similarity between the standard and shifted conditions was maximized closer to the actual shift of the platform (gray area, right) for a majority of cells, suggesting a bias in the binding strength of place-specific activity to the platform-based coordinate frame relative to that of the room (gray area, left). A paired comparison for each cell was created by calculating the distance between the number of pixels shifted to maximize the r-value and the platform-based coordinate frame, and then separately for the room-based coordinate frame. A Wilcoxon signed rank test confirmed the observation that most cells displayed activity that was significantly closer to the platform-based coordinate frame than the room-based coordinate frame for all conditions (Z-values > 3.40, p < 0.001 for each histogram's data).
Figure 6.
The number of pixel-shifts necessary to maximize rate map similarity (r-values) between the standard and platform-shifted conditions for the pre-task and task epochs was used to examine the strength with which place cell activity was bound to either the room- or platform-based coordinate frame across experiences (only significant r-values are included).A Histograms based on Shift 1 data (light gray, top) reveal that during task conditions a majority of cells displayed place-specific activity that was more strongly bound to the platform-based coordinate frame (”Platform”) but that also was pulled in the direction of the room-based coordinate frame (”Room”). Histograms based on Shift 2 data (dark gray, bottom) reveal that during task conditions fewer observations fell in between the room- and platform-based coordinate frame, with most cells bound to either the room or platform coordinate frame with less interaction between the two. No differences were observed between r-values corresponding to the room- or platform-based coordinate frame, or at pixel-shifts that reflected an interaction between the two (scatter plots). B CA1 and CA3 cells did not respond differently to either the first or second platform-shift manipulation, and so were combined for further analysis (see text). C Notched box plots showing the binding strength of place cell activity to either the room- or platform-based coordinate frame (i.e., the difference between the number of pixels shifted to maximize r-values and the closest reference frame) for the pre-task epochs and task epochs of the first and second platform-shift experience. Note that lower values are indicative of stronger binding. Notched box plots were constructed from the median ± 25th and 75th IQRs (with bars giving the range and notches indicating 95% confidence intervals), and show the significantly skewed distributions observed in some cases. The mean ± SEM are also given (white markers). A significant difference in the binding of place-specific activity to either the platform- or room-based reference frame was observed between Shift 1 and Shift 2 only during task conditions. Although violations of the assumptions of a normal distribution and completely independent samples complicate the interpretation, a two-way ANOVA revealed a significant interaction (F1 = 8.02, p = .005) and a trend toward a main effect of Shift (F1 = 3.27, p = .07), in support of the analogous nonparametric tests (see text). The main effect of pre-task x task was not significant (F1 = 0.05, p = .82). D Limiting the analysis to cells that did not remap across task conditions (between the pre-task and task epochs, allowing for a repeated measure) revealed that on average individual cells became more strongly bound to either the room- or platform-based coordinate frame during Shift 2 with the initiation of task conditions. Horizontal bars reflect median ± confidence intervals. Means ± SEMs are given to the side (light and dark gray markers).
Although a bias in favor of platform-associated activity was observed, during Shift 1 task conditions (Fig. 6A,top right histogram) a substantial number of cells displayed maximum similarity at pixel-shifts that were intermediate to the platform-(gray, right) or room-based coordinate frames (gray, left) during task conditions. The data suggest an interaction in which platform-associated activity was pulled toward the room-based coordinate frame (see example cells given in Fig. 4A and B). It should also be noted that a majority of the cells that displayed maximum similarity that was biased toward the room-based coordinate frame also displayed significant correlations corresponding to the actual shift of the platform, and were considered ambiguous in the categorization scheme described above (3/4 cells within 2 pixel-shifts of the room-based coordinate frame). In contrast, a more bimodal distribution was observed during task conditions for Shift 2 (Fig. 6A, bottom right), with most cells displaying maximum similarity at pixel-shifts distributed around the platform- or room-based coordinate frame. None of the cells associated with the room-based coordinate frame were categorized as ambiguous for Shift 2. Histograms based on data from the pre-task epochs of Shift 1 and Shift 2 (Fig. 6A, left top and bottom) both reveal patterns of predominantly platform-associated activity, as suggested above. Similarly, many of the cells that displayed apparent room-associated activity during the pre-task epoch of Shift 1 also displayed significant correlations at the platform-based coordinate frame (3/5 cells).
Previous research has revealed greater coherence in the responses of CA3 cell ensembles to the dissociation of distal (room-associated) versus proximal (apparatus-associated) cues, relative to that observed for CA1 cell ensembles in the double-rotation paradigm (Lee et al. 2004). Figure 6B shows the number of pixels shifted to maximize correlation values for the pre-task foraging and task epochs of Shift 1 and Shift 2, separately for CA1 and CA3 cells. Interestingly, CA3 and CA1 did not differ in terms of the proportions of cells bound more strongly to the room- and/or platform-based coordinate frames, or that displayed an interaction between the two (based on binding error to either the room- or platform-based coordinate frame, see below; Kolmogorov-Smirnov 2-group tests, p > .5 for each CA1-CA3 comparison). Simultaneously recorded CA1 and CA3 ensembles from a given session both displayed discordant responses. Therefore, analyses and statistical tests were pooled across CA1 and CA3 cells for presentation of the results.
To quantify changes in the location of place-specific activity relative to the two possible coordinate frames between the first and second platform-shift manipulations, the difference between the number of pixels shifted to maximize r-values and the closest reference frame (relative to either the room or the platform) was calculated and compared across conditions. Place-specific activity was more strongly bound to either the platform- or room-based coordinate frame during the task for Shift 2 relative to Shift 1 (Fig. 6C, right pair of box plots; Kolmogorov-Smirnov 2-group test, χ2 = 10.31, df = 2, p = .01; note that lower values indicate greater binding strength). No difference in binding strength to the platform- or room-based coordinate frame was observed during the pre-task epochs between the first and second platform-shift experience (χ2 = 1.08, df = 2, p > .99; Fig. 6C, left pair of box plots). The increased binding of place-specific activity to either the platform- or room-based coordinate frame suggests the emergence of independent representations with the initiation of task conditions during the second platform-shift experience, with the platform-based representation dominant over the room-based representation.
The apparent increase in the binding of place-specific activity to either the room- or platform-based reference frame during the second platform-shift experience may have been due to the remapping of cells that were only weakly bound to either coordinate frame, rather than an increase in binding strength per se. To test whether the increase in binding strength resulted from selective remapping between the standard and platform-shifted condition we used a repeated measures test between the pre-task and task epoch for each platform-shift manipulation, limiting the analysis to cells that did not remap across conditions relative to standard sessions (i.e., analysis was limited to cells that displayed neither task- nor shift-associated remapping). A significant increase in binding strength to the platform- or room-based coordinate frame between the pre-task and task epochs was observed for Shift 2 (Fig. 6D, dark gray bars; n = 21 cells, Tie-corrected Z = −2.20, p = 0.03, ties = 4), with no significant difference for Shift 1 (light gray bars; n = 23 cells, Tie-corrected Z = 0.94, p = 0.35, ties = 4). Although the assumption of normality is violated, a repeated measures ANOVA supported a significant effect of Shift (F1 = 6.94, p = .01), in addition to a significant interaction (F1 = 10.32, p = .002; the main effect of pre-task x task was not significant, F1 = 0.14, p = .71; means are given by circle markers in Fig. 6D). The results suggest that individual cells displayed increased binding to either the platform- or room-based coordinate frame with the initiation of task conditions during the second shift experience.
Control comparisons for the shifted correlation analysis
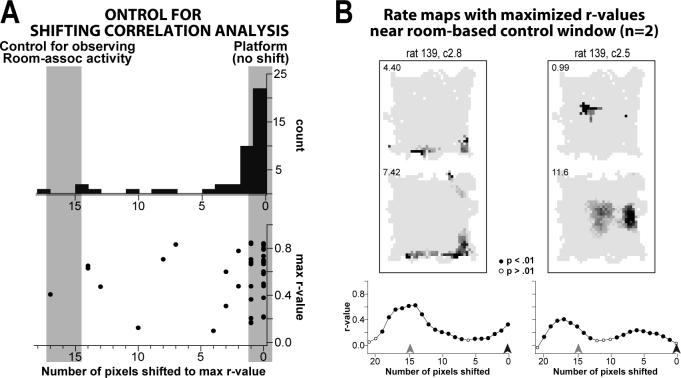
Figure 6A shows that the activity of a number of place cells was maximally correlated at pixel-shifts in-between the platform- and room-based coordinate frames, suggesting an interaction between the two reference frames. It is possible that the apparent interaction may be an artefact of the shifting correlation analysis, or may represent typical variability in the location of place-specific activity whenever an animal is reintroduced into an environment. To assess the likelihood of such artefacts, and to examine the degree of variability expected when an animal is placed back into an environment under platform-stable conditions, we reintroduced 4 of the rats back into the environment without shifting the platform following a standard training session. (Two rats were tested after the standard training session on the day after Shift 1, and 2 rats were tested after standard training on the day after Shift 2. Place cells from the two variations responded similarly.) We also wanted to assess whether the number of cells displaying room-associated activity during platform-shift manipulations could have been observed by chance in the shifting correlation analysis, due to the fewer number of pixels included in the correlation for the room-based coordinate frame. Because the platform was not shifted relative to the standard condition, the number of pixel-shifts representing the most accurately reproduced pattern of place-specific activity (corresponding to the position of the stable platform) was 0. A pixel-shift of 15 or 17 provides a control comparison to determine the likelihood of observing putative room-associated activity in the shifting correlation analysis.
The number of pixel-shifts necessary to maximize rate map similarity (r-values) between the standard and platform-stable conditions for each cell are plotted in Fig. 7A (the x-axis is reversed to facilitate visual comparisons with histograms for platform-shifted data given in Fig. 6A). Kolmogorov-Smirnov 2-group tests confirmed that the distribution given by the platform-stable control comparisons was significantly different than the distributions observed for pre-task and task conditions in both Shift 1 and Shift 2 (Fig. 7A and 6A; χ2 > 11.0 and p < .01 for all 4 comparisons; note that the control distribution was reflected for statistical tests). In the platform-stable control analysis, over 70% of rate map comparisons were maximized within 1 pixel-shift (32/44 cells; Fig. 7A, gray area to the right), suggesting that cells can accurately reproduce place-specific activity patterns to ±1 pixel in spatially stable conditions. Additionally, relatively few comparisons yielded maximized r-values greater than 2 pixel-shifts from platform-aligned comparisons. The latter observations suggest that the proportion of cells displaying significant maximized r-values in between the room- and platform-based coordinate frame observed for Shift 1 was unlikely to be the result of expected variability between sessions (compare Fig. 7A to histograms in Fig. 6A,top).
Figure 7.
Results of a control comparison for the shifting correlation analysis in which rats were reintroduced back into the environment with the platform still in its standard condition (i.e., the platform was not shifted within the room-based coordinate frame, see text), used to examine the degree to which accurate place-specific activity could be reproduced under spatially stable conditions (”Platform”) and as a control to investigate the likelihood that room-associated activity could have been observed by chance in the shifting correlation analysis (”Control”; x-axis is reversed to facilitate comparisons with data given in Fig. 6A). A Histogram of the number of pixel-shifts necessary to maximize rate map similarity (r-values) between the standard and platform-stable conditions (plotted in association with the corresponding r-values, scatter plot below). Most cells displayed place-specific activity that was accurate to within 1 pixel-shift under stable conditions (“Platform, no shift”). Additionally, few significant r-values were maximized near the control for room-based comparisons (at 15 pixels for one group of rats and 17 pixels for the second group, “Control”), suggesting that spurious correlations were unlikely to occur by chance. B Rate maps and graphs of shifting-correlation r-values for the 2 cells that displayed maximum r-values within 2 pixel-shifts of the control comparison for the room-based coordinate frame. The shifting correlation graphs for room-associated cells typically did not show the same pattern of results as the control comparisons yielded here (compare the graphs given here with those given in Fig. 4C), suggesting that most cells categorized as room-associated were not the result of similar spurious correlations.
Furthermore, only two of the platform-stable shifted correlations yielded maximized r-values within 2 pixel-shifts of the control for room-associated activity (gray area to the left; note that the control was 15 for one group of rats and 17 for the other group), only one of which would have been considered as displaying room-associated activity (the other cell would have been considered ambiguous; rate maps for those cells are given in Fig. 7B). The latter result further supports the interpretation of the previous control analysis (Fig. 5B) suggesting that even the relatively low number of cells displaying maximized r-values associated with the room-based coordinate frame in the task epoch of Shift 2 was unlikely to have been observed by chance.
Absence of goal-related activity
Some studies have shown that hippocampal place cells display goal-related activity (Breese et al. 1989; Kobayashi et al. 1997; Hollup et al. 2001; Hok et al. 2007). To examine whether cells displayed goal-associated activity in the present study, peri-event raster plots and cumulative histograms were examined for the 10 sec before and after feeder activations (which only occurred when a rat was at the goal location). The few cells that displayed consistent feeder-associated activity also displayed place-specific activity at the rewarded location in tone-off (inter-trial foraging) rate maps, suggesting that the apparent reward- or goal-associated increases in activity could be explained by a place field that happened to be at the goal location. Thus, in contrast to previous reports (e.g., Hok et al. 2007), we did not detect a preference for cells to fire at the goal location. Peri-event raster plots and cumulative histograms were also examined for the 10 sec before and after tone onsets. Peri-event raster plots and cumulative histograms did not reveal any tone-specific responses that were consistent across trials for either the early or late phases of task acquisition (data not shown).
DISCUSSION
Previous studies in which a circular or rectangular track was translated within the environment resulted in place-specific activity that was bound to the track, with little evidence of room-associated activity (Knierim and Rao 2003; Yoganarasimha and Knierim 2005). However, the behavioral tracks used in those studies provided little opportunity to investigate possible room-associated activity or interactions between the track- and room-based coordinate frames. The open field platform used in the current study allowed for at least a portion of room-associated space to be sampled in both the standard and platform-shifted conditions, and revealed place cell responses that initially reflected an interaction between a dominant, platform-based (proximal) coordinate frame and a weaker, room-based (distal) coordinate frame (Shift 1). With a rat's second experience (Shift 2) place cell activity became bound to either the platform- or room-based coordinate frame, but not both, suggesting the emergence of two independent spatial frames of reference (with the large majority of cells participating in the platform-based representation).
Evidence regarding the existence of multiple independent spatial reference frames represented simultaneously in the hippocampus has been previously observed (Shapiro et al. 1997; Tanila et al. 1997; Zinyuk et al. 2000; Knierim 2002). Zinyuk and colleagues (2000) dictated one spatial reference frame as relevant for successful task performance, yet evidence for both task-relevant (room-associated) and task-irrelevant (platform-associated) reference frames were observed within rats. The results of the current study suggest that this can occur with a single exposure to the dissociated condition. The present study also supported a substantial bias in favor of a platform-based representation during both pre-task foraging and navigational task epochs. Similarly, Zinyuk et al. reported that approximately one-third of active cells displayed platform-associated activity in rats navigating on a slowly rotating arena, even though only the room-based coordinate frame was relevant to successful task performance. In contrast to the current study, Zinyuk et al. reported that more than two-thirds of the cells displaying platform-associated activity in experienced, navigating rats also displayed room-associated activity. The latter observation may have resulted from dictating the room-based coordinate frame as task-relevant or from the extended experience of the rats in their rotating-platform task. Alternatively, room-associated activity may have been observed because that representation was directionally anchored to distal cues, with the precise location of place field activity still dictated by the boundaries of the circular apparatus even though the platform itself was spinning (i.e., the relationship between the boundaries of the platform and the distal cues actually did not change when the apparatus was rotating). Nevertheless, the current study supports previous observations that multiple frames of reference can be represented simultaneously in the hippocampus, and that each reference frame may not be given the same neural weight. It is currently unclear what the effects of such biases may be in regards to spatial cognition and/or behavioral choices, given that most rats made equally tentative choices between the platform- and room-associated goals during probe trials in the platform-shifted condition in the current study (but see Weisend et al. 1995).
The results reported here can be interpreted in the framework of the Boundary Vector Cell (BVC) model of Burgess, O'Keefe, and colleagues (O'Keefe and Burgess 1996; Hartley et al. 2000; Barry et al. 2006). The BVC model postulates a class of cells that encode the distance and allocentric bearing of the rat relative to environmental boundaries. According to the model, a BVC is active whenever the rat is a specific distance from its associated boundary, and thus would display an apparent stripe-like “place field” parallel to that boundary at that distance. The theory proposes that each place cell receives inputs from a number of BVCs. The restricted place field of a hippocampal cell results from a thresholded sum of multiple BVC inputs. Although the existence of putative BVCs has not been conclusively demonstrated (but see Barry et al. 2006), the model accounts for an impressive number of experimental findings and has been shown to predict the responses of place fields to novel manipulations (Hartley et al. 2000). For our purposes, a key component of the model is that boundaries that are closer to the rat have more influence on place cells than boundaries that are farther away. This bias may explain the results of the current experiment. The majority of place fields followed the platform because they were most strongly bound to the boundaries of the platform. The data further suggest that at least a few place cells receive inputs only from BVCs that encode the room-associated (distal) boundaries, because a small minority of place cells displayed room-associated activity in the shifted condition (O'Keefe and Burgess 1996). Similarly, cells that receive inputs from both proximal- and distal-associated BVCs may show an interaction between both the room and platform frames of reference. The changes in platform- and room-associated activity between Shift1 and Shift2 and between the pre-task foraging and task epochs suggests that the relative strength of inputs from different BVCs can be modified due to experience or changes in attention (Barry et al. 2006; see also Lever et al., 2002; Rivard et al., 2004).
Interestingly, we did not observe a dissociation between the responses of CA1 and CA3 cells to the platform-shift manipulation. Previous reports from this lab observed greater concordance within CA3 ensembles in response to a double-rotation of local versus distal cue sets, relative to CA1 cell ensembles (Lee et al. 2004). In the previous work, the place fields of some CA1 cells rotated with local cues, while others rotated with distal cues or displayed activity associated with both cue sets within ensembles (also see Knierim 2002). In contrast, the place fields of CA3 cells rotated more coherently within ensembles, most often rotating with the local cue set. The dissociation in the way CA3 and CA1 ensembles responded to the double-rotation paradigm suggest that CA1 cells were not heavily influenced by their CA3 afferents under those experimental conditions. In the current study, CA1 and CA3 cells displayed similar degrees of concordance in response to the platform-shift manipulations. Both subregions were clearly dominated by platform-associated activity, and both contained a minority of cells that fired in association with the room-based coordinate frame within ensembles. Because a majority of CA3 and CA1 cell responses were associated with local cues (i.e., the platform), it is possible that the paradigm used here represents a case in which CA1 cells were influenced by Schaffer collateral input from CA3. Unfortunately, the high degree of remapping within ensembles during the pre-task epoch in this study makes it difficult to determine if the difference was due to the use of a navigational task versus the track-running paradigm used in the previous study because individual ensembles did not have enough active cells from both subregions during the pre-task epoch to allow for a proper comparison across ensembles. Alternatively, the absence of a CA1-CA3 dissociation in the current study may have been due to differences in the nature of the manipulation (cue rotations versus platform translations). The CA1 region receives input from Layer III cells of the entorhinal cortex, which have been shown to display space-specific (grid cells) and/or orientation-specific (head direction cells) activity. In contrast, the CA3 region receives its major entorhinal cortex input from Layer II cells, which display space-specific (grid cell) activity but no head direction specificity (Sargolini et al. 2006). This pattern of connectivity is consistent with the idea that the discordant responses observed within CA1 ensembles in double-rotation paradigms (Shapiro et al. 1997; Tanila et al. 1997; Knierim 2002; Lee et al. 2004) may have been due to the influence of the distal cue set in orienting representations of space via head direction activity (Yoganarasimha and Knierim 2005; Yoganarasimha et al. 2006). Based on this hypothesis, a similar response would not be predicted for CA1 ensembles in the paradigm used here because the orientation of the distal cue set was not manipulated when the platform was translated within the environment, resulting in a coherent platform-dominated ensemble response in CA1 similar to that consistently observed for CA3 across paradigms.
Partial remapping of hippocampal representations with changes in task demands
Similar to previous studies, changes in task demands or spatial manipulations within the same environment induced partial remapping in ensembles of hippocampal place cells recorded from rats (Markus et al. 1995; Knierim et al. 1995; Tanila et al., 1997; Shapiro et al. 1997; Skaggs and McNaughton 1998; Wood et al. 2000; Knierim and McNaughton 2001; Anderson and Jeffery 2003; Ferbinteanu and Shapiro 2003; Knierim and Rao 2003; Lee et al. 2004; Smith and Mizumori 2006). The observed rates of remapping reported here across the different conditions are in general agreement with previous observations (Markus et al. 1995; Shapiro et al. 1997; Knierim and Rao 2003; Lee et al. 2004). It is noteworthy that partial remapping was observed between the pre-task and task epochs even though most of the task epoch was actually dominated by the same kind of unstructured foraging behavior that occurred in the pre-task epoch. Partial remapping between the pre-task and task epochs suggests that the behavioral context, and not the behavior per se, was represented by different but overlapping spatial reference frames in the hippocampus. Furthermore, the relative absence of partial remapping between periods of navigation and foraging for reward within the task epoch provides further support that remapping within the hippocampus does not necessarily reflect momentary changes in behavioral state. Rather, the observed pattern of partial remapping more likely reflects the cognitive organization of ongoing experience within a given environment. In theory, a partial change in the spatial reference frame associated with a given environment/condition could serve to disambiguate experiences that occur within the same environment while still providing access to the learned associations that previously occurred in that environment. The failure to observe deficits in task performance under conditions of substantial partial remapping has been given as evidence to the latter (Jeffery et al. 2003; Kentros et al. 2004).
In addition to task-associated partial remapping, researchers have also reported within-task partial remapping, which we did not observe here (Wood et al. 2000; Frank et al. 2000; Lee et al. 2006; but see Lenck-Santini et al. 2001; Bower et al. 2005; Griffin et al. 2007). Additionally, we did not observe goal-related activity similar to that reported in previous studies (Hollup et al. 2001; Ferbinteanu and Shapiro 2003; Smith and Mizumori 2006). In a place preference task similar to our paradigm (minus the tone initiation of trials), Hok and colleagues (2007) observed a number of place fields at the goal location during navigation that were not observed during random foraging between trials. However, we closely examined peri-event histograms time-locked to reward delivery and saw very few cells with activity peaks around this time. Moreover, navigating and task-foraging rate map comparisons for those cells yielded relatively high r-values with comparably sized place fields, suggesting that neither trial- nor goal-specificity was observed in the paradigm used here. The differences between the present results and the results of Hok et al. (2007) likely reflect differences in how rats cognitively organized their experiences, perhaps established very early in training (e.g., also compare Wood et al. 2000, with Lenck-Santini et al. 2001, and Bower et al. 2005).
Summary
In previous research, the results of translating a behavioral apparatus within the experimental room suggested that distal cues may be used as a directional frame of reference, with local cues (the apparatus boundaries) preferentially used to determine momentary position in space. The current study supports the dominance of the local coordinate frame in defining the precise locations of place-specific activity. However, the results reported here also show that place field activity can be bound to distal cues as well, although more weakly, because shifting the behavioral apparatus resulted in platform-associated activity that was pulled toward the room-based coordinate frame. Furthermore, the results provide evidence regarding ongoing plasticity in hippocampal representations of space, even in well-experienced environments. We show here that a single experience (e.g., the dissociation of cue sets) can induce a restructuring of the spatial framework associated with that environmental context, resulting in the development of an independent spatial frame of reference as a result of that experience.
Acknowledgements
The authors gratefully acknowledge Geeta Rao, Inah Lee, Eric Roth and Xintian Yu for expert technical assistance, and the Neural Systems & Behavior “Space Rats” students of 2004 for collaboration in behavioral training and data acquisition. This work was supported by the National Institutes of Health grants R01 NS39456, K02 MH63297, T32 NS41226, and T32 NS007467-07.
REFERENCES
- Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. J Neurosci. 2003;23:8827–8835. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MI, Killing S, Morris C, O'Donoghue A, Onyiagha D, Stevenson R, Verriotis M, Jeffery KJ. Behavioral correlates of the distributed coding of spatial context. Hippocampus. 2006;16:730–742. doi: 10.1002/hipo.20206. [DOI] [PubMed] [Google Scholar]
- Barry C, Lever C, Hayman R, Hartley T, Burton S, O'Keefe J, Jeffery K, Burgess N. The boundary vector cell model of place cell firing and spatial memory. Rev Neurosci. 2006;17:71–97. doi: 10.1515/revneuro.2006.17.1-2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett HC, McCutchan K, Matthews R. Spatial learning in the T-maze; the influence of direction, turn, and food location. J Exp Psychol. 1949;39:800–809. doi: 10.1037/h0058978. [DOI] [PubMed] [Google Scholar]
- Bower MR, Euston DR, McNaughton BL. Sequential-context-dependent hippocampal activity is not necessary to learn sequences with repeated elements. J Neurosci. 2005;25:1313–1323. doi: 10.1523/JNEUROSCI.2901-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Hampson RE, Deadwyler SA. Hippocampal place cells: stereotypy and plasticity. J Neurosci. 1989;9:1097–1111. doi: 10.1523/JNEUROSCI.09-04-01097.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bures J, Fenton AA. Neurophysiology of Spatial Cognition. News Physiol Sci. 2000;15:233–240. doi: 10.1152/physiologyonline.2000.15.5.233. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Griffin AL, Eichenbaum H, Hasselmo ME. Spatial representations of hippocampal CA1 neurons are modulated by behavioral context in a hippocampus-dependent memory task. J Neurosci. 2007;28:2416–2423. doi: 10.1523/JNEUROSCI.4083-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hartley T, Burgess N, Lever C, Cacucci F, O'Keefe J. Modelling place fields in terms of the cortical inputs to the hippocampus. Hippocampus. 2000;10:369–379. doi: 10.1002/1098-1063(2000)10:4<369::AID-HIPO3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Hok V, Lenck-Santini PP, Roux S, Save E, Muller RU, Poucet B. Goal-related activity in hippocampal place cells. J Neurosci. 2007;27:472–482. doi: 10.1523/JNEUROSCI.2864-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollup SA, Molden S, Donnett JG, Moser MB, Moser EI. Accumulation of hippocampal place fields at the goal location in an annular watermaze task. J Neurosci. 2001;21:1635–1644. doi: 10.1523/JNEUROSCI.21-05-01635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne MR, Martin GM, Harley CW, Skinner DM. Where am I? Distal cue use requires sensitivity to start location change in the rat. J Exp Psychol Anim Behav Process. 2007;33:92–99. doi: 10.1037/0097-7403.33.2.92. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ, Gilbert A, Burton S, Strudwick A. Preserved performance in a hippocampal-dependent spatial task despite complete place cell remapping. Hippocampus. 2003;13:175–189. doi: 10.1002/hipo.10047. [DOI] [PubMed] [Google Scholar]
- Jeffery K, O'Keefe J. Learned interaction of visual and idiothetic cues in the control of place field orientation. Exp Brain Res. 1999;127:151–161. doi: 10.1007/s002210050785. [DOI] [PubMed] [Google Scholar]
- Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42:283–295. doi: 10.1016/s0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- Knierim JJ. Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. J Neurosci. 2002;22:6254–6264. doi: 10.1523/JNEUROSCI.22-14-06254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS, McNaughton BL. Place cells, head direction cells, and the learning of landmark stability. J Neurosci. 1995;15:1648–1659. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, McNaughton BL. Hippocampal place-cell firing during movement in three-dimensional space. J Neurophysiol. 2001;85:105–116. doi: 10.1152/jn.2001.85.1.105. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Rao G. Distal landmarks and hippocampal place cells: effects of relative translation versus rotation. Hippocampus. 2003;13:604–617. doi: 10.1002/hipo.10092. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Nishijo H, Fukuda M, Bures J, Ono T. Task-dependent representations in rat hippocampal place neurons. J Neurophysiol. 1997;78:597–613. doi: 10.1152/jn.1997.78.2.597. [DOI] [PubMed] [Google Scholar]
- Lee I, Griffin AL, Zilli EA, Eichenbaum H, Hasselmo ME. Gradual translocation of spatial correlates of neuronal firing in the hippocampus toward prospective reward locations. Neuron. 2006;51:639–650. doi: 10.1016/j.neuron.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- Lenck-Santini PP, Save E, Poucet B. Place-cell firing does not depend on the direction of turn in a Y-maze alternation task. Eur J Neurosci. 2001;13:1055–1058. doi: 10.1046/j.0953-816x.2001.01481.x. [DOI] [PubMed] [Google Scholar]
- Leonard BW, McNaughton BL. Spatial representation in the rat: conceptual, behavioral, and neurophysiological perspectives. In: Olton DS, Kesner RP, editors. Neurobiology of comparative cognition. Erlbaum; Hillsdale, NJ: 1990. [Google Scholar]
- Lever C, Willis T, Cacucci F, Burgess N, O'Keefe J. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature. 2002;416:90–94. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Barnes CA, McNaughton BL, Gladden VL, Skaggs WE. Spatial information content and reliability of hippocampal CA1 neurons: effects of visual input. Hippocampus. 1994;4:410–421. doi: 10.1002/hipo.450040404. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Qin YL, Leonard B, Skaggs WE, McNaughton BL, Barnes CA. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, O'Keefe J, Barnes CA. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J Neurosci Methods. 1983;8:391–397. doi: 10.1016/0165-0270(83)90097-3. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature. 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Conway DH. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; Oxford: 1978. [Google Scholar]
- Pisula W, Siegel J. Exploratory behavior as a function of environmental novelty and complexity in male and female rats. Psychol Rep. 2005;97:631–638. doi: 10.2466/pr0.97.2.631-638. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Muller RU, Kubie JL. The firing of hippocampal place cells in the dark depends on the rat's recent experience. J Neurosci. 1990;10:2008–2017. doi: 10.1523/JNEUROSCI.10-06-02008.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudineau S, Poucet B, Save E. Flexible use of proximal objects and distal cues by hippocampal place cells. Hippocampus. 2007;17:381–395. doi: 10.1002/hipo.20277. [DOI] [PubMed] [Google Scholar]
- Rivard B, Li Y, Lenck-Santini PP, Poucet B, Muller RU. Representation of objects in space by two classes of hippocampal pyramidal cells. J Gen Physiol. 2004;124:9–25. doi: 10.1085/jgp.200409015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonovich A, McNaughton BL. Path integration and cognitive mapping in a continuous attractor neural network model. J Neurosci. 1997;17:5900–5920. doi: 10.1523/JNEUROSCI.17-15-05900.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B. Hippocampal-partietal cortical interactions in spatial cognition. Hippocampus. 2000;10:491–499. doi: 10.1002/1098-1063(2000)10:4<491::AID-HIPO16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Tanila H, Eichenbaum H. Cues that hippocampal place cells encode: dynamic and hierarchical representation of local and distal stimuli. Hippocampus. 1997;7:624–642. doi: 10.1002/(SICI)1098-1063(1997)7:6<624::AID-HIPO5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Siegel JJ, Nitz D, Bingman VP. Spatial-specificity of single-units in the hippocampal formation of freely moving homing pigeons. Hippocampus. 2005;15:26–40. doi: 10.1002/hipo.20025. [DOI] [PubMed] [Google Scholar]
- Siegel JJ, Nitz D, Bingman VP. Lateralized functional components of spatial cognition in the avian hippocampal formation: evidence from single-unit recordings in freely moving homing pigeons. Hippocampus. 2006;16:125–140. doi: 10.1002/hipo.20139. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Spatial firing properties of hippocampal CA1 populations in an environment containing two visually identical regions. J Neurosci. 1998;18:8455–8466. doi: 10.1523/JNEUROSCI.18-20-08455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Gothard KM, Markus EJ. An information-theoretic approach to deciphering the hippocampal code. In: Hanson SJ, Cowan JD, Giles CL, editors. Advances in neural information processing systems. Morgan Kaufman; San Mateo, CA: 1993. [Google Scholar]
- Smith DM, Mizumori SJ. Learning-related development of context-specific neuronal responses to places and events: the hippocampal role in context processing. J Neurosci. 2006;26:3154–3163. doi: 10.1523/JNEUROSCI.3234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanila H, Shapiro ML, Eichenbaum H. Discordance of spatial representation in ensembles of hippocampal place cells. Hippocampus. 1997;7:613–623. doi: 10.1002/(SICI)1098-1063(1997)7:6<613::AID-HIPO4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr. Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. JNeurosci. 1990;10:436–447. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A, Wolfowitz J. On a Test Whether Two Samples are from the Same Population. Ann of Math Stats. 1940;11:147–162. [Google Scholar]
- Weisend MP, Klein RL, Hoesing JM, Astur RS, Koerner A, McDonald RJ, Geving T, Peinado J, Biela J, McWhorter J, Weems M, Schlegelmilch J, Yeo R, Sutherland RJ. Morris water task: which cues define locations? Soc Neurosci Abs. 1995;21:1939. [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Yoganarasimha D, Knierim JJ. Coupling between place cells and head direction cells during relative translations and rotations of distal landmarks. Exp Brain Res. 2005;160:344–359. doi: 10.1007/s00221-004-2016-9. [DOI] [PubMed] [Google Scholar]
- Yoganarasimha D, Yu X, Knierim JJ. Head direction cell representations maintain internal coherence during conflicting proximal and distal cue rotations: comparison with hippocampal place cells. J Neurosci. 2006;26:622–631. doi: 10.1523/JNEUROSCI.3885-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BJ, Fox GD, O'Keefe JM. Correlates of hippocampal complex-spike cell activity in rats performing a nonspatial radial maze task. J Neurosci. 1994;14:6553–6563. doi: 10.1523/JNEUROSCI.14-11-06553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinyuk L, Kubik S, Kaminsky Y, Fenton AA, Bures J. Understanding hippocampal activity by using purposeful behavior: place navigation induces place cell discharge in both task-relevant and task-irrelevant spatial reference frames. Proc Natl Acad Sci USA. 2000;97:3771–3776. doi: 10.1073/pnas.050576397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugaro MB, Berthoz A, Wiener SI. Background, but not foreground, spatial cues are taken as references for head direction responses by rat anterodorsal thalamus neurons. J Neurosci. 2001;21:RC154. doi: 10.1523/JNEUROSCI.21-14-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]