Abstract
Changes in dietary sodium intake are associated with changes in vascular volume and reactivity that may be mediated, in part, by alterations in endothelial nitric oxide synthase (eNOS) activity. Caveolin-1 (Cav-1), a transmembrane anchoring protein in the plasma membrane caveolae, binds eNOS and limits its translocation and activation. To test the hypothesis that endothelial Cav-1 participates in the dietary sodium-mediated effects on vascular function, we assessed vascular responses and nitric oxide (NO)-medicated mechanisms of vascular relaxation in Cav-1 knockout mice (KO, Cav-1-/-) and wild type control mice (WT, Cav-1+/+) placed on high salt (HS, 4% NaCl) or low salt (LS, 0.08% NaCl) diet for 16 days. After measuring the systolic blood pressure (BP), the thoracic aorta was isolated for measurement of vascular reactivity and NO production, and the heart was used for measurement of eNOS expression and activity. The BP was elevated in HS mice treated with L-NAME and more so in Cav-1-/- than WT mice, and was significantly reduced during LS diet. Phenylephrine (Phe) caused vascular contraction that was significantly reduced in Cav-1-/- (max 0.25±0.06 g/mg) compared with WT (0.75±0.22 g/mg) on HS diet, and the differences were eliminated with LS diet. Also, vascular contraction in response to membrane depolarization by high KCl (96 mM) was reduced in Cav-1-/- (0.27± 0.05 g/mg) compared with WT mice (0.53±0.12 g/mg) on HS diet, suggesting that the reduced vascular contraction is not limited to a particular receptor. Acetylcholine (Ach) (10-5 M) caused aortic relaxation in WT mice on HS (23.6±3.5%) and LS (23.7±5.5%), that was enhanced in Cav-1-/- HS (72.6±6.1%) and more so in Cav-1-/- LS mice (93.6±3.5%). RT-PCR analysis indicated increased eNOS mRNA expression in the aorta and heart, and Western blots indicated increased total eNOS and phosphorylated eNOS in the heart of Cav-1-/- compared to WT mice on HS diet, and the genotypic differences were less apparent during LS diet. Thus, Cav-1 deficiency during HS diet is associated with decreased vasoconstriction, increased vascular relaxation, and increased eNOS expression/activity, and these effects are altered during LS diet. The data support the hypothesis that endothelial Cav-1, likely through an effect on eNOS activity, plays a prominent role in the regulation of vascular function during substantial changes in dietary sodium intake.
Introduction
High salt (HS) diet is often associated with increased vascular volume (de Wardener et al., 2004). The volume overload triggers suppression of the renin-angiotensin-aldosterone system (RAAS), leading to increased salt and water excretion and restoration of vascular volume towards normal (Guyton, 1991; Hall, 1986; Hall et al. 1980; Meneton et al., 2005). High dietary sodium may also promote vasoconstriction by changing plasma osmolarity, nuclear localization and increased nuclear expression of vasoconstrictive stimuli such as endothelin-1, release of ouabain-like factor, and activation of the sodium/calcium exchange mechanisms (Arnon et al., 2000; Barron et al., 2002; Iwamoto et al., 2004; Payne et al., 2004; Khalil, 2006). Other studies have suggested that high dietary sodium may also affect vascular nitric oxide (NO) production (Giardina et al., 2001).
Caveolin-1 (Cav-1) is a transmembrane protein identified in the plasma membrane caveolae of many cell types including endothelial cells, vascular smooth muscle (VSM), and the heart (Feron et al., 1996; Minshall et al., 2003; Schnitzer et al., 1995; Stan et al., 1997; Ishizaka et al., 1998). Cav-1 is an important regulator of vasoconstrictive signaling via α-adrenergic, angiotensin II (AngII) and endothelin receptors (Ushio-Fukai et al., 2005; Zuo et al., 2005; Ushio-Fukai & Alexander, 2006; Shakirova et al, 2006). Evidence also suggests a role of Cav-1 in the anchoring of eNOS to caveolae, thus limiting its translocation and phosphoactivation (Feron et al., 1998; Segal et al., 1999; Batova et al., 2006). These studies have suggested a role of Cav-1 in the regulation of vascular function and growth (Drab et al., 2001; Razani et al., 2001; Yu et al., 2006) as well as in the normal systolic and diastolic cardiac function (Wunderlich et al., 2006).
Although both HS and Cav-1 appear to affect vascular function and the NO pathway, their potential interactions on the NO-dependent mechanism of vascular relaxation and blood pressure (BP) regulation are unclear. We reasoned that if Cav-1 and HS function through independent mechanisms, then changing dietary sodium intake should have similar vascular effects in wild-type (WT, Cav-1+/+) and Cav-1 knock-out mice (KO, Cav-1-/-) mice. On the other hand, if Cav-1 and dietary sodium intake are functionally-linked then the effects of changing dietary sodium on vascular function should differ in Cav-1 KO and WT mice. If the latter possibility is true, then the HS-related differences in vascular function between the Cav-1 KO and WT mice should be minimized/improved with salt restriction.
The purpose of this study was to test the hypothesis that Cav-1, by affecting eNOS activity, plays a role in modulating vascular function during substantial changes in dietary sodium intake. We used WT and Cav-1 KO mice placed on a HS or a low salt (LS) diet to investigate: 1) whether the BP and vascular contraction are altered in Cav-1-/- versus WT mice on HS diet, 2) whether vascular relaxation is modified in Cav-1-/- versus WT mice on HS diet, 3) whether the changes in BP, vascular contraction and relaxation in Cav-1-/- versus WT mice during HS diet reflect changes in the expression/activity of eNOS and NO production, and 4) whether the effects on BP, vascular function and the NO pathway associated with Cav-1 deficiency and HS diet are minimized/improved during salt restriction.
Material and Methods
Animals
Twelve-week-old KO (Cav-1-/-) and genetically matched WT (Cav-1+/+) male mice (stock number: 004585 and 101045, respectively) were purchased from Jackson Laboratories, Bar Harbor, ME. The genotypes were confirmed by PCR according to Jackson Laboratories’ guidelines. Animals were housed in the animal facility in 12 hr/12 hr light/dark cycle, at an ambient temperature of 22±1°C and were maintained on ad libitum Purina Rodent Chow (5053, 0.8% NaCl, Purina, St. Louis, MO) and tap water. After 3 days of acclimatization, mice from each genotype were randomized to either HS (4% NaCl) or LS (0.08% NaCl) diets for 5 days to achieve sodium balance (Holtzman et al., 1988; Oliverio et al., 2000), then maintained on the respective diets for 11 more days. A subgroup of the HS mice was treated with the NOS inhibitor L-NAME (0.2 mg/ml in drinking water) for 11 days. All experimental procedures followed the guidelines of, and were approved by, the Institutional Animal Care and Use Committee at Harvard Medical School.
Blood pressure (BP)
Systolic BP was measured in conscious mice after reaching sodium balance on days 0, 7 and 11 using tail-cuff plethysmography (blood pressure analyzer, model 179, IITC LifeScience, Woodland Hills, CA). Mice were kept warm at 37°C for 10 min and allowed to rest quietly before BP measurements. The BP measurements were taken in mice kept calm and handled by the same person. No sedation was used.
Tissue preparation
After measuring the BP on day 11, mice were euthanized under deep anesthesia with isofluorane, the thoracic and abdominal cavity was opened, and the aorta and the heart were rapidly excised. The thoracic aorta was placed in oxygenated Krebs solution, carefully dissected and cleaned of connective tissue under microscopic visualization, and cut into 3 mm-wide rings. The heart and abdominal aorta were placed in liquid nitrogen immediately after collection in preparation for mRNA and protein analysis.
Isometric contraction
Aortic segments were suspended between two tungsten wire hooks, one hook is fixed at the bottom of a tissue bath and the other hook is connected to a Grass force transducer (FT03, Astro-Med Inc., West Warwick, RI). Aortic segments were stretched under 0.5 g of resting tension and allowed to equilibrate for 45 min in a temperature controlled, water-jacketed tissue bath, filled with 50 ml Krebs solution continuously bubbled with 95% O2 5% CO2 at 37° C. The changes in isometric contraction were recorded on a Grass polygraph (Model 7D, Astro-Med).
After tissue equilibration, a control contraction in response to 96 mM KCl was elicited. Once maximum KCl contraction was reached the tissue was rinsed with Krebs 3 times, 10 min each. The control KCl-induced contraction followed by rinsing in Krebs was repeated twice. Aortic segments were stimulated with increasing concentrations of phenylephrine (Phe, 10-9-10-5 M), concentration-contraction curves were constructed, and the maximal Phe contraction (in g/mg tissue weight) and ED50 were calculated. In other experiments the tissues were contracted with a submaximal concentration of Phe, increasing Ach concentrations were added (10-9-10-5 M) and the relaxation of Phe contraction was observed. In another set of experiments, Phe concentration-contraction curves and Ach concentration-relaxation curves were constructed in endothelium-denuded aortic segments, and the responses were compared with those in endothelium-intact segments. Other tissues were stimulated with AngII (10-7 M) and the maximal contraction was measured.
Analysis of eNOS mRNA expression by real time RT-PCR
Total mRNA was extracted from the aorta and heart using the RNeasy mini kit (QIAGEN Sciences, Germantown, MD). cDNA was synthesized from 1.5 μg RNA with the first-strand cDNA synthesis kit (GE Healthcare, Piscataway, NJ). PCR amplification reactions were performed in duplicate using the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) and using the ΔΔCT method to determine mRNA levels. The gene expression data were normalized to 18S rRNA levels. PCR amplification to detect eNOS and 18S rRNA was performed with TaqMan gene expression assays (proprietary primers and probes designed and synthesized by Applied Biosystems). Data are presented as fold increase relative to the measurement in WT mice on HS diet.
Western blot analysis
Protein was extracted by homogenizing cardiac tissue with RIPA lysis buffer (Santa Cruz Biotechnology Inc, Santa Cruz, CA). Protein extracts (40 μg) were combined with an equal volume of 2X Laemmli loading buffer, boiled for 5 min, and size-fractionated by electrophoresis on 7.5% SDS-polyacrylamide gels. Proteins were transferred from the gel to a nitrocellulose membrane by electroblotting. Membranes were incubated with 5% non-fat dried milk in TBS-Tween (USB Corporation, Cleveland, OH) for 1h and then incubated overnight at 4°C with mouse anti-eNOS antibody (1:2500, BD Transduction Laboratories, San Diego, CA) or rabbit anti-peNOS antibody (1:1000, Cell Signaling Technology, Danvers, MA). Cav-1 was detected using a mouse anti-Cav-1 antibody (1:1000, BD Transduction Laboratories). After incubation in the primary antibody solution, samples were washed, incubated with peroxidase-conjugated secondary antibody, and analyzed using enhanced chemiluminescence (Perkin-Elmer Life Sciences, Boston, MA). The blots were subsequently re-probed for β-actin (1:5000 dilution) and the results for eNOS, peNOS and Cav-1 were normalized to β-actin to correct for loading. The immunoreactive bands were analyzed quantitatively by optical densitometry, and the densitometry values represented the pixel intensity.
Solutions and drugs
Krebs solution contained (in mM): NaCl 120, KCl 5.9, NaHCO3 25, NaH2PO4 1.2, dextrose 11.5, CaCl2 2.5, MgCl2 1.2, at pH 7.4, and bubbled with 95% O2 and 5% CO2. 96 mM KCl was prepared as Krebs solution with equimolar substitution of NaCl with KCl. Stock solutions of Phe, acetylcholine (Ach), and AngII (Sigma, St. Louis, MO) were prepared in distilled water. All other chemicals were of reagent grade or better.
Statistical Analysis
The data were analyzed using 2-way ANOVA (Cav-1 status vs. salt intake) and presented as means±SEM. Scheffe’s F test was used for comparison of multiple means. Pairwise comparison was made when a significant interaction effect was noted. Student’s t-test for unpaired data was used for comparison of two means. Differences were considered statistically significant if P < 0.05. All studies were completed with the individual performing the study blinded as to the treatment group and genotype of the animal from which the tissue was isolated.
RESULTS
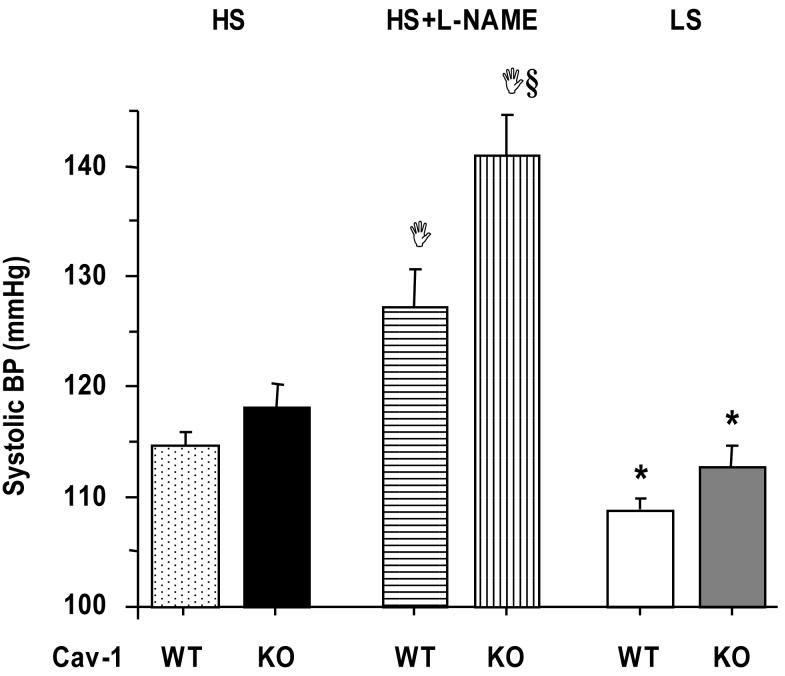
The systolic BP was not significantly different between Cav-1-/- and WT mice on HS diet (Fig. 1). However, when the HS mice were treated with L-NAME a dramatic increase in BP was observed particularly in the Cav-1 KO mice, suggesting increased NOS activity in association with Cav-1 deficiency and HS diet. The BP was significantly reduced in Cav-1-/- and WT mice on LS compared with HS diet, and no differences were observed between the WT and Cav-1 KO mice on LS diet (Fig. 1).
Fig.1.
Systolic BP in WT and Cav-1-/- mice on HS and LS diet, and on HS diet plus L-NAME in drinking water. Data represent means±SEM of measurements in 6 to 31 mice.
§ Measurements in Cav-1-/- are significantly different (p<0.05) from corresponding measurements in WT.
Ι Measurements in HS+L-NAME are significantly different (p<0.05) from corresponding measurements in HS.
* Measurements in LS are significantly different (p<0.05) from corresponding measurements in HS.
The total body weight was significantly reduced in the KO as compared to the WT mice on both HS and LS diet (Table 1). The body weight was slightly but not significantly greater in mice on HS diet as compared to LS diet. The weight gain with HS was ∼2g and appeared to be similar in both the WT and KO mice. The weights of the isolated aortic rings were significantly greater in Cav-1 KO on HS diet as compared to the WT on HS diet or the Cav-1 KO on LS diet (Table 1). Interestingly, the Cav-1 KO mice on HS diet have also shown the most significant increase in BP, suggesting a possible relationship between the aortic mass and the increased BP. Although the precision by which the lengths of aortic segments are cut may make the weight be different, whether the increased tissue weight in the Cav-1 KO on HS diet reflect increases in the thickness of the total or certain layers of the vascular wall thickness warrant further histological examination and quantitative morphometric analysis in future studies.
Table 1.
Body weight and aortic ring mass, and Phe Contraction and Ach relaxation in aortic rings of WT and Cav-1-/- mice on HS and LS diets.
| Diet | HS | LS | ||
|---|---|---|---|---|
| Cav-1 | WT | KO | WT | KO |
| Body weight (g) | 27.5±0.5 (21) | 24.3±0.6 (18) § | 25.8±1.0 (6) | 22.3±0.9 (6) § |
| Aortic Ring Mass (mg) | 0.45±0.06 (14) | 0.62±0.04 (16) § | 0.56±0.07 (21) | 0.44±0.07 (8) * |
| Phe (10-5 M) Max Contraction (g/mg) |
0.75±0.22 (14) | 0.25±0.06 (16) § | 0.70±0.17 (21) | 0.56±0.16 (8) * |
| pED50 (-log M) | 6.77±0.14 (14) | 7.05±0.07 (16) | 7.00±0.09 (21) | 7.10±0.05 (8) |
| Ach (10-6 M) % Relaxation |
23.6±3.5 (15) | 72.57±6.1 (16) § | 23.7±5.5 (21) | 93.6±3.5 (8) §* |
Data represent means±SEM (n).
Measurements in Cav-1-/- are significantly different (p<0.05) from corresponding measurements in WT.
Measurements in LS are significantly different (p<0.05) from corresponding measurements in HS.
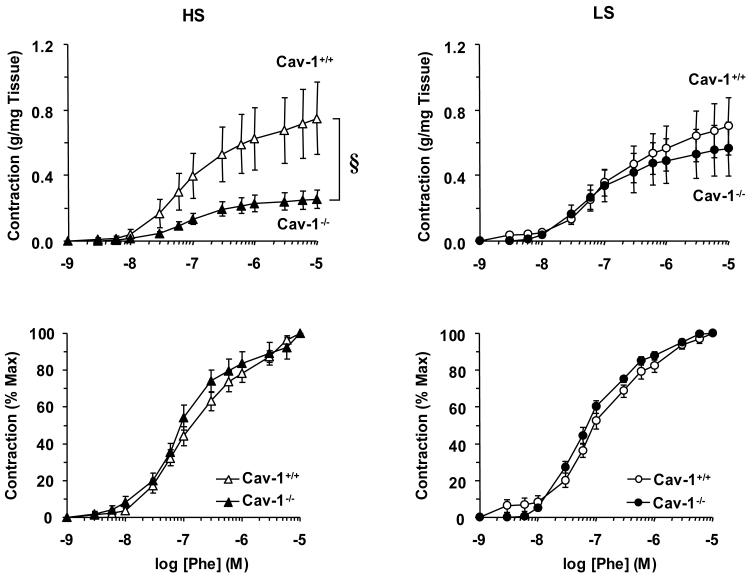
In aortic segments incubated in normal Krebs solution, the α-adrenergic agonist Phe caused concentration-dependent contraction that reached a maximum of 0.75±0.22 g/mg in WT mice on HS diet. The maximum Phe contraction was reduced in Cav-1-/- compared with WT mice on HS diet (Fig. 2, top panels). The Phe contraction was not significantly different between Cav-1-/- and WT mice on LS diet. Also, maximal Phe contraction was reduced in Cav-1 KO mice on HS diet as compared to those on LS diet (Table 1). However, when the Phe contraction was presented as % of maximum and the Phe ED50 was calculated, no significant difference was observed between Cav-1-/- and WT mice on either diet (Fig. 2, bottom panels, Table 1), suggesting that neither Cav-1 deficiency nor dietary sodium affect the sensitivity of the α-adrenergic receptors to Phe.
Fig. 2.
Phe-induced contraction in aortic segments of WT and Cav-1-/- mice on HS and LS diet. Aortic rings were stimulated with increasing concentrations of Phe, the contractile response was measured and presented as g/mg tissue weight (Upper panels) or as % of maximum Phe contraction (Bottom panels). Data represent means±SEM of measurements in 8 to 21 experiments. § p<0.05
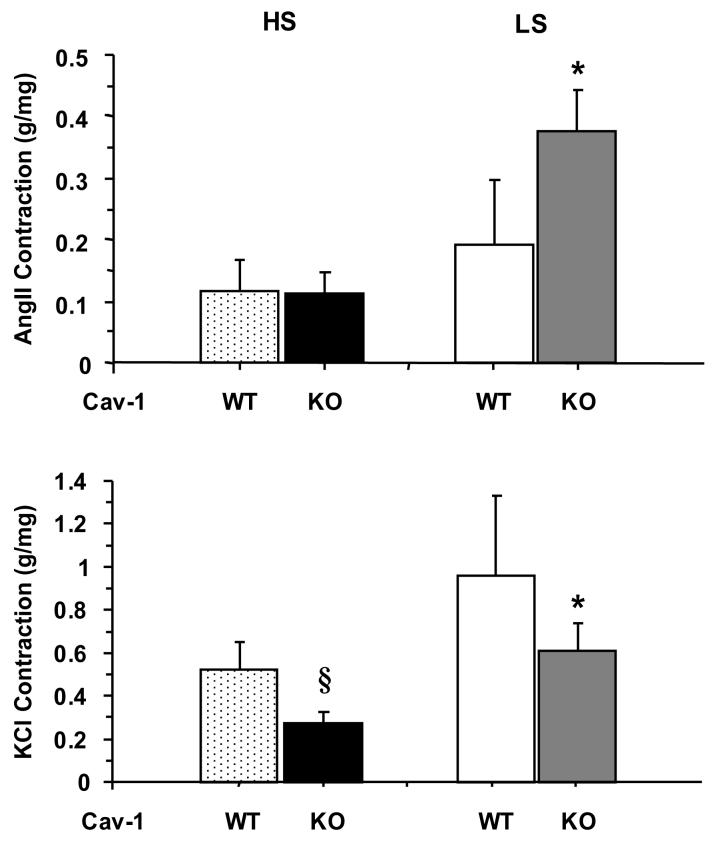
To determine whether the reduced aortic contraction in Cav-1-/- mice is specific to a particular agonist or receptor, the vascular response to AngII (10-7 M) was measured. AngII caused a small and transient contraction in aortic rings of all groups. The peak AngII contraction was not significantly different between the Cav-1-/- and WT mice on HS diet. However, AngII contraction was enhanced in aortic rings of Cav-1-/- mice on LS compared to those on HS diet (Fig. 3, Top Panel). Because of the difficulty in measuring a maintained contractile response to AngII, we measured the aortic contraction in response to high KCl (96 mM). Comparison of the KCl-induced contraction, a membrane depolarization-dependent and receptor-independent mechanism, indicated that it was reduced in Cav-1-/- compared with WT particularly during HS diet (Fig. 3, Bottom Panel). The KCl contraction data are consistent with the Phe data and suggest that the reduced vascular contraction in the Cav-1-/- mice on HS diet is not limited to a particular receptor, and may involve a common downstream signaling or contraction pathway that is inhibited in the absence of Cav-1, possibly due to activation of an endothelium-dependent vascular relaxation pathway.
Fig. 3.
AngII- and KCl-induced contraction in aortic rings of WT and Cav-1-/- mice on HS and LS diet. Aortic rings were stimulated with AngII (10-7 M) or KCl (96 mM) and the contractile response was presented as g/mg tissue weight. Data represent means±SEM of measurements in 7 to 16 experiments.
* Measurements in LS are significantly different (p<0.05) from corresponding measurements in HS.
§ Measurements in Cav-1-/- are significantly different (p<0.05) from corresponding measurements in WT.
The maximal Phe (10-5 M) contraction was enhanced in endothelium-denuded (1.73±0.85) compared to intact aortic segments from WT HS mice (0.73±0.22 g/mg tissue). Also, the Phe concentration-contraction curve was enhanced in endothelium-denuded (maximum: 0.42±0.15) compared to endothelium-intact aortic segments from Cav-1 KO mice on HS diet (maximum: 0.29±0.06 g/mg tissue). Removal of the endothelium in aortic segments of cav-1-/- HS mice made the Phe response (maximum: 0.42±0.15) not significantly different from that in endothelium-intact WT HS mice (maximum: 0.73±0.22 g/mg tissue). Additional experiments revealed that maximal Phe contraction was enhanced in endothelium-denuded (1.35±0.5) compared with intact aortic segments from Cav-1+/+ mice on LS diet (0.70±0.17 g/mg tissue). To test for potential role of endothelium-derived NO in the reduced aortic contraction in mice on LS diet, the effects of the NOS inhibitor L-NAME were examined. Treatment of aortic segments from Cav-1+/+ mice on LS diet with L-NAME (10-4 M) alone did not cause any detectable constriction. On the other hand, the maximal Phe contraction was enhanced in endothelium-intact aortic segments treated with L-NAME (0.94±0.67) as compared to non-treated segments from Cav-1+/+ mice on LS diet (0.70±0.17 g/mg tissue). These data suggest prominent effects of endothelium-derived NO in the mouse aortic segments, and that NO production may be increased more during agonist stimulation as compared to basal resting conditions.
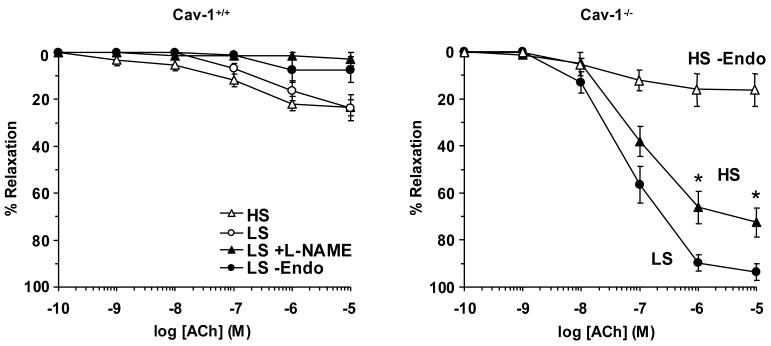
In aortic segments from WT mice on either HS or LS diet and precontracted with Phe, Ach caused concentration-dependent relaxation that reached a maximum at 10-5 M concentration (Fig. 4). Ach-induced relaxation was nearly abolished in aortic segments from WT mice on LS when the segments were treated ex vivo with the NOS inhibitor L-NAME (10-4 M) or when the endothelium was removed by scraping the interior of the vessel (Fig. 4). Ach-induced relaxation was significantly greater in Cav-1-/- compared with WT mice on HS and LS diet (Table 1). Also, Ach-induced relaxation was significantly reduced in endothelium-denuded aortic segments from Cav-1-/- mice (Fig. 4), suggesting activated endothelium-dependent vascular relaxation pathway(s) in the Cav-1 KO mice. While LS diet did not improve Ach relaxation in the WT, it significantly enhanced Ach relaxation in the Cav-1 KO (Fig. 4, Table 1).
Fig. 4.
Ach-induced relaxation in aortic rings of WT and Cav-1-/- mice on HS and LS diet. Aortic rings were contracted with Phe (10-5 M), increasing concentrations of Ach were added and the % relaxation of Phe contraction was measured. Data represent means±SEM of measurements in 4 to 16 experiments.
* Measurements in LS are significantly different (p<0.05) from corresponding measurements in HS.
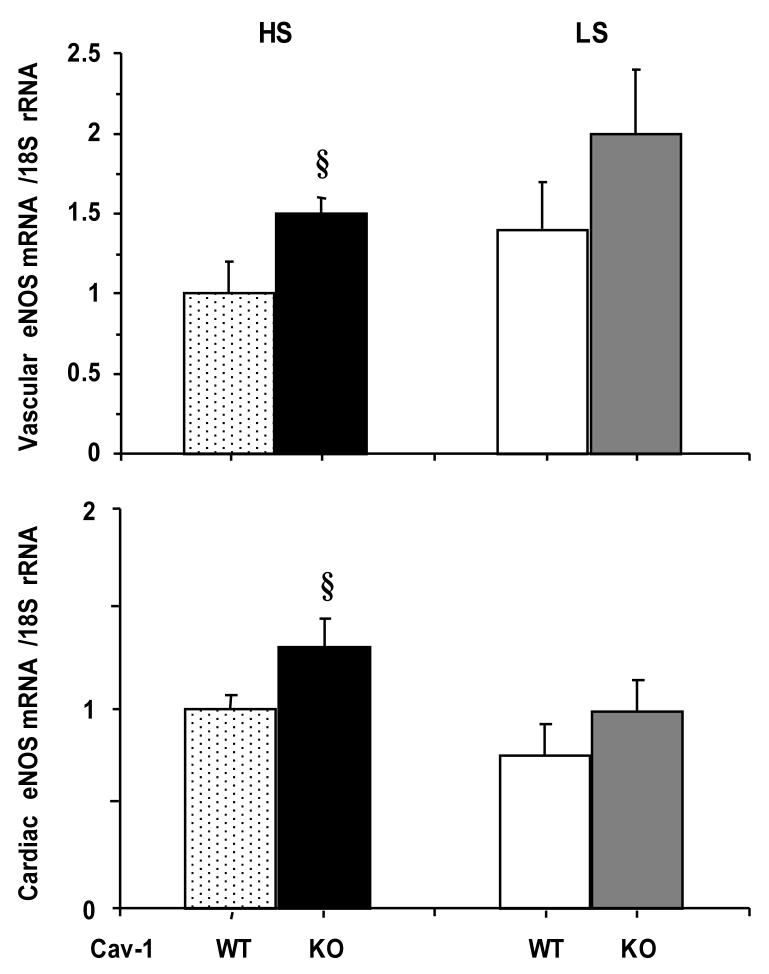
RT-PCR analysis showed significant amounts of eNOS mRNA expressed in the aorta and heart of WT mice. The aortic eNOS mRNA expression was significantly greater in Cav-1-/- as compared to WT on HS diet (Fig. 5). In contrast, eNOS mRNA expression was not significantly different between Cav-1-/- and WT mice on LS diet. Similar data were obtained for eNOS mRNA expression in the mouse heart (Fig, 5). The eNOS expression was not different between the HS and LS groups.
Fig. 5.
RT-PCR eNOS in aortic (upper panel) and cardiac tissue (bottom panel) of WT and Cav-1-/- mice on HS and LS diet
§ Measurements in Cav-1-/- are significantly different (p<0.05) from corresponding measurements in WT.
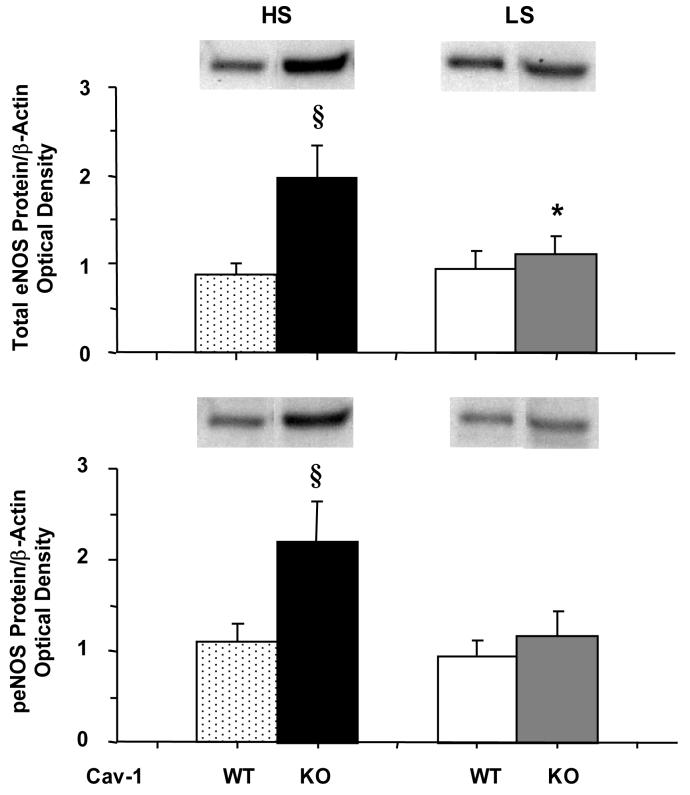
Western blot analysis revealed that the amount of total eNOS protein in the heart was significantly greater in Cav-1-/- compared with WT on HS diet, which is in agreement with the eNOS mRNA expression data. Total eNOS protein was slightly, but not significantly greater in Cav-1-/- compared with WT on LS diet (Fig. 6). A marked 2-fold increase in peNOS could be observed in Cav-1-/- as compared with WT on HS diet. In contrast, the amount of activated peNOS was not significantly different between Cav-1-/- and WT on LS diet (Fig. 6).
Fig. 6.
Western blot analysis of total and peNOS in cardiac tissue of WT and Cav-1-/- mice on HS and LS diet
§ Measurements in Cav-1-/- are significantly different (p<0.05) from corresponding measurements in WT.
* Measurements in LS are significantly different (p<0.05) from corresponding measurements in HS.
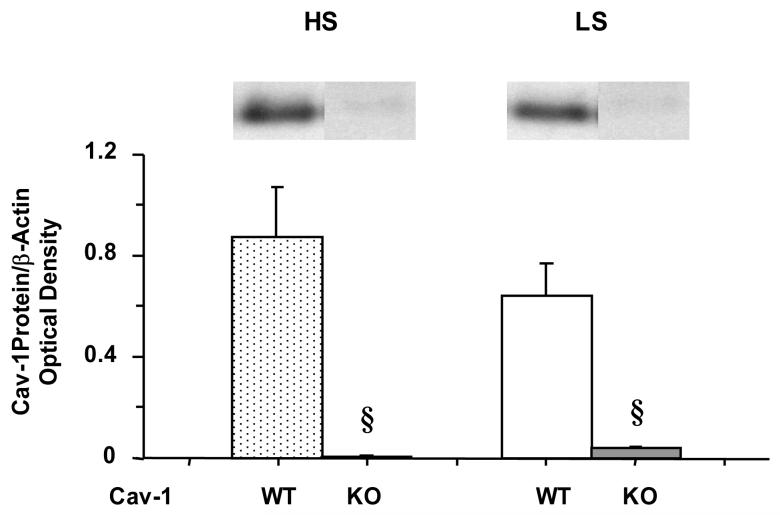
Western blot analysis of heart tissue revealed that the amount of Cav-1 protein was significantly greater in WT as compared to Cav-1-/- on HS or LS diet, confirming the genotype specifications obtained from Jackson Laboratories. The amount of Cav-1 was slightly but not significantly greater in WT mice on HS compared to those on LS diet (Fig. 7).
Fig. 7.
Western blot analysis of Cav-1 in cardiac tissue of WT and Cav-1-/- mice on HS and LS diet
§ Measurements in Cav-1-/- are significantly different (p<0.05) from corresponding measurements in WT.
DISCUSSION
The present study demonstrates that: 1) vascular contraction is reduced in Cav-1-/- compared to WT mice on HS diet, and the differences are eliminated during LS diet, 2) endothelium-dependent vascular relaxation is enhanced in Cav-1-/- compared to WT mice on HS diet, 3) LS diet enhances endothelium-dependent vascular relaxation in Cav-1-/- but not WT, and 4) eNOS mRNA and protein are elevated in the heart of Cav-1-/- compared to WT mice on HS diet, and the genotypic differences are less apparent during LS diet.
Cav-1 is a transmembrane anchoring protein and major regulator of signaling transduction in many tissues including the vasculature. In VSM, Cav-1 plays a role in the coupling of α-adrenergic, AngII and endothelin receptors to VSM contraction and growth (Ushio-Fukai et al., 2005; Zuo et al., 2005; Ushio-Fukai & Alexander, 2006; Shakirova et al, 2006). In the endothelium, Cav-1 anchors eNOS to caveolae, thus preventing its activation, and reducing NO production and vascular relaxation (Feron et al., 1998; Segal et al., 1999; Batova et al., 2006). Previous studies have used Cav-1 KO mice on normal rodent chow (∼0.8% NaCl) to examine the role of Cav-1 in mechanotransduction, vascular remodeling, and cardiovascular function (Drab et al., 2001; Razani et al., 2001; Yu et al., 2006; Wunderlich et al., 2006). In one study, Cav-1 null mice showed evidence of hyperproliferative and vascular abnormalities (Razani et al., 2001). Other studies have shown loss of caveolae, vascular dysfunction, and pulmonary defects in Cav-1 gene-disrupted mice (Drab et al, 2001). Also, disruption of Cav-1 led to enhanced nitrosative stress and severe systolic and diastolic heart failure (Wunderlich et al., 2006). Our present observation that vascular relaxation was greater in Cav-1 KO mice compared with WT is in general agreement with these reports.
Although previous studies support a role of Cav-1 in vascular signaling in mice on regular salt diet, little is known regarding its role during substantial changes in dietary sodium intake. We have previously shown that the effects of high dietary salt as compared to normal salt on vascular function could be very subtle or undetectable in Sprague-Dawley rats (Giardina et al, 2001; Smith et al., 2003). In contrast, we have documented that there are substantial differences in vascular function when animals on a normal/HS diet are compared to those on a sodium restricted diet (Williams et.al, 1976). Therefore, in order to detect measurable effects of dietary sodium it was important to examine the vascular function in mice at the two ends of the spectrum i.e. under the dietary sodium loading condition (HS) as compared to the sodium restriction condition (LS).
Chronic HS diet is often associated with vascular damage and/or remodeling, and Cav-1 could be one of the targets affected. We reasoned that if Cav-1 and HS function through independent mechanisms, then changing dietary sodium intake should have similar vascular effects in WT and Cav-1 KO mice. On the other hand, if Cav-1 and dietary sodium intake are functionally-linked then the effects of high dietary sodium on vascular function should differ in Cav-1 KO and WT mice. Also, if the latter possibility is correct, then the HS-related differences in vascular function between the Cav-1 KO and WT mice should be minimized/improved with salt restriction. We observed that the magnitude of Phe-induced contraction was reduced in Cav-1-/- compared to WT mice on HS diet, suggesting a role of Cav-1 in the control of vascular function during HS diet. The reduced Phe contraction in Cav-1-/- HS mice can be in part explained by interruption of Phe-α-adrenergic receptor interaction. However, analysis of Phe ED50 indicated lack of statistical difference between aortic segments of Cav-1-/- and WT, suggesting that the reduced Phe contraction in Cav-1-/- mice is not due to changes in the α-adrenergic receptor binding/sensitivity to Phe.
The renin-angiotensin system is modified during changes in dietary sodium intake (Meneton et al., 2005). Therefore, it is important to investigate the vascular response to AngII in animal models on different dietary salt intake. Previous studies have shown that the contraction induced by AngII was decreased in Cav-1 KO compared to WT mice under normal salt diet (Drab et al, 2001). This is different from the present observation that AngII contraction was not statistically different between Cav-1 KO and WT mice on HS. This can be related to the possibility that the reduction in AngII response due to Cav-1 deficiency is masked by enhanced vascular sensitivity to AngII associated with changes in dietary sodium intake. This is supported by the observation that the AngII contraction was significantly increased in Cav-1 KO compared to WT mice on LS diet, a condition known to induce the rennin-angiotensin system and enhance the vascular sensitivity to AngII (Khalil & Hall, 1999). We should caution that analysis of the AngII vasoconstrictor response in the mouse tissues was difficult. AngII is notoriously tachyphylactic in vascular tissues. Application of AngII 10-7 M caused a transient constriction that rapidly returned to basal levels. Also, a concentration-response curve to AngII in mouse tissue was not possible to construct. On the other hand, high KCl is known to cause membrane depolarization and to induce vascular contraction via receptor-independent mechanisms (Khalil & van Breemen, 1988; Murphy & Khalil, 2000). Consistent with the Phe contraction data, the KCl-induced contraction was reduced in Cav-1-/- compared to WT mice on HS diet. These data suggest that the reduction in vasoconstriction in Cav-1 deficient mice on HS diet is not specific to a particular agonist/receptor coupling mechanism in VSM. Alternatively, the receptor-nonspecific reduction in vascular contraction in Cav-1-/- mice on HS diet could be related to enhancement of the endothelium-dependent mechanisms of vascular relaxation.
The endothelium releases various vasodilator and constricting factors, and NO is a major endothelium-derived vasodilator (Fleming & Busse, 1999; Ignarro, 2002; Murad, 2006). Under basal conditions eNOS is bound to Cav-1 at the endothelial cell caveolae. An increase in endothelial cell Ca2+ by agonists such as Ach is thought to induce the release from Cav-1 of eNOS to the cytosolic compartment where it is phosphorylated and fully activated (Ferron et al, 1996; 1998; Minshal et al., 2003).
Several lines of evidence point to a role of endothelium-derived NO in the present BP and vascular function measurements in mice. 1) With L-NAME treatment BP increased substantially, but more so in the Cav-1-/- mice, suggesting an increase in NO production compared to WT mice. 2) Phe contraction appeared to be enhanced in endothelium-denuded versus intact aortic segments and in L-NAME treated versus nontreated segments. Importantly, the Phe response in endothelium-denuded aortic segments of Cav-1-/- HS mice was enhanced to levels not significantly different from those observed in endothelium-intact WT HS mice, suggesting a role of endothelium-derived vasodilators in the reduced Phe contraction in the Cav-1-/- HS mice. 3) Ach-induced relaxation was nearly abolished in endothelium-denuded aortic segments, and in vascular segments treated with L-NAME. 4) Ach-induced relaxation was enhanced in Cav-1-/- compared with WT mice on HS diet mice, suggesting an increased amount/activity of eNOS and increased endothelium-derived NO.
To further test whether the enhanced vascular relaxation in vessels from Cav-1-/- mice involves eNOS activation and increased NO release, we attempted to measure NO production using two methods. In our hands, measuring nitrite/nitrate using Griess reagent or NO release using DAF-FM fluorescence in the relatively small tissue of mouse aorta was associated with high background signals that limited our ability to discern differences in NO production between the WT and Cav-1 KO mice.
We chose to further test the NO pathway using the more sensitive RT-PCR technique and using larger mouse tissues such as the heart. We have previously shown that chronic HS diet in WT mice or in rats is not associated with cardiac tissue damage (Turchin et al., 2006; Rocha et al., 2000; Martinez et al., 2002; Oestreicher et al., 2003). We hypothesized that Cav-1 deficiency during HS diet may affect eNOS expression/activity not only in the blood vessels, but also in the heart. The RT-PCR experiments indicated increased expression of eNOS mRNA in the aorta and heart of Cav-1-/- mice on HS diet. Also, immunoblot analysis revealed that Cav-1 KO mice under HS diet present more eNOS than WT mice. Additionally, a 2-fold increase in the amount of phospho-eNOS was observed in Cav-1-/- mice on HS diet, suggesting increased eNOS activity. Based on our preliminary observations that Cav-1-/- mice have intact levels of Cav-3 expression in the cardiac myocytes (data not shown), the present results suggest that the observed effects in the heart are a reflection of changes of NOS expression/activity in the cardiac vessels and not the cardiac myocytes. Collectively, these observations suggest that Cav-1 may limit the expression/activity of vascular eNOS during HS diet, and therefore the lack of Cav-1 is associated with constitutively larger amounts of eNOS and greater eNOS activation during HS diet. We should note that Cav-1 KO mice under HS diet present more eNOS than WT mice. However, no differences in the expression of total eNOS or phospho-eNOS were observed between KO and WT mice during LS diet despite an increased relaxation to Ach in aorta from KO mice under HS compared to LS diet. This could be related to the possibility that eNOS expression may represent a compensatory protective mechanism that is activated to minimize vascular damage during HS diet, but may not be needed during LS diet. Also, biochemical measurement of phospho-eNOS may not be as sensitive predictor of eNOS activity as the vascular relaxation assays. Additionally, phospho-eNOS was measured under basal conditions, while vascular relaxation was measured during stimulation by Ach. Furthermore, Ach-induced vascular relaxation involves activation of other pathways in addition to eNOS/NO, including cyclooxygenase/prostacyclin and endothelium-derived hyperpolarizing factor(s). Whether Cav-1 binds to and inhibits additional vascular relaxation pathways, and whether these pathways are activated in Cav-1 deficiency states is an intriguing possibility that should be further examined in future studies.
Our previous studies in Sprague-Dawley rats have shown that changes in dietary salt alone are associated with slight or no change in BP or aortic vascular reactivity (Giardina et al, 2001; Smith et al., 2003). The lack of salt sensitivity has been related to the possibility that the vasoconstrictive effects of HS are counterbalanced by HS-induced increase in NO production and vascular relaxation (Giardina et al, 2001; Smith et al., 2003). The association between changes in NO and sensitivity to sodium has been supported by studies showing salt sensitivity in rats or mice treated with L-NAME, which inhibits NOS (Hodge et al., 2002; Mattson et al., 2006; Rudd et al., 1999) or with ETB receptor antagonist, which blocks endothelial ETB receptor-mediated NOS activation and NO production (Giardina et al., 2001). The present study demonstrates significant differences in the BP and aortic constriction to Phe and KCl in Cav-1-/- mice on LS compared with HS diets. Also, endothelium-dependent NO-mediated vascular relaxation is enhanced in Cav-1-/- versus WT mice on HS diet, and further enhanced in Cav-1-/- mice on LS diet compared to those on HS diet. Additionally, eNOS expression/activity is elevated in the heart of Cav-1-/- compared to WT mice on HS diet, and the genotypic differences are less apparent during LS diet. These data demonstrate salt sensitivity of the vascular mechanisms controlling BP, the vasoconstriction, the vascular relaxation and the NO pathway in the Cav-1 null mouse.
The present data specifically addresses the salt sensitivity of the Cav-1 NO pathway and demonstrate the novel finding that LS diet does not improve vascular relaxation in the WT, but significantly improves vascular function and Ach relaxation in the Cav-1 KO. The molecular interaction between Cav-1 and HS is unclear at the present time, but does not appear to involve changes in Cav-1 expression (see Fig. 7). However, the present data do not rule out the interesting possibility that the binding and inhibition of eNOS by Cav-1 may be more pronounced during HS diet. We should note that if binding of eNOS by Cav-1 is more pronounced during HS diet, some differences in the WT mice on HS compared to LS diet should also be observed. The present data did not show significant differences in vascular contraction or Ach-induced relaxation in WT mice on HS compared to LS diet, suggesting that LS alone may not be sufficient to reverse the binding and inhibition of eNOS by Cav-1. On the other hand, a significant decrease in vasoconstriction and enhanced Ach-induced relaxation were observed in KO mice on LS compared to HS diet. These data raise the exciting possibility that the beneficial effects of LS diet may be more pronounced when eNOS is unlocked from its inhibition by Cav-1.
In conclusion, during HS diet Cav-1 deficiency is associated with decreased vasoconstriction and increased vascular relaxation and eNOS expression/activity, and these effects are altered during salt restriction. The data supports the hypothesis that Cav-1, likely through an effect on eNOS activity, plays a prominent role in the regulation of vascular function, particularly when dietary sodium intake is substantially modified.
ACKNOWLEDGEMENTS
This work was supported by grants from National Heart, Lung, and Blood Institute (HL-65998 and HL-70659 to RAK, and HL 069208 to GHW). Support to LHP was provided by a NHLBI training grant (T32 HL07609). We also acknowledge the helpful technical assistance of Paul Loutraris and the advice provided by Drs. C. Guo, V. Ricchiuti and J. Romero.
List of Abbreviations
- Ach
Acetylcholine
- AngII
Angiotensin II
- Cav-1
Caveolin-1
- eNOS
endothelial nitric oxide synthase
- HS
high salt
- KO
Knockout
- LS
low salt
- NO
nitric oxide
- Phe
phenylephrine
- WT
wild-type
REFERENCES
- Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. Am J Physiol Cell Physiol. 2000;278(1):C163–73. doi: 10.1152/ajpcell.2000.278.1.C163. [DOI] [PubMed] [Google Scholar]
- Barron LA, Green GM, Khalil RA. Gender differences in vascular smooth muscle reactivity to increases in extracellular sodium salt. Hypertension. 2002;39(2 Pt 2):425–32. doi: 10.1161/hy02t2.102779. [DOI] [PubMed] [Google Scholar]
- Batova S, DeWever J, Godfraind T, Balligand JL, Dessy C, Feron O. The calcium channel blocker amlodipine promotes the unclamping of eNOS from caveolin in endothelial cells. Cardiovasc Res. 2006;71(3):478–85. doi: 10.1016/j.cardiores.2006.04.013. [DOI] [PubMed] [Google Scholar]
- de Wardener HE, He FJ, MacGregor GA. Plasma sodium and hypertension. Kidney Int. 2004;66(6):2454–66. doi: 10.1111/j.1523-1755.2004.66018.x. [DOI] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293(5539):2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271(37):22810–4. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- Feron O, Saldana F, Michel JB, Michel T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. J Biol Chem. 1998;273(6):3125–8. doi: 10.1074/jbc.273.6.3125. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98(1):121–8. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- Fleming I, Busse R. NO: the primary EDRF. J Mol Cell Cardiol. 1999;31(1):5–14. doi: 10.1006/jmcc.1998.0839. [DOI] [PubMed] [Google Scholar]
- Giardina JB, Green GM, Rinewalt AN, Granger JP, Khalil RA. Role of endothelin B receptors in enhancing endothelium-dependent nitric oxide-mediated vascular relaxation during high salt diet. Hypertension. 2001;37(2 Part 2):516–23. doi: 10.1161/01.hyp.37.2.516. [DOI] [PubMed] [Google Scholar]
- Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252(5014):1813–6. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- Hall JE, Guyton AC, Smith MJ, Jr, Coleman TG. Blood pressure and renal function during chronic changes in sodium intake: role of angiotensin. Am J Physiol. 1980;239(3):F271–80. doi: 10.1152/ajprenal.1980.239.3.F271. [DOI] [PubMed] [Google Scholar]
- Hall JE. Control of sodium excretion by angiotensin II: intrarenal mechanisms and blood pressure regulation. Am J Physiol. 1986;250(6 Pt 2):R960–72. doi: 10.1152/ajpregu.1986.250.6.R960. [DOI] [PubMed] [Google Scholar]
- Hodge G, Ye VZ, Duggan KA. Salt-sensitive hypertension resulting from nitric oxide synthase inhibition is associated with loss of regulation of angiotensin II in the rat. Exp Physiol. 2002;87(1):1–8. doi: 10.1113/eph8702322. [DOI] [PubMed] [Google Scholar]
- Holtzman EJ, Braley LM, Williams GH, Hollenberg NK. Kinetics of sodium homeostasis in rats: rapid excretion and equilibration rates. Am J Physiol. 1988;254(6 Pt 2):R1001–6. doi: 10.1152/ajpregu.1988.254.6.R1001. [DOI] [PubMed] [Google Scholar]
- Hurwitz S, Fisher ND, Ferri C, Hopkins PN, Williams GH, Hollenberg NK. Controlled analysis of blood pressure sensitivity to sodium intake: interactions with hypertension type. J Hypertens. 2003;21(5):951–9. doi: 10.1097/00004872-200305000-00020. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53(4 Pt 1):503–14. [PubMed] [Google Scholar]
- Ishizaka N, Griendling KK, Lassegue B, Alexander RW. Angiotensin II type 1 receptor: relationship with caveolae and caveolin after initial agonist stimulation. Hypertension. 1998;32(3):459–66. doi: 10.1161/01.hyp.32.3.459. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Kita S, Zhang J, Blaustein MP, Arai Y, Yoshida S, Wakimoto K, Komuro I, Katsuragi T. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med. 2004;10(11):1193–9. doi: 10.1038/nm1118. [DOI] [PubMed] [Google Scholar]
- Joffe HV, Adler GK. Effect of aldosterone and mineralocorticoid receptor blockade on vascular inflammation. Heart Fail Rev. 2005;10(1):31–7. doi: 10.1007/s10741-005-2346-0. [DOI] [PubMed] [Google Scholar]
- Khalil RA, Crews JK, Carroll JF, Hall JE. Enhanced vascular reactivity and Ca2+ entry with low-salt diet: effect of obesity. Hypertension. 1999;34(4 Pt 2):882–8. doi: 10.1161/01.hyp.34.4.882. [DOI] [PubMed] [Google Scholar]
- Khalil RA. Dietary salt and hypertension: new molecular targets add more spice. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R509–13. doi: 10.1152/ajpregu.00600.2005. [DOI] [PubMed] [Google Scholar]
- Kosachunhanun N, Hunt SC, Hopkins PN, Williams RR, Jeunemaitre X, Corvol P, Ferri C, Mortensen RM, Hollenberg NK, Williams GH. Genetic determinants of nonmodulating hypertension. Hypertension. 2003;42:901–8. doi: 10.1161/01.HYP.0000095615.83724.82. [DOI] [PubMed] [Google Scholar]
- Li L, Yang G, Ebara S, Satoh T, Nasu Y, Timme TL, Ren C, Wang J, Tahir SA, Thompson TC. Caveolin-1 mediates testosterone-stimulated survival/clonal growth and promotes metastatic activities in prostate cancer cells. Cancer Res. 2001;61(11):4386–92. [PubMed] [Google Scholar]
- Li T, Sotgia F, Vuolo MA, Li M, Yang WC, Pestell RG, Sparano JA, Lisanti MP. Caveolin-1 mutations in human breast cancer: functional association with estrogen receptor alpha-positive status. Am J Pathol. 2006;168(6):1998–2013. doi: 10.2353/ajpath.2006.051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez DV, Rocha R, Matsumura M, Oestreicher E, Ochoa-Maya M, Roubsanthisuk W, Williams GH, Adler GK. Cardiac damage prevention by eplerenone: comparison with low sodium diet or potassium loading. Hypertension. 2002;39(2 Pt 2):614–8. [PubMed] [Google Scholar]
- Mattson DL, Meister CJ. Sodium sensitivity of arterial blood pressure in L-NAME hypertensive but not eNOS knockout mice. Am J Hypertens. 2006;19(3):327–9. doi: 10.1016/j.amjhyper.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85(2):679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol. 2003;285(6):L1179–83. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- Murad F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006;355(19):2003–11. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- Oestreicher EM, Martinez-Vasquez D, Stone JR, Jonasson L, Roubsanthisuk W, Mukasa K, Adler GK. Aldosterone and not plasminogen activator inhibitor-1 is a critical mediator of early angiotensin II/NG-nitro-L-arginine methyl ester-induced myocardial injury. Circulation. 2003;108:2517–2523. doi: 10.1161/01.CIR.0000097000.51723.6F. [DOI] [PubMed] [Google Scholar]
- Oliverio MI, Best CF, Smithies O, Coffman TM. Regulation of sodium balance and blood pressure by the AT(1A) receptor for angiotensin II. Hypertension. 2000;35(2):550–4. doi: 10.1161/01.hyp.35.2.550. [DOI] [PubMed] [Google Scholar]
- Payne JA, Alexander BT, Khalil RA. Decreased endothelium-dependent NO-cGMP vascular relaxation and hypertension in growth-restricted rats on a high-salt diet. Hypertension. 2004;43(2):420–7. doi: 10.1161/01.HYP.0000111832.47667.13. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276(41):38121–38. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Rocha R, Stier CT, Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141(10):3871–8. doi: 10.1210/endo.141.10.7711. [DOI] [PubMed] [Google Scholar]
- Rudd MA, Trolliet M, Hope S, Scribner AW, Daumerie G, Toolan G, Cloutier T, Loscalzo J. Salt-induced hypertension in Dahl salt-resistant and salt-sensitive rats with NOS II inhibition. Am J Physiol. 1999;277(2 Pt 2):H732–9. doi: 10.1152/ajpheart.1999.277.2.H732. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47(3):312–8. doi: 10.1161/01.HYP.0000201443.63240.a7. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Jacobson BS, Dvorak AM. Caveolae from luminal plasmalemma of rat lung endothelium: microdomains enriched in caveolin, Ca2+-ATPase, and inositol trisphosphate receptor. Proc Natl Acad Sci U S A. 1995;92(5):1759–63. doi: 10.1073/pnas.92.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SS, Brett SE, Sessa WC. Codistribution of NOS and caveolin throughout peripheral vasculature and skeletal muscle of hamsters. Am J Physiol. 1999;277:H1167–77. doi: 10.1152/ajpheart.1999.277.3.H1167. [DOI] [PubMed] [Google Scholar]
- Shakirova Y, Bonnevier J, Albinsson S, Adner M, Rippe B, Broman J, Arner A, Sward K. Increased Rho activation and PKC-mediated smooth muscle contractility in the absence of caveolin-1. Am J Physiol Cell Physiol. 2006;291(6):C1326–35. doi: 10.1152/ajpcell.00046.2006. [DOI] [PubMed] [Google Scholar]
- Sheng JZ, Wang D, Braun AP. DAF-FM (4-amino-5-methylamino-2′,7′-difluorofluorescein) diacetate detects impairment of agonist-stimulated nitric oxide synthesis by elevated glucose in human vascular endothelial cells: reversal by vitamin C and L-sepiapterin. J Pharmacol Exp Ther. 2005;315(2):931–40. doi: 10.1124/jpet.105.087932. [DOI] [PubMed] [Google Scholar]
- Smith L, Payne JA, Sedeek MH, Granger JP, Khalil RA. Endothelin-induced increases in Ca2+ entry mechanisms of vascular contraction are enhanced during high-salt diet. Hypertension. 2003;41(3 Pt 2):787–93. doi: 10.1161/01.HYP.0000051643.05700.56. [DOI] [PubMed] [Google Scholar]
- Stan RV, Roberts WG, Predescu D, Ihida K, Saucan L, Ghitescu L, Palade GE. Immunoisolation and partial characterization of endothelial plasmalemmal vesicles (caveolae) Mol Biol Cell. 1997;8(4):595–605. doi: 10.1091/mbc.8.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn WB. Eplerenone antagonizes atherosclerosis, but what is the agonist? Hypertension. 2005;46(5):1093–4. doi: 10.1161/01.HYP.0000184652.93583.77. [DOI] [PubMed] [Google Scholar]
- Turchin A, Guo CZ, Adler GK, Ricchiuti V, Kohane IS, Williams GH. Effect of acute aldosterone administration on gene expression profile in the heart. Endocrinology. 2006;147(7):3183–9. doi: 10.1210/en.2005-1674. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander RW. Caveolin-dependent angiotensin II type 1 receptor signaling in vascular smooth muscle. Hypertension. 2006;48(5):797–803. doi: 10.1161/01.HYP.0000242907.70697.5d. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Zuo L, Ikeda S, Tojo T, Patrushev NA, Alexander RW. cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: role in vascular hypertrophy. Circ Res. 2005;97(8):829–36. doi: 10.1161/01.RES.0000185322.46009.F5. [DOI] [PubMed] [Google Scholar]
- Williams GH, Hollenberg NK, Braley LM. Influence of sodium intake on vascular and adrenal angiotensin II receptors. Endocrinology. 1976;98:1343–50. doi: 10.1210/endo-98-6-1343. [DOI] [PubMed] [Google Scholar]
- Wunderlich C, Schober K, Lange SA, Drab M, Braun-Dullaeus RC, Kasper M, Schwencke C, Schmeisser A, Strasser RH. Disruption of caveolin-1 leads to enhanced nitrosative stress and severe systolic and diastolic heart failure. Biochem Biophys Res Commun. 2006;340(2):702–8. doi: 10.1016/j.bbrc.2005.12.058. [DOI] [PubMed] [Google Scholar]
- Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116(5):1284–91. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Ushio-Fukai M, Ikeda S, Hilenski L, Patrushev N, Alexander RW. Caveolin-1 is essential for activation of Rac1 and NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells: role in redox signaling and vascular hypertrophy. Arterioscler Thromb Vasc Biol. 2005;25(9):1824–30. doi: 10.1161/01.ATV.0000175295.09607.18. [DOI] [PubMed] [Google Scholar]