Abstract
The association between activation of the immune system and mood disorders has been reported by several studies. However, the mechanisms by which the immune system affects mood are only partially understood. In the present study, we detected depressive-like behavior in a rat animal model which involves the induction of inflammation in the nasal cavities by intranasal (i.n.) instillation of bacterial lipopolysaccharides (LPS). Female rats showed depressive-like behavior as evidenced by the forced swim test after repeated i.n. administration of LPS. These responses were not paralleled by alterations in motor activity as measured by the open field test. In the same animals, corticosterone responses after the swimming sessions were the highest of all the groups evaluated. Real-time RT PCR was used to analyze the transcriptional regulation of the cytokines interleukin-1β, tumor necrosis factor-α, and interleukin-6 in several brain regions. Increased tumor necrosis factor-α was detected in the hippocampus and brainstem of female rats challenged with i.n. LPS. These results suggest that peripheral inflammation in the upper respiratory tract is an immune challenge capable of inducing depressive-like behavior, promoting exaggerated glucocorticoid responses to stress, and increasing cytokine transcription in the brain. These results further our understanding of the role that the immune system may play in the pathophysiology of depression.
Keywords: forced swim test, real-time RT-PCR, hippocampus, brainstem, corticosterone, cytokines, depression
INTRODUCTION
Evidence obtained from clinical human studies suggest that activation of the immune system and the cytokine network may be related to at least some aspect of the complex etiology of major depression (Anisman et al, 2005; Capuron et al, 2001; Capuron and Dantzer, 2003; Dantzer and Kelley, 2007; Dantzer et al, 2002; Prolo and Licinio, 1999; Raison et al, 2006; Schiepers et al, 2005). It is known that treatment of hepatitis-C with the cytokine interferon-α resulting in the reduction of viral titers (Kobayashi et al, 2006) induces depression in a large number of patients (Crone et al, 2004; Dieperink et al, 2000; Janssen et al, 1994; Raison et al, 2005; Schaefer et al, 2002). Treatment with the cytokine interleukin-2 (IL-2) in melanoma patients produces similar psychiatric side effects (Capuron et al, 2000; Denicoff et al, 1987). Moreover, several studies have reported the presence of elevated proinflammatory cytokines in depressed patients (Frommberger et al, 1997; Levine et al, 1999) and the reduction in these mediators of inflammation after treatment with antidepressants (Yirmiya et al, 1999). Understanding the relationship and mechanistic basis of interactions between the immune system and mood disorders could provide novel insights into the etiology and treatment of depression.
A well-established model to study behavioral and physiological responses under activation of the immune system is the administration of bacterial lipopolysaccharides (LPS). LPS are components of the cell wall of gram negative bacteria that generate a dose-dependent activation of the innate immune response and secretion of proinflammatory cytokines (Miller et al, 2005). LPS administered intravenously (i.v.) or intraperitoneally (i.p.) have been shown to induce changes indicative of depression in human subjects and affect behaviors related to mood in rodent animal models (De La Garza, 2005; Engeland et al, 2003; Reichenberg et al, 2001). Although the degree to which sickness, rather than depression, is involved in these behavioral changes is under debate, studies show that healthy individuals receiving low doses of i.v. LPS had elevated anxiety and depressive scores (Reichenberg et al, 2001). These effects were not accompanied by manifest symptoms of systemic sickness such as changes in heart rate but by increased circulating cytokines. In addition, in rodents, i.p. LPS have been found to elicit behaviors related to anxiety and depression, including reduced exploratory behavior (Engeland et al, 2003), reduced social interaction, and increased anhedonia (De La Garza, 2005). However, i.p. administration of LPS failed to increase immobility time in the forced swim test (FST) in rats (Deak et al, 2005), or it did in mice only when the doses were high enough to also affect motor activity (Dunn and Swiergiel, 2005). In rodents, increased immobility time in the FST without changes in motor activity is often interpreted as a measure of depressive-like behavior (Cryan and Holmes, 2005; Cryan et al, 2005; Porsolt, 1979). Because the FST has been validated for its sensitivity to the effect of clinically efficacious antidepressants, these findings may limit further studies on mechanisms linking activation of the immune system with mood disorders and the evaluation of new antidepressant interventions related to this phenomenon.
In the present study, we sought to advance further the understanding of the relationship between immune function and depression using the LPS model and the FST in rats. The first objective was to examine the effect of the route of LPS administration. While i.p. administration of LPS is a good model for sepsis and systemic inflammatory reaction, there is some evidence indicating that other types of inflammatory processes such as upper respiratory tract illnesses also induce negative mood, reduced alertness, and impaired psychomotor functions (Capuron et al, 1999; Hall and Smith, 1996; Smith et al, 1998). Inflammation in the upper respiratory airways such as those triggered by airborne pathogens may be an important immune challenge for brain function because of the unique characteristics of the nasal neuro-epithelium that is exposed to the environment, and the known transfer of large molecules and pathogens into the brain via the intranasal (i.n.) pathway (Illum, 2004; Jin et al, 2001; Loftus et al, 2006; Mori et al, 2005; Thorne et al, 2004). This raises the possibility that transfer of LPS or cytokines into the brain via the i.n. pathway may exert effects on brain function and behavior that differ from the inflammation caused by i.p. LPS administration. To test, we studied the effects of i.n. and i.p. LPS administration on depression related behavior in the FST and neuroendocrine responses to the stress of forced swimming. In addition, because peripheral administration of LPS has been shown to induce cytokine expression in the brain (Buttini et al, 1996; Quan et al, 1999; Tonelli et al, 2003; Tonelli and Postolache, 2005) we examined the effects of i.n. LPS delivery on gene expression of the cytokines interleukin-1β (IL-1β), tumor necrosis factor-α, and interleukin-6 (IL-6) in the olfactory bulbs, prefrontal cortex, hippocampus and brainstem. These areas were selected based on their involvement in depression (Arango et al, 2002; Drevets, 2000; Song and Leonard, 2005) and for being a target of i.n. transfer of molecules (Fliedner et al, 2006; Thorne et al, 2004).
There is increased prevalence of depression in women relative to men (Noble, 2005) and similar sex-related differences have been reported in depression-related behavior in rats (Dalla et al, 2005). Moreover, female rats exhibit greater sensitivity than males to LPS and/or cytokines in several behaviors including locomotor activity, sucrose intake and mating behavior (Avitsur and Yirmiya, 1999a, b; Engeland et al, 2003; Merali et al, 2003). Therefore, the second objective of the present study was to test the hypothesis that females are more sensitive to the depressogenic effects of i.p. and/or i.n. LPS.
MATERIALS AND METHODS
Animals
A total of 192 inbred Fischer F344 (F344/NHsd) rats divided into equal parts of males and females were used in these studies. This strain was selected based on previous studies showing strong cytokine expression in cortical regions of the brain after LPS injection in combination with highstress system reactivity (Tonelli et al, 2001, 2003).
The animals were obtained from Harlan-Sprague Dawley (Indianapolis, IN) and shipped to our animal facility at 2 months of age. Animals were housed in groups of three in Plexiglas cages with standard food pellets and water available ad libitum in a room at a constant temperature of 23°C. All animals were maintained on a 12:12 light dark cycle (lights on at 0700 h). The animals were left undisturbed for 1 week and then handled daily for an additional week before starting with the experimental procedures. Weight was monitored daily starting with the handling and continued until the experiments were terminated. Vaginal smears were taken to determine the phase of the ovarian estrous cycle as described (Becker et al, 2005). All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore.
Intraperitoneal LPS Studies
Rats were injected with 1 or 2 mg/kg LPS (Sigma, St Louis, MO, serotype 055:B5) or 0.9% saline between 1900 and 2000 h or between 0900 and 1000 h and evaluated in the open field and FST at 12 and 24 h after administration respectively. These doses and time points were selected based on our previous studies showing peak of expression of IL-1β mRNA in the brain parenchyma after 12 h of LPS administration (Tonelli et al, 2003) and studies showing measurable levels of this cytokine in the brain parenchyma at 24 h (Buttini and Boddeke, 1995) correlated with maximal microglial activation at this time point (Buttini et al, 1996).
Intranasal LPS Studies
Rats were slightly anesthetized with isofluorane in induction chambers and administered in the nasal cavities with either a single dose of LPS (100 μg/rat in 100 μl saline) and evaluated 24 h later or they were administered with two doses of LPS (100 μg/rat in 100 μl saline) on 2 consecutive days and evaluated 24 h after the last administration (48 h after the first administration). Control animals were subjected to the same schedule but administered with 100 μl of saline solution. All i.n. instillations were made between 0800 and 1000 h. These doses and schedule of administration were based in previous studies in mice (Delayre-Orthez et al, 2005a, b; Tesfaigzi et al, 2001) and the volumes were adjusted for the rat to avoid reaching the lungs (KleinJan et al, 2006). Groups of animals were used for behavioral testing or they were used for brain cytokine determination without behavioral testing. All groups of animals were used for corticosterone determinations. All animals were killed by rapid decapitation without anesthesia. Trunk blood was collected in EDTA containing tubes and the plasma immediately frozen until corticosterone determinations. For cytokine determinations, the brains were immediately removed and frozen by immersion in isopentane and stored at -80°C.
Open Field Testing
Ambulatory motor activity was monitored in two square Plexiglas enclosures (40.1 × 40.1 cm) maintained under constant illumination by four overhead 34 W fluorescent lights. The floor was divided in 16 equal squares and a digital video camera was placed on top and recorded the session. The animals were placed in one corner of the enclosures and measurement of horizontal motor activity over 5 min was determined as the number of times the animal entered a square measured by a blind rater. The number of crossings of the center field (crossing any of the four center squares) and rearing were also measured. At the completion of the session, the animals were returned to their cages, left undisturbed for 15 min and then tested in the FST.
Forced Swim Test
On day 1, rats were placed in a vertical glass cylinder (diameter 22.5 cm and height 60 cm) containing 35 cm of water maintained at 25°C for 10 min. Twenty-four hours later, the animals were forced to swim for 5 min (day 2). Behavior during the full 10 min (day 1) and 5 min (day 2) was recorded as digital video by the Forced SwimScan system (Cleversys Inc., Reston, VA). The system automatically analyzed immobility, swimming, and escape behaviors as described by Detke et al (1995), and Detke and Lucki (1996). This system has been validated in mice (Hines et al, 2006) and was also validated in our laboratory by blind raters (Supplementary Figure S1).
Corticosterone Determinations
Determinations were performed in the i.n. group of animals. All groups of animals were killed between 1200 and 1300 h. For those rats that were behaviorally tested, at the end of the FST they were removed from the cylinder, dried with paper towels, and placed in an individual cage to rest and recover for 1 h before termination of the experiment. Plasma corticosterone levels were determined by radioimmunoassay using a double antibody kit (ImmuChem, MP Biomedicals, Orangeburg, NY) according to manufacturer’s instruction. Intra assay variation was less than 8%.
Real-Time RT-PCR
These determinations were performed in the brain of i.n. treated animals that were not behaviorally tested. Frozen brains were allowed to reach ice-cold temperature and the olfactory bulbs, frontal cortex (Bregma 5.2 to 4.2 mm), hippocampus, and brain stem (Bregma -9 to -14mm) were dissected and processed for mRNA extraction using the Trizol reagent (Invitrogen) as described (Tonelli et al, 2004). Five hundred nanograms of total RNA per sample was reverse-transcribed into cDNA in a 20 μl reaction mixture using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to manufacturer’s instructions. Real-time RT-PCR was conducted using the iQ SYBR Green Supermix (Bio-Rad) in a 50 μl reaction mixture using the set of primers listed in Table 1. All sets of primers were tested in 0.9% agarose gel to confirm a single amplification product. The amplified products for cytokines were directly cloned into the pCRII-Topo vector (Invitrogen, Paisley, Scotland, UK) and sequenced to confirm their identity. All the primer pairs were designed using the Accelrys Gene 2.0v software.
Table 1.
Primer Sequences Used for Real-Time RT-PCR Determinations
| Gene | Accession | Primer sequence | Region | Product length | |
|---|---|---|---|---|---|
| IL-1β | NM_031512 | Fwd | 5′-AATGCCTCGTGCTGTCTGACC-3′ | 471-588 | 118 |
| Rev | 5′-TTGTCGTTGCTTGTCTCTCCTTG-3′ | ||||
| TNF-α | NM_012675 | Fwd | 5′-TCTTCTGTCTACTGAACTTCGGGG-3′ | 284-365 | 82 |
| Rev | 5′-ATGGAACTGATGAGAGGGAGCC-3′ | ||||
| IL-6 | NM_012589 | Fwd | 5′-CAAGAGACTTCCAGCCAGTTGC-3′ | 81-191 | 111 |
| Rev | 5′-TGTTGTGGGTGGTATCCTCTGTG-3′ | ||||
| 18S | X01117 | Fwd | 5′-CCAGTAAGTGCGGGTCATAAGC-3′ | 1650-1732 | 83 |
| Rev | 5′CCATCCAATCGGTAGTAGCGAC-3′ |
IL-1β, interleukin-1 β; TNF-α, tumor necrosis factor α; IL-6, interleukin-6; 18S, rat ribosomal RNA subunit.
The real-time PCR was run on a MyiQ instrument (Bio-Rad) with a three-step cycling program as follows: an initial hot start for 5 min at 95°C followed by 40 cycles with a denaturation step of 15 s at 95°C, an annealing step of 30 s at 55°C, an extension step of 30 s at 72°C with the optics on at this last step. In preparation of a melt curve, the samples were heated for 1 min at 95°C then cooled for 1 min at 55°C, and the melt curve was executed in 10 s increments of 0.5°C with the temperature increasing from 55 to 95°C with the optics on. All the primers used were selected and tested for the described amplification conditions. Efficiency and consistency of the cDNA synthesis was determined by amplification of the rat 18S gene as a control. For each round of amplifications, only those samples that were within 1 cycle of difference, with respect to the mean for 18S were considered for further analysis. For each sample of a specific target gene, each cycle threshold was normalized with respect to 18S. Relative expression was determined using the 2-ΔΔCt method (Livak and Schmittgen, 2001). The following genes were analyzed according to their published sequences: interleukin-1β (IL-1β, NM_031512); tumor necrosis factor-α (TNF-α, NM_012675); interleukin-6 (IL-6, NM_012589); rat ribosomal RNA subunit 18S (18S, X01117).
Statistics
Open field and the FST data were analyzed with a two-way analysis of variance (ANOVA) with sex (two levels: female/male) and treatment (three levels: control; LPS 12 h; LPS 24 h) as factors for i.p. studies. For i.n. studies the same model was used with the levels: control; i.n. single dose; i.n. repeated dose.
Corticosterone data were analyzed with a three-way ANOVA with sex (two levels: female/male), treatment (two levels: control; i.n. LPS) and condition (two levels: subjected to forced swim test; not subjected to forced swim test) as factors.
Cytokine expression data were analyzed with a two-way ANOVA with sex (two levels: female/male) and treatment (three levels: control; LPS single dose; LPS repeated dose) as factors.
Tukey’s multiple pair-wise comparisons were used as post hoc test for all the models. Significance level was set at p<0.05.
RESULTS
Oscillation in Swimming Behavior Across the Ovarian Estrous Cycle in Fischer 344 Rats
It has been reported that female rats subjected to the FST display variations in swimming behavior during different phases of the estrous cycle (Barros and Ferigolo, 1998; Consoli et al, 2005; Contreras et al, 1998; Frye and Walf, 2002; Marvan et al, 1996). Increased immobility time has been reported during both the proestrus/estrus phase (Contreras et al, 1998; Frye and Walf, 2002; Marvan et al, 1996) and during the diestrus 1 and 2 phases (Barros and Ferigolo, 1998; Consoli et al, 2005). These differences were reported in out-bred and inbred rat strains. An early study in F344/NHsd rats did not detect differences in swimming behavior between males and females (Armario et al, 1995); however, the phase of the estrous cycle in those experiments were not considered. We therefore examined the behavioral responses in saline-treated animals on the FST in females during the ovarian estrous cycle (n = 24) and compared the response with males (n = 21). During the first day of testing, a significant effect for immobility time (F(1, 41) = 20.1; p<0.001) and swimming (F(1, 41) = 12.6; p<0.001), and escape (F(1, 41) = 13.24; p<0.008) behaviors were detected (Figure 1a). Post hoc analysis showed that females during the proestrus/estrus phase had increased immobility time and decreased swimming and escape time with respect to both females on diestrus 1 and 2 and to males (Figure 1a). No differences were observed when comparing male with female rats tested during the diestrus phase. On day 2, a significant sex effect in immobility (F(1, 41) = 25.4; p<0.0001) and swimming (F(1, 41) = 10.7; p<0.001), and escape (F(1, 41) = 19.79; p<0.0001) behavior was detected (Figure 1b). Post hoc analysis showed that females had increased immobility time, reduced swimming, and escape behaviors as compared to males regardless of the phase of the estrous cycle (Figure 1b). To avoid introducing this variable when studying the effects of LPS in the FST on day 1, animals were administered and tested during the diestrus phase.
Figure 1.
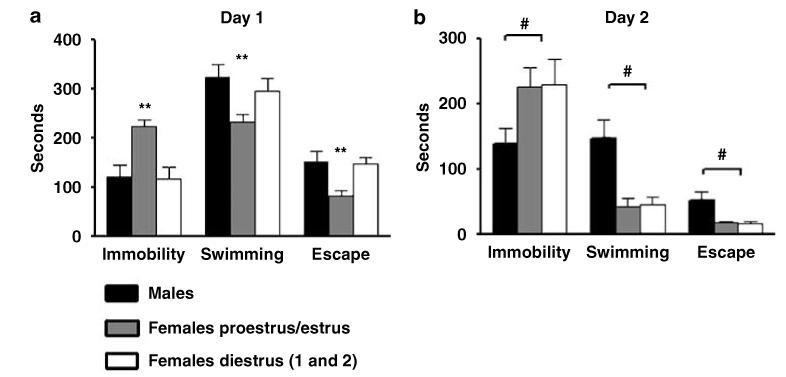
Immobility, swimming, and escape behaviors in inbred Fischer (F344/NHsd) rats in control conditions during 10 min of FST on day 1 (a) and during 5 min of re-exposure on day 2 (b). On day 1 (a), significant increases in immobility and decrease in swimming, and escape times were detected in females evaluated during the proestrus-estrus phase (n = 12) respect to females during diestrus 1 and 2 (n = 12) and to males (n = 21). **Significant proestrus/estrus differences p<0.01. On day 2 (b) significantly higher immobility time and reduced swimming time were detected in female with respect to male rats regardless the phase of the estrous cycle. Values are means±SEM. #Significant sex differences p<0.01.
Behavioral Effects of i.p. LPS Administration
Open field test
Female and male rats-treated i.p. with 2 mg/kg of LPS and tested at 12 (n = 24) or 24 (n = 24) hours after administration showed reduced levels of horizontal exploratory motor activity [F(2, 42) = 16,36; p<0.0001] (Figure 2a). In addition, we detected decreased activity in the open field with the dose of 1 mg/kg at 12 and 24 h after administration (data not shown). Overall, the activity of female and male rats treated with 2 mg/kg and tested at 24 h was very low (Figure 3a). Significant differences in all the parameters analyzed in the open field including total activity, center crossings, and rears were detected (Figure 3a). Additional signs of sickness were also evident including piloerection at all times tested and red tears at 24 h after i.p. LPS administration. No differences were observed when comparing sexes. These data indicate that i.p. LPS at these doses affects motor exploratory behavior in F344/Nhsd rats as shown previously in mice (Dunn and Swiergiel, 2005).
Figure 2.
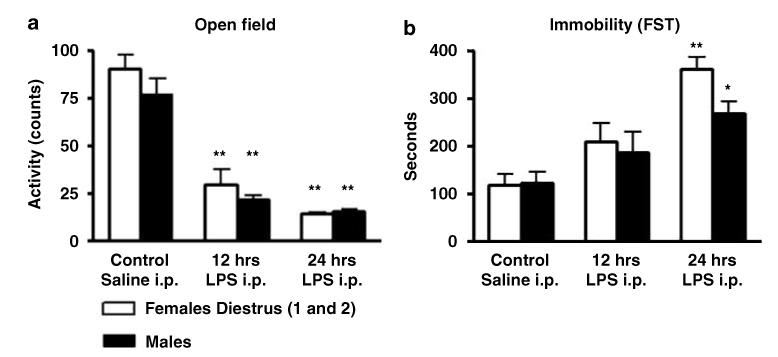
Total motor horizontal activity during 5 min of test in an open field arena (a) and total immobility time during 10 min of FST (b) in male and female rats administered intraperitoneally (i.p.) with control saline solution (n = 24) or LPS (2 mg/kg). The animals were tested after 12 h (n = 24) or 24 h (n = 24) after administration. Values are means±SEM. *Significant differences with respect to control saline conditions (*p<0.05; **p<0.01).
Figure 3.
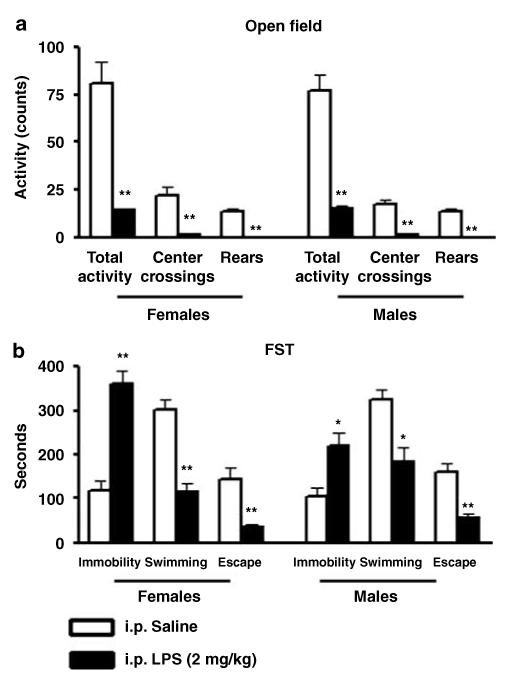
Total motor horizontal activity, center crossings, and total rears during 5 min of open field test (a) and immobility, swimming, and escape time during 10 min of FST (b) on day 1 in female and male rats in control saline conditions (n = 24) or after 24 h (n = 24) of intraperitoneal (i.p.) administration of LPS (2 mg/kg). Values are means±SEM. *Significant differences with respect to control (*p<0.05; **p<0.01).
Forced Swim Test
Intraperitoneal administration of 2 mg/kg of LPS produced a significant effect on immobility (F(1, 44) = 19.7; p<0.0001), swimming (F(1, 44) = 8.34; p<0.006), and escape (F(1, 44) = 11.7; p<0.001) behaviors in both male and female rats at 24 h after administration when tested on day 1 (Figures 2b and 3b). Post hoc analysis indicated that LPS treated animals had increased immobility and decreased swimming and escape behavior with respect to saline treated rats. No interactions of gender treatment was observed for any parameter; however, LPS-treated females showed higher immobility values with respect to LPS-treated males (Figures 2b and 3b). No significant differences were observed with respect to saline-treated rats when the animals were tested at 12 h (Figure 2b). Similarly, no differences in immobility were observed in rats that received an i.p. dose of 1 mg/kg and were tested at 12 or 24 h after LPS administration. Neither dose had any significant effect when the rats were tested on day 2 (Supplementary Figure S2).
Behavioral Effects of i.n. LPS Administration
Open field test
Administration of LPS in the nasal cavity had no effect on ambulatory motor activity, number of crossings of the center field or rearing in any dose or schedule of administration tested in this series of experiments (Figures 4a and 5a). In addition, no significant changes in body weight were detected (Supplementary Figure S3).
Figure 4.
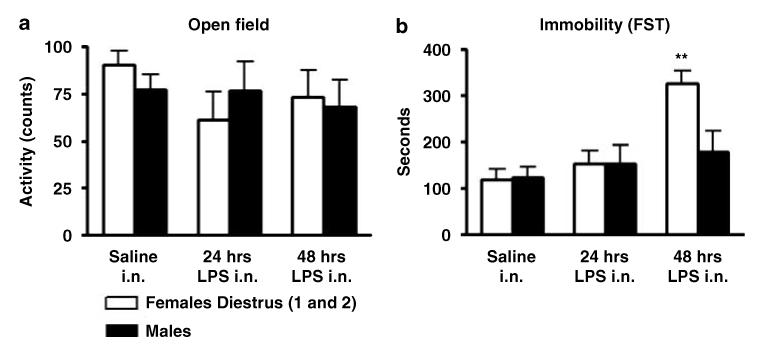
Total motor horizontal activity during 5 min of test in an open field arena (a) and total immobility time during 10 min of FST (b) in male and female rats administered with a single intranasal (i.n.) dose of LPS (100 μg/rat) (n = 24) or with 2 i.n. instillations of LPS (100 μg/rat each day) in 2 consecutive days (n = 24) or with respective volumes of control saline solution (n = 24). The animals were tested 24 h after the single dose or 24 h after the second dose respectively. Values are means±SEM. **Significant differences with respect to control saline conditions (**p<0.01).
Figure 5.

Total motor horizontal activity, center crossings and total rears during 5 min of open field test (a) and immobility, swimming, and escape time during 10 min of FST in day 1 (b) in female and male rats in control saline conditions (n = 24) or after two doses of intranasal (i.n.) administration of LPS (100 μg/rat each day) (n = 24). Values are means±SEM. **Significant differences with respect to control **p<0.01. #Significant sex differences (#p<0.05).
Forced swim test
Repeated i.n. administration of 100 μg/rat of LPS induced a sex × treatment effect in immobility (F(1, 41) = 5.5; p<0.024) and escape (F(1, 41) = 5.6; p<0.032) behaviors respectively on the first day of the FST (Figures 4b and 5b). Post hoc analysis indicated that LPS treated females had increased immobility and reduced escape behavior with respect to control conditions and to males treated with LPS. No differences in swimming time were detected in LPS-treated animals with respect to control saline (Figures 4b and 5b). Administration of single doses of LPS did not induce statistically significant differences in any parameter analyzed (Figure 4b). Similar to i.p. LPS, i.n. LPS did not induce significant differences with respect to control saline when the animals were evaluated on the second day of the test.
These data indicate that repeated LPS administration in the nasal cavities of female rats over a period of 2 days induces behavioral changes indicative of depression as revealed by the FST without affecting overall motor activity. Moreover, the increased immobility time was paralleled by a reduction in escape behavior without affecting swimming behavior.
Effects of i.n. LPS on Corticosterone Release
Figure 6 shows plasma corticosterone concentrations in male and female rats (sex factor) challenged i.n. with two doses of LPS (100 μg/rat) (treatment factor) and subjected or not to the FST (condition factor). Statistical analysis showed a significant sex × treatment × condition effect. Sex effect (F(1, 74) = 60.02; p<0.0001); treatment effect (F(1, 74) = 50.90; p<0.0001); condition effect (F(1, 74) = 37.89; p<0.0001). Post hoc analyses indicated that females had higher values as compared to males in all the conditions tested. The highest plasma corticosterone concentrations were observed in females administered i.n. with LPS and subjected to the FST (Figure 6). Post hoc comparisons revealed that females under this condition had significantly increased values with respect to any other condition tested and with respect to males.
Figure 6.
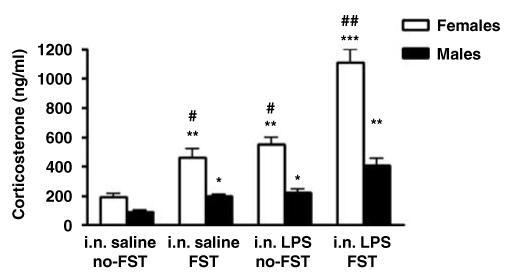
Plasma corticosterone concentrations (ng/ml) as determined by radioimmunoassay in F344/NHsd rats. Values are means±SEM (i.n. saline no-FST): animals administered i.n. with saline and not subjected to the forced swim test (n = 16). (i.n. saline FST): animals administered intranasally with saline and evaluated in the forced swim test (n = 24). (i.n. LPS no-FST): animals administered i.n. with LPS (two doses of 100 μg/rat) and not subjected to the FST (n = 16). (i.n. LPS FST): animals administered i.n. with LPS (two doses of 100 μg/rat) and evaluated in the FST (n = 24). *Significant differences with respect to control (*p<0.05; **p<0.001). #Significant sex differences (#p<0.01; ##p<0.001).
Effects of i.n. LPS on Cytokine Gene Expression
Interleukin 1-β
No differences with respect to control saline were observed in any of the brain regions analyzed after a single or repeated i.n. administration of LPS in both male and female rats. In addition, no sex differences were detected in any of the regions analyzed.
Tumor necrosis factor-α
A significant sex × treatment effect was detected in the hippocampus (F(1, 21) = 4.25; p<0.04) and brainstem (F(1, 21) = 4.89; p<0.02) after two doses of 100 μg/rat i.n. LPS administration (Figures 7b and e). Post hoc analysis revealed that in the brainstem, two doses of i.n. LPS induced a significant increase in both male and female rats with respect to their own control and this increase was higher in females compared to males (Figure 7e). In the hippocampus, a significant increase with respect to controls was observed only in females after two i.n. doses of LPS (Figure 7b). No significant differences were observed in the brainstem and hippocampus after a single dose of i.n. LPS. Similar to IL-1β, no differences were detected in the frontal cortex and olfactory bulbs under any condition tested.
Figure 7.
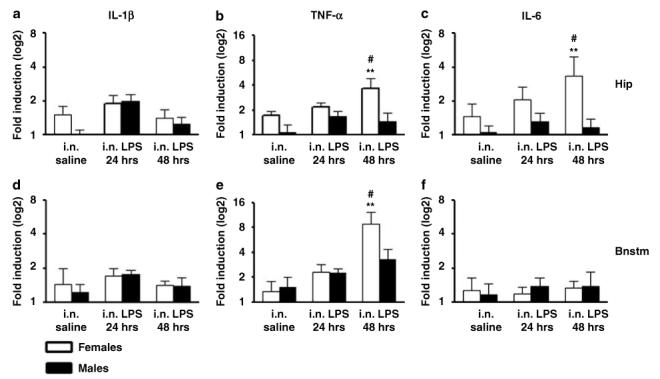
Relative mRNA cytokine expression in the hippocampus and brainstem of female and male F344/NHsd rats in control saline conditions (n = 12), 24 h after a single intranasal (i.n.) administration of LPS (100 μg/rat) (n = 12) or after two instillations of LPS (100 μg/rat each day) in 2 consecutive days (n = 12). The determinations for IL-1β (a, d), TNF-α (b, e), and IL-6 (c, f) were done by triplicates obtained from the same cDNA preparation for each individual brain region. Values are means±SEM. Values are fold induction with respect to control saline displayed in a logarithmic scale. Hip: hippocampus; Bnstm: brainstem. **Significant differences with respect to control p<0.01. #Significant sex-difference, p<0.05.
Interleukin-6
A significant sex × treatment effect was detected in the hippocampus of females (F(1, 21) = 4.05; p<0.05) but not in the brainstem (Figures 7c and f). Post hoc analysis showed that in the hippocampus females had increased levels of IL-6 with respect to males after repeated administration of i.n. LPS (Figure 7c). As it was the case for IL-1β and TNF-α, no differences with respect to control saline was detected for IL-6 in the olfactory bulbs and frontal cortex after i.n. LPS.
DISCUSSION
The data presented here show depressive-like behaviors induced by peripheral LPS when administered in the nasal cavities of female rats. These effects were paralleled by exaggerated corticosterone responses after the swimming sessions suggesting altered glucocorticoid responses to stress. Finally, elevated TNF-α in the hippocampus and brainstem of females challenged with i.n., LPS was also observed. Furthermore, these data confirm previous observations indicating that the induction of depressive-like behaviors in rodents measured by the FST after i.p. administration of LPS are dose-dependent and are paralleled by motor dysfunction (Deak et al, 2005; Dunn and Swiergiel, 2005).
Intranasal administration of LPS-induced measurable increases in immobility time in female rats 48 h after the initial instillation, and after two consecutive administrations of LPS. No alterations in total exploratory activity in the open field were detected and no significant changes in body weight were observed. This is suggestive that the behavioral effect is related to the local inflammatory process in the nasal cavities without inducing evident signs of sickness. It also indicates that i.n. LPS requires a certain time to affect behavior and that it is sex-specific. Moreover, it has to be considered that the effect was restricted to the first day of test, and that it did not affect swimming behavior but did affect escape behavior. On the other hand, depressive-like behavior measured by increased immobility was observed in both male and female rats administered i.p. with the higher dose tested in this study (2 mg/kg) at 24 h after administration. The same dose tested at earlier time points or lower doses at 24 h did not produce significant changes in immobility, but did affect exploratory responses in the open field test. These data indicate that although signs of sickness induced by i.p. LPS are measurable, rats are capable of mounting a behavioral response to a stressor such as the FST during the acute phase response to systemic inflammation. It also indicates that there is a threshold in which rats can cope with the FST challenge during sepsis which is dose- and time-dependent. An important consideration of the present i.p. studies is that only single injections of LPS were tested. It is possible that other schedules of i.p. LPS injections such as repeated lower doses, for example, may still provide a model to differentiate sickness from depression using the i.p. LPS and the FST.
Although there is some controversy about the FST as a model of depressive-like behavior, it is accepted that the test models certain aspects of depression involving the uncontrollability of a stressor (the rat cannot escape from the cylinder) evaluating passive (immobility or floating) vs active (struggling and swimming) coping responses to stress (Cryan and Holmes, 2005). The type of response observed after i.n. LPS may indicate a specific neurochemical system involved in response to the i.n. LPS challenge since different types of antidepressants have been shown to have different effects on active behaviors in the FST (Cryan et al, 2005). While the present studies may be far from providing specific mechanisms of immune function leading to depression at the individual level, the i.n. LPS immune challenge indicates that upper respiratory infections may be one factor related to the increased incidence of mood disorders during certain times of the year (Nelson et al, 2002). On average, children have between six and eight, and adults between two and four, upper respiratory infections each year (Monto, 1994; Monto and Ullman, 1974). Despite their usually benign course, upper respiratory infections place an enormous economic burden on society; they are responsible for 20 million days absence from work and 22 million days of absence from school in the USA (Adams et al, 1999).
Intranasal instillation of LPS is known to induce airway inflammation characterized by neutrophil and macrophage infiltration and the production of chemokines, and cytokines including TNF-α (Delayre-Orthez et al, 2005a, b). Moreover, i.n. instillation of recombinant cytokines including IL-6 and IL-12 have been shown to affect the course of experimentally induced neurological disorders in rats (Kalueff et al, 2004; Pelidou et al, 2000). Although the mechanisms on how cytokines applied i.n. relate to brain function are unknown, these studies suggest that cytokines in the nasal cavities secreted as part of an inflammatory process have the potential to influence brain function. In support of this observation, the present data show measurable induction of cytokine transcription in the brain after 48 h of the initial i.n. LPS administration. From the brain regions examined, the highest reactivity corresponded to the brainstem and the hippocampus, and no changes were detected in the frontal cortex and olfactory bulbs. With the exception of the olfactory bulbs, the cytokine response of these brain structures is in agreement with the regions in which i.n. administration of radiolabelled insulin-like growth factor-I (a 7.65 kDa protein) distributes in the brain (Thorne et al, 2004). In addition, the involvement of these regions in the modulation of mood has been reported (Arango et al, 2002; Drevets, 2000). However, it has to be considered that other important regions including the hypothalamus and amygdaloid complex that are involved in mood regulation and are targets of i.n. delivered molecules were not analyzed in the present study. Another consideration is that changes in cytokine expression in the olfactory bulbs were expected but not detected. It is possible that this may be due to a differential immune response of this area involving a different set of cytokines and chemokines depending on the type of antigen (Shwe et al, 2006). The close relationship of the olfactory bulbs with sensory neurons exposed to the environment is likely to play a role in a differential cytokine response of this structure.
We obtained in i.n. challenged animals a dimorphic response in the hippocampus and brainstem in the induction of TNF-α transcription. It is known that females of many species mount a stronger immune response than males after immunization and have increased survival ratio to infections (Verthelyi, 2001; Weinstein et al, 1984). Increased humoral responses and production of antibodies as compared to males have been proposed to participate in this increased survival ratio (Verthelyi, 2001). In the brain, enhanced immune responses in female with respect to male were reported in mice after i.n. inoculation with vesicular stomatitis virus (Barna et al, 1996), herpes simple virus-2 or intracerebral administration of LPS (Soucy et al, 2005). This has been proposed to be related to a stronger cytokine expression and transfer from innate to adaptive immune responses in females (Soucy et al, 2005) in which TNF-α have been shown to play an important role (Rahman and McFadden, 2006). Relevant to this point, it has been recently shown that mice deficient in TNF-α receptors show reduced immobility time in the FST supporting a role for TNF-α in behavioral responses of depression (Simen et al, 2006). Moreover, the authors report that TNF-α receptors in the wild type animal are highly expressed in the brainstem and hippocampus, the 2 areas in which the present study reports increased transcription of TNF-α after i.n. LPS challenge.
In the hippocampus, but not in the other brain regions studied, increased IL-6 expression in females after i.n. LPS was also detected. IL-6 receptors in this region are abundant and IL-6 has been shown to interfere with memory consolidation (Balschun et al, 2004). However, the present study did not address whether this profile of cytokine expression in these brain regions is causally related to behavioral symptoms observed. Moreover, there is the possibility that circulating levels of cytokines induced by the i.n. treatment may be contributing to the observed effects. Also, the role of sex hormones in mediating the behavioral, glucocorticoid and cytokine responses observed in the present study has not been addressed. These issues will be matter of future studies.
Another dimorphic factor induced by i.n. LPS that may be contributing to the behavioral symptoms independent of brain cytokines is the glucocorticoid response to the FST. It is known that female F344/NHsd rats show the highest hypothalamic-pituitary-adrenal (HPA) axis reactivity from all of the known inbred and outbred rat strains tested (Tonelli et al, 2001). Nevertheless, the concentrations detected in the present study for female F344/NHsd rats challenged with i.n. LPS and subjected to the FST are likely to represent maximal corticosterone responses for this strain (Armario et al, 1995; Grota et al, 1997; Tonelli et al, 2001, 2003). Corticosterone concentrations in i.n. treated animals were more than double with respect to control and similar to values after the stress of forced swimming. More importantly, values in i.n. LPS animals exposed to the FST were double in respect to those induced by i.n. LPS alone or by the FST alone. These findings may also be of relevance for human depression. Although there is no conclusive evidence on the exact mechanisms on how glucocorticoids and depression relate to each other, studies performed in depressed individuals strongly suggest that altered glucocorticoid levels and/or HPA axis responses are a hallmark of human depression (Belanoff et al, 2002; Gillespie and Nemeroff, 2005; Gold and Chrousos, 2002; Gold et al, 2002; McEwen, 2002; Swaab et al, 2005). Both human studies and animal models show that hypercortisolemia and/or altered glucocorticoid responses to stress are found in humans suffering from depression and in animals that have developed depressive-like behaviors. Several mechanisms are under investigation to explain this association, including interaction with neurotransmitter and neuropeptides (Leonard, 2005; Schulkin et al, 1998), structural remodeling of the brain (McEwen, 2005), loss of glucocorticoid receptor function (Pariante, 2004; Pariante and Miller, 2001) and interactions with the immune system (Himmerich et al, 2006; Leonard, 2005).
In summary, repeated i.n. administration of LPS in female rats produced depressive-like behavior, maximal corticosterone responses to stress, and increased TNF-α transcription in the brain. With additional studies to elucidate their mechanisms, these findings may further our understanding of the role that the immune system may play in the pathophysiology of depression.
Supplementary Material
ACKNOWLEDGEMENTS
Supported in part by NIH K12 HD43489, Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) award and by R21MH075905-01A1 to LH Tonelli. We thank Dr Drew E Carlson, PhD, for his help with the corticosterone determinations and Dr Hegang Cheng, PhD, for his help with the statistical analyses.
Footnotes
DISCLOSURE/CONFLICTS OF INTEREST
The authors do not have any conflict of interest to report.
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
REFERENCES
- Adams PF, Hendershot GE, Marano MA. Current estimates from the National Health Interview Survey, 1996. Vital Health Stat. 1999;10:1–203. [PubMed] [Google Scholar]
- Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11:963–972. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- Armario A, Gavalda A, Marti J. Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology. 1995;20:879–890. doi: 10.1016/0306-4530(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Yirmiya R. Cytokines inhibit sexual behavior in female rats: I. Synergistic effects of tumor necrosis factor alpha and interleukin-1. Brain Behav Immun. 1999a;13:14–32. doi: 10.1006/brbi.1999.0555. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Yirmiya R. The immunobiology of sexual behavior: gender differences in the suppression of sexual activity during illness. Pharmacol Biochem Behav. 1999b;64:787–796. doi: 10.1016/s0091-3057(99)00165-3. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, et al. Interleukin-6: a cytokine to forget. FASEB J. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Barna M, Komatsu T, Bi Z, Reiss CS. Sex differences in susceptibility to viral infection of the central nervous system. J Neuroimmunol. 1996;67:31–39. doi: 10.1016/0165-5728(96)00022-7. [DOI] [PubMed] [Google Scholar]
- Barros HM, Ferigolo M. Ethopharmacology of imipramine in the forced-swimming test: gender differences. Neurosci Biobehav Rev. 1998;23:279–286. doi: 10.1016/s0149-7634(98)00029-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Belanoff JK, Rothschild AJ, Cassidy F, DeBattista C, Baulieu EE, Schold C, et al. An open label trial of C-1073 (mifepristone) for psychotic major depression. Biol Psychiatry. 2002;52:386–392. doi: 10.1016/s0006-3223(02)01432-4. [DOI] [PubMed] [Google Scholar]
- Buttini M, Boddeke H. Peripheral lipopolysaccharide stimulation induces interleukin-1 beta messenger RNA in rat brain microglial cells. Neuroscience. 1995;65:523–530. doi: 10.1016/0306-4522(94)00525-a. [DOI] [PubMed] [Google Scholar]
- Buttini M, Limonta S, Boddeke HW. Peripheral administration of lipopolysaccharide induces activation of microglial cells in rat brain. Neurochem Int. 1996;29:25–35. doi: 10.1016/0197-0186(95)00141-7. [DOI] [PubMed] [Google Scholar]
- Capuron L, Bluthe RM, Dantzer R. Cytokines in clinical psychiatry. Am J Psychiatry. 2001;158:1163–1164. doi: 10.1176/appi.ajp.158.7.1163. [DOI] [PubMed] [Google Scholar]
- Capuron L, Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav Immun. 2003;17(Suppl 1):S119–S124. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- Capuron L, Lamarque D, Dantzer R, Goodall G. Attentional and mnemonic deficits associated with infectious disease in humans. Psychol Med. 1999;29:291–297. doi: 10.1017/s0033291798007740. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- Consoli D, Fedotova J, Micale V, Sapronov NS, Drago F. Stressors affect the response of male and female rats to clomipramine in a model of behavioral despair (forced swim test) Eur J Pharmacol. 2005;520:100–107. doi: 10.1016/j.ejphar.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Contreras CM, Martinez-Mota L, Saavedra M. Desipramine restricts estral cycle oscillations in swimming. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:1121–1128. doi: 10.1016/s0278-5846(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Crone CC, Gabriel GM, Wise TN. Managing the neuropsychiatric side effects of interferon-based therapy for hepatitis C. Cleve Clin J Med. 2004;71(Suppl 3):S27–32. doi: 10.3949/ccjm.71.suppl_3.s27. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, et al. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Wollman EE, Yirmiya R. Cytokines and depression: an update. Brain Behav Immun. 2002;16:501–502. doi: 10.1016/s0889-1591(02)00002-8. [DOI] [PubMed] [Google Scholar]
- De La Garza R., II Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neurosci Biobehav Rev. 2005;29:761–770. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D’Agostino LG, Rosanoff M, McElderry NK, Bordner KA. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav Brain Res. 2005;160:125–134. doi: 10.1016/j.bbr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Delayre-Orthez C, Becker J, de Blay F, Frossard N, Pons F. Exposure to endotoxins during sensitization prevents further endotoxin-induced exacerbation of airway inflammation in a mouse model of allergic asthma. Int Arch Allergy Immunol. 2005a;138:298–304. doi: 10.1159/000088867. [DOI] [PubMed] [Google Scholar]
- Delayre-Orthez C, Becker J, Guenon I, Lagente V, Auwerx J, Frossard N, et al. PPARalpha downregulates airway inflammation induced by lipopolysaccharide in the mouse. Respir Res. 2005b;6:91. doi: 10.1186/1465-9921-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denicoff KD, Rubinow DR, Papa MZ, Simpson C, Seipp CA, Lotze MT, et al. The neuropsychiatric effects of treatment with interleukin-2 and lymphokine-activated killer cells. Ann Intern Med. 1987;107:293–300. doi: 10.7326/0003-4819-107-2-293. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: a review. Am J Psychiatry. 2000;157:867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav. 2005;81:688–693. doi: 10.1016/j.pbb.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland CG, Kavaliers M, Ossenkopp KP. Sex differences in the effects of muramyl dipeptide and lipopolysaccharide on locomotor activity and the development of behavioral tolerance in rats. Pharmacol Biochem Behav. 2003;74:433–447. doi: 10.1016/s0091-3057(02)01024-9. [DOI] [PubMed] [Google Scholar]
- Fliedner S, Schulz C, Lehnert H. Brain uptake of intranasally applied radioiodinated leptin in Wistar rats. Endocrinology. 2006;147:2088–2094. doi: 10.1210/en.2005-1016. [DOI] [PubMed] [Google Scholar]
- Frommberger UH, Bauer J, Haselbauer P, Fraulin A, Riemann D, Berger M. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci. 1997;247:228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med. 2005;67(Suppl 1):S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gold PW, Drevets WC, Charney DS. New insights into the role of cortisol and the glucocorticoid receptor in severe depression. Biol Psychiatry. 2002;52:381–385. doi: 10.1016/s0006-3223(02)01480-4. [DOI] [PubMed] [Google Scholar]
- Grota LJ, Bienen T, Felten DL. Corticosterone responses of adult Lewis and Fischer rats. J Neuroimmunol. 1997;74:95–101. doi: 10.1016/s0165-5728(96)00209-3. [DOI] [PubMed] [Google Scholar]
- Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59:569–577. doi: 10.1016/0031-9384(95)02112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmerich H, Binder EB, Kunzel HE, Schuld A, Lucae S, Uhr M, et al. Successful antidepressant therapy restores the disturbed interplay between TNF-alpha system and HPA axis. Biol Psychiatry. 2006;60:882–888. doi: 10.1016/j.biopsych.2006.03.075. [DOI] [PubMed] [Google Scholar]
- Hines LM, Hoffman PL, Bhave S, Saba L, Kaiser A, Snell L, et al. A sex-specific role of type VII adenylyl cyclase in depression. J Neurosci. 2006;26:12609–12619. doi: 10.1523/JNEUROSCI.1040-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56:3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- Janssen HL, Brouwer JT, van der Mast RC, Schalm SW. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J Hepatol. 1994;21:241–243. doi: 10.1016/s0168-8278(05)80402-7. [DOI] [PubMed] [Google Scholar]
- Jin Y, Dons L, Kristensson K, Rottenberg ME. Neural route of cerebral Listeria monocytogenes murine infection: role of immune response mechanisms in controlling bacterial neuroinvasion. Infect Immun. 2001;69:1093–1100. doi: 10.1128/IAI.69.2.1093-1100.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Lehtimaki KA, Ylinen A, Honkaniemi J, Peltola J. Intranasal administration of human IL-6 increases the severity of chemically induced seizures in rats. Neurosci Lett. 2004;365:106–110. doi: 10.1016/j.neulet.2004.04.061. [DOI] [PubMed] [Google Scholar]
- KleinJan A, Willart M, van Rijt LS, Braunstahl GJ, Leman K, Jung S, et al. An essential role for dendritic cells in human and experimental allergic rhinitis. J Allergy Clin Immunol. 2006;118:1117–1125. doi: 10.1016/j.jaci.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Ueno Y, Kobayashi Y, Akahane T, Satoh S, Kikuchi K, et al. Th1 response during ribavirin and interferon-alpha combination therapy in chronic hepatitis C. Hepatol Res. 2006;34:104–110. doi: 10.1016/j.hepres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Leonard BE. The HPA and immune axes in stress: the involvement of the serotonergic system. Eur Psychiatry. 2005;20(Suppl 3):S302–S306. doi: 10.1016/s0924-9338(05)80180-4. [DOI] [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40:171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loftus LT, Li HF, Gray AJ, Hirata-Fukae C, Stoica BA, Futami J, et al. In vivo protein transduction to the CNS. Neuroscience. 2006;139:1061–1067. doi: 10.1016/j.neuroscience.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Marvan ML, Chavez-Chavez L, Santana S. Clomipramine modifies fluctuations of forced swimming immobility in different phases of the rat estrous cycle. Arch Med Res. 1996;27:83–86. [PubMed] [Google Scholar]
- McEwen BS. Cortisol, Cushing’s syndrome, and a shrinking brain-new evidence for reversibility. J Clin Endocrinol Metab. 2002;87:1947–1948. doi: 10.1210/jcem.87.5.9999. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Merali Z, Brennan K, Brau P, Anisman H. Dissociating anorexia and anhedonia elicited by interleukin-1beta: antide-pressant and gender effects on responding for ‘free chow’ and ‘earned’ sucrose intake. Psychopharmacology (Berl) 2003;165:413–418. doi: 10.1007/s00213-002-1273-1. [DOI] [PubMed] [Google Scholar]
- Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16:351–373. doi: 10.1093/oxfordjournals.epirev.a036158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227:164–169. [PubMed] [Google Scholar]
- Mori I, Goshima F, Ito H, Koide N, Yoshida T, Yokochi T, et al. The vomeronasal chemosensory system as a route of neuroinvasion by herpes simplex virus. Virology. 2005;334:51–58. doi: 10.1016/j.virol.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Klein SL, Kriegsfeld LJ. Seasonal Patterns of Stress, Immune Function & Disease. Cambridge University Press; Cambridge, UK: 2002. pp. 58–88. [Google Scholar]
- Noble RE. Depression in women. Metabolism. 2005;54:49–52. doi: 10.1016/j.metabol.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Pariante CM. Glucocorticoid receptor function in vitro in patients with major depression. Stress. 2004;7:209–219. doi: 10.1080/10253890500069650. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Pelidou SH, Zou LP, Deretzi G, Nennesmo I, Wei L, Mix E, et al. Intranasal administration of recombinant mouse interleukin-12 increases inflammation and demyelination in chronic experimental autoimmune neuritis in Lewis rats. Scand J Immunol. 2000;51:29–35. doi: 10.1046/j.1365-3083.2000.00636.x. [DOI] [PubMed] [Google Scholar]
- Porsolt RD. Animal model of depression. Biomedicine. 1979;30:139–140. [PubMed] [Google Scholar]
- Prolo P, Licinio J. Cytokines in affective disorders and schizophrenia: new clinical and genetic findings. Mol Psychiatry. 1999;4:109–111. doi: 10.1038/sj.mp.4000523. [DOI] [PubMed] [Google Scholar]
- Quan N, Stern EL, Whiteside MB, Herkenham M. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol. 1999;93:72–80. doi: 10.1016/s0165-5728(98)00193-3. [DOI] [PubMed] [Google Scholar]
- Rahman MM, McFadden G. Modulation of tumor necrosis factor by microbial pathogens. PLoS Pathog. 2006;2:e4. doi: 10.1371/journal.ppat.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Schmidt F, Neumer R, Scholler G, Schwarz M. Interferon-alpha, cytokines and possible implications for mood disorders. Bipolar Disord. 2002;4(Suppl 1):111–113. doi: 10.1034/j.1399-5618.4.s1.52.x. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Shwe TW, Yamamoto S, Ahmed S, Kakeyama M, Kobayashi T, Fujimaki H. Brain cytokine and chemokine mRNA expression in mice induced by intranasal instillation with ultrafine carbon black. Toxicol Lett. 2006;163:153–160. doi: 10.1016/j.toxlet.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Simen BB, Duman CH, Simen AA, Duman RS. TNFα signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Smith A, Thomas M, Kent J, Nicholson K. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23:733–739. doi: 10.1016/S0306-4530(98)00042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosc Behav Rev. 2005;29:627–646. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Soucy G, Boivin G, Labrie F, Rivest S. Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. J Immunol. 2005;174:6391–6398. doi: 10.4049/jimmunol.174.10.6391. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Tesfaigzi Y, Rudolph K, Fischer MJ, Conn CA. Bcl-2 mediates sex-specific differences in recovery of mice from LPS-induced signs of sickness independent of IL-6. J Appl Physiol. 2001;91:2182–2189. doi: 10.1152/jappl.2001.91.5.2182. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., II Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Postolache TT. Tumor necrosis factor alpha, interleukin-1 beta, interleukin-6 and major histocompatibility complex molecules in the normal brain and after peripheral immune challenge. Neurol Res. 2005;27:679–684. doi: 10.1179/016164105X49463. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Gunsolly CA, Belyavskaya E, Atwood AR, Sternberg EM. Increased pro-thyrotropin-releasing hormone transcription in hypophysiotropic neurons of Lewis rats. J Neuroimmunol. 2004;153:143–149. doi: 10.1016/j.jneuroim.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Maeda S, Rapp KL, Sternberg EM. Differential induction of interleukin-I beta mRNA in the brain parenchyma of Lewis and Fischer rats after peripheral injection of lipopolysaccharides. J Neuroimmunol. 2003;140:126–136. doi: 10.1016/s0165-5728(03)00171-1. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Webster JI, Rapp KL, Sternberg E. Neuroendocrine responses regulating susceptibility and resistance to autoimmune/inflammatory disease in inbred rat strains. Immunol Rev. 2001;184:203–211. doi: 10.1034/j.1600-065x.2001.1840118.x. [DOI] [PubMed] [Google Scholar]
- Verthelyi D. Sex hormones as immunomodulators in health and disease. Int Immunopharmacol. 2001;1:983–993. doi: 10.1016/s1567-5769(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Weinstein Y, Ran S, Segal S. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J Immunol. 1984;132:656–661. [PubMed] [Google Scholar]
- Yirmiya R, Weidenfeld J, Pollak Y, Morag M, Morag A, Avitsur R, et al. Cytokines, ‘depression due to a general medical condition,’ and antidepressant drugs. Adv Exp Med Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


