Abstract
Serotonergic dysfunction is present in mood disorders and suicide. Brainstem 5-HT1A somatodendritic autoreceptors regulate serotonin neuron firing but studies of autoreceptor binding in the dorsal raphe nucleus (DRN) in depressed suicides report conflicting results. We sought to determine: (1) the anatomical distribution of 5-HT1A receptor binding in the DRN in depressed suicides and psychiatrically normal controls; and (2) whether sex differences in 5-HT1A binding in the DRN contribute to differences between depressed suicides and controls. Previously collected quantitative receptor autoradiograms of [3H]8-hydroxy-2-(di-n-propyl)aminotetralin (3H-8-OH-DPAT) in postmortem tissue sections containing the DRN from drug-free suicide victims (n = 10) and matched controls (n = 10) were analyzed. Less total receptor binding (fmol/mg tissue × mm3) was observed in the entire DRN in depressed suicides compared with controls (p < 0.05). Group differences along the rostrocaudal extent of the DRN were observed for cross-sectional 5-HT1A binding (fmol/mg tissue) and receptor binding (fmol/mg × mm3, p < 0.05). Cross-sectional 5-HT1A DRN binding in depressed suicides compared with controls was higher rostrally and lower caudally. The differences between depressed suicides and controls were present in males and females, although females had more binding than males. Less autoreceptor binding in the DRN of depressed suicides may represent a homeostatic response to less serotonin release, increasing serotonin neuron firing. More autoreceptor binding in rostral DRN might contribute to deficient serotonin release in ventromedial prefrontal cortex by lower neuronal firing.
Keywords: Serotonin, Suicide, 5-HT1A autoreceptors, Postmortem, Dorsal raphe nucleus
1. Introduction
1.1. Background
Serotonergic abnormalities in major depressive disorder (MDD) and suicide include low serotonin (5-HT) and 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the brainstem, and fewer serotonin transporter (SERT) sites in cortex, hypothalamus and brainstem, suggesting less serotonin release in widespread areas of the brain (see, Mann, 2003 for review). Suicide is distinguished from MDD by the localization of pre- and postsynaptic serotonergic changes. In suicide, there is less SERT and more 5-HT1A binding in the ventral prefrontal cortex (PFC, Arango et al., 1995; Mann et al., 2000), a region involved in behavioral inhibition (Bechara et al., 2000). Using the 5-HT1A antagonist 11-C-WAY100635, some studies report lower 5-HT1A binding potential (BP) by positron emission tomography (PET) in the raphe and cortex in MDD (Drevets et al., 1999; Sargent et al., 2000), although medication-naïve MDD patients have more 5-HT1A binding in the midbrain and other brain regions (Parsey et al., 2006). Studies in non-human primates support the involvement of 5-HT1A receptors in mood. In behaviorally depressed cynomolgus monkeys, 5-HT1A BP measured with the 5-HT1A antagonist, 4,2″-(methoxyphenyl)-1-[2″-(N-2″-pyridinyl)-p-fluorobenzamido]ethylpiperazine, was lower in raphe and other brain regions. (Shively et al., 2006). No in vivo studies have reported on suicidal behavior and 5-HT1A binding.
The 5-HT synthesizing neurons in the brainstem dorsal (DRN) and median (MRN) raphe nuclei give rise to the serotonergic innervation of the forebrain. In the raphe, the 5-HT1A receptor is a somatodendritic inhibitory autoreceptor on 5-HT neurons (Verge et al., 1985; Middlemiss and Fozard, 1983). Locally released 5-HT acts on the autoreceptor inhibiting neuronal firing (Wang and Aghajanian, 1977; De Montigny et al., 1984; Artigas et al., 1996; Hensler, 2003; Celada et al., 2001) and reducing 5-HT release in all projection targets (Aghajanian et al., 1987).
1.2. Objectives of the study
Previous studies yield conflicting results. Stockmeier et al. (1998) reported more 5-HT1A binding in depressed suicides vs. controls in the ventrolateral and dorsal subnuclei of the DRN in rostral midbrain. When calculating binding density × total volume of the nucleus (binding capacity), we found lower 5-HT1A receptor binding in depressed suicides (Arango et al., 2001). Differences in the study populations and different regions of the DRN being sampled between the two studies could account for the discrepant findings. We (Arango et al., 2001) included more female subjects and analyzed the caudal subnucleus, that was omitted by Stockmeier et al. (1998). Finally, we estimated the total amount of 5-HT1A binding in the volume of the DRN and/or MRN using “binding capacity” as an index of the total number of receptors in the DRN or MRN where: binding capacity = (cross-sectional receptor binding in fmol/mg tissue) × (region volume). In suicides, the DRN binding capacity, but not cross-sectional receptor binding, was lower in suicides.
In postmortem (Arango et al., 1995) and in vivo PET studies, (Parsey et al., 2002, 2005) females have more 5-HT1A receptor binding in PFC than males. No postmortem studies have considered sex in comparing 5-HT1A binding in suicide and controls in the brainstem.
Given the conflicting results about 5-HT1A receptor binding in MDD suicide victims, we sought to determine the possible effects of sex and of region of the DRN through an expanded regional analysis of 5-HT1A receptor binding, assayed previously (Arango et al., 2001).
2. Methods
2.1. Subjects
The present study was carried out on subjects previously collected and reported on Arango et al. (2001) and their characteristics are only summarized here. The investigation was carried out in accordance with the latest version of the Declaration of Helsinki. Brain samples were obtained in accordance with Institutional Review Board requirements. Next-of-kin consented to tissue donation after full disclosure of the nature of the procedures.
All cases died suddenly. A review of available records and an interview with at least one informant per case was performed according to our psychological autopsy method (Kelly and Mann, 1996) to determine axis I and axis II diagnoses and other clinical information according to DSM-IV criteria.
Brains were collected at autopsy, dissected into blocks and flash-frozen in Freon (−20 °C) and stored at −80 °C until sectioning. Samples from the left cerebral hemisphere were fixed for neuropathologic examination. Individuals with a history of cerebral trauma, central nervous system disease, chronic alcoholism, or AIDS use were excluded. Body fluids (blood, bile, aqueous humor and urine) were used for toxicological screening for cocaine, opiates, alcohol, antidepressants and other acidic and basic drugs. The brain samples were coded and sections collected and assayed by personnel blind to the cause of death.
Suicides (n = 10) and controls (n = 10) were matched for age (±5 years), sex (same sex) and postmortem interval (PMI, ±5 h) and assayed as pairs. Controls did not meet criteria for axis I or axis II diagnosis during their lifetime (see Table 1). A history of prescribed medications during the last three months of life was also taken. Detailed demographic information on individuals is reported elsewhere (Arango et al., 2001).
Table 1.
Demographics, diagnosis and toxicological screening results of suicides and controls
| Controls (n = 10) | Suicides (N = 10) | |
|---|---|---|
| Sex (M:F) | 6:4 | 6:4 |
| Age (years) | 45.4 ± 18.6 | 44.1 ± 7.7 |
| PMI (h) | 12.7 ± 4.9 | 15.8 ± 2.2 |
| Brain pH | 6.4 ± 0.3 | 6.4 ± 0.3 |
| Race (C:H:AA:As) | 7:1:2:0 | 6:3:0:1 |
| Toxicology | 10 = nothing detected | 8 = nothing detected |
| 1 = carbon monoxide | 1 = opiates | |
| 1 = analgesic | ||
| 1 = opiates, analgesics | ||
| Alcoholism | 0 | 0 |
| Axis I Diagnosis | None | 7 = MDD |
| 1 = Schizoaffective, | ||
| MDD | ||
| 1 = MDD, Bulimia | ||
| Axis II Diagnosis | None | 1 = Schizotypical |
| Personality Disorder | ||
| Cause of death | 5 = cardiovascular | 5 = gun shot wound |
| 4 = motor vehicle accident (pedestrian) | 2 = hanging | |
| 1 = motor vehicle accident (passenger) | 3 = fall from height |
PMI, postmortem interval; C, caucasian; H, hispanic; AA, African American; As, Asian; MDD, Major Depressive Disorder.
2.2. Brainstem
Three sets of DRN sections every mm, previously assayed for 5-HT1A receptors (2 slides; total and non-specific binding) by quantitative receptor autoradiography using 3H-8-OH-DPAT (Fig. 1) and Nissl stain (thionin, 1 slide) were used. Each slide used for receptor autoradiography was also stained for Nissl substance.
Fig. 1.
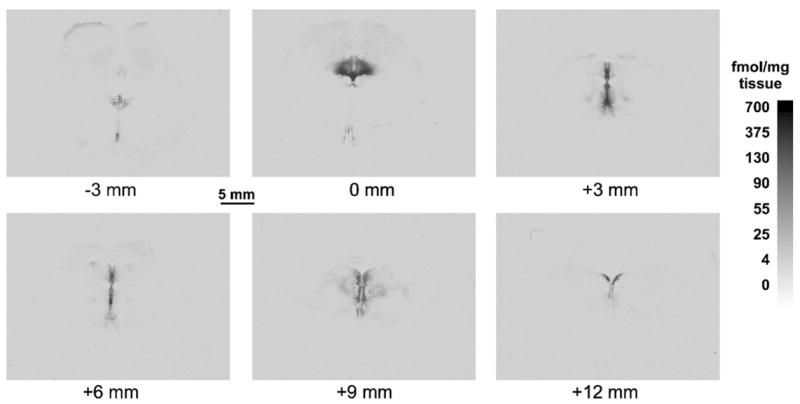
A series of immunoautoradiograms illustrating the rostrocaudal distribution of 5-HT1A receptor binding in a representative brain. The largest part of the dorsal raphe nucleus is indicated as the origin (0 mm) and the other five levels are measured as actual distance (in mm) rostral and caudal to the origin.
Comparable rostrocaudal anatomical levels were identified. For each case, the tissue section with the maximum DRN binding area, measured in a 3H-8-OH-DPAT autoradiogram, was identified (see Fig. 1, Section 2 in upper panel) and used to align the other sections every 1 mm (missing sections were considered as missing and the interval was calculated accordingly). For statistical analysis of the rostrocaudal distribution, the DRN was divided into 6 levels relative to the section with the greatest cross sectional area as described in a previous study (Boldrini et al., 2005): −3 mm (3 mm rostral from peak area), 0 mm (DRN peak area), 3 mm (3 mm caudal from peak area), 6 mm (6 mm caudal from peak area), 9 mm (9 mm caudal from peak area), 12 mm (12 mm caudal from peak area). The cross-sectional binding value for each level was derived from the average of three slides except for the peak level (0 mm) and the second minor peak level (6 mm), which contained two slides. Plotting DRN 5-HT1A binding area on a graph with distance in mm from peak on the abscissa, we obtained a curve (Fig. 3A) which shows the same pattern of rostrocaudal variation of the DRN area obtained in previous studies performing TPH immunostaining (Underwood et al., 1999) and TPH immunoautoradiography (Boldrini et al., 2005). This was taken as a confirmation that the method of section alignment allowed for a representative across subject representation of DRN anatomical location.
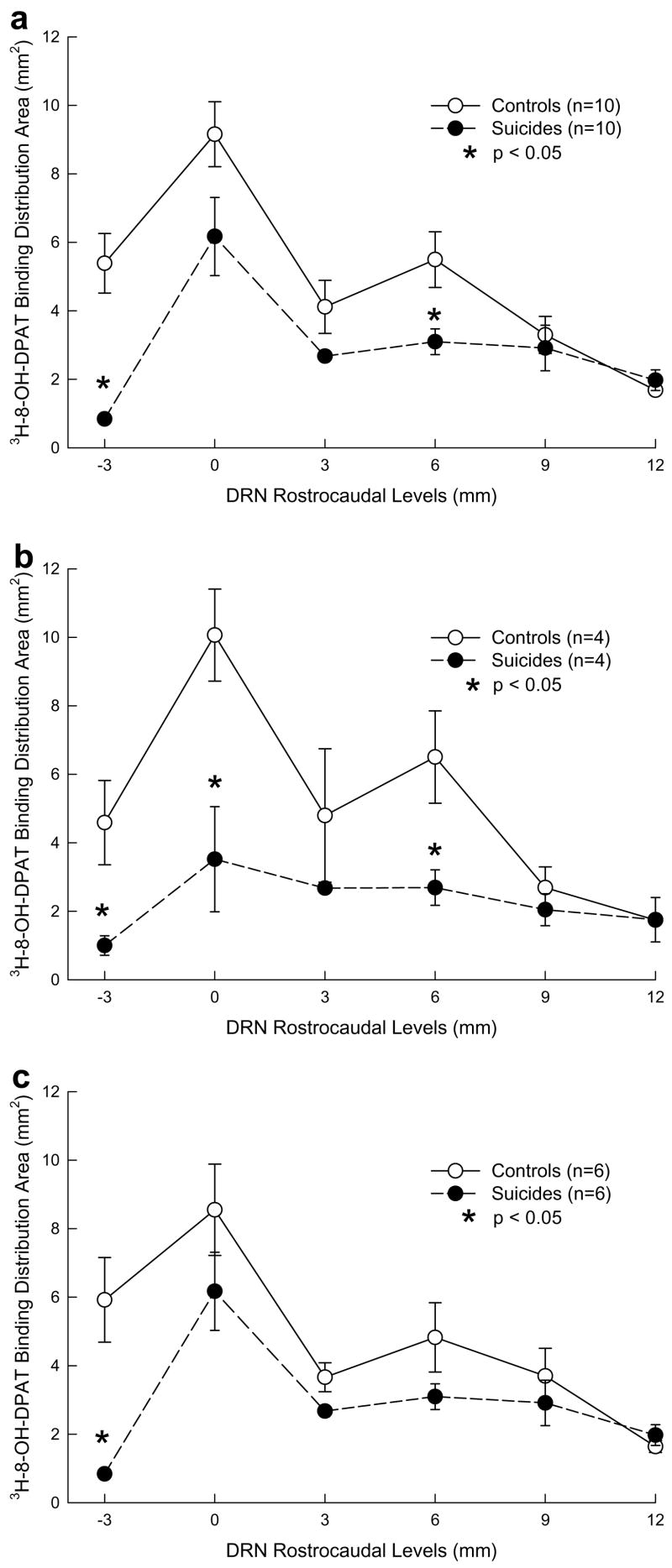
Fig. 3.
5-HT1A receptor ([3H]-8-OH0DPAT) binding distribution area in controls and depressed suicides in total sample (a), in females (b) and in males (c) at each dorsal raphe nucleus rostrocaudal level. Binding distribution area is lower in depressed suicides than in controls. In females, Area is lower in depressed suicides at most rostrocaudal levels compared to controls. In males, Area is lower only at the most rostral level in suicides versus controls. Distribution area is not affected by sex in controls. Female suicides have lower distribution area than male suicides.
2.3. Quantitative receptor autoradiography of [3H]8-OH-DPAT to the 5-HT1A autoreceptor
Autoradiography of 5-HT1A receptors was performed as previously described, including exposure for two weeks and development (Arango et al., 1995, 2001). Tissue sections were fixed in 10% buffered formalin and stained for Nissl substance with thionin.
2.4. Densitometry of 5-HT1A receptor autoradiograms
Receptor autoradiograms were quantified using a computer-based image analysis system (MCID, Imaging Research, Inc.), as previously described (Arango et al., 1995, 2001). Images of [3H]8-OH-DPAT autoradiograms were used to outline the boundaries of the DRN since there is a high degree of correspondence between the outline of the DRN based on sections immunostained for tryptophan hydroxylase (PH8, Törk and Hornung, 1990; Stockmeier et al., 1996) and the outline based on [3H]8-OH-DPAT autoradiograms (Arango et al., 2001).
2.5. Binding capacity
We previously introduced the concept of binding capacity (Arango et al., 2001) as an index of the total binding in a brain area of interest. This was considered to be a more physiologically-relevant measure than the cross-sectional binding in a region of interest (e.g. fmol of radioactive ligand/mg of tissue) because it estimates the total amount of receptors and is also more comparable with data obtained in vivo by brain imaging. The total amount of binding is calculated as: binding capacity = (cross-sectional receptor binding) × (binding area × distance between sections) (fmol/mg tissue × mm3).
2.6. Statistical analysis
Ten matched pairs of controls and suicide victims were used for the group by level analysis of [3H]8-OH-DPAT binding density, binding area and binding capacity. ANOVA analysis for repeated measures was performed using, as the grouping variable, suicide/non-suicide and as a within-subjects factor, the six rostrocaudal levels measured. Univariate tests performed at each level were used to analyze group differences for [3H]8-OH-DPAT binding density, binding area and binding capacity. The same analysis was also performed within male and female groups to analyze control-suicide differences in the two sexes.
Linear correlation analysis was used to analyze the relationship between film autoradiography measures and pH, age and PMI. Data are expressed as mean ± SD and all p values are 2-tailed.
3. Results
3.1. Cross-sectional binding
5-HT1A receptor cross-sectional binding (fmol/mg tissue) varies along the rostrocaudal axis of the DRN (F = 11.08 df = 5,14; p = 0.0002; Table 2; Fig. 2a). Binding is greatest rostrally from 0 to 6 mm and relatively less in caudal portions (Fig. 2a).
Table 2.
5-HT1A binding density, binding distribution area and binding capacity changes in the dorsal raphe nucleus (DRN) depending on group membership (depressed suicides versus controls) and DRN rostrocaudal level
| Measure | Factors | df | F | p |
|---|---|---|---|---|
| 5-HT1A binding (fmol/mg tissue) | Group | 1,18 | 2.20 | .1555 |
| Level | 5,14 | 11.08 | .0002 | |
| Group * Level | 5,14 | 11.61 | .0001 | |
| 5-HT1A binding distribution area (mm2) | Group | 1,18 | 14.76 | .0012 |
| Level | 5,15 | 14.85 | <.0001 | |
| Group * Level | 5,14 | 4.19 | .0154 | |
| 5-HT1A binding capacity (fmol/mg tissue* mm3) | Level | 5,14 | 26.62 | <.0001 |
| Group | 1,18 | 18.06 | .0005 | |
| Group * Level | 5,14 | 5.05 | .0075 |
Group = depressed suicides vs. controls; Level = DRN rostrocaudal level of tissue sections; Group * Level = group by rostrocaudal level interaction.
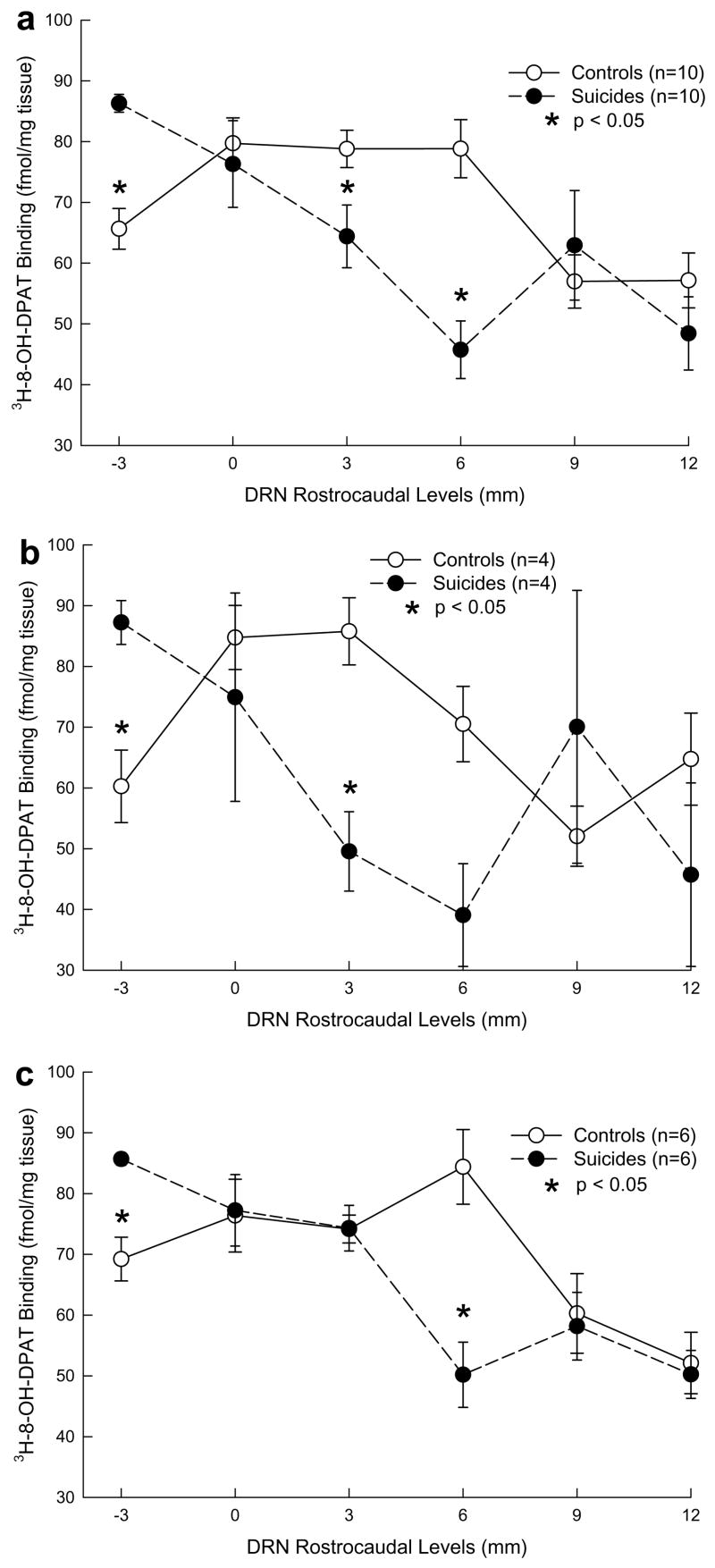
Fig. 2.
5-HT1A receptor ([3H]-8-OH DPAT) binding density in controls and depressed suicides in total sample (a), in females (b) and in males (c) at each dorsal raphe nucleus rostrocaudal level. Binding density is higher in the rostral raphe and lower in the caudal raphe in depressed suicides compared to controls. In controls, 5-HT1A receptor binding is higher in females than in males. In suicides, 5-HT1A binding is lower in females than in males.
The suicide group shows a decline in cross-sectional binding proceeding from rostral to caudal compared with controls (Fig. 2a). The distribution of cross-sectional binding along the rostrocaudal axis of the DRN differs between depressed suicides and controls (Table 2). Univariate tests performed at each level show higher binding in depressed suicides at the most rostral −3 mm level (F = 39.63; df = 1,18; p = 0.0001), and lower binding at the 3 mm level (F = 5.75; df = 1,18; p = 0.0276) and the 6 mm level (F = 24.23; df = 1,18; p = 0.0001; Fig. 2a). At other levels, the differences are not statistically significant.
In females, a difference in cross-sectional binding was also found (F = 28.47; df = 5,2; p = .0343) and post hoc tests revealed higher [3H]8-OH-DPAT binding in female depressed suicides compared to female controls at the most rostral DRN level (−3 mm, F = 19.60; df = 1,6; p = 0.0044), and lower binding at the more caudal 3 mm level (F = 17.94; df = 1,6; p = 0.0055) and 6 mm level (F = 9.00; df = 1,6; p = 0.0240; Fig. 2b). In males (Fig. 2c), cross-sectional binding differed between depressed suicides and controls (F = 8.59; df = 5,6; p = .0105) and upon post hoc testing, the depressed suicides had higher binding at the most rostral DRN level (−3 mm) (F = 23.69; df = 1,10; p = 0.0007) and lower at the more caudal end at the 6 mm level (F = 17.61; df = 1, 10; p = 0.0018; Fig. 2c).
When looking at sex differences within controls and within depressed suicides, we found that, in controls, there was a difference between males and females (F = 22.24; df = 5,4; p = .0051; Fig. 2b and c). Control females have higher binding than control males, and the control male and female patterns differ (F = 22.24; df = 5,4; p = .0050; Fig. 2b and c). In depressed suicides, there was no sex difference in rostrocaudal distribution of cross-sectional binding (F = 0.53; df = 1,8; p = .4892) with binding decreasing caudally in both males and females. There was no sex by level interaction (F = 4.33; df = 5,4; p = .0901).
3.2. Binding area
A rostrocaudal variation in the area occupied by 5-HT1A receptors exists in the DRN (Table 2; Fig. 3a). The binding area is greatest rostrally; there is a smaller peak at the 6 mm level. In depressed suicides there is less binding area from 0 to −3, and the second peak at 6 mm is almost missing compared with controls (Table 2; Fig. 3a). The total binding area is an approximation of DRN volume and is smaller in depressed suicides compared with controls (Table 2). Post hoc tests confirm smaller binding area in depressed suicides compared with controls at the most rostral DRN level (−3 mm) (F = 26.79; df = 1,18; p < .0001) and at the 6 mm level (F = 7.19; df = 1,18; p = 0.0152; Fig. 3a).
Analyzing sex differences in controls we found that [3H]8-OH-DPAT binding area shows a comparable pattern of the area variation in the rostrocaudal axis (F = 4.83; df = 5,4; p = 0.0762), with no male to female differences in the total DRN area (F = 0.14; df = 1,8; p = 0.7174; Fig. 3b and c).
In depressed suicides however we found less [3H]8-OH-DPAT binding area at all rostrocaudal levels in females compared to males (F = 9.33; df = 1,8; p = 0.0157) and the post-hoc test shows that the largest deficit in DRN area in female depressed suicides compared to male depressed suicides is at the level of greatest DRN area, rostrally at level 0 (F = 5.33; df = 1,8; p = 0.0490; Fig. 3b and c).
3.3. Binding capacity
A rostrocaudal variation in 5-HT1A receptor binding capacity in the DRN is observed (Table 2; Fig. 4a) reflecting different areas and densities in the different DRN subregions. Binding capacity is lower in depressed suicides compared with controls (Table 2; Fig. 4a).
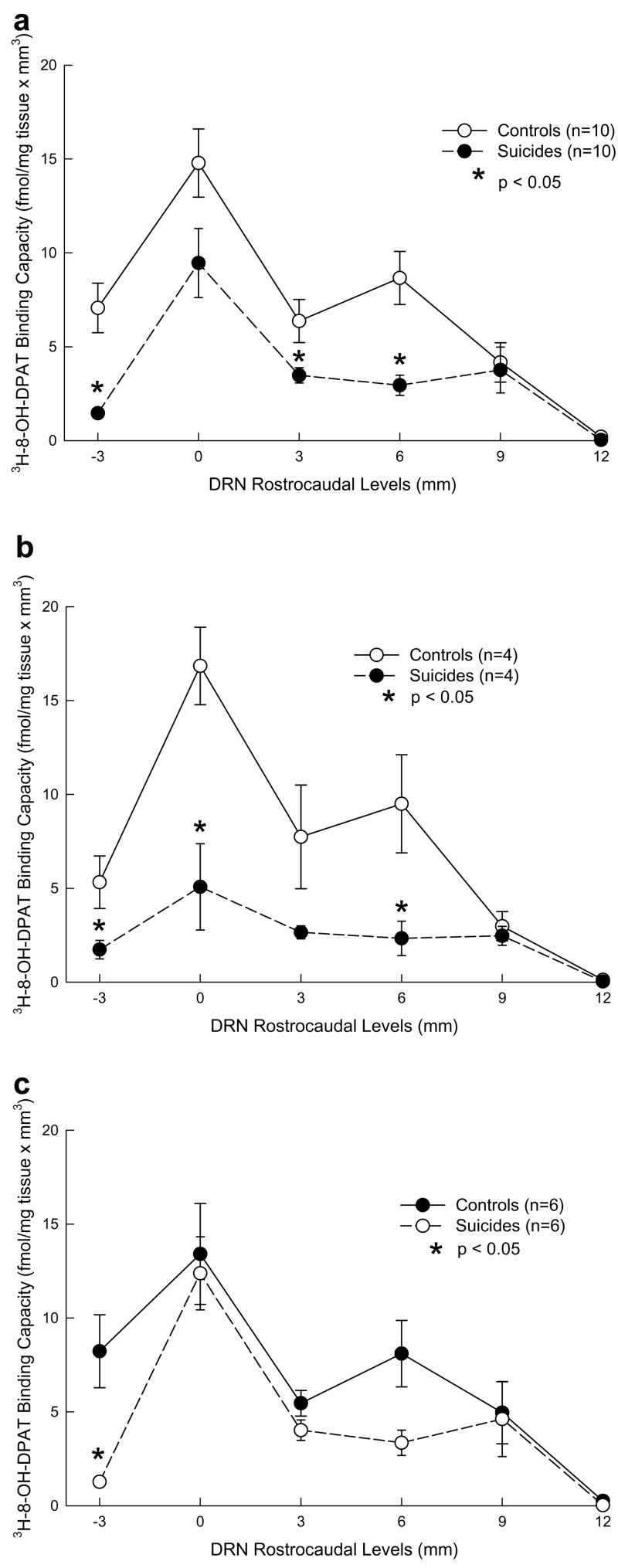
Fig. 4.
5-HT1A receptor [3H]-8-OH DPAT binding capacity in controls and depressed suicides in total sample (a), in females (b) and in males (c) at each dorsal raphe nucleus rostrocaudal level. Binding capacity is lower in depressed suicides than in controls, and this is true at all levels in females, and more selectively in males. In controls, binding capacity is higher in females than in males, but in suicides, binding capacity is lower in females than in males.
Among females, MDD suicides have lower [3H]8-OH-DPAT binding capacity than controls (F = 12.38, df = 1,6; p = .0125) at the rostral DRN region (0 mm level) and at the level of enlargement of the caudal DRN subnucleus (6 mm level; Fig. 4b). In males, lower [3H]8-OH-DPAT binding capacity was found in depressed suicides compared with controls (F = 7.04; df = 1, 10; p = .0242) at the most rostral −3 mm level and at the enlargement in the caudal portion of the DRN within the caudal subnucleus (6 mm level; Fig. 4c).
Binding capacity (F = 0.06, df = 1,8; p = .8167) was comparable in control males and females (Fig. 4b and c). In contrast, in depressed suicides females [3H]8-OH-DPAT binding capacity was lower compared to suicide males (F = 10.88; df = 1, 8; p = .0109), showing a total flattening at the rostral DRN region (Fig. 4b and c).
3.4. Effect of clinical diagnosis, brain pH, postmortem interval (PMI) and age on 5-HT1A receptor binding, binding area and binding capacity
No effects of brain pH (range 6.23–6.77; p > 0.05), PMI (time in hours from death to collection of brain tissue) or age were observed on 5-HT1A receptor binding density, binding capacity or DRN binding area. Since all but one suicide had a diagnosis of MDD and was in a depressive episode around the time of death, we could not determine whether our findings are due to major depression or suicide.
4. Discussion
A difference in the anatomical distribution of 5-HT1Areceptor binding along the rostrocaudal axis of the DRN was observed in depressed suicides and non-suicide, control group, which is dependent on sex. The variation of 5-HT1A receptor density along the rostrocaudal axis of the DRN was previously recognized (Stockmeier et al., 1996). We confirm and extend the observations of Stockmeier et al. (1996), examining the area of distribution of 5-HT1A receptor binding (mm2) and the binding capacity as the product of cross sectional area, distance between the sections of each level and binding density at that level [binding capacity = (cross-sectional receptor binding) × (region volume) = (fmol/mg tissue) × mm3] together with the 5-HT1A autoreceptor binding amount per unit tissue (fmol/mg tissue) along the full rostrocaudal extent of the DRN. This is a new observation since previous studies did not quantify area or volume in considering the total receptor binding, but only measured binding per mg protein (Stockmeier et al., 1998) or per mg tissue (Arango et al., 2001). 5-HT1A receptor binding capacity varies along the rostrocaudal extent of the raphe and in depressed suicides is lower than in controls in both the rostral and the caudal DRN.
We found higher 5-HT1A receptor cross-sectional binding (fmol/mg tissue) in the rostral DRN in depressed suicides compared to controls, consistently with the results obtained by Stockmeier et al. (1998) in the rostral raphe. 5-HT1A receptor binding was lower in suicides compared to controls in the caudal subnucleus, thereby explaining why there was no between groups differences when averaging across the whole raphe values (Arango et al., 2001).
4.1. Effect of rostrocaudal DRN level
The rostralcaudal difference in cross-sectional binding could explain the apparent discrepancies in the literature (Stockmeier et al., 1998 versus Arango et al., 2001). Our previous study included the rostrocaudal extent of the raphe, from the superior colliculus to the middle cerebellar peduncle, but did not divide the DRN into anatomical regions as we have done in the present study. Consistent with Stockmeier et al. (1998) we find higher 5-HT1A binding at the most rostral level of the DRN, which includes the interfascicular, ventral, ventrolateral and dorsal subnuclei (Boldrini et al., 2005) in depressed suicides compared with controls. In contrast, we also find 5-HT1A binding is lower in depressed suicides at more caudal levels, where the caudal subnucleus of the DRN is located within the pontine portion of the raphe (Boldrini et al., 2005). The lower binding in depressed suicides relative to controls at more caudal levels may therefore offset the higher binding in depressed suicides at rostral levels, explaining why no difference was detected between depressed suicides and controls when the rostrocaudal level is not considered (Arango et al., 2001).
The observation of more or fewer 5-HT1A receptors at discrete rostrocaudal levels of the DRN of depressed suicides compared to controls may be related to differences in the topographical pattern of efferent projections of the rostral versus caudal DRN. Most data about anatomical projections of the DRN come from rodent and monkey studies (Wilson and Molliver, 1991a,b). The elaboration of the DRN phylogenetically warrants caution in extrapolating these results to man. Nevertheless, in the rodent, neurons projecting to the caudate-putamen and substantia nigra are located in the rostral portion of the DRN, whereas neurons projecting to the hippocampus and locus coeruleus are more caudal (Imai et al., 1986). Neurons projecting to the medial prefrontal cortex in the rat are clustered in the medial DRN (Van Bockstaele et al., 1993). The anterior thalamic nucleus receives a serotonergic projection from the ventromedial and ventrolateral part of the ipsilateral DRN (Gonzalo-Ruiz et al., 1995), whereas the pyriform cortex is innervated by neurons in the ventromedial DRN (Datiche et al., 1995) The caudal DRN includes projections to the pineal gland in the golden hamster (Leander et al., 1998). In macaque monkeys, a coarse rostrocaudal topographic relationship is reported between dorsal raphe subnuclei and cortical targets. Projections to dorsolateral prefrontal cortex are concentrated in the rostral part of the dorsal raphe nucleus (Wilson and Molliver, 1991b). Thus, overall, it is likely that rostral neurons in the DRN provide most projection to cortical regions. We hypothesize that the rostral elevation of DRN 5-HT1A autoreceptor binding in depressed suicides is functionally related to our finding of more 5-HT1A binding in the ventral and lateral prefrontal cortex (Arango et al., 1995) because an excess of autoreceptors would tend to lower firing rate and reduce serotonin release. Less serotonin release may result in potential upregulation of post-synaptic receptors in target regions such as prefrontal cortex.
The DRN 5-HT1A receptor distribution corresponds closely with tryptophan hydroxylase protein immunoreactivity (Underwood et al., 1999) which also shows the larger cross-sectional area at the rostral end of the DRN complex. TPH2 mRNA (Bach-Mizrachi et al., 2006) and protein immunoreactivity are greater in the DRN of depressed suicides (Boldrini et al., 2005) although not all studies agree (Bonkale et al., 2004) and we (Underwood et al., 1999), but not others (Baumann et al., 2002) find more serotonin neurons in the DRN. The DRN 5-HT1A receptor cross sectional area shows one large rostral peak and a second peak, approximately 6 mm caudal (caudal subnucleus). Depressed suicides in comparison to controls have a sharper drop off of 5-HT1A receptor binding area starting from the most rostral portion of the DRN, and the second area enlargement is almost missing (Fig. 3A). The reason for fewer autoreceptors more caudally appears related to the presence of fewer DRN neurons expressing the 5-HT1A receptor more caudally. What we cannot determine in this study is whether the binding level per neuron varies in the rostral-caudal axis or between depressed suicides and controls. A future study looking at binding levels per cell is needed to address this question.
Lower 5-HT1A receptor expression, indicating less autoreceptor inhibition, despite more TPH biosynthetic enzyme and more TPH2 mRNA (Bach-Mizrachi et al., 2006), could suggest upregulatory effects at a neuron level with DRN neurons having more capacity for serotonin synthesis and less autoinhibition. Such an effect would enhance neuronal firing and may be a potential compensatory response for a lack of serotonin. The decrease in 5-HT1A autoreceptor binding is also a similar effect as that of chronic SSRI administration to rodents (Blier and De Montigny, 1994). It has been hypothesized that this autoreceptor downregulation or desensitization effect is the basis for the therapeutic action of SSRIs (Blier and De Montigny, 1994). Perhaps the decrease in autoreceptor binding we observe in depressed suicides is a homeostatic effect functionally similar to that produced by SSRIs, although not sufficient, to restore serotonin levels.
4.2. Binding capacity
Cross-sectional binding and area vary along the rostrocaudal axis of the DRN. The rostral portion of the DRN contains the dorsal, ventral, ventrolateral and Interfascicular subnuclei at levels −3 mm to 3 mm, while the caudal DRN contains the caudal and interfascicular subnuclei (levels 3–12 mm). We calculated binding capacity as an index of the total amount of binding in the rostral and caudal DRN to account for variations between subnuclei.
In depressed suicides, binding capacity in the DRN is lower than in controls in both the rostral and the caudal DRN, This is consistent with the in vivo PET finding that 5-HT1A receptor binding potential was 42% lower in the raphe nuclei of depressed subjects compared to controls (Drevets et al., 1999). This PET study used the antagonist 11-C-WAY100635, while we used the agonist 8-OH-DPAT in our in vitro studies. If we assume that both the PET and our postmortem studies are correct, depressed suicides have both lower high affinity agonist and low affinity antagonist binding. Since the high affinity conformation of the receptor is coupled to the G protein, agonist binding is a measure of the signal transduction capacity and lower agonist binding is also consistent with lower sensitivity of the autoreceptor. Lower 5-HT1A receptor binding potential was also associated with life-time aggression (Parsey et al., 2002), and since greater aggression is associated with suicidal behavior, that finding is also consistent with our postmortem findings, but suggests the relationship is with suicidal behavior rather than with major depression. That conclusion is also supported by our PET studies finding higher binding in medication-naïve major depression patients (Parsey et al., 2006). This may suggest that suicide or aggression may explain the low postmortem 5-HT1A binding observed in our sample. Future studies on suicides with a different psychiatric diagnosis and depressed subjects not dying by suicide are necessary to test this hypothesis.
4.3. Effect of sex
We found a sex effect on 5-HT1A binding that was independent of the difference between groups. Within controls, females have more 5-HT1A binding than males. Within suicides, 5-HT1A binding is lower in females than in males.
No previous postmortem study has analyzed suicide/control differences on 5-HT1A receptors binding between sexes in the brainstem. In cerebral cortex, we previously reported higher prefrontal [3H]8-OH-DPAT binding in females compared to males (Arango et al., 1995) with the difference being up to 40% in some prefrontal cortical sub regions. A PET study showed that healthy females have higher 5-HT1A [11C]WAY-100635 BP than males in the cortex and DRN (Parsey et al., 2002) and in the cerebellum (Parsey et al., 2005) a finding consistent with our results here. Since estradiol increases 5-HT1A binding (Biegon et al., 1982; Biegon and McEwen, 1982), such an effect may explain the sex differences of higher 5-HT1A receptor binding seen in females in postmortem and PET imaging studies (Parsey et al., 2002, 2005).
It appears that these binding differences have functional consequences. Ipsapirone, a 5-HT1A partial agonist, stimulates GH secretion more in male than in female subjects indicating greater responsivity in females (Newman et al., 1999). Animal studies suggest different 5-HT1A responses in males and females (Gelfin et al., 1995; Ebenezer and Tite, 1994; Haleem et al., 1989, 1990; Joppa et al., 1997; Uphouse et al., 1991; Mendelson and McEwen, 1991; Curzon, 1989). 5-HT transporter knockout mice have lower 5-HT1A receptor binding in the dorsal raphe and this is more extensive in females compared with males (Li et al., 2000) suggesting that females may have a stronger compensatory response to higher intra-synaptic serotonin levels.
Due to the limited number of subjects studied, conclusion regarding the male/female differences should be considered as tentative and the findings need to be replicated in larger samples.
4.4. Limitations
Confounding factors potentially influencing 5-HT1A binding results include age and PMI. However, we matched control and suicide subjects for these variables and did not find significant correlations of either of these parameters with binding measures. Another confounding factor may be the use of drugs or medications that may desensitize 5-HT1A receptors or alter 5-HT1A receptor density and their G-protein coupling (Le Poul et al., 1995; Blier and De Montigny, 1994; Davidson and Stamford, 1998; Stockmeier et al., 1998; Rossi et al., 2006). In the present study, no subjects were taking antidepressants at the time of death, therefore the observed differences are not likely attributable to medication. We found higher binding in the rostral DRN, which is the opposite direction of change that would be predicted from animal studies of the effects of SSRIs on 5-HT1A autoreceptors (Riad et al., 2004; Aznavour et al., 2006). Also, the direction of change was different in the caudal DRN where depressed suicides had less binding, a complex pattern of abnormality making a medication effect an unlikely explanation.
Since 90% of the suicides in our sample also had a history of major depression, our findings may be due to major depression or the diathesis for suicide. Most postmortem brainstem studies of suicide analyzed subjects that, in life, had experienced an episode of major depression (Arango et al., 2001; Underwood et al., 1999; Stockmeier et al., 1998) but the serotonin system abnormalities associated with mood disorders and with suicide may be different as seen for example in the prefrontal cortex. A diffuse decrease in serotonin transporter binding throughout the dorsoventral extent of the prefrontal cortex was found in association with major depression, while a localized reduction in ventral prefrontal cortex transporter binding was associated with suicide (Mann et al., 2000). Future studies must determine whether serotonergic alterations observed at the level of the DRN are specific for suicide or related to mood disorders and whether different parts of the DRN or components of the serotonin system are related to depression or suicide.
A major limitation of the study is the small sample size. Given the complexity of 5-HT1A receptor changes due to anatomical location and sex, future studies should seek to replicate the present findings.
5. Conclusions
The current study demonstrated anatomically distinct changes in DRN 5-HT1A receptor binding of MDD suicides compared with controls. 5-HT1A receptor binding is higher in MDD suicides in the rostral DRN and lower in the caudal DRN compared to controls. This indicates that 5-HT1A receptor expression is heterogeneously affected in MDD and/or suicide, and that the level of autoreceptor expression within functionally diverse DRN subregions may have consequences for projections to specific brain regions. 5-HT1A receptor binding is higher in females than males suggesting greater autoreceptor inhibition in females, reduced serotonergic neurotransmission and the potential for greater risk for effects on mood related to 5-HT function. MDD suicide females have fewer 5-HT1A receptors than MDD suicide males and this may suggest greater homeostatic responsivity in females than males.
Acknowledgments
Role of funding source
Supported by PHS Grants MH40210 and MH62105, The Diane Goldberg Foundation, NARSAD, AFSP and the Paul Janssen Fellowship in Translational Neuroscience. The funding sources had no involvement in the study design or in the collection, analysis or interpretation of the data; they were not involved in the writing of the report or in the decision to submit the paper for publication.
Mihran J. Bakalian assisted with data management and preparation of figures. Suham A. Kassir assisted with receptor autoradiography.
Footnotes
Conflict of interest
All of the authors declare that they have no actual or potential conflicts of interest within three (3) years of beginning the work submitted that could inappropriately influence, or be perceived to influence, their work.
References
- Aghajanian GK, Sprouse JS, Rasmussen K. Physiology of the midbrain serotonin system. In: Meltzer HY, editor. Psychopharmacology. The third generation of progress. New York: Raven Press; 1987. pp. 41–149. [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Research. 1995;688:121–33. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Artigas F, Romero L, De Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends in Neurosciences. 1996;19:378–83. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- Aznavour N, Rbah L, Riad M, Reilhac A, Costes N, Descarries L, et al. A PET imaging study of 5-HT(1A) receptors in cat brain after acute and chronic fluoxetine treatment. Neuroimage. 2006;33:834–42. doi: 10.1016/j.neuroimage.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, et al. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology. 2006;31:814–24. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- Baumann B, Bielau H, Krell D, Agelink MW, Diekmann S, Wurthmann C, et al. Circumscribed numerical deficit of dorsal raphe neurons in mood disorders. Psychological Medicine. 2002;32:93–103. doi: 10.1017/s0033291701004822. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Biegon A, McEwen BS. Modulation by estradiol of serotonin receptors in brain. Journal of Neuroscience. 1982;2:199–205. doi: 10.1523/JNEUROSCI.02-02-00199.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A, Fischette CT, Rainbow TC, McEwen BS. Serotonin receptor modulation by estrogen in discrete brain nuclei. Neuroendocrinology. 1982;35:287–91. doi: 10.1159/000123396. [DOI] [PubMed] [Google Scholar]
- Blier P, De Montigny C. Current advances and trends in the treatment of depression. Trends in Pharmacological Sciences. 1994;15:220–6. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Research. 2005;1041:19–28. doi: 10.1016/j.brainres.2005.01.083. [DOI] [PubMed] [Google Scholar]
- Bonkale WL, Murdock S, Janosky JE, Austin MC. Normal levels of tryptophan hydroxylase immunoreactivity in the dorsal raphe of depressed suicide victims. Journal of Neurochemistry. 2004;88:958–64. doi: 10.1046/j.1471-4159.2003.02225.x. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. Journal of Neuroscience. 2001;21:9917–29. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon G. 5-Hydroxytryptamine and corticosterone in an animal model of depression. Progress in Neuro-psychopharmacology & Biological Psychiatry. 1989;13:305–10. doi: 10.1016/0278-5846(89)90119-x. [DOI] [PubMed] [Google Scholar]
- Datiche F, Luppi PH, Cattarelli M. Serotonergic and non-serotonergic projections from the raphe nuclei to the piriform cortex in the rat: a cholera toxin B subunit (CTb) and 5-HT immunohistochemical study. Brain Research. 1995;671:27–37. doi: 10.1016/0006-8993(94)01293-q. [DOI] [PubMed] [Google Scholar]
- Davidson C, Stamford JA. Contrasting effects of chronic paroxetine on 5-HT1A control of dorsal raphe cell firing and 5-HT release. Neuroreport. 1998;9:2535–8. doi: 10.1097/00001756-199808030-00020. [DOI] [PubMed] [Google Scholar]
- De Montigny C, Blier P, Chaput Y. Electrophysiologically-identified serotonin receptors in the rat CNS. Effect of antidepressant treatment. Neuropharmacology. 1984;23:1511–20. doi: 10.1016/0028-3908(84)90095-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biological Psychiatry. 1999;46:1375–87. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Ebenezer IS, Tite R. Sex difference in the feeding responses of non-deprived rats to the 5-HT1A agonists 8-OH-DPAT and gepirone. Methods and Findings in Experimental and Clinical Pharmacology. 1994;16:91–6. [PubMed] [Google Scholar]
- Gelfin Y, Lerer B, Lesch KP, Gorfine M, Allolio B. Complex effects of age and gender on hypothermic, adrenocorticotrophic hormone and cortisol responses to ipsapirone challenge in normal subjects. Psychopharmacology (Berlin) 1995;120:356–64. doi: 10.1007/BF02311184. [DOI] [PubMed] [Google Scholar]
- Gonzalo-Ruiz A, Lieberman AR, Sanz-Anquela JM. Organization of serotoninergic projections from the raphe nuclei to the anterior thalamic nuclei in the rat: a combined retrograde tracing and 5-HT immunohistochemical study. Journal of Chemical Neuroanatomy. 1995;8:103–15. doi: 10.1016/0891-0618(94)00039-v. [DOI] [PubMed] [Google Scholar]
- Haleem DJ, Kennett GA, Whitton PS, Curzon G. 8-OH-DPAT increases corticosterone but not other 5-HT1A receptor-dependent responses more in females. European Journal of Pharmacology. 1989;164:435–43. doi: 10.1016/0014-2999(89)90251-3. [DOI] [PubMed] [Google Scholar]
- Haleem DJ, Kennett GA, Curzon G. Hippocampal 5-hydroxytryptamine synthesis is greater in female rats than in males and more decreased by the 5-HT1A agonist 8-OH-DPAT. Journal of Neural Transmission General Section. 1990;79:93–101. doi: 10.1007/BF01251004. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Regulation of 5-HT1A receptor function in brain following agonist or antidepressant administration. Life Sciences. 2003;72:1665–82. doi: 10.1016/s0024-3205(02)02482-7. [DOI] [PubMed] [Google Scholar]
- Imai H, Park MR, Steindler DA, Kitai ST. The morphology and divergent axonal organization of midbrain raphe projection neurons in the rat. Brain and Development. 1986;8:343–54. doi: 10.1016/s0387-7604(86)80054-7. [DOI] [PubMed] [Google Scholar]
- Joppa MA, Rowe RK, Meisel RL. Effects of serotonin 1A or 1B receptor agonists on social aggression in male and female Syrian hamsters. Pharmacology, Biochemistry and Behavior. 1997;58:349–53. doi: 10.1016/s0091-3057(97)00277-3. [DOI] [PubMed] [Google Scholar]
- Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: a comparison with clinician ante-mortem diagnosis. Acta Psychiatrica Scandinavica. 1996;94:337–43. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- Leander P, Vrang N, Moller M. Neuronal projections from the mesencephalic raphe nuclear complex to the suprachiasmatic nucleus and the deep pineal gland of the golden hamster (Mesocricetus auratus) Journal of Computational Neurology. 1998;399:73–93. [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L. Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedeberg’s Archives of Pharmacology. 1995;352:141–8. doi: 10.1007/BF00176767. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch K-P, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. Journal of Neuroscience. 2000;20:7888–95. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ. Neurobiology of suicidal behaviour. Nature Reviews Neuroscience. 2003;4:819–28. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, et al. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Archives of General Psychiatry. 2000;57:729–38. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- Mendelson SD, McEwen BS. Autoradiographic analyses of the effects of restraint-induced stress on 5-HT1A, 5-HT1C and 5-HT2 receptors in the dorsal hippocampus of male and female rats. Neuroendocrinology. 1991;54:454–61. doi: 10.1159/000125951. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Fozard JR. 8-Hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. European Journal of Pharmacology. 1983;90:151–3. doi: 10.1016/0014-2999(83)90230-3. [DOI] [PubMed] [Google Scholar]
- Newman ME, Li Q, Gelfin Y, Van de Kar LD, Lerer B. Low doses of ipsapirone increase growth hormone but not oxytocin secretion in normal male and female subjects. Psychopharmacology (Berlin) 1999;145:99–104. doi: 10.1007/s002130051037. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5- HT(1A) receptor binding potential measured by PET using [C-11]WAY-100635. Brain Research. 2002;954:173–82. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, Mann JJ. Regional heterogeneity of 5-HT(1A) receptors in human cerebellum as assessed by positron emission tomography. Journal of Cerebral Blood Flow and Metabolism. 2005;25:785–93. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. Higher 5-HT(1A) receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006;31:1745–9. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- Riad M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe dorsalis of rat. Journal of Neuroscience. 2004;24:5420–6. doi: 10.1523/JNEUROSCI.0950-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DV, Valdez M, Gould GG, Hensler JG. Chronic administration of venlafaxine fails to attenuate 5-HT1A receptor function at the level of receptor-G protein interaction. International Journal of Neuropsychopharmacology. 2006;9:393–406. doi: 10.1017/S1461145705005754. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Archives of General Psychiatry. 2000;57:174–80. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, et al. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Archives of General Psychiatry. 2006;63:396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Haycock JW, Thompson PA, Lowy MT. Quantitative subregional distribution of serotonin1A receptors and serotonin transporters in the human dorsal raphe. Brain Research. 1996;727:1–12. doi: 10.1016/0006-8993(96)00239-9. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. Journal of Neuroscience. 1998;18:7394–401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törk I, Hornung J-P. Raphe nuclei and the serotonergic system. In: Paxinos G, editor. The human nervous system. San Diego: Academic Press; 1990. pp. 1001–22. [Google Scholar]
- Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, et al. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biological Psychiatry. 1999;46:473–83. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Uphouse L, Salamanca S, Caldarola-Pastuszka M. Gender and estrous cycle differences in the response to the 5-HT1A agonist 8-OH-DPAT. Pharmacology, Biochemistry and Behavior. 1991;40:901–6. doi: 10.1016/0091-3057(91)90104-a. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Research. 1993;624:188–98. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- Verge D, Daval G, Patey A, Gozlan H, El Mestikawy S, Hamon M. Presynaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites but not terminals are of the 5-HT1A subtype. European Journal of Pharmacology. 1985;113:463–4. doi: 10.1016/0014-2999(85)90099-8. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK. Antidromically identified serotonergic neurons in the rat midbrain raphe: Evidence for collateral inhibition. Brain Research. 1977;132:186–93. doi: 10.1016/0006-8993(77)90719-3. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Molliver ME. The organization of serotonergic projections to cerebral cortex in primates: regional distribution of axon terminals. Neuroscience. 1991a;44:537–53. doi: 10.1016/0306-4522(91)90076-z. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Molliver ME. The organization of serotonergic projections to cerebral cortex in primates: retrograde transport studies. Neuroscience. 1991b;44:555–70. doi: 10.1016/0306-4522(91)90077-2. [DOI] [PubMed] [Google Scholar]