Abstract
Ethanol-induced oxidative stress appears to play a major role in mechanisms by which ethanol causes liver injury. Many pathways have been suggested to contribute to the ability of ethanol to induce a state of oxidative stress. One central pathway appears to be the induction of cytochrome P450 2E1 (CYP2E1) by ethanol. CYP2E1 metabolizes and activates many toxicological substrates, including ethanol, to more reactive, toxic products. Levels of CYP2E1 are elevated under a variety of physiological and pathophysiological conditions, and after acute and chronic alcohol treatment. CYP2E1 is also an effective generator of reactive oxygen species such as the superoxide anion radical and hydrogen peroxide, and in the presence of iron catalysts, produces powerful oxidants such as the hydroxyl radical. This Review Article summarizes some of the biochemical and toxicological properties of CYP2E1, and briefly describes the use of cell lines developed to constitutively express CYP2E1 in assessing the actions of CYP2E1. Possible therapeutic implications for treatment of alcoholic liver injury by inhibition of CYP2E1 or CYP2E1-dependent oxidative stress will be discussed, followed by some future directions which may help to understand the actions of CYP2E1 and its role in alcoholic liver injury.
Introduction-cytochrome P450, oxidative stress, and alcoholic liver injury
The cytochrome P450 enzymes are a superfamily of hemeproteins that serve as terminal oxidases in the mixed function oxidase system for metabolizing various endogenous substrates such as steroids and fatty acids, and xenobiotics including drugs, toxins and carcinogens (1). Many different enzymes belong to this P450 family; P450s are present in virtually all living organisms. A systematic nomenclature system was developed for the P450 family which is based on the sequence identity of the different P450 enzymes (2, 3). The enzymes are named CYP for cytochrome P450, followed by an Arabic number denoting the family (more than 40% identity on the amino acid sequence level), a letter designating the subfamily (more than 55% identity) and finally an Arabic numeral representing the individual gene in the subfamily. The P450s catalyze many different chemical reactions including monooxygenation (insertion of an atom of oxygen into the substrate), peroxidation, reduction, dealkylation, epoxidation, and dehalogenation (4–6). Many different compounds of diverse structure can be metabolized by P450 enzymes. A major function of P450-catalyzed reactions is to convert a compound into a more polar metabolite that can be easily excreted directly by the organism or conjugated by phase II enzymes into more polar excretable metabolites. With some compounds e.g. carbon tetrachloride or acetaminophen, metabolism by P450 can give rise to toxic metabolites which damage cells. For P450s to function catalytically, flavoprotein reductases such as NAPDH-cytochrome P450 reductase, adrenodoxin, adrenodoxin reductase, are necessary to transfer electrons from NAPDH or NADH to reduce the heme from the ferric redox state to the ferrous state. The latter is necessary to bind molecular oxygen to form the oxygenated P450 complex that catalyzes the diverse chemical reactions mentioned above (7). Cytochrome b5 may also play an important role in electron transfer to certain P450s.
It is important to recognize that oxygen activation by P450, necessary for the enzymes catalytic function, can also result in the production of reactive oxygen species (ROS). Small amounts of the superoxide anion radical (O2·̄ ) can be produced from decay of the oxygenated P450 complex, while hydrogen peroxide (H2O2) can form from either dismutation of O2·̄ or from decay of the peroxy P450 complex (8–10). ROS have been implicated in many of the major diseases that plague mankind, including the toxicity of O2 itself; hyperbaric O2; ischemia-reperfusion injury; cardiovascular diseases; atherosclerosis; carcinogenesis; diabetes; neurodegenerative diseases, including Parkinson’s disease and Alzheimer’s disease; toxicity of heavy metals, e.g., iron; asbestos injury; radiation injury; vitamin deficiency; drug (e.g., redox cycling agents) toxicity; aging; inflammation; smoking toxicity; emphysema; and toxicity of acute and chronic ethanol treatment (11–15). ROS can be produced from many systems in cells including the mitochondrial respiratory chain (16); the cytochrome P450s (10,17); oxidative enzymes such as xanthine oxidase, aldehyde oxidase, cyclooxygenase, monoamine oxidase, the NADPH oxidase complex (18, 19); autooxidation of heme proteins such as ferrohemoglobin or myoglobin or biochemicals such as catecholamines, quinones or tetrahydrobiopterins. In addition to these cellular sources of ROS, environmental sources of ROS include radiation, UV light, smoke and certain drugs which can redox cycle. ROS are toxic to cells because they can react with most cellular macromolecules inactivating enzymes or denaturing proteins, causing DNA damage such as strand breaks, base removal or base modifications which can result in mutation, peroxidation of lipids which can result in destruction of biological membranes and produce reactive aldehydic products such as malondialdehyde or 4-hydroxynonenal (20, 21). A variety of enzymatic and non-enzymatic mechanisms have evolved to protect cells against ROS, including the superoxide dismutases, which remove O2·̄; catalase and the glutathione (GSH) peroxidase system which remove H2O2; glutathione transferases which can remove reactive intermediates and lipid aldehydes; metallothioneins, heme oxygenase, thioredoxin which remove various ROS; ceruloplasmin and ferritin which help remove metals such as iron which promote oxidative stress reactions; non-enzymatic, low molecular weight antioxidants such as GSH itself, vitamin E, ascorbate (vitamin C), vitamin A, ubiquinone, uric acid, bilirubin (22,23). Oxidative stress or toxicity by ROS reflects a balance between the rates of production of ROS compared to the rates of removal of ROS plus repair of damaged cellular macromolecules. While excess ROS can cause toxicity, macrophages and neutrophils contain an NADPH oxidase which produces ROS to destroy foreign organisms (24), and the enzyme myeloperoxidase catalyzes a reaction between H2O2 and chloride to produce the powerful oxidant hypochlorite (bleach) to help destroy foreign invaders. In addition, ROS at low concentrations, especially H2O2, may be important in signal transduction mechanisms in cells, and thus be involved in cellular physiology and metabolism (25).
The ability of acute and chronic ethanol treatment to increase production of reactive oxygen species and enhance peroxidation of lipids, protein, and DNA has been demonstrated in a variety of systems, cells, and species, including humans. Much has been learned about alcohol metabolism, the various enzymes and pathways involved, and how alcohol, directly via its metabolism, or indirectly via its solvent-like action affecting cellular membranes impacts on cell function. Yet, despite this tremendous growth in understanding alcohol metabolism and actions, the mechanism(s) by which alcohol causes cell injury are still not clear. A variety of leading mechanisms have been briefly summarized (13–15), and it is likely that many of them ultimately converge as they reflect a spectrum of the organism’s response to the myriad of direct and indirect actions of alcohol. A major mechanism that is a focus of considerable research is the role of lipid peroxidation and oxidative stress in alcohol toxicity. Many pathways have been suggested to play a key role in how ethanol induces “oxidative stress” (reviewed in 13–15). Again, many of these pathways are not exclusive of one another and it is likely that several, indeed many, systems contribute to the ability of alcohol to induce a state of oxidative stress.
What is the evidence that ethanol-induced oxidative stress plays a role in cell injury? There are many studies which show that administration of antioxidants or iron chelators or GSH-replenishing agents can prevent or ameliorate the toxic action of ethanol. The most convincing data that oxidative stress contributes to alcohol-induced liver injury comes from the studies using the intragastric infusion model of alcohol administration. In these studies, alcohol-induced liver injury was associated with enhanced lipid peroxidation, protein carbonyl formation, formation of the 1-hydroxyethyl radical, formation of lipid radicals, decreases in hepatic antioxidant defense, especially GSH (26–30). Replacement of polyunsaturated fat (required for lipid peroxidation to occur) with saturated fat or medium chain triglycerides in the diets fed to rats intragastrically, lowered or prevented the lipid peroxidation, and the alcohol-induced liver injury (29, 30). Thus, alcohol plus polyunsaturated fat was required for the injury to occur. Addition of iron, known to generate OH and promote oxidative stress, to these diets exacerbated the liver injury (31). Importantly, addition of antioxidants such as vitamin E, ebselen, superoxide dismutase (SOD), GSH precursors, prevented the alcohol-induced liver injury (28). Because alcohol-induced liver injury has been linked to oxidative stress, we investigated the effect of a compromised antioxidant defense system, copper-zinc superoxide dismutase (SOD1) deficiency on alcohol-induced liver injury (32, 33). A rather moderate ethanol consumption promoted oxidative stress and liver injury in SOD1 knockout mice indicating that compromised antioxidant defense promotes alcohol liver injury.
In addition to these in vivo studies, in vitro studies with hepatocytes also showed that ethanol can produce oxidative stress and hepatocyte toxicity. Studies with isolated hepatocytes from control rats or chronic ethanol-fed rats indicated that ethanol metabolism via alcohol dehydrogenase results in an increase in ROS production, hepatocyte injury, and apoptosis, reactions blocked by antioxidants (34, 35). Studies in our laboratory with HepG2 cell lines expressing CYP2E1 showed that addition of ethanol or polyunsaturated fatty acids or iron, or depletion of GSH, resulted in cell toxicity, increased oxidative stress and mitochondrial damage, reactions prevented by antioxidants (36) Recent reviews on the roles of oxidative stress in alcoholic liver disease can be found in (37, 38). Since CYP2E1 plays a role in ethanol-induced oxidant stress and is a minor pathway of ethanol oxidation, the biochemical and toxicological properties of CYP2E1 will form the basis for much of the remainder of this review.
CYP2E1 and the microsomal ethanol oxidizing system
Alcohol dehydrogenase is the major enzyme pathway for oxidizing ethanol to acetaldehyde. The morphological observations that chronic-ethanol treatment causes proliferation of the liver smooth endoplasmic reticulum suggested that ethanol, similar to certain xenobiotics which are metabolized by cytochrome P450, may also be metabolized by P450 (39). A microsomal ethanol oxidizing system (MEOS) was characterized by Lieber and associates and shown to be dependent on P450 (40). The Km for ethanol oxidation by MEOS (about 10 mM) was about an order of magnitude greater than the Km for ethanol by alcohol dehydrogenase. Acetaldehyde is the product resulting from ethanol oxidation by MEOS, and it is clear that MEOS represents a minor pathway of ethanol oxidation, probably accounting for less than 10 percent of the liver capacity to oxidize ethanol (41). Importantly, activity of MEOS is enhanced after chronic ethanol treatment, partly due to an increased total content of P450, and partly due to induction of CYP2E1, a member of the P450 family with high catalytic activity with ethanol (40). Induction of MEOS may play an important role in the metabolic tolerance found after chronic ethanol treatment, i.e., the increased capacity to oxidize ethanol. While there was early controversy over the nature of MEOS, the purification of an ethanol-inducible form of P450 from rabbit liver microsomes firmly established the role of P450 in MEOS (42). Ethanol-inducible P450s have been isolated from many species and while several P450s may be induced by ethanol, the major inducible P450 is now referred to as CYP2E1.
CYP2E1 substrates
CYP2E1 metabolizes a variety of small, hydrophobic substrates and drugs (reviewed in 40, 43–46). Possible physiological substrates are acetone and fatty acids such as linoleic and arachidonic acid (47). From a toxicological point of view, interest in CYP2E1 revolves around the ability of this enzyme to metabolize and activate many toxicologically important compounds such as ethanol, carbon tetrachloride, acetaminophen, benzene, halothane and many other halogenated substrates. Procarcinogens including nitrosamines and azo compounds are effective substrates for CYP2E1 e.g., CYP2E1 is a low Km dimethylnitrosamine demethylase (48). Toxicity by the above compounds is enhanced after induction of CYP2E1 e.g. by ethanol treatment, and toxicity is reduced by inhibitors of CYP2E1 or in CYP2E1 knockout mice (49). Of the substrates, chlorzoxazone is of special value as its hydroxylated product can readily be assayed in the blood and the ratio of 6-hydroxychlorzoxazone/chlorzoxazone is widely used to assess the approximate levels of CYP2E1 in humans, including alcoholics (50).
Molecular oxygen itself is likely to be a most important substrate for CYP2E1. CYP2E1, relative to several other P450 enzymes, displays high NADPH oxidase activity as it appears to be poorly coupled with NADPH-cytochrome P450 reductase (51, 52). CYP2E1 was the most efficient P450 enzyme in the initiation of NADPH-dependent lipid peroxidation in reconstituted membranes among five different P450 forms investigated. Furthermore, anti-CYP2E1 IgG inhibited microsomal NADPH oxidase activity and microsomal lipid peroxidation dependent on P450, but not lipid peroxidation initiated by the action of NADPH-cytochrome P450 reductase (52). In our laboratory, we found that microsomes isolated from rats fed ethanol chronically were about twofold to threefold more reactive in generating superoxide radical and H2O2 and in the presence of ferric complexes, in generating hydroxyl radical and undergoing lipid peroxidation compared to microsomes from pair-fed controls (53–56). CYP2E1 levels were elevated about threefold to fivefold in the liver microsomes after feeding rats the Lieber-DeCarli diet for four weeks. The enhanced effectiveness of microsomes isolated from the ethanol-fed rats was prevented by addition of chemical inhibitors of CYP2E1 and by polyclonal antibody raised against CYP2E1, confirming that the increased activity in these microsomes was due to CYP2E1.
CYP2E1 is a minor pathway of ethanol oxidation as it catalyzes the two electron oxidation of ethanol to acetaldehyde. Interestingly, acetaldehyde is also a substrate for CYP2E1 and is oxidized to acetate, thus CYP2E1 can, at least theoretically, catalyze the oxidation of ethanol to acetate (57). However, this oxidation is likely to be negligible in the presence of ethanol, the substrate which generates acetaldehyde (58). CYP2E1 can also promote the one electron oxidation of ethanol to the 1-hydroxyethyl radical. Detection of the 1-hydroxyethyl radical in the bile after administration of ethanol to rodents has been a most valuable assay for determining ethanol-induced radical formation and oxidant stress in vivo (26, 59).
Mitochondrial CYP2E1
CYP2E1 is mainly found in the liver but significant amounts are also found in most organs, including the brain (60). CYP2E1 is expressed mainly in the hepatocytes of the liver, however, significant amounts are also found in the Kupffer cells (61), and hepatocyte and Kupffer cell CYP2E1 are inducible e.g. by ethanol. CYP2E1, like other xenobiotic metabolizing P450s, is mainly located in the membrane of the endoplasmic reticulum (ER). CYP2E1 has also been detected in other cellular compartments such as the plasma membrane (62–64). CYP2E1 located at the plasma membrane has been suggested to play a role in the immune mediated hepatotoxicity observed in patients suffering from drug toxicity and ALD (65–67). CYP2E1 was shown to be transported out of the ER to the Golgi apparatus, with subsequent transfer to the plasma membrane (68,69).
Ingelman-Sundberg and co-workers, and Avadhani and co-workers have shown that CYP2E1 is also present in the mitochondria (70–75). Essentially 2 forms of CYP2E1 are present in the mitochondria, a highly phosphorylated form mediated via cAMP-dependent protein kinase A, and a shortened 40 kDa amino terminal-truncated form which lacks the N-terminal amino acids, and which can be further NH2-terminally truncated to produce a mature mitochondrial form of CYP2E1 lacking about 100 amino acids. The phosphorylation and amino terminal truncation are hypothesized to cause conformational changes and altered interactions with molecular chaperones and signal recognition particles and direct the CYP2E1 to the mitochondria. The mitochondrial CYP2E1 is catalytically active with typical substrates but requires, as do the other mitochondrial P450s, adrenodoxin and adrenodoxin reductase (and NADPH) as electron donors (70, 73). It is not clear what regulates either the phosphorylation or the amino-terminal truncation which directs CYP2E1 to the mitochondria. Importantly, the mitochondria isolated from rat liver and highly purified and essentially devoid of endoplasmic reticulum contamination, contained CYP2E1 indicating the in vivo presence of mitochondrial CYP2E1 (70, 71, 73). Robin et al (73) showed pyrazole treatment not only elevated microsomal CYP2E1, but also mitochondrial CYP2E1. The mitochondrial CYP2E1 was present at about 30% of the level of the microsomal CYP2E1 under basal conditions, and at 40% of the level of the microsomal CYP2E1 after pyrazole treatment (73). In a similar manner, streptozotocin-induced diabetes elevated microsomal CYP2E1 2- to 3-fold, and mitochondrial CYP2E1 5- to 6-fold (75); mitochondrial CYP2E1 protein and catalytic activity was 25 to 35% that of microsomal CYP2E1 after treatment with streptozotocin. Raza & John (76) recently reported that 4-hydroxynonenal increased CYP2E1 activity in the mitochondria and postmitochondrial supernatant of PC12 cells, in association with elevated mitochondrial oxidative stress.
To evaluate functional consequences associated with expression of mitochondrial CYP2E1, we established a HepG2 cell line which expresses CYP2E1 in the mitochondria (77). A CYP2E1 expression vector lacking the coding sequences for amino acids 2–34 was generated, cloned into a pCI-neo expression vector, transfected into HepG2 cells, and stable cell lines established by selection for G418 resistance. Western blot analysis of whole cell extracts and of isolated mitochondria, and immunofluoresence of permeabilized cells showed the presence of CYP2E1 in the mitochondrial fraction of mE10 and mE27 cells (HepG2 cells transfected with the amino terminal depleted CYP2E1) but not in pCI vector transfected HepG2 cells or E47 HepG2 cells which express CYP2E1 in the endoplasmic reticulum. Treatment with 0.1 mM BSO for 48 h to lower GSH levels caused a striking loss of cell viability in mE10 and mE27 cells which contain mtCYP2E1 but not in the pCI-neo cells. Toxicity could be prevented by antioxidants such as glutathione ethyl ester and trolox, suggesting enhanced oxidant stress plays a role in the toxicity. Indeed, ROS production (DCF fluorescence) was elevated after BSO addition to the mE10 and mE27 cells. There was an increase in 3-nitrotyrosine protein adducts and 4-hydroxynonenal protein adducts in the mE10 and mE27 cells treated with BSO compared to plasmid vector controls. Mitochondrial membrane potential slightly declined in BSO-treated pCI-neo cells but dramatically declined in the mE10 and mE27 cells. This decline in MMP was prevented by cyclosporine A, and the BSO-induced loss of cell viability in the mE10 and mE27 cells was prevented by cyclosporine A. Importantly, ethanol was shown to elevate the levels of mitochondrial CYP2E1 in addition to the well known increase in microsomal CYP2E1 (78). It is interesting to speculate that damage to mitochondrial function and membrane potential produced by mitochondrial CYP2E1 may be an early event in liver cell injury and that mitochondrial CYP2E1 may contribute to the biochemical and toxicological effects which were previously ascribed to CYP2E1 in the endoplasmic reticulum. However, at present it is not obvious how effects contributed by mitochondrial CYP2E1 versus microsomal CYP2E1 in vivo or in primary hepatocytes can be distinguished from each other e.g. lack of specific inhibitors.
Induction and regulation of CYP2E1
Many of the substrates for CYP2E1 can induce their own metabolism. This was initially observed with ethanol, which is a substrate for CYP2E1 and elevates CYP2E1 levels (39, 40). In fact, these two properties explain the ability of ethanol to inhibit the metabolism of certain substrates when the alcohol is present, i.e., ethanol and the substrate compete for oxidation by CYP2E1, and for ethanol to increase the metabolism of substrates when it is no longer present to compete, but the ethanol treatment elevated the levels of the CYP2E1 catalyst. Ethanol can be oxidized by other P450s besides CYP2E1, notably CYPs 3A and 1A, and ethanol treatment can elevate the levels of these CYPs (79, 80). A variety of heterocyclic compounds such as imidazole, pyrazole, 4-methylpyrazole, thiazole, isoniazid have been shown to elevate CYP2E1 levels as do solvents such as dimethylsulfoxide, various alcohols, benzene and acetone (81–83). These low molecular weight compounds have been used in vivo or in vitro to elevated or help prevent loss of CYP2E1 under tissue culture conditions and their mode of mechanism will be discussed below.
CYP2E1 can also be induced under a variety of metabolic or nutritional conditions. For example, CYP2E1 levels were elevated in chronically obese, overfed rats and in rats fed a high-fat diet (84). Somewhat paradoxical, in rats levels of CYP2E1 were increased by fasting and by prolonged starvation (85, 86). Diabetes has been reported to increase the expression of CYP2E1 mRNA and protein levels several fold (87). This may be related to actions of insulin which downregulated CYP2E1 expression at the posttranscriptional level in a rat hepatoma cell line (88, 89) and in rat hepatocyte culture (90). CYP2E1 levels were elevated in liver and kidney microsomes of rats treated with streptozotocin.
CYP2E1 induction in diabetes may be associated with the elevated production of ketone bodies (91). The carbohydrate content of the diet influences CYP2E1 levels as a low carbohydrate diet increased the extent of induction of MEOS by ethanol (92) and high fat/low carbohydrate diets resulted in the highest levels of CYP2E1 induced by ethanol (93). In this respect, it is interesting that alcohol-induced liver is magnified in diets with very low levels of carbohydrate and high levels of fat (94).
Besides insulin, other hormones can affect CYP2E1 levels. Hypophysectomy and triiodothyronine increase CYP2E1 protein and mRNA levels in contrast to insulin which lowers them (89, 95). In primary rat hepatocyte cultures, glucagon lowered CYP2E1 levels by accelerating turnover of the CYP2E1 protein by a cyclic AMP-dependent process (96). Testosterone increased renal but not hepatic CYP2E1 levels (97).
Considerable data have been reported elucidating the molecular mechanism of CYP2E1 regulation by exogenous compounds as well as during pathophysiologiccal conditions. CYP2E1 is regulated by multiple, distinct regulatory mechanisms (83, 98, 99). The CYP2E1 gene is under transcriptional control during development. In rats, immediately after birth, it is activated and is maximally transcribed within the first week. Upon fasting or induced diabetes, the mRNA for CYP2E1 is increased several fold due to posttranscriptional mRNA stabilization (100). After administration of ethanol, acetone or pyrazole to rats, Song et al found that CYP2E1 mRNA levels did not increase (81). The mechanism of induction was, therefore, suggested to be at the level of protein degradation. CYP2E1 is not transcriptionally activated by an acute bolus dose or chronic administration of ethanol, acetone, or other exogenous inducing agents. Although elevation of CYP2E1 mRNA levels has been reported (101), most investigators have found little induction or slight reduction of CYP2E1 mRNA level after ethanol administration (81, 83, 102). From in vivo data of CYP2E1 turnover in rats chronically treated with acetone (103) and in vitro hepatocyte culture systems (104,105), exogenous CYP2E1 inducers such as acetone, ethanol, imidazole and 4-methylpyrazole (4-MP) were shown to increase CYP2E1 by protein stabilization. Roberts et al (106,107) reported that ethanol increases CYP2E1 by protein stabilization. This phenomenon was observed not only in the liver but also other extra-hepatic tissues such as kidney, brain and intestine. In addition, CYP2E1 protein stabilization appeared dependent on blood ethanol or acetone concentration. Furthermore, a turnover study, using in vivo radiolabeling of CYP2E1 with (14C)NaHCO3 and immuno-purification, demonstrated that ethanol treatment abolished the rapid phase of CYP2E1 degradation while biphasic degradation of CYP2E1 was observed in the control animals (108). Pyrazole and 4-MP elevated liver and kidney CYP2E1 immunoreactive protein and catalytic activity in the absence of an increase in CYP2E1 mRNA levels (109–111). In isolated rat hepatocyte cultures, CYP2E1 mRNA and protein levels and CYP2E1 catalytic activity rapidly declined with time in culture. Addition of pyrazole or 4-MP slowed the decline in CYP2E1 protein and activity, without any effect on CYP2E1 mRNA levels (105). Similarly, McGhee et al (112) reported the half-life of CYP2E1 in a hepatoma cell line to be 1.8 h in the absence of ethanol and 45 h in the presence of ethanol. It is clear that a major level of regulation of CYP2E1 formation appear to be posttranscriptional as various substrates and ligands increase the content of CYP2E1 by protection against rapid degradation by intracellular proteolytic pathways.
What are the proteolytic systems responsible for CYP2E1 turnover and prevented from their action on CYP2E1 by ethanol? Roberts (113) provided evidence for a role of the proteasome in the degradation of several cytochrome P450s including CYP2E1. Huan et al (114) showed that in a Hela cell line, inhibitors of the proteasome decreased the degradation of CYP2E1 and CYP2B1. They found that ubiquitination of CYP2E1 was not required for its degradation by the proteasome. However, Banerjee et al (115) using molecular models predicted a cytosolic domain of CYP2E1 which would function as a putative ubiquitination-target/substrate interaction structure. An antibody recognizing this domain (amino acids 317–340) quenched CYP2E1 ubiquinitation and inhibited CYP2E1 catalytic activity. They suggested that substrate binding shields the CYP2E1 protein from turnover by blocking the ubiquitination domain. Morishima et al (116) reported that a HSP90 inhibitor promoted CYP2E1 degradation by the proteasome. They found that purified bacterially expressed truncated CYP2E1 (Δ3–29) is ubiquitylated by the E3 ubiquitin ligase CHIP and concluded that CYP2E1 is a HSP90 “client” protein. In contrast, Huan et al (114) found that three HSP90 inhibitors had no effect on CYP2E1 turnover. We found that in an in vitro reconstituted system containing microsomes or cytosol or purified 20S proteasome, geldanamycin, an inhibitor of HSP90, decreased CYP2E1 degradation and suggested that HSP90 helps present oxidized CYP2E1 to the proteasome for degradation (117). The proteasome complex was important in the degradation of CYP2E1 in HepG2 cells, as proteasome inhibitors proved to be effective in preventing CYP2E1 degradation. Importantly, Bardag-Gorce et al (118) showed that the rapid loss of CYP2E1, which occurs in vivo after the ethanol inducer is withdrawn, could be blocked by the proteasome inhibitor PS-341, thus establishing the critical role of the proteasome in regulating CYP2E1 turnover in vivo.
CYP2E1 and alcohol-induced liver injury
Since CYP2E1 can generate ROS during its catalytic circle, and its levels are elevated by chronic treatment with ethanol, CYP2E1 has been suggested as a major contributor to ethanol-induced oxidant stress, and to ethanol-induced liver injury. Initial suggestions for a role for CYP2E1 in alcoholic liver injury arose from studies with the intragastric model of ethanol feeding in which prominent induction of CYP2E1 occurs and in which significant liver injury occurs (29–31). In these models, large increases in microsomal lipid peroxidation have been observed and the ethanol-induced liver pathology has been shown to correlate with CYP2E1 levels and elevated lipid peroxidation (29, 30, 119, 120). Experimentally, a decrease in CYP2E1 induction was found to be associated with a reduction in alcohol-induced liver injury (121,122). CYP2E1 inhibitors such as diallyl sulfide (DAS) (123), phenethyl isothiocyanate (PIC) (124,125) and chlormethiazole (126), blocked the lipid peroxidation and ameliorated the pathologic changes in ethanol-fed rats. Polyenylphosphatidylcholine (PPC), another compound exerting anti-CYP2E1 properties (127) was effective in opposing alcohol-induced oxidative stress (40). A strong association between dietary carbohydrate, enhanced CYP2E1 induction and hepatic necrosis was observed. No liver injury was found if carbohydrate levels were elevated (128). It was concluded that diet is an important factor in toxicity mediated by ethanol because of modulation of the levels of CYP2E1 (128). Ethanol consumption in oral liquid diets does not cause significant liver injury. However, micro and macrovesicular steatosis, occasional inflammatory foci and a three-fold increase in transaminase levels was observed in a nutritional adequate ethanol containing liquid diet with a carbohydrate content of 5.5%; no changes were found if the level of carbohydrate was elevated to 11% (94,129). Thus dietary and nutritional factors play a key role in the toxic actions of ethanol to the liver, in part, due to modulation of the levels of CYP2E1. Recently, A CYP2E1 transgenic mouse model was developed that overexpressed CYP2E1. When treated with ethanol, the CYP2E1 overexpressing mice displayed higher transaminase levels and histological features of liver injury compared with the control mice (130). We developed an adenoviral vector which expresses human CYP2E1 and showed that infection of HepG2 cells with this adenovirus potentiated acetaminophen toxicity as compared to HepG2 cells infected with a LacZ expressing adenovirus (131). Administration of CYP2E1 adenovirus in vivo to mice produced significant liver injury compared to the LacZ-infected mice as reflected by histopathology, markers of oxidative stress and elevated transaminase levels (132).
On the other hand, studies by Thurman and colleagues have presented powerful support for a role for endotoxin, activation of Kupffer cells and cytokines such as TNFα in the alcohol-induced liver injury found with the intragastric infusion model (133,134). They suggested that CYP2E1 may not play a role in alcohol liver injury based upon studies with gadolinium chloride or CYP2E1 knockout mice (135,136). Female CYP2E1 wild type mice or knockout mice were given a high fat liquid diet intragastrically with either ethanol or isocaloric maltose-dextrin for 4 weeks. Mice given ethanol had elevated transaminases, mild steatosis and slight inflammation and necrosis with no differences in pathology between the wild type and the knockouts (135). However, Bardag-Gorce et al (137) using the same model reported that ethanol-induced oxidative stress and inactivation of the proteasome complex was completely prevented in these mice. They concluded that CYP2E1 induction by chronic ethanol treatment was responsible for the decrease in proteasome activity and accumulation of oxidized proteins in the liver. They speculated the pathology found in the CYP2E1 knockouts by Kono et al (135) may be due to upregulation of NADPH-cytochrome P450 reductase and other CYPs such as CYP3A and 4A (137) (see below). As to their observations with gadolinium chloride, others have reported that gadolinium chloride does indeed decrease levels of several P450 enzymes including CYP2E1, and lowered the induction of CYP2E1 by ethanol. Moreover, Leclercq et al. (138) using the same knockout mice observed that other CYPs, notably CYP4A10 and CYP4A14, were upregulated in the CYP2E1 knockout but not the wild type mice; these CYPs were, like CYP2E1, active generators of ROS and catalysts of lipid peroxidation, and in the absence of CYP2E1 served as alternative initiators of oxidative stress. Bradford et al (139) using CYP2E1 and NADPH oxidase knockout mice concluded that CYP2E1 was required for ethanol induction of oxidative stress to DNA, whereas NADPH oxidase was required for ethanol-induced liver injury. Clearly, further studies are necessary to resolve the above discrepancies. As mentioned earlier, it is likely that several mechanisms contribute to alcohol-induced liver injury, and that ethanol-induced oxidant stress is likely to arise from several sources, including CYP2E1, mitochondria and activated Kupffer cells.
Cell lines expressing CYP2E1
To characterize the biochemical and toxicological properties of CYP2E1, several investigators have developed cell lines to express CYP2E1. The first cell line to be developed was to introduce human CYP2E1 into NIH 3T3 mouse fibroblasts via retroviral infection followed by selection via G418 resistance (140). Southern blot analysis showed the viral DNA was integrated into the cellular DNA. The transduced CYP2E1 was catalytically active, oxidizing ethoxycoumarin to 7-hydroxycoumarin, and forming labeled covalent DNA adducts after the incubation with (14C)-nitroso-dimethylamine (140). The cytotoxic effect of N-nitrosodimethylamine was studied in the P450-expressing human fibroblast cell line GM2E1, which express the rat CYP2E1 (141). N-nitrosomethylamine was toxic to the cells expressing CYP2E1; toxicity was decreased by CYP2E1 inhibitors and was partially prevented by antioxidants (141). Toxicity was apoptotic in nature and could be prevented by caspase inhibitors (142). A V79 Chinese Hamster cell line expressing human CYP2E1 was used in toxicological studies involving CYP2E1-mediated activation of N-nitrosodimethylamine and p-nitrophenol and for mutagenicity studies with the former substrate (143). Human CYP2E1 was also expressed in the rat adrenal pheochromocytoma cell line, PC12 (144). CYP2E1 metabolism of several different substrates was characterized in these cells; levels of enzyme activities were about 10% that of human liver microsomes, similar to what our lab found with HepG2 cells expressing CYP2E1 (145). The PC12 cells were shown to metabolize acetaminophen, and activation of this protoxin caused a loss of viability to the cells (144). Acetaminophen toxicity was also characterized in a human hepatoman cell line, HLE, expressing human CYP2E1 (146). Treating these cells with buthionine-sulfoximine to lower GSH levels also produced a decrease in cell viability which could be inhibited by ethanol or vitamin E. This cell line has been used to examine changes of heme metabolism via assays of δ-aminoleulinic acid synthase and heme oxygenase-1, the rate limiting enzymes in heme synthesis and heme breakdown (147). Both enzymes were upregulated in the HLE cells expressing CYP2E1 perhaps due to the demand for increased heme synthesis for holoCYP2E1 formation and perhaps increased availability of heme to induce heme oxygenase. Our lab has also observed an increase in heme oxygenase-1 mRNA, protein and activity in HepG2 cells expressing CYP2E1, perhaps an increase in response to CYP2E1-generated oxidant stress (148). Huan and Koop (149) established a tetracycline-controlled rabbit CYP2E1-expressing system in Hela cells in culture. This system was used to evaluate turnover of the rabbit CYP2E1, which was rapid with a half-life of 3.9 h in the absence of a stabilizing substrate or ligand. Addition of the latter, 4-methylpyrazole, decreased the degradation of CYP2E1. We observed similar results in HepG2 cells expressing CYP2E1 as the half-life of human CYP2E1 was about 3–6 h in the absence of substrate or ligand, and was elevated in the presence of various substrates and ligands (150). The CYP2E1 half-life was also elevated by an inhibitor of the proteasome complex. Recently, a comparison of mouse, rat and human CYP2E1 activities in V79 Chinese Hamster cell lines was made to study possible species differences in toxicity and metabolism of CYP2E1 substrates (151).
A HepG2 cell model expressing human CYP2E1 was established by Patten et al (152) using the vaccinia virus expression system. The oxidation of several typical substrates of CYP2E1 was evaluated in these cells and the ability of cytochrome b5 to elevate CYP2E1 activity was shown. As briefly mentioned above, an approach that our laboratory has utilized to try to understand basic effects and actions of CYP2E1 was to establish cell lines that constitutively express human CYP2E1. HepG2 cell lines, which overexpress CYP2E1, were established either by retroviral infection methods (MV2E1-9 cells, or E9 cells) or by plasmid transfection methods (E47 cells) (145,153). Results utilizing E9 or E47 cells to study CYP2E1-generated oxidant stress have been summarized in recent reviews (154–156). We have characterized the toxicity of ethanol, polyunsaturated fatty acids (PUFA) such as arachidonic acid (AA) and iron in E9 and E47 cells. Concentrations of ethanol or AA or iron which were toxic to the CYP2E1-expressing cells had no effect on control HepG2 cells not expressing CYP2E1 or to HepG2 cells expressing a different P450, CYP3A4 (3A4 cells). Toxicity to CYP2E1-expressing cells was found when GSH was depleted by treatment with L-buthionine sulfoximine (157). Inhibitors of CYP2E1 prevented the toxicity by the above treatments. Antioxidants such as vitamin E, trolox and ascorbate also prevented toxicity found when the CYP2E1-expressing E9 HepG2 cells were treated with either ethanol or AA. The above treatment of CYP2E1-expressing cells with ethanol, AA, iron or BSO resulted in an increase in oxidative stress to the cells as reflected by increased lipid peroxidation and enhanced dichloroflurorescein fluorescence. Low concentrations of iron and AA that are not cytotoxic by themselves can act as priming or sensitizing factors for CYP2E1-dependent loss of viability in HepG2 cells or rat hepatocytes. This synergistic toxicity was associated with elevated lipid peroxidation and could be prevented by antioxidants which prevent lipid peroxidation. Damage to mitochondria by CYP2E1-derived oxidants seems to be an early event in the overall pathway of cellular injury.
Adaptation to oxidant stimuli is critical for short- and long-term survival of cells exposed to oxidative stress. We found that the levels of GSH and several antioxidant enzymes such as glutathione transferase, catalase, and heme oxygenase-1 were up-regulated in the CYP2E1-expressing cells. This upregulation was prevented by antioxidants, suggesting that ROS generated by CYP2E1 were responsible for the transcriptional activation of these antioxidant genes. Because of this activation of antioxidant genes, the CYP2E1-expressing cells were less sensitive to toxicity to H2O2, menadione, or HNE than control cells. We believe that the upregulation of these antioxidant genes reflect an adaptive mechanism to remove CYP2E1-derived oxidants. Recent experiments suggested that Nrf2 plays a key role in the adaptive response against the increased oxidative stress caused by CYP2E1 (158)(reviewed in 159).
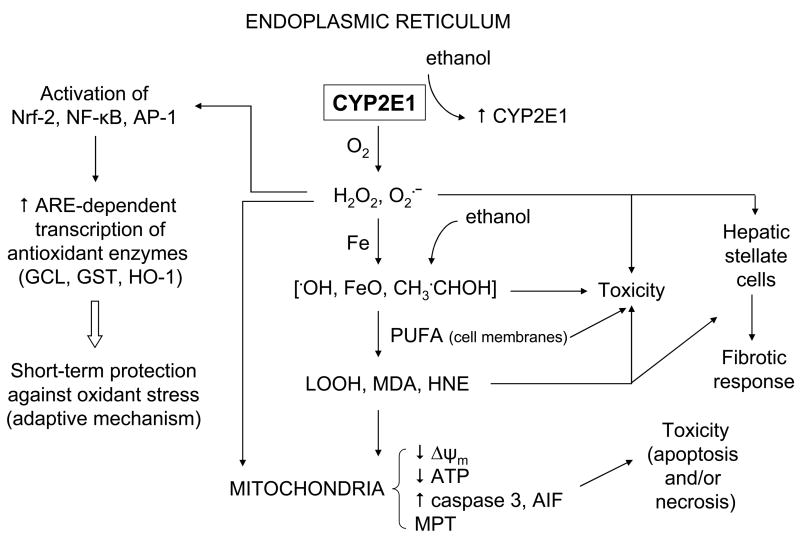
A working model of CYP2E1-dependent oxidative stress and toxicity is shown in Figure 1. Ethanol increases levels of CYP2E1, largely by a posttranscriptional mechanism involving enzyme stabilization against degradation. CYP2E1, a loosely coupled enzyme, generates ROS such as O2·̄ and H2O2 during its catalytic cycle. In the presence of iron, which is increased after ethanol treatment, more powerful oxidants including OH, ferryl species, and 1-hydroxyethyl radical are produced. Initially, the liver cells respond to the CYP2E1-related oxidative stress by transcriptionally inducing various antioxidant enzymes via their antioxidant response elements. Ultimately, these protective mechanisms are overwhelmed and the cells become sensitive to the CYP2E1-generated oxidants. These various oxidants can promote toxicity by protein oxidation and enzyme inactivation, oxidative damage to the DNA, and disturbing cell membranes via lipid peroxidation and production of reactive lipid aldehydes, such as malondialdehyde and 4-hydroxynonenal. Mitochondria appear to be among the critical cellular organelles damaged by CYP2E1-derived oxidants. A decrease of ΔΨm, likely due to the mitochondrial membrane permeability transition, causes release of proapoptotic factors resulting in apoptosis. A decrease in ATP levels will cause necrosis. Some CYP2E1-derived ROS, such as H2O2, LOOH, and HNE, are diffusible and may exit hepatocytes and enter other liver cell types such as stellate cells and stimulate these cells to produce collagen and elicit a fibrotic response (160,161). We believe that the linkage between CYP2E1-derived oxidative stress, mitochondrial injury, and GSH homeostasis contribute to the toxic actions of ethanol on the liver.
Fig. 1.
Working model of CYP2E1-dependent oxidative stress and cytotoxicity. Please see the text for discussion.
Other investigators have utilized E9 and E47 HepG2 cells expressing CYP2E1 in a variety of studies including evaluating the effects of ethanol and acetaldehyde on activation of the transcriptional factors AP-1 and NF-kB (162), in proteomic studies on ethanol-induced oxidation of mitochondrial and cytosolic proteins (163,164), on ethanol-induced inhibition of the proteasome and cytokeratin aggresome formation (165), on comparison of gene expression patterns induced by alcohol in vivo and in vitro (166), on CYP2E1-hepatitis C virus (167) or hepatitis B virus (168) interactions, on acetaminophen alterations of the microsomal ryanodine calcium channel (169), on fatty acid ethyl ester toxicity (170), and in ethanol potentiation of TNFα cytotoxicity (171) and the role of P38 MAPK pathways in ethanol plus TNFα toxicity (172). A rather interesting HepG2 cell culture model in which CYP2E1 and alcohol dehydrogenase are both expressed has been effectively utilized to study ethanol-CYP2E1-proteasome interactions, interferon gamma induction of the proteasome and interferon gamma signaling and antigen processing (173–175). The use of these combined cell lines as a model of ethanol-elicited cytotoxicity has recently been reported (176). Thus, CYP2E1 biochemistry, oxidant stress and toxicology have been extensively studied in a variety of stable cell lines.
The CYP2E1 knockout mouse
CYP2E1 knockout mice were developed by Gonzalez and colleagues to determine the role of CYP2E1 in xenobiotic metabolism and toxicity (49, 177,178). The development of the CYP2E1 knockout mouse has been of great value in establishing the role of CYP2E1 in the metabolism and toxicity of various hepatotoxins. For example, there was no liver pathology or elevation of transaminases induced by CCl4 in CYP2E1 knockout mice compared to wild type mice, leading to the conclusion that CYP2E1 is the major factor in CCl4 hepatotoxicity (179). Blood acetone levels were elevated 2.5–4 times in wild type mice after 48 h fasting but elevated 28-fold in the CYP2E1 knockout mice, leading to the conclusion that CYP2E1 plays a critical role in catabolism of acetone after fasting (180). Formation of benzene metabolites such as hydroquinone, catechol and phenol were lowered more than 90% with microsomes from CYP2E1 knockout mice compared to microsomes from controls (181). The CYP2E1 knockout mice have been used to validate the important role of CYP2E1 in metabolism of thioacetamide, trichloroethylene, acrylonitrile and urethane (182–186). The half-life for urethane was 0.8 h in wild type mice expressing CYP2E1 and 22 h in CYP2E1 knockout mice (186). Interestingly, no difference in oxidation of styrene to styrene oxide was observed between microsomes from wild type mice and knockout mice (187). Since the styrene metabolite styrene oxide was comparably toxic in wild type and knockout mice, the decreased sensitivity of the CYP2E1 knockout mice to styrene likely should be due to decreased bioactivation of styrene to styrene oxide. Yet no differences in styrene metabolism were found, disconnecting the metabolism from the toxicity for unknown reasons (187). The CYP2E1 knockout mice have been used to validate that hydroxylation of p-nitrophenol may be used as a specific probe for CYP2E1 (188).
The metabolism of acetaminophen has been widely studied. CYP2E1 knockout mice were highly resistant to liver toxicity as compared to wild type mice treated with acetaminophen (49). Mice lacking both CYP2E1 and 1A2 were almost completely resistant to acetaminophen toxicity (189). The combination of ethanol plus isopentanol caused an increase in acetaminophen hepatotoxicity in CYP2E1 knockout mice which was sensitive to the CYP3A inhibitor, triacetyloleandomycin, leading to the suggestion that both CYP2E1 and CYP3A contribute to acetaminophen toxicity in ethanol plus isopentanol-treated mice (190). Recently, a CYP2E1-humanized transgenic mouse model that expresses functional and inducible human CYP2E1 was described (191). Comparisons between CYP2E1-humanized mice, CYP2E1 knockout mice and wild type mice will allow a determination whether actions of human CYP2E1 are similar to those of mouse CYP2E1 in vivo. Indeed, the CYP2E1-humanized mouse model was successfully used to characterize acetaminophen toxicity by human CYP2E1 (191).
With respect to the role of CYP2E1 in alcohol-induced liver injury, as discussed above, Bardag-Gorce et al (137) reported that ethanol-induced oxidative stress and inactivation of the proteasome complex was completely prevented in CYP2E1 knockout mice. They concluded that CYP2E1 induction by chronic ethanol treatment was responsible for the decrease in proteasome activity and accumulation of oxidized proteins in the liver. In a very interesting study, Bradford et al (139) found that ethanol treatment for four weeks led to an increase in oxidative DNA damage and induction of expression of basic excision DNA repair genes in wild type mice but not in CYP2E1 knockout mice. The increase in DNA repair genes in wild type mice was abolished by treatment with a P450 inhibitor. The induction and the DNA damage induced by ethanol was the same in wild type mice and NADPH oxidase deficient mice. The authors concluded that CYP2E1 but not NADPH oxidase is required for the ethanol induction of oxidative stress to DNA and thus CYP2E1 may play a key role in ethanol-associated hepatocarcinogenesis (139). On the other hand, as mentioned above, studies by Thurman and colleagues suggests that CYP2E1 may not play a role in alcohol-induced liver injury (135). Instead, their studies have presented powerful support for a role for endotoxin (LPS), activation of Kupffer cells and cytokines such as TNFα in the alcohol-induced liver injury found with the intragastric infusion model.
LPS/TNFα-CYP2E1 Interactions
Abnormal cytokine metabolism is a major feature of alcoholic liver disease as described in many review articles (192–195). Rats chronically fed ethanol were more sensitive to the hepatotoxic effects of administration of LPS and had higher plasma levels of TNFα than control rats (196–198). In the intragastric model of chronic ethanol administration, the development of liver injury coincided with an increase in TNFα, associated with an increase in serum LPS (195, 197–199). The pioneering studies of Thurman and collaborators showed that anti-TNFα antibody prevented alcohol liver injury in rats (200) and mice lacking the TNFR1 receptor did not develop alcohol liver injury (133). Taken as a whole, these and other studies clearly implicate TNFα as a major risk factor for the development of alcoholic liver injury. One complication in this central role for TNFα is that hepatocytes are normally resistant to TNFα induced toxicity. This led to the hypothesis that besides elevating TNFα, alcohol somehow sensitizes or primes the liver to become susceptible to TNFα (201,202). Known factors which sensitize the liver to TNFα are inhibitors of mRNA or protein synthesis, which likely prevent the synthesis of protective factors, inhibition of NF-κB activation to lower synthesis of such protective factors, depletion of GSH, especially mitochondrial GSH, lowering of s-adenosyl methionine (SAM) coupled to elevation of S-adenosyl homocysteine (SAH) i.e. a decline in the SAM/SAH ratio, or inhibition of the proteasome (203–210). Of major relevance to this review, is the work by Hoek and collaborators that combined treatment with ethanol plus TNFα is more toxic to hepatocytes and HepG2 E47 cells which express high levels of CYP2E1 than control hepatocytes with lower levels of CYP2E1 or HepG2 C34 cells which do not express CYP2E1 (171). The ethanol sensitization of TNFα toxicity in E47 cells and hepatocytes from chronic ethanol fed rats also depended on P38 MAPK signaling since SB203580, a P38 MAPK inhibitor, prevented this enhanced toxicity (172). In a RALA hepatocyte cell line model, Czaja and collaborators showed that hepatocytes with increased expression of CYP2E1 were sensitized to TNFα mediated cell death (211). Toxicity was a mixture of necrosis and apoptosis, was associated with prolonged activation of JNK and phosphorylation of c-Jun, and could be prevented by a dominant negative c-Jun construct (211). These results suggest that increased oxidant stress from CYP2E1 may sensitize isolated hepatocytes to TNFα-induced toxicity.
Since CYP2E1 and LPS/TNFα are believed to be key risk factors in the development of alcoholic liver injury, we evaluated possible interactions in promoting liver injury between them in vivo (212, 213). Sprague-Dawley rats were treated with 200 mg/kg body weight pyrazole in the absence or presence of LPS (10 mg/kg) and killed at 8 h after LPS (212). C57BL/6 mice were treated with 150 mg/kg body weight pyrazole in the absence or presence of LPS (4 mg/kg) and killed at 24 h after LPS (213). The combination of LPS plus pyrazole treatment resulted in elevated ALT and AST levels in rats and mice. Liver injury was confirmed by H&E staining. LPS alone or pyrazole alone did not elevate transaminase levels and did not produce liver injury under these conditions. Increased 3-Nitrotyrosine protein adducts were observed at 8 (in rats) and 24 h (in mice) after LPS plus pyrazole treatment (212, 213). Positive staining for 4-hydroxynonenal adducts was found at 24 h in the LPS plus pyrazole mice (213). The CYP2E1 inhibitor chlormethiazole (CMZ) protected against the elevation in ALT and AST in mice and the histopathology changes. CYP2E1 catalytic activity was decreased about 50% by the CMZ treatment (213). We obtained CYP2E1 knockout mice from Dr. Frank Gonzalez, NCI. These mice were treated with pyrazole plus LPS. Compared to SV/129 wild type mice, ALT and AST levels were lower in the CYP2E1 null mice, histopathology was normal and TUNEL staining was much less (213). Western blot analysis confirmed the absence of CYP2E1 in the CYP2E1 knockout mice (213). Based on such studies, we hypothesize (212, 213) that increased production of ROS by CYP2E1 may prime or sensitize the liver to LPS/TNFα, and such interactions may be important in alcohol-induced liver injury.
Nonalcoholic fatty liver disease, steatohepatitis and CYP2E1
Nonalcoholic steatohepatitis (NASH) is a progressive liver disorder that occurs in patients without significant alcohol consumption. The pathogenesis of NASH is not well understood, although it has been suggested that oxidative stress and lipid peroxidation may play key roles in the pathogenesis of NASH (214–217). Elevated CYP2E1 was observed in conditions such as obesity and high fat/low carbohydrate diets (84). Weltman et al (218) reported increased liver expression of CYP2E1 in the methionine-choline deficient model of NASH. CYP2E1 activity, protein level and mRNA levels were all elevated in this experimental model of NASH, although total P450s content was decreased. Inhibitor studies further suggested that CYP2E1 was the major catalyst of lipid peroxidation in mice fed the methionine-choline deficient diet (138). However, CYP2E1 knockout mice fed with this diet still displayed elevated lipid peroxidation and NASH (138). Under these conditions, CYP4A10 and CYP4A14 but not CYP1A or CYP3A were upregulated and could replace the deficient CYP2E1 as catalysts for microsomal lipid peroxidation. Thus, while CYP2E1 contributes to the pathogenesis of NASH, it is not unique among P450 enzymes in promoting oxidant stress as some CYP4A enzymes can serve as alternative initiators of oxidant stress in the liver. Interestingly, antibody against CYP2E1 strongly inhibited lipid peroxidation by microsomes from wild type mice but antibody against CYP4A had little effect (138). The opposite was found with microsomes from CYP2E1 knockout mice as antibody against CYP4A blocked lipid peroxidation whereas antibody against CYP2E1 had no effect. Thus CYP4A can mediate lipid peroxidation as an alternative pathway when CYP2E1 is absent (219). This can partially explain the observations by Kono et al (135) that in the intragastric infusion model of alcohol-induced liver injury, injury persisted in CYP2E1 knockout mice ie, possible upregulation of CYP4A or other enzymes could replace CYP2E1 as initiators or catalysts of oxidative stress.
Hepatic CYP2E1 levels were increased in patients with NASH (220). Chalasani et al (221) measured liver CYP2E1 activity in a cohort of nondiabetic patients with NASH and controls. They found that chlorzoxazone clearance was greater in the NASH patients compared with controls and lymphocyte CYP2E1 mRNA levels were also higher in the NASH patients. Increases in CYP2E1 correlated with increases in the ketone body β-hydroxybutyrate. They suggested that although more studies were necessary, CYP2E1 is a reasonable candidate in the pathogenesis of human NASH (221). In a recent study involving obese patients with nonalcoholic liver disease, increased CYP2E1 protein content and activity correlated with the development of liver injury (222).
Since CYP2E1 is elevated in pathophysiological conditions such as obesity and diabetes, we recently evaluated the effects of CYP2E1 induction on promoting oxidative and nitrosative stress and liver injury in ob/ob mice, an experimental model of obesity (223). Ob/ob mice and lean controls were treated with pyrazole or acetone to induce CYP2E1. CYP2E1 protein and activity were elevated in acetone or pyrazole-treated obese and lean mice. Acetone or pyrazole induced distinct histological changes in liver and significantly higher aminotransferase enzymes in obese mice compared to obese controls or acetone-or pyrazole-treated lean mice (223). Increased malondialdehyde, protein carbonyls, 4-hydroxynonenal-protein adducts, elevated levels of inducible nitric oxide synthase, and higher 3-nitrotyrosine protein adducts were found in livers of pyrazole-treated obese animals, suggesting elevated oxidative and nitrosative stress (223). Liver TNFα levels were higher in pyrazole-treated animals. The CYP2E1 inhibitor CMZ and iNOS inhibitor N-(3-(amino-methyl)-benzyl) acetamidine (1400W) abrogated the elevated toxicity (transaminases, caspase 3, triglyceride) and the oxidative stress (protein carbonyl, HNE adduct formation, malondialdehyde) elicited by the induction of CYP2E1 (223). Peroxynitrite (ONOO−), formed by the rapid reaction between NO and O2·̄ has been shown to nitrate free and protein-associated tyrosine residues and produce nitrotyrosine (212), therefore, either decreased NO by 1400W or declined O2·̄ by CMZ prevented 3-NT formation (223). These results show that obesity contributes to oxidative/nitrtosative stress and liver injury and that induction of CYP2E1 may synergize with high fat in obesity to promote liver cell injury.
Future Perspectives
Alcohol-induced liver injury is probably a multifactorial process involving several mechanisms. Future studies are required to further clarify how alcohol produces oxidative stress in various tissues. Some of the major proposed systems require more detail about mechanism, e.g., how ethanol-derived NADH, itself or when reoxidized in the mitochondrial respiratory chain, produces ROS. What is the role of ethanol metabolism or ethanol metabolites like acetaldehyde in the production of ROS, and how is oxidative stress produced by ethanol in tissues with limited ethanol metabolism? What are the priming or sensitizing factors for ethanol-induced oxidant stress and cell injury? Can markers predictive of individuals particularly sensitive to ethanol-induced oxidant stress and liver injury be developed?
The role of CYP2E1 in the toxic effects of ethanol requires further study as this remains a controversial issue. This is significant not only from a mechanistic point of view but perhaps from a therapeutic treatment approach. If indeed CYP2E1-induced oxidative stress plays a central role in alcohol-induced liver damage, possible strategies for preventing this stress may be effective in attempts to minimize the hepatotoxicity of ethanol in humans. The CYP2E1 inhibitors which were partially effective in preventing ethanol-induced liver injury are not entirely selective and may be toxic, although chlormethiazole (CMZ) (126) or polyenylphosphatidylcholine (PPC) (127) may merit further consideration. YH439 is a novel synthetic compound inhibiting CYP2E1 (but also other P450s) that is being evaluated as a hepatoprotective agent (224). Actually, natural agents inhibiting CYP2E1, including dially sulfide (from garlic) mentioned above, phenylethyl isothiocyanate and sulforaphane (present in cruciferous vegetables) and bergamottin (found in the essential oils of grapefruit and certain oranges) have been proposed as possible candidates for minimizing the ethanol-induced hepatotoxicity (225). In addition, trans-1,2-dichloroethylene (DCE) was reported to be a selective inhibitor of CYP2E1 (226).
Regulation of CYP2E1 protein levels is complex, with transcriptional, translational, and posttranscriptional effects observed; more mechanistic details as to how ethanol modulates CYP2E1 levels are required to define, e.g. effects on activity of the proteasome, ubiquitination, how ethanol stabilizes CYP2E1. What are the factors which trigger the rapid turnover of CYP2E1? Most studies on the biochemical and pharmacological actions of CYP2E1 are derived from studies with rodents and rabbits and cultured hepatocytes: extrapolation to human studies is obviously necessary. The role of polymorphic forms of CYP2E1 on CYP2E1 expression, activity, and action requires further understanding, as current literature suggests some possible relationships with certain types of cancers but not with alcohol toxicity. Are there endogenous substrates for CYP2E1? At present, acetone and some fatty acids (omega-1 hydroxylase activity) appears to be physiological substrates for CYP2E1, but further studies should be carried out because altered metabolism of such putative endogenous substrates, if any, could contribute to the cellular actions associated with CYP2E1. CYP2E1 is present, although at relatively low levels, in other tissues, e.g. kidney, lung, brain, gastrointestinal tract. Much less is known about the actions of CYP2E1 under various pathophysiological conditions or after chronic ethanol exposure in these tissues. CYP2E1-nutritional interactions require further study, especially interactions with prooxidants, such as iron; polyunsaturated fatty acids; or reagents that lower oxidant defense, e.g., lower GSH levels. There is much current interest in synergistic interactions between alcohol and hepatitis B or hepatitis C virus, especially with respect to generating oxidative stress. The role of CYP2E1 in such synergistic interactions, if any, would be important to explore in view of the many chemicals and conditions that are known to elevate CYP2E1.
The ability of alcohol to promote oxidative stress and the role of free radicals in alcohol-induced tissue injury clearly are important areas of research, particularly because such information may be of major therapeutic significance in attempts to prevent or ameliorate alcohol’s toxic effects, e.g., by antioxidants, iron chelators, inhibitors of CYP2E1 or of cytokine production/actions, and GSH replenishment. As basic information continues to emerge regarding the role of oxidative stress in disease development and the mechanisms underlying ROS-related cellular toxicity, these findings will lead to more rational antioxidant therapeutic approaches. Moreover, these finding could result in the development of more effective and selective new medications capable of blocking the actions of CYP2E1 and ROS and, consequently, the toxic effects of alcohol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guengerich FP. Oxidative cleavage of carboxylic esters by cytochrome P-450. J Biol Chem. 1987;262:8459–8462. [PubMed] [Google Scholar]
- 2.Nebert DW, Nelson DR, Coon MJ, Estabrook RW, Feyereisen R, Fujii-Kuriyama Y, Gonzalez FJ, Guengerich FP, Gunsalus IC, Johnson EF, et al. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991;10:1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- 3.Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, Waterman MR, Gotoh O, Coon MJ, Estabrook RW, Gunsalus IC, Nebert DW. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Porter TD, Coon MJ. Cytochrome P-450. Multiplicity of isoforms, substrates, and catalytic and regulatory mechanisms. J Biol Chem. 1991;266:13469–13472. [PubMed] [Google Scholar]
- 5.Rendic S, Di Carlo FJ. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev. 1997;29:413–580. doi: 10.3109/03602539709037591. [DOI] [PubMed] [Google Scholar]
- 6.Guengerich FP. Uncommon P450-catalyzed reactions. Curr Drug Metab. 2001;2:93–115. doi: 10.2174/1389200013338694. [DOI] [PubMed] [Google Scholar]
- 7.Lewis DF, Pratt JM. The P450 catalytic cycle and oxygenation mechanism. Drug Metab Rev. 1998;30:739–786. doi: 10.3109/03602539808996329. [DOI] [PubMed] [Google Scholar]
- 8.Loida PJ, Sligar SG. Molecular recognition in cytochrome P-450: mechanism for the control of uncoupling reactions. Biochemistry. 1993;32:11530–11538. doi: 10.1021/bi00094a009. [DOI] [PubMed] [Google Scholar]
- 9.Kuthan H, Ullrich V. Oxidase and oxygenase function of the microsomal cytochrome P450 monooxygenase system. Eur J Biochem. 1982;126:583–588. doi: 10.1111/j.1432-1033.1982.tb06820.x. [DOI] [PubMed] [Google Scholar]
- 10.White RE. The involvement of free radicals in the mechanisms of monooxygenases. Pharmacol Ther. 1991;49:21–42. doi: 10.1016/0163-7258(91)90020-m. [DOI] [PubMed] [Google Scholar]
- 11.Knight JA. Free radicals: their history and current status in aging and disease. Ann Clin Lab Sci. 1998;28:331–346. [PubMed] [Google Scholar]
- 12.Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol. 1993;23:21–48. doi: 10.3109/10408449309104073. [DOI] [PubMed] [Google Scholar]
- 13.Bondy SC. Reactive oxygen species: relation to aging and neurotoxic damage. Neurotoxicology. 1992;13:87–100. [PubMed] [Google Scholar]
- 14.Nordmann R, Ribiere C, Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic Biol Med. 1992;12:219–240. doi: 10.1016/0891-5849(92)90030-k. [DOI] [PubMed] [Google Scholar]
- 15.Cederbaum AI. Microsomal generation of reactive oxygen species and their possible role in alcohol hepatotoxicity. Alcohol Alcohol Suppl. 1991;(1):291–296. [PubMed] [Google Scholar]
- 16.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 17.Blanck J, Ristau O, Zhukov AA, Archakov AI, Rein H, Ruckpaul K. Cytochrome P-450 spin state and leakiness of the monooxygenase pathway. Xenobiotica. 1991;21:121–135. doi: 10.3109/00498259109039456. [DOI] [PubMed] [Google Scholar]
- 18.Toyokuni S. Reactive oxygen species-induced molecular damage and its application in pathology. Pathol Int. 1999;49:91–102. doi: 10.1046/j.1440-1827.1999.00829.x. [DOI] [PubMed] [Google Scholar]
- 19.De Groot H. Reactive oxygen species in tissue injury. Hepato-gastroenterology. 1994;41:328–332. [PubMed] [Google Scholar]
- 20.Nakazawa H, Genka C, Fujishima M. Pathological aspects of active oxygens/free radicals. Jpn J Physiol. 1996;46:15–32. doi: 10.2170/jjphysiol.46.15. [DOI] [PubMed] [Google Scholar]
- 21.McCord JM. Iron, free radicals, and oxidative injury. Semin Hematol. 1998;35:5–12. [PubMed] [Google Scholar]
- 22.Halliwell B. Antioxidant defence mechanisms: from the beginning to the end (of the beginning) Free Radic Res. 1999;31:261–272. doi: 10.1080/10715769900300841. [DOI] [PubMed] [Google Scholar]
- 23.Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 24.Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. Free radicals and phagocytic cells. FASEB J. 1995;9:200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 25.Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- 26.Knecht KT, Adachi Y, Bradford BU, Iimuro Y, Kadiiska M, Xuang QH, Thurman RG. Free radical adducts in the bile of rats treated chronically with intragastric alcohol: inhibition by destruction of Kupffer cells. Mol Pharmacol. 1995;47:1028–1034. [PubMed] [Google Scholar]
- 27.Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- 28.Iimuro Y, Bradford BU, Yamashina S, Rusyn I, Nakagami M, Enomoto N, Kono H, Frey W, Forman D, Brenner D, Thurman RG. The glutathione precursor L-2-oxothiazolidine-4-carboxylic acid protects against liver injury due to chronic enteral ethanol exposure in the rat. Hepatology. 2000;31:391–398. doi: 10.1002/hep.510310219. [DOI] [PubMed] [Google Scholar]
- 29.Morimoto M, Zern MA, Hagbjork AL, Ingelman-Sundberg M, French SW. Fish oil, alcohol, and liver pathology: role of cytochrome P450 2E1. Proc Soc Exp Biol Med. 1994;207:197–205. doi: 10.3181/00379727-207-43807. [DOI] [PubMed] [Google Scholar]
- 30.Nanji AA, Zhao S, Sadrzadeh SM, Dannenberg AJ, Tahan SR, Waxman DJ. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol Clin Exp Res. 1994;18:1280–1285. doi: 10.1111/j.1530-0277.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 31.Tsukamoto H, Horne W, Kamimura S, Niemela O, Parkkila S, Yla-Herttuala S, Brittenham GM. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995;96:620–630. doi: 10.1172/JCI118077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kessova IG, Ho YS, Thung S, Cederbaum AI. Alcohol-induced liver injury in mice lacking Cu, Zn-superoxide dismutase. Hepatology. 2003;38:1136–1145. doi: 10.1053/jhep.2003.50450. [DOI] [PubMed] [Google Scholar]
- 33.Kessova IG, Cederbaum AI. Mitochondrial alterations in livers of Sod1−/− mice fed alcohol. Free Radic Biol Med. 2007;42:1470–1480. doi: 10.1016/j.freeradbiomed.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adachi M, Ishii H. Role of mitochondria in alcoholic liver injury. Free Radic Biol Med. 2002;32:487–491. doi: 10.1016/s0891-5849(02)00740-2. [DOI] [PubMed] [Google Scholar]
- 35.Bailey SM, Cunningham CC. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:11–16. doi: 10.1016/s0891-5849(01)00769-9. [DOI] [PubMed] [Google Scholar]
- 36.Wu D, Cederbaum AI. Ethanol-induced apoptosis to stable HepG2 cell lines expressing human cytochrome P-4502E1. Alcohol Clin Exp Res. 1999;23:67–76. [PubMed] [Google Scholar]
- 37.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 38.Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778–790. doi: 10.1053/gast.2003.50087. [DOI] [PubMed] [Google Scholar]
- 39.Lieber CS. Microsomal ethanol-oxidizing system (MEOS): the first 30 years (1968–1998)—a review. Alcohol Clin Exp Res. 1999;23:991–1007. [PubMed] [Google Scholar]
- 40.Lieber CS. Cytochrome P4502E1: its physiological and pathological role. Physiol Rev. 1997;77:517–544. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 41.Lieber CS, DeCarli LM. The role of the hepatic microsomal ethanol oxidizing system (MEOS) for ethanol metabolism in vivo. J Pharmacol Exp Ther. 1972;181:279–287. [PubMed] [Google Scholar]
- 42.Koop DR, Morgan ET, Tarr GE, Coon MJ. Purification and characterization of a unique isozyme of cytochrome P-450 from liver microsomes of ethanol-treated rabbits. J Biol Chem. 1982;257:8472–8480. [PubMed] [Google Scholar]
- 43.Koop DR. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J. 1992;6:724–730. doi: 10.1096/fasebj.6.2.1537462. [DOI] [PubMed] [Google Scholar]
- 44.Raucy JL, Kraner JC, Lasker JM. Bioactivation of halogenated hydrocarbons by cytochrome P4502E1. Crit Rev Toxicol. 1993;23:1–20. doi: 10.3109/10408449309104072. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka E, Terada M, Misawa S. Cytochrome P450 2E1: its clinical and toxicological role. J Clin Pharm Ther. 2000;25:165–175. doi: 10.1046/j.1365-2710.2000.00282.x. [DOI] [PubMed] [Google Scholar]
- 46.Bolt HM, Roos PH, Thier R. The cytochrome P-450 isoenzyme CYP2E1 in the biological processing of industrial chemicals: consequences for occupational and environmental medicine. Int Arch Occup Environ Health. 2003;76:174–185. doi: 10.1007/s00420-002-0407-4. [DOI] [PubMed] [Google Scholar]
- 47.Laethem RM, Balazy M, Falck JR, Laethem CL, Koop DR. Formation of 19(S)-, 19(R)-, and 18(R)-hydroxyeicosatetraenoic acids by alcohol-inducible cytochrome P450 2E1. J Biol Chem. 1993;268:12912–12918. [PubMed] [Google Scholar]
- 48.Yang CS, Yoo JS, Ishizaki H, Hong JY. Cytochrome P450IIE1: roles in nitrosamine metabolism and mechanisms of regulation. Drug Metab Rev. 1990;22:147–159. doi: 10.3109/03602539009041082. [DOI] [PubMed] [Google Scholar]
- 49.Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 50.Girre C, Lucas D, Hispard E, Menez C, Dally S, Menez JF. Assessment of cytochrome P4502E1 induction in alcoholic patients by chlorzoxazone pharmacokinetics. Biochem Pharmacol. 1994;47:1503–1508. doi: 10.1016/0006-2952(94)90524-x. [DOI] [PubMed] [Google Scholar]
- 51.Gorsky LD, Koop DR, Coon MJ. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. J Biol Chem. 1984;259:6812–6817. [PubMed] [Google Scholar]
- 52.Ekstrom G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1) Biochem Pharmacol. 1989;38:1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- 53.Dicker E, Cederbaum AI. Hydroxyl radical generation by microsomes after chronic ethanol consumption. Alcohol Clin Exp Res. 1987;11:309–314. doi: 10.1111/j.1530-0277.1987.tb01314.x. [DOI] [PubMed] [Google Scholar]
- 54.Klein SM, Cohen G, Lieber CS, Cederbaum AI. Increased microsomal oxidation of hydroxyl radical scavenging agents and ethanol after chronic consumption of ethanol. Arch Biochem Biophys. 1983;223:425–432. doi: 10.1016/0003-9861(83)90606-9. [DOI] [PubMed] [Google Scholar]
- 55.Puntarulo S, Cederbaum AI. Increased NADPH-dependent chemiluminescence by microsomes after chronic ethanol consumption. Arch Biochem Biophys. 1988;266:435–445. doi: 10.1016/0003-9861(88)90275-5. [DOI] [PubMed] [Google Scholar]
- 56.Rashba-Step J, Turro NJ, Cederbaum AI. Increased NADPH- and NADH-dependent production of superoxide and hydroxyl radical by microsomes after chronic ethanol treatment. Arch Biochem Biophys. 1993;300:401–408. doi: 10.1006/abbi.1993.1054. [DOI] [PubMed] [Google Scholar]
- 57.Terelius Y, Norsten-Hoog C, Cronholm T, Ingelman-Sundberg M. Acetaldehyde as a substrate for ethanol-inducible cytochrome P450 (CYP2E1) Biochem Biophys Res Commun. 1991;179:689–694. doi: 10.1016/0006-291x(91)91427-e. [DOI] [PubMed] [Google Scholar]
- 58.Wu YS, Salmela KS, Lieber CS. Microsomal acetaldehyde oxidation is negligible in the presence of ethanol. Alcohol Clin Exp Res. 1998;22:1165–1169. [PubMed] [Google Scholar]
- 59.Reinke LA, Lai EK, DuBose CM, McCay PB. Reactive free radical generation in vivo in heart and liver of ethanol-fed rats: correlation with radical formation in vitro. Proc Natl Acad Sci U S A. 1987;84:9223–9227. doi: 10.1073/pnas.84.24.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hansson T, Tindberg N, Ingelman-Sundberg M, Kohler C. Regional distribution of ethanol-inducible cytochrome P450 IIE1 in the rat central nervous system. Neuroscience. 1990;34:451–463. doi: 10.1016/0306-4522(90)90154-v. [DOI] [PubMed] [Google Scholar]
- 61.Koop DR, Chernosky A, Brass EP. Identification and induction of cytochrome P450 2E1 in rat Kupffer cells. J Pharmacol Exp Ther. 1991;258:1072–1076. [PubMed] [Google Scholar]
- 62.Loeper J, Descatoire V, Maurice M, Beaune P, Feldmann G, Larrey D, Pessayre D. Presence of functional cytochrome P-450 on isolated rat hepatocyte plasma membrane. Hepatology. 1990;11:850–858. doi: 10.1002/hep.1840110521. [DOI] [PubMed] [Google Scholar]
- 63.Loeper J, Descatoire V, Maurice M, Beaune P, Belghiti J, Houssin D, Ballet F, Feldmann G, Guengerich FP, Pessayre D. Cytochromes P-450 in human hepatocyte plasma membrane: recognition by several autoantibodies. Gastroenterology. 1993;104:203–216. doi: 10.1016/0016-5085(93)90853-5. [DOI] [PubMed] [Google Scholar]
- 64.Wu D, Cederbaum AI. Presence of functionally active cytochrome P-450IIE1 in the plasma membrane of rat hepatocytes. Hepatology. 1992;15:515–524. doi: 10.1002/hep.1840150326. [DOI] [PubMed] [Google Scholar]
- 65.Eliasson E, Kenna JG. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Mol Pharmacol. 1996;50:573–582. [PubMed] [Google Scholar]
- 66.Bourdi M, Chen W, Peter RM, Martin JL, Buters JT, Nelson SD, Pohl LR. Human cytochrome P450 2E1 is a major autoantigen associated with halothane hepatitis. Chem Res Toxicol. 1996;9:1159–1166. doi: 10.1021/tx960083q. [DOI] [PubMed] [Google Scholar]
- 67.Lytton SD, Helander A, Zhang-Gouillon ZQ, Stokkeland K, Bordone R, Arico S, Albano E, French SW, Ingelman-Sundberg M. autoantibodies against cytochromes P-4502E1 and P-4503A in alcoholics. Mol Pharmacol. 1999;55:223–233. doi: 10.1124/mol.55.2.223. [DOI] [PubMed] [Google Scholar]
- 68.Neve EP, Eliasson E, Pronzato MA, Albano E, Marinari U, Ingelman-Sundberg M. Enzyme-specific transport of rat liver cytochrome P450 to the Golgi apparatus. Arch Biochem Biophys. 1996;333:459–465. doi: 10.1006/abbi.1996.0415. [DOI] [PubMed] [Google Scholar]
- 69.Neve EP, Ingelman-Sundberg M. Molecular basis for the transport of cytochrome P450 2E1 to the plasma membrane. J Biol Chem. 2000;275:17130–17135. doi: 10.1074/jbc.M000957200. [DOI] [PubMed] [Google Scholar]
- 70.Neve EP, Ingelman-Sundberg M. Identification and characterization of a mitochondrial targeting signal in rat cytochrome P450 2E1 (CYP2E1) J Biol Chem. 2001;276:11317–11322. doi: 10.1074/jbc.M008640200. [DOI] [PubMed] [Google Scholar]
- 71.Neve EP, Ingelman-Sundberg M. A soluble NH(2)-terminally truncated catalytically active form of rat cytochrome P450 2E1 targeted to liver mitochondria(1) FEBS Lett. 1999;460:309–314. doi: 10.1016/s0014-5793(99)01361-7. [DOI] [PubMed] [Google Scholar]
- 72.Neve EP, Hidestrand M, Ingelman-Sundberg M. Identification of sequences responsible for intracellular targeting and membrane binding of rat CYP2E1 in yeast. Biochemistry. 2003;42:14566–14575. doi: 10.1021/bi035193s. [DOI] [PubMed] [Google Scholar]
- 73.Robin MA, Anandatheerthavarada HK, Fang JK, Cudic M, Otvos L, Avadhani NG. Mitochondrial targeted cytochrome P450 2E1 (P450 MT5) contains an intact N terminus and requires mitochondrial specific electron transfer proteins for activity. J Biol Chem. 2001;276:24680–24689. doi: 10.1074/jbc.M100363200. [DOI] [PubMed] [Google Scholar]
- 74.Robin MA, Anandatheerthavarada HK, Biswas G, Sepuri NB, Gordon DM, Pain D, Avadhani NG. Bimodal targeting of microsomal CYP2E1 to mitochondria through activation of an N-terminal chimeric signal by cAMP-mediated phosphorylation. J Biol Chem. 2002;277:40583–40593. doi: 10.1074/jbc.M203292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raza H, Prabu SK, Robin MA, Avadhani NG. Elevated mitochondrial cytochrome P450 2E1 and glutathione S-transferase A4–4 in streptozotocin-induced diabetic rats: tissue-specific variations and roles in oxidative stress. Diabetes. 2004;53:185–194. doi: 10.2337/diabetes.53.1.185. [DOI] [PubMed] [Google Scholar]
- 76.Raza H, John A. 4-hydroxynonenal induces mitochondrial oxidative stress, apoptosis and expression of glutathione S-transferase A4–4 and cytochrome P450 2E1 in PC12 cells. Toxicol Appl Pharmacol. 2006;216:309–318. doi: 10.1016/j.taap.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 77.Bai J, Cederbaum AI. Overexpression of CYP2E1 in mitochondria sensitizes HepG2 cells to the toxicity caused by depletion of glutathione. J Biol Chem. 2006;281:5128–5136. doi: 10.1074/jbc.M510484200. [DOI] [PubMed] [Google Scholar]
- 78.Robin MA, Sauvage I, Grandperret T, Descatoire V, Pessayre D, Fromenty B. Ethanol increases mitochondrial cytochrome P450 2E1 in mouse liver and rat hepatocytes. FEBS Lett. 2005;579:6895–6902. doi: 10.1016/j.febslet.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 79.Asai H, Imaoka S, Kuroki T, Monna T, Funae Y. Microsomal ethanol oxidizing system activity by human hepatic cytochrome P450s. J Pharmacol Exp Ther. 1996;277:1004–1009. [PubMed] [Google Scholar]
- 80.Salmela KS, Kessova IG, Tsyrlov IB, Lieber CS. Respective roles of human cytochrome P-4502E1, 1A2, and 3A4 in the hepatic microsomal ethanol oxidizing system. Alcohol Clin Exp Res. 1998;22:2125–2132. [PubMed] [Google Scholar]
- 81.Song BJ, Gelboin HV, Park SS, Yang CS, Gonzalez FJ. Complementary DNA and protein sequences of ethanol-inducible rat and human cytochrome P-450s. Transcriptional and post-transcriptional regulation of the rat enzyme. J Biol Chem. 1986;261:16689–16697. [PubMed] [Google Scholar]
- 82.Park KS, Sohn DH, Veech RL, Song BJ. Translational activation of ethanol-inducible cytochrome P450 (CYP2E1) by isoniazid. Eur J Pharmacol. 1993;248:7–14. doi: 10.1016/0926-6917(93)90019-m. [DOI] [PubMed] [Google Scholar]
- 83.Song BJ. Ethanol-inducible cytochrome P450 (CYP2E1): biochemistry, molecular biology and clinical relevance: 1996 update. Alcohol Clin Exp Res. 1996;20:138A–146A. doi: 10.1111/j.1530-0277.1996.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 84.Raucy JL, Lasker JM, Kraner JC, Salazar DE, Lieber CS, Corcoran GB. Induction of cytochrome P450IIE1 in the obese overfed rat. Mol Pharmacol. 1991;39:275–280. [PubMed] [Google Scholar]
- 85.Hong JY, Pan JM, Gonzalez FJ, Gelboin HV, Yang CS. The induction of a specific form of cytochrome P-450 (P-450j) by fasting. Biochem Biophys Res Commun. 1987;142:1077–1083. doi: 10.1016/0006-291x(87)91525-7. [DOI] [PubMed] [Google Scholar]
- 86.Johansson I, Lindros KO, Eriksson H, Ingelman-Sundberg M. Transcriptional control of CYP2E1 in the perivenous liver region and during starvation. Biochem Biophys Res Commun. 1990;173:331–338. doi: 10.1016/s0006-291x(05)81061-7. [DOI] [PubMed] [Google Scholar]
- 87.Woodcroft KJ, Hafner MS, Novak RF. Insulin signaling in the transcriptional and posttranscriptional regulation of CYP2E1 expression. Hepatology. 2002;35:263–273. doi: 10.1053/jhep.2002.30691. [DOI] [PubMed] [Google Scholar]
- 88.De Waziers I, Garlatti M, Bouguet J, Beaune PH, Barouki R. Insulin down-regulates cytochrome P450 2B and 2E expression at the post-transcriptional level in the rat hepatoma cell line. Mol Pharmacol. 1995;47:474–479. [PubMed] [Google Scholar]
- 89.Peng HM, Coon MJ. Regulation of rabbit cytochrome P450 2E1 expression in HepG2 cells by insulin and thyroid hormone. Mol Pharmacol. 1998;54:740–747. [PubMed] [Google Scholar]
- 90.Woodcroft KJ, Novak RF. Insulin effects on CYP2E1, 2B, 3A, and 4A expression in primary cultured rat hepatocytes. Chem Biol Interact. 1997;107:75–91. doi: 10.1016/s0009-2797(97)00075-6. [DOI] [PubMed] [Google Scholar]
- 91.Bellward GD, Chang T, Rodrigues B, McNeill JH, Maines S, Ryan DE, Levin W, Thomas PE. Hepatic cytochrome P-450j induction in the spontaneously diabetic BB rat. Mol Pharmacol. 1988;33:140–143. [PubMed] [Google Scholar]
- 92.Teschke R, Moreno F, Petrides AS. Hepatic microsomal ethanol oxidizing system (MEOS): respective roles of ethanol and carbohydrates for the enhanced activity after chronic alcohol consumption. Biochem Pharmacol. 1981;30:1745–1751. doi: 10.1016/0006-2952(81)90004-6. [DOI] [PubMed] [Google Scholar]
- 93.Yoo JS, Ning SM, Pantuck CB, Pantuck EJ, Yang CS. Regulation of hepatic microsomal cytochrome P450IIE1 level by dietary lipids and carbohydrates in rats. J Nutr. 1991;121:959–965. doi: 10.1093/jn/121.7.959. [DOI] [PubMed] [Google Scholar]
- 94.Lindros KO, Jarvelainen HA. A new oral low-carbohydrate alcohol liquid diet producing liver lesions: a preliminary account. Alcohol Alcohol. 1998;33:347–353. doi: 10.1093/oxfordjournals.alcalc.a008403. [DOI] [PubMed] [Google Scholar]
- 95.Hong JY, Ning SM, Ma BL, Lee MJ, Pan JM, Yang CS. Roles of pituitary hormones in the regulation of hepatic cytochrome P450IIE1 in rats and mice. Arch Biochem Biophys. 1990;281:132–138. doi: 10.1016/0003-9861(90)90422-u. [DOI] [PubMed] [Google Scholar]
- 96.Eliasson E, Mkrtchian S, Ingelman-Sundberg M. Hormone- and substrate-regulated intracellular degradation of cytochrome P450 (2E1) involving MgATP-activated rapid proteolysis in the endoplasmic reticulum membranes. J Biol Chem. 1992;267:15765–15769. [PubMed] [Google Scholar]
- 97.Hoivik DJ, Manautou JE, Tveit A, Hart SG, Khairallah EA, Cohen SD. Gender-related differences in susceptibility to acetaminophen-induced protein arylation and nephrotoxicity in the CD-1 mouse. Toxicol Appl Pharmacol. 1995;130:257–271. doi: 10.1006/taap.1995.1031. [DOI] [PubMed] [Google Scholar]
- 98.Koop DR, Tierney DJ. Multiple mechanisms in the regulation of ethanol-inducible cytochrome P450IIE1. Bioessays. 1990;12:429–435. doi: 10.1002/bies.950120906. [DOI] [PubMed] [Google Scholar]
- 99.Ronis MJ, Lumpkin CK, Ingelman-Sundberg M, Badger TM. Effects of short-term ethanol and nutrition on the hepatic microsomal monooxygenase system in a model utilizing total enteral nutrition in the rat. Alcohol Clin Exp Res. 1991;15:693–699. doi: 10.1111/j.1530-0277.1991.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 100.Song BJ, Matsunaga T, Hardwick JP, Park SS, Veech RL, Yang CS, Gelboin HV, Gonzalez FJ. Stabilization of cytochrome P450j messenger ribonucleic acid in the diabetic rat. Mol Endocrinol. 1987;1:542–547. doi: 10.1210/mend-1-8-542. [DOI] [PubMed] [Google Scholar]
- 101.Tsutsumi M, Lasker JM, Takahashi T, Lieber CS. In vivo induction of hepatic P4502E1 by ethanol: role of increased enzyme synthesis. Arch Biochem Biophys. 1993;304:209–218. doi: 10.1006/abbi.1993.1341. [DOI] [PubMed] [Google Scholar]
- 102.Johansson I, Ekstrom G, Scholte B, Puzycki D, Jornvall H, Ingelman-Sundberg M. Ethanol-, fasting-, and acetone-inducible cytochromes P-450 in rat liver: regulation and characteristics of enzymes belonging to the IIB and IIE gene subfamilies. Biochemistry. 1988;27:1925–1934. doi: 10.1021/bi00406a019. [DOI] [PubMed] [Google Scholar]
- 103.Song BJ, Veech RL, Park SS, Gelboin HV, Gonzalez FJ. Induction of rat hepatic N-nitrosodimethylamine demethylase by acetone is due to protein stabilization. J Biol Chem. 1989;264:3568–3572. [PubMed] [Google Scholar]
- 104.Eliasson E, Johansson I, Ingelman-Sundberg M. Ligand-dependent maintenance of ethanol-inducible cytochrome P-450 in primary rat hepatocyte cell cultures. Biochem Biophys Res Commun. 1988;150:436–443. doi: 10.1016/0006-291x(88)90539-6. [DOI] [PubMed] [Google Scholar]
- 105.Wu DF, Clejan L, Potter B, Cederbaum AI. Rapid decrease of cytochrome P-450IIE1 in primary hepatocyte culture and its maintenance by added 4-methylpyrazole. Hepatology. 1990;12:1379–1389. doi: 10.1002/hep.1840120620. [DOI] [PubMed] [Google Scholar]
- 106.Roberts BJ, Shoaf SE, Jeong KS, Song BJ. Induction of CYP2E1 in liver, kidney, brain and intestine during chronic ethanol administration and withdrawal: evidence that CYP2E1 possesses a rapid phase half-life of 6 hours or less. Biochem Biophys Res Commun. 1994;205:1064–1071. doi: 10.1006/bbrc.1994.2774. [DOI] [PubMed] [Google Scholar]
- 107.Roberts BJ, Song BJ, Soh Y, Park SS, Shoaf SE. Ethanol induces CYP2E1 by protein stabilization. Role of ubiquitin conjugation in the rapid degradation of CYP2E1. J Biol Chem. 1995;270:29632–29635. doi: 10.1074/jbc.270.50.29632. [DOI] [PubMed] [Google Scholar]
- 108.Roberts BJ, Shoaf SE, Song BJ. Rapid changes in cytochrome P4502E1 (CYP2E1) activity and other P450 isozymes following ethanol withdrawal in rats. Biochem Pharmacol. 1995;49:1665–1673. doi: 10.1016/0006-2952(95)00098-k. [DOI] [PubMed] [Google Scholar]
- 109.Winters DK, Cederbaum AI. Time course characterization of the induction of cytochrome P-450 2E1 by pyrazole and 4-methylpyrazole. Biochim Biophys Acta. 1992;117:15–24. doi: 10.1016/0304-4165(92)90156-o. [DOI] [PubMed] [Google Scholar]
- 110.Wu D, Cederbaum AI. Induction of liver cytochrome P4502E1 by pyrazole and 4-methylpyrazole in neonatal rats. J Pharmacol Exp Ther. 1993;264:1468–1473. [PubMed] [Google Scholar]
- 111.Wu D, Cederbaum AI. Characterization of pyrazole and 4-methylpyrazole induction of cytochrome P4502E1 in rat kidney. J Pharmacol Exp Ther. 1994;270:407–413. [PubMed] [Google Scholar]
- 112.McGehee RE, Jr, Ronis MJ, Cowherd RM, Ingelman-Sundberg M, Badger TM. Characterization of cytochrome P450 2E1 induction in a rat hepatoma FGC-4 cell model by ethanol. Biochem Pharmacol. 1994;48:1823–1833. doi: 10.1016/0006-2952(94)90469-3. [DOI] [PubMed] [Google Scholar]
- 113.Roberts BJ. Evidence of proteasome-mediated cytochrome P-450 degradation. J Biol Chem. 1997;272:9771–9778. doi: 10.1074/jbc.272.15.9771. [DOI] [PubMed] [Google Scholar]
- 114.Huan JY, Streicher JM, Bleyle LA, Koop DR. Proteasome-dependent degradation of cytochromes P450 2E1 and 2B1 expressed in tetracycline-regulated HeLa cells. Toxicol Appl Pharmacol. 2004;199:332–343. doi: 10.1016/j.taap.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 115.Banerjee A, Kocarek TA, Novak RF. Identification of a ubiquitination-Target/Substrate-interaction domain of cytochrome P-450 (CYP) 2E1. Drug Metab Dispos. 2000;28:118–124. [PubMed] [Google Scholar]
- 116.Morishima Y, Peng HM, Lin HL, Hollenberg PF, Sunahara RK, Osawa Y, Pratt WB. Regulation of cytochrome P450 2E1 by heat shock protein 90-dependent stabilization and CHIP-dependent proteasomal degradation. Biochemistry. 2005;44:16333–16340. doi: 10.1021/bi0515570. [DOI] [PubMed] [Google Scholar]
- 117.Goasduff T, Cederbaum AI. CYP2E1 degradation by in vitro reconstituted systems: role of the molecular chaperone hsp90. Arch Biochem Biophys. 2000;379:321–330. doi: 10.1006/abbi.2000.1870. [DOI] [PubMed] [Google Scholar]
- 118.Bardag-Gorce F, Li J, French BA, French SW. Ethanol withdrawal induced CYP2E1 degradation in vivo, blocked by proteasomal inhibitor PS-341. Free Radic Biol Med. 2002;32:17–21. doi: 10.1016/s0891-5849(01)00768-7. [DOI] [PubMed] [Google Scholar]
- 119.Castillo T, Koop DR, Kamimura S, Triadafilopoulos G, Tsukamoto H. Role of cytochrome P-450 2E1 in ethanol-, carbon tetrachloride- and iron-dependent microsomal lipid peroxidation. Hepatology. 1992;16:992–996. doi: 10.1002/hep.1840160423. [DOI] [PubMed] [Google Scholar]
- 120.Ronis MJ, Huang J, Crouch J, Mercado C, Irby D, Valentine CR, Lumpkin CK, Ingelman-Sundberg M, Badger TM. Cytochrome P450 CYP 2E1 induction during chronic alcohol exposure occurs by a two-step mechanism associated with blood alcohol concentrations in rats. J Pharmacol Exp Ther. 1993;264:944–950. [PubMed] [Google Scholar]
- 121.French SW, Morimoto M, Reitz RC, Koop D, Klopfenstein B, Estes K, Clot P, Ingelman-Sundberg M, Albano E. Lipid peroxidation, CYP2E1 and arachidonic acid metabolism in alcoholic liver disease in rats. J Nutr. 1997;127:907S–911S. doi: 10.1093/jn/127.5.907S. [DOI] [PubMed] [Google Scholar]
- 122.Kim ND, Kwak MK, Kim SG. Inhibition of cytochrome P450 2E1 expression by 2-(allylthio)pyrazine, a potential chemoprotective agent: hepatoprotective effects. Biochem Pharmacol. 1997;53:261–269. doi: 10.1016/s0006-2952(96)00647-8. [DOI] [PubMed] [Google Scholar]
- 123.Morimoto M, Hagbjork AL, Nanji AA, Ingelman-Sundberg M, Lindros KO, Fu PC, Albano E, French SW. Role of cytochrome P4502E1 in alcoholic liver disease pathogenesis. Alcohol. 1993;10:459–464. doi: 10.1016/0741-8329(93)90065-v. [DOI] [PubMed] [Google Scholar]
- 124.Morimoto M, Reitz RC, Morin RJ, Nguyen K, Ingelman-Sundberg M, French SW. CYP-2E1 inhibitors partially ameliorate the changes in hepatic fatty acid composition induced in rats by chronic administration of ethanol and a high fat diet. J Nutr. 1995;125:2953–2964. doi: 10.1093/jn/125.12.2953. [DOI] [PubMed] [Google Scholar]
- 125.Albano E, Clot P, Morimoto M, Tomasi A, Ingelman-Sundberg M, French SW. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology. 1996;23:155–163. doi: 10.1002/hep.510230121. [DOI] [PubMed] [Google Scholar]
- 126.Gouillon Z, Lucas D, Li J, Hagbjork AL, French BA, Fu P, Fang C, Ingelman-Sundberg M, Donohue TM, Jr, French SW. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med. 2000;224:302–308. doi: 10.1046/j.1525-1373.2000.22435.x. [DOI] [PubMed] [Google Scholar]
- 127.Aleynik MK, Leo MA, Aleynik SI, Lieber CS. Polyenylphosphatidylcholine opposes the increase of cytochrome P-4502E1 by ethanol and corrects its iron-induced decrease. Alcohol Clin Exp Res. 1999;23:96–100. [PubMed] [Google Scholar]
- 128.Korourian S, Hakkak R, Ronis MJ, Shelnutt SR, Waldron J, Ingelman-Sundberg M, Badger TM. Diet and risk of ethanol-induced hepatotoxicity: carbohydrate-fat relationships in rats. Toxicol Sci. 1999;47:110–117. doi: 10.1093/toxsci/47.1.110. [DOI] [PubMed] [Google Scholar]
- 129.Li J, French BA, Riley N, Bardag-Gorce F, Fu P, French SW. Oral low-carbohydrate alcohol liquid diet induces experimental steatohepatitis in the rat. Exp Mol Pathol. 2001;71:132–136. doi: 10.1006/exmp.2001.2396. [DOI] [PubMed] [Google Scholar]
- 130.Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36:122–134. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- 131.Bai J, Cederbaum AI. Adenovirus mediated overexpression of CYP2E1 increases sensitivity of HepG2 cells to acetaminophen induced cytotoxicity. Mol Cell Biochem. 2004;262:165–176. doi: 10.1023/b:mcbi.0000038232.61760.9e. [DOI] [PubMed] [Google Scholar]
- 132.Bai J, Cederbaum AI. Adenovirus-mediated expression of CYP2E1 produces liver toxicity in mice. Toxicol Sci. 2006;91:365–371. doi: 10.1093/toxsci/kfj165. [DOI] [PubMed] [Google Scholar]
- 133.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 134.Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, Gabele E, Rusyn I, Yamashina S, Froh M, Adachi Y, Iimuro Y, Bradford BU, Smutney OM, Connor HD, Mason RP, Goyert SM, Peters JM, Gonzalez FJ, Samulski RJ, Thurman RG. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544–1549. doi: 10.1016/s0891-5849(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 135.Kono H, Bradford BU, Yin M, Sulik KK, Koop DR, Peters JM, Gonzalez FJ, McDonald T, Dikalova A, Kadiiska MB, Mason RP, Thurman RG. CYP2E1 is not involved in early alcohol-induced liver injury. Am J Physiol. 1999;277:G1259–G1267. doi: 10.1152/ajpgi.1999.277.6.G1259. [DOI] [PubMed] [Google Scholar]
- 136.Koop DR, Klopfenstein B, Iimuro Y, Thurman RG. Gadolinium chloride blocks alcohol-dependent liver toxicity in rats treated chronically with intragastric alcohol despite the induction of CYP2E1. Mol Pharmacol. 1997;51:944–950. doi: 10.1124/mol.51.6.944. [DOI] [PubMed] [Google Scholar]
- 137.Bardag-Gorce F, Yuan QX, Li J, French BA, Fang C, Ingelman-Sundberg M, French SW. The effect of ethanol-induced cytochrome p4502E1 on the inhibition of proteasome activity by alcohol. Biochem Biophys Res Commun. 2000;279:23–29. doi: 10.1006/bbrc.2000.3889. [DOI] [PubMed] [Google Scholar]
- 138.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bradford BU, Kono H, Isayama F, Kosyk O, Wheeler MD, Akiyama TE, Bleye L, Krausz KW, Gonzalez FJ, Koop DR, Rusyn I. Cytochrome P450 CYP2E1, but not nicotinamide adenine dinucleotide phosphate oxidase, is required for ethanol-induced oxidative DNA damage in rodent liver. Hepatology. 2005;41:336–344. doi: 10.1002/hep.20532. [DOI] [PubMed] [Google Scholar]
- 140.Nouso K, Thorgeirsson SS, Battula N. Stable expression of human cytochrome P450IIE1 in mammalian cells: metabolic activation of nitrosodimethylamine and formation of adducts with cellular DNA. Cancer Res. 1992;52:1796–1800. [PubMed] [Google Scholar]
- 141.Lin HL, Roberts ES, Hollenberg PF. Heterologous expression of rat P450 2E1 in a mammalian cell line: in situ metabolism and cytotoxicity of N-nitrosodimethylamine. Carcinogenesis. 1998;19:321–329. doi: 10.1093/carcin/19.2.321. [DOI] [PubMed] [Google Scholar]
- 142.Lin HL, Parsels LA, Maybaum J, Hollenberg PF. N-Nitrosodimethylamine-mediated cytotoxicity in a cell line expressing P450 2E1: evidence for apoptotic cell death. Toxicol Appl Pharmacol. 1999;157:117–124. doi: 10.1006/taap.1999.8651. [DOI] [PubMed] [Google Scholar]
- 143.Schmalix WA, Barrenscheen M, Landsiedel R, Janzowski C, Eisenbrand G, Gonzalez F, Eliasson E, Ingelman-Sundberg M, Perchermeier M, Greim H, et al. Stable expression of human cytochrome P450 2E1 in V79 Chinese hamster cells. Eur J Pharmacol. 1995;293:123–131. doi: 10.1016/0926-6917(95)00008-9. [DOI] [PubMed] [Google Scholar]
- 144.Mapoles J, Berthou F, Alexander A, Simon F, Menez JF. Mammalian PC-12 cell genetically engineered for human cytochrome P450 2E1 expression. Eur J Biochem. 1993;214:735–745. doi: 10.1111/j.1432-1033.1993.tb17975.x. [DOI] [PubMed] [Google Scholar]
- 145.Dai Y, Rashba-Step J, Cederbaum AI. Stable expression of human cytochrome P4502E1 in HepG2 cells: characterization of catalytic activities and production of reactive oxygen intermediates. Biochemistry. 1993;32:6928–6937. doi: 10.1021/bi00078a017. [DOI] [PubMed] [Google Scholar]
- 146.Nozaki I, Tsuji T, Sakaguchi M, Inoue Y, Hirai R, Andou A, Miyazaki M, Shimizu N, Namba M. Establishment of a human hepatoma cell line, HLE/2E1, suitable for detection of p450 2E1-related cytotoxicity. In Vitro Cell Dev Biol Anim. 2000;36:566–570. doi: 10.1007/BF02577524. [DOI] [PubMed] [Google Scholar]
- 147.Takahashi S, Takahashi T, Mizobuchi S, Matsumi M, Yokoyama M, Morita K, Miyazaki M, Namba M, Akagi R, Sassa S. CYP2E1 overexpression up-regulates both non-specific delta-aminolevulinate synthase and heme oxygenase-1 in the human hepatoma cell line HLE/2E1. Int J Mol Med. 2003;11:57–62. [PubMed] [Google Scholar]
- 148.Gong P, Cederbaum AI, Nieto N. Increased expression of cytochrome P450 2E1 induces heme oxygenase-1 through ERK MAPK pathway. J Biol Chem. 2003;278:29693–29700. doi: 10.1074/jbc.M304728200. [DOI] [PubMed] [Google Scholar]
- 149.Huan JY, Koop DR. Tightly regulated and inducible expression of rabbit CYP2E1 using a tetracycline-controlled expression system. Drug Metab Dispos. 1999;27:549–554. [PubMed] [Google Scholar]
- 150.Yang MX, Cederbaum AI. Characterization of cytochrome P4502E1 turnover in transfected HepG2 cells expressing human CYP2E1. Arch Biochem Biophys. 1997;341:25–33. doi: 10.1006/abbi.1997.9907. [DOI] [PubMed] [Google Scholar]
- 151.Bernauer U, Glatt H, Heinrich-Hirsch B, Liu Y, Muckel E, Vieth B, Gundert-Remy U. Heterologous expression of mouse cytochrome P450 2e1 in V79 cells: construction and characterisation of the cell line and comparison with V79 cell lines stably expressing rat P450 2E1 and human P450 2E1. Altern Lab Anim. 2003;31:21–30. doi: 10.1177/026119290303100104. [DOI] [PubMed] [Google Scholar]
- 152.Patten CJ, Ishizaki H, Aoyama T, Lee M, Ning SM, Huang W, Gonzalez FJ, Yang CS. Catalytic properties of the human cytochrome P450 2E1 produced by cDNA expression in mammalian cells. Arch Biochem Biophys. 1992;299:163–171. doi: 10.1016/0003-9861(92)90258-x. [DOI] [PubMed] [Google Scholar]
- 153.Chen Q, Cederbaum AI. Cytotoxicity and apoptosis produced by cytochrome P450 2E1 in Hep G2 cells. Mol Pharmacol. 1998;53:638–648. doi: 10.1124/mol.53.4.638. [DOI] [PubMed] [Google Scholar]
- 154.Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 155.Kessova I, Cederbaum AI. CYP2E1: biochemistry, toxicology, regulation and function in ethanol-induced liver injury. Curr Mol Med. 2003;3:509–518. doi: 10.2174/1566524033479609. [DOI] [PubMed] [Google Scholar]
- 156.Jimenez-Lopez JM, Cederbaum AI. CYP2E1-dependent oxidative stress and toxicity: role in ethanol-induced liver injury. Expert Opin Drug Metab Toxicol. 2005;1:671–685. doi: 10.1517/17425255.1.4.671. [DOI] [PubMed] [Google Scholar]
- 157.Wu D, Cederbaum AI. Removal of glutathione produces apoptosis and necrosis in HepG2 cells overexpressing CYP2E1. Alcohol Clin Exp Res. 2001;25:619–628. [PubMed] [Google Scholar]
- 158.Gong P, Cederbaum AI. Nrf2 is increased by CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology. 2006;43:144–153. doi: 10.1002/hep.21004. [DOI] [PubMed] [Google Scholar]
- 159.Cederbaum AI. Cytochrome P450 2E1-dependent oxidant stress and upregulation of anti-oxidant defense in liver cells. J Gastroenterol Hepatol. 2006;21(Suppl 3):S22–S25. doi: 10.1111/j.1440-1746.2006.04595.x. [DOI] [PubMed] [Google Scholar]
- 160.Nieto N, Friedman SL, Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology. 2002;35:62–73. doi: 10.1053/jhep.2002.30362. [DOI] [PubMed] [Google Scholar]
- 161.Nieto N, Friedman SL, Cederbaum AI. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. J Biol Chem. 2002;277:9853–9864. doi: 10.1074/jbc.M110506200. [DOI] [PubMed] [Google Scholar]
- 162.Roman J, Colell A, Blasco C, Caballeria J, Pares A, Rodes J, Fernandez-Checa JC. Differential role of ethanol and acetaldehyde in the induction of oxidative stress in HEP G2 cells: effect on transcription factors AP-1 and NF-kappaB. Hepatology. 1999;30:1473–1480. doi: 10.1002/hep.510300623. [DOI] [PubMed] [Google Scholar]
- 163.Suh SK, Hood BL, Kim BJ, Conrads TP, Veenstra TD, Song BJ. Identification of oxidized mitochondrial proteins in alcohol-exposed human hepatoma cells and mouse liver. Proteomics. 2004;4:3401–3412. doi: 10.1002/pmic.200400971. [DOI] [PubMed] [Google Scholar]
- 164.Kim BJ, Hood BL, Aragon RA, Hardwick JP, Conrads TP, Veenstra TD, Song BJ. Increased oxidation and degradation of cytosolic proteins in alcohol-exposed mouse liver and hepatoma cells. Proteomics. 2006;6:1250–1260. doi: 10.1002/pmic.200500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bardag-Gorce F, French BA, Nan L, Song H, Nguyen SK, Yong H, Dede J, French SW. CYP2E1 induced by ethanol causes oxidative stress, proteasome inhibition and cytokeratin aggresome (Mallory body-like) formation. Exp Mol Pathol. 2006;81:191–201. doi: 10.1016/j.yexmp.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 166.Bardag-Gorce F, French BA, Dedes J, Li J, French SW. Gene expression patterns of the liver in response to alcohol: in vivo and in vitro models compared. Exp Mol Pathol. 2006;80:241–251. doi: 10.1016/j.yexmp.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 167.Wen F, Abdalla MY, Aloman C, Xiang J, Ahmad IM, Walewski J, McCormick ML, Brown KE, Branch AD, Spitz DR, Britigan BE, Schmidt WN. Increased prooxidant production and enhanced susceptibility to glutathione depletion in HepG2 cells co-expressing HCV core protein and CYP2E1. J Med Virol. 2004;72:230–240. doi: 10.1002/jmv.10567. [DOI] [PubMed] [Google Scholar]
- 168.Kim WH, Hong F, Jaruga B, Hu Z, Fan S, Liang TJ, Gao B. Additive activation of hepatic NF-kappaB by ethanol and hepatitis B protein X (HBX) or HCV core protein: involvement of TNF-alpha receptor 1-independent and -dependent mechanisms. FASEB J. 2001;15:2551–2553. doi: 10.1096/fj.01-0217. [DOI] [PubMed] [Google Scholar]
- 169.Holownia A, Braszko JJ. Acetaminophen alters microsomal ryanodine Ca2+ channel in HepG2 cells overexpressing CYP2E1. Biochem Pharmacol. 2004;68:513–521. doi: 10.1016/j.bcp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 170.Wu H, Cai P, Clemens DL, Jerrells TR, Ansari GA, Kaphalia BS. Metabolic basis of ethanol-induced cytotoxicity in recombinant HepG2 cells: role of nonoxidative metabolism. Toxicol Appl Pharmacol. 2006;216:238–247. doi: 10.1016/j.taap.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 171.Pastorino JG, Hoek JB. Ethanol potentiates tumor necrosis factor-alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology. 2000;31:1141–1152. doi: 10.1053/he.2000.7013. [DOI] [PubMed] [Google Scholar]
- 172.Pastorino JG, Shulga N, Hoek JB. TNF-alpha-induced cell death in ethanol-exposed cells depends on p38 MAPK signaling but is independent of Bid and caspase-8. Am J Physiol Gastrointest Liver Physiol. 2003;285:G503–G516. doi: 10.1152/ajpgi.00442.2002. [DOI] [PubMed] [Google Scholar]
- 173.Osna NA, Clemens DL, Donohue TM., Jr Interferon gamma enhances proteasome activity in recombinant Hep G2 cells that express cytochrome P4502E1: modulation by ethanol. Biochem Pharmacol. 2003;66:697–710. doi: 10.1016/s0006-2952(03)00252-1. [DOI] [PubMed] [Google Scholar]
- 174.Osna NA, Clemens DL, Donohue TM., Jr Ethanol metabolism alters interferon gamma signaling in recombinant HepG2 cells. Hepatology. 2005;42:1109–1117. doi: 10.1002/hep.20909. [DOI] [PubMed] [Google Scholar]
- 175.Osna NA, White RL, Todero S, McVicker BL, Thiele GM, Clemens DL, Tuma DJ, Donohue TM., Jr Ethanol-induced oxidative stress suppresses generation of peptides for antigen presentation by hepatoma cells. Hepatology. 2007;45:53–61. doi: 10.1002/hep.21442. [DOI] [PubMed] [Google Scholar]
- 176.Donohue TM, Osna NA, Clemens DL. Recombinant Hep G2 cells that express alcohol dehydrogenase and cytochrome P450 2E1 as a model of ethanol-elicited cytotoxicity. Int J Biochem Cell Biol. 2006;38:92–101. doi: 10.1016/j.biocel.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 177.Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res. 2005;569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 178.Gonzalez FJ. The 2006 Bernard B. Brodie Award Lecture. Cyp2e1. Drug Metab Dispos. 2007;35:1–8. doi: 10.1124/dmd.106.012492. [DOI] [PubMed] [Google Scholar]
- 179.Wong FW, Chan WY, Lee SS. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998;153:109–118. doi: 10.1006/taap.1998.8547. [DOI] [PubMed] [Google Scholar]
- 180.Bondoc FY, Bao Z, Hu WY, Gonzalez FJ, Wang Y, Yang CS, Hong JY. Acetone catabolism by cytochrome P450 2E1: studies with CYP2E1-null mice. Biochem Pharmacol. 1999;58:461–463. doi: 10.1016/s0006-2952(99)00111-2. [DOI] [PubMed] [Google Scholar]
- 181.Powley MW, Carlson GP. Hepatic and pulmonary microsomal benzene metabolism in CYP2E1 knockout mice. Toxicology. 2001;169:187–194. doi: 10.1016/s0300-483x(01)00519-4. [DOI] [PubMed] [Google Scholar]
- 182.Chilakapati J, Korrapati MC, Shankar K, Hill RA, Warbritton A, Latendresse JR, Mehendale HM. Role of CYP2E1 and saturation kinetics in the bioactivation of thioacetamide: Effects of diet restriction and phenobarbital. Toxicol Appl Pharmacol. 2007;219:72–84. doi: 10.1016/j.taap.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 183.Kim D, Ghanayem BI. Comparative metabolism and disposition of trichloroethylene in Cyp2e1−/−and wild-type mice. Drug Metab Dispos. 2006;34:2020–2027. doi: 10.1124/dmd.106.010538. [DOI] [PubMed] [Google Scholar]
- 184.Hoffler U, Ghanayem BI. Increased bioaccumulation of urethane in CYP2E1−/− versus CYP2E1+/+ mice. Drug Metab Dispos. 2005;33:1144–1150. doi: 10.1124/dmd.105.003806. [DOI] [PubMed] [Google Scholar]
- 185.El Hadri L, Chanas B, Ghanayem BI. Comparative metabolism of methacrylonitrile and acrylonitrile to cyanide using cytochrome P4502E1 and microsomal epoxide hydrolase-null mice. Toxicol Appl Pharmacol. 2005;205:116–125. doi: 10.1016/j.taap.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 186.Hoffler U, El-Masri HA, Ghanayem BI. Cytochrome P450 2E1 (CYP2E1) is the principal enzyme responsible for urethane metabolism: comparative studies using CYP2E1-null and wild-type mice. J Pharmacol Exp Ther. 2003;305:557–564. doi: 10.1124/jpet.102.049072. [DOI] [PubMed] [Google Scholar]
- 187.Carlson GP. Comparison of the susceptibility of wild-type and CYP2E1 knockout mice to the hepatotoxic and pneumotoxic effects of styrene and styrene oxide. Toxicol Lett. 2004;150:335–339. doi: 10.1016/j.toxlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 188.Wolf KK, Wood SG, Bement JL, Sinclair PR, Wrighton SA, Jeffery E, Gonzalez FJ, Sinclair JF. Role of mouse CYP2E1 in the O-hydroxylation of p-nitrophenol: comparison of activities in hepatic microsomes from Cyp2e1(−/−) and wild-type mice. Drug Metab Dispos. 2004;32:681–684. doi: 10.1124/dmd.32.7.681. [DOI] [PubMed] [Google Scholar]
- 189.Zaher H, Buters JT, Ward JM, Bruno MK, Lucas AM, Stern ST, Cohen SD, Gonzalez FJ. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol Appl Pharmacol. 1998;152:193–199. doi: 10.1006/taap.1998.8501. [DOI] [PubMed] [Google Scholar]
- 190.Wolf KK, Wood SG, Allard JL, Hunt JA, Gorman N, Walton-Strong BW, Szakacs JG, Duan SX, Hao Q, Court MH, von Moltke LL, Greenblatt DJ, Kostrubsky V, Jeffery EH, Wrighton SA, Gonzalez FJ, Sinclair PR, Sinclair JF. Role of CYP3A and CYP2E1 in alcohol-mediated increases in acetaminophen hepatotoxicity: comparison of wild-type and Cyp2e1(−/−) mice. Drug Metab Dispos. 2007;35:1223–1231. doi: 10.1124/dmd.107.014738. [DOI] [PubMed] [Google Scholar]
- 191.Cheung C, Yu AM, Ward JM, Krausz KW, Akiyama TE, Feigenbaum L, Gonzalez FJ. The cyp2e1-humanized transgenic mouse: role of cyp2e1 in acetaminophen hepatotoxicity. Drug Metab Dispos. 2005;33:449–457. doi: 10.1124/dmd.104.002402. [DOI] [PubMed] [Google Scholar]
- 192.McClain CJ, Song Z, Barve SS, Hill DB, Deaciuc I. Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;287:G497–G502. doi: 10.1152/ajpgi.00171.2004. [DOI] [PubMed] [Google Scholar]
- 193.Nagy LE. Molecular aspects of alcohol metabolism: transcription factors involved in early ethanol-induced liver injury. Annu Rev Nutr. 2004;24:55–78. doi: 10.1146/annurev.nutr.24.012003.132258. [DOI] [PubMed] [Google Scholar]
- 194.Hoek JB, Pastorino JG. Cellular signaling mechanisms in alcohol-induced liver damage. Semin Liver Dis. 2004;24:257–272. doi: 10.1055/s-2004-832939. [DOI] [PubMed] [Google Scholar]
- 195.Thurman RG. Mechanisms of hepatic toxicity II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 196.Honchel R, Ray MB, Marsano L, Cohen D, Lee E, Shedlofsky S, McClain CJ. Tumor necrosis factor in alcohol enhanced endotoxin liver injury. Alcohol Clin Exp Res. 1992;16:665–669. doi: 10.1111/j.1530-0277.1992.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 197.Kamimura S, Tsukamoto H. Cytokine gene expression by Kupffer cells in experimental alcoholic liver disease. Hepatology. 1995;22:1304–1309. [PubMed] [Google Scholar]
- 198.Nanji AA, Zhao S, Sadrzadeh SM, Waxman DJ. Use of reverse transcription-polymerase chain reaction to evaluate in vivo cytokine gene expression in rats fed ethanol for long periods. Hepatology. 1994;19:1483–1487. [PubMed] [Google Scholar]
- 199.Barve S, Joshi-Barve S, Song Z, Hill D, Hote P, Deaciuc I, McClain C. Interactions of cytokines, S-Adenosylmethionine, and S-Adenosylhomocysteine in alcohol-induced liver disease and immune suppression. J Gastroenterol Hepatol. 2006;21(Suppl 3):S38–S42. doi: 10.1111/j.1440-1746.2006.04590.x. [DOI] [PubMed] [Google Scholar]
- 200.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 201.Tsukamoto H. How is the liver primed or sensitized for alcoholic liver disease? Alcohol Clin Exp Res. 2001;25:171S–181S. doi: 10.1097/00000374-200105051-00029. [DOI] [PubMed] [Google Scholar]
- 202.Purohit V, Brenner DA. Mechanisms of alcohol-induced hepatic fibrosis: a summary of the Ron Thurman Symposium. Hepatology. 2006;43:872–878. doi: 10.1002/hep.21107. [DOI] [PubMed] [Google Scholar]
- 203.Bradham CA, Plumpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387–G392. doi: 10.1152/ajpgi.1998.275.3.G387. [DOI] [PubMed] [Google Scholar]
- 204.Bradham CA, Qian T, Streetz K, Trautwein C, Brenner DA, Lemasters JJ. The mitochondrial permeability transition is required for tumor necrosis factor alpha-mediated apoptosis and cytochrome c release. Mol Cell Biol. 1998;18:6353–6364. doi: 10.1128/mcb.18.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Colell A, Garcia-Ruiz C, Miranda M, Ardite E, Mari M, Morales A, Corrales F, Kaplowitz N, Fernandez-Checa JC. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–1551. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 206.Song Z, Zhou Z, Uriarte S, Wang L, Kang YJ, Chen T, Barve S, McClain CJ. S-adenosylhomocysteine sensitizes to TNF-alpha hepatotoxicity in mice and liver cells: a possible etiological factor in alcoholic liver disease. Hepatology. 2004;40:989–997. doi: 10.1002/hep.20412. [DOI] [PubMed] [Google Scholar]
- 207.Joshi-Barve S, Barve SS, Butt W, Klein J, McClain CJ. Inhibition of proteasome function leads to NF-kappaB-independent IL-8 expression in human hepatocytes. Hepatology. 2003;38:1178–1187. doi: 10.1053/jhep.2003.50470. [DOI] [PubMed] [Google Scholar]
- 208.Liu H, Lo CR, Jones BE, Pradhan Z, Srinivasan A, Valentino KL, Stockert RJ, Czaja MJ. Inhibition of c-Myc expression sensitizes hepatocytes to tumor necrosis factor-induced apoptosis and necrosis. J Biol Chem. 2000;275:40155–40162. doi: 10.1074/jbc.M001565200. [DOI] [PubMed] [Google Scholar]
- 209.Liu H, Lo CR, Czaja MJ. NF-kappaB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology. 2002;35:772–778. doi: 10.1053/jhep.2002.32534. [DOI] [PubMed] [Google Scholar]
- 210.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 211.Liu H, Jones BE, Bradham C, Czaja MJ. Increased cytochrome P-450 2E1 expression sensitizes hepatocytes to c-Jun-mediated cell death from TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2002;282:G257–G266. doi: 10.1152/ajpgi.00304.2001. [DOI] [PubMed] [Google Scholar]
- 212.Lu Y, Wang X, Cederbaum AI. Lipopolysaccharide-induced liver injury in rats treated with the CYP2E1 inducer pyrazole. Am J Physiol Gastrointest Liver Physiol. 2005;289:G308–G319. doi: 10.1152/ajpgi.00054.2005. [DOI] [PubMed] [Google Scholar]
- 213.Lu Y, Cederbaum AI. Enhancement by pyrazole of lipopolysaccharide-induced liver injury in mice: role of cytochrome P450 2E1 and 2A5. Hepatology. 2006;44:263–274. doi: 10.1002/hep.21241. [DOI] [PubMed] [Google Scholar]
- 214.Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–210. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 215.Diehl AM. Lessons from animal models of NASH. Hepatol Res. 2005;33:138–144. doi: 10.1016/j.hepres.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 216.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 217.Angulo P, Lindor KD. Insulin resistance and mitochondrial abnormalities in NASH: a cool look into a burning issue. Gastroenterology. 2001;120:1281–1285. doi: 10.1053/gast.2001.23591. [DOI] [PubMed] [Google Scholar]
- 218.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 219.Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1135–G1139. doi: 10.1152/ajpgi.2001.281.5.G1135. [DOI] [PubMed] [Google Scholar]
- 220.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 221.Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, Crabb DW. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544–550. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 222.Orellana M, Rodrigo R, Varela N, Araya J, Poniachik J, Csendes A, Smok G, Videla LA. Relationship between in vivo chlorzoxazone hydroxylation, hepatic cytochrome P450 2E1 content and liver injury in obese non-alcoholic fatty liver disease patients. Hepatol Res. 2006;34:57–63. doi: 10.1016/j.hepres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 223.Dey A, Cederbaum AI. Induction of cytochrome P450 2E1 promotes liver injury in ob/ob mice. Hepatology. 2007;45:1355–1365. doi: 10.1002/hep.21603. [DOI] [PubMed] [Google Scholar]
- 224.Jeong KS, Lee IJ, Roberts BJ, Soh Y, Yoo JK, Lee JW, Song BJ. Transcriptional inhibition of cytochrome P4502E1 by a synthetic compound, YH439. Arch Biochem Biophys. 1996;326:137–144. doi: 10.1006/abbi.1996.0057. [DOI] [PubMed] [Google Scholar]
- 225.McCarty MF. Inhibition of CYP2E1 with natural agents may be a feasible strategy for minimizing the hepatotoxicity of ethanol. Med Hypotheses. 2001;56:8–11. doi: 10.1054/mehy.1999.1015. [DOI] [PubMed] [Google Scholar]
- 226.Mathews JM, Etheridge AS, Raymer JH, Black SR, Pulliam DW, Jr, Bucher JR. Selective inhibition of cytochrome P450 2E1 in vivo and in vitro with trans-1,2-dichloroethylene. Chem Res Toxicol. 1998;11:778–785. doi: 10.1021/tx970227g. [DOI] [PubMed] [Google Scholar]