Abstract
Previous investigations demonstrated that repeated stresses before an ethanol exposure sensitize ethanol withdrawal-induced anxiety-like behavior (‘anxiety’). In addition to activating the hypothalamic–pituitary–adrenal axis, acute stress also elevates cytokines in brain. Initially, to test possible cytokine involvement in this stress/withdrawal protocol, cytokines were increased in brain with 2 weekly repeated lipopolysaccharide (LPS) administrations (1000 μ/kg) (LPS/withdrawal protocol) or with twice weekly intracerebroventricular (i.c.v.) administrations of the cytokines IL-1β, CCL2 (MCP-1) or TNFα (cytokine/withdrawal protocol) before exposure and withdrawal from a 5-day cycle of chronic ethanol diet. Both protocols sensitized withdrawal-induced anxiety and confirm cytokine involvement in the sensitized anxiety response. Testing of various doses of LPS (16–1000 μg/kg) and TNFα (3–100 ng, i.c.v.) demonstrated the dose-related nature of these protocols to sensitize withdrawal-induced anxiety. The sensitized anxiety was not produced by a single 5-day ethanol diet cycle or by repeated LPS or cytokine treatments alone. Likewise, sensitized anxiety in these protocols could not be attributed to differences in ethanol ingestion. When challenged with a subsequent re-exposure to a 5-day ethanol diet cycle 16 days after completion of the LPS/withdrawal or cytokine/withdrawal protocols, an increase in withdrawal-induced anxiety was observed—an indication of induction of an underlying persistent adaptive change. Finally, just as found previously with the stress/withdrawal protocol, administration of the benzodiazepine receptor antagonist flumazenil before the LPS or TNF treatments prevented anxiety sensitization. Together, these findings indicate that increased cytokine activity induces adaptive change that supports sensitization of ethanol withdrawal-induced anxiety that may be linked to GABAA-receptor function.
Keywords: cytokine, ethanol, anxiety, flumazenil, sensitization, withdrawal
INTRODUCTION
Based upon the observation that symptoms of withdrawal increase over time in alcoholics (Ballenger and Post, l978), McCown and Breese (1990) found that repeated withdrawals from chronic ethanol diet facilitated kindling of seizure activity from the inferior colliculus—a finding consistent with induction of cumulative adaptation by the repeated ethanol exposures. Subsequent experiments explored whether emotional symptoms were sensitized by repeated withdrawals (Overstreet et al, 2002). In these latter investigations, just three withdrawals from 5-day cycles of chronic ethanol diet sensitized anxiety-like behavior (‘anxiety’) during the final withdrawal (Overstreet et al, 2002; Breese et al, 2004). Because this sensitization was not observed after exposure to continuous ethanol diet for the period the cycled rats experienced ethanol, it was hypothesized that the repeated withdrawals caused a cumulative adaptation that supported sensitization of this negative emotional response (Overstreet et al, 2002; Breese et al, 2005a).
Because reports implied that stress increases the risk of relapse in alcoholics (Breese et al, 2005c; Brown et al, 1995; Pohorecky, 1991; Sinha, 2001), the ability of restraint stress (substituted for the initial withdrawals in the repeated withdrawal protocol) to sensitize anxiety-like behavior following withdrawal from a single 5-day exposure to ethanol diet (stress/withdrawal protocol) was tested. Breese et al (2004) reported that this repeated exposure to restraint stress followed by a limited ethanol diet regimen did sensitize anxiety upon withdrawal. Importantly, withdrawal from a single 5-day exposure to the 4.5% ethanol liquid diet itself did not elevate anxiety (Breese et al, 2004). Therefore, since stress alone was not able to sensitize anxiety, a synergistic interaction between the previous stresses and the subsequent chronic ethanol exposure was proposed to account for this sensitization.
Based upon evidence that stress and chronic ethanol had common influences on the central nervous system to influence emotional behavior associated with withdrawal from chronic ethanol, mechanisms that might explain this common influence were sought. One overlapping aspect of acute stress (Carrasco and Van de Kar, 2003; Vale et al, 1981; Rivest and Rivier, 1994) and acute ethanol (Rivier et al, 1984; Rivier, 1996) is an effect on the hypothalamic–pituitary–adrenal (HPA) axis. Although early studies implicated cytokines in the release of CRF and HPA axis activation (Berkenbosch et al, 1987; Sapolsky et al, 1987; Takemura et al, 1997; Turnbull et al, 1997; see review in Turnbull and Rivier, 1995, 1999a, b), the possibility that cytokines might be involved in the adaptive change responsible for withdrawal-induced anxiety following the stress/withdrawal protocol had not been considered. Nonetheless, previous work demonstrated that chronic ethanol exposure increased cytokines in brain (Kiefer et al, 2002; Emanuele et al, 2005) and affected cytokine action on the HPA axis (Ogilvie et al, 1998). Therefore, the possibility that cytokines could be involved in the adaptive change responsible for sensitization of anxiety during withdrawal was explored with the use of LPS.
Because the stress/withdrawal protocol sensitized anxiety following withdrawal from a single re-exposure to 5 days of ethanol (Overstreet et al, 2002; Breese et al, 2004), experiments were conducted to determine if LPS (which is known to increase brain cytokines—Breder et al, 1994; Buttini et al, 1997; Hagan et al, 1993; Hillhouse and Mosley, 1993; Ilyin et al, 1998; Matalka et al, 2005; Quan et al, 1994; unpublished data) administered repeatedly before a single ethanol diet exposure would produce a similar effect as stress to sensitize withdrawal-induced anxiety. In this report, new data demonstrate that these two systemic administrations of varying LPS doses at weekly intervals before a single 5-day exposure to ethanol diet sensitized withdrawal-induced anxiety. Subsequently, to address the possibility that a central action of cytokines by the repeated LPS exposure could be responsible for its sensitization of withdrawalinduced anxiety, experiments were conducted to determine whether repeated central administration of IL-1β, CCL2 (MCP-1), or TNF-α before a single exposure to chronic ethanol would, like LPS, sensitize withdrawal-induced anxiety. Finally, given that flumazenil administered during the repeated withdrawal and repeated stress/withdrawal protocols prevented the cumulative adaptation that supported withdrawal-induced anxiety (Breese et al, 2004; Knapp et al, 2004, 2005; Overstreet et al, 2003), we examined whether administration of this drug before the initial exposures to LPS or TNF-α would have a similar inhibitory effect on anxiety during withdrawal and whether the flumazenil would prevent the persistent sensitivity to withdrawal from a future re-exposure to 5 days of ethanol diet (Overstreet et al, 2003; Breese et al, 2004; Knapp et al, 2004).
METHODS
Animals
Male Sprague–Dawley rats (Charles-River, Raleigh, NC) approaching 6 weeks of age (180–200 g) were initially housed in groups of three or four and fed normal chow for several days to adapt to the animal facility conditions (light:dark cycle of 12:12, with lights on at 0700 hours). Subsequently, the rats were individually housed and exposed to control and ethanol-containing diets. For implanting injection cannulae into the ventricles for intracerebroventricular (i.c.v.) administration, the rats were anesthetized with pentobarbital sodium (50 mg/kg). The surgical procedure involved opening the skin over the skull, drilling a small hole in the skull directly over the ventricle, placing the cannulae stereotaxically over the ventricle, and placing screws in the skull before addition of cranioplastic cement to secure the entire head apparatus. Post-surgical care required to minimize discomfort included application of local anesthetic to the wound area along with antibiotic cream and administration of sweetened pediatric Tylenol (100–300 mg/kg acetaminophen per day) in their drinking water for 2 days after surgery. It was confirmed at the end of the procedure for each rat that the injector needle tip was in the ventricle. The Institutional Animal Care & Use Committee (IACUC) at the University of North Carolina approved all procedures described.
Ethanol and Control Diets
After placement in individual cages, rats received a lactalbumin/dextrose-based nutritionally complete liquid diet (with concentrations of vitamins, minerals, and other nutrients derived from Dyets Inc., Bethlehem, PA) (Frye et al, 1983; Overstreet et al, 2002). Dextrose calories in the control diet were equated with calories for ethanol in the ethanol-containing diet. After 3 days of accommodation to control diet, a portion of rats continued on this diet for the duration of the experiment. Other rats were placed on the cycled regimen of 4.5% (w/v) ethanol diet (ethanol-treated groups). A modified pair-feeding design was used for control rats to provide a volume of control diet equivalent to the average volume of ethanol diet consumed the previous day by the rats maintained on ethanol diet. Rats were weighed at weekly intervals to establish groups with similar body weights. After withdrawal, some animals were returned to normal chow for 16 days, at which time they were re-exposed to 5 days of the 4.5% ethanol diet or control diet.
Repeated Exposure to LPS and Selected Cytokines before Chronic Ethanol
These experiments evaluated the effect of repeated LPS (Calbiochem, La Jolla, CA) or repeated proinflammatory cytokine administration (rat IL-1β; TNFα: R&D Systems, Minneapolis, MN, or MCP-1: Leinco Technologies, St Louis, MO) on social interaction after exposure to the repeated withdrawal protocol. Rats on control diet were treated with either varying doses of LPS (i.p., saline vehicle) or designated cytokines (100 ng/5 μl) administered i.c.v. over a 2-min period at 1-week intervals before either continuing on control diet or switching to 5 days of ethanol-containing liquid diet (Figure 1). This 100-ng dose of the cytokines was initially chosen from literature as one having consistent effects on other functions (Ufnal et al, 2006; Plata-Salaman and Borkoski, 1994; Plata-Salaman et al, 1996). After showing that the 100-ng dose of TNFα sensitized withdrawal-induced anxiety, several doses of TNFα were chosen to determine if lower doses would have a similar consequence. For i.c.v. administrations, the cytokines were dissolved in artificial cerebrospinal fluid vehicle. Rats not receiving cytokines received vehicle. The 2-day delay (before ethanol diet was administered) was included in order to prevent any effect of the LPS or cytokines on ethanol diet intake (Figure 1). As noted above, some rats exposed to the LPS/withdrawal and cytokine/withdrawal protocols and control diet were also given an additional 5-day exposure to 4.5% ethanol diet 16 days after the protocols to test for persistence of adaptation that can sustain withdrawal-induced anxiety-like behavior (Overstreet et al, 2002; Breese et al, 2004).
Figure 1.
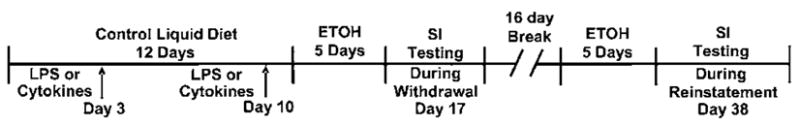
Protocol for repeated administration of the LPS or a cytokine (TNF-α, MCP-1 (CCL2), and IL-1β) before 5 days of chronic ethanol (ETOH) diet to assess social interaction deficits (increased anxiety-like behavior). At the arrows on days 3 and 10, LPS or one of the cytokines was administered weekly followed 2 days later by 5 days of ethanol liquid diet (ED 4.5%). The LPS was given i.p. and the cytokines were administered i.c.v. at the indicated time points. SI indicates testing of social interaction on day 17, 5–6 h after removal of the final ED exposure. Reinstatement animals were returned to chow food for 16 days, then exposed again to 5 days of the 4.5% ED before being withdrawn and tested.
Flumazenil Treatment before Repeated LPS Administration
To determine if the repeated LPS/withdrawal and the TNFα/withdrawal protocols share sensitivity to flumazenil inhibition of withdrawal-induced anxiety-like behavior after the repeated withdrawal and repeated stress/withdrawal protocols (Breese et al, 2004; Knapp et al, 2004, 2005), rats were treated with flumazenil (5 mg/kg, 2 ml/kg i.p.; Roche, Basel, Switzerland) or 0.5% carboxymethylcellulose vehicle 30 min before each of the two weekly doses of LPS (250 μ; i.p.) or TNFα (30 ng, i.c.v.), but not during withdrawal from the 5 days of ethanol diet. This dose of flumazenil was chosen based upon effectiveness in previous studies to diminish the adaptive change induced by the repeated withdrawal protocol (Knapp et al, 2004, 2005) and the repeated stress/withdrawal protocol (Breese et al, 2004). Subsequently, social interaction was measured in controls and drug-treated groups as noted above.
Measurement of Social Interaction
The social interaction test introduced by File and Hyde (1978) has been validated as a measure of anxiety-like behavior in many investigations (File, 1980; Guy and Gardner, 1985; Irvine et al, 2001; see review by File and Seth, 2003). In this test, the amount of time animals actively interacted (ie, grooming, sniffing, boxing, or crawling over/under each other) during a 5-min session is measured. Testing of rats unfamiliar to the environment was carried out under relatively low lighting in a square open field (60 × 60 cm2, with 16 squares marked on the floor; Overstreet et al, 2002). Social interaction was determined utilizing data from individual rats in the pair (Overstreet et al, 2002, 2003; Breese et al, 2004), a proven modification of the standard social interaction test (File, 1980; File and Seth, 2003). For this investigation, the time rats spent in social interaction was measured 5–6 h after removal of the ethanol diet to assess the degree of withdrawal-induced anxiety-like behavior. Generally, each squad of 40 rats was tested in subgroups of 20 with balanced numbers of rats in each treatment group. Rat pairs were matched on the basis of body weights and treatment conditions and placed simultaneously in the open field (Overstreet et al, 2002). Line crosses (by two forepaws), which served as a measure of locomotor activity independent of social interaction (File, 1980; Overstreet et al, 2002), were recorded simultaneously with social interaction. Experienced observers who scored rats were ‘blind’ to treatment conditions. In some cases, controls within groups that had no surgery were also tested. Because these data did not differ from that of rats with surgery, these data were combined.
Data Analysis
Statistical analyses were carried out using the GBStat software package. The data were initially analyzed by ANOVAs. If the main effects were statistically significant, post hoc analyses were performed using Tukey’s protected t-tests, as described previously (Breese et al, 2004; Knapp et al, 2004, 2005; Overstreet et al, 2002). As noted in the tables, there were typically at least six or more rats in each group being investigated.
RESULTS
Dose-Effect of Repeated LPS on Withdrawal-Induced Anxiety-Like Behavior
In the initial experiment, it was demonstrated that twiceweekly administrations of various LPS doses before a single exposure to a single 5-day 4.5% ethanol diet (ED LPS; LPS/withdrawal protocol) significantly reduced social interaction upon withdrawal (F(8,87) = 22.89, p < 0.0001; Figure 2). Repeated weekly administration of LPS alone (250 or 1000 μg/kg to rats maintained on control diet (CD LPS 1000/250)) did not affect social interaction. Likewise, as previously reported (Breese et al, 2004; Overstreet et al, 2004), 5 days of ethanol liquid diet in animals that received vehicle (ED Veh) did not change social interaction compared with control diet (CD Veh) animals. Ethanol intake for the rats in these groups is presented in Table 1. In each case of the LPS/withdrawal protocol treatments, the ethanol intake of the rats did not differ across the groups that received ethanol. These findings are consistent with previous data showing blood ethanol concentrations in animals exposed to repeated withdrawals or repeated stress/withdrawal protocols were equivalent to rats that received continuous ethanol (Breese et al, 2004; Overstreet et al, 2002). Importantly, the body weights of the animals also did not differ. However, locomotor activity was significantly reduced in all groups of rats that received ethanol diet but was somewhat higher in the control group that received LPS alone.
Figure 2.
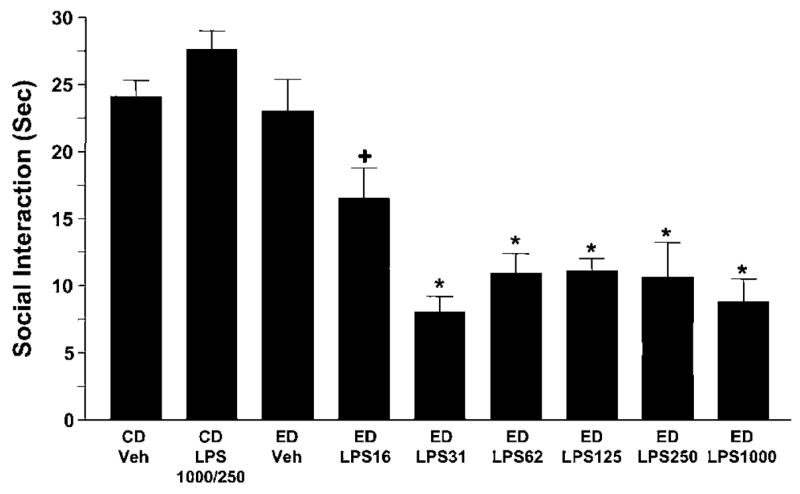
Repeated LPS reduces social interaction behaviors during withdrawal from chronic ethanol. Dose response (16–1000 μg/kg) for LPS given twice at weekly intervals before exposure to 5 days of 4.5% ethanol diet (ED; see Figure 1 for illustration of the protocol), sensitized anxiety (ie, reduced social interaction behavior). No such effect was observed in rats that received only 5 days of ethanol diet with vehicle injections (ED-Veh) or that received LPS (1000 or 250 μg/kg) before control diet (CD). N for each group listed in Table 1. *Significantly different from CD-Veh, p < 0.001. +Significantly different from CD-Veh, p < 0.05.
Table 1.
Effect of the Repeated LPS/Withdrawal Protocol on Ethanol Intake, Locomotor Activity and Body Weight
| Treatments | Alcohol intake (g/kg/day) | Locomotor activity (crosses/5 min) | Body weight (g) |
|---|---|---|---|
| CD Veh (0) (16) | — | 105±9 | 299±3 |
| CD LPS (250) (8) | — | 142±9+ | 285±4 |
| ED Veh (0) (16) | 9.2±0.3 | 69±9** | 303±4 |
| ED LPS (16) (8) | 8.9±0.2 | 55±6** | 296±4 |
| ED LPS (31) (8) | 8.9±0.4 | 54±8** | 291±5 |
| ED LPS (62) (8) | 8.9±0.3 | 57±11** | 299±3 |
| ED LPS (125) (16) | 9.2±0.3 | 85±11* | 291±4 |
| ED LPS (250) (8) | 9.5±0.3 | 83±15* | 294±5 |
| ED LPS (1000) (8) | 9.5±0.4 | 81±13* | 286±7 |
| F | NS | 4.45 | NS |
p < 0.05,
p < 0.01 compared to vehicle-control diet;
p < 0.01 compared to vehicle-control diet or the LPS–ethanol groups; these data are associated with Figure 2.
In the Treatments column, the first number in parentheses is the i.p. dose in μg and the second number is the n per group.
Effect of Repeated Cytokine Administration before 5-Days of Ethanol Diet on Withdrawal-Induced Reduction in Social Interaction
As LPS increases cytokines in brain (Breder et al, 1994; Buttini et al, 1997; Hagan et al, 1993; Hillhouse and Mosley, 1993; Ilyin et al, 1998; Matalka et al, 2005; Quan et al, 1994), it was next determined if cytokines could indeed be responsible for the LPS/withdrawal protocol sensitization of withdrawal-induced anxiety. To test this possibility, the cytokines (IL-1β, CCL2 (MCP-1), and TNFα (100 ng)) or vehicle were given i.c.v. at weekly intervals (instead of systemic LPS) before the 5-day exposure to ethanol diet (cytokine/withdrawal protocol). As shown in Figure 3, repeated administration of a 100-ng dose of each of these cytokines before ethanol-diet exposure, like LPS, reduced social interaction during withdrawal (F(5,59) = 22.41, p < 0.0001), a reflection of the ability of the cytokine/withdrawal protocol to sensitize withdrawal-induced anxiety-like behavior.
Figure 3.
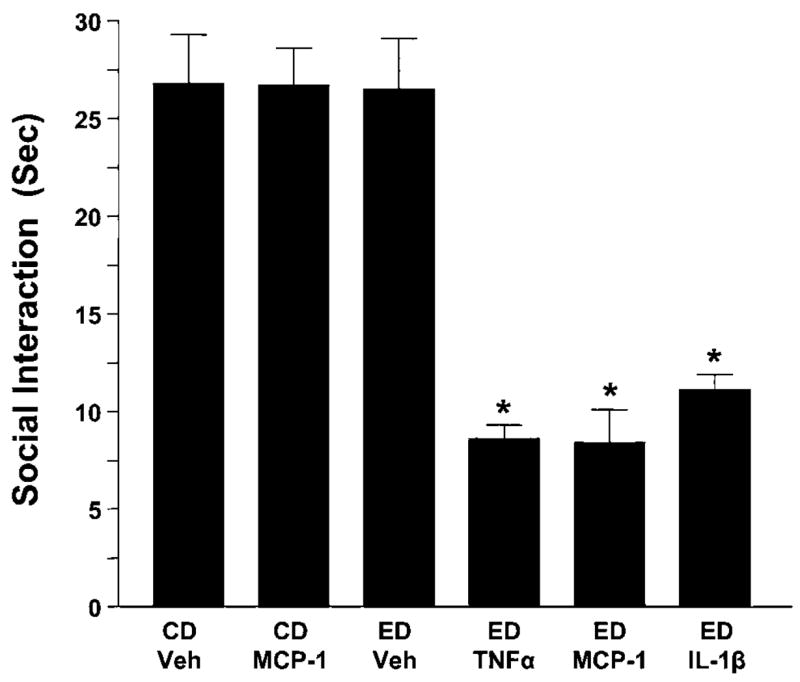
Repeated IL-1β, TNFα, or MCP-1 (100 ng/5 μl, i.c.v.) sensitize ethanol withdrawal-induced anxiety. Rats were injected twice at weekly intervals with either vehicle or cytokine (IL-1β, TNFα, or MPC-1) while drinking control diet (CD) and then were either continued on CD or switched to a 4.5% ethanol diet (ED) for 5 days. The cytokine (MCP-1) was also given twice in a group exposed only to CD to test for an effect on social interaction in the absence of the ethanol exposure. Social interaction for all groups was measured 5–6 h after removal of the ED. MCP-1 = monocyte chemo-attractant protein-1; IL-1β = interleukin-1β; TNFα = tumor necrosis factor-α; CD = control diet; Veh = artificial cerebrospinal fluid. N for each group listed in Table 2. *p < 0.001 compared to CD-Veh or ED-Veh groups.
In order to test for dose–response effects of cytokine influences on sensitization of withdrawal-induced anxiety, various doses of TNFα (3–100 ng) were administered i.c.v. twice at weekly intervals before a 5-day cycle of 4.5% ethanol diet. As shown in Figure 4, TNFα was again active (F(5,58) = 24.99, p < 0.0001) at doses as low as 3–10 μg with the effect of the 3 μg dose being significantly different from all other groups. Finally, there was no difference in ethanol intake, body weight, or locomotor behavior after the various cytokine treatments (Tables 2 and 3).
Figure 4.
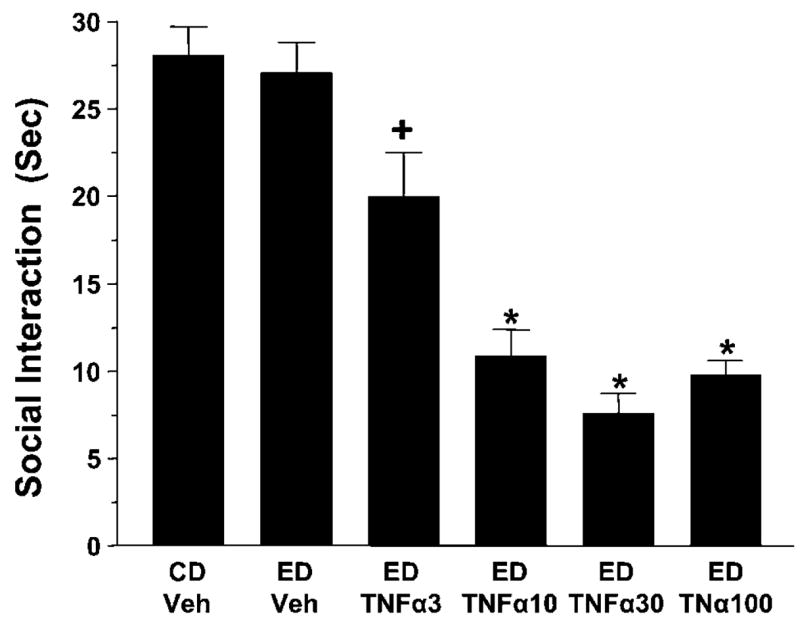
Dose response effect for repeated TNFα sensitization of ethanol withdrawal induced anxiety. In this case, various doses of TNFα, including the 100 ng dose tested in Figure 3, were given twice before exposure to 5 days of 4.5% ethanol diet (ED). As shown, all doses induced a significant sensitization of anxiety-like behavior. No such effect on anxiety-like behavior was observed in rats that received only 5 days of ethanol diet with vehicle (ED-Veh). CD = control diet. N for each group listed in Table 3. *p < 0.01 compared to CD-Veh or ED-Veh. + p < 0.05 compared to all other groups.
Table 2.
Effect of Cytokine Administration on Ethanol Intake, Locomotor Activity, and Body Weights for Rats Exposed to 5 Days Ethanol Diet Starting Immediately after Repeated i.c.v. Administration of 100 ng IL-1β, TNF-α, or MCP-1a
| Treatments | Ethanol intake (g/kg/day) | Locomotor activity (crosses/5 min) | Body weight (g) |
|---|---|---|---|
| CD Veh (15) | — | 82±6 | 312±5 |
| CD MCP-1 (10) | — | 83±7 | 306±9 |
| ED Veh (16) | 9.1±0.2 | 68±5 | 308±7 |
| ED TNFα (7) | 9.0±0.2 | 58±5 | 323±8 |
| ED MCP-1 (10) | 8.8±0.2 | 74±9 | 303±9 |
| ED IL1β (7) | 9.2±0.1 | 65±9 | 309±5 |
| F | NS | NS | NS |
No significant differences noted among the groups for any of the measures. These data relate to work completed in Figure 3. In the Treatments column, the numbers in parentheses are the n sizes per group.
Table 3.
Ethanol Intake, Locomotor Activity, and Body Weights in Rats Exposed to Different Doses of TNF-α before Exposure to Ethanol Dieta
| Treatments | Ethanol intake (g/kg) | Locomotor activity (lines/5 min) | Body weight (g) |
|---|---|---|---|
| CD Veh (0) (16) | — | 102±8 | 315±6 |
| ED Veh (0) (16) | 8.7±0.2 | 87±8 | 313±6 |
| ED TNFα (3) (9) | 8.4±0.2 | 81±5 | 305±10 |
| ED TNFα (10) (9) | 8.6±0.3 | 85±9 | 313±8 |
| ED TNFα (30) (5) | 8.2±0.2 | 99±26 | 296±4 |
| ED TNFα (100) (9) | 8.9±0.2 | 71±9 | 320±7 |
| F | NS | NS | NS |
No significant differences noted among the groups for any of the measures; these data relate to work in Figure 4. In the Treatments column, the first number is the i.p. dose in μg and the second number is the n per group.
Effect of the LPS/Withdrawal or Cytokine/Withdrawal Protocols on Withdrawal-Induced Anxiety from Re-Exposure to Chronic Ethanol Diet
Previous data show that rats previously exposed to the repeated withdrawal (Overstreet et al, 2002) or the stress/withdrawal (Breese et al, 2005b) protocols exhibited sensitized anxiety-like behavior upon withdrawal from a future re-exposure to an additional 5 days of ethanol diet 16 days after the initial withdrawal. Therefore, the possibility that the repeated LPS/withdrawal and the cytokine/withdrawal protocols would produce a similarly persistent sensitization of withdrawal-induced anxiety after re-exposure to an additional 5 days of ethanol diet was assessed. As shown in Figure 5, reinstatement of the ethanol diet 16 days after the initial LPS or cytokine (IL-1β or TNFα)/withdrawal protocols reduced social interaction equivalent to that observed during withdrawal from the initial treatments (F(4,35) = 13.93, p < 0.0001; compare to Figures 3 and 4). Finally, no differences among the groups were found on ethanol intake, locomotor behavior, or body weight (Table 4) despite the persistent effect of cytokines on social interaction behavior (Figure 5).
Figure 5.
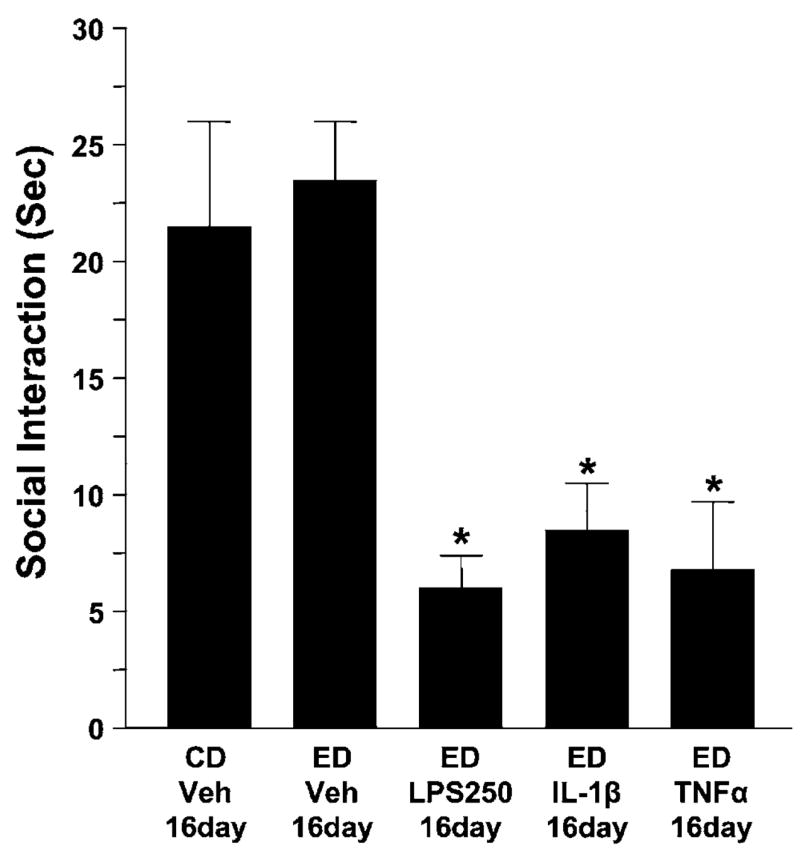
Persistent effect of repeated IL-1β, TNFα, or LPS on withdrawal-induced anxiety following re-exposure to 5 days of chronic ethanol starting 16 days later. Subgroups of rats from Figures 2 and 3 were treated with an additional 5 days of ethanol diet (4.5%) 16 days after having been exposed earlier to the initial repeated LPS or to the cytokines TNFα or IL-1β. All groups treated earlier with LPS or the cytokines exhibited a significant reduction of social interaction. Abbreviations for treatments are as per those in Figures 2 and 3, while the 16-day notation refers to the 16- day delay between the two ethanol diet cycles. N for each group listed in Table 4. *p < 0.01 compared to CD-Veh-16-day or ED-Veh-16day groups.
Table 4.
Ethanol Intake, Locomotor Activity, and Body Weights for Rats Exposed to 5 Days of Ethanol Re-Exposure 16 Days after the Repeated LPS/Withdrawal or Repeated Cytokine Protocolsa
| Treatments | Ethanol intake (g/kg/day) | Locomotor activity (crosses/5 min) | Body weight (g) |
|---|---|---|---|
| CD Veh 16day (8) | — | 43±7 | 415±9 |
| ED Veh 16day (8) | 7.3±0.3 | 63±16 | 429±12 |
| ED LPS250 16day (8) | 7.3±0.6 | 35±14 | 414±15 |
| ED IL-1β 16day (8) | 7.3±0.4 | 45±8 | 413±13 |
| ED TNFα 16day (8) | 7.4±0.4 | 37±8 | 409±17 |
| F | NS | NS | NS |
No significant differences noted among the groups for any of the measures. These data relate to work in Figure 5. In the Treatments column, the number in parentheses represents the n size per group.
Flumazenil Treatment before LPS and TNFα Blocks Sensitized Withdrawal-Induced Anxiety Induced by the LPS/and TNFα/Withdrawal Protocols
Previous work demonstrates that flumazenil given before initial repeated withdrawals or repeated stresses, but not the final withdrawal, minimized withdrawal-induced anxiety-like behavior (Breese et al, 2004; Knapp et al, 2004, 2005; Overstreet et al, 2003). Based upon these findings, flumazenil (5 mg/kg, i.p.) was given before each repeated dose of LPS or TNFα to determine if the withdrawal-induced anxiety-like behavior that accompanies the LPS/and TNFα/withdrawal protocols would be affected. As shown in Figure 6a, prior flumazenil treatment significantly reduced the deficit in social interaction induced by these protocols (F(4,44) = 24.79, p < 0.0001). Previous results indicate that two injections of flumazenil to rats receiving control liquid diet had no effect on social interaction (Knapp et al, 2004, 2005). In addition, when retested after re-exposure to an additional cycle of ethanol diet 16 days later, the prior flumazenil treatment retained activity to limit the magnitude of ‘reinstated’ ethanol withdrawal-induced anxiety response (Figure 6b) (F(4,26) = 7.02, p < 0.001). However, no differences among the groups were found on ethanol intake, locomotor behavior, or body weight (Tables 5 and 6).
Figure 6.
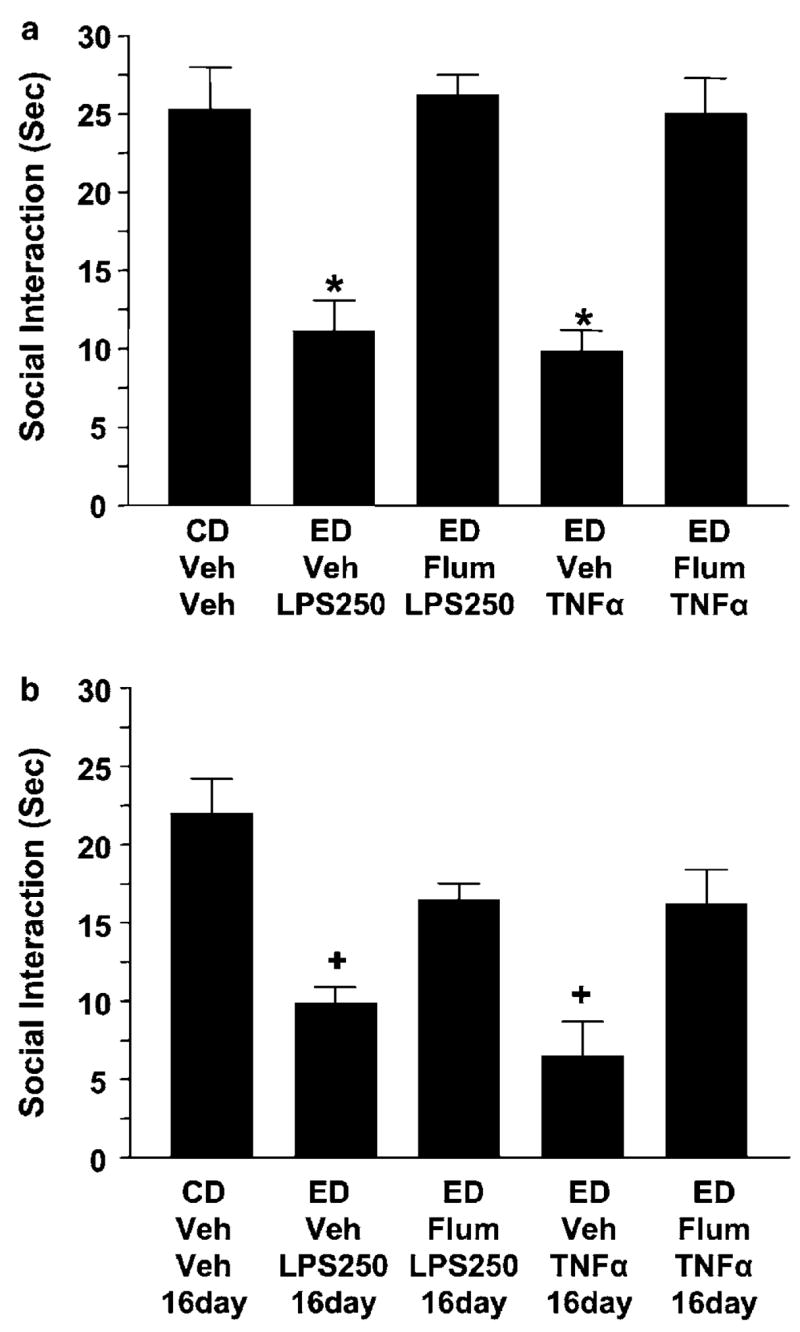
Pretreatment with flumazenil prevented the acute as well as the persistent sensitization of withdrawal-induced anxiety induced by LPS and TNFα. In accordance with previous studies (Breese et al, 2004; Knapp et al, 2005), flumazenil (Flum; 5 mg/kg, 2.5 mg/ml in carboxymethylcellulose) was injected before LPS (250 μg/kg, i.p., 2 ml/kg in saline) or TNFα (10 ng/5 μg). Assessment of anxiety-like behavior (ie, social interaction deficit) was measured 5–6 h after withdrawal from the initial 5 days of ethanol and again after re-exposure to an additional 5 days of ethanol. Flumazenil (ED-Flum- LPS250; ED-Flum-TNFα) prevented the reduced social interaction observed during the initial withdrawal (a) as well as the withdrawal from the re-exposure to another ethanol diet cycle 16 days later (b). The 16-day notation refers to the 16-day delay between the two ethanol diet cycles. N for each group listed in Tables 5 and 6. *p < 0.001 compared to the CD-Veh-Veh or flumazenil-treated groups. +p < 0.05 compared to the CD-Veh-Veh-16day or flumazenil-treated groups.
Table 5.
Ethanol Intake, Locomotor Activity, and Body Weights for Rats Pretreated with Flumazenil or Vehicle before LPS or TNFα and 5 Days of Ethanol Dieta
| Treatments | Ethanol intake (g/kg/day) | Locomotor activity (crosses/5 min) | Body weight (g) |
|---|---|---|---|
| CD Veh Veh (10) | — | 74±6 | 322±5 |
| ED Veh LPS250 (8) | 9.0±0.5 | 61±10 | 319±6 |
| ED Flum LPS250 (8) | 9.1±0.1 | 73±9 | 325±8 |
| ED Veh TNFα (11) | 8.4±0.2 | 74±11 | 311±7 |
| ED Flum TNFα (12) | 8.4±0.2 | 69±7 | 318±6 |
| F | NS | NS | NS |
No significant differences noted among the groups for any of the measures. These data relate to work in Figure 6a. In the Treatments column, the number in parentheses represents the n size per group.
Table 6.
Ethanol Intake, Locomotor Activity, and Body Weights in Rats Pretreated with Flumazenil or Vehicle before LPS or TNFα and 5 Days of Ethanol Dieta
| Treatments | Ethanol intake (g/kg/day) | Locomotor activity (crosses/5 min) | Body weight (g) |
|---|---|---|---|
| CD Veh Veh 16 day (6) | — | 42±8 | 415±9 |
| ED Veh LPS250 16 day (6) | 7.3±0.5 | 36±11 | 412±12 |
| ED Flum LPS250 16 day (7) | 7.0±0.4 | 52±11 | 445±13 |
| ED Veh TNFα 16 day (7) | 6.7±0.2 | 70±7 | 420±20 |
| ED Flum TNFα 16 day (6) | 6.3±0.2 | 69±10 | 418±15 |
| F | NS | NS | NS |
Reinstatement of ethanol diet 16 days later. No significant differences noted among the groups for any of the measures. These data relate to work in Figure 6b. In the Treatments column, the number in parentheses represents the n size per group. See Table 5 for similar measures in these animals 16 days before the start of the 5-day dietary reinstatement cycle.
DISCUSSION
Repeated stresses before a single 5-day cycle of ethanol diet sensitize withdrawal-induced anxiety (Breese et al, 2004). Stress not only can increase cytokine levels (Black, 2002; Deak et al, 2005; Minami et al, 1991; Nguyen et al, 1998; O’Connor et al, 2003; Shintani et al, 1995a, b; Shizuya et al, 1997; Suzuki et al, 1997) but also can augment the effects of a subsequent increase in cytokines by LPS or stress (Johnson et al, 2004; Munhoz et al, 2006; Takemura et al, 1997). These findings led to the hypothesis that cytokines are involved in depressive illness (Anisman and Merali, 2003; Dunn et al, 2005; Hayley et al, 2005; Raison et al, 2006; Simen et al, 2006) and addictive behavior (Crews et al, 2006; Friedman and Eisenstein, 2004). Further, the release of CRF by stress partly depends on cytokines (Sapolsky et al, 1987; Turnbull and Rivier, 1995). Therefore, the potential existed that cytokines could substitute for stress to sensitize withdrawal-induced anxiety that follows 5 days of alcohol diet (Breese et al, 2004), particularly given the view that cytokines can act as neuromodulators (Adler et al, 2006; Bauer et al, 2007). The present work demonstrates that repeated administration of LPS before 5 days of chronic ethanol exposure sensitized withdrawal-induced anxiety. It is well established that this amount of ethanol exposure itself does not normally induce this behavioral syndrome upon withdrawal (Breese et al, 2004; Overstreet et al, 2002; Knapp et al, 2004, 2005; Figures 2 and 3). Likewise, LPS exposure in the absence of the chronic ethanol exposure did not affect this measure of anxiety-like behavior. As LPS is known to increase cytokines in brain (Grinevich et al, 2001; Hagan et al, 1993; Hillhouse and Mosley, 1993; Ilyin et al, 1998; Matalka et al, 2005; Munhoz et al, 2006; Obuchowicz et al, 2006; Quan et al, 1994; Zujovic et al, 2001), it was speculated that the ability of the repeated LPS exposure to affect withdrawal-induced anxiety is related to a central expression of cytokines.
To test this latter view, the cytokines IL-1β, CCL2 (MCP-1), and TNFα were administered i.c.v. at weekly intervals before 5 days of ethanol diet to substitute for the LPS exposure. Like LPS, the repeated exposure to these cytokines induced an adaptive change as evidenced by emergence of sensitized anxiety-like behavior upon withdrawal from a single 5-day exposure to chronic ethanol diet. The fact that repeated systemic LPS and repeated i.c.v. cytokine administration did not affect the amount of ethanol ingested seems to eliminate the possibility that an increase in blood ethanol concentration is responsible for this sensitization. Additionally, a reduction in locomotor activity did not consistently relate to the change in withdrawal-induced anxiety in the various LPS/ethanol or cytokine/ethanol groups. Further, ameliorative drug treatments (eg, flumazenil) dramatically altered anxiety-like behavior with little impact on locomotor behavior. Thus, as consistently established (Overstreet et al, 2002), locomotor activity in this social interaction test appears to be differentially regulated from anxiety-like behavior. Conversely, while reduced locomotor activity was observed on occasion (Table 1), the relative independence of the two behaviors was further underscored by the reduced locomotor activity in an ethanol diet-treated group with no concurrent change in anxiety-like behavior (Figure 2). Previous observations showing no correlation between social interaction behavior and locomotor behavior or between the social interaction behavior of members in a socially interacting pair further emphasize this point (Overstreet et al, 2002). It should also be noted that behavioral testing was conducted in a drug-free state; therefore, acute nonspecific effect of the treatments was not operating. Overall, the present results are consistent with previous findings that overexpression of cytokines in autoimmune mice is associated with anxiety (Schrott and Crnic, 1996) and reports of cytokine administration to rodents producing anxiety (Cragnolini et al, 2006).
Earlier studies from our laboratory demonstrate that the repeated withdrawal and the stress/withdrawal protocols have an extended maladaptive influence (Overstreet et al, 2002; Breese et al, 2004). This persistent effect of these protocols is emphasized by induction of anxiety-like behavior following a subsequent withdrawal from a future re-exposure to 5 days of ethanol diet (Overstreet et al, 2003; Breese et al, 2004). Such an ethanol challenge to rats previously treated with only control diet did not affect the social interaction measure, a finding suggesting that the sensitization depends on the previous repeated exposure to the withdrawal and the stress/withdrawal protocols (Overstreet et al, 2002; Breese et al, 2004). In the present investigation, rats exposed to the LPS/withdrawal protocol showed a similar cumulative persistent adaptive effect on social interaction when a future re-exposure to ethanol was delayed for 16 days—a result comparable to that observed previously (Overstreet et al, 2002; Breese et al, 2004). Likewise, the repeated IL-1β and TNF-α/withdrawal protocols induced an anxiety-like behavioral response upon withdrawal following a similar 16-day delay in re-exposure to chronic ethanol diet. This latter observation suggests that the persistent adaptation observed with the LPS/withdrawal protocol depends upon its influence on cytokines (Munhoz et al, 2006; Obuchowicz et al, 2006).
To gain further evidence that the LPS/withdrawal and the TNFα/withdrawal protocols shared an additional common relationship with the stress/withdrawal protocol, flumazenil (which prevents sensitization of anxiety-like behavior induced by the stress/withdrawal protocol; Breese et al, 2004) was administered before each of the two LPS or the TNFα treatments given before the 5 days of ethanol diet. Flumazenil prevented the anxiety-like behavior in both protocols. Further, flumazenil prevented the reappearance of anxiety upon withdrawal from an additional cycle of ethanol diet introduced 16 days later. Thus, the induction and the prevention of the sensitized response appear to reflect modulation of a persistent maladaptive change. Although the mechanism responsible for this effect of flumazenil is unknown, this pharmacological finding is consistent with the view that stress and the ability of cytokines to facilitate an adaptive change responsible for the sensitization of anxiety-like behavior may be linked to GABAA receptor function. Importantly, serotonin and CRF are also closely related to cytokine function (Hayley et al, 2002; Zhu et al, 2006; Sapolsky et al, 1987) as well as the stress/withdrawal protocol (Breese et al, 2004). Therefore, future investigations should determine whether drugs affecting CRF and serotonergic function that minimize the adaptation induced by the stress/withdrawal protocol will have a similar effect on the sensitization of withdrawal- induced anxiety induced by the cytokine/withdrawal protocols.
Cytokines and LPS have been associated with immunity and inflammation and mediate a variety of CNS-induced responses including ‘sickness behavior’ (Kent et al, 1992; Bluthe et al, 1992; Fiore et al, 1998), fever, altered emotional behavior (Anisman and Merali, 2003; Yamada et al, 2000), and changes in endocrine function (Connor et al, 1998; Dunn et al, 2005; Turnbull and Rivier, 1999a, b; Watkins et al, 1995). In addition to the involvement of cytokines in these acute symptoms, cytokines may be involved in symptoms of depressive illness and negative affect (Anisman and Merali, 1999, 2003; Bonaccorso et al, 2003; Dunn et al, 2005; Millan, 2003; Pucak and Kaplin, 2005; Smith, 1991) and addictive behavior (Friedman and Eisenstein, 2004). Leonard (2005) has implicated interactions of the HPA axis, the immune system, and serotonergic mechanisms as contributors to induction of anxiety-associated behavior and depression. Kiefer et al (2002) associated elevated TNFα levels in the alcoholic to factors that may facilitate craving and relapse to drinking. It is emphasized that the anxiety-like behavior, which occurs with exposure to the LPS/withdrawal and cytokine/withdrawal protocols, does not occur when the repeated LPS or the repeated cytokines are administered in the absence of a subsequent ethanol challenge. Because withdrawal from the 5 days of ethanol diet alone does not effect anxiety-like behavior, it appears that adaptive changes responsible for withdrawal-induced anxiety follows prior exposures to LPS and cytokines, just as occurred with prior stress experience (Breese et al, 2004).
The present investigation provides the first evidence for a potentially new mechanism that is based upon the action of cytokines to contribute to a cumulative adaptation that sensitizes anxiety-like behavior upon withdrawal from exposure to chronic ethanol diet. The persistent adaptation associated with the LPS/withdrawal and cytokine/withdrawal protocols is proposed to be related to behaviors associated with negative affect—a response thought to facilitate relapse to drinking (Breese et al, 2005a, c). Consequently, the argument that cytokines are involved in the neural disruption and addictive behaviors induced by other drugs of abuse (Friedman and Eisenstein, 2004; Yamada and Nabeshima, 2004; Nakajima et al, 2004) may apply to an association of cytokines to alcohol abuse (Kiefer et al, 2002). The results of the present work would be consistent with this view. Future work is required to further establish this association.
Acknowledgments
We acknowledge the assistance of Alia Dickinson and Kui-Ling Huang for preparation and administration of liquid diets. This work was supported by grants from NIAAA—AA11605, AA12655, AA14949, AA014284, AA-007573, and AA16704.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The author(s) declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Adler M, Geller E, Chen X, Rogers T. Viewing chemokines as a third major system of communication in the brain. AAPS J. 2006;7:E865–E870. doi: 10.1208/aapsj070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Merali Z. Anhedonic and anxiogenic effects of cytokine exposure. Adv Exp Med Biol. 1999;461:199–233. doi: 10.1007/978-0-585-37970-8_12. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z. Cytokines, stress and depressive illness: brain–immune interactions. Ann Med. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bauer S, Kerr B, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat Neurosci Rev. 2007;8:221–232. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Maier SF, Meltzer HY, Maes M. Behavioral changes in rats after acute, chronic and repeated administration of interleukin-1beta: relevance for affective disorders. J Affect Disord. 2003;77:143–148. doi: 10.1016/s0165-0327(02)00118-0. [DOI] [PubMed] [Google Scholar]
- Breder CD, Hazuka C, Ghayur T, Klug C, Huginin M, Yasuda K, et al. Regional induction of tumor necrosis factor alpha expression in the mouse brain after systemic lipopolysaccharide administration. Proc Natl Acad Sci. 1994;91:11393–11397. doi: 10.1073/pnas.91.24.11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a ‘kindling’/stress hypothesis. Psychopharmacology. 2005a;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005b;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, et al. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005c;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Buttini M, Mir A, Appel K, Wiederhold KH, Limonta S, Gebicke-Haerter PJ, et al. Lipopolysaccharide induces expression of tumour necrosis factor alpha in rat brain: inhibition by methyl-prednisolone and by rolipram. Br J Pharmacol. 1997;122:1483–1489. doi: 10.1038/sj.bjp.0701502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Song C, Leonard BE, Merali Z, Anisman H. An assessment of the effects of central interleukin-1beta, -2, -6, and tumor necrosis factor-alpha administration on some behavioural, neurochemical, endocrine and immune parameters in the rat. Neuroscience. 1998;84:923–933. doi: 10.1016/s0306-4522(97)00533-2. [DOI] [PubMed] [Google Scholar]
- Cragnolini AB, Schioth HB, Scimonelli TN. Anxiety-like behavior induced by IL-1beta is modulated by alpha-MSH through central melanocortin-4 receptors. Peptides. 2006;27:1451–1456. doi: 10.1016/j.peptides.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, et al. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Jr, Deak MM, et al. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bull. 2005;646:541–556. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Emanuele N, LaPaglia N, Kovacs EJ, Emanuele MA. Effects of chronic ethanol (ETOH) on pro-inflammatory cytokines of the hypothalamic–pituitary–gonadal (HPG) axis in female rats. Endocr Res. 2005;31:9–16. doi: 10.1080/07435800500228930. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Meth. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Fiore M, Alleva E, Probert L, Kollias G, Angelucci F, Aloe L. Exploratory and displacement behavior in transgenic mice expressing high levels of brain TNF-alpha. Physiol Behav. 1998;63:571–576. doi: 10.1016/s0031-9384(97)00514-3. [DOI] [PubMed] [Google Scholar]
- Friedman H, Eisenstein TK. Neurological basis of drug dependence and its effects on the immune system. J Neuroimmunol. 2004;147:106–108. doi: 10.1016/j.jneuroim.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Differential sensitivity of ethanol withdrawal signs in the rat to γ-aminobutyric acid (GABA) mimetics: blockade of audiogenic seizures but not forelimb tremors. J Pharmacol Exp Ther. 1983;226:720–725. [PubMed] [Google Scholar]
- Grinevich V, Ma X-M, Herman JP, Jezova D, Akmayev I, Aguilera G. Effect of repeated lipopolysaccharide administration on tissue cytokine expression and hypothalamic–pituitary–adrenal axis activity in rats. J Neuroendocrinol. 2001;13:711–723. doi: 10.1046/j.1365-2826.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- Guy AP, Gardner CR. Pharmacological characterisation of a modified social interaction model of anxiety in the rat. Neuropsychobiology. 1985;13:194–200. doi: 10.1159/000118187. [DOI] [PubMed] [Google Scholar]
- Hagan P, Poole S, Bristow AF. Endotoxin-stimulated production of rat hypothalamic interleukin-1 beta in vivo and in vitro, measured by specific immunoradiometric assay. J Mol Endocrinol. 1993;11:31–36. doi: 10.1677/jme.0.0110031. [DOI] [PubMed] [Google Scholar]
- Hayley S, Poulter MO, Merali Z, Anisman H. Pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Hayley S, Wall P, Anisman H. Sensitization to the neuroendocrine, central monoamine and behavioural effects of murine TNF-α: peripheral and central mechanisms. Eur J Neurosci. 2002;15:1061–1076. doi: 10.1046/j.1460-9568.2002.01936.x. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Mosley K. Peripheral endotoxin induces hypothalamic immunoreactive interleukin-1 beta in the rat. Br J Pharmacol. 1993;109:289–290. doi: 10.1111/j.1476-5381.1993.tb13567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyin SE, Gayle D, Glynn MC, Plata-Salaman CR. Interleukin-1beta system (ligand, receptor type I, receptor accessory protein and receptor antagonist), TNF-alpha, TGF-beta1 and neuro-peptide Y mRNAs in specific brain regions during bacterial LPS-induced anorexia. Brain Res Bull. 1998;45:507–515. doi: 10.1016/s0361-9230(97)00437-1. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Bagnalasta M, Marcon C, Motta C, Tessari M, File SE, et al. Nicotine self-administration and withdrawal: modulation of anxiety in the social interaction test in rats. Psychopharmacology. 2001;153:315–320. doi: 10.1007/s002130000586. [DOI] [PubMed] [Google Scholar]
- Johnson J, O’Connor K, Watkins L, Maier S. The role of IL-1β in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127:569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe R-M, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Schick M, Wiedeman K. Alcohol intake, tumour necrosis factor-alpha, leptin and craving: factors of a possibly vicious circle? Alcohol Alcohol. 2002;37:401–404. doi: 10.1093/alcalc/37.4.401. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Modulation of ethanol withdrawal-induced anxiety-like behavior during later withdrawals by treatment of early withdrawals with benzodiazepine/gamma-aminobutyric acid ligands. Alcohol Clin Exp Res. 2005;29:553–563. doi: 10.1097/01.alc.0000158840.07475.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE. The HPA and immune axes in stress: the involvement of the serotonergic system. Eur Psychiatry. 2005;3:302–306. doi: 10.1016/s0924-9338(05)80180-4. [DOI] [PubMed] [Google Scholar]
- Matalka KZ, Tutunji MF, Abu-Baker M, Abu Baker Y. Measurement of protein cytokines in tissue extracts by enzyme-linked immunosorbent assays: application to lipopolysaccharide-induced differential milieu of cytokines. Neuroendocrinol Lett. 2005;26:231–236. [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol ‘kindles’ inferior collicular seizure activity: evidence for kindling of seizures associated with alcoholism. Alcohol Clin Exp Res. 1990;14:394–399. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Minami M, Kuraishi Y, Yamaguchi T, Nakai S, Hirai Y, Satoh M. Immobilization stress induces interleukin-1 beta mRNA in the rat hypothalamus. Neurosci Lett. 1991;123:254–256. doi: 10.1016/0304-3940(91)90944-o. [DOI] [PubMed] [Google Scholar]
- Munhoz C, Lepsch L, Kawamoto E, Malta M, Lima L, Avellar M, et al. Chronic unpredictable stress exacerbates LPS-induced activation of NFκB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Yamada K, Nagai T, Uchiyama T, Miyamoto Y, Mamiya T, et al. Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity. J Neurosci. 2004;24:2212–2225. doi: 10.1523/JNEUROSCI.4847-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, et al. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuchowicz E, Marcinowska A, Drzyzga L, Wojcikowski J, Daniel WA, Herman ZS. Effect of chronic treatment with perazine on lipopolysaccharide-induced interleukin-1beta levels in the rat brain. NS Arch Pharmacol. 2006;373:79–84. doi: 10.1007/s00210-006-0058-1. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, et al. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991:123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Weiss B, Rivier C. Mechanisms mediating the influence of alcohol on the hypothalamic–pituitary–adrenal axis response to immune and nonimmune signals. Alcohol Clin Exp Res. 1998;22:243s–247s. doi: 10.1097/00000374-199805001-00005. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2c antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacology. 2003;167:344–352. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata-Salaman CR, Borkoski JP. Chemokines/intercrines and central regulation of feeding. Am J Physiol. 1994;266(Part 2):R1711–R1715. doi: 10.1152/ajpregu.1994.266.5.R1711. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Sonti G, Borkoski JP, Wilson CD, French-Mullen JM. Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol Behav. 1996;60:867–875. [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: an update of human research. Alcohol Clin Exp Res. 1991;15:438–459. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Pucak ML, Kaplin Al. Unkind cytokines: current evidence for the potential role of cytokines in immune-mediated depression. Int Rev Psychiatry. 2005;17:477–483. doi: 10.1080/02646830500381757. [DOI] [PubMed] [Google Scholar]
- Quan N, Sundar SK, Weiss JM. Induction of interleukin-1 in various brain regions after peripheral and central injections of lipopolysaccharide. J Neuroimmunol. 1994;49:125–134. doi: 10.1016/0165-5728(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Raison C, Capuron L, Miller A. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S, Rivier C. Stress and interleukin-1 beta-induced activation of c-fos, NGFI-B and CRF gene expression in the hypothalamic PVN: comparison between Sprague–Dawley, Fisher-344 and Lewis rats. J Neuroendocrinol. 1994;6:101–117. doi: 10.1111/j.1365-2826.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res. 1996;20:240–254. doi: 10.1111/j.1530-0277.1996.tb01636.x. [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic–pituitary–adrenal axis in the rat: role of corticotropin- releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–131. [PubMed] [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin- releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Schrott LM, Crnic LS. Increased anxiety behaviors in autoimmune mice. Behav Neurosci. 1996;110:492–502. doi: 10.1037//0735-7044.110.3.492. [DOI] [PubMed] [Google Scholar]
- Shintani F, Nakaki T, Kanba S, Kato R, Asai M. Role of interleukin-1 in stress responses. A putative neurotransmitter. Mol Neurobiol. 1995a;10:47–71. doi: 10.1007/BF02740837. [DOI] [PubMed] [Google Scholar]
- Shintani F, Nakaki T, Kanba S, Sato K, Yagi G, Shiozawa M, et al. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. Neuroscience. 1995b;15:1961–1970. doi: 10.1523/JNEUROSCI.15-03-01961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizuya K, Komori T, Fujiware R, Miyahara S, Ohmori M, Nomura J. The influence of restraint stress on the expression of mRNAs for IL-6 and the IL-6 receptor in the hypothalamus and midbrain of the rat. Life Sci. 1997;61(PL):135–140. doi: 10.1016/s0024-3205(97)00608-5. [DOI] [PubMed] [Google Scholar]
- Simen B, Duman C, Simen A, Duman R. TNFα signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Shintani F, Kanba S, Asai M, Nakaki T. Immobilization stress increases mRNA levels of interleukin-1 receptor antagonist in various rat brain regions. Cell Mol Neurobiol. 1997;17:557–562. doi: 10.1023/A:1026319107528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura T, Makino S, Takao T, Asaba K, Suemaru S, Hashimoto K. Hypothalamic–pituitary–adrenocortical responses to single vs repeated endotoxin lipopolysaccharide administration in the rat. Brain Res. 1997;767:181–191. doi: 10.1016/s0006-8993(97)00460-5. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Regulation of the HPA axis by cytokines. Brain Behav Immun. 1995;9:253–275. doi: 10.1006/brbi.1995.1026. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Regulation of the hypothalamic– pituitary–adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999a;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Sprague–Dawley rats obtained from different vendors exhibit distinct adrenocorticotropin responses to inflammatory stimuli. Neuroendocrinology. 1999b;70:186–195. doi: 10.1159/000054475. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Pitossi FJ, Leburn JJ, Lee S, Meltzer JC, Nance DM, et al. Inhibition of tumor necrosis factor-alpha action within the CNS markedly reduces the plasma adrenocorticotropin response to peripheral local inflammation in rats. J Neurosci. 1997;17:3262–3273. doi: 10.1523/JNEUROSCI.17-09-03262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufnal M, Dudek M, Zera T, Szczepanska-Sadowska E. Centrally administered interleukin-1 beta sensitizes to the central pressor action of angiotensin II. Brain Res. 2006;19:64–72. doi: 10.1016/j.brainres.2006.04.122. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- Yamada K, Lida R, Miyamoto Y, Saito K, Sekikawa K, Seishima M, et al. Neurobehavioral alterations in mice with a targeted deletion of the tumor necrosis factor-alpha gene: implications for emotional behavior. J Neuroimmunol. 2000;111:131–138. doi: 10.1016/s0165-5728(00)00375-1. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Pro- and anti-addictive neurotropic factors and cytokines in psychostimulant addiction: Mini Review. Ann NY Acad Sci. 2004;1025:198–204. doi: 10.1196/annals.1316.025. [DOI] [PubMed] [Google Scholar]
- Zhu C, Blakely R, Hewlett W. The pro-inflammatory cytokines IL-1β and TNF-α activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- Zujovic V, Schussler N, Jourdain D, Duverger D, Taupin V. In vivo neutralization of endogenous brain fractalkine increases hippocampal TNFalpha and 8-isoprostane production induced by intracerebroventricular injection of LPS. J Neuroimmunol. 2001;115:135–143. doi: 10.1016/s0165-5728(01)00259-4. [DOI] [PubMed] [Google Scholar]


