Abstract
Background
Major depression is characterized by a negativity bias: an enhanced responsiveness to, and memory for, affectively negative stimuli. However it is not yet clear whether this bias represents (1) impaired top-down cognitive control over affective responses, potentially linked to deficits in dorsolateral prefrontal cortex function; or (2) enhanced bottom-up responses to affectively-laden stimuli that dysregulate cognitive control mechanisms, potentially linked to deficits in amygdala and anterior cingulate function.
Methods
We used an attentional interference task using emotional distracters to test for top-down versus bottom-up dysfunction in the interaction of cognitive-control circuitry and emotion-processing circuitry. A total of 27 patients with major depression and 24 controls were tested. Event-related functional magnetic resonance imaging was carried out as participants directly attended to, or attempted to ignore, fear-related stimuli.
Results
Compared to controls, patients with depression showed an enhanced amygdala response to unattended fear-related stimuli (relative to unattended neutral). By contrast, control participants showed increased activity in right dorsolateral prefrontal cortex (Brodmann areas 46/9) when ignoring fear stimuli (relative to neutral), which the patients with depression did not. In addition, the depressed participants failed to show evidence of error-related cognitive adjustments (increased activity in bilateral dorsolateral prefrontal cortex on post-error trials), but the control group did show them.
Conclusions
These results suggest multiple sources of dysregulation in emotional and cognitive control circuitry in depression, implicating both top-down and bottom-up dysfunction.
Introduction
A primary feature of major depression (MDD) is a preoccupation with negative ideation. Many behavioral studies have documented an enhanced attention to, and memory for, negative emotional stimuli in depression [1–4]. However, the source of this bias is unclear. One possibility is that this negativity bias reflects a top-down deficit in the control of attention (for example, a failure to suppress distracting emotional influences), potentially linked to deficits in brain regions supporting cognitive control such as dorsolateral prefrontal cortex (DLPFC) or dorsal anterior cingulate (dorsal ACC) [5, 6]. Alternatively, this bias may reflect an enhanced bottom-up response to emotional stimuli that dysregulates cognitive control mechanisms, potentially linked to deficits in amygdala and ventromedial prefrontal cortex function.
Recent research [7] has identified a network of emotion-processing areas that might drive bottom-up influences of emotion on cognitive functioning in depression. These include the amygdala and ventromedial prefrontal cortex (subgenual and pregenual cingulate). It has been proposed that these areas are involved in the perception, evaluation and response to emotion-inducing stimuli and that they mediate the experience of fear, sadness and other negative emotions [8–10]. Both ventromedial areas and the amygdala are normally deactivated during cognitive processing, and increase activation during the experience of fear, anxiety or sadness [11]. Individuals with major depression show hyperactivity of the amygdala when processing emotionally evocative information [12–14]. In addition, resting-state overactivity in the subgenual cingulate is consistently found in major depression [9, 15, 16]. If these emotion regions are hyper-responsive in major depression, they may bias individuals towards the processing of affectively negative stimuli.
A negativity bias might also reflect primary dysfunction in cognitive control areas of the brain. Some studies have shown that the DLPFC plays an important role in the top-down regulation of emotional processing [5, 17]. In addition, the dorsal ACC is thought to monitor for errors or processing conflicts that could disrupt performance and to recruit the DLPFC to reallocate attentional resources as needed [18–25]. Importantly, some research suggests that MDD is characterized by hypoactivity in DLFPC and dorsal ACC [6, 26] as well as in rostral cingulate [27].
As noted above, both excessive activity in the amygdala and reduced activity in DLPFC have been documented in MDD patients [6, 12, 13]. For example, MDD patients have long been found to show elevated activity in the amygdala during passive resting or during sleep [9, 28]. They have also shown excessive amygdala activity when exposed to stimuli with negative valence that are presented outside of conscious awareness [12]. However, less is known about amygdala function in depression when patients are actively engaged in demanding cognitive processing. In such situations, processing in cognitive-control regions of the brain may suppress emotion-processing regions such as the amygdala, since these two circuits are known to work in opposition to each other (Drevets & Raichle, 1998). Recently Siegle and colleagues [29] tested MDD patients on a demanding executive task and a separate emotion-processing task. They found reduced activation in dorsolateral prefrontal cortex in the executive task, as well as increased amygdala activity in the emotional task. However, these findings do not address the issue of amygdala reactivity in MDD when there could be direct competition between cognitive and emotion circuitry. Such conflict can occur in cognitive tasks that include task-irrelevant emotional information, since these tasks should evoke activity in two networks that would normally suppress each other. Thus the goal of the current study is to investigate the pattern of recruitment seen in these two networks when individuals with MDD were asked to either ignore, or directly attend to, emotionally negative stimuli. In doing so, we hoped to examine top-down and bottom-up influences when cognitive control was needed, and when it was not.
To investigate these questions, we performed an event-related fMRI study in which MDD patients and controls performed a matching task while exposed to emotional interference [30, 31]. Stimuli were fearful or neutral faces, or houses, and the face stimuli were either targets or distracters. Trials with fearful faces as distracters were considered to generate emotional interference and would therefore require cognitive control. In addition, we considered error trials as possible sources of emotional conflict. As a second test of cognitive control, we examined activation on trials following emotional conflict trials, since in healthy controls, both errors and conflict trials usually induce increased cognitive control on subsequent trials [32–34].
We made several predictions based on the two hypotheses about the source of negative bias in depression. If this bias reflects deficits in the top down control of attention, then compared to healthy controls, individuals with MDD should show: 1) on correct trials, impaired activity in DLPFC and the dorsal ACC on all trials; (2) on correct trials, enhanced activity in the amygdala and ventromedial PFC when ignoring fearful faces, (and possibly also when attending to them) because of inadequate suppression by cognitive control regions; 3) on error trials, enhanced activity in the amygdala and ventromedial PFC, because negative affect associated with errors could not be appropriately regulated by the DLPFC and the dorsal cingulate; 4) on error trials, reduced dorsal ACC responses and 5) on trials following errors, reduced DLPFC response, reflecting impaired control recruitment.
If the negativity bias in MDD reflects abnormal bottom-up responses to emotional stimuli, we would predict: 1) on correct trials, enhanced activity in the amygdala and subgenual/pregenual ACC when ignoring fearful faces, and possibly also when attending to them (similar to the top down model, but not because of reduced cognitive control); 2) in contrast to the top-down model, for correct trials, impaired DLPFC, but only on trials in which the participant shows enhanced amygdala response to negative stimuli; 3) on error trials, possibly either reduced or enhanced dorsal ACC responses to errors, depending on whether MDD participants experience suppressed cognitive control, or instead more readily detect conflict from emotionally evocative events; and 4) on post-error trials, impaired recruitment of DLPFC if the enhanced bottom-up processing of the negative stimuli impairs DLPFC recruitment.
Amongst all these predictions, we viewed the behavior of DLPFC as key to the distinction between top-down and bottom-up influences, since it might show dysfunction either on all trials (top-down), or only when the amygdala was over-active (bottom-up).
Methods
Participants
Participants were 27 patients with major depression (M/F: 10/17, mean age: 33.4 years (SD 8), mean education: 15 years (SD 2.2)), and 24 demographically matched controls (M/F: 12/12, mean age: 36.4 years (SD 9), mean education: 16 years (SD 2.3)). Inclusion criteria for depressed subjects were a current episode of unipolar recurrent major depression by DSM-IV criteria [35]. All participants were free of psychotropic medication for a minimum of four weeks and were administered a 17-item Hamilton Rating Scale for Depression (HRSD) [36] to determine depression severity. Depressed participants were included with HRSD scores 18 or above (mean: 20, SD 2.3) and control participants with scores less than 8 (mean: 0.3, SD .6). Patients were excluded for any Axis I disorder (other than MDD) that preceded the onset of MDD. Additional exclusion criteria were acute physical illness, history of trauma resulting in loss of consciousness, current neurological disorder, lifetime psychiatric disorder (other than major depression for the patients). All participants provided written informed consent in accordance with criteria established by the Washington University Human Subjects Committee. Seven additional participants (6 patients, 1 control) completed behavioral testing but withdrew from the study before undergoing scanning. The two groups did not differ significantly in age or gender (proportion of females). However, the controls showed a tendency to have greater educational attainment (p=.07). Participants were paid $25.00 per hour for their participation.
Procedure
The emotional interference experiment was carried out as part of a larger study that included two other scanning tasks (data for which will be reported separately). Scanning for the emotional interference task occurred on a second day, and was always carried out before the other tasks. At the beginning of the session, participants were instructed on how to do the task, to emphasize speed and not worry about mistakes. They were given practice trials inside the scanner, using neutral faces only.
The emotion-interference task [30, 31] presented participants with a pair of houses and a pair of faces in each trial, with one pair arranged horizontally and the other vertically around a central fixation cross. Participants were instructed to fixate on the cross and attend to the horizontal or vertical axis for a given block (4 blocks total, counterbalanced order). Positioning of face-pairs or house-pairs was random. For each trial, the task was to tell whether the two items in the target axis were the same or different. Participants responded by button-press on a fiber optic response box interfaced with PsyScope [37]. Each block contained 13 trials for each attention × emotion condition, pseudo-randomly interleaved throughout the block. Thus trial types were: attend-fearful-faces, attend-neutral-faces, ignore-fearful-faces (attend-houses), and ignore-neutral-faces (attend-houses). For each trial, the two faces displayed were either both neutral or both fearful, with the two expression types occurring equally often in a block. Each trial lasted 3200 milliseconds, starting with a fixation (displayed for 1000 milliseconds), after which the four stimuli appeared for 250 milliseconds. Participants had 2200 milliseconds to make a response. An ITI then took place that varied randomly between five possible lengths (2150, 4660, 7170, 9680, or 12190 milliseconds).
fMRI imaging and analysis
Image acquisition
fMRI images were collected on a Siemens 3T Allegra MRI scanner (Erlangen, Germany). The protocol included localizer images, a high-resolution structural image (MPRAGE), and a series of functional images. The structural images were acquired with 1 × 1 × 1.25 resolution using a sagittal 3-D T1-weighted sequence with repetition time (TR) of 1.9 seconds, time-to-echo (TE) of 3.93 ms flip angle = 7 degrees, and inversion time (TI) of 1000 milliseconds. Functional images were collected using an asymmetric spin-echo echo-planar sequence with volume TR=2.5 seconds (slice TR= 64.10 ms), TE=25 ms flip angle=90 degrees and field of view (FOV) of 205 cm. One acquisition consisted of 39 transverse slices, 3.2 mm thick (no gap), and with an in-plane resolution of 3.2 × 3.2 mm. Each functional run began with four volume images that were not analyzed, followed by 160 acquisitions for the paradigm.
Image analysis
The functional imaging data were preprocessed to correct for asynchronous slice acquisition and odd/even slice intensity differences caused by interleaving. Following this, the data were rigid body motion corrected [38, 39]. Atlas transformation (12 parameter affine) of the functional data was computed via the structural images. Our atlas representative target image conforms to the space of Talairach & Tournoux [40] as defined by Lancaster and colleagues [41]. The final preprocessing step combined motion correction and atlas transformation in one resampling to 3 mm isotropic voxels. Before statistical analysis, the data were smoothed using a Gaussian filter with 9 mm full-width half-maximum.
For each participant, a General Linear Model (GLM) was used to estimate hemodynamic model-independent [42] event related responses over 17.5-seconds (7 frames). Separate regressors were used to estimate response to each facial emotion (fear vs. neutral), attention condition (attend to house, attend to face) and trial type (same or different), yielding a total of 8 response types. Preliminary analyses showed no effects attributable to the same versus different dimension. Accordingly, all present analyses were collapsed across this dimension, leaving four main conditions: 1) attend to fearful faces, 2) attend to neutral faces, 3) ignore fearful faces and 4) ignore neutral faces. We computed a response magnitude estimate for each condition based on the cross-correlation of the time-series with an assumed canonical hemodynamic response shape [43]. All analyses reported below were based on analyses of variance (ANOVA) and t-tests conducted with subject as a random factor.
ROI identification
To test our hypotheses, we used a priori defined regions of interest (ROI) including left and right amygdala, subgenual cingulate, pregenual cingulate, more superior rostral cingulate, dorsal anterior cingulate, and right and left dorsolateral prefrontal cortex. The dorsolateral ROIs were defined on an atlas-representative image using the boundaries described by [44]. The more superior area of rostral ACC (which we call “superior rostral” in this paper) has been implicated in cognitive control, especially the detection of errors, while the more ventral pregenual region has been linked with more overtly emotional processes [45]. To separate these two, we used a Talairach z-coordinate of 6 as a boundary. Voxels within the a priori defined ROIs showing effects of interest were identified using a two-stage process. 1) To protect against Type II error, we required voxels to show significant effects at p <.025 and to belong to clusters of at least 9 contiguous voxels [46]. 2) We then conducted regional analyses based on the clusters identified in the previous step, and (to protect against Type I error) required region results to show post-hoc effects at p <.006. Exploratory analyses. To look for non-predicted effects in regions outside the a priori ROIs, we conducted a whole-brain three-way ANOVA. The ANOVA results were thresholded to obtain a whole brain false positive rate of .05 (p <.0001 and a minimum-cluster extent of 14 or more contiguous voxels). As this ANOVA revealed no significant group-related effects, the whole-brain analysis was not pursued further.
Results
Behavioral results
To examine data, we used three-way repeated-measures analyses of variance (ANOVA) with attention (attended or ignored faces), emotion (fearful or neutral), and group (controls or depressed) as factors. No significant group-related effects were found for accuracy: main effect of group and all group interactions, p>.2. There was a main effect of group (F(1,49)= 6.575, p=.013, η2=.118) on response times, with depressed participants slower than controls. Behavioral results are summarized in table 1. We also carried out post-error analyses to examine group differences in error-related performance adjustment (see table 1). Post-error trials were significantly faster than post-correct trials (F(1,49)= 6.110, p=.017, η2=.111). There was also a trend toward a significant interaction of trial-type by group (F(1,49)= 2.692, p=.107, η2=.052), such that depressed patients showed a speed-up of approximately 60 msec for post-error versus post-correct trials, while controls showed almost no difference between the two trial types. Effects of group, trial-type, or their interaction on accuracy were non-significant. Thus, no conventional post-error effects were found for this task, but instead a speed-up on post-error trials that was mostly driven by performance in the depressed group.
Table 1.
Performance in the conflict task and the post-error analysis. (Means and SEs).
| Conflict task | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Percent accuracy | Response times | ||||||||
| Group | AF | AN | IF | IN | AF | AN | IF | IN | |
| Controls | .83±.02 | .82±.02 | .85±.02 | .85±.02 | 805±36 | 770±32 | 754±35 | 753±38 | |
| Depressed | .80±.02 | .80±.02 | .81±.02 | .83±.02 | 922±34 | 888±30 | 883±33 | 871±36 | |
| Post-error analysis | |||||||||
| Percent accuracy | Response times | ||||||||
| Group | Post-correct | Post-error | Post-correct | Post-error | |||||
| Controls | .85 ±.02 | .84 ±.02 | 777 ± 37 | 763 ± 29 | |||||
| Depressed | .80 ± .02 | .81 ± .02 | 906 ± 35 | 839 ± 27 | |||||
fMRI results
In this section we describe findings for all regions showing significant group-related effects. Significant effects that were not group-related are presented in table 2 but not further discussed here.
Table 2.
Group and non-group-related effects for the conflict task.
| Attention x Emotion x Group | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxels | Side | x | y | z | Z-val. | Effect |
| Amygdala | 28 | L | −18 | −5 | −19 | 3.02 | (see text) | |
| Middle frontal G. | 9/46 | 41 | R | 36 | 27 | 29 | 2.58 | (see text) |
| Attention x Group | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxels | Side | x | y | z | Z-val. | Effect |
| Pregenual cing. | 24 | 33 | L | −10 | 35 | −2 | 2.73 | Cont: Attend>Ignore Depr: Ignore>Attend |
| Emotion x Group | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxels | Side | x | y | z | Z-val. | Effect |
| Dorsal cingulate | 32 | 57 | R | 4 | 17 | 41 | 2.52 | Cont: Neutral> Fear Depr: Fear>Neutral |
| Main effect of group | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxels | Side | x | y | z | Z-val. | Effect |
| Subgenual cingulate | 25 | 40 | L | −6 | 13 | −13 | 2.66 | Depr > Cont |
| Superior-rostral cing. | 24 | 25 | 0 | 13 | 29 | 2.92 | Cont > Depr | |
| Dorsal cingulate | 24 | 29 | 0 | 13 | 34 | 2.92 | Cont > Depr | |
| Effect of Attention | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxels | Side | x | y | z | Z-val. | Effect |
| Amygdala | 67 | L | −21 | −6 | −14 | 3.34 | Attend > Ignore | |
| Amygdala | 59 | R | 21 | −6 | −12 | 4.08 | Attend > Ignore | |
| Middle frontal G. | 9 | 128 | R | 40 | 20 | 27 | 3.75 | Attend > Ignore |
| Superior frontal G. | 9 | 65 | R | 18 | 40 | 30 | 2.7 | Attend > Ignore |
| Inferior frontal G. | 44 | 16 | L | −38 | 16 | 26 | 2.61 | Attend > Ignore |
| Middle frontal G. | 9,8 | 36 | L | −39 | 22 | 42 | 3.25 | Ignore>Attend |
| Dorsal cingulate | 32 | 141 | R | 3 | 21 | 38 | 3.13 | Attend > Ignore |
| Dorsal cingulate | 32 | 57 | R | 4 | 17 | 41 | 2.83 | Attend > Ignore |
| Effects of Emotion | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxels | Side | x | y | z | Z-val. | Effect |
| Pregenual cing. | 32 | 83 | L | −3 | 40 | −3 | 3.24 | Neutral > Fear |
| Superior-rostral cing. | 32 | 23 | R | 11 | 39 | 20 | 2.63 | Neutral > Fear |
| Superior frontal G. | 8 | 14 | L | −20 | 30 | 43 | 3.01 | Neutral > Fear |
| Mid./sup.frontal G. | 8 | 181 | L | −24 | 24 | 44 | 3.6 | Neutral > Fear |
| Middle frontal G. | 8,32 | 202 | R | 20 | 28 | 41 | 3.79 | Neutral > Fear |
| Attention x Emotion | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxels | Side | x | y | z | Z-val. | Effect |
| Superior-rostral cing. | 24 | 63 | R | 4 | 28 | 21 | 2.61 | Attend: Neut > Fear Ignore: Fear > Neut |
Group effects of depression
We found (table 2, figure 4) a significant main effect of group in subgenual cingulate (BA 25) and in a second region spanning from superior rostral cingulate (BA 24, at Talairach z-coordinate 28–30) up into the lower dorsal ACC (BA 32, z =34). In the subgenual area, depressed patients showed significantly greater activation (less deactivation) than controls. In the superior rostral ACC region, activation was significantly lower for the depressed than controls.
Figure 4.
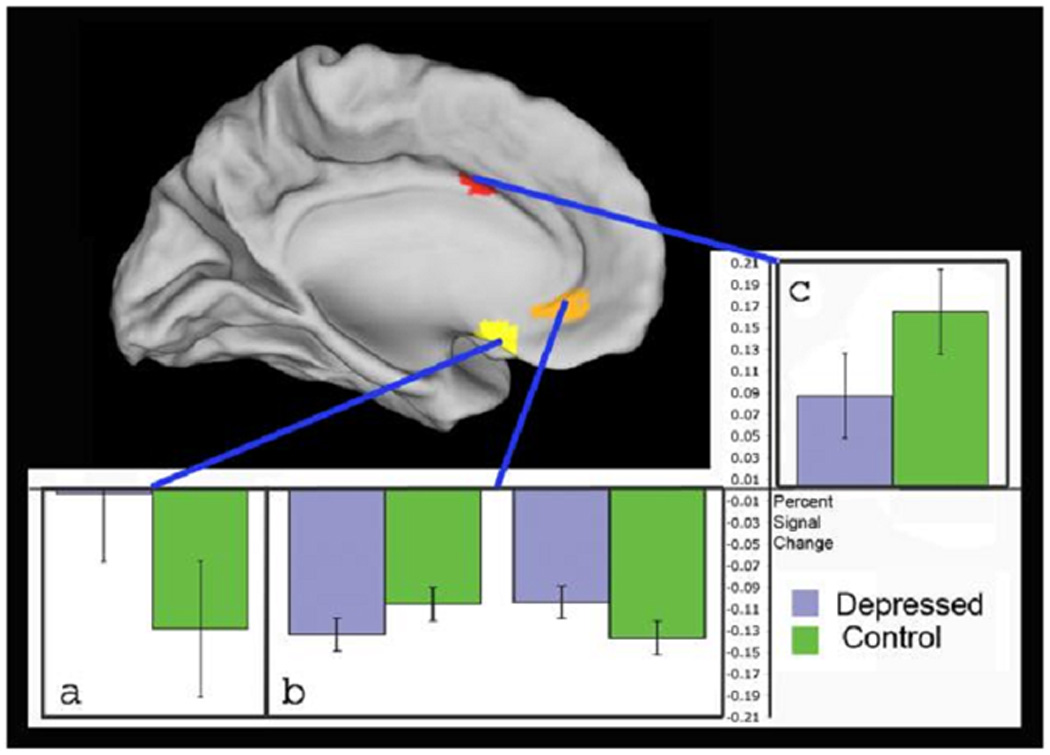
Areas in the subgenual anterior cingulate (a) and superior-rostral anterior cingulate (c) show significant group differences across all conditions. Areas in pregenual cingulate (b) show significant differences in a group × attention interaction, where controls had less deactivation in the attend-to-faces conditions (left side of graph) while depressed had less deactivation in the ignore-faces conditions (right side of graph). Graphs show percent change in signal magnitude for each region. Error bars show standard errors of the mean.
Effects of attended and unattended fear
To test the hypothesis that depressed participants are more sensitive than controls to unattended fear-related stimuli, we looked at activation in the three-way interaction of attention × emotion × group. Only two regions showed significant three-way effects (figure 2, with details provided in the supplementary information). As predicted, we found that the MDD patients (p=.05), but not the controls (p>.1), showed significantly increased activation in the left amygdala in the contrast of the ignore-fear versus ignore-neutral conditions. Further, this fear-related increase in the ignore condition was significantly larger for the MDD patients than controls (p<.05). The opposite pattern was found for the attend condition. Controls (p<.05), but not MDD (p>.1) showed significant activation of left amygdala in the contrast of attend-fear versus attend-neutral. In a direct contrast of the two groups, this fear-related increase in the attend condition was significantly larger in controls than in MDD (p<.01). We found a different pattern in the right DLPFC. The controls (p<.01), but not the MDD (p>.10) showed a significant increase in right DLPFC activity for ignore-fear versus ignore neutral, with this increase significantly greater in controls than MDD (p<.05). Neither group showed a significant difference in right DLPFC activity for the attend-fear versus attend-neutral contrast (p>.1), nor were there significant group differences for this contrast.
Figure 2.
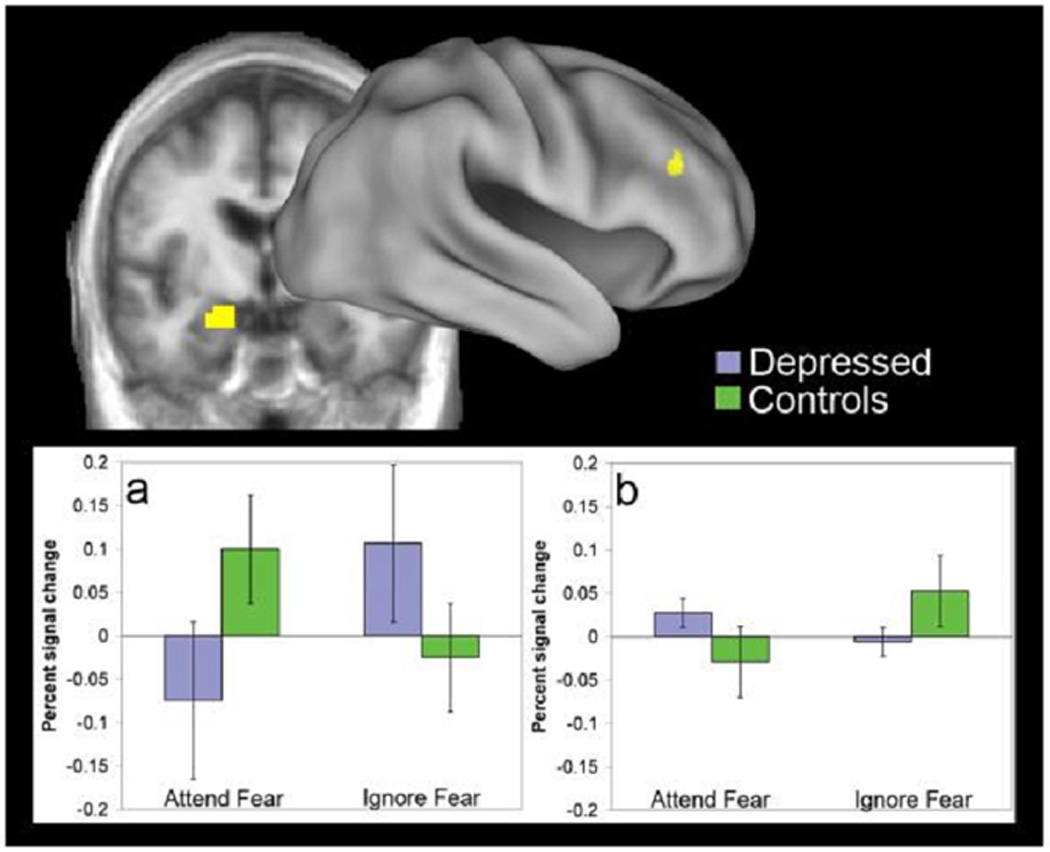
Areas in the left amygdala (a) and right dorsolateral prefrontal cortex (b) showing a significant three-way interaction of attention × emotion × group. Graphs show percent change in signal magnitude for the fear-minus-neutral contrast in each region. Error bars show standard errors of the mean.
Effects of attending versus ignoring faces
One region showed a significant interaction between attention and group (table 2, figure 4). This was an area in pregenual cingulate that showed deactivations overall. The interaction with group reflected a cross-over pattern such that the control group showed greater deactivation (lower activation) in pregenual ACC than the depressed in the ignore face conditions, while in the attend conditions, the control group showed less deactivation than the depressed.
Error analysis
We looked for group differences in the contrast of correct-trial processing versus error-trial processing. An area in the dorsal cingulate region (BA 32 and 24:Talairach z =33–45) showed significantly increased activation for error versus correct trials, but this effect did not differ by group. Only one area, in the pregenual cingulate, showed an interaction between trial-type and group. In this region, both groups showed deactivation for correct trials, and both increased activation (lost deactivation) on errors; however the depressed group increased activation more sharply than the controls.
Post-error analysis
All correct trials were classified as either post-correct (following a correct trial) or post-error (following an error trial). We found two areas in right and left DLPFC (figure 3 and table 3) that showed significant group differences in the contrast of post-correct versus post-error processing. Both areas showed the same pattern. Both controls and depressed showed a modest deactivation on post-correct trials. However, for the post-error trials, the controls increased activation significantly (into the positive range), consistent with recruiting stronger cognitive control, while the depressed did not change.
Figure 3.
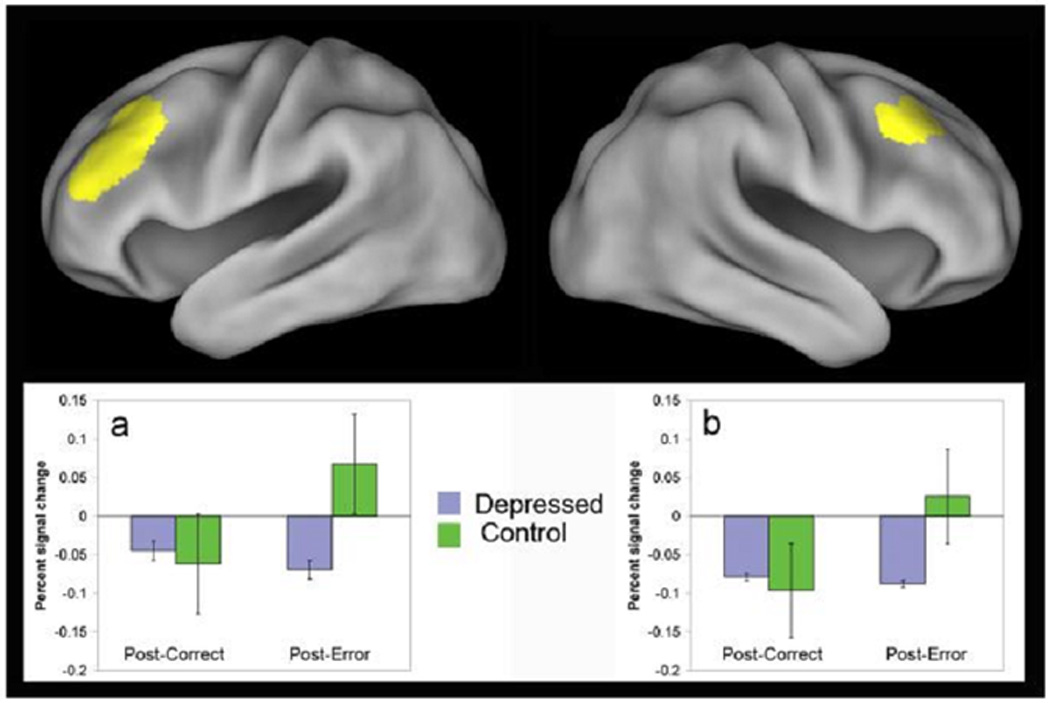
Areas in left (a) and right (b) dorsolateral prefrontal cortex showing significant group differences in the post-error effect: interaction of trial-type (post-correct versus post-error) × group. Graphs show percent change in signal magnitude for each region. Error bars show standard errors of the mean.
Table 3.
Error-related effects in the neuro-imaging data.
| Correct trials vs error trials: Trial-type x Group | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxels | Side | x | y | z | Z-val. | Effect |
| Pregenual cing. | 11 | 36 | L | −8 | 36 | −12 | 2.55 | Cont: Error>Corr Depr: Error>>Corr |
| Correct trials vs error trials: Effects of Trial-type | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxels | Side | x | y | z | Z-val. | Effect |
| Dorsal cingulate | 32 | 287 | 0 | 13 | 40 | 5.29 | Error>Correct | |
| Pregenual cing. | 24 | 393 | L | −1 | 36 | 6 | 3.85 | Correct>Error |
| Middle frontal G. | 9,46 | 126 | R | 42 | 26 | 26 | 3.95 | Error>Correct |
| Middle frontal G. | 9,46 | 77 | L | −40 | 24 | 28 | 3.67 | Error>Correct |
| Inferior frontal G. | 10,46 | 39 | L | −33 | 37 | 10 | 3.05 | Correct>Error |
| Middle frontal G. | 8,9 | 407 | L | −23 | 35 | 38 | 4.69 | Correct>Error |
| Mid/Sup frontal G. | 8 | 428 | R | 20 | 34 | 38 | 4.69 | Correct>Error |
| Post-correct vs post-error trials: Trial-type x Group | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxels | Side | x | y | z | Z-val. | Effect |
| Middle frontal G. | 9 | 198 | L | −38 | 31 | 33 | 3.48 | Depr: Posterr=Postcorr Cont: Posterr>Postcorr |
| Middle frontal G. | 8,9 | 52 | R | 37 | 19 | 42 | 2.83 | Depr: Posterr=Postcorr Cont: Posterr>Postcorr |
| Post-correct vs post-error trials: Effect of Trial-type | ||||||||
| Talairach coordinates | ||||||||
| Brain region | BA | Voxels | Side | x | y | z | Z-val. | Effect |
| Pregenual cing. | 24 | 32 | L | −1 | 26 | −2 | 2.56 | Posterr > Postcorr |
| Superior-rostral cing. | 9,32 | 27 | L | −5 | 38 | 30 | 2.5 | Posterr > Postcorr |
| Inferior frontal G. | 10,46 | 10 | R | 31 | 31 | 13 | 2.55 | Posterr > Postcorr |
| Mid/Sup.frontal G. | 9 | 334 | L | −26 | 34 | 32 | 3.65 | Posterr > Postcorr |
| Middle frontal G. | 9 | 438 | R | 27 | 29 | 32 | 3.65 | Posterr > Postcorr |
Correlational analyses
We conducted correlational analyses to look for similarities in fear-related activation between right DLPFC and left amygdala. We found a significant negative correlation between activity in these regions only in the depressed, and then only in the attend condition, not in the ignore condition, r= −.726, p=.000.
Discussion
This study investigated the negativity bias in depression by asking whether this bias reflected dysfunction in emotional processing or impaired cognitive control over emotion. The main result was a depression-related difference in both the right DLPFC and the left amygdala in response to fearful versus neutral stimuli, an effect strongly modulated by attention. As predicted for the emotional interference condition, the depressed showed enhanced amygdala responses to unattended fear-related stimuli while the controls did not. In the same conditions, the controls recruited the DLPFC while the depressed did not. The enhanced amygdala activation seen in the depressed while ignoring fear-related stimuli, and their failure to recruit DLPFC, suggest that the patients did not suppress emotional responses to fear-related distracters. This is consistent with findings suggesting that depression entails a particular sensitivity to negative stimuli that are unattended [12]. The controls’ robust recruitment of right DLPFC in the same condition suggests that cognitive control recruitment is normally increased in response to fear distracters. Enhanced DLPFC activation could be recruited to increase selective attention, consistent with theories of attentional control. Increased attention to houses could decrease activation in face-processing areas, perhaps reducing amygdala activation as a side effect. Alternatively, DLPFC might be recruited to directly suppress amygdala activity, consistent with theories of emotion regulation [17, 47, 48].
An opposite and more surprising result was found in the attend condition. The controls showed no DLPFC increases in this condition, (perhaps because emotional stimuli facilitated attention) but did increase amygdala activation. This suggests that amygdala responses are normally not suppressed when fear stimuli are directly attended. By contrast, the depressed in this condition showed a pattern normally associated with affect regulation: increased DLPFC and deactivated amygdala indicating they were able to suppress amygdala responses when directly attending to fear stimuli. A supposition of explicit affect regulation in the patients is supported by the strong negative correlation between fear-related activity in the right DLPFC and left amygdala in the attend conditions.
While amygdala and DLPFC activation depended on attention and facial expressions, other regions showed robust main effects. Subgenual ACC activation was increased in the depressed patients, consistent with findings of elevated resting metabolism in this region in depression [16]. The dorsal and superior-rostral cingulate showed decreased activation in the depressed patients. Given the proposed role for these areas in conflict monitoring and error-processing [25, 49, 50], hypoactivity here could predispose depressed patients to deficits in cognitive control. Nevertheless, the dorsal cingulate increased activity on error trials in the depressed, consistent with unimpaired error processing. On the other hand, in the pregenual cingulate during error trials, the depressed patients failed to increase activation as the controls did. Moreover, on post-error trials, the patients failed to increase activity in DLPFC as the controls did, suggesting an impairment in recruiting post-error cognitive adjustments. This effect is consistent with their hypoactivity in superior-rostral cingulate, a region where dysfunction has been linked to failure to increase cognitive control after committing errors [27].
A primary goal of this study was to determine whether abnormal function in depression implicates dysfunction in cognitive or emotional circuitry. In the depressed patients, reduced recruitment in right DLPFC was only present when the amygdala was over-active, arguing against a primary dysfunction in the DLPFC. This finding suggests that amygdala over-activity had a bottom-up influence on the level of activity in DLPFC. In contrast, the dorsal cingulate showed global deficits independent of emotional condition or level of amygdala activity. Importantly however, the depressed participants showed enhanced amygdala activity only when ignoring fearful faces, not when attending to them. This suggests that when negative stimuli were explicitly attended, amygdala responsiveness could be modulated. Thus, depression may involve a primary dysfunction in both cognitive control and emotion areas, but the two systems may also modulate each other. Indeed, in the development of early depressive episodes, compromises in one system could lead to and lock in dysregulation of the other.
The design of this study involves some limitations. First, contrary to the findings of Vuilleumier and colleagues [30], our study did not find increased amygdala activation to unattended fear stimuli in controls. However, increased amygdala activation is not found consistently in healthy people [51], and may depend on other factors such as cognitive load [52] or anxiety level [31, 53]. Indeed, in the current study, removal of anxiety variance from our analyses (see supplementary information) weakened or abolished some of our depression effects, consistent with the known importance of anxiety in mood disorders. Future studies might pursue the separate contributions of anxiety and mood symptoms by comparing responses of depressed patients to sadness-related versus fear-related distracters.
Secondly, although the MDD participants were overall slower, we found no performance deficits related to task factors, despite clear evidence for functional brain changes that were specific to certain task conditions. Functional brain activity may have more sensitivity to detect cognitive or emotional processing changes in MDD than purely behavioral measures. In addition, we found no post-error slowing, even in the controls. This may have been an artifact of the rapid inter-trial intervals used, which may have prevented participants from being able to slow down after errors. We believe that the non-significant tendency of the patients to speed up after errors is consistent with their failure to increase DLFPC activation in post-error trials. However, given the lack of performance effects, our brain activation findings, while suggesting a basis for the negativity bias in depression, must be interpreted with caution.
Conclusion
Depressed subjects exhibited a bottom-up impairment in emotional processing, as summarized above. In addition, depressed patients showed impaired top-down cognitive control over affective interference. When exposed to emotional distracters during a cognitive task, control participants were able to recruit dorsolateral PFC and suppress amygdala activation. By contrast, depressed individuals showed exaggerated amygdala response to such distracters, and a failure to recruit DLPFC. Simultaneously, the patients in this study showed error-processing abnormalities that may reflect downstream effects of insufficient error monitoring in dorsal cingulate cortex.
Supplementary Material
Figure 1.

Example of a stimulus screen used in the emotional conflict task.
Acknowledgements
This work was supported by National Institute of Mental Health RO1 MH64821 and K24 RR18192 awarded to Y.I.S. and National Institutes of Health R01 MH06603101 awarded to D.M.B. The granting agencies had no role in any of the following aspects of this study: design and conduct of the study; collection, management, analysis and interpretation of the data; or preparation, review, or approval of the manuscript. Authors C.L.F., D.M.B., and Y.I.S. are independent of any commercial provider, had full access to all of the data in this study, and take responsibility for the integrity of the data and the accuracy of the data analysis. No author named on the title page of this study has any financial interest in the results of the study, nor any other conflict of interest relevant to the subject matter of this manuscript. We thank Anthony Durbin and Adrian Epstein for help with data acquisition and data processing. Correspondence concerning this article should be addressed to Dr. Yvette I. Sheline, Department of Psychiatry, Campus Box 8134, Washington University School of Medicine, 660 S. Euclid Ave, St. Louis MO 63110 (email: yvette@npg.wustl.edu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The contents of this paper were initially reported in a poster presented at the 36th meeting of the Society for Neuroscience, October 2006, held in Atlanta, Georgia. This work was supported by a National Institutes of Mental Health grant RO1 MH64821 and K24 RR18192 awarded to Y.I.S.
References
- 1.Williams JMG, Oaksford M. Cognitive science, anxiety, and depression: From experiments to connectionism. In: Young S, editor. Cognitive science and the clinical disorders. San Diego, CA: Academic Press; 1992. [Google Scholar]
- 2.Norman WH, Miller IW, Dow MG. Characteristics of depressed patients with elevated levels of dysfunctional cognitions. Cognitive Therapy and Research. 1988;12:39–52. [Google Scholar]
- 3.Wenzlaff RM, Wegner DM, Roper DW. Depression and mental control: The resurgence of unwanted negative thoughts. Journal of Personality and Social Psychology. 1988;55:882–892. doi: 10.1037//0022-3514.55.6.882. [DOI] [PubMed] [Google Scholar]
- 4.Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: The role of awareness. British Journal of Clinical Psychology. 1995;34(1):17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 5.Ochsner K, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Mayberg HS, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 7.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 8.Bechara A, et al. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- 9.Drevets WC. Neuroimaging and neuropathological studies of depression: Implications for the cognitive-emotional features of mood disorders. Current Opinion in Neurobiology. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 10.Morris JS, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implication for interactions between emotion and cognition. Cognition and Emotion. 1998;12(3):353–385. [Google Scholar]
- 12.Sheline YI, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 13.Siegle GJ, et al. Can't shake that feeling: Event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 14.Fu CHY, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 15.Drevets WC, et al. A functional anatomical study of unipolar depression. Journal of Neuroscience. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Ochsner KN, et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Botvinick MM, et al. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 19.Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- 20.Bunge SA, et al. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17(3):1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- 21.Yeung N, Cohen JD, Botvinick MM. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111(4):931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- 22.Carter CS, et al. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 23.Davis KD, et al. Human anterior cingulate cortex neurons encode cognitive and emotional demands. Journal of Neuroscience. 2005;25(37):8402–8406. doi: 10.1523/JNEUROSCI.2315-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerns JG, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 25.Braver TS, et al. Anterior cingulate cortex and response conflict: Effects of frequency, inhibition and errors. Cerebral Cortex. 2001;11(9):825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- 26.Davidson RJ, et al. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 27.Pizzagalli DA, et al. Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: A 128-channel EEG study. Human Brain Mapping. 2006;27:185–201. doi: 10.1002/hbm.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Progress in Brain Research. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 29.Siegle GJ, et al. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 30.Vuilleumier P, et al. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 31.Bishop S, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. Journal of Neuroscience. 2004;24(26):10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grattan G, Coles MG, Donchin E. Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- 33.Laming D. Information theory of choice response times. London, UK: Academic Press; 1968. [Google Scholar]
- 34.Rabbitt PMA. Errors and error correction in choice-response tasks. Journal of Experimental Psychology: General. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- 35.Association AP, editor. DSM-IV-R: Diagnostic and statistical manual of mental disorders. 4th ed. Washington, D.C.: American Psychiatric Association; 2000. [Google Scholar]
- 36.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen J, et al. PsyScope: An interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavior Research Methods, Instruments, & Computers. 1993;25:257–271. [Google Scholar]
- 38.Friston KJ, et al. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 39.Snyder AZ. Difference image versus ratio image error function forms in PET-PET realignment. In: Bailer D, Jones T, editors. Quantification of Brain Functions using PET. San Diego, CA: Academic Press; 1996. pp. 131–137. [Google Scholar]
- 40.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Georg Thiem Verlag; 1988. [Google Scholar]
- 41.Lancaster J, et al. A modality-independent approach to spatial normalization of tomographic images of the human brain. Human Brain Mapping. 1995;3:209–223. [Google Scholar]
- 42.Ollinger J, Shulman G, Corbetta M. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13(1):210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- 43.Boynton GM, et al. Linear systems analysis of functional magnetic resonance imaging in human VI. Journal of Neuroscience. 1996;16(13):4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach coordinate system. Cerebral Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- 45.Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and medial prefrontal cortex, a substrate for emotional behavior? Progress in Brain Research. 1996;107:523–536. doi: 10.1016/s0079-6123(08)61885-3. [DOI] [PubMed] [Google Scholar]
- 46.Forman SD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 47.Phan KL, et al. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 48.Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. The Journal of Neuroscience. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Science. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307(5712):1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- 51.Anderson AK, et al. Neural correlates of the automatic processing of threat facial signals. Journal of Neuroscience. 2003;23:5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pessoa L, et al. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences, U.S.A. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bishop S, et al. Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7(2):184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


