Abstract
Rates of brain loss have been shown to accelerate over time in early Alzheimer’s disease (AD); however the trajectory of change in frontotemporal lobar degeneration with ubiquitin immunoreactive-changes (FTLD-U) is unknown. This study compared the progression of atrophy over multiple MRI in subjects with autopsy confirmed AD and FTLD-U. Nine subjects with autopsy confirmed FTLD-U, and nine with autopsy confirmed AD, were identified that had three or more serial MRI. The boundary-shift integral was used to calculate change over time in whole brain and ventricular volume. A hierarchical regression model was used to estimate the slope of volume change in AD and FTLD-U over time and to estimate differences in the slopes across the subject groups. Whole brain volume loss did not deviate from a linear rate over time in both AD and FTLD-U subjects, although this may be due to limited sample size. The FTLD-U subjects had a faster rate (23ml/yr) than the AD subjects (10ml/yr). The rate of ventricular expansion accelerated over time. At the point when each subject had a Clinical Dementia Rating Sum-of-Boxes score of 6 the annual rate was 7ml/yr in FTLD-U and 5ml/yr in AD. These rates of change increased by an estimated 1.66ml/yr in FTLD-U, and 0.44ml/yr in AD, although these estimates were not significantly different between the two groups. The trajectory of brain and ventricular changes were similar in AD and FTLD-U suggesting that it is independent of pathology, although subjects with FTLD-U show a more rapidly progressive decline.
INTRODUCTION
Rates of cerebral atrophy measured over serial MRI have increasingly become useful markers of disease progression in neurodegenerative dementias and are already routinely being used as outcome measures in clinical trials (Fox, et al., 2005, Jack, et al., 2003). Increased rates of whole brain atrophy have been observed in subjects with both Alzheimer’s disease (AD) and frontotemporal lobar degeneration (FTLD) when compared to controls (Chan, et al., 2001, Ezekiel, et al., 2004, Fox and Freeborough, 1997, Jack, et al., 2004, Wang, et al., 2002, Whitwell, et al., 2007), and rates have been shown to be higher in subjects with FTLD compared to AD (Chan, et al., 2001, Whitwell, et al., 2007). Longitudinal studies using multiple serial MRI have also shown that the rates of atrophy in subjects with familial AD increase over the course of the disease as subjects progress from presymptomatic to moderate AD (Chan, et al., 2003, Ridha, et al., 2006). However, the extent to which atrophy rates change over time in FTLD, and whether it is different from that observed in AD, is not known. Furthermore no studies have assessed the trajectory of brain loss in pathologically confirmed subjects. Pathological confirmation in AD and FTLD is important since both diseases have overlapping clinical features. The pathological diagnosis of AD is made according to the presence of amyloid plaques and neurofibrillary tangles (Braak and Braak, 1996). The pathological diagnoses underlying FTLD are heterogeneous, although the most common pathological subtype is frontotemporal lobar degeneration with ubiquitin-immunoreactive changes (FTLD-U) (Josephs, et al., 2004). Understanding the natural progression of these diseases will be important for the assessment of future disease modifying treatments. The aim of this study was therefore to assess and compare the progression of brain atrophy over time using multiple serial MRI in pathologically confirmed subjects with FTLD-U and AD.
METHODS
Subject selection
The neuropathology files of the Mayo Clinic were used to identify all cases that had come to autopsy and had been given a diagnosis of FTLD-U or AD that had at least three volumetric head MRI scans of adequate quality for the boundary-shift integral (BSI) measurements. All subjects had been studied prospectively in the Mayo Clinic Alzheimer’s Disease Research Center or Alzheimer’s Disease Patient Registry. Informed consent was obtained for participation in the studies, which were approved by the Mayo Institutional Review Board. The medical records of all cases were reviewed by a behavioral neurologist (KAJ). All FTLD-U subjects included in the study fulfilled clinical criteria for one of the disorders considered to lie under the frontotemporal dementia spectrum at the time of the first scan (Neary, et al., 1998). All AD subjects fulfilled clinical criteria for AD (McKhann, et al., 1984). All MRI scans were visually assessed (JLW and CRJ). Scans were rejected for poor quality (such as motion artifact) or the presence of other pathologies (such as stroke, tumor or other structural lesion) that may influence either the structural analysis or clinical presentation. Nine subjects with FTLD-U were identified that fulfilled our inclusion criteria. Five of the FTLD-U subjects had a clinical diagnosis of behavioral variant FTD and four had primary progressive aphasia. These cases were then frequency matched by age to nine subjects with AD that fulfilled our inclusion criteria. Cognitive ability across groups was assessed using the Clinical Dementia Rating scale Sum of Boxes (CDR-SB) (Hughes, et al., 1982).
Pathological Diagnoses
Neuropathological examinations were performed according to the recommendations of the Consortium to Establish a Registry for Alzheimer’s Disease. In all cases pathological assessment and diagnosis was conducted by one of two expert neuropathologists (DWD or JEP). The pathological methods have been described in detail previously (Whitwell, et al., 2007). Frontotemporal lobar degeneration with ubiquitin-only-immunoreactive changes was diagnosed if there were inclusions that stained positive for ubiquitin, yet stained negative for tau, neurofilament, and α-synuclein, in frontal or temporal cortex, and the hippocampus dentate granular cells without any evidence of motor neuron degeneration (amyotrophic lateral sclerosis) as previously described (FTLD-U) (Josephs, et al., 2006), and meeting established research criteria (McKhann, et al., 2001). Cases with pathological evidence of motor neuron disease (amyotrophic lateral sclerosis) were excluded. Subjects were classified as AD if the subject had frequent neuritic plaques and met CERAD diagnostic criteria C for definite AD (Mirra, et al., 1991). Plaque count was done with the modified Bielschowsky silver stain. In addition, the subject had to have had a Braak neurofibrillary stage of V or VI (Braak and Braak, 1996) and therefore fulfill NIA-Reagan criteria for high probability AD (NIA, 1997). We excluded any subjects with Lewy bodies including amygdala only Lewy bodies (Uchikado, et al., 2006). None of the FTLD-U subjects had AD-type pathology. Vascular pathology was only identified on autopsy in one subject with FTLD-U (infarct) and one subject with AD (microvascular disease).
Image analysis
All MRI studies were performed at 1.5T with a standardized imaging protocol that included a coronal T1-weighted 3-dimensional volumetric spoiled gradient echo (SPGR) sequence with 124 contiguous partitions and 1.6mm slice thickness (22×16.5cm FOV, 25° flip angle). Different scanners were used, but all were GE Signa 1.5T with body resonance module gradient sets and transmit-receive single channel head coils. All scanners undergo a standardized quality control calibration procedure daily, which monitors geometric fidelity over a 200mm volume along all three cardinal axes, signal-to-noise, and transmit gain, and maintains the scanner within a tight calibration range.
Change in the whole brain and ventricle volumes were calculated using a software algorithm that has been developed in our lab and described in detail elsewhere (Gunter, et al., 2003). Whole brain masks were created for all baseline scans using MIDAS image analysis software (Freeborough, et al., 1997). This is a semi-automated brain segmentation algorithm that performs an intensity thresholding step, and a series of erosions and dilations to remove non-brain structures. Manual editing was then performed in order to remove any “non-brain” structures from the segmentation. Ventricular segmentations were performed on all repeat scans using the autotrace subprogram in Analyze/AVW plus manual editing of anatomic outlines. The ventricular region included the lateral ventricles, the temporal horn of the lateral ventricles, and the third ventricle. Repeat scans were then registered, i.e. spatially matched, using 9 degrees of freedom (dof) rigid body registration, to the baseline scan for each subject. This algorithm combines a 6dof rigid registration (three translations, three rotations), which matches the position and orientation of the two brain volumes, with an additional three scaling parameters measured using the skull which is invariant over time to correct for drifts in gradient calibration. The algorithm also incorporates a correction for intensity inhomogeneity (Sled, et al., 1998). Change in brain and ventricle volume was calculated automatically from each registered scan pair using the BSI (Freeborough and Fox, 1997, Gunter, et al., 2003). The BSI results between each interval were then used to calculate the brain and ventricular volumes at each time-point. Total intracranial volume (TIV) was measured on the baseline scan for each subject using a technique that has been previously described (Whitwell, et al., 2001).
Statistical methods
The majority of the baseline demographic features of the FTLD-U and AD subjects were summarized by their medians and ranges, and comparisons between the two groups were made using Mann-Whitney U statistics. The gender breakdown was summarized with counts of male and female subjects in the two study groups, and the comparison between these groups was achieved through the use of Fisher’s exact test.
Trends in whole-brain volumes and ventricle volumes were assessed individually in regression models that accounted for the presence of longitudinally measured data for each individual. The volume measurements, in ml, served as the dependent variable in these models. The time scale used in these regression models reflected the length of time that had elapsed from the first record at the time each individual was assessed to be at a CDR-SB of 6. All subjects had passed through a score of 6 during their disease progression, even if they were not given a score of exactly 6 on any one assessment. For those whose time at a CDR-SB of 6 was not known (n=10), an a priori decision was made to use quadratic interpolation to obtain an estimate of this time point on a per subject basis. In the regression models, repeated measurements within a person were accounted for by the inclusion of random effects in the models to account for potential subject-specific differences in baseline volumes, and overall linear trends in the volumes. An additional repeated measure effect was incorporated into the models to account for temporal correlations among the longitudinal measures.
Of primary interest in these models were estimates of trends in whole-brain and ventricle volumes for subjects with FTLD-U and AD, and comparisons of the trends between the two study groups. Estimates of linear and quadratic trend were therefore obtained, and tested for significance within, and between those with FTLD-U and AD. When quadratic trends were evident, suggesting that the rates of change were not constant, estimates of the time-specific rates of change were obtained for various time points of interest, along with corresponding 95% confidence intervals. P-values assessing the significance of the difference between study groups were also obtained.
While assessing the trends in volumes as a function of time, other important correlates of the volumes, such as TIV, age, education, CDR-SB and gender, were included in the models in order to adjust for potentially confounding effects. These variables were identified through modelling efforts that paralleled those described for the primary outcomes of interest. Results were also assessed without adjustment for age, gender, or education. All statistical tests were two-sided, and all statistical analyses were conducted using SAS (The SAS Institute, Cary NC).
RESULTS
The demographic features of the AD and FTLD-U groups are shown in Table 1. There was no significant difference in age at baseline, years of education, time from disease onset to baseline scan, or gender ratio between the groups. There was also no significant difference in the cognitive scores, whole brain volume, ventricular volume or TIV at the baseline scan. Both groups had a median of three serial scans per subject (range 3-4 in AD and 3-6 in FTLD-U) and the scans spanned an average 3.1 years (range 1.9-5.2) in the FTLD-U group, and 3.5 years (range 2.7-6.7) in the AD group.
Table 1.
Subject demographics
| AD (n=9) | FTLD-U (n=9) | |
|---|---|---|
| No. females (%) | 4 (44) | 7 (78) |
| Age at Baseline (years) | 75 (50, 81) | 73 (55, 79) |
| Education (years) | 14 (12, 17) | 12 (8, 18) |
| No. with family history (%) | 5 (56) | 3 (33) |
| CDR-SB at baseline (/18) | 3.5 (1.5, 6.0) | 3.5 (0.5, 7.0) |
| CDR at baseline | 0.5 (0.5-1.0) | 0.8 (0.5-1.0) |
| MMSE at baseline (/30) | 25 (23, 29) | 25 (19, 29) |
| Brain Volume at baseline (ml) | 1242 (1114, 1355) | 1192 (1041, 1368) |
| Ventricle Volume at baseline (ml) | 54 (28, 119) | 54 (30, 81) |
| TIV (ml) | 1445 (1299, 1564) | 1466 (1209, 1539) |
| Number of Scans | 3 (3, 4) | 3 (3, 6) |
| Years from onset to first scan | 2.0 (0.05, 4.4) | 2.1 (0.9, 7.1) |
| Years between first and last scan | 3.5 (2.7, 6.7) | 3.1 (1.9, 5.2) |
Data is shown as median (range)
AD = Alzheimer’s disease; FTLD-U = frontotemporal lobar degeneration; CDR-SB = Clinical Dementia Rating scale-sum of the boxes score; MMSE = Mini Mental State Examination; TIV = total intracranial volume
There were no significant differences between the AD and FTLD-U groups based on the Mann-Whitney U test
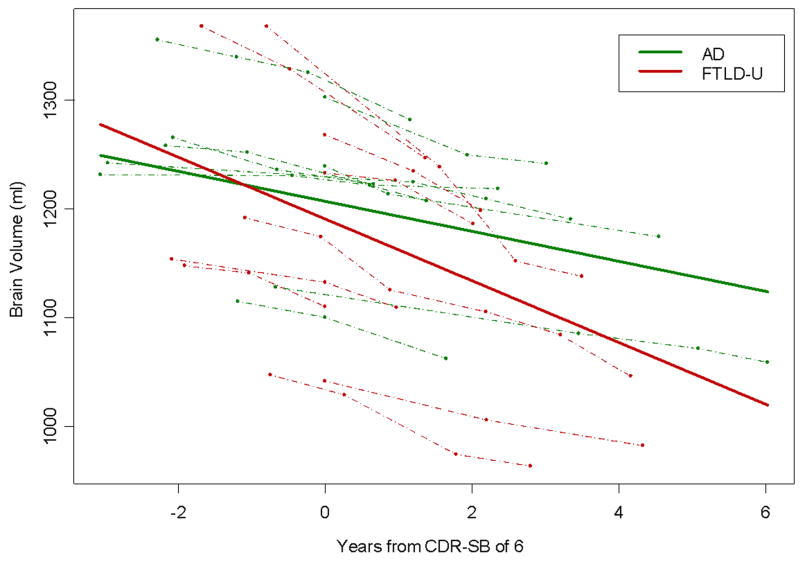
The adjusted models showed that there was a significant linear decrease in brain volume over time in both the AD (p=0.01) and FTLD-U (p<0. 001) subjects, with no evidence from the statistical model that the slope of change in brain volume was non-linear in either group (p=0.23). The rate of linear decline was significantly larger in the FTLD-U group compared to the AD (p=0.01), with an average rate of decline of 23ml (95% Confidence Interval [CI]: 15ml – 31ml) per year in the FTLD-U group versus 10ml (95% CI: 2ml – 18ml) per year in the AD group. The modeled slopes for both groups are shown in Figure 1. The unadjusted models showed the same trends, although the rate estimates were somewhat higher, with an average rate of decline of 28ml (95% CI: 21ml – 36ml) per year in the FTLD-U group versus 14ml (95% CI: 6ml – 21ml) per year in the AD group.
Figure 1.
Plot showing the change in brain volume over time in each of the AD and FTLD-U subjects (shown by dashed lines) and the model estimated best-fit slope for both the AD and FTLD-U groups (shown by a solid line). The AD subjects are represented by green lines and the FTLD-U subjects by red lines. The models were adjusted for TIV, age, gender, and education.
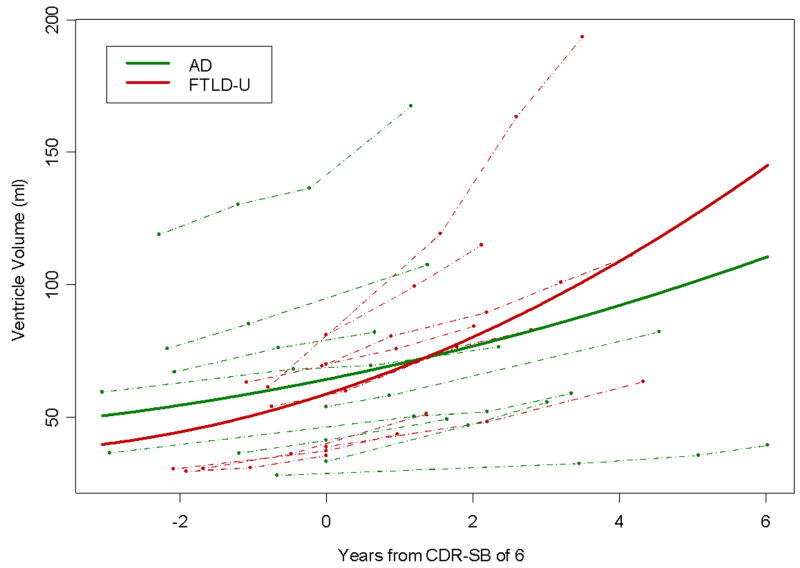
There was evidence from the adjusted models that expansion of the ventricular volumes accelerated over time across all subjects (p=0.03). This acceleration was significant in the FTLD-U subjects (p=0.007). The degree of acceleration did not reach significance in the AD subjects (p=0.28). The rise, or change, per year in the annual rate of ventricular expansion was 1.66ml (95% CI: 0.5ml – 2.8ml) in the FTLD-U group and 0.44ml (95% CI: -0.38ml – 1.26ml) in the AD group. This degree of difference in the curvature between the AD and FTLD-U subjects did not reach statistical significance (p=0.09). The modeled slopes using the adjusted data for both groups are shown in Figure 2. The same trends were observed in the unadjusted models, with a rise per year in the annual rate of 1.78ml (95% CI: 0.4ml – 3.2ml) in the FTLD-U group, and 0.70ml (95% CI: -0.30ml – 1.70ml) in the AD group. The estimated slopes over time using both the adjusted and unadjusted models are shown in Table 2.
Figure 2.
Plot showing the change in ventricular volume over time in each of the AD and FTLD-U subjects (shown by dashed lines) and the model estimated best-fit slope for both the AD and FTLD-U groups (shown by a solid line). The AD subjects are represented by green lines and the FTLD-U subjects by red lines. The models were adjusted for TIV, age, gender, and education.
Table 2.
Estimated yearly rates of ventricular expansion for subjects with AD and FTLD-U
| Time from CDR-SB of 6 | Time-specific slope in ml per year (95% confidence interval) | |||||
|---|---|---|---|---|---|---|
| Adjusted | Unadjusted | |||||
| AD | FTLD-U | p value* | AD | FTLD-U | p value* | |
| 2 years prior | 3.6 (-1.1, 8.3) | 3.9 (-1.4, 9.3) | 0.924 | 4.2 (-0.9, 9.3) | 5.4 (-0.1, 11.3) | 0.749 |
| 1 year prior | 4.0 (-0.4, 8.5) | 5.6 ( 0.8, 10.4) | 0.626 | 4.9 (0.2, 9.5) | 7.2 (2.1, 12.2) | 0.497 |
| 0 years | 4.5 ( 0.2, 8.8) | 7.2 ( 2.8, 11.7) | 0.358 | 5.6 (1.1, 10.0) | 9.0 (4.4, 13.5) | 0.285 |
| 1 year post | 4.9 ( 0.6, 9.3) | 8.9 ( 4.5, 13.3) | 0.186 | 6.3 (1.8, 10.7) | 10.7 (6.3, 15.2) | 0.157 |
| 2 years post | 5.4 ( 0.9, 9.9) | 10.6 ( 5.9, 15.2) | 0.102 | 7.0 (2.3, 11.6) | 12.5 (7.7, 17.3) | 0.100 |
| 3 years post | 5.8 ( 1.0, 10.6) | 12.2 ( 7.0, 17.4) | 0.066 | 7.7 (2.6, 12.7) | 14.3 (8.8, 19.8) | 0.078 |
| 4 years post | 6.2 ( 1.0, 11.5) | 13.9 ( 8.0, 19.7) | 0.050 | 8.4 (2.8, 14.0) | 16.1 (9.7, 22.5) | 0.073 |
Tests the null hypothesis that the slopes are different between AD and FTLD-U at the time indicated
Models are shown with and without adjustment for age, gender, and education
AD = Alzheimer’s disease; FTLD-U = frontotemporal lobar degeneration; CDR-SB = Clinical Dementia Rating scale-sum of the boxes score
There were significant associations between brain volume and age, education, gender, TIV and CDR-SB across both groups, however the only variable to show an association with ventricular volume was CDR-SB. The CDR-SB significantly increased over time across all subjects (p<0.001), although there was no evidence for a non-linear increase (p=0.75), and there was no difference across the two groups in the rate of linear change (p=0.16).
DISCUSSION
This study has analyzed serial MRI data spanning approximately three years in subjects with pathologically and clinically confirmed FTLD-U and AD and has shown that the whole brain volume decreases at an approximately linear rate over this time interval in both subject groups. The annual rate of decline in the FTLD-U subjects was significantly larger than the rate observed in the AD subjects. The rates observed were similar to those that we have previously reported in a larger cohort of pathologically confirmed FTLD-U and AD subjects (Whitwell, et al., 2007). The majority of our subjects were included in the previous study; however the rates of loss were previously calculated over only two serial scans, with an approximate scan interval of one year, and the previous study failed to find a significant difference between FTLD-U and AD. The inclusion of multiple serial MRI and hence a longer assessment period in this study most likely reduced the within-subject variability (Schott, et al., 2006) and increased the power to differentiate these two disorders. The rate of atrophy observed in the AD subjects was however lower than has been previously reported in clinical cohorts of AD subjects (Fox and Freeborough, 1997, Schott, et al., 2005, Wang, et al., 2002). This difference could be due to a combination of factors. Firstly, the majority of our subjects are elderly sporadic AD whereas many of the studies reporting higher rates of atrophy assessed younger familial AD cases. In addition, the fact that we have selected subjects with more than three scans means we would by definition be including subjects whose disease progression and duration allowed for them to obtain three scans. It is therefore possible that this cohort may represent a subset of AD patients with a slower progressive rate. Therefore, rates of atrophy obtained from cohorts of young subjects with familial AD (Fox and Freeborough, 1997) and rates of atrophy obtained from our cohort will have good internal validity but may not necessarily generalize to all AD patients.
The fact that we failed to find a non-linear trend in the rate of brain volume loss in the AD subjects contrasts with the results of previous studies that have assessed multiple scans in familial AD and shown acceleration in the rate of decline over time (Chan, et al., 2001, Ridha, et al., 2006). There are two possible explanations for this discrepancy. First, it is possible that while the rate of brain loss accelerates over time in the early phases of the disease it may slow and follow a more linear trajectory as the disease progresses. The previous studies that have shown acceleration have assessed subjects at an earlier phase of the disease in which some patients were presymptomatic at the time of the first scan. The subjects in this study all fulfilled clinical criteria for AD or FTLD-U at the time of the first scan and were approximately two years from onset. Second, it is possible that acceleration in the rate of brain volume loss was also occurring in our data but we have failed to detect it due to limitations in statistical power. The number of available subjects with multiple MRI and pathologically confirmed disease was relatively small and the magnitude of non-linear trends may be less than that associated to the linear component of decline. There was also no evidence for a non-linear trend in the FTLD-U subjects. No previous studies have assessed the longitudinal trajectory of groups of subjects with FTLD-U, although the results are consistent with a study that looked at gross atrophy at post mortem in FTLD and found a linear rate of change over the first five years from diagnosis (Broe, et al., 2003).
Interestingly a different trend was observed for the ventricular volumes in which the rate of loss appeared to accelerate over time. This quadratic trend reached significance for the FTLD-U subjects but not for the AD subjects. However, there was no significant difference in the curvature observed between the two groups suggesting that the difference in significance of the quadratic trends does not reflect differences in the disease process between AD and FTLD-U, but most likely reflects lack of statistical power. This result suggests a possible disassociation between the degree of whole brain atrophy and ventricular expansion, with the percentage of brain volume loss attributable to ventricular expansion increasing as the disease progresses. A similar trend has been previously suggested by a study that looked at rates of atrophy over two serial MRI (Schott, et al., 2005). This phenomenon could have a biological explanation. For example, it is possible that the regions surrounding the ventricles, i.e. the subcortical grey matter structures, atrophy later in the disease course than the cortex and therefore cause a disproportionate amount of ventricular expansion later in the disease. Alternatively, there may be greater inertia to inward collapse of the outer surface of the brain than outward expansion of the inner surface, perhaps due to restrictions posed by external structures. However, as discussed above it is possible that acceleration in the rate of brain volume loss was also occurring but we have failed to detect it due to limitations in statistical power. The ventricular brain/CSF boundary is well defined and is centered deep within the brain and is therefore less susceptibility to inhomogeneity artifacts caused from variation in position within the scanners magnetic field than the whole brain volume. The ventricular measurements may therefore be less variable than the whole brain volumes and have more statistical power to detect non-linearity. In addition, it is possible that the brain volumes do slightly accelerate by the same degree as the vent volumes (i.e. 1.66ml per year) but since this is a much smaller proportion of the total brain volume change than the total ventricular volume it does not reach significance.
Although there was no significant difference between the modeled slopes for ventricular volume in the AD and FTLD-U groups, the FTLD-U subjects had larger rates of ventricular expansion at any one time-point than the AD subjects. For example, at a CDR-SB score of six the estimated rates of ventricular expansion were 4.5ml per year in AD compared to 7.2ml per year in FTLD-U. These rates fit well with those previously reported in subjects with AD and FTLD-U (Jack, et al., 2004, Schott, et al., 2005, Whitwell, et al., 2007). Therefore both the rates of brain volume loss and ventricular expansion suggest more rapid brain loss in FTLD-U than AD. The fact that AD and FTLD-U differ more in the rate of whole brain volume loss than ventricular expansion probably reflects the fact that these diseases show very different and severe patterns of regional cortical atrophy (Boccardi, et al., 2003). In fact, assessments of rates of atrophy of regional structures are likely to be very useful in the differentiation of these two subject groups.
Both the AD and FTLD-U groups also showed a decline in cognition and function over the study period. The CDR-SB score increased over time with an approximately linear slope across all subjects. The CDR-SB scores were also significantly associated with both the whole brain volume and the ventricular volume in both groups suggesting that rates of cerebral atrophy provide excellent markers of disease progression in both AD and FTLD-U. However, a possible limitation of the study is that clinical measures, such as the CDR-SB, may not measure comparable degrees of disease advancement across clinically diverse groups such as FTD and AD. However, the CDR-SB does assess functional aspects of the disease such as judgement, problem solving, and personal interactions, which are features of behavioral variant FTD and may therefore be a more reliable assessment of disease state in FTD and AD than other measures of cognitive impairment that do not assess function, such as the mini-mental state examination.
These results suggest that the general shape of the trend, i.e. linear vs. quadratic, for brain and ventricular loss may be similar in both AD and FTLD-U subjects and may therefore not be disease specific. Once subjects have fulfilled clinical criteria for either AD or FTLD the brain volumes decrease at an approximately linear rate while the ventricular expansion accelerates over time. The rates of atrophy were however faster in the FTLD-U subjects. However, these results apply to subjects with mild dementia, that is, around a CDR-SB of six, and should not be generalized to all stages of the disease. These results will have important implications for the design of future disease modifying trials which aim to assess changes in brain volume over time in subjects with clinically diagnosed AD or FTLD-U.
Acknowledgments
This study was supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHD)-HD49078, by grants P50 AG16574, U01 AG06786, and R01 AG11378 from the National Institute on Aging, Bethesda MD, NIRG-03-4842 from the Alzheimer’s Association, and the generous support of the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program of the Mayo Foundation, U.S.A. DSK has been a consultant to GE HealthCare, GlaxoSmithKline and Myriad Pharmaceuticals, has served on a Data Safety Monitoring Board for Neurochem Pharmaceuticals, and is an investigator in a clinical trial sponsored by Elan Pharmaceuticals. BFB is an investigator in a clinical trial sponsored by Myriad Pharmaceuticals. RCP has been a consultant to GE Healthcare and has served on a data safety monitoring board in a clinical trial sponsored by Elan Pharmaceuticals.
We would like to thank Professor Nick Fox at the Dementia Research Centre, London, UK, for providing the MIDAS image analysis software.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boccardi M, Laakso MP, Bresciani L, Galluzzi S, Geroldi C, Beltramello A, Soininen H, Frisoni GB. The MRI pattern of frontal and temporal brain atrophy in fronto-temporal dementia. Neurobiol Aging. 2003;24:95–103. doi: 10.1016/s0197-4580(02)00045-3. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Broe M, Hodges JR, Schofield E, Shepherd CE, Kril JJ, Halliday GM. Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology. 2003;60:1005–11. doi: 10.1212/01.wnl.0000052685.09194.39. [DOI] [PubMed] [Google Scholar]
- Chan D, Fox NC, Jenkins R, Scahill RI, Crum WR, Rossor MN. Rates of global and regional cerebral atrophy in AD and frontotemporal dementia. Neurology. 2001;57:1756–63. doi: 10.1212/wnl.57.10.1756. [DOI] [PubMed] [Google Scholar]
- Chan D, Janssen JC, Whitwell JL, Watt HC, Jenkins R, Frost C, Rossor MN, Fox NC. Change in rates of cerebral atrophy over time in early-onset Alzheimer’s disease: longitudinal MRI study. Lancet. 2003;362:1121–2. doi: 10.1016/S0140-6736(03)14469-8. [DOI] [PubMed] [Google Scholar]
- Ezekiel F, Chao L, Kornak J, Du AT, Cardenas V, Truran D, Jagust W, Chui H, Miller B, Yaffe K, Schuff N, Weiner M. Comparisons between global and focal brain atrophy rates in normal aging and Alzheimer disease: Boundary Shift Integral versus tracing of the entorhinal cortex and hippocampus. Alzheimer Dis Assoc Disord. 2004;18:196–201. [PMC free article] [PubMed] [Google Scholar]
- Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, Koller M. Effects of A{beta} immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–72. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer’s disease. J Magn Reson Imaging. 1997;7:1069–75. doi: 10.1002/jmri.1880070620. [DOI] [PubMed] [Google Scholar]
- Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans Med Imaging. 1997;16:623–9. doi: 10.1109/42.640753. [DOI] [PubMed] [Google Scholar]
- Freeborough PA, Fox NC, Kitney RI. Interactive algorithms for the segmentation and quantitation of 3-D MRI brain scans. Comput Methods Programs Biomed. 1997;53:15–25. doi: 10.1016/s0169-2607(97)01803-8. [DOI] [PubMed] [Google Scholar]
- Gunter JL, Shiung MM, Manduca A, Jack CR., Jr Methodological considerations for measuring rates of brain atrophy. J Magn Reson Imaging. 2003;18:16–24. doi: 10.1002/jmri.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Shiung MM, Gunter JL, O’Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, Tangalos EG, Petersen RC. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Slomkowski M, Gracon S, Hoover TM, Felmlee JP, Stewart K, Xu Y, Shiung M, O’Brien PC, Cha R, Knopman D, Petersen RC. MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD. Neurology. 2003;60:253–60. doi: 10.1212/01.wnl.0000042480.86872.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Holton JL, Rossor MN, Godbolt AK, Ozawa T, Strand K, Khan N, Al-Sarraj S, Revesz T. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathology & Applied Neurobiology. 2004;30:369–73. doi: 10.1111/j.1365-2990.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW. Clinically undetected motor neuron disease in pathologically proven frontotemporal lobar degeneration with motor neuron disease. Arch Neurol. 2006;63:506–12. doi: 10.1001/archneur.63.4.506. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–9. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- NIA. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- Ridha BH, Barnes J, Bartlett JW, Godbolt A, Pepple T, Rossor MN, Fox NC. Tracking atrophy progression in familial Alzheimer’s disease: a serial MRI study. Lancet Neurol. 2006;5:828–34. doi: 10.1016/S1474-4422(06)70550-6. [DOI] [PubMed] [Google Scholar]
- Schott JM, Frost C, Whitwell JL, Macmanus DG, Boyes RG, Rossor MN, Fox NC. Combining short interval MRI in Alzheimer’s disease: Implications for therapeutic trials. J Neurol. 2006;253:1147–53. doi: 10.1007/s00415-006-0173-4. [DOI] [PubMed] [Google Scholar]
- Schott JM, Price SL, Frost C, Whitwell JL, Rossor MN, Fox NC. Measuring atrophy in Alzheimer disease: a serial MRI study over 6 and 12 months. Neurology. 2005;65:119–24. doi: 10.1212/01.wnl.0000167542.89697.0f. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–97. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Chalk JB, Rose SE, de Zubicaray G, Cowin G, Galloway GJ, Barnes D, Spooner D, Doddrell DM, Semple J. MR image-based measurement of rates of change in volumes of brain structures. Part II: application to a study of Alzheimer’s disease and normal aging. Magn Reson Imaging. 2002;20:41–8. doi: 10.1016/s0730-725x(02)00472-1. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Crum WR, Watt HC, Fox NC. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. AJNR Am J Neuroradiol. 2001;22:1483–9. [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Ferman TJ, Dickson DW, Josephs KA. Rates of cerebral atrophy differ in different degenerative pathologies. Brain. 2007;130:1148–58. doi: 10.1093/brain/awm021. [DOI] [PMC free article] [PubMed] [Google Scholar]