Dermatokinetics determine the efficacy and toxicity of drugs used to treat skin disorders. The time course of drug in the dermal extracellular fluid (ECF) could be used as a tool for assessing the concentration dependent activity/toxicity of the drugs in the skin tissue. Currently dermatokinetic studies of drugs are carried out by tissue biopsy or skin blister sampling or by microdialysis. However, all these methods are invasive and causes significant discomfort to the patients. Microdialysis technique has been used for determination glucose concentration (1,2), percutaneous absorption (3) of drugs and histamine release in the cutaneous tissue (4). Microdialysis has the advantage of sampling only the free drug (5,6). ETS is a noninvasive method of reversible permeabilization of stratum corneum and sampling of drugs from the ECF by diffusion. The diffusion flux of drug is governed by the unbound drug concentration gradient between the dermal tissue fluid and the sampling medium. Similar to microdialysis, the ETS samples are from the interstitial space, which is a defined, anatomical compartment and there is no net loss of body fluid (7). The hypothesis is that the transcutaneous flux of drugs is proportional to the concentration of unbound drug in the dermal ECF. Knowing the “reciprocal of Permeability coefficient (1/Pin vivo)” of skin, the unbound drug concentration in the dermal ECF could be determined. The objective of this project was to assess the feasibility of using ETS technique for studying dermatokinetics of acyclovir. Acyclovir, an antiviral agent used in the treatment of herpes simplex infection that occurs at the lowest epidermis. The time course of acyclovir in the dermal ECF is critical in determining the efficacy of acyclovir therapy of herpes simplex. In the present work, Acyclovir was administered to hairless rats and the time course of drug concentration in the dermal ECF was determined by ETS and microdialysis sampling.
The experiment was carried out in hairless rats (∼300 g) under ketamine (80 mg/kg) and xylazine (20 mg/kg) anesthesia. For ETS sampling, the shaved portion of the back skin was folded and clamped on to a custom made in vivo electroporation cell. The cell contains a sample collection chamber with platinum wire electrode. A stainless steel plate clamped across the skin fold served as the counter electrode (7,8). The collection chamber was filled with 200 μl of 0.05% w/v SDS solution prepared in phosphate buffered saline (PBS, pH 7.1) and 15 pulses each of 1 ms duration at 120 V and 1 Hz were applied. The SDS solution in the collection chamber was immediately replaced with 500 μl of PBS, which remained in the collection chamber during the extraction time (15 min). Such samples were obtained before administration of drug and at different time points after administration of the drug.
Microdialysis was carried out on the same rats and the samples were obtained at the same time points as that of the ETS sampling. For microdialysis, the dialysis probes (CMA 20 with mol. wt cut off 6000 Da from CMA Microdialysis, Sweden) was implanted into the dermal tissue horizontal to the skin surface. The inlet and outlet tubing were positioned and glued. The inlet tube was connected to a microinjection (CMA/100 from CMA Microdialysis, Sweden) pump and perfused with isotonic saline at a 2 μl/min. The equilibration time was 30 minutes before administration of drug.
The experiments were carried out in two stages. In the first stage the in vivo recovery of drug by the microdialysis procedure as well as by ETS was determined under steady state concentration conditions. The drug was administered as an i.v. bolus (5 mg/ml) and the drug was infused (0.1 to 1 mg/ml concentration) at 100 μl/h to achieve different steady state concentrations. Sampling by microdialysis was carried out continuously and the samples were collected at different time intervals. The constant drug levels in the microdialysate confirmed the steady state concentration. At steady state drug levels, while the infusion was still continued, the blood sampling, microdialysis sampling and the transcutaneous sampling was carried out at the same time points.. The recovery by ETS was the (1/slope) × 100 of the linear plot of flux (ng/min) versus subdermal drug concentration (ng/ml). The recovery by microdialysis protocol was the % fraction of subdermal drug concentration obtained in the microdialysate.
The second stage experiments were carried out to assess the feasibility of utilizing ETS technique for dermatokinetic studies. The drug was administered as i.v bolus (10 mg/kg) to a group of six animals and the ETS and microdialysis samples were collected at different time points. The concentration of drug in the samples was measured by HPLC (9). The unbound/ free drug concentration in the dermal extracellular fluid was calculated from the recovery factor. The drug levels in the dermal ECF determined by ETS and microdialysis and the pharmacokinetic parameters (calculated using WINNONLIN software). were compared by ANOVA.
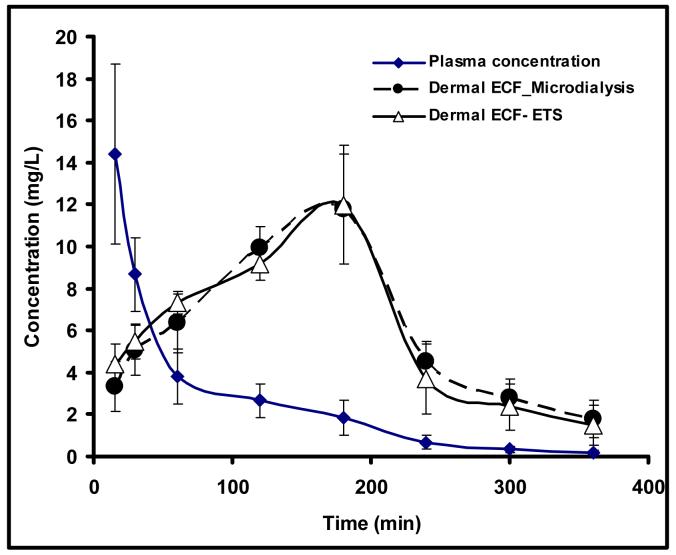
The in vivo passive permeability coefficient of hairless rat skin was found to be in 2.33± 0.38 × 10 -5 cm/min. Electroporation increased the in vivo permeability by two orders of magnitude (permeability coefficient 8.99± 1.21 × 10-2 cm/min). The in vivo recovery by free drug by microdialysis was ∼22±4.7%. When administered by i.v. bolus, acyclovir was found to undergo biexponential disposition. The time course of drug in the dermal ECF determined by ETS and microdialysis coincided well (Figure 1). The pharmacokinetic parameters calculated in the dermal ECF by noncompartmental model are given in table 1. The concentrations at each time-point and the pharmacokinetic parameters, i.e. Tmax, Cmax, AUC or T1/2, between microdialysis group and ETS group did not show any significant difference at the level of P < 0.05. Therefore it is likely that ETS could be utilized for noninvasive dermatokinetic studies of acyclovir and other drugs used in treating skin diseases.
Figure 1.
The time course of acyclovir in plasma (◆), derma ECF determined by microdialysis (●) and dermal ECF determined by ETS techniques (▵).
Table 1.
Mean dermatokinetic parameters of acyclovir determined by microdialysis and ETS techniques (n= 6 ± s.d.)
| Parameter | Microdialysis | ETS |
|---|---|---|
| Tmax (min) | 180 | 180 |
| Cmax (mg/L) | 11.77±2.62 | 11.98± 2.82 |
| AUC (min*mg/L) | 2263.13 ±326.12 | 2257.87±254.38 |
| T1/2 (min) | 78.74±19.26 | 63.86±15.41 |
Acknowledgement
This publication was made possible by Grant Number AR053097 from the National Institute of Arthritis, Musculoskeletal and Skin diseases (NIAMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petersen LJ, Kristensen, Bullow J. Microdialysis of the interstitial water space in human skin in vivo: Quantitative measurement of cutaneous glucose concentrations. J.Investig.Dermatol. 1992;99:357–360. doi: 10.1111/1523-1747.ep12616676. [DOI] [PubMed] [Google Scholar]
- 2.Boschmann M, Murphy FP, Krueger JG. Microdialysis can detect age-related differences in glucose distribution within the dermis and subcutaneous adipose tissue. Dermatology. 2001;202:207–210. doi: 10.1159/000051638. [DOI] [PubMed] [Google Scholar]
- 3.Ault JM, Lunte C, Meltzer NM, Riley CM. Microdialysis sampling for the investigation of dermal drug transport. Pharm.Res. 1992;9:1256–1261. doi: 10.1023/a:1015892914649. [DOI] [PubMed] [Google Scholar]
- 4.Anderson C, Anderson T, Anderson RCG. In vivo microdialysis estimation of histamine in human skin. Skin. Pharmacol. 1992;5:177–183. doi: 10.1159/000211035. [DOI] [PubMed] [Google Scholar]
- 5.Trampuz A, Wenk M, Rojacic Z, Zimmerli W. Pharmacokinetics and pharmacodynamics of levofloxacin against streptococcus pneumoniae and staphylococcus aureus in human skin blister fluid. Antimicrob. Agents and Chemother. 2000;44:1352–1355. doi: 10.1128/aac.44.5.1352-1355.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner M, Hollenstein U, Delacher S, Jager D, Schmid R, Lackner E, Georgopoulos, Eichler H, Muller M. Distribution and antimicrobial activity of ciprofloxacin in human soft tissues. Antimicrob. Agents Chemother. 1999;43:1307–1309. doi: 10.1128/aac.43.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murthy SN, Zhao Y, Hui SW, Sen A. Electroporation and transcutaneous extraction for pharmacokinetics of salicylate. J.Control.Release. 2005;105:132–141. doi: 10.1016/j.jconrel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Murthy SN, Manjili A, Guan LJ, Sen A, Hui SW. Induction of cytotoxic T-lymphocytes by electroporation-enhanced needle-free skin immunization. Vaccine. 2006;24:1282–1290. doi: 10.1016/j.vaccine.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Stagni G, Ali M, Weng D. Pharmacokinetics of acyclovir in rabbit skin after IV-bolus, ointment, and iontophoretic administrations. Int. j. Pharm. 2004;274:201–211. doi: 10.1016/j.ijpharm.2004.01.024. [DOI] [PubMed] [Google Scholar]