Abstract
Deoxymugineic acid (DMA) is a member of the mugineic acid family phytosiderophores (MAs), which are natural metal chelators produced by graminaceous plants. Rice secretes DMA in response to Fe deficiency to take up Fe in the form of Fe(III)–MAs complex. In contrast with barley, the roots of which secrete MAs in response to Zn deficiency, the amount of DMA secreted by rice roots was slightly decreased under conditions of low Zn supply. There was a concomitant increase in endogenous DMA in rice shoots, suggesting that DMA plays a role in the translocation of Zn within Zn-deficient rice plants. The expression of OsNAS1 and OsNAS2 was not increased in Zn-deficient roots but that of OsNAS3 was increased in Zn-deficient roots and shoots. The expression of OsNAAT1 was also increased in Zn-deficient roots and dramatically increased in shoots; correspondingly, HPLC analysis was unable to detect nicotianamine in Zn-deficient shoots. The expression of OsDMAS1 was increased in Zn-deficient shoots. Analyses using the positron-emitting tracer imaging system (PETIS) showed that Zn-deficient rice roots absorbed less 62Zn-DMA than 62Zn2+. Importantly, supply of 62Zn-DMA rather than 62Zn2+ increased the translocation of 62Zn into the leaves of Zn-deficient plants. This was especially evident in the discrimination center (DC). These results suggest that DMA in Zn-deficient rice plants has an important role in the distribution of Zn within the plant rather than in the absorption of Zn from the soil.
Electronic supplementary material
The online version of this article (doi:10.1007/s11103-008-9292-x) contains supplementary material, which is available to authorized users.
Keywords: Deoxymugineic acid, Iron, Nicotianamine, Rice, Transport, Zinc
Introduction
Zn, an essential element for plant growth, is absorbed from the rhizosphere by higher plants through specific transporters. The Zinc-regulated and Iron-regulated transporter Proteins (ZIP) have been isolated from both graminaceous (Ramesh et al. 2003; Ishimaru et al. 2005) and non-graminaceous plants (Grotz et al. 1998; Lasat et al. 2000; Moreau et al. 2002). Several ZIP transporters have been shown to be transcriptionally upregulated in Zn-deficient roots, but their contribution to the absorption of Zn from the rhizosphere into roots has not yet been demonstrated. Zn is also absorbed from the rhizosphere as complexes of Zn(II)–mugineic acid family phytosiderophores (MAs). The maize protein YS1 transports Fe(III)–MAs complexes (Curie et al. 2001), as well as other micronutrient-MAs complexes, including Zn(II)–MAs (Schaaf et al. 2004). von Wirén et al. (1996) reported that the ys1 mutant absorbed less 65Zn(II)-MAs than did the wild type, and Suzuki et al. (2006) showed that Zn-deficient barley absorbed more 62Zn(II)-DMA than 62Zn2+. These two studies also showed that free Zn2+ ions are absorbed into roots to some extent, suggesting that graminaceous plant roots absorb both Zn-DMA and Zn2+.
Graminaceous plants secrete MAs into the rhizosphere from their roots, although the amount differs among species in the order of: barley > wheat = rye > oat > maize > sorghum > rice (Takagi 1993). While the amount of MAs secreted is increased dramatically by Fe deficiency, it is not clear if Zn deficiency increases the secretion of MAs. Zn deficiency has been reported to increase (Cakmak et al. 1994; Walter et al. 1994; Zhang et al. 1989) or have no effect on the secretion of MAs from wheat and barley roots into the rhizosphere (Gries et al. 1995; Pedler et al. 2000).
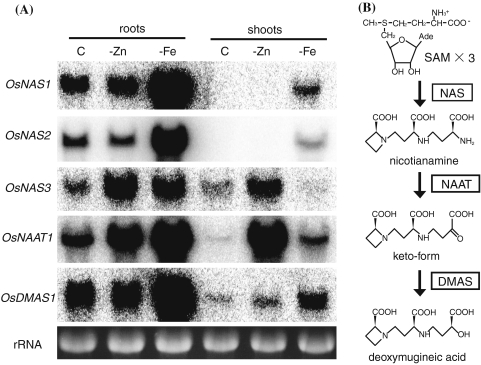
The biosynthesis of MAs and their corresponding genes have been characterized (Fig. 3b) as being synthesized from methionine (Mori and Nishizawa 1987). Three molecules of S-adenosyl-l-methionine (SAM) are combined to form one molecule of nicotianamine (NA) by nicotianamine synthase (NAS). NA is then converted to the 3′′-keto acid by NA aminotransferase (NAAT), and 2′-deoxymugineic acid (DMA) is synthesized by DMA synthase (DMAS). In some graminaceous species, including barley, DMA is further hydroxylated by two dioxygenases, IDS2 and IDS3 (Kobayashi et al. 2001; Nakanishi et al. 2000). The genes encoding the enzymes involved in DMA synthesis have been well-characterized in Fe-deficient rice and barley. The expression of HvNAS1, HvNAAT-A, HvNAAT-B, HvDMAS1, HvIDS2, and HvIDS3 is increased in Fe-deficient barley roots (Nakanishi et al. 1993; Okumura et al. 1994; Higuchi et al. 1999; Takahashi et al. 1999; Bashir et al. 2006). In rice, the expression of OsNAS1, OsNAS2, OsNAAT1, and OsDMAS1 is increased in both roots and shoots by Fe deficiency (Inoue et al. 2003; Bashir et al. 2006). The expression of OsNAS3 is increased in Fe-deficient roots, but decreased in Fe-deficient shoots (Inoue et al. 2003). Promoter-GUS analysis suggests that OsNAS1 and OsNAS2 are involved in DMA secretion, because those genes are expressed in all root cells. On the other hand, OsNAS3 may not be involved in DMA secretion because its expression is restricted to the pericycle and companion cells of the roots (Inoue et al. 2003).
Fig. 3.
Expression patterns of genes involved in DMA synthesis (a) Northern blot analysis of each gene involved in DMA synthesis. Each lane contained 10 μg of total RNA. C, control; -Zn, zinc deficiency; -Fe, iron deficiency. (b) DMA synthesis from S-adenosyl-l-methionine (SAM)
Recently, we showed that the expression of NASHOR2, a NAS gene in barley (Herbik et al. 1999), and HvNAAT-B was increased in Zn-deficient barley shoots, but that the expression of IDS2 and IDS3 was not detected in Zn-deficient shoots (Suzuki et al. 2006). On the other hand, the expression of HvNAS1, HvNAAT-A, HvNAAT-B, IDS2, and IDS3 was increased in both Zn- and Fe-deficient barley roots, while the expression of these genes was not evident in Fe-deficient shoots (Suzuki et al. 2006). This suggests that in barley Zn deficiency induces DMA synthesis in shoots, while both Zn and Fe deficiency induce MAs synthesis and secretion in roots.
The aims of the present study were to examine if the stimulation of MAs secretion by Zn deficiency is common in graminaceous plants and roles of MAs in Zn homeostasis of rice plants. In this report, we shows that DMA secretion is decreased in rice roots, while the concentration of endogenous DMA is increased in Zn-deficient rice shoots.
Materials and methods
Plant material and growth conditions
Rice seeds (Oryza sativa L. cv. Nipponbare) were germinated for 7 days at room temperature on paper towels soaked with distilled water. After germination, the seedlings were transferred to a Saran net floating on nutrient solution (Higuchi et al. 2001) and grown in a glasshouse. After 4 days, 270 seedlings were transferred to six 20-l plastic containers containing nutrient solution. After a further 5 days, 90 seedlings were transferred to two 20-l plastic containers containing nutrient solution without Zn followed 7 days later when a further 90 seedlings were transferred to two 20-l plastic containers containing nutrient solution without Fe. All samples were harvested 5 days later for analysis. Consequently, the Zn-deficient treatment was performed for 12 days, and Fe-deficient treatment for 5 days since Fe deficiency has a much more rapid deleterious effect. The nutrient solutions were renewed weekly with adjustment to pH 5.5 every 2 days with KOH.
Measurement of DMA secretion and endogenous levels of DMA and NA
DMA secretion was measured as described by Suzuki et al. (2006) and the concentrations of endogenous DMA and NA were measured as described by Higuchi et al. (2001). Briefly, the roots of 12 plants were rinsed with deionized water and then placed in 600 ml of deionized water at sunrise. Root exudates were collected for two periods of 2 and 3 h. After collection, Micropur (Katadyn, Switzerland), an antimicrobial agent, was added to prevent microbial degradation of the MAs Both solutions containing root exudates were combined and filtered (Advantec 5C, Toyo Roshi Kaisha, Ltd., Japan).
The shoots of the plants from which root exudates were collected were ground in liquid nitrogen using a mortar and pestle. Approximately 1 g of material was suspended in 40 ml of distilled water at 80°C for 30 min and filtered. The cationic fraction of the samples was prepared as a 2-M NH4OH eluate from Amberlite IR(H+)120 (Rohm and Haas Co., USA). The condensed and microfiltered samples were subjected to HPLC analysis, as described previously (Mori et al. 1987; Shojima et al. 1989). To ensure better separation of DMA, the pH of 0.15-N Li-citrate buffer was decreased to 2.97 and the pH of 0.2-N Li-citrate buffer increased to 3.30 for better separation of NA.
Northern blot analysis
The samples for Northern blot analysis were harvested 3 h after sunrise. The roots and shoots were separated immediately and stored at −80°C. Total RNA was extracted from the roots and shoots using SDS-phenol. Ten micrograms of total RNA were electrophoresed in 1.2% (w/v) agarose gels containing 0.66 M formaldehyde, then transferred to a Hybond-N+ membrane (Amersham, USA) and hybridized with probes at 65°C. The blots were analyzed using a Bio-imaging Analyzer System (BAS) 3000 (Fuji Film, Japan). The following specific primers were used to prepare specific probes: OsNAS1, 5′-GTCTAACAGCCGGACGATCGAAAGG-3′ and 5′-TTTCTCACTGTCATACACAGATGGC-3′; OsNAS2, 5′-TGAGTGCGTGCATAGTAATCCTGGC-3′ and 5′-CAGACGGTCACAAACACCTCTTGC-3′; OsNAS3, 5′-GACTGCTTCCATCGCTTGCTACCTC-3′ and 5′-CGCAACAGAGACAATGGTTGATTGT-3′; OsNAAT1, 5′-TAAGAGGATAATTGATTTGCTTAC-3′ and 5′-CTGATCATTCCAATCCTAGTACAAT-3′; and OsDMAS1, 5′-GCCGGCATCCCGCAGCGGAAGATCA-3′ and 5′-CTCTCTCTCTCGCACCTGCTAGCGT-3′. The accession numbers of the genes are AB021746 (OsNAS1), AB023818 (OsNAS2), AB023819 (OsNAS3), AB206814 (OsNAAT1), and AB269906 (OsDMAS1).
PETIS analysis of 62Zn translocation
PETIS experiment was established by Kume et al. (1997). 62Zn (half life: 9.13 h) was produced by the method of Watanabe et al. (2001). After adjustment of the 62Zn2+ solution to about pH 4 with 1 M KOH, 62Zn2+ was chelated with DMA in darkness for more than 3 h. Prior to the analysis, Zn-deficient rice plants were grown as described above but in a growth chamber (day: 30°C, 14 h of light at 320 μmol photons m−2 sec−1; night: 10 h at 25°C). The Zn-deficient rice plants were each supplied with 15 ml of culture solution lacking Zn in a polyethylene bag. The plants and bags were fixed between two acrylic boards and placed between a pair of PETIS detectors in a chamber set at 30°C, 65% humidity, and a light density of 320 μmol m−2 s−1. In the root absorption experiment, 62Zn (0.6 nmol) with DMA (79 nmol) was dissolved in 1 ml of distilled water and added to the culture solution. In the leaf absorption experiment, 62Zn (0.5 nmol) with DMA (33 nmol) was dissolved in 4.5 ml of nutrient solution containing cold Zn (2.5 μmol) and additional DMA (308 μmol), and supplied at the cut end of the second newest leaf. The individual leaves were cut to ensure a constant distance from discrimination center (DC) which is the junction of shoot and root (Kiyomiya et al. 2001). The γ-rays emitted from decaying positrons from 62Zn were counted over time using the coincident method with the paired detectors. The data were automatically corrected using 9.13 h as the half-life of 62Zn. The radioactivity of selected region was measured as region of interest (ROI) analysis. After PETIS analysis for 8 h, the plants were removed from the polyethylene bags, and the roots were gently washed for 1 min in 50 ml of 0.01 mM EDTA. Next, each plant was placed under a Bio-Imaging plate inside a cassette. The plate was scanned using an image analysis system (BAS-1500, Fuji Film, Japan). The plants were then cut into pieces, and the absolute amount of radioactivity was analyzed using γ-ray spectrometry with an ORTEC HPGe detector (Seiko EG & G Co., Ltd., Japan). Each experiment was conducted in triplicate. Due to the very short half-life of 62Zn, the radioactivity was different in each experiment. Therefore, images of individual plants and PETIS results are representative of those obtained.
Results
Plant growth, DMA synthesis and gene expression
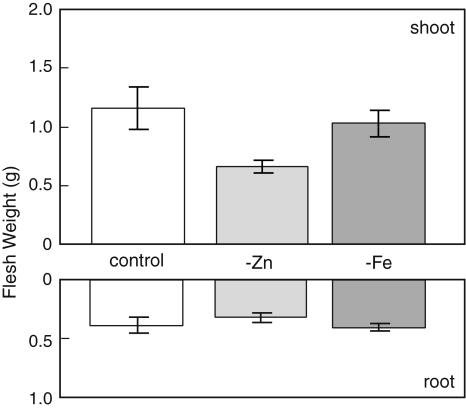
In contrast with the good growth of the control rice plants, Zn or Fe deficiency had a markedly detrimental effect when plants were grown for 12 d or 5 d in solutions containing no Zn or Fe, respectively. Shoot growth was reduced by 50% in the absence of Zn and by 10% without Fe (Fig. 1). The corresponding figure for the Zn-deficient roots was a reduction of 20%, while the absence of Fe supply for 5 d had no significant effect on root growth. As previously established in our studies (Suzuki et al. 2006), there was no measurable effect of no Zn supply during the first week of growth (data not shown), but thereafter plants developed symptoms of Zn deficiency and growth declined. Chlorotic symptoms of Fe deficiency developed after 5 days of treatment while the fresh weights of the shoots and roots were almost the same as in the control plants.
Fig. 1.
Fresh weights of rice shoots and roots. 31-days old rice seedlings grown in hydroponically were harvested. Zn-deficient treatment (-Zn) was performed for 12 days, and Fe-deficient treatment (-Fe) was performed for 5 days. Values are mean ± SD (n = 4)
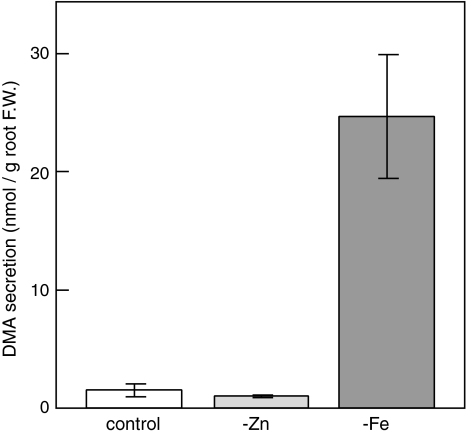
The amount of DMA secreted from the roots of Fe-deficient rice was much higher than that of the control plants (Fig. 2). On the other hand, Zn deficiency slightly decreased the level of DMA secretion. In fact, the amount of DMA secreted per plant in Zn-deficient rice was significantly decreased compared to that in the control plants (P < 0.05).
Fig. 2.
Amount of DMA secreted from rice roots. The growth condition and harvest time are the same in Fig. 1. Root exudates were collected for 5 h after sunrise. -Zn, zinc deficiency; -Fe, iron deficiency. Values are mean ± SD (n = 4)
We next analyzed the expression pattern of the genes participated in DMA synthesis in roots and shoots under Zn or Fe deficiency (Fig. 3a, b). As reported previously, the transcription of OsNAS1, OsNAS2, OsNAS3, OsNAAT1, and OsDMAS1 in roots was increased by Fe deficiency (Inoue et al. 2003, 2008; Bashir et al. 2006). On the other hand, Zn deficiency enhanced the transcription of OsNAS3 and OsNAAT1, but not that of OsNAS1, OsNAS2, and OsDMAS1. The expression of OsNAS2 in roots was slightly decreased by Zn deficiency. The expression patterns of OsNAS1 and OsNAS2 were well correlated with the amount of DMA secreted from the roots of Fe-deficient or Zn-deficient rice plants (Figs. 2, 3a).
In the shoots, Fe deficiency increased the expression of OsNAS1, OsNAS2, OsNAAT1, and OsDMAS1, but decreased that of OsNAS3. In comparison, Zn deficiency increased the expression of OsNAS3 and especially that of OsNAAT1. The expression of OsDMAS1 was slightly increased in Zn-deficient shoots. Transcripts of OsNAS1 and OsNAS2 were not detected in control or Zn-deficient shoots (Fig. 3a).
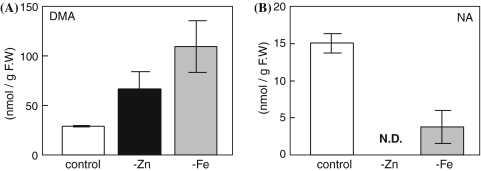
Higuchi et al. (2001) reported that the concentration of endogenous DMA was increased in Fe-deficient rice shoots. We confirmed that the concentration of endogenous DMA in Fe-deficient shoots was about three times higher than that in control shoots (Fig. 4a). The concentration of endogenous DMA in Zn-deficient shoots was about two times higher than that in control shoots (Fig. 4b). Although the concentration of endogenous DMA in Zn-deficient shoots was lower than that in Fe-deficient shoots, the expression of OsNAAT1, which encodes the enzyme that converts nicotianamine to its 3′′-keto acid, in Zn-deficient shoots was much higher than that in Fe-deficient shoots (Fig. 3a). Therefore, we measured the endogenous NA, which is an intermediate of DMA. Corresponding to the expression pattern of OsNAAT1, endogenous NA in Zn-deficient shoots was under the detection limit by HPLC, while in Fe-deficient shoots NA was lower than in the control shoots (Fig. 4b).
Fig. 4.
Concentration of endogenous DMA and NA in rice shoots (a) Concentration of endogenous DMA. (b) Concentration of endogenous NA. The plants were harvested after collection of root exudates (Fig. 2), then DMA or NA was measured using HPLC. -Zn, zinc deficiency; -Fe, iron deficiency; N.D., not detected. Values are mean ± SD (n = 4)
Contribution of DMA to Zn translocation in Zn-deficient rice
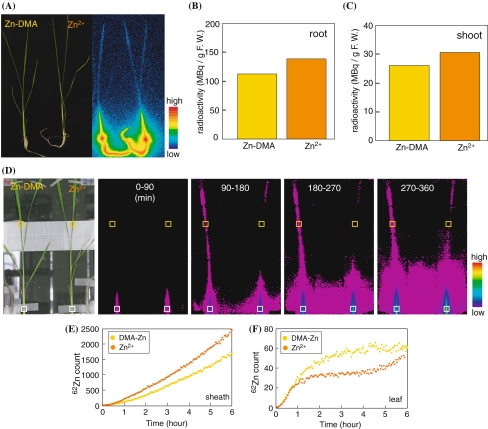
To investigate whether DMA is involved in Zn absorption and translocation, a PETIS experiment was performed using Zn-deficient rice (Fig. 5; supplemental movie 1, supplemental Fig. 1). 62Zn chelated with DMA (62Zn-DMA) or 62Zn without DMA (62Zn2+) was supplied to Zn-deficient rice roots. Images of the plants and the localization of 62Zn after 6 h of absorption are shown in Fig. 5a. In both plants, most of the 62Zn absorbed into the plant was observed at the DC and in the bottom of the leaf sheath (Fig. 5a). The radioactivity of the plant supplied with 62Zn2+ was higher than that of the plant supplied with 62Zn-DMA both in the roots and shoots (Fig. 5b, c). This indicates that compared to Zn-DMA, Zn2+ is the preferred form for root absorption in Zn-deficient rice. Real-time imaging of the 62Zn absorption was observed by PETIS analysis (Fig. 5d). More 62Zn was observed in the bottom of the leaf sheath supplied with 62Zn2+ than in that supplied with 62Zn-DMA. This observation was quantified by ROI analysis (Fig. 5e) On the other hand, beginning at 90 min after the start of the experiment, more 62Zn was observed in the newest leaf of the plant supplied with 62Zn-DMA than in that supplied with 62Zn2+ (Fig. 5d, f). This indicates that Zn-DMA is preferred over Zn2+ for translocation within Zn-deficient rice plants. Continuous PETIS imaging is shown in supplemental movie 1. We performed three experiments of 62Zn absorption. Due to the very short half-life of 62Zn, however, the radioactivity was different in each experiment. Therefore, we showed the relative radioactivity of each experiment in supplemental Fig. 1. In each experiment, 62Zn2+ was more absorbed than 62Zn-DMA.
Fig. 5.
62Zn movement from roots in Zn-deficient rice using PETIS (a) Plant image (left) and BAS image (right). (b) Radioactivity in roots after 6 h of absorption. (c) Radioactivity in shoots after 6 h of absorption. (d) Time course of the accumulation of radioactivity as determined by PETIS analysis. The data were scored every 3 min, and the images shown were taken at 90-min intervals for 6 h after supplying 62Zn through the roots. (e) Region of interest (ROI) analysis of 62Zn movement in the bottom of the sheath white boxed in (d). (f) ROI analysis in the newest leaf yellow boxed in (d)
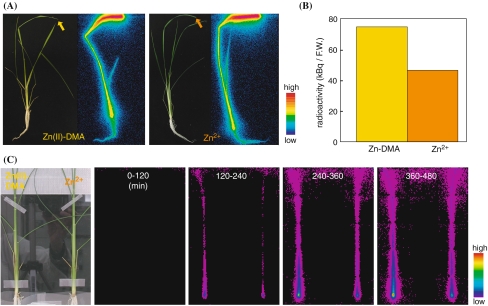
We conducted an additional PETIS experiment in which Zn-DMA or Zn2+ was supplied to Zn-deficient rice at the cut second newest leaf (Fig. 6; supplemental movie 2, supplemental Fig. 2). Plant images and the localization of 62Zn after 6 h of absorption are shown in Fig. 6a. Most of the 62Zn absorbed from the second newest leaf remained in that leaf, but some 62Zn was translocated to the other leaves and roots as well as to the DC and leaf sheath. The level of radioactivity in the entire plant, except for the second newest leaf, was higher in the plant supplied with 62Zn-DMA than in the plant supplied with 62Zn2+ (Fig. 6b), while that of the second newest leaf was not higher in the plant supplied with 62Zn-DMA than in the plant supplied with 62Zn2+ (supplemental Fig. 3). PETIS analysis showed that more 62Zn appeared continuously in the plant supplied with 62Zn-DMA than in the plant supplied with 62Zn2+. This suggests that 62Zn-DMA is translocated more rapidly than 62Zn2+ within the plant.
Fig. 6.
62Zn movement from cut leaf in Zn-deficient rice using PETIS (a) Plant image (left) and BAS image (right). Arrows indicate the point where 62Zn was absorbed. (b) Radioactivity in the entire plant except for the second newest leaf after 8 h of absorption. (c) Time course of the accumulation of radioactivity as determined by PETIS analysis. The data were scored every 3 min, and the images shown were taken at 120-min intervals for 8 h after supplying 62Zn through the shoots
Discussion
The concentration of endogenous DMA is increased in Zn-deficient shoots, but DMA secretion from the roots is not increased
Previously we reported increased MAs secretion in Zn-deficient barley (Suzuki et al. 2006); however, in rice, Zn deficiency did not increase the secretion of DMA, but rather slightly decreased it (Fig. 2). Fe deficiency increased MAs secretion in both rice and barley (Suzuki et al. 2006; Fig. 2). In barley, expression of the genes encoding the MAs biosynthetic enzymes was increased in the roots of both Zn- and Fe-deficient plants (Suzuki et al. 2006). On the other hand, in rice, the expression pattern of the genes involved in DMA synthesis in roots was increased in Fe-deficient roots, while the expression of OsNAS1, OsNAS2, and OsDMAS1 was not increased in Zn-deficient roots (Fig. 3a). Inoue et al. (2003) suggested that OsNAS1 and OsNAS2 are involved in DMA secretion. Supporting this idea, the expression pattern of OsNAS1 and OsNAS2 in roots was in good correlation with the amount of DMA secreted (Figs. 2, 3a). On the other hand, OsNAS3 is thought not to be involved in DMA secretion, because the expression of OsNAS3 is restricted to the pericycle cells adjacent to the protoxylem and companion cells but not in other cortical cells (Inoue et al. 2003). The expression of OsNAS3 is observed in whole leaf tissues in the control plants (Inoue et al. 2003). The expression of OsNAS3 was increased in Zn-deficient roots and shoots (Fig. 3a); therefore, OsNAS3 may function to synthesize NA or subsequent components, such as DMA, for the distribution of Zn within Zn-deficient plants. It is noteworthy that Zn deficiency enhanced the expression of OsNAS3 in shoots, while Fe deficiency enhanced the expression of OsNAS1 and OsNAS2. This suggests that NA and DMA are involved both in Fe and Zn homeostasis, but that their synthesis under conditions of Fe and Zn deficiency is catalyzed by different isozymes.
The concentration of endogenous DMA in the shoots was increased by Zn and Fe deficiencies (Fig. 4a). Although the induction of OsNAAT1 in Zn-deficient shoots was much higher than that in Fe-deficient shoots, the concentration of DMA in Zn-deficient shoots was lower than that in Fe-deficient shoots. In accordance with the high level of OsNAAT1 expression in Zn-deficient shoots, the concentration of NA in Zn-deficient shoots was low (Fig. 4b). This result led to us hypothesize that OsDMAS1 is a rate-limiting enzyme for DMA synthesis in Zn-deficient rice.
Zn-DMA is the preferred form for long-distance transport of Zn in Zn-deficient rice
Recently, we showed that Zn-deficient barley absorbs more Zn-DMA than Zn2+ from its roots (Suzuki et al. 2006). In the present paper, however, less Zn-DMA than Zn2+ was absorbed from the roots of Zn-deficient rice (Fig. 5). These findings are correlated with the change in the amount of MAs secreted under Zn deficiency. In barley, MAs secretion is increased by Zn-deficiency, and this contributes to the absorption of Zn from the soil. On the other hand, in rice, the level of DMA secretion was slightly decreased (Fig. 2). This suggested that rice may prefer to absorb Zn2+ rather than Zn-DMA.
Our PETIS experiment clearly showed that more 62Zn was translocated to the newest leaf when 62Zn-DMA was supplied than when 62Zn2+ was supplied, while the opposite tendency was observed at the bottom of the leaf sheath (Fig. 5, supplemental movie 1). This finding suggests that Zn-DMA is the preferred form for long-distance transport of Zn in Zn-deficient rice. The preferential translocation of 62Zn-DMA was also observed in the PETIS experiment in which 62Zn-DMA and 62Zn2+ were supplied from the Zn-deficient leaf (Fig. 6, supplemental movie 2). These data also support the idea that Zn-DMA is the preferred form for long-distance transport of Zn in Zn-deficient rice.
Since DMA synthesis might be promoted by Zn deficiency in barley shoots (Suzuki et al. 2006), graminaceous plants have a common system for Zn translocation within the plant, while the contribution of MAs to Zn uptake from the soil differs among species. Further studies will enable us to understand the mechanism of Zn transport, to manipulate the distribution of Zn in order to produce crops that tolerate Zn-deficient stress, and to enhance the Zn content of crop seeds.
Electronic supplementary material
Acknowledgments
We thank Dr. Yoshiaki Nagamura (National Institute of Agrobiological Sciences, Tsukuba, Japan) for providing the rice cDNA clones. We also thank to Dr. Pax Blamey (University of Queensland, Brisbane, Australia) and Dr. Takanori Kobayashi (University of Tokyo, Tokyo, Japan) for careful reading of the manuscript. This work was supported by a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Green Technology Project IP-5003).
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- DMA
Deoxymugineic acid
- Mas
Mugineic acid family phytosiderophores
- NA
Nicotianamine
- PETIS
Positron-emitting tracer imaging system
- DC
Discrimination center
- ZIP
Zinc-regulated and Iron-regulated transporter proteins
- ROI
Region of interest
Footnotes
Motofumi Suzuki and Takashi Tsukamoto equally contributed to this work.
Electronic supplementary material
The online version of this article (doi:10.1007/s11103-008-9292-x) contains supplementary material, which is available to authorized users.
References
- Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem 43:32395–32402 [DOI] [PubMed]
- Cakmak I, Gülüt KY, Marschner H, Graham RD (1994) Effect of zinc and iron deficiency on phytosiderophore release in wheat genotypes differing in zinc deficiency. J Plant Nutr 17:1–17
- Curie C, Panavience Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL (2001) Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409:346–349 [DOI] [PubMed]
- Gries D, Klatt S, Crowley DE, Parker DR (1995) Phytosiderophore release in relation to micronutrient metal deficiencies in barley. Plant Soil 172:299–308 [DOI]
- Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide D (1998) Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc Natl Acad Sci USA 95:7220–7224 [DOI] [PMC free article] [PubMed]
- Herbik A, Koch G, Mock HP, Dushkov D, Czihal A, Thielmann J, Stephan UW, Baumlein H (1999) Isolation, characterization and cDNA cloning of nicotianamine synthase from barley. A key enzyme for iron homeostasis in plants. Eur J Biochem 265:231–239 [DOI] [PubMed]
- Higuchi K, Suzuki K, Nakanishi H, Yamaguchi H, Nishizawa NK, Mori S (1999) Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiol 119:471–479 [DOI] [PMC free article] [PubMed]
- Higuchi K, Watanabe S, Takahashi M, Kawasaki S, Nakanishi H, Nishizawa NK, Mori S (2001) Nicotianamine synthase gene expression differs in barley and rice under Fe-deficient conditions. Plant J 25:159–167 [DOI] [PubMed]
- Inoue H, Higuchi K, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2003) Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J 36:366–381 [DOI] [PubMed]
- Inoue H, Takahashi M, Kobayashi T, Suzuki M, Nakanishi H, Mori S, Nishizawa NK (2008) Identification and localisation of the rice nicotianamine transferase gene OsNAAT1 expression suggests the site of phytosiderophore synthesis in rice. Plant Mol Biol 66:193–203 [DOI] [PubMed]
- Ishimaru Y, Suzuki M, Kobayashi T, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2005) OsZIP4, a novel zinc-regulated zinc transporter in rice. J Exp Bot 56:3207–3214 [DOI] [PubMed]
- Kiyomiya S, Nakanishi H, Uchida H, Tsuji A, Nishiyama S, Futatsubashi M, Tsukada H, Ishioka NS, Watanabe S, Osa A, Matsuhashi S, Hashimoto S, Sekine T, Mori S (2001) Real time visualization of 13N translocation in rice under different environmental conditions using positron emitting tracer imaging system. Plant Physiol 125:1743–1753 [DOI] [PMC free article] [PubMed]
- Kobayashi T, Nakanishi H, Takahashi M, Kawasaki S, Nishizawa NK, Mori S (2001) In vivo evidence that Ids3 from Hordeum vulgare encodes a dioxygenase that converts 2’-deoxymugineic acid to mugineic acid in transgenic rice. Planta 212:864–871 [DOI] [PubMed]
- Kume T, Matsuhashi S, Shimazu M, Ito H, Fujimura T, Adachi K, Uchida H, Shigeta N, Matsuoka H, Osa A (1997) Uptake and transport of positron-emitting tracer (18F) in plants. Appl Radiot Isot 48: 1035–1043 [DOI]
- Lasat MM, Pence NS, Garvin DF, Ebbs SD, Kochian LV (2000) Molecular physiology of zinc transport in the Zn hyperaccumulator Thlaspi caerulescens. J Exp Bot 51:71–79 [DOI] [PubMed]
- Moreau S, Thomson RM, Kaiser BN, Trevaskis B, Guerinot ML, Udvardi MK, Puppo A, Day DA (2002) GmZIP1 encodes a symbiosis-specific zinc transporter in soybean. J Biol Chem 277:4738–4746 [DOI] [PubMed]
- Mori S, Nishizawa N (1987) Methionine as a dominant precursor of phytosiderophores in Graminaceae plants. Plant Cell Physiol 28:1081–1092
- Mori S, Nishizawa N, Kawai S, Sato Y, Takagi S (1987) Dynamic state of mugineic acid and analogous phytosiderophores in Fe-deficient barley. J Plant Nutr 10:1013–1020
- Nakanishi H, Okumura N, Umehara Y, Nishizawa NK, Chino M, Mori S (1993) Expression of a gene specific for iron deficiency (Ids3) in the roots of Hordeum vulgare. Plant Cell Physiol 34:401–410 [PubMed]
- Nakanishi H, Yamaguchi H, Sasakuma T, Nishizawa NK, Mori S (2000) Two dioxygenase genes, Ids3 and Ids2, from Hordeum vulgare are involved in the biosynthesis of mugineic acid family phytosiderophores. Plant Mol Biol 44:199–207 [DOI] [PubMed]
- Okumura N, Nishizawa NK, Umehara Y, Ohata T, Nakanishi H, Yamaguchi T, Chino M, Mori S (1994) A dioxygenase gene (Ids2) expressed under iron deficiency conditions in the roots of Hordeum vulgare. Plant Mol Biol 25:705–719 [DOI] [PubMed]
- Pedler JF, Parker DR, Crowley DE (2000) Zinc deficiency-induced phytosiderophore release by the Triticaceae is not consistently expressed in solution culture. Planta 211:120–126 [DOI] [PubMed]
- Ramesh SA, Shin R, Eide DJ, Schachtman DP (2003) Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiol 133:126–134 [DOI] [PMC free article] [PubMed]
- Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, von Wirén N (2004) ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J Biol Chem 279:9091–9096 [DOI] [PubMed]
- Shojima S, Nishizawa NK, Fushiya S, Nozoe S, Kumashiro T, Nagata T, Ohata T, Mori S (1989) Biosynthesis of nicotianamine in the suspension-cultured cells of tobacco (Nicotiana megalosiphon). Biol Metals 2:142–145 [DOI]
- Suzuki M, Takahashi M, Tsukamoto T, Watanabe S, Matsuhashi S, Yazaki J, Kishimoto N, Kikuchi S, Nakanishi H, Mori S, Nishizawa NK (2006) Biosynthesis and secretion of mugineic acid family phytosiderophores in zinc-deficient barley. Plant J 48:85–97 [DOI] [PubMed]
- Takagi S (1993) Production of phytosiderophores. In: Barton LL, Hemming BC (eds) Iron chelation in plants and soil microorganisms. Academic Press, San Diego
- Takahashi M, Yamaguchi H, Nakanishi H, Shioiri T, Nishizawa NK, Mori S (1999) Cloning two genes for nicotianamine aminotransferase, a criticalenzyme in iron acquisition (Strategy II) in graminaceous plants. Plant Physiol 121:947–956 [DOI] [PMC free article] [PubMed]
- von Wirén N, Marschner H, Römheld V (1996) Roots of iron-efficient maize also absorb phytosiderophore-chelated zinc. Plant Physiol 111:1119–1125 [DOI] [PMC free article] [PubMed]
- Walter A, Römheld V, Marschner H, Mori S (1994) Is the release of phytosiderophores in zinc-deficient wheat plants a response to impaired iron utilization? Physiol Plant 92:493–500 [DOI]
- Watanabe S, Ishioka NS, Osa A, Sekine T, Kiyomiya S, Nakanishi H, Mori S (2001) Production of positron emitters of metallic elements to study plant uptake and distribution. Radiochim Acta 89:853–858 [DOI]
- Zhang F, Römheld V, Marschner H (1989) Effect of zinc deficiency in wheat on the release of zinc and iron mobilizing root exudates. Z Pflanzenernhr Bodenkd 152:205–210 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.