Abstract
Peripheral exposure to LPS induces a biphasic fever thought to be initiated via vagal afferents to the preoptic area of the anterior hypothalamus (POAH), an important thermoregulatory control center in the brain. Previous studies have shown norepinephrine synaptically mediates this Prostaglandin E2 (PGE2)-dependent change in temperature through the selective activation of alpha-2 adrenoreceptors (AR). However, there is clear evidence that alpha-1 AR activation of thermoregulatory hypothalamic neurons will result in a rapid hyperthermia that is not dependent on PGE2. This direct action of norepinephrine in the POAH was tested in the present study by recording the single-unit activity of POAH neurons in a tissue slice preparation from the adult male rat, in response to temperature and the selective alpha-1 AR agonist Cirazoline (1−100 μM). Neurons were classified as either warm sensitive or temperature insensitive. Warm sensitive neurons responded to Cirazoline with a decrease in firing rate, while temperature insensitive neurons showed a firing rate increase. These responses are similar to those reported for PGE2 and suggest that both warm sensitive and temperature insensitive neurons in the POAH are important in mediating this alpha-1 AR dependent hyperthermic shift in body temperature.
Keywords: Hypothalamus, Thermoregulation, Hyperthermia, Norepinephrine, Cirazoline
1. Introduction
The preoptic area of the anterior hypothalamus (POAH), considered part of the body's central thermostat, acts as an integrating center of somatosensory input from skin and spinal thermoreceptors (Boulant, 2000). Present in this region are neurons that can be defined by their intrinsic ability to respond to changes in temperature. The majority of these neurons are insensitive to temperature, but there are thermosensitive populations which modify their firing rates as a result of local cooling or warming. These neurons are consequently able to compare central and peripheral information and establish a set-point temperature. Through their synapses with effector outputs, neurons of the POAH can manage thermoregulatory processes to maintain proper body temperature, such as vasomotion and thermogenesis.
Fever, which is the temporary elevation of the thermoregulatory set-point, is the result of various events coordinated by the hypothalamus (Saper and Breder, 1994). The adjustments made by thermoregulatory neurons in the POAH are responsible for raising body temperature into a hyperthermic range (Boulant, 1998). According to a current model of thermoregulation (Boulant, 2006), the contrasting responses of temperature insensitive and warm sensitive neurons are integral in mediating this shift. A decrease in the firing rate of warm sensitive neurons and concurrent increase in the firing rate of temperature insensitive neurons would inhibit heat loss responses, stimulate heat production, and elevate hypothalamic set-point temperature, as is the case during invasion of a pathogen. Once pathogen levels diminish, the firing rates of these neurons would return to baseline levels and a normal set-point temperature would be re-established.
The immune system responding to an invading pathogen, through a response to an endotoxin such as lipopolysaccharide (LPS), is responsible for communicating with the hypothalamus to adjust set-point temperature as described above. The indirect mechanism through which this occurs is well established to depend on LPS induced production of circulating cytokines such as tumor necrosis factor-alpha and interleukin-1beta, and the stimulated release of the endogenous pyrogen Prostaglandin E2 (PGE2) within the POAH, which will lead to fever production through its selective effects on thermoregulatory neurons (Ranels and Griffin, 2003). Yet the traditional mechanism of the PGE2-dependent febrile response has been re-evaluated because of time course discrepancies. The appearance of these circulating cytokines lags the onset of the febrile response when LPS is intravenously injected, and the synthesis of cyclooxygenase-2 (COX-2), responsible for PGE2 production, only appears well after the onset of fever (Blatteis, 2006). New evidence suggests this peripheral febrile message is relayed to the hypothalamus via a much faster neural route, through the hepatic vagus to the nucleus tractus solitarius (NST; Blatteis, 2007). Upon arrival in the liver, LPS is taken up by Kupffer cells (Kc) and a complement cascade is activated, promoting Kc to release PGE2 and stimulate hepatic vagal afferents that project to the POAH (Li et al., 2006). It is then the release of norepinephrine via the hepatic vagus which mediates this response to peripheral LPS (Feleder et al., 2007).
It is clear that norepinephrine has a thermogenic effect in the POAH, resulting in a core temperature rise (Zeisberger, 1987), as well as promoting PGE2 release (Feleder et al., 2004). Specifically, the microdialysis of norepinephrine in the POAH results in two unique thermal responses which are mediated through the actions of two adrenoreceptors (AR; Feleder et al., 2004). The alpha-1 AR, a G-protein coupled receptor, is present on thermosensitive neurons of the POAH (Mallick et al., 2002), and is thought to increase sodium pump activity (Mallick et al., 2000). It appears that POAH neurons can be affected by the alpha-1 AR agonist Cirazoline, since its microdialysis in the POAH produces a prompt rise in body temperature. This is in contrast to the delayed temperature rise mediated by the alpha-2 AR receptors that induces and is dependent on PGE2 production, and is important in the late phase of the febrile response (Feleder et al., 2004).
Because this alpha-1 AR mediated response results in an increase of core body temperature, we hypothesize that norepinephrine acts to decrease the firing rates of warm sensitive neurons and increase the firing rates of insensitive neurons in the POAH. These contrasting effects would stimulate heat production effector neurons according to current thermoregulatory models. The alpha-1 AR mediated, PGE2 independent response appears to be a significant factor in initiating a quick, initial hyperthermia. We tested the direct affect of norepinephrine on thermally classified neurons in the POAH using the alpha-1 AR agonist Cirazoline to validate norepinephrine's role in the initial hypothalamic response to LPS.
2. Results
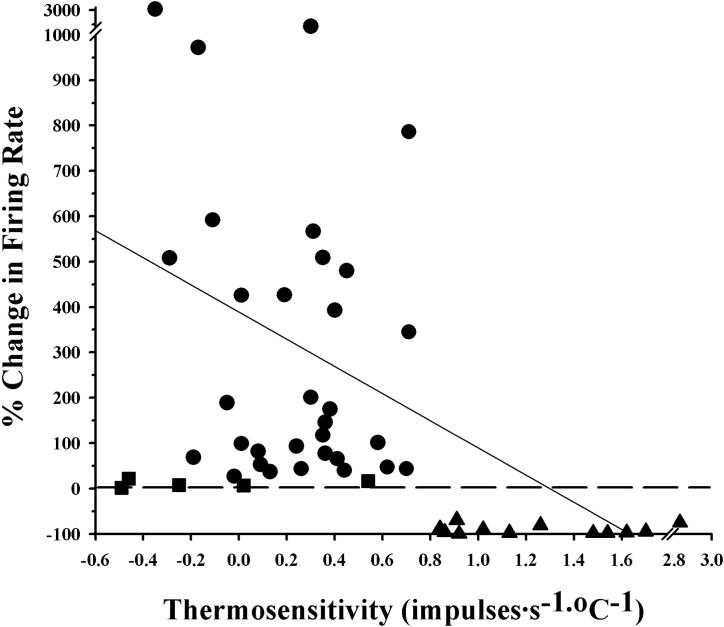
The firing rates of forty-nine POAH neurons were recorded during changes in temperature and perfusion with the alpha-1 AR agonist Cirazoline at concentrations that ranged from 1−100μM. Prior to perfusion with Cirazoline, neuronal thermosensitivity (impulses·s−1·°C−1) was determined by recording firing rate while the temperature within the recording chamber was varied by several degrees (See Methods). A neurons was classified as warm sensitive if it had a thermosensitivity of at least 0.8 impulses·s−1·°C−1. All other neurons in this study were classified as temperature insensitive. While twelve neurons were classified as warm sensitive, the majority of neurons in this study were temperature insensitive (n = 37). Fig. 1 shows the percent change in firing rate responses to Cirazoline for each neuron, plotted as a function of the neuron's thermosensitivity. Thirty-three temperature insensitive neurons showed significant increases in firing rate during perfusion with Cirazoline. All of the warm sensitive neurons responded to Cirazoline with a significant decrease in firing rate. While a negative regression could be fit to these data, there is little correlation between the magnitude of the response and thermosensitivity (r = 0.1). However, there is a correlation in the direction of response (i.e. increase, decrease in firing rate) and thermal classification (mentioned above). As a population (Table 1), temperature insensitive neurons significantly increased their firing rates from a mean of 3.4 impulses.s−1 during baseline conditions, to 10.5 impulses.s−1 during perfusion with Cirazoline. In contrast, the mean firing rate of the warm sensitive neurons decreased from 3.7 impulses.s−1 to 0.4 impulses.s−1.
Fig 1. The firing rate responses of POAH neurons to Cirazoline.
The percent change in firing rate (absolute value) for all neurons (N = 49) in response to Cirazoline is plotted against thermosensitivity. A neuron was classified as warm sensitive if it had a thermosensitivity ≥ 0.8 impulses·s−1·°C−1. All other neurons in this study were classified as temperature insensitive. Circles = insensitive neurons which showed a significant increase in firing rate, Squares = insensitive neurons that did not show a change in firing rate, Triangles = warm sensitive neurons which showed a significant decrease in firing rate. Dotted line indicates 0% change in firing rate. The solid diagonal line represents a regression fit to the data (r = 0.1).
Table 1.
Effects of Cirazoline on the firing rates of thermally classified POAH neurons
| Firing Rate (impulses.s−1 ± S.E.) |
||||
|---|---|---|---|---|
| Thermosensitivity (impulses.s−1.oC) | N | Baseline | Cirazoline | Washout |
| ≤0.79 | 37 | 3.41 ± 0.38 | 10.49 ± 1.40* | 2.68 ± 0.84 |
| ≥0.8 | 12 | 3.71 ± 0.50 | 0.44 ± 0.19* | 0.41 ± 0.00 |
Significantly different from Baseline Firing Rate (Paired T-test p < 0.05)
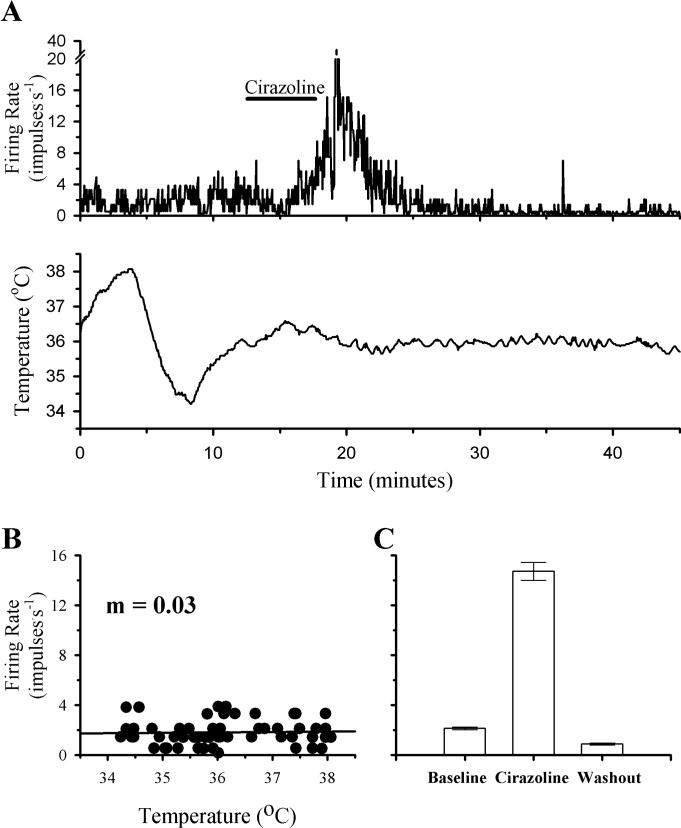
The firing rate activity of a temperature insensitive POAH neuron during a change in temperature and perfusion with Cirazoline is shown in Fig. 2. With respect to a variation in temperature (∼4°C), firing rate remained constant (m = 0.03). In response to perfusion with Cirazoline, firing rate increased from a baseline mean of 2.1 impulses.s−1 to 14.7 impulses.s−1, peaking at a firing rate of 36.8 impulses.s−1 as the perfusion medium containing Cirazoline was clearing the recording chamber. Within 10 minutes from the time perfusion with Cirazoline was stopped, firing rate had returned to baseline levels.
Fig 2. The effects of temperature and Cirazoline on the firing rate activity of a POAH temperature insensitive neuron.
A shows the firing rate of this neuron during changes in temperature and Cirazoline (10 μM; indicated by the solid bar above the graph). In B, firing rate (60 Hz) is plotted as a function of temperature. A linear regression is indicated by the solid line. In C, one minute segments of firing rate activity are plotted as individual bar graphs, just before perfusion with Cirazoline (Baseline; 2.1 ± 0.1), during the peak of the response (Cirazoline; 14.7 ± 0.7), and several minutes after Cirazoline perfusion had stopped (Washout; 0.9 ± 0.1). For plots in C, error bars may be difficult to visualize.
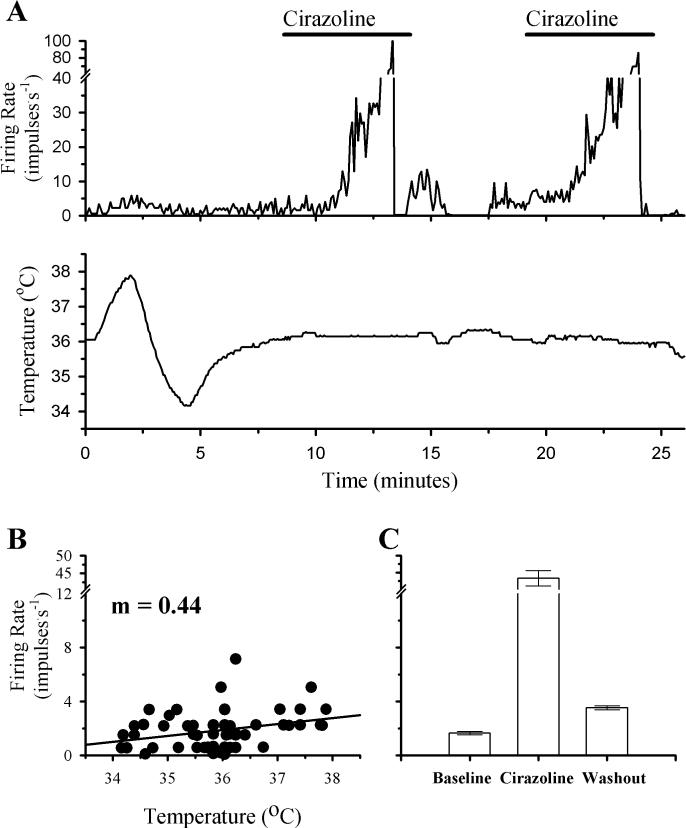
Fig. 3 shows that firing rate activity of a temperature insensitive neuron during a change in temperature and two successive perfusions with Cirazoline. While there was some correlation between temperature and firing rate, it was not large enough to classify this neuron as warm sensitive (m = 0.44). In response to Cirazoline, firing rate increased from a mean of 1.7 impulses.s−1 to 43.3 impulses.s−1. As shown in fig. 3A, this response was repeated during a second perfusion with Cirazoline.
Fig 3. The effects of temperature and successive perfusions with Cirazoline on the firing rate activity of a POAH temperature insensitive neuron.
A shows the firing rate of this neuron during changes in temperature and perfusion with Cirazoline (1 μM; indicated by the solid bars above the graph). In B, firing rate (60 Hz) is plotted as a function of temperature. A linear regression is indicated by the solid line. In C, one minute segments of firing rate activity are plotted as individual bar graphs, just before the initial perfusion with Cirazoline (Baseline; 1.7 ± 0.1), during the peak of the response (Cirazoline; 43.3 ± 2.3), and several minutes after Cirazoline perfusion had stopped (Washout; 3.5 ± 0.2). For plots in C, error bars may be difficult to visualize.
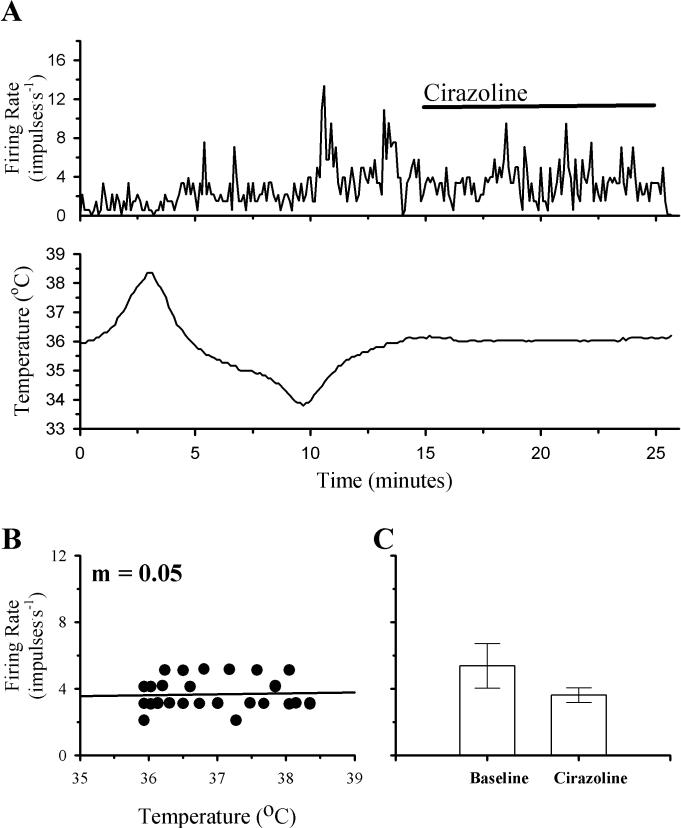
Four temperature insensitive neurons did not show significant changes in firing rate in response to perfusion with Cirazoline. The firing rate activity of one of these neurons is shown in Fig. 4. Firing rate did not change in response to a change in temperature (m = 0.05). Additionally, a ten minute perfusion with Cirazoline did not produce a significant change in this neuron's firing rate.
Fig 4. The effects of temperature and Cirazoline on the firing rate activity of a non-responsive POAH temperature insensitive neuron.
A shows the firing rate of this neuron during changes in temperature and Cirazoline (1 μM; indicated by the solid bar above the graph). In B, firing rate (60 Hz) is plotted as a function of temperature. A linear regression is indicated by the solid line. In C, one minute segments of firing rate activity are plotted as individual bar graphs, just before perfusion with Cirazoline (Baseline; 5.0 ± 0.30), and during the peak of the response (Cirazoline; 3.2 ± 0.2).
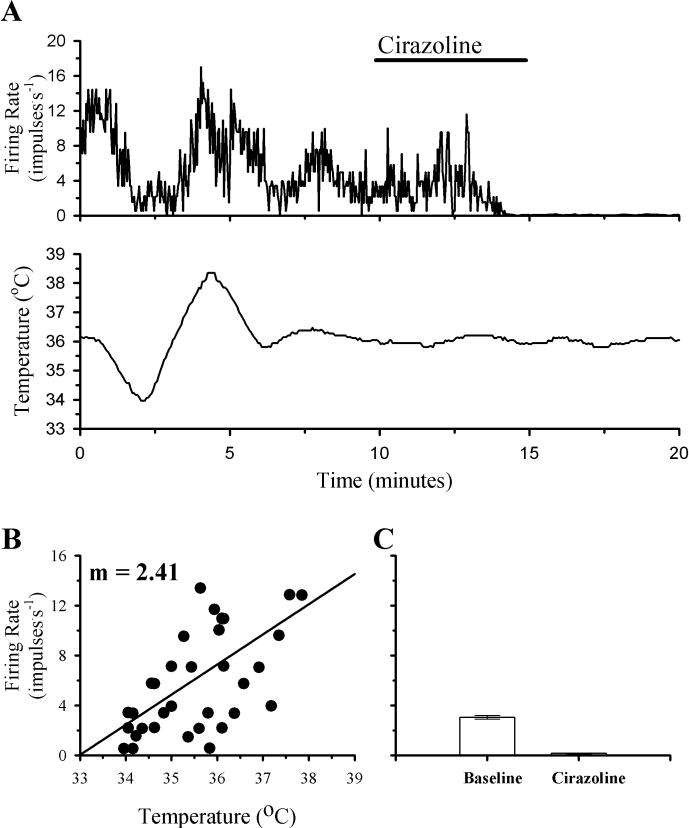
In contrast to the increases in firing rate that Cirazoline perfusion produced in the majority of temperature insensitive neurons, all of the warm sensitive neurons decreased their firing rates in response to this alpha-1 AR agonist. Fig. 5 shows the effects of Cirazoline on a warm sensitive neuron. The firing rate of this neuron showed a clear correlation with temperature (m = 2.41). During perfusion with Cirazoline, the firing rate decreased from a mean of 3.3 impulses.s−1 to 0.2 impulses.s−1. Firing rate remained low during the washout period with only the occasional production of an action potential.
Fig 5. The effects of temperature and Cirazoline on the firing rate activity of a POAH warm sensitive neuron.
A shows the firing rate of this neuron during changes in temperature and Cirazoline (1 μM; indicated by the solid bar above the graph). In B, firing rate (60 Hz) is plotted as a function of temperature. A linear regression is indicated by the solid line. In C, one minute segments of firing rate activity are plotted as individual bar graphs, just before perfusion with Cirazoline (Baseline; 3.1 ± 0.1), and during the peak of the response (Cirazoline; 0.2 ± 0.0). For plots in C, error bars may be difficult to visualize.
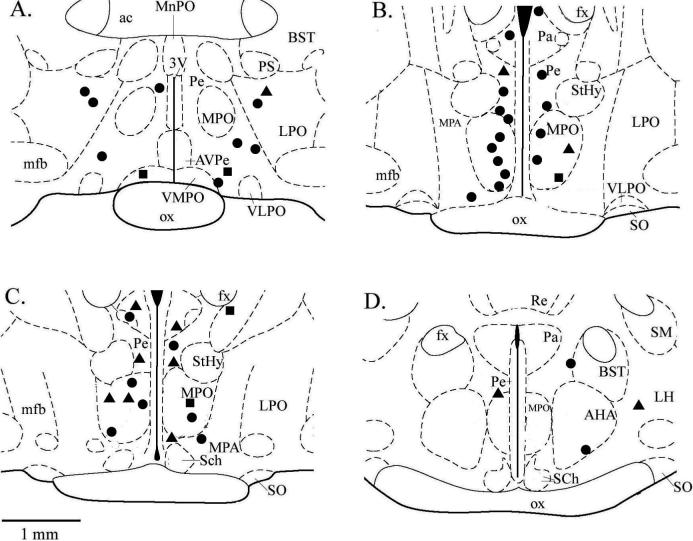
Fig. 6 provides a detailed anatomical view of the location of each recording presented in this study. The majority of recordings were made from neurons in the medial preoptic and anterior hypothalamic areas, with a few recordings from neurons in more lateral and dorsal areas of the areas of the hypothalamus. While there was no specific area of the hypothalamus in which temperature insensitive or warm sensitive neurons were found in higher proportions, it does appear that the majority of temperature insensitive neurons were located in close proximity to the midline. In addition, temperature insensitive neurons which did not respond to Cirazoline, were found more laterally. However, responses to Cirazoline were recorded from neurons in all areas where recordings were made.
Fig 6. The electrode locations for recordings of single neuron activity in response to temperature and Cirazoline.
Section diagrams are shown in the coronal plane and ordered from rostral to caudal, beginning with the upper left section and moving clockwise. Distance from bregma: A = −0.3 mm; B = −0.8 mm; C = −0.92 mm; D = −1.4 mm. Sections were adapted from an atlas of the rat brain (Paxinos and Watson, 1998). Circles = insensitive neurons which showed a significant increase in firing rate, Squares = insensitive neurons that did not show a change in firing rate, Triangles = warm sensitive neurons which showed a significant decrease in firing rate. 3V, third ventricle; ac, anterior commissure; AHA, anterior hypothalamic area; AVPe, anteroventral periventricular nucleus; BST, bed nucleus stria terminalis; fx, fornix; LH, lateral hypothalamus; LPO, lateral preoptic area; MnPO, median preoptic nucleus; MPO, medial preoptic nucleus; MPA, medial preoptic area; mfb, median forebrain bundle; ox, optic chiasm; Pa, paraventricular nucleus; Pe periventricular nucleus; PS, parastrial nucleus; Re, reunions thalamic nucleus; Sch, suprachiasmatic nuceus; SM, stria medullaris of thalamus; SO, supraoptic nucleus; StHy, striohypothalamic nuc.; VLPO, ventrolateral preoptic area; VMPO, ventromedial preoptic area.
3. Discussion
Temperature sensitive and insensitive POAH neurons form synaptic networks affecting the responses that regulate body temperature around a set-point. During fever, the thermoregulatory set-point is shifted into the hyperthermic range by the actions of these neurons. In 1965, Hammel proposed a hypothalamic neuronal model suggesting that set-point temperature is controlled by opposing synaptic inputs of warm sensitive and temperature insensitive neurons upon heat-loss and heat-production effector neurons. This model is useful in interpreting our findings. In Hammel's model, for example, warm sensitive neurons synaptically excite heat loss effector neurons and inhibit heat production effector neurons. Therefore, Cirazoline's attenuation of warm sensitive neurons should increase body temperature via reduced heat loss and increased heat production. Similarly, in Hammel's model, temperature insensitive neurons synaptically inhibit heat loss effector neurons and excite heat production effector neurons. Therefore, Cirazoline's stimulation of temperature insensitive neurons should also increase body temperature, once again, by reducing heat loss and increasing heat production.
The novel finding that Cirazoline induced a rapid-onset hyperthermia independent of PGE2 production suggested that alpha-1 ARs have an important role in the early development of a febrile response (Feleder et al., 2007). Antagonism of the alpha-1 ARs adds support for this observation, ultimately hindering rapid onset of fever and reducing its high point (Feleder, 2004). It has also been established that hepatic vagal afferents are necessary to promptly convey information from the immune system to the hypothalamus and initiate a fever in response to peripheral LPS exposure (Simons et al., 1998; MohanKumar et al., 2000). In this regard it appears that interleukin-1beta or PGE2 stimulates the vagus (Li et al., 2006; Wieczorek and Dunn, 2006), and these noradrenergic pathways project from the brainstem through the ventral noradrenergic bundle and synapse in the POAH, where there are numerous noradrenergic terminals (Kumar et al., 2007). Our work suggests that norepinephrine released from these projections may act quickly and directly at alpha-1 ARs to modulate the activity of thermosensitive and insensitive neurons to shift the set-point into a hyperthermic range. Alpha-1 AR are widely distributed in the anterior hypothalamus (Nicholas et al., 1996), and the present study indicates that these adrenoreceptors occur in both temperature sensitive and insensitive neuronal populations.
Since norepinephrine also acts at alpha-2 ARs to produce an initial hypothermic effect (Quan et al., 1992), it may be presumed that their activation results in responses for these two populations of POAH neurons that contrast those we have shown for the alpha-1 AR agonist Cirazoline, but this remains to be tested. This early hyperthermia likely attenuates due to eventual desensitization at the postsynaptic alpha-1 ARs, the induction of COX-2 expression by activation of alpha-2 ARs, ensuing PGE2 synthesis, and action at Prostaglandin E receptors (EP; presumably the EP3 subtype) which contribute the late phase of fever (Feleder et al., 2007; Lazarus et al., 2007; Oka, 2004). The application of norepinephrine in the POAH has been shown to increase the frequency of spontaneous, miniature inhibitory postsynaptic currents (IPSCs) via the alpha-1 AR, and likewise decrease the frequency of IPSCs via the alpha-2 AR (Kolaj and Renaud, 2007). These observations indicate that these two ARs can modify rapid GABA-mediated inhibitory transmission and thus have a central role in regulating excitability within this noradrenergic network of thermoregulatory neurons.
There have been some discrepancies in previous in vivo studies regarding thermal responses in the presence of norepinephrine. An early investigation found that norepinephrine produced a variety of effects in thalamic neurons, with alpha-1 AR antagonists tending to depress activity (Phillis and Tebecis, 1967). Additionally, a study to confirm the presence of alpha-1 ARs on thermosensitive neurons using in vivo single unit recordings in the presence of the alpha-1 AR agonist Prazosin, found that all thermosensitive neurons and insensitive neurons were significantly inhibited (Mallick et al., 2002). These inconsistent responses appear to be a manifestation of the surgical method used to deliver norepinephrine. It has since been demonstrated that two different responses, a temperature fall or rise, could be seen when norepinephrine was microdialyzed or microinjected respectively, and that PGE2 is a contaminant of microinjection (Quan and Blatteis, 1989). On the other hand, microdialysis of norepinephrine produces consistent physiological responses that are not artifacts of the procedure (Quan et al., 1991).
The above notwithstanding, the noradrenergic pathways in the POAH are intricately involved in thermoregulation (Blatteis, 2007; Mallick et al., 2002). As the alpha-1 AR has been implicated in initiating a rapid, PGE2-independent early hyperthermia (Feleder et al., 2004), we argued that based on the body of knowledge supporting Hammel's model of thermoregulation (Hammel, 1965), Cirazoline would result in a decrease and increase of firing rates respectively of warm sensitive and temperature insensitive neurons in the POAH. Our findings clearly demonstrate the alpha-1 AR agonist produces these contrasting effects in the two distinct populations of neurons. It lends support to this mechanism underlying febrigenesis; initiated by the immune system responding to an invading pathogen and rapidly communicated to the hypothalamus via the hepatic vagus and noradrenergic projections.
4. Experimental Procedure
4.1 Tissue Preparation and Solutions
To record the single-unit activity of POAH neurons, brain tissue slices containing the POAH were prepared from male Sprague-Dawley rats (Harlan; 100−150 grams), which were housed under standard conditions and provided unlimited food and water. Before each recording session, a rat was anesthetized using isoflurane and promptly decapitated, following procedures that have been approved by the Animal Care and Use Committee of the College of William and Mary. After dissection of the brain, a tissue block containing the hypothalamus was mounted on a vibratome and bathed in artificial cerebral spinal fluid (aCSF). One to three 400 μm thick tissue slices (coronal or saggital plane) were cut and then placed in an interface style recording chamber. Tissue slices were continually perfused with normal aCSF, which consisted of (in mM): 124 NaCl, 26 NaHCO3, 10 glucose, 5 KCl, 2.4 CaCl2, 1.3 MgSO4, and 1.24 KH2PO4. After gentle aeration (95% O2 – 5% CO2), the aCSF (300 mOsM; pH 7.5 at 36−37°C) was allowed to gravity flow at 1−2 ml.minute−1 into the recording chamber (volume = 2 ml). Temperature was maintained ∼36°C except for periodic warming and cooling to characterize the thermosensitivity of recorded neurons. A thermocouple was placed below the tissue slices to constantly monitor temperature. Cirazoline (Sigma Chemicals) was diluted to 1−100 μM and oxygenated in a separate perfusion tube (at 100 μM ; 305 mOsM; pH 7.46 at 36−37°C), which could be switched to the primary perfusion line in place of the normal aCSF medium by means of a valve control system.
4.2 Electrophysiology
To characterize the firing rate activity of individual neurons in the POAH, single-unit recordings were made of electrical activity. Recordings were made using glass microelectrodes pulled to a tip diameter of ∼1 μm and filled with a 3 M NaCl solution. Electrical activity was amplified and filtered using a Xcell-3 microelectrode amplifier and APM System (FHC Inc.). An acceptable single-unit recording required potential amplitudes with a signal-to-noise ratio greater than 3:1. Firing rate was determined through the use of a Rate / Interval Monitor (FHC Inc.) and was continuously recorded, along with temperature, on a computer using Axoscope software (Molecular Devices).
Once the activity of a single neuron had been isolated and firing rate recorded for an initial period of stability, temperature was varied 1−3 °C above and below the baseline temperature (∼36 °C) by changing the input voltage to a thermoelectric heating assembly through which the perfusion media flowed before entering the chamber. Neuronal thermosensitivity (impulses·s−1·°C−1) was later characterized by plotting firing rate as a function of temperature to determine the regression coefficient (m) of this plot. As in previous studies (Kelso et al., 1982, Fetsch et al., 2006), warm sensitivity was characterized by a regression coefficient of at least 0.8 impulses·s−1·°C−1. All other neurons in this study were classified as temperature insensitive.
Tissue temperature was again stabilized at a baseline level and the perfusion medium was switched from normal aCSF to one containing Cirazoline (1−100 μM). Perfusion with Cirazoline continued for 10 minutes or until a change in firing rate of at least 15% occurred (Fetsch et al., 2006). Cirazoline was then removed from the recording chamber by perfusion with normal aCSF for at least 10 minutes. Occasionally, after a neuron's firing rate activity recovered to a stable level for at least five minutes, a second perfusion with Cirazoline was performed.
4.3 Data Analysis
To determine if there was a significant change in a neuron's firing rate in response to Cirazoline, firing rate measurements were collected for three segments (at 60 Hz): one minute before the beginning of perfusion with Cirazoline (Baseline), one minute during the peak of the response or immediately preceding the end of the experimental perfusion (Cirazoline), and one minute at the end of the following perfusion with normal aCSF (Washout). For each segment, a mean and standard error were determined. A change in firing rate was considered significant between Baseline and Cirazoline if it was at least 15% and there was a significant difference at p < 0.05 (Student's t-test; Fetsch et al., 2006).
4.4 Histology
After each recording, the location and depth of the electrode was noted on a section diagram adapted from an atlas of the rat brain (Paxinos and Watson, 1998). At the end of a recording session, tissue slices were removed from the chamber, fixed in a formalin solution, and sectioned to a thickness of 40 − 50 μm. Sections were then mounted on gelatin coated slides and stained with giemsa to identify specific hypothalamic areas so the location of each recording within the POAH could be reconfirmed.
Acknowledgements
We wish to thank Katy Bowman, Connie Chung, and Swati Mishra for their assistance with histological processing of brain tissue. Support for this study was provided by the National Science Foundation: IBN-9983624, the National Institutes of Health: NS053794, and in part by a Howard Hughes Medical Institute grant through the Undergraduate Science Program to the College of William and Mary.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blatteis CM. Endotoxic Fever: New Concepts of its Regulation suggest new approaches to its management. Pharmacol Ther. 2006;111(1):194–223. doi: 10.1016/j.pharmthera.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Blatteis CM. The Onset of Fever: New Insights into its Mechanism. Prog Brain Res. 2007;162:3–14. doi: 10.1016/S0079-6123(06)62001-3. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Handbook of Physiology. Environmental Physiology. Am. Physiol. Soc. I. Bethesda: 1996. Hypothalamic neurons regulating body temperature. pp. 105–126. [Google Scholar]
- Boulant JA. Hypothalamic neurons: Mechanism of sensitivity to temperature. Ann. NY Acad. Sci. 1998;856:108–1153. doi: 10.1111/j.1749-6632.1998.tb08319.x. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Role of Preoptic-Anterior Hypothalamus in Thermoregulation and Fever. Clinical Infectious Diseases. 2000;31:S157–S161. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Neuronal Basis of Hammel's Model for Set-Point Thermoregulation. J. Appl. Physiol. 2006;100:1347–1354. doi: 10.1152/japplphysiol.01064.2005. [DOI] [PubMed] [Google Scholar]
- Feleder C, Perlik V, Blatteis CM. Preoptic alpha 1- and alpha 2-noradrenergic agonists induce, respectively, PGE2-independent and PGE2-dependent hyperthermic responses in guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R1156–66. doi: 10.1152/ajpregu.00486.2003. [DOI] [PubMed] [Google Scholar]
- Feleder C, Perlik V, Blatteis CM. Preoptic norepinephrine mediates the febrile response of guinea pigs to lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1135–43. doi: 10.1152/ajpregu.00067.2007. [DOI] [PubMed] [Google Scholar]
- Fetsch CR, Heideman PD, Griffin JD. Effects of melatonin on thermally classified anterior hypothalamic neurons in the white-footed mouse (peromyscus leucopus). Journal of Thermal Biology. 2006;31:40–49. [Google Scholar]
- Hammel HT. Neurons and temperature regulation. In: Yamamoto WS, Brobeck JR, editors. Physiological Controls and Regulations. Saunders; Philadelphia: 1965. pp. 71–97. [Google Scholar]
- Kelso SR, Perlmutter MN, Boulant JA. Thermosensitive single-unit activity of in vitro hypothalamic slices. Am J Physiol. 1982;242(1):R77–84. doi: 10.1152/ajpregu.1982.242.1.R77. [DOI] [PubMed] [Google Scholar]
- Kolaj M, Renaud LP. Presynaptic adrenoceptors in median preoptic nucleus modulate inhibitory neurotransmission from subfornical organ and organum vasculosum lamina terminalis. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1907–R1915. doi: 10.1152/ajpregu.00763.2006. [DOI] [PubMed] [Google Scholar]
- Kumar VM, Vetrivelan R, Mallick HN. Noradrenergic afferents and receptors in the medial preoptic area: neuroanatomical and neurochemical links between the regulation of sleep and body temperature. Neurochem Int. 2007;250(6):783–90. doi: 10.1016/j.neuint.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nature Neuroscience. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Li Z, Perlik V, Feleder C, Tang Y, Blatteis CM. Kupffer cell-generated PGE2 triggers the febrile response of guinea pigs to intravenously injected LPS. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1262–70. doi: 10.1152/ajpregu.00724.2005. [DOI] [PubMed] [Google Scholar]
- Mallick BN, Adya HV, Faisal M. Norepinephrine-stimulated increase in Na+K+−ATPase activity in the rat brain is mediated through 1A-adrenoceptor possibly by dephosphorylation of the enzyme. J Neurochem. 2000;74:1574–1578. doi: 10.1046/j.1471-4159.2000.0741574.x. [DOI] [PubMed] [Google Scholar]
- Mallick BN, Jha SK, Islam F. Presence of alpha-1 adrenoreceptors on thermosensitive neurons in the medial preoptico-anterior hypothalamic area in rats. Neuropharmacology. 2002;42:697–705. doi: 10.1016/s0028-3908(02)00016-3. [DOI] [PubMed] [Google Scholar]
- MohanKumar SMJ, MohanKumar PS, Quadri SK. Effects of bacterial lipopolysaccharide on central monoamines and fever in the rat: involvement of the vagus. Neuroscience Letters. 2000;284:159–162. doi: 10.1016/s0304-3940(00)01025-9. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Hokfelt T, Pieribone VA. The distribution and significance of CNS adrenoceptors examined with in situ hybridization. Trends Pharmacol. Sci. 1996;17:245–255. doi: 10.1016/0165-6147(96)10022-5. [DOI] [PubMed] [Google Scholar]
- Oka T. Prostaglandin E2 as a mediator of fever: the role of prostaglandin E (EP) receptors. Front Biosci. 2004;9:3046–3057. doi: 10.2741/1458. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. Academic Press; San Diego: 1998. [Google Scholar]
- Phillis JW, Tebecis AK. The responses of thalamic neurones to iontophoretically applied monoamines. J. Physiol. 1967;192:715–745. doi: 10.1113/jphysiol.1967.sp008327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Blatteis CM. Intrapreoptically microdialyzed and microinjected norepinephrine evokes different thermal responses. Am J Physiol Regulatory Integrative Comp Physiol. 1989;257:816–821. doi: 10.1152/ajpregu.1989.257.4.R816. [DOI] [PubMed] [Google Scholar]
- Quan N, Xin L, Blatteis CM. Microdialysis of norepinephrine into preoptic areaof guinea pigs: characteristics of hypothermic effect. Am J Physiol Regul Integr Comp Physiol. 1991;261:R378–R385. doi: 10.1152/ajpregu.1991.261.2.R378. [DOI] [PubMed] [Google Scholar]
- Quan N, Xin L, Ungar AL, Blatteis CM. Preoptic norepinephrine-induced hypothermia is mediated by a-2 adrenoreceptors. Am J Physiol Regulatory Integrative Comp Physiol. 1992;262:407–411. doi: 10.1152/ajpregu.1992.262.3.R407. [DOI] [PubMed] [Google Scholar]
- Ranels HJ, Griffin JD. The effects of prostaglandin E2 on the firing rate activity of thermosensitive and temperature insensitive neurons in the ventromedial preoptic area of the rat hypothalamus. Brain Research. 2003;964:42–50. doi: 10.1016/s0006-8993(02)04063-5. [DOI] [PubMed] [Google Scholar]
- Reimann W, Steinhauer HB, Hedler L, Starke K, Hertting G. Effect of prostaglandins D2, E2 and F2 on catecholamine release from slices of rat and rabbit brain. Eur J Pharmacol. 1981;69:421–427. doi: 10.1016/0014-2999(81)90445-3. [DOI] [PubMed] [Google Scholar]
- Saper CB, Breder CD. The neurological basis of fever. New Engl. J. Med. 1994;330:1880–1886. doi: 10.1056/NEJM199406303302609. [DOI] [PubMed] [Google Scholar]
- Simons CT, Kulchitsky VA, Sugimoto N, Homer LD, Szekely M, Romanovsky AA. Signaling the brain in systemic inflammation: which vagal branch is involved in fever genesis? Am J Physiol Regul Integr Comp Physiol. 1998;275:R63–R68. doi: 10.1152/ajpregu.1998.275.1.R63. [DOI] [PubMed] [Google Scholar]
- Wieczorek M, Dunn AJ. Effect of subdiaphragmatic vagotomy on the noradrenergic and HPA axis activation induced by intraperitoneal interleukin-1administration in rats. Brain Research. 2006;1101:73–84. doi: 10.1016/j.brainres.2006.04.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberger E. The roles of monoaminergic neurotransmitters in thermoregulation. Can J Physiol Pharmacol. 1987;65:1395–1401. doi: 10.1139/y87-219. [DOI] [PubMed] [Google Scholar]