Abstract
Background
Abnormalities in the amygdala and hippocampus have been implicated in the pathogenesis of major depressive disorder (MDD). To our knowledge, no prior study has examined amygdala-hippocampus anatomy in pediatric patients with familial MDD (at least one first degree relative with MDD).
Methods
32 psychotropic-naïve patients with familial MDD, aged 8 − 21 years (12 males and 20 females), and 35 group-matched healthy participants (13 males and 22 females) underwent volumetric magnetic resonance imaging (MRI) in order to evaluate hippocampal and amygdala volumes.
Results
Patients with familial MDD had significantly smaller left hippocampal (p = 0.007, d = 0.44) and right hippocampal volumes (p = 0.025, d = 0.33) than controls. No differences were noted in amygdala volumes between groups (right: p > 0.05, left: p > 0.05). No correlations between hippocampal or amygdala volumes and demographic or clinical variables were noted.
Conclusions
Reduced hippocampal volume may be suggestive of a risk factor for developing MDD.
Keywords: Adolescents, Amygdala, Depression, Familial, Hippocampus, Magnetic Resonance Imaging
INTRODUCTION
Major depressive disorder (MDD) is a common, debilitating and often severe illness with frequent onset in childhood and adolescence. The lifetime prevalence of pediatric MDD is 15 to 20%, consistent with rates reported in adult MDD samples (1). Studies of pediatric patients with MDD may minimize potential confounds such as treatment intervention and illness duration. Investigations of pediatric MDD may also clarify potential neurodevelopmental abnormalities related to the pathogenesis of the disease.
Temporal-limbic structures, such as the amygdala and hippocampus, are critical in regulating emotion (2). Dysfunction in the hippocampus and amygdala has been hypothesized to be involved in causing depressive symptoms. Decreased hippocampal volumes have been reported in adults with MDD compared to healthy controls (3-6). Increased amygdala volume has also been observed in first episode adults with MDD as compared to healthy controls (7). Frodl et al (8) extended this finding by noting significantly larger amygdala volumes in patients with a first episode of MDD compared to patients with recurrent MDD and healthy controls. Enlarged amygdala volumes were found in patients with temporal lobe epilepsy and comorbid depression as compared to patients with temporal lobe epilepsy without comorbid depression and healthy controls (9). MacMillan et al (10) reported increased amygdala to hippocampal volume ratios in psychotropic-naïve pediatric patients with MDD compared to age and sex-matched pediatric controls. While amygdala volumes tended to be larger and hippocampal volumes smaller in pediatric patients with MDD vs. controls, these differences were not statistically significant. MacMaster and Kusumakar (11) did, however, observe significant reductions in hippocampal volume in adolescents with MDD compared with age and sex matched controls. In contrast, Rosso et al (12) reported significantly decreased amygdala volume with no differences in hippocampal volume in pediatric patients with MDD vs. healthy pediatric controls. Methodological differences in measurements of the regions of interest and sample characteristics could account for varying/discrepant results in the literature, e.g, psychotropic-naïve, more comorbid anxiety disorders in the MacMillan et al (10) investigation, while in the Rosso et al (12) report, some patients were on medication and there was less comorbid anxiety. Re-analysis of the 23 pediatric patients with MDD reported in the MacMillan et al (10) investigation suggested that volumetric alterations were more prominent in the 13 pediatric patients with familial MDD (e.g, patients with at least one first degree relative with MDD) compared to the 10 pediatric patients with nonfamilial MDD (no obvious family history of mood disorder; unpublished observation). Prior investigation in the subgenual region of the prefrontal cortex has also demonstrated volumetric alterations that are most prominent in patients with familial MDD as compared to both patients with nonfamilial MDD and healthy controls (13-16).
Interestingly, prefrontal-limbic alterations have been noted in other affective disorders. In first-episode bipolar disorder, Rosso et al (17) noted smaller amygdala volume as compared to controls, similar to what was noted in MDD (12). Chang et al (18) found smaller amygdala volumes but no difference in hippocampal volume between pediatric bipolar subjects and controls. Using voxel based morphometry, Dickstein et al (19) noted reductions in amygdala and left dorsolateral prefrontal cortex volumes in pediatric bipolar disorder as compared to controls.
Twenty to 46% of MDD patients have a first degree relative with the disorder. An inverse relationship has been noted between age of onset of MDD and the density of familial loading of the disease (20, 21). The amygdala and hippocampus undergo striking maturational changes during childhood and adolescence (22-24). The current volumetric MRI investigation was conducted to evaluate amygdala and hippocampal volume in a large sample of psychotropic-naïve pediatric patients with familial MDD. We predicted reduced hippocampal volumes (3-6, 11) and increased amygdala volumes (7, 8) in pediatric patients with familial MDD compared to matched healthy volunteers. A secondary analysis used the subjects not included in our previous report (10) to determine if our results held with just the novel sample.
METHODS
Subjects
Sixteen right hand–dominant (25), psychotropic-naive patients with MDD, aged 8 to 21 years (7 males, 9 females), and 17 healthy controls (7 males, 10 females) were matched group-wise for age. These subjects have not been previously reported. For the larger analysis, an additional 32 subjects, reported previously (10, 26) were added (controls: 6 males and 12 females; MDD patients: 6 males and 12 females) (see table 1 for summary). Participants were recruited after being referred to the Pediatric Mood and Anxiety Disorders Program at Wayne State University and the Children's Hospital of Michigan. Controls were recruited via advertisement. Patients and controls were paid an honorarium for their participation in this clinical research study. The Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL) (27) was administered to all participants and their parent(s) by the child psychologists and psychiatrists. A board-certified pediatric psychiatrist (D.R.R. or Y.M.) reviewed all clinical information and confirmed DSM-IV (28) diagnostic criteria as well as associated medical or neurologic conditions. Exclusion criteria included lifetime history of psychosis, bipolar disorder, obsessive-compulsive disorder, anorexia or bulimia nervosa, posttraumatic stress disorder, substance abuse or dependence, Tourette's syndrome or other tic-related conditions, autism, mental retardation or learning disabilities, or significant medical or neurologic conditions. As determined by Family History–Research Diagnostic Criteria (29), all patients with MDD had at least one first-degree relative with MDD. No patients had a first-degree relative with bipolar disorder, using the same criteria. Controls had no history of psychiatric illness and no first degree relative with a DSM-IV (28) Axis I disorder. Written informed consent was obtained from legal guardians and written assent was obtained from all participants prior to initiating the study in compliance with the regulations of the Wayne State University Human Investigation Committee.
Table 1.
Demographic Characteristics for Patients with Familial MDD and Healthy Comparison Subjects*
| Healthy Comparison Subjects | Patients with Familial MDD | |||
|---|---|---|---|---|
| Item | N = 35 | N = 32 | T | p value |
| Age (years) | 14.51 (2.72) | 14.08 (2.88) | 0.64 | 0.53 |
| Hamilton Anxiety | 2.26 (2.65) | 14.38 (6.58) | 10.04 | <0.0001 |
| CDRS | 12.17 (10.53) | 55.34 (8.83) | 15.48 | <0.0001 |
| Illness Duration (months) | - | 27.70 (27.68) | - | - |
| Sex | 13 male, 22 female | 12 male, 20 female | - | - |
Data are given as mean (SD). MDD indicates major depressive disorder; Unpaired t-tests
Assessments
Depressive symptom severity was measured using the Childhood Depression Rating Scale-Revised (CDRS-R) (30). All patients had a CDRS-R score of at least 40, which is indicative of significant dysfunction. Severity of anxiety was assessed with the Hamilton Anxiety Rating Scale (HAMA, mean ± SD score, new subjects: 14.50 ± 7.24; old subjects: 14.25 ± 6.09; overall: 14.38 ± 6.58) (31). A score of 14 or higher is considered clinically significant (32) and 18 (56%) of the MDD patients scored 14 or greater. Duration of illness was also recorded.
MRI Acquisition & Analysis
MRI studies were conducted with a 1.5-Tesla (version 5.7; GE Signa; General Electric, Milwaukee, Wisconsin) magnetic resonance system (General Electric). Image acquisition and analysis are described in detail in our prior reports (10, 26, 33-37). Briefly, a sagittal scout series was obtained to determine image quality and clarity. A 3-dimensional spoiled gradient echo-pulse (SPGR) sequence obtained one hundred twenty-four 1.5-mm-thick coronal contiguous slices through the entire brain, perpendicular to the anterior commissure–posterior commissure line (TE = 5 milliseconds; TR = 25 milliseconds; acquisition matrix = 256 × 256 pixels; field of view = 24 cm; and flip angle =10°). All MRI scans were reviewed to rule out clinically significant abnormalities. Images were exported from the MRI unit to a computer workstation (MacIntosh Personal Computer; Apple Computer, Cupertino, California). Anatomical boundaries (detailed definitions are available on request) were determined from neuroanatomical atlases (38). Descriptions of the measurement methods have been detailed previously for intracranial volume (33), amygdala and hippocampus (10, 35). Briefly, the measurement of the posterior hippocampus starts when an ovoid mass of gray matter appeared inferiomedially to the trigone of the lateral ventricle. All cornu ammonis (CA) segments, dentate gyrus, alveus, parasubiculum, subiculum proper, and prosubiculum were included in the measurement of the posterior hippocampus after the interruption of the pulvinar by the crus of the fornix. The anterior and posterior hippocampi are separated by the appearance of the cistern pontis. The anterior hippocampus measurement began on the first slice where the cistern pontis was visible. The amygdala measurement began when it first appeared posteriorly. We separated the anterior portion of the hippocampus and the amygdala by following the alveus when visible. If this was not readily seen, a straight line was drawn from the most superiomedial portion of the temporal horn laterally to the most medial part of the ambient gyrus (10). Measurement of amygdala, hippocampus, and intracranial volume was made by well-trained and reliable raters (0.94 and 0.98, 0.95 and 0.99, 0.99 and 0.99, respectively, PE and LK), blind to any identifying clinical information. Manual measurement of the amygdala and hippocampus was performed using MED× 3.30 software (Sensor Systems). One MDD patient was judged to not have amygdala measurements of sufficient quality due to subject motion to be included in the analysis. Their hippocampal measurement was judged to be sufficient for inclusion.
Data Analysis
We conducted analysis of covariance (ANCOVA) with age, ICV and sex as covariates. As the groups were not pairwise matched for age or sex, it was felt that adding them as covariates would help limit their impact on the effects of interest. As intracranial volume demonstrated a trend to be larger in familial patients with MDD than in controls overall (1137.36 ± 108.19 controls vs. 1189.97 ± 139.18 MDD patients, t = 1.74, df = 65, p = 0.09), ICV was added as a covariate as well. For the primary analysis, based on the directional hypotheses (3-8, 11), significance was set at p = 0.03. For the secondary, exploratory analysis, significance was set at alpha = 0.05. Pearson correlations were used to determine the relationships between the regions of interest and clinical/demographic variables (i.e. age, CDRS, HAMA, duration of illness). The potential confound of comorbid conditions were addressed by comparing patients with comorbidity to those patients without and controls (t-tests). The potential influence of anxiety disorders were also examined in this manner.
RESULTS
General
Of the 32 patients, 20 had comorbid anxiety disorders, 5 had oppositional defiant disorder, 4 had attention-deficit disorder without hyperactivity, 1 had dysthymia, 1 had conduct disorder and 8 had MDD as their sole diagnosis. Patients and control groups did not differ with regard to age (new subjects; t = 0.50, df = 31, p = 0.62; old subjects: t = 0.44, df = 32, p = 0.67; overall: t = 0.64, df = 65, p = 0.53). Mean (±SD) age of onset of the first clinical presentation in the patients with MDD was 11.77 ± 2.92 years (new subjects: 11.55 ± 3.41 years; old subjects: 11.99 ± 2.44 years). CDRS (mean ± SD) scores were 54.38 ± 9.95 for new subjects and 56.31 ± 7.76 for the old subjects (overall: 55.34 ± 8.83). HAMA (mean ± SD) scores were 14.50 ± 7.24 for the new subjects and 14.25 ± 6.09 for the old subjects (overall: 14.38 ± 6.58).
Primary Analysis
Both left hippocampal (3.15 ± 0.46cc controls vs. 2.95 ± 0.44cc MDD patients; F = 7.93, df = 1, 62, p = 0.007; d = 0.44) and right hippocampal volume (3.16 ± 0.42cc controls vs. 3.00 ± 0.50cc MDD patients; F = 5.27, df = 1, 62, p = 0.025, d = 0.33) was significantly smaller in pediatric patients with familial MDD as compared to controls. Neither right amygdala (1.36 ± 0.30cc controls vs. 1.52 ± 0.27cc MDD patients; F = 3.68, df = 1, 61, p = 0.06) nor left amygdala volume (1.36 ± 0.29cc controls vs. 1.49 ± 0.36cc MDD patients; F = 0.03, df = 1, 61, p = 0.86), was significantly different in pediatric patients with familial MDD as compared to controls (see table 2). No relationship between hippocampus and amygdala with age was noted in either the group. No relationships (correlations) between the regions of interest and the clinical variables (CDRS, duration of illness) were noted in the familial MDD patients as well. When comparing MDD patients with one year or less duration of illness to controls, right amygdala was significantly larger in patients (t = 2.37, df = 45, p = 0.02) with a strong trend in the same direction in the left amygdala (t = 1.96, df = 45, p = 0.06). In the more chronic cases (> 1 year of illness), no difference was noted in amygdala volumes in our sample.
Table 2.
Summary of Volumetric Results
| Region of Interest | All Familial MDD* | New Sample | ||
|---|---|---|---|---|
| Right Hippocampus | p = 0.025 | Smaller in MDD | p = n.s. | - |
| Left Hippocampus | p = 0.007 | Smaller in MDD | p = 0.017 | Smaller in MDD |
| Right Amygdala | p = n.s. | - | p = n.s. | - |
| Left Amygdala | p = n.s. | - | p = n.s. | - |
MDD indicates major depressive disorder
Secondary Analysis
In the subgroup of novel subjects, the difference noted in the left hippocampal volume (3.06 ± 0.49cc controls vs. 2.76 ± 0.27cc MDD patients; F = 6.50, df = 1, 28, p = 0.017; d = 0.66) held with comparable (medium) effect sizes and differences noted in the expected direction. With the larger sample, a difference was noted in the right hippocampus. In the novel sample, the difference between the groups did not hold for the right hippocampus, but the effect size was very similar and in the expected direction (2.99 ± 0.27cc controls vs. 2.84 ± 0.46cc MDD patients; F = 2.11, df = 1, 28, p = 0.18, d = 0.39). No differences were noted (as with the larger sample) in amygdala volume. No relationship between hippocampus and amygdala with age was noted in either the group. No relationships (correlations) between the regions of interest and the clinical variables were noted in the familial MDD patients as well.
Sex Differences
With the larger sample in the secondary analysis, an exploratory analysis of possible sex differences was conducted. We found that the right amygdala was smaller in control females as compared to control males (t = 2.12, df = 33, p = 0.04) but not in MDD patients (t = 1.70, df = 29, p = 0.10). Left amygdala did not significantly differ in control females as compared to control males (t = 1.77, df = 33, p = 0.09) or in MDD patients (t = 0.90, df = 29, p = 0.38). Left hippocampal volumes were smaller in females than males in both controls (t = 3.21, df = 33, p = 0.003) and MDD patients (t = 3.08, df = 30, p = 0.004). Right hippocampal volumes were smaller in females than males in MDD patients (t = 3.68, df = 30, p = 0.001) but not controls (t = 1.62, df = 33, p = 0.11).
Effect of Comorbidity
As 20 patients had a comorbid anxiety disorder, it is especially noteworthy that anxiety did not correlate with any region of interest volume in MDD patients, even when splitting the MDD patient group by presence of an anxiety disorder or not. Indeed, when comparing the left hippocampus of the 8 MDD patients without a comorbid diagnosis to the 35 controls, the effect size remained similar (d = 0.62) and in the expected direction but lacked statistical power to demonstrate more than a trend (t = 1.75, df = 41, p = 0.09). No differences were noted between the 8 MDD patients without a comorbid disorder and the 24 MDD patients with a comorbid disorder in any of the regions of interest (right amygdala: t = 1.72, df = 29, p = 0.10; left amygdala: t = 0.28, df = 29, p = 0.78; right hippocampus: t = 0.42, df = 30, p = 0.68; left hippocampus: t = 0.99, df = 30, p = 0.33).
COMMENT
This hypothesis driven preliminary investigation extends previous investigation in adults and pediatric patients with MDD that have included patients with familial and nonfamilial MDD (3-6, 10, 11, 39) by finding smaller hippocampal volumes in pediatric patients with familial MDD patients as compared to case matched controls. Given normative developmental changes in hippocampal anatomy (22-24), the volumetric alterations observed in the present study may result from altered brain development. It is interesting that the difference in the right hippocampus noted in the larger analyses were not seen in the secondary analysis. The right hippocampus was smaller in MDD patients as compared to controls in the larger sample with the effect sizes for both analyses (primary and secondary) being similar (effect size [d] = 0.33 and 0.39). This similarity held for the left hippocampus (d = 0.44 and 0.66). For the amygdala, its small size and difficulty in its measurement may explain why even with a strong effect sizes (right: d = 0.56 and left: d = 0.37), no significant difference was noted in the larger sample. Indeed, the inherent variance in the amygdala measure (coefficient of variation = 22%) as compared to that of the hippocampus (coefficient of variation = 15%) indicates that larger samples are required in order to establish robust differences in amygdala volume in pediatric MDD.
The present study's finding of no significant difference in amygdala volume between psychotropic-naïve patients with familial MDD and controls is not consonant with the investigation of Frodl et al (8) which showed an increased amygdala volume in adults with a first episode of MDD. Interestingly, when comparing MDD patients with one year or less duration of illness to controls, right amygdala was significantly larger in patients with a strong trend in the same direction in the left amygdala. In the more chronic cases (> 1 year of illness), no difference was noted in amygdala volumes in our sample. It may be that the enlargement is transitory in depression. Longitudinal, course of illness studies are needed to clarify this. It also differs from the finding of Rosso et al (12) in pediatric MDD noting a smaller amygdala volume as compared to controls. Discrepant findings may be due to differences in image acquisition, analysis and measurement of regions of interest as well as sample characteristics including family history, gender, age, duration of illness, medication status and comorbidity. There is growing evidence for distinct neuroanatomic patterns, particularly in the prefrontal cortex, in familial vs. nonfamilial patients with MDD (13-16, 26). Prior investigation has also shown that treatment with a selective serotonin reuptake inhibitor (SSRI) can reduce amygdala volumes in pediatric patients with obsessive-compulsive disorder (37). That report found that greater reductions in amygdala volume were correlated with higher SSRI dosages and longer cumulative exposure to the medication. Hence, medication status may indeed play a role in the discrepancies noted in the literature. Aberrations in the developmental maturation of the amygdala and hippocampus in patients with MDD vs. controls may also be involved.
Giedd et al (22) noted sex-specific maturational changes in the volumes of medial temporal structures. The left amygdala increased significantly only in males with age and the right hippocampus increased significantly only in females. We did not note this effect here. We did note a smaller right amygdala in females than males in controls and a smaller left hippocampus in females than males in both groups. Our sample size is cross-sectional and may be too small to demonstrate a similar set of correlations to Giedd et al (22). Given our findings and those of Giedd et al (22), a further exploration of sex differences in relation to regional brain volumes in mood disorders is warranted.
Comorbid and/or sub-clinical states may influence the results presented here. Indeed, the mean HAMA score reported is considered in the clinically significant range for anxiety (32). Although all patients in this report had a primary diagnosis of MDD, comorbidity is common in pediatric MDD (21, 22). Rusch et al (39) found a positive correlation between hippocampal volume and trait anxiety. Altered hippocampal volumes have been observed in hyperanxious rats (40). Additionally, early childhood anxiety has also been shown to predict later emergence of depression (41). However, no differences were noted between the MDD patients with a comorbid anxiety disorder and MDD patients without such comorbidity on any of the regions of interest, nor were any correlations noted between HAMA and any of the regions of interest. However, given the smaller groups used when sub-dividing, our power to assess the effect of comorbidity is not ideal. Interestingly, our results indicate that pediatric MDD may indeed differ from pediatric bipolar disorder; as a smaller amygdala volumes have been reported in pediatric bipolar disorder (17-19). Smaller hippocampal volumes may be indicative of mood disorders in general; as they have been noted in bipolar disorder (42, 43).
A trend for a larger ICV in psychotropic-naïve pediatric patients with familial MDD than in controls was found in this report. It should be noted that Steingard et al (44) reported smaller whole brain volumes in adolescents with MDD as compared to controls. Some patients in that study had been treated with psychotropic medication previously and familial vs. nonfamilial status was not reported. It is a strength of our report that all MDD patients were psychotropic naïve at the time of their scan.
These results should be considered preliminary given several limitations. We defined familial MDD as a patient having at least one first-degree relative with MDD. While consistent with prior reports (13, 14), a potential limitation of this definition is that it did not include patients who may have had other relatives (e.g. grandparents, cousins, etc) who have MDD. The method for determining the degree of family history (29) is also limited by the number and veracity of the interviewed family members. Given the heterogeneity of pediatric MDD, future studies are needed to more precisely delineate familial and non-familial subtypes of MDD. It is also not possible to tease out whether a smaller hippocampus volume and enlarged amygdala are a function of the patients being depressed or due to a genetic loading (due to afflicted first degree relatives) or both. These findings, in conjunction with other markers, could help delineate an endophenotype for genetic studies of MDD. Indeed, high-risk approaches may also serve to clarify if these differences are prodromal. However, smaller hippocampal volume may not be a risk factor for MDD. Hippocampal volume changes may also be a result of the illness itself with repeated episodes causing a reduction in volume (6, 45, 46). Additionally, these findings may not be specific to MDD, as many other conditions affect/involve changes to hippocampal volume (i.e. 45).
These findings suggest smaller left hippocampal volume in psychotropic-naïve pediatric patients with familial MDD. These differences may represent an early neurobiological marker of familial MDD. It may not be specific to mood disorders as smaller hippocampal volumes have been noted in schizophrenia as well (47, 48). It could also be that the smaller volume is actually secondary to stress process (46).
These alterations may also be an epiphenomenon of the underlying pathology of the illness. In view of the fact that SSRI treatment can lead to regional change in brain volumes (34, 36), longitudinal MRI studies of the regions of interest may help identify neuroanatomic targets for treatment. Neuroimaging studies may also better define clinical endophenotypes for MDD facilitating candidate gene studies. Neuroimaging studies of unaffected offspring or siblings of patients with MDD at increased genetic risk for developing MDD may also be helpful given recent neuroendocrine studies demonstrating abnormalities in this population (49).
Figure 1.
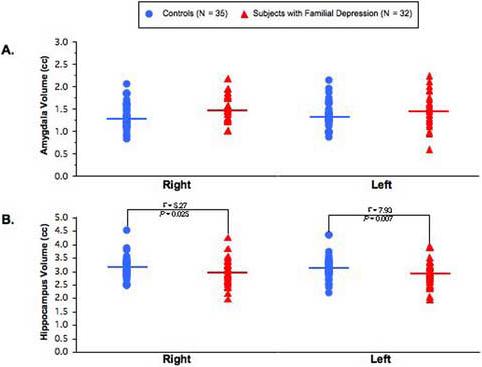
Scatter plots of right and left (A) amygdala and (B) hippocampal volume in patients with familial major depressive disorder and healthy comparison subjects (means are represented by straight line).
Acknowledgment
This work was supported in part by the State of Michigan Joe F. Young Sr. Psychiatric Research and Training Program, the Miriam L. Hamburger Endowed Chair at Children's Hospital of Michigan and Wayne State University, Detroit, MI, and grants from the National Institute of Mental Health (R01MH59299, R01MH65122, K24MH02037 and MH01990 (Szeszko)) and the Mental Illness Research Association (MIRA). Sponsors played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
IN THIS ISSUE
The amygdala and hippocampus are thought to be involved with depression. We looked at these brain regions using volumetric magnetic resonance imaging in healthy and depressed youth. Depressed youth had smaller hippocampal volumes than healthy children. A smaller hippocampal volume may be a risk factor for developing depression.
Financial Disclosures: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Lewinsohn PM, Duncan EM, Stanton AK, Hautzinger M. Age at first onset for nonbipolar depression. J Abnorm Psychol. 1986;95:378–383. doi: 10.1037//0021-843x.95.4.378. [DOI] [PubMed] [Google Scholar]
- 2.LeDoux JE. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. Simon & Schuster; New York: 1996. [Google Scholar]
- 3.Frodl T, Meisenzahl EM, Zetzsche T, Hohne T, Banac S, Schorr C, et al. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159:1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- 4.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 5.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheline Y, Sanghavi M, Mintun M, Gado M. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C, et al. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- 8.Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy controls. Biol Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- 9.Tebartz van Elst L, Woermann F, Lemieux L, Trimble MR. Increased amygdala volumes in female and depressed humans. A quantitative magnetic resonance imaging study. Neurosci Lett. 2000;281:103–106. doi: 10.1016/s0304-3940(00)00815-6. [DOI] [PubMed] [Google Scholar]
- 10.MacMillan S, Szeszko PR, Moore GJ, Madden R, Lorch E, Ivey J, et al. Increased amygdala: hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J Child Adolesc Psychopharmacol. 2003;13:65–73. doi: 10.1089/104454603321666207. [DOI] [PubMed] [Google Scholar]
- 11.MacMaster FP, Kusumakar V. Hippocampal volume in early onset depression. BMC Med. 2004;2:2. doi: 10.1186/1741-7015-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA. Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry. 2005;57:21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 15.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- 17.Rosso IM, Kilgore WD, Cintron CM, Gruber SA, Tohen M, Yurgelun-Todd DA. Reduced amygdala volumes in first episode bipolar disorder and correlation with cerebral white matter. Biol Psychiatry. 2007;61:743–749. doi: 10.1016/j.biopsych.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:565–73. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 19.Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, Pine DS, et al. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62:734–41. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 20.Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, et al. Childhood and adolescent depression, I: A review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1996a;35:1427–1439. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J. Childhood and adolescent depression, II: A review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1996b;35:1575–1583. doi: 10.1097/00004583-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4−18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Pfluger T, Weil S, Weis S, Vollmar C, Heiss D, Egger J, et al. Normative volumetric data of the developing hippocampus in children based on magnetic resonance imaging. Eilepsia. 1999;40:414–423. doi: 10.1111/j.1528-1157.1999.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 24.Yurgelun-Todd DA, Killgore WD, Cintron CB. Cognitive correlates of medial temporal lobe development across adolescence: A magnetic resonance imaging study. Percept Mot Skills. 2003;96:3–17. doi: 10.2466/pms.2003.96.1.3. [DOI] [PubMed] [Google Scholar]
- 25.Annett M. The binomial distribution of right, mixed and left handedness. Q J Exp Psychol. 1967;19:327–333. doi: 10.1080/14640746708400109. [DOI] [PubMed] [Google Scholar]
- 26.Nolan CL, Moore GJ, Madden R, Farchione T, Bartoi M, Lorch E, et al. Prefrontal cortical volume in childhood-onset major depression: Preliminary findings. Arch Gen Psychiatry. 2002;59:173–179. doi: 10.1001/archpsyc.59.2.173. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Press; Washington, DC: 1994. (DSMIV) [Google Scholar]
- 29.Andreasen N, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: Reliability and validity. Arch Gen Psychiatry. 1977;34:1229–1335. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 30.Poznanski EO, Freman LN, Mokros HB. Children's depression rating scale-revised. Psychopharmacol Rev. 1985;21:979–989. [Google Scholar]
- 31.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 32.Kobak KA, Reynolds WM, Greist JH. Development and validation of a computer-administered version of the Hamilton Anxiety Scale. Psychol Assess. 1993;5:487–492. [Google Scholar]
- 33.Rosenberg DR, Keshavan MS, O'Hearn KM, Dick EL, Bagwell WW, Seymour AB, et al. Frontostriatal measurement of treatment-naive pediatric obsessive-compulsive disorder. Arch Gen Psychiatry. 1997;54:824–830. doi: 10.1001/archpsyc.1997.01830210068007. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert AR, Moore GJ, Keshavan MS, Paulson LA, Narula V, MacMaster FP, et al. Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder taking paroxetine. Arch Gen Psychiatry. 2000;57:449–456. doi: 10.1001/archpsyc.57.5.449. [DOI] [PubMed] [Google Scholar]
- 35.Szeszko PR, Goldberg E, Gunduz-Bruce H, Ashtari M, Robinson D, Malhotra AK, et al. Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:2190–2197. doi: 10.1176/appi.ajp.160.12.2190. [DOI] [PubMed] [Google Scholar]
- 36.Szeszko PR, MacMillan S, McMeniman M, Chen S, Baribault K, Lim KO, et al. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. Am J Psychiatry. 2004;161:1049–1056. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- 37.Szeszko PR, MacMillan S, McMeniman M, Lorch E, Madden R, Ivey J, et al. Amygdala volume reductions in pediatric patients with obsessive-compulsive disorder treated with paroxetine: preliminary findings. Neuropsychopharmacology. 2004;29:826–832. doi: 10.1038/sj.npp.1300399. [DOI] [PubMed] [Google Scholar]
- 38.Daniels DL, Haughton VM, Naidich TP. Cranial and Spinal Magnetic Resonance Imaging: An Atlas and Guide. Raven Press; New York, NY: 1987. [Google Scholar]
- 39.Rusch BD, Abercrombie HC, Oakes TR, Schaefer SM, Davidson RJ. Hippocampal morphometry in depressed patients and control subjects: Relations to anxiety symptoms. Biol Psychiatry. 2001;50:960–964. doi: 10.1016/s0006-3223(01)01248-3. [DOI] [PubMed] [Google Scholar]
- 40.Kalisch R, Schubert M, Jacob W, Kessler MS, Hemauer R, Wigger A, et al. Anxiety and Hippocampus Volume in the Rat. Neuropsychopharmacology. 2006;31:925–932. doi: 10.1038/sj.npp.1300910. [DOI] [PubMed] [Google Scholar]
- 41.Pine DS, Cohen P, Brook J. Adolescent fears as predictors of depression. Biol Psychiatry. 2001;50:721–724. doi: 10.1016/s0006-3223(01)01238-0. [DOI] [PubMed] [Google Scholar]
- 42.Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 43.Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 44.Steingard RJ, Renshaw PF, Hennen J, Lenox M, Cintron CB, Young AD, et al. Smaller frontal lobe white matter volumes in depressed adolescents. Biol Psychiatry. 2002;52:413–417. doi: 10.1016/s0006-3223(02)01393-8. [DOI] [PubMed] [Google Scholar]
- 45.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54: 200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 47.Narr KL, van Erp TG, Cannon TD, Woods RP, Thompson PM, Jang S, et al. A twin study of genetic contributions to hippocampal morphology in schizophrenia. Neurobiology. 2002;11:83–95. doi: 10.1006/nbdi.2002.0548. [DOI] [PubMed] [Google Scholar]
- 48.Seidman LJ, Faraone SV, Goldstein JM, Kremen WS, Horton NJ, Makris N, et al. Left hippocampal volume as a vulnerability indicator for schizophrenia: A magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Arch Gen Psychiatry. 2002;59:839–849. doi: 10.1001/archpsyc.59.9.839. [DOI] [PubMed] [Google Scholar]
- 49.Birmaher B, Dahl RE, Williamson DE, Perel JM, Brent DA, Axelson DA, et al. Growth hormone secretion in children and adolescents at high risk for major depressive disorder. Arch Gen Psychiatry. 2000;57:867–872. doi: 10.1001/archpsyc.57.9.867. [DOI] [PubMed] [Google Scholar]


