Abstract
Numerical and functional defects in plasmacytoid dendritic cells (pDCs) are an important hallmark of progressive HIV-1 infection, yet its etiology remains obscure. HIV-1 p17 matrix protein (p17) modulates a variety of cellular responses, and its biological activity depends on the expression of p17 receptors (p17Rs) on the surface of target cells. In this study, we show that peripheral blood pDCs express p17Rs on their surface and that freshly isolated pDCs are sensitive to p17 stimulation. Upon p17 treatment, pDCs undergo phenotypic differentiation with up-regulation of CCR7. A chemotaxis assay reveals that p17-treated pDCs migrate in response to CCL19, suggesting that these cells may acquire the ability to migrate to secondary lymphoid organs. In contrast, p17 does not induce release of type I IFN nor does it enhance pDC expression of CD80, CD86, CD83, or MHC class II. Microarray gene expression analysis indicated that p17-stimulated pDCs down-regulate the expression of molecules whose functions are crucial for efficient protein synthesis, protection from apoptosis, and cell proliferation induction. Based on these results, we propose a model where p17 induces immature circulating pDCs to home in lymph nodes devoid of their ability to serve as a link between innate and adaptative immune systems.
Keywords: immune deregulation, CCR7 chemokine receptor
Plasmacytoid dendritic cells (pDCs) are a subset of dendritic cells (DCs) present in the peripheral blood and secondary lymphoid organs (1). As compared with other DC subsets, pDCs display unique features in terms of surface receptor phenotype (2, 3) and capacity to migrate directly from blood to lymphoid tissues, where they play an essential role in innate antiviral immunity, being the natural IFN type I producing cells (3, 4). Moreover, pDCs may switch their function from professional effector cells of the innate system to professional antigen-presenting cells, thus directly linking the innate and adaptive immune system.
The role of pDCs in HIV-1 disease is of great interest. In fact, recent studies indicate that pDCs are directly involved in the immunopathogenesis of HIV-1 infection. During chronic HIV-1 infection, reduced blood pDC frequency correlates with high viral load, reduced CD4 count, and susceptibility to opportunistic infections (5–7). Along with their quantitative loss during HIV-1 disease, pDCs also display an altered phenotype (5, 8) and an impaired functionality (9, 10). pDCs can be infected with HIV-1 in vitro, but productive infection is achieved only by using high titer stocks (11, 12), and in vivo direct infection is unlikely to account for the altered pDCs functions described above. This hypothesis is further supported by the finding that the decrease of pDCs, as well as their dysfunction, are not completely reversed in HIV-1 seropositive subjects despite long-term successful highly active antiretroviral therapy (HAART) (13, 14), suggesting that mechanisms other than active viral replication are involved in HIV-1-induced pDC perturbation. Popovic et al. (15) have recently reported a long-term persistence of HIV-1 structural proteins in the lymph nodes (LNs) of patients under HAART even in the absence of detectable virus replication. HIV-1 structural proteins have been associated with immune activation and loss of functional competence for different immune cells. It is therefore possible that interactions between pDCs and virion components may impact the number and functions of pDCs.
The HIV-1 matrix protein p17 (p17) is a 17-kDa myristoylated protein derived from the extreme N terminus of the Gag precursor polyprotein p55. It is a key component of the HIV-1 preintegration complex and performs other crucial functions throughout the HIV-1 life cycle (16). In addition to its viral replication supporting role, p17 exhibits different immunomodulatory properties that may be relevant in the context of viral pathogenesis. In fact, p17 has been shown to enhance HIV-1 replication in vitro, influencing the activation and differentiation status—as well as the proliferative capacity—of viral target cells (17). Therefore, p17 may be considered as a viral cytokine whose functional activities are exerted after binding to a yet uncharacterized cellular receptor (p17R), which is expressed on different immune cells (18, 19).
The present study reports that circulating pDCs express p17Rs and that p17 induces the expression of functional CCR7 molecules, allowing pDCs to migrate in response to specific chemotactic stimuli. The acquisition of a migratory phenotype by p17-stimulated pDCs is not followed by cell maturation and type I IFN release. These results are consistent with the very restricted gene program induced in pDCs by p17. These data allow us to propose a model where p17 induces immature circulating pDCs to home in LNs, devoid of their ability to serve as a link between innate and adaptative immune systems.
Results
p17 Receptor Molecules Are Specifically Expressed by pDCs.
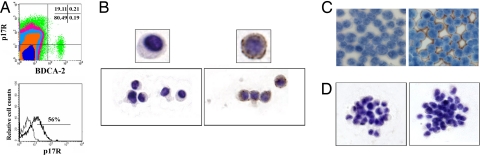
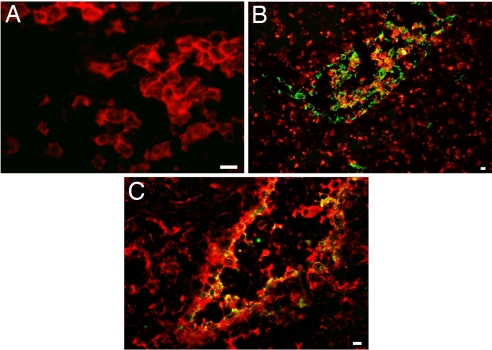
Recently, we demonstrated that p17Rs are expressed on different human immune cell subsets (18, 19). The present study was designed to delineate the significance of p17R expression for pDCs. When peripheral blood mononuclear cell (PBMCs) were double-stained with a mAb against the pDC marker BDCA-2 and biotinylated p17 (b-p17), phenotypic analysis showed that ≈50% of circulating pDCs were p17R+ (Fig. 1A). The presence of p17Rs on pDCs was then evaluated on cells isolated by magnetic sorting. Purified pDCs were obtained by a combined negative and positive magnetic selection, which allowed us to obtain a >98% pure population. The absence of cellular contaminants was assessed by flow cytometry (data not shown) and by the absence of IL-12 and IL-10 release upon cell stimulation with CpG and inactivated influenza virus (FLU) (20). Flow cytometric analysis on freshly isolated pDCs confirmed that a high percentage of cells expresses p17Rs. Cytospin-collected purified pDCs showed a robust and homogeneous surface expression upon binding with b-p17 and staining with HRP-conjugated streptavidin (Fig. 1B). Human lymphoblastoid cell lines H9 and Raji served as negative and positive controls, respectively (Fig. 1C). After being cultured with IL-3, purified pDCs exhibited their conventional aggregated morphology (2) and, in contrast to freshly collected cells, they displayed a p17Rneg phenotype within 24 h of culture (Fig. 1D). In keeping with the lack of p17R occurring on mature pDCs, no expression of p17R was found on CD123+ pDCs in reactive LNs (Fig. 2A) and tonsils (not shown). Interestingly, strong p17 labeling was detected on sinusoidal CD68+/DC-SIGN+ lining cells, whereas no p17 binding was observed in perisinusoidal macrophages and myeloid DCs, strongly labeled by CD68 or DC-SIGN, respectively (Fig. 2 B and C).
Fig. 1.
p17R expression on human pDCs. (A) Flow cytometry was used to detect the expression of p17R on BDCA-2+ pDCs within freshly isolated PBMCs. (Upper) Results are displayed as bivariate dot plots. (Lower) pDCs were then purified, and p17R expression on their surface was evaluated on BDCA-2-gated cells (thick line). The thin line shows isotype control mAb. Immunocytochemical staining of p17R on purified pDCs is shown. (B) The expression of p17R was evident in freshly isolated cells. (C) The lymphoblastic cell lines H9 and Raji served as negative (Left) and positive (Right) control, respectively. (D) Lack of staining was evident when pDCs were cultured for 18–24 h. (B Left and D Left) Unbiotinylated protein was used as specificity control. Representative results from one of three independent experiments are shown.
Fig. 2.
Immunohistochemical analysis of p17R expression in reactive LN. Double immunofluorescence for CD123 (A), CD68 (B), and DC-SIGN (C) (all revealed by Texas red) and b-p17 (revealed by FITC). CD123+ pDCs do not express p17R, whereas coexpression is obvious on DC-SIGN+ and CD68+ sinusoidal lining cells. Note that perisinusoidal macrophages and myeloid DC, stained by CD68 and DC-SIGN, respectively, are p17-negative. (Magnification: A, ×400; B, ×200; C, ×300.) (Scale bars: 10 μm.)
pDCs Matured by p17 Acquire Chemotactic Capacity Toward CCL19.
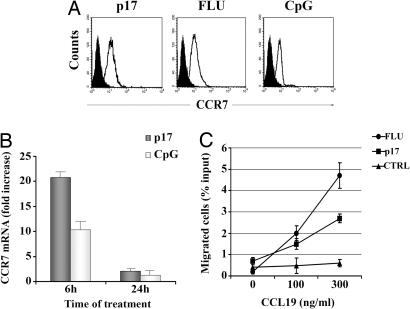
A complex modulation of chemokine responsiveness and production is responsible for activated pDC migration from the peripheral tissues into the T cell areas of secondary lymphoid organs where DCs exert their functions (21, 22). After exposure to a variety of stimuli, pDCs undergo up-regulation of CCR7, which is one of the first events during their transition from immature to mature pDCs. The presence of p17R on the pDC cell surface made us speculate that pDCs may undergo an activation and/or maturation program upon exposure to the viral protein. Treatment of pDCs with exogenous p17 at different doses ranging from 10 ng/ml to 1 μg/ml induced a clear up-regulation of CCR7 expression as compared with cells incubated 24 h in the presence of IL-3 alone. The maximum increase in CCR7 expression was reached when p17 was used at 500 ng/ml, being similar to that observed by using Toll-like receptor (TLR)9 (CpG) and TLR7 (FLU) agonists as specific pDC stimuli (Fig. 3A).
Fig. 3.
p17-treated pDCs express functional CCR7. (A) Phenotypic expression of CCR7 was analyzed on pDCs cultured for 24 h in medium (black histograms) or in the presence of the indicated stimuli (white histograms). (B) Quantitative real-time PCR analysis of CCR7 gene expression in p17- and CpG-treated pDCs. CCR7 mRNA levels are expressed as fold induction of the levels detected in untreated cells at the same time points. Bars represent the mean ± SD of triplicate samples. (C) Migration of pDCs to the indicated concentration of CCL19 was measured as described in Materials and Methods. Results are expressed as mean percentage of migration of input cells ± SD from triplicate wells. A representative experiment of five performed is shown. CTRL, control.
In a subsequent set of experiments, we used a quantitative RT-PCR method to confirm that CCR7 induction by p17 occurred at the mRNA level. After 6 and 24 h of p17 incubation, CCR7 messengers were quantified in relation to the β-actin mRNA expression in each sample. As shown in Fig. 3B, a significant induction of CCR7 transcripts, equivalent to that observed in CpG-stimulated cells, was found in p17-treated pDCs (P < 0.01 as compared with unstimulated pDCs). Expression of CCR7 alone, however, is not sufficient for DC migration, because it can be expressed in a biologically insensitive state, such that CCR7+ DCs either fail to undergo chemotaxis toward CCR7 ligands (23) or require a high concentration of CCR7 ligands before responding (24). Because pDCs express CCR7 following exposure to p17, we tested their capacity to migrate toward the CCL19 chemokine, the CCR7 ligand, which is secreted in the lymphoid compartment. After 24 h of culture with medium, p17, or CpG, the chemotactic activity of pDCs toward CCL19 was measured in a 4-h transwell assay. CCL19 induced a dose-dependent migration of pDCs cultured with p17. At the optimal CCL19 concentration tested (300 ng/ml), migration of p17-treated pDCs was 4.6-fold higher than that observed in untreated cells (P < 0.05). On the other hand, p17 treatment resulted in a migration that was 57.4% of that obtained after stimulation with FLU, whereas comparable migration rates were observed at a lower CCL19 dosage. These data indicate that p17 not only up-modulates CCR7 expression but also sensitizes CCR7 to its ligand CCL19. The expression of CCR7 induced by p17 in pDCs is therefore functional and may facilitate pDC homing to secondary lymphoid tissues.
p17 Does Not Induce pDC Maturation.
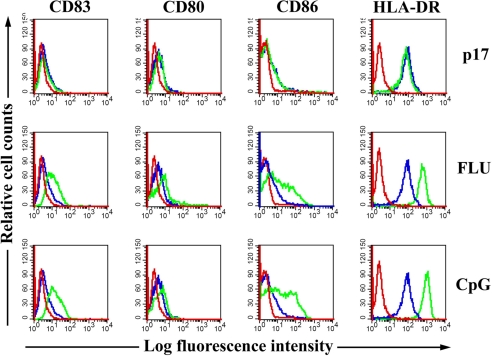
CCR7 expression typically correlates with the up-regulation of MHC class II, activation markers, and costimulatory molecules that occur when DCs mature (25). We therefore attempted to verify whether the p17 binding to its receptor could induce a phenotypic maturation of pDCs. Cells were treated or not with p17 and stained for HLA-DR, CD80, CD86,and CD83. Upon p17 exposure, pDCs maintained levels of HLA-DR expression levels comparable with those observed on untreated cells. Moreover, p17-treated pDCs showed no expression of the molecules CD83, CD80, and CD86 (Fig. 4). The expression of all of the tested surface markers, on the other hand, was up-regulated in pDCs treated with either FLU or CpG. Taken together, these data show that the migratory phenotype induced by p17 is not followed by a maturation to a fully differentiated phenotype.
Fig. 4.
FACS analysis of expression of pDC maturation molecules. pDCs were cultured 24 h in the presence or absence of p17 (500 ng/ml), FLU (20 ng/ml), or CpG (6 μg/ml). Surface expressions of CD83, CD80, CD86, and HLA-DR on treatments (green histograms) are presented in comparison with untreated cells (blue histograms). Red lines represent the matched isotype control. Each image is representative of at least three different experiments.
Cytokine Production by pDCs in Response to p17 Treatment.
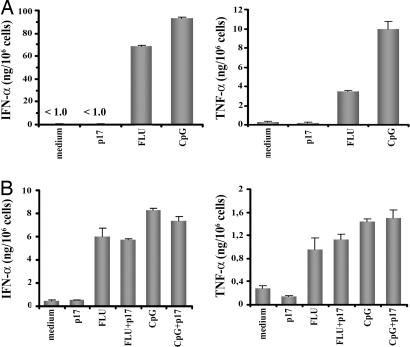
pDCs exposed to viruses such as FLU or HSV are known to secrete IFN-α and TNF-α, which promote their viability and induce their maturation. Experiments were thus performed to quantify IFN-α and TNF-α in the supernatant of p17-treated pDCs. As shown in Fig. 5A, p17 was unable to induce a clear-cut production of IFN-α (460 ± 237 pg per 106 cells) and TNF-α (380 ± 336 pg per 106 cells). Significantly, the induction of cytokine secretion by stimulation with the influenza virus or the TLR9 ligand was not modulated by the presence of p17. In fact, pDCs promptly produced considerable amounts of IFN-α and TNF-α after stimulation by low doses of FLU or CpG (0.5 ng/ml and 0.5 μg/ml, respectively), and neither the IFN-α nor the TNF-α secretion induced by such stimuli was affected significantly upon costimulation with p17 (Fig. 5B). It follows that p17 does not seem to affect the capacity of pDCs to respond to other stimuli.
Fig. 5.
Production of IFN-α and TNF-α by stimulated pDCs. Production of IFN-α and TNF-α by pDCs in the presence p17 (500 ng/ml), FLU (20 ng/ml), or CpG (6 μg/ml) (A) or suboptimal doses of CpG (0.5 μg/ml) and FLU (0.5 ng/ml) alone or in combination with p17 (500 ng/ml) (B). Results represent the mean ± SD of triplicate samples. Data are from one experiment representative of eight with similar results.
HIV-p17 Triggers a Highly Restricted Gene Program in pDCs.
Upon p17 treatment, pDCs showed an uncoupling of the expression of functional CCR7 from both their cytokine production and their phenotypic maturation. To investigate the global gene program triggered by p17, we analyzed by microarrays the transcriptional profiles of pDCs purified from two healthy donors 18 h after in vitro exposure to medium, p17, or CpG. In agreement with previous studies (20), CpG induced changes in 416 probe sets and sharply up-regulated genes involved in pDCs activation. Nineteen of these differentially expressed transcripts encoded IFN type I-related genes [supporting information (SI) Table 1]. On the other hand, the transcriptional profile of p17-treated pDCs showed that only 48 probe sets were modulated with a program that significantly differs from that induced in CpG-activated cells. In agreement with the lack of IFN-α release, p17 did not trigger the induction of any IFN type I-related gene. It is worth noting that p17 down-regulated the expression of different molecules involved in the control of anti-apoptotic functions and cell survival and proliferation (i.e., nucleophosmin, hsp70, and eukaryotic translation factor 5B). These results point to a possible role for p17 in rendering cells susceptible to apoptotic signals (SI Table 1).
Detection of p17 Antigen in Sera.
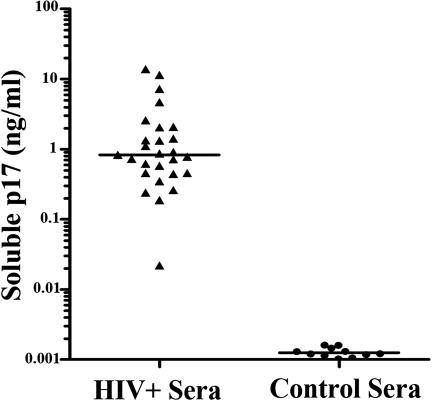
p17 has been shown to exert its biological activity on different cell types at doses as low as 1 ng/ml, reaching an optimal in vitro activity at concentrations ranging from 50 to 500 ng/ml (17–19). Despite the fact that p17 was detected in tissues from HIV-1+ patients, it was still unknown whether p17 is available in sera to exert its modulatory activity onto circulating pDCs. Therefore, the level of p17 was monitored in sera of HIV-l-infected individuals by antigen capture ELISA (Fig. 6). Twenty-six of 27 sera obtained from HIV-1+ patients contained p17 at concentrations ranging from 0.19 to 13.9 ng/ml (median 0.83 ng/ml). Nonspecific reactions were not present in the 11 control sera obtained from healthy volunteers. All of the sera also contained detectable titers of anti-p17 Abs (more than 1:1,000 by ELISA, not shown).
Fig. 6.
Detection of p17 in the sera of HIV-1-infected patients. Sera from 27 individuals infected with HIV-1 and 11 healthy volunteers were examined for the presence of p17 by antigen capture ELISA. Recombinant p17 was added to control sera and was used for the standard curve.
Discussion
A crucial role in the generation and regulation of immunity is played by pDCs, and evidences of their relevance in the pathogenesis of AIDS are rapidly accumulating. Deregulation of the immune system and, in particular, of DC biology is a strategic mechanism whereby HIV-1 escapes immune attack. This phenomenon is directly linked to the capacity of HIV-1 to target immune cells but is also driven by the presence, in the infected host, of HIV-1 viral proteins whose contribution to immune dysregulation has been widely investigated. In the present study, we characterize the biological response of pDCs to the HIV-1 matrix protein p17, and we delineate a pDC activation pathway where the acquisition of a migratory phenotype is not followed by the release of IFN-α or by other biological effects relevant to the competence of pDCs.
Our results show that two pDC cell subsets can be identified in human blood based on the differential expression of p17R on their surface. Given the evidence that pDCs become p17Rneg after culture, it can be hypothesized that loss of p17R expression by pDCs reflects a different stage in cell maturation. However, it cannot be ruled out that the p17R+ cell subset may be characterized by different biological features and may have a different impact on the development of HIV-1-related pDC dysfunction.
After activation with microbial stimuli (TLR ligands), blood pDCs up-regulate functional CCR7, thereby acquiring responsiveness toward CCL19 and CCL21 expressed by high endothelial venules (HEVs) and LN constituents (21–25). Because of the critical role of CCR7 in mediating pDC homing, we investigated whether p17 has the ability to induce the expression of the chemokine receptor. We found that CCR7 mRNA transcription was markedly induced at 6 h after p17 exposure and declined thereafter, whereas the presence of CCR7 on the cell surface was clearly detectable within 18–24 h of culture in the presence of p17. Notably, the kinetics, as well as the intensity, of p17-driven CCR7 expression were comparable with those displayed after treatment with potent CCR7 inducers such as FLU and CpGs. Moreover, CCR7 molecules were functional because p17-treated pDCs were able to undergo chemotaxis toward CCL19. These findings suggest that p17 can act as an immune modulator capable of changing peripheral pDC phenotype in a way that supports migration to secondary lymphoid organs. Several studies have shown that during HIV-1 infection, pDCs are substantially reduced in the blood of patients (5–7, 13). Different hypotheses have been put forward to account for the disappearance of pDCs from the peripheral blood of HIV-1-positive patients, such as their death because of HIV-1 infection (11), the failure of DC precursors to differentiate, or their relocalization in secondary lymphoid tissues as a consequence of an increased homing potential (8, 26). This hypothesis would be consistent with the in vitro induction by p17 of CCR7 expression and of migration toward CCL19 and suggests that exposure to p17 may contribute to the loss of pDCs observed during HIV-1 infection through a relocalization of pDCs to secondary lymphoid tissues.
CCR7 expression typically correlates with increased expression levels of HLA-DR and activation molecules as well as with induction of cytokine waves occurring when pDCs mature (25). This study reports that pDC migration and IFN type I production and/or maturation can be independently regulated events. The induction of CCR7 expression in the absence of cell maturation is not, however, an unknown phenomenon in other DC types. Geissmann et al. (27) have observed that immature Langerhans cells migrate and accumulate in LNs draining chronically inflamed skin, but that they nevertheless lack costimulatory molecules. Verbovetski et al. (28) have shown that immature DCs respond to apoptotic cells opsonized by iC3b acquiring CCR7 expression despite the absence of cell maturation. The fact that p17-stimulated pDCs exhibit a typical immature phenotype (i.e., do not express CD80, CD83, and CD86 or up-regulate HLA-DR) raises questions as to their role in the regulation of immune response at the LN level, considering that immature pDCs have been proposed to mediate tolerance (29). It will be of interest to delineate the molecular mechanisms, which may differentially trigger pDC migration and maturation as well as determine the role and fate of such immature p17-triggered cells within LNs.
Stimulation of pDCs with p17 did not induce IFN-α production. Moreover, interaction of p17 with its receptor did not trigger the transcriptional activation of any IFN-α-related genes, thus confirming that type I IFNs were not produced following p17 stimulation. Instead, the same cells were fully competent to respond to CpG by releasing high amounts of IFN-α and to strongly activate a complex IFN-α-related gene program. To understand whether p17 promotes signaling events aimed at blocking IFN-α production, we stimulated pDCs with low doses of TLR7 or TLR9 agonists together with p17. In both cases, we observed that p17 does not synergize or counteract the induction of cytokine release by FLU- and CpG-treated cells. This finding clearly demonstrates that p17 does not impair the capability of pDCs to produce type I IFNs following appropriate stimulation. Moreover, our data suggest that p17 and TLR7 or TLR9 pathways do not intersect at the molecular level in human pDCs.
Data obtained from p17-triggered gene expression program identify candidate genes that may be important to pDC biology. In the examination of host genes modulated by the viral protein, a down-regulation of nucleophosmin, heat shock protein 70, and the eukaryotic translation initiation factor 5B was observed. Nucleophosmin is a major nucleolar phosphorprotein involved in many cellular activities whose expression strongly stimulates E2F1-mediated transcriptional activity, promotes cell survival, and confers resistance to apoptotic stimuli. It is now well established that nucleophosmin acts as a physiologically important anti-apoptotic protein and that its knock-down induces cell death (30). Also, the molecular chaperone hsp70 has general cytoprotective properties. Cell lines engineered to overexpress hsp70 have been shown to resist apoptosis, and its elevated expression is associated with increased proliferation (31). Although the significance of down-regulating these genes is not known, deregulation of these factors may be detrimental for the surviving of migrated pDCs. Recent studies have shown that pDC depletion is a dramatic event occurring not only in the periphery but also in LNs (32), and this event can be ascribed to: (i) the presence in LNs of pDCs that are primed for apoptosis; and (ii) the internal deficit of IFN-α, which is known to be a potent survival factor for pDCs (26, 33). Impaired IFN-α production and depletion of pDCs are common features of viral infections, including HIV-1, human T cell leukemia virus type 1, hepatitis B virus, and hepatitis C virus (26, 34, 35). In some cases, the viral protein responsible for activity on pDCs has been identified: e.g., the hepatitis C core protein. The common exploitation of diverting the pDC functions further points to the central role of these cells in antiviral immunity and of viral proteins in escaping pDC surveillance. In the case of HIV-1, subverting pDC biology is a redundant phenomenon, performed by such viral proteins as gp120 (12) and, as shown here, by the matrix protein p17. The major role of HIV-1 structural proteins is enforced by the finding that following HAART, the restoration of pDC numbers and function is a belated and often incomplete event (13). Popovic et al. (15) have highlighted the long-term persistence of HIV-1 structural proteins in tissue specimens of HIV-1-infected patients who received antiviral treatment, even in the absence of any viral replication. Moreover, amounts of HIV-1 proteins did not noticeably differ in specimens either before initiation of therapy or during treatment where the virus burden significantly decreased. This finding strongly suggests that HIV-1 structural proteins persist in patients under HAART for a long time in the absence of detectable virus replication and makes it conceivable that the chronic activity of such proteins on immune cells could lead to pathogenic events and to the HIV-1-related immune dysfunction.
p17 is present in the serum of HIV-1+ patients at nanomolar concentrations. Abs to p17 were also present in all of the sera tested, making it possible that the amount of p17 detected by capture ELISA could be lowered by the presence of p17-anti-p17 immune complexes. Because p17 binds to its receptor on pDCs, the protein concentration on the cell surface could even be higher, as has been hypothesized for the chemokine family and the HIV-1 protein Tat (36, 37). These findings suggest that the viral protein may likely exert biologically relevant activities on circulating pDCs. p17 may access the extracellular space via alternative secretion pathways (38, 39). Because two subsets of the matrix protein exist, one cleaved in the virions and the other cleaved in the host cell (40), it is conceivable that p17 may be generated and released along all of the virus life cycle by infected cells. Concurrently, extracellular p17 can reach consistent concentrations in HIV-1-infected hosts through mechanisms of virus disintegration or cell lysis.
Extrapolating our data, we envisage a scenario where p17 can trigger circulating pDCs to express CCR7, thus helping their recruitment, and eventually, their trapping in the lymphoid organs. In the LN environment, pDCs may be altered in their functions and possibly be driven to apoptosis and cell death. Our results, showing that p17 induces pDC migration to LNs without IFN-α production and phenotype maturation, offer insight into HIV-1 pathogenesis and reveal a target for immune-based intervention as a way to slow down HIV-1 disease progression.
Materials and Methods
Recombinant Proteins.
Recombinant p17 and GST proteins were expressed and purified as described previously (18). The absence of endotoxin contamination (<0.1 endotoxin units/100 μg of recombinant protein) in the p17 preparation was assessed by Limulus amoebocyte assay (BioWhittaker), according to the manufacturer's recommendations. Purified p17 and GST were also biotinylated by using AH-NHS-Biotin (SPA).
Peripheral Blood pDC Purification and Culture.
PBMC were isolated from buffy coats (through the courtesy of the Centro Trasfusionale, Spedali Civili, Brescia) by Ficoll gradient (Sigma), and pDCs were magnetically sorted to a purity of 98% by using the Diamond plasmacytoid dendritic cells isolation kit (Miltenyi Biotec). pDCs (106 cells per milliliter) were then cultured in RPMI medium 1640 10% FCS plus 20 ng/ml IL-3 (ProSpec) (culture medium). Where indicated, cells were stimulated with p17 (500 ng/ml), 6 μg/ml CpG type A oligonucleotides 2216 (CpG) (MGW Biotech), and 20 ng/ml hemagglutinin-inactivated influenza virus strain A/Moscow/10/99 (FLU) (a kind gift from T. De Magistris, Istituto Superiore di Sanità, Rome, Italy). In some experiments, CpG and FLU were used at 0.5 μg/ml and 0.5 ng/ml, respectively.
RNA Preparation and Microarray Hybridization.
Total RNA from pDCs was isolated after cell culture with the RNeasy kit and used to generate cDNA and cRNA to hybridize on Human Genome U133 plus 2.0 array according to the manufacturer's protocol (Affymetrix). Microarray hybridization and analysis are described in the SI Materials and Methods.
Real-Time PCR.
The relative amounts of CCR7 and β-actin mRNAs were determined by TaqMan-based real-time PCR assays (Hs00171054_m1 and Hs99999903_m1 gene expression assays; Applied Biosystems). Analysis of data was performed with the 2−ΔΔCt method. Quantification of CCR7 cDNA was normalized in each reaction according to the internal β-actin control. Levels of CCR7 mRNA were expressed as fold difference of treated cells as compared with untreated cells (calibrator sample).
Immunocytochemistry and Immunofluorescence Stainings.
Lymphoblastoid cell lines H9 (ATCC no. HTB-176), Raji (ATCC no. CCL-86), and magnetically purified pDCs were centrifuged onto microscope slides (200 × g for 3 min), fixed with 95% ethanol, and used for immunocytochemical staining. p17R expression was analyzed by using b-p17 (3.5–4.0 mg/ml) for 30 min at room temperature followed by the streptavidin–biotin complex immunoperoxidase technique (streptavidin–HRP; Biogenex), with diaminobenzidine as chromogen. At least five fields for each sample were randomly selected and analyzed. False positive reactions were excluded, staining all of the cells with unlabeled p17 or with biotinylated-GST replacing the b-p17.
Double immunofluorescence was performed on frozen sections of tonsils and LNs; sections were first incubated with anti-CD123 (Pharmingen/BD Biosciences), anti-DC-SIGN (Pharmingen/BD Biosciences), and anti-CD68 (Dakocytomation) as primary Abs followed by Texas red-conjugated anti-IgG1 secondary Ab (Southern Biotechnology); subsequently, b-p17 was applied and revealed by streptavidin FITC (Southern Biotechnology). Sections were examined with an Olympus BX60 microscope, equipped with fluorescence and with a DP-70 digital camera (Olympus). Images were acquired by using analysis ImageProcessing software (Soft Imaging System GmbH). Immunofluorescence images were merged as described previously (41).
Flow Cytometric Analysis.
p17 binding to the pDC surface was evaluated as described with minor modifications (18). Briefly, PBMCs were suspended in PBS/1% FCS (107 cells per milliliter), incubated with b-p17 (400 ng/ml) at 4°C for 30 min, and then counterstained with streptavidin–allophycocyanin (Caltag/Valter Occhiena) together with anti-BDCA-2-phycoerythrin mAb (Miltenyi Biotech). For phenotypic determination, purified pDCs were stained with anti-CD80, anti-CD83, anti-CD86 (Pharmingen) anti-CCR7 (R&D System) FITC, anti-HLA-DR phycoerythrin (Pharmingen), or the respective FITC- or phycoerythrin-conjugated isotype mAbs (Pharmingen). Fluorescence was analyzed by flow cytometry on a FACScalibur by using CellQuest software (BD Biosciences).
Migration Assay.
pDC migration was measured by chemotaxis through a 5-μm pore polycarbonate filter in 24-well transwell chambers (6.5-mm diameter; Costar). Five-hundred microliters of RPMI medium 1640 containing serial dilutions of CCL19 (PeproTech) were added to the lower chamber. Differentially treated pDCs (5 × 104), resuspended in 100 μl of RPMI medium 1640 containing 0.5% BSA, were then added to the transwell insert. After incubation for 4 h at 37°C, the migrated cells were harvested and counted. The number of cells in the starting population and the migrated population was calculated, and the percentage of migration was determined from these values. Each experiment was performed in triplicate.
Measurement of Cytokines and Chemokines.
Supernatants were collected 24 h after stimulation and assayed for the presence of IFN-α and TNF-α by using a quantitative sandwich ELISA system (Endogen). Release of IL-12 and IL-10 was evaluated by using the SearchLight protein arrays (Endogen).
Measurement of p17 in Sera of HIV-1-Infected Patients.
p17 antigen in sera was measured by sandwich-type capture ELISA. Wells of the microplates (Maxisorp; Nunc) were coated with 10 μg per well of purified anti-p17 mAb MBS-3 (18) overnight at 4°C. After incubation with blocking buffer (PBS/0.1% Tween-20/3% BSA), sample sera and purified p17 (diluted in pooled sera obtained from HIV− donors) as a standard were added to the wells in duplicate at 2-fold dilutions. After a 2-h incubation at room temperature, plates were washed (PBS/0.1% Tween-20), and 100 μl of 10 μg/ml biotinylated anti-p17 rabbit polyclonal Ab was used to detect captured p17. The reactive Abs were detected with a 1,000-fold dilution of HRP-conjugated streptavidin (Sigma) (30 min at room temperature). This enzyme reaction was started by adding the specific substrate 3,3′,5,5′-tetramethylbenzidine (Sigma) and stopped with 1 M sulfuric acid. The concentration of p17 in the samples was calculated by interpolation of OD values from the standard curve.
Statistical Analysis.
The statistical significance of the differences was determined from the means and standard deviations by using the Student's two-tailed t test. Differences were considered significant at P < 0.05.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Francesca Gentili and Wilma Pellegrini for excellent technical assistance and Robert Geffers for expert support in array experiments. This work was supported by the Italian Ministry of University and Scientific Research (Programmi di Ricerca di Rilevante Interesse Nazionale projects) and Istituto Superiore di Sanitá (AIDS Grant 40G.16).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE10147).
This article contains supporting information online at www.pnas.org/cgi/content/full/0800370105/DC1.
References
- 1.Colonna M, Krug A, Cella M. Interferon-producing cells: On the front line in immune responses against pathogens. Curr Opin Immunol. 2002;14:373–379. doi: 10.1016/s0952-7915(02)00349-7. [DOI] [PubMed] [Google Scholar]
- 2.Facchetti F, Vermi W, Mason D, Colonna M. The plasmacytoid monocyte/interferon producing cells. Virchows Arch. 2003;443:703–717. doi: 10.1007/s00428-003-0918-8. [DOI] [PubMed] [Google Scholar]
- 3.Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 4.Cella M, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 5.Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in HIV-1-infected individuals. J Infect Dis. 2003;187:26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- 6.Donaghy H, et al. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 7.Soumelis V, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 8.Loré K, et al. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS. 2002;16:683–692. doi: 10.1097/00002030-200203290-00003. [DOI] [PubMed] [Google Scholar]
- 9.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–4511. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 10.Almeida M, Cordero M, Almeida J, Orfao A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS. 2005;19:261–271. [PubMed] [Google Scholar]
- 11.Schmidt B, et al. Low-level HIV infection of plasmacytoid dendritic cells: Onset of cytopathic effects and cell death after PDC maturation. Virology. 2004;329:280–288. doi: 10.1016/j.virol.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Martinelli E, et al. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-α secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2007;104:3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chehimi J, et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, et al. Differential restoration of myeloid and plasmacytoid dendritic cells in HIV-1-infected children after treatment with highly active antiretroviral therapy. J Immunol. 2006;176:5644–5651. doi: 10.4049/jimmunol.176.9.5644. [DOI] [PubMed] [Google Scholar]
- 15.Popovic M, et al. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2005;102:14807–14812. doi: 10.1073/pnas.0506857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorentini S, Marini E, Caracciolo S, Caruso A. Functions of the HIV-1 matrix protein p17. New Microbiol. 2006;29:1–10. [PubMed] [Google Scholar]
- 17.De Francesco MA, et al. HIV p17 enhances lymphocyte proliferation and HIV-1 replication after binding to a human serum factor. AIDS. 1998;12:245–252. doi: 10.1097/00002030-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 18.De Francesco MA, et al. HIV-1 matrix protein p17 increases the production of proinflammatory cytokines and counteracts IL-4 activity by binding to a cellular receptor. Proc Natl Acad Sci USA. 2002;99:9972–9977. doi: 10.1073/pnas.142274699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitale M, et al. HIV-1 matrix protein p17 enhances the proliferative activity of natural killer cells and increases their ability to secrete proinflammatory cytokines. Br J Haematol. 2003;120:337–343. doi: 10.1046/j.1365-2141.2003.04053.x. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 21.Forster R, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 22.Penna G, Sozzani S, Adorini L. Selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol. 2001;167:1862–1866. doi: 10.4049/jimmunol.167.4.1862. [DOI] [PubMed] [Google Scholar]
- 23.Scandella E, Men Y, Gillessen S, Förster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–1361. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 24.Robbiani DF, et al. The leukotriene C (4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 25.Sozzani S. Dendritic cell trafficking: More than just chemokines. Cytokine Growth Factor Rev. 2005;16:581–592. doi: 10.1016/j.cytogfr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Hosmalin A, Lebon P. Type I interferon production in HIV-infected patients. J Leukoc Biol. 2006;80:984–993. doi: 10.1189/jlb.0306154. [DOI] [PubMed] [Google Scholar]
- 27.Geissmann F, et al. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J Exp Med. 2002;196:417–430. doi: 10.1084/jem.20020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verbovetski I, et al. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR, CD86, and up-regulates CC chemokine receptor 7. J Exp Med. 2002;196:1553–1561. doi: 10.1084/jem.20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohl L, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, et al. Sumoylation of nucleophosmin/B23 regulates its subcellular localization, mediating cell proliferation and survival. Proc Natl Acad Sci USA. 2007;104:9679–9684. doi: 10.1073/pnas.0701806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosser DD, et al. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biancotto A, et al. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–4279. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito T, et al. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–2969. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 34.van der Molen RG, et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40:738–746. doi: 10.1002/hep.20366. [DOI] [PubMed] [Google Scholar]
- 35.Anthony DD, et al. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907–4916. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 36.Hoogewerf AJ, et al. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry. 1997;36:13570–13578. doi: 10.1021/bi971125s. [DOI] [PubMed] [Google Scholar]
- 37.Albini A, et al. HIV-1 Tat protein mimicry of chemokines. Proc Natl Acad Sci USA. 1998;95:13153–13158. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nickel W. The mystery of nonclassical protein secretion: A current view on cargo proteins and potential export routes. Eur J Biochem. 2003;270:2109–2119. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- 39.Gould SJ, Booth AM, Hildreth JEK. The Trojan exosome hypothesis. Proc Natl Acad Sci USA. 2003;100:10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan AH, Swanstrom R. HIV-1 Gag proteins are processed in two cellular compartments. Proc Natl Acad Sci USA. 1991;88:4528–4532. doi: 10.1073/pnas.88.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marafioti T, et al. Phenotype and genotype of interfollicular large B cells, a subpopulation of lymphocytes often with dendritic morphology. Blood. 2003;102:2868–2876. doi: 10.1182/blood-2003-03-0692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.