Abstract
NF-κB inducing kinase (NIK) is required for osteoclastogenesis in response to pathologic stimuli, and its loss leads to functional blockade of both alternative and classical NF-κB caused by cytoplasmic retention by p100. We now show that deletion of p100 restores the capacity of NIK-deficient osteoclast (OC) precursors to differentiate and normalizes RelB and p65 signaling. Differentiation of NIK−/− precursors is also restored by overexpression of RelB, but not p65. Additionally, RelB−/− precursors fail to form OCs in culture, and this defect is rescued by re-expression of RelB, but not by overexpression of p65. To further support the role of RelB in OCs, we challenged RelB−/− mice with TNF-α in vivo and found a diminished osteoclastogenic response. We then examined tumor-induced osteolysis in both RelB−/− and NIK−/− mice by using the B16 melanoma model. Growth of tumor cells in the bone marrow was similar to WT controls, but the absence of either RelB or NIK completely blocked the tumor-induced loss of trabecular bone. Thus, the alternative NF-κB pathway, culminating in activation of RelB, has a key and specific role in the differentiation of OCs that cannot be compensated for by p65.
Keywords: bone, metastasis, receptor activator of NF-κB ligand
Activation of NF-κB transcription factors is critical for inflammation, immune responses, cell survival, and tumorigenesis (reviewed in ref. 1). All five NF-κB family members (p65, RelB, c-rel, p50, and p52) contain the Rel homology domain (RHD), but only the first three bear transcriptional activation domains. Within the RHD are subdomains mediating DNA binding and dimerization. Although the overall homology among NF-κB proteins is highest in the RHD, there are differences in patterns of promoter activity (DNA binding) and in dimerization. RelB is unique in that it also includes a leucine zipper domain required for full transcriptional activity (2). Additionally, RelB, unlike p65 and c-rel, is unable to form a stable homodimer (1, 3).
NF-κB signaling can occur through two distinct pathways with a common pattern: dimers of the transcription complex are retained in the cytoplasm by the IκBs, until an activating signal is received (1). In the classical pathway, IKKβ phosphorylates IκBα, leading to its ubiquitination and proteolysis. The degradation of IκBα releases NF-κB complexes (predominantly p65:p50 dimers) for nuclear translocation. This classical pathway is activated within minutes, is usually transient, and is independent of de novo protein translation. In the alternative pathway, NF-κB-inducing kinase (NIK) and IKKα are required for the processing of p100, the only IκB that binds RelB, allowing nuclear translocation of RelB, which may heterodimerize with either p52 or p50. Activation of this alternative pathway requires longer times (several hours), is typically sustained for several days, and requires nascent protein synthesis. Lastly, the classical pathway is induced at least to some extent by all NF-κB-activating stimuli, whereas the alternative pathway is limited to a subset of the TNF family of cytokines [lymphotoxin-β, BAFF, CD40 ligand, and receptor activator for NF-κB ligand (RANKL)], rendering this pathway a more attractive drug target.
The existence of highly distinct classical and alternative NF-κB pathways, leading to activation p65 and RelB, respectively, suggests that these two subunits in particular have different biological functions. p65−/− mice show embryonic lethality, secondary to hepatocyte apoptosis (4, 5), whereas RelB−/− mice are viable (6). Studies of RelB−/− mice or radiation chimeras bearing p65−/− marrow indicate that these subunits have unique functions in lymphocyte development (7, 8) and in the stromal compartment of secondary lymphoid organs (9, 10). However, although the activities of p65 and c-rel have been directly compared in several contexts (11–14), comparisons with RelB have been much more limited (12, 15).
RelB-deficient mice have altered lymph node development and defective splenic and thymic stromal cells, with abnormal immune responses and multiorgan inflammation (10). Similar defects in lymph organ development, lymphocyte function, and chemokine expression are present in mice lacking functional NIK and IKKα (10, 15–18), indicating that models lacking RelB share most features of those lacking more upstream elements. Therefore, RelB−/− mice are likely to be useful in ascertaining specific functions of the alternative NF-κB pathway, in the absence of effects on the classical pathway that are seen in mice lacking upstream components of the alternative pathway (19, 20). To date, however, no defects in RelB−/− mice have been described outside of the immune system.
In addition to RelB, p52 is often considered to be a subunit specific to the alternative NF-κB pathway. Although the generation of p52 from its precursor p100 is controlled by the upstream alternative kinases, NIK and IKKα, the nuclear role of p52 is not limited to dimerization with RelB. p52 can dimerize with both p65 and c-Rel (21), whose nuclear translocation are controlled by the classical NF-κB pathway. Additionally, the RelB:p50 dimer is transcriptionally active and has been crystallized in complex with κB DNA (22). As a DNA binding subunit, p52 bears a RHD, but does not have a transactivation domain, which is the region responsible for interactions with coactivators that may play a significant role in the selection of specific gene targets by NF-κB complexes. In the nucleus, p52 is not required for RelB activity and may participate in the transactivation of genes associated with the classical NF-κB pathway.
Osteoclasts (OCs) are the cells responsible for bone resorption, a process critical for maintenance of bone structure and calcium homeostasis, but their pathological activation results in bone loss associated with inflammatory arthritis, periodontal disease, and cancer metastasis to bone. They are derived from monocyte/macrophage lineage progenitors under the influence of the cytokine RANKL, which is one of the few known stimuli of the alternative NF-κB pathway. Global NF-κB blockade, including p50/p52 double-deficient (23, 24) mice and treatment with an IκBα superrepressor (25) or NEMO-binding peptide (26, 27), prevents OC differentiation. Furthermore, NIK and IKKα are required for RANKL-mediated OC differentiation in vitro, indicating a specific role for the alternative NF-κB pathway in osteoclastogenesis (19, 20, 28). On the other hand, loss of NIK inhibits nuclear translocation of both p65 and RelB caused by the IκB activity of unprocessed p100, raising the possibility that the loss of classical, p65-mediated signaling could be responsible for some of the observed defects (19). Thus, NIK-deficient animals do not distinguish the relative contribution of the classical and alternative NF-κB pathways to RANKL signal transduction.
To determine whether blockade in classical or alternative NF-κB pathways is responsible for the osteoclastogenic defect in the absence of NIK, we overexpressed p65 and RelB in NIK−/− OC precursors and found that RelB, but not p65, rescued differentiation. Using RelB−/− mice, we showed that RelB itself is required for RANKL-induced osteoclastogenesis, in vitro, and for TNF-induced bone resorption, in vivo. Both RelB−/− and NIK−/− mice are resistant to tumor-mediated osteolysis. Thus, the NIK-activated alternative NF-κB pathway, via RelB, plays an essential and unique role in RANKL signaling toward OC development. We have, therefore, defined a function for RelB and the alternative NF-κB pathway in an important physiological context and demonstrate that isolated blockade of this pathway prevents pathological bone loss.
Results
Deletion of p100 Rescues NIK−/− Osteoclastogenesis.
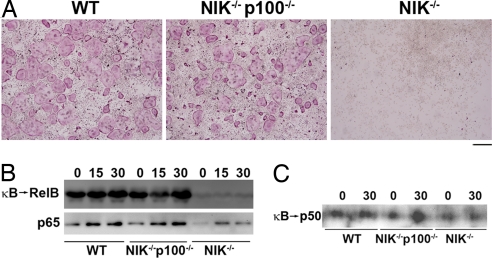
NIK−/− progenitors fail to undergo OC differentiation caused by cytoplasmic retention of p65 and RelB by elevated levels of p100 (19). p100 and its processed product p52 are not required for osteoclastogenesis (19, 23, 24). Therefore, we hypothesized that precursors deficient for both NIK and p100 would undergo normal differentiation, with restoration of p65 and RelB signaling. We crossed p100−/− mice (29) with NIK−/− mice and treated isolated bone marrow macrophages (BMMs) with macrophage colony-stimulating factor (M-CSF) and RANKL to induce OC differentiation. Osteoclastogenesis in the NIK−/−p100−/− culture was similar to the WT littermate control, in contrast to the complete absence of mature tartrate-resistant acid phosphatase (TRAP)+ cells in the NIK−/− culture (Fig. 1A). To determine whether the absence of p100 restored NF-κB subunits to the nucleus, we generated nuclear extracts from preOCs (BMMs treated with RANKL for 48 h). The amount of RelB capable of binding to a κB oligo was extremely low in the NIK−/− cultures, but was normalized to WT levels in NIK−/−p100−/− cultures (Fig. 1B Upper). Similarly, nuclear translocation of p65 was diminished in NIK−/− cultures and was restored in NIK−/−p100−/− cultures (Fig. 1B Lower). Because p52, the product of p100 processing, is absent in p100−/− cultures, we also examined nuclear extracts for κB binding of p50, the most likely dimerization partner for RelB in the absence of p52. Levels of κB-bound p50 paralleled those of RelB (Fig. 1C). We conclude that processing of p100, thereby reducing its IκB activity, appears to be the primary function of NIK for OC differentiation.
Fig. 1.
Deletion of p100 restores osteoclastogenesis in NIK−/− cultures. (A) WT, NIK−/−p100−/−, or NIK−/− BMMs were cultured in RANKL for 7 days, then fixed and stained for TRAP. (Scale bar: 500 μm.) (B) Equal amounts of nuclear extracts derived from pre-OCs (48 h RANKL), starved, and restimulated with RANKL for the indicated times (min) were incubated with biotinylated κB oligos bound to streptavidin-coated beads. Beads were washed to isolate κB-bound proteins. (Upper) They were analyzed by immunoblot for RelB (κB → RelB). (Lower) Nuclear extracts were also immunoblotted for p65. (C) κB-bound proteins from pre-OC nuclear extracts were examined for the presence of p50.
Overexpression of RelB, but Not p65, Rescues NIK−/− Osteoclastogenesis.
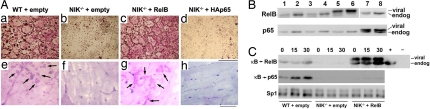
Because removal of p100 restored both RelB and p65 to the nucleus of NIK-deficient cells, the previous result did not allow us to determine whether one or both of these subunits is required for osteoclastogenesis. We hypothesized that retrovirus-mediated overexpression of p65 or RelB in NIK−/− cells would overwhelm the binding capacity of p100 and permit their nuclear translocation, allowing us to determine which subunit is required for OC differentiation. We therefore transduced NIK−/− BMMs with retrovirus directing expression of either HA-tagged p65 (HAp65) or RelB, using the pMX system, and generated osteoclastogenic cultures. TRAP stains of cells grown on plastic showed a complete rescue of OC differentiation by RelB, to levels comparable with WT empty vector control (Fig. 2A, a–d). In contrast, HAp65 failed to rescue, with OC numbers comparable with empty virus controls, despite expression at levels 3- to 5-fold over endogenous (Fig. 2B) and RANKL-induced κB binding by HAp65 (SI Fig. 6 in supporting information (SI) Appendix). The same RelB-transduced cells also excavated pits on bone slices (Fig. 2A, e–h). To demonstrate that the viral RelB was able to travel to the nucleus and bind DNA, we examined the κB-binding activity of nuclear extracts from pre-OCs, treated with RANKL for 0, 15, or 30 min (Fig. 2C). In agreement with our previous study (19), control empty vector NIK−/− cultures had undetectable levels of RelB and p65 DNA binding activity. In NIK−/− cultures expressing RelB, both viral RelB and endogenous RelB DNA binding activity was detectable, exceeding that in WT cultures. Interestingly, levels of p65 κB binding were not significantly affected by viral RelB expression in NIK−/− cultures, with levels remaining significantly below WT. Thus, overexpression of RelB in NIK−/− cultures rescues differentiation independent of p65 activation, leading to the formation of fully functional, bone-resorbing OCs.
Fig. 2.
RelB rescues OC differentiation of NIK-deficient precursors. NIK−/− progenitors were transduced with either empty retrovirus, RelB retrovirus, or HA-tagged p65 (with empty vector-transduced WT progenitors as control) and selected in blastocydin before plating for osteoclastogenic cultures. (A) Osteoclastogenic cultures were grown on plastic and TRAP-stained (a–d) or on bovine bone for analysis of pit formation (e–h). Arrows indicate resorbed areas. (Scale bars: a–d, 500 μm; e–h, 250 μm.) (B) Immunoblot of total lysates from macrophages (no RANKL; lanes 1, 3, 5, and 7) or pre-OCs (48 h RANKL; lanes 2, 4, 6, and 8) for RelB or p65. (C) Equal amounts of nuclear extracts derived from transduced pre-OCs (48 h RANKL), starved, and restimulated with RANKL for the indicated times (min) were incubated with biotinylated κB oligos bound to streptavidin-coated beads. Beads were washed to isolate κB-bound proteins, and these were analyzed by immunoblot. Total lysates from WT and RelB−/− BMMs are shown in the last two lanes, identifying the endogenous RelB band. Sp1 is used as a loading control.
RelB Is Required for Full OC Differentiation in Vitro.
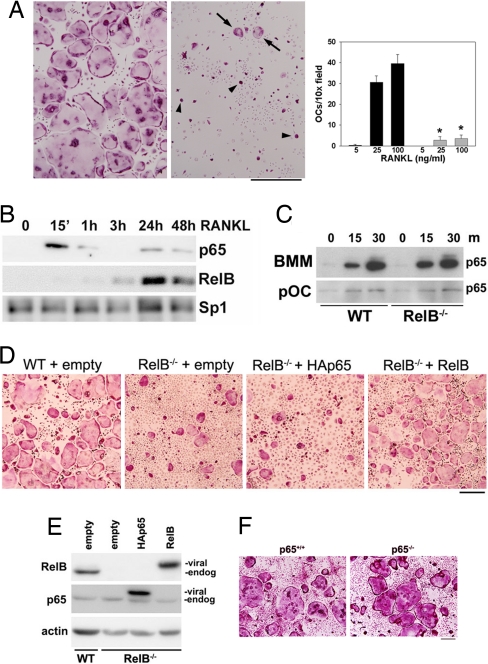
To definitively demonstrate that loss of RelB signaling is responsible for the failure of NIK−/− precursors to become OCs, we turned to the RelB−/− mouse model. BMMs derived from RelB−/− mice treated with M-CSF and RANKL generate significantly less mature, spread OCs than BMMs from WT littermates at doses of 25 or 100 ng/ml of RANKL, although some TRAP+ mononuclear cells, and a few poorly spread multinucleated cells are present (Fig. 3A). Quantitative real-time PCR analysis indicates weak induction of TRAP, MMP9, cathepsin K, calcitonin receptor, and DC-STAMP by RANKL in the absence of RelB (SI Fig. 7 in SI Appendix). However, expression of RANK, the receptor for RANKL, is intact. Therefore, although RelB−/− precursors have some osteoclastogenic response when treated with RANKL, they do not undergo full differentiation, despite continued expression of RANK. These data suggest that RelB controls expression of some key factors for terminal OC differentiation.
Fig. 3.
RelB is required for full RANKL-induced OC maturation, in vitro. (A) (Left and Center) TRAP stain of WT and RelB−/− BMM cultures treated with 100 ng/ml RANKL for 6 days. Arrowheads indicate TRAP+ mononuclear OC precursors. Arrows indicate small poorly spread multinucleated OCs. (Scale bar: 500 μm.) (Right) Quantitation of spread, TRAP+ OCs at 5, 25, or 100 ng/ml RANKL (WT, black bars; RelB−/−, gray bars). *, P < 0.0001, RelB−/− compared with WT at doses of both 25 and 100 ng/ml. (B) Analysis of WT nuclear extracts by immunoblot shows translocation of both p65 and RelB in response to RANKL, but with different kinetics. Sp1 was used as a loading control. (C) Levels of p65 in nuclear extracts from BMMs or pre-OCs (pOC) starved and treated with RANKL for the indicated minutes in WT and RelB−/− cultures. (D) Osteoclastogenic cultures from WT or RelB−/− precursors transduced with empty, HAp65, or RelB retrovirus. (Scale bar: 250 μm.) (E) Immunoblot analysis of total lysates from transduced cultures in D, after drug selection but before RANKL treatment. Viral-encoded proteins (both p65 and RelB) migrate more slowly than their endogenous counterparts. (F) BMMs from p65+/+ and p65−/− mice, both on the tnfr1−/− background, were cultured for 7 days in RANKL at 100 ng/ml, then fixed and stained for TRAP. (Scale bar: 250 μm.)
RelB and p65 Are Not Functionally Equivalent in Osteoclastogenesis.
Upon activation of the alternative NF-κB pathway, RelB is released from its IκB, namely p100, and translocates to the nucleus. We have previously demonstrated that RANKL leads to p100 processing in the OC lineage, and that RelB is present in the nucleus of committed OC precursors (BMMs exposed to RANKL for 48 h) but not in naïve BMMs, exposed to the cytokine for <1 h (19). A more detailed time course of RANKL treatment, beginning with naïve BMMs, showed that RelB nuclear translocation began at 3 h and persisted for at least 48 h (Fig. 3B). In contrast, p65 nuclear translocation was biphasic, with peak levels at 15 min, and a second, smaller peak at 24 h. This difference in kinetics suggests that p65 and RelB may have distinct roles in OC differentiation.
Having found that RelB−/− OC precursors fail to fully differentiate in vitro, we wanted to establish that classical NF-κB signaling is intact in this model. In both BMMs and pre-OCs, levels of nuclear p65 were the same in WT and RelB−/− cells treated with RANKL (Fig. 3C), suggesting that there are unique functions of RelB in OC differentiation, which are not performed by p65. To confirm this idea that p65 cannot compensate for the absence of RelB, we asked whether overexpression of p65 in RelB−/− precursors could restore full OC differentiation. We therefore transduced RelB−/− BMMs with retrovirus directing expression of either HAp65 or RelB. As expected, expression of exogenous RelB in RelB−/− cells restored osteoclastogenesis to WT levels (Fig. 3D). In contrast, expression of HAp65 had no effect, despite expression at levels significantly higher than endogenous p65 (Fig. 3E). Further supporting the hypothesis that RelB, and not p65, contributes to OC differentiation, RANKL can induce osteoclastogenesis in p65−/− cultures at levels equivalent to p65+/+ controls (Fig. 3F), although cells must be plated at high density to overcome an apoptotic phenotype (S.V. and D.V.N., unpublished observation). We conclude that RelB is specifically required for RANKL-mediated OC differentiation, in vitro, and that p65 is unable to compensate for its absence.
Inflammatory Osteolysis Is Blunted in RelB−/− Mice.
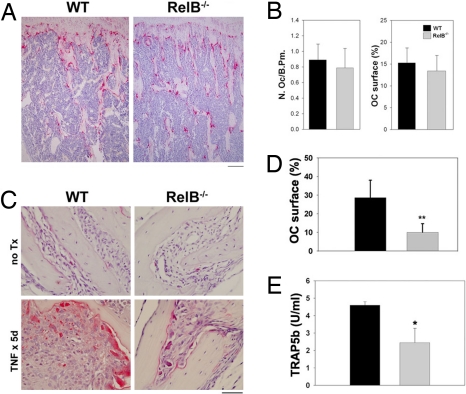
We previously showed that NIK is not required for basal osteoclastogenesis in vivo, but that it is critical in pathological states of osteolysis (19, 30). However, absence of NIK leads to blockade of both classical and alternative NF-κB pathways (19), leaving open the possibility that global NF-κB blockade is required to significantly impact OCs in vivo. Therefore, we turned to RelB−/− mice to specifically examine the role of the alternative pathway in OCs. Similar to NIK−/− mice, 8-week-old unmanipulated RelB−/− mice showed no differences in OC numbers or OC surface (Fig. 4 A and B). We next stimulated osteoclastogenesis by treating the mice with TNFα (1.5 μg per day for 5 days s.c. over the calvarium) (Fig. 4C). In this model of inflammatory osteolysis, the effect on osteoclastogenesis is largely mediated by induction of RANKL production by stromal cells (31). In both WT and RelB−/− mice, TNF-α treatment increases the number of OCs along the calvarial suture lines. However, the WT OCs are much larger and, in keeping with the increased size of the cells, the surface of bone covered by OCs is significantly higher in WT mice (Fig. 4D). Measurement of serum TRAP5b, a marker of systemic OC activity, after TNF-α treatment demonstrates that RelB−/− mice have less bone resorption than WT controls (Fig. 4E), indicating that the small TRAP+ OCs in RelB−/− mice are less active and may represent a population of cells that is not fully differentiated. Thus, RelB is required for optimal OC induction by inflammation, in vivo.
Fig. 4.
RelB is important for TNF-stimulated bone resorption in vivo. (A) TRAP staining of tibias from 2-month-old unmanipulated WT and RelB−/− mice. OCs are stained red. (Scale bar: 200 μm.) (B) Quantitation of OC number (Left) and OC surface (Right). (C) TRAP staining of calvaria from mice with and without TNF treatment (1.5 μg per day × 5 days). (Scale bar: 50 μm.) (D) Histomorphometric analysis of TNF-treated mice from C. OC surface is the percentage of bone/suture interface covered by OCs. WT, black bars, n = 7; RelB−/−, gray bars, n = 8; **, P < 0.0003 compared with WT. (E) Analysis of serum TRAP5b levels after TNF treatment. n = 4 per group; *, P < 0.01.
Both NIK−/− and RelB−/− Mice Are Resistant to Tumor-Induced Osteolysis.
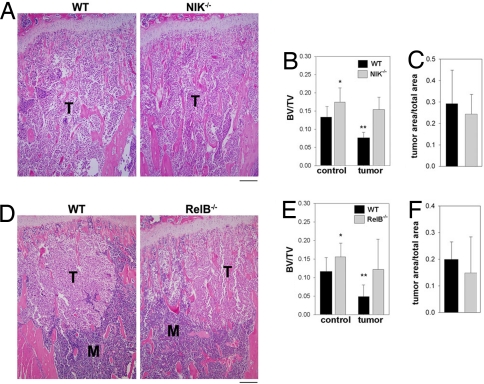
Another context in which the OC is responsible for clinically significant bone loss is tumor-mediated osteolysis. Metastatic tumor cells within the bone marrow secrete factors that stimulate OC differentiation and activity, leading to loss of trabecular bone. The B16 murine melanoma cell line is capable of forming osteolytic bone lesions upon injection into the left cardiac ventricle, even in immunocompetent C57BL/6 mice. We therefore used this model in both NIK−/− and RelB−/− mice, and their respective littermate WT controls, injecting tumor cells at 42 ± 4 days of age. The B16 cells were tagged with firefly luciferase (B16-FL), and mice were imaged at 7 and 10 days after tumor injection to determine tumor burden in the leg bones. With tumor bioluminescence, there were no significant differences in tumor burden in either mutant, compared with its WT control (data not shown). Mice were killed at day 12, and leg bones were dissected, processed, and sectioned for histomorphometric analysis, along with a control group killed at 56 days of age (Fig. 5 A and D). At baseline, in both NIK−/− and RelB−/− control mice, there was a small, but statistically significant (P < 0.05), difference in trabecular bone volume compared with WT littermates (Fig. 5 B and E). After B16-FL tumor injection, WT mice in both cohorts lost ≈50% of their trabecular bone volume. In contrast, neither NIK−/− nor RelB−/− mice lost a significant amount of trabecular bone volume compared with nontumor controls (Fig. 5 B and E), despite having a tumor burden comparable with WT controls (Fig. 5 C and F). These data demonstrate that the alternative NF-κB pathway, mediated by NIK and RelB, is required for tumor-mediated osteolysis.
Fig. 5.
Absence of either NIK or RelB blocks tumor-induced osteolysis. (A) Tibial sections from NIK−/− and WT mice, 12 days after injection of B16-FL tumor cells stained with H&E show the marrow cavity replaced by tumor cells (T) in both genotypes. (Scale bar: 200 μm.) (B) Quantitative histomorphometry was performed on control and tumor-injected bones to determine trabecular bone volume (BV/TV); n = 9–14 bones per group. *, P < 0.05 compared with WT control; **, P < 0.0005 compared with WT control. There was no significant difference between NIK−/− control and tumor-injected mice. (C) Histomorphometric analysis of tumor area in tibial sections shows no significant difference between NIK−/− and WT mice. (D) Tibial sections from RelB−/− mice and WT littermate controls injected with B16-FL tumor cells, as in A. H&E-stained sections of tibias show marrow (M) replacement by tumor cells beneath the growth plate. (Scale bar: 200 μm.) (E) Quantitative histomorphometry was performed on control and tumor-injected bones to determine trabecular bone volume (BV/TV); n = 8–11 bones per group. *, P < 0.05 compared with WT control; **, P < 0.0005 compared with WT control. There was no significant difference between RelB−/− control and tumor-injected mice. (F) Histomorphometric analysis of tumor area in tibial sections shows no significant difference between RelB−/− and WT groups.
Discussion
The definition of two distinct NF-κB activation pathways has provided a partial explanation for functional differences between subunits controlled by each of the pathways, as indicated by distinct phenotypes in single and multiple subunit-deficient mice (32). The classical pathway, induced by all signals that mediate NF-κB, culminates in activation of p65, the most ubiquitous and abundant subunit, as well as c-rel. Within the OC lineage, numerous studies have shown RANKL-induced p65 activation (28, 33, 34), and inhibition of p65 with IκBα super repressor or NEMO-binding peptide (25–27) blocks osteoclastogenesis. The role of c-rel in this context has not been examined, although RANKL does induce its nuclear translocation (28). Thus, the classical pathway, via p65, has been thought of as the dominant mediator of NF-κB effects in OCs. We have shown previously that RANKL is one of only a few cytokines that signals to the alternative NF-κB pathway, leading to activation of RelB (19). However, the role of the alternative pathway in osteoclastogenesis is less clear. Ablation of NIK, the upstream mediator of this pathway, blocks activation of both RelB and p65 in pre-OCs (19).
The goal of this study was to define the role of the alternative pathway in OC differentiation by examining RelB−/− mice, which we confirm have intact classical, p65-mediated NF-κB signaling. We have found that BMMs lacking RelB, but not p65, fail to undergo RANKL-mediated osteoclastogenesis, in vitro. Furthermore, overexpression of p65 cannot rescue the blockade in RelB−/− OC differentiation, whereas overexpression of RelB in NIK−/− cultures rescues osteoclastogenesis, without affecting nuclear p65 levels. Thus, RelB has a unique role in the developmental program of OCs, a role that cannot be fulfilled by p65.
In our model, NIK is required to process the IκB domain of p100, the specific inhibitor of RelB, allowing transactivation of RelB-responsive genes important for OC differentiation. In the absence of NIK, p100 levels are elevated and its IκB domain retains RelB in the cytoplasm, inhibiting osteoclastogenesis. When p100 is deleted, removing both p100 and its processing product p52, RelB is again bound to κB sites in the nucleus of OC precursors, most likely in complex with p50, and OC differentiation is restored. Thus, RelB is the NF-κB subunit responsible for transactivating genes specific to the alternative pathway.
In the absence of both the p50 and p52 NF-κB subunits, mice have osteopetrosis caused by a complete lack of OCs (23, 24), but in this case, overall NF-κB signaling, through both classical and alternative pathways, is inhibited. Ruocco et al. (28) compared mice lacking IKKβ or bearing mutant IKKα and found that only the former mice were osteopetrotic. They concluded that the classical pathway was more important for osteoclastogenesis, in vivo, than the alternative pathway. However, given that IKKβ-deficient OC precursors had a much more global defect in NF-κB activation than their IKKα-defective counterparts (including a lack of nuclear RelB) this study did not truly differentiate the relative contributions of the classical and alternative pathways. Despite its robust induction by RANKL, we find that p65 is not required for OC differentiation, in vitro. c-rel is also activated by the classical pathway, but this subunit is not required for OC differentiation in vitro (S.V. and D.V.N., unpublished observation).
We have now demonstrated that mice with aberrant alternative NF-κB signaling, via ablation of NIK or RelB, have relatively normal OC function at baseline, but show dramatically reduced bone resorption in response to inflammatory conditions or tumor in the marrow cavity. Therefore, the alternative NF-κB pathway, via RelB, appears to be more critical for abnormal, pathological osteoclastogenesis than for basal osteoclastogenesis. This is an important distinction because OCs are required to maintain calcium homeostasis and bone microarchitecture. Targeting pathological osteolysis, without blocking normal bone homeostasis, would have significant advantages over global OC blockade. Thus, the alternative NF-κB pathway and RelB represent promising therapeutic targets for the treatment of osteolytic diseases.
Materials and Methods
Mice.
RelB−/− mice (6) were maintained by heterozygote mating in a specific pathogen-free facility. NIK−/− mice (35) were backcrossed eight generations to C57BL/6 mice, and then maintained by heterozygote mating in the same facility. NIK−/−p100−/− mice, and their littermate controls, were generated by crossing NIK+/− mice on the C57BL/6 background (backcrossed eight generations) to p100 (nfkb2)+/− mice (36), also on a C57BL/6 background [backcrossed eight generations (29)], with subsequent double heterozygote mating. The genotype of all mice used was confirmed by PCR analysis of genomic DNA extracted from tail biopsies. RelB−/−, NIK−/−, and NIK−/−p100−/− mice were 4–8 weeks old at the time of bone marrow harvest for in vitro culture. All experimental protocols were approved by the Institutional Animal Studies Committee at the Washington University School of Medicine.
OC Culture and Retroviral Transduction.
BMMs were obtained and differentiated into OCs as described (19) using CMG14–12 supernatant as a source of M-CSF and recombinant GST-RANKL. For osteoclastogenesis in 96-well plates, BMMs were plated at 5 × 103 per well for RelB−/− and NIK−/− cells and their littermate controls or at 20 × 103 per well for p65−/− and controls. Retrovirus was generated by cloning murine relB or p65 cDNAs into the pMX-IRES-bsr vector and transfecting platE packaging cells. Replication incompetent viral supernatant was added to BMMs for 24 h, along with 4 μg/ml polybrene (Sigma), before selection with blastocydin (1 μg/ml) for 3 days. Surviving transduced BMMs were then cultured in a similar fashion to primary unmanipulated BMMs. OCs were visualized by histochemical staining for TRAP activity, according to the manufacturer's instructions (Sigma).
In Vivo TNF-α Treatment.
WT and RelB −/− mice (2–3 months old) were injected s.c. with recombinant TNF-α (1.5 μg per day for 5 days) or PBS (sham) (31). At the time of death, serum was collected and subjected to the MouseTRAP assay (IDT) for measurement of serum TRAP5b activity. Calvaria were fixed overnight in 10% buffered formalin, decalcified in 14% EDTA, embedded in paraffin, sectioned, and stained for TRAP with a hematoxylin counterstain. OC surface was evaluated by using the Osteomeasure system (Osteometrics). Statistics were performed with the unpaired Student's t test.
Intrarterial Bone Metastasis Model.
The B16-FL murine melanoma cell line, which expresses firefly luciferase, was cultured as described (37). Mice at 38–46 days of age were anesthetized and inoculated intra-arterially via the left cardiac ventricle with 105 B16-FL cells in 50 μl of PBS as described (38). Mice were killed on day 12 after tumor cell injection and underwent blinded necropsy. Mice were discarded from the final analysis if the animal died before day 10 or if necropsy demonstrated a large mediastinal tumor indicative of injection of tumor cells injected into chest cavity, not the left ventricle. Unmanipulated mice killed at 8 weeks of age were used as noninjected controls.
In Vivo Bioluminescence Imaging.
Mice were imaged for tumor-expressed bioluminescence at days 7 and 10 after tumor cell injection. Mice were anesthetized by isofluorane (2% vaporized in O2), and shaved to minimize attenuation of light by pigmented hair, then injected i.p. with 150 mg/kg d-luciferin (Biosynth) in PBS, and imaged 10 min later. Imaging was performed with a CCD camera (IVIS 100: exposure time 1 or 5 min, binning 8, field of view 15 cm, f/stop 1, and open filter) in the Molecular Imaging Center at Washington University. For analysis, total photon flux (photons per second) was measured from a fixed region of interest in the tibia by using Living Image 2.50 and IgorPro software (Wavemetrics) (39).
Bone Histomorphometry.
Mouse tibias were fixed in formalin overnight and decalcified in 14% EDTA for 10–14 days. Paraffin-embedded sections were stained with hematoxylin and eosin. Trabecular bone volume was measured from 120 to 1,080 μm distal to the growth plate, and tumor area/total area was measured from 120 to 4,800 μm from the growth plate by using Bioquant Osteo (Bioquant Image Analysis). OC number and OC surface were assessed on TRAP-stained sections of control RelB−/− mice by using the same software.
Subcellular Fractionation and Immunoblotting.
To obtain nuclear extract from RANKL-treated cells, plates were washed with water and cells were lysed with hypotonic buffer (10 mM HEPES, 1.5 mM MgCl2, 1 mM KCl, 1 mM DTT, and protease and phosphatase inhibitors), followed by the addition of Nonidet P-40 at 0.1%. After centrifugation, the pellets (nuclear fraction) were suspended in high-salt buffer (hypotonic buffer + 400 mM NaCl). Extracts were quantitated by a modified Coomassie method (Pierce). For immunoblots, 10 μg of nuclear extract or 40 μg of total lysate was used, and equivalent loading was verified by Ponceau red staining after transfer to PVDF membrane. Antibodies to RelB, p65, Sp1, and β-actin were from Santa Cruz Biotechnology; antibody to p50 was from Millipore. For κB oligonucleotide pull-down assays, 20 μg of nuclear extract was incubated with streptavidin-coated agarose beads preincubated with biotinylated κB3 oligonucleotide (19) for 30 min at room temperature on a rotator in 1× binding buffer [30 mM NaCl, 10 mM Tris (pH 7.4), 1 mM EDTA, 5% glycerol, 1 mg/ml BSA, and 1 mM DTT] with 1 μg of poly(dIdC). Beads were then washed in 1× binding buffer three times before SDS/PAGE and immunoblot for RelB, p65, and/or p50.
Supplementary Material
ACKNOWLEDGMENTS.
We thank C. Hunter (University of Pennsylvania, Philadelphia) for p100−/− mice, Crystal Idleberg and Pat Keller for expert animal histology, and Erin Jackson for bioluminescent imaging. This work was supported by National Institutes of Health Grants AR47846, CA103035, and AR52705 (to D.V.N.), AR52921 (to R.F.), CA097250 (to K.N.W.), and CA94056 (to Molecular Imaging Center) and the Arthritis Foundation (R.F.). A.C.H. was supported by Hematology Training Grant T32 HL007088).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708576105/DC1.
References
- 1.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 2.Dobrzanski P, Ryseck R, Bravo R. Both N- and C-terminal domains of RelB are required for full transactivation: Role of the N-terminal leucine zipper-like motif. Mol Cell Biol. 1993;13:1572–1582. doi: 10.1128/mcb.13.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryseck R, et al. RelB, a new Rel family transcription activator that can interact with p50-NF-κB. Mol Cell Biol. 1992;12:674–684. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 5.Alcamo E, et al. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for NF-κB in leukocyte recruitment. J Immunol. 2001;167:1592–1600. doi: 10.4049/jimmunol.167.3.1592. [DOI] [PubMed] [Google Scholar]
- 6.Weih F, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 7.Guerin S, et al. RelB reduces thymocyte apoptosis and regulates terminal thymocyte maturation. Eur J Immunol. 2002;32:1–9. doi: 10.1002/1521-4141(200201)32:1<1::AID-IMMU1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Prendes M, Zheng Y, Beg AA. Regulation of developing B cell survival by RelA-containing NF-κB complexes. J Immunol. 2003;171:3963–3969. doi: 10.4049/jimmunol.171.8.3963. [DOI] [PubMed] [Google Scholar]
- 9.Alcamo E, et al. Requirement for the NF-κB family member RelA in the development of secondary lymphoid organs. J Exp Med. 2002;195:233–244. doi: 10.1084/jem.20011885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weih DS, Yilmaz ZB, Weih F. Essential role of RelB in germinal center and marginal zone formation and proper expression of homing chemokines. J Immunol. 2001;167:1909–1919. doi: 10.4049/jimmunol.167.4.1909. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann A, Leung TH, Baltimore D. Genetic analysis of NF-κB/Rel transcription factors defines functional specificities. EMBO J. 2003;22:5530–5539. doi: 10.1093/emboj/cdg534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saccani S, Pantano S, Natoli G. Modulation of NF-κB activity by exchange of dimers. Mol Cell. 2003;11:1563–1574. doi: 10.1016/s1097-2765(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 13.Fan Y, Rayet B, Gelinas C. Divergent C-terminal transactivation domains of Rel/NF-B proteins are critical determinants of their oncogenic potential in lymphocytes. Oncogene. 2004;23:1030–1042. doi: 10.1038/sj.onc.1207221. [DOI] [PubMed] [Google Scholar]
- 14.Sanjabi S, et al. A c-Rel subdomain responsible for enhanced DNA-binding affinity and selective gene activation. Genes Dev. 2005;19:2138–2151. doi: 10.1101/gad.1329805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonizzi G, et al. Activation of IKKα target genes depends on recognition of specific κB binding sites by RelB:p52 dimers. EMBO J. 2004;23:4202–4210. doi: 10.1038/sj.emboj.7600391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platzer B, Jorgl A, Taschner S, Hocher B, Strobl H. RelB regulates human dendritic cell subset development by promoting monocyte intermediates. Blood. 2004;104:3655–3663. doi: 10.1182/blood-2004-02-0412. [DOI] [PubMed] [Google Scholar]
- 17.Yamada T, et al. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-κB-inducing kinase. J Immunol. 2000;165:804–812. doi: 10.4049/jimmunol.165.2.804. [DOI] [PubMed] [Google Scholar]
- 18.Fagarasan S, et al. Alymphoplasia (aly)-type nuclear factor κB-inducing kinase (NIK) causes defects in secondary lymphoid tissue chemokine receptor signaling and homing of peritoneal cells to the gut-associated lymphatic tissue system. J Exp Med. 2000;191:1477–1486. doi: 10.1084/jem.191.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novack DV, et al. The IκB function of NF-κB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003;198:771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaisson ML, et al. Osteoclast differentiation is impaired in the absence of inhibitor of κB kinase α. J Biol Chem. 2004;279:54841–54848. doi: 10.1074/jbc.M406392200. [DOI] [PubMed] [Google Scholar]
- 21.Thanos D, Maniatis T. NF-κB: A lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 22.Moorthy AK, Huang DB, Wang VYF, Vu D, Ghosh G. X-ray structure of a NF-κB p50/RelB/DNA complex reveals assembly of multiple dimers on tandem κB sites. J Mol Biol. 2007;373:723–734. doi: 10.1016/j.jmb.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franzoso G, et al. Requirement for NF-κB in osteoclast and B cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iotsova V, et al. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 25.Abbas S, Abu-Amer Y. Dominant-negative IκB facilitates apoptosis of osteoclasts by tumor necrosis factor α. J Biol Chem. 2003;278:20077–20082. doi: 10.1074/jbc.M208619200. [DOI] [PubMed] [Google Scholar]
- 26.Jimi E, et al. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10:617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 27.Dai S, Hirayama T, Abbas S, Abu-Amer Y. The IκB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J Biol Chem. 2004;279:37219–37222. doi: 10.1074/jbc.C400258200. [DOI] [PubMed] [Google Scholar]
- 28.Ruocco MG, et al. IκB kinase (IKK)β, but not IKKα, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med. 2005;201:1677–1687. doi: 10.1084/jem.20042081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speirs K, Lieberman L, Caamano J, Hunter CA, Scott P. Cutting edge: NF-κB2 is a negative regulator of dendritic cell function. J Immunol. 2004;172:752–756. doi: 10.4049/jimmunol.172.2.752. [DOI] [PubMed] [Google Scholar]
- 30.Aya K, et al. NF-κB-inducing kinase controls lymphocyte and osteoclast activities in inflammatory arthritis. J Clin Invest. 2005;115:1848–1854. doi: 10.1172/JCI23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitaura H, et al. Marrow stromal cells and osteoclast precursors differentially contribute to TNF-α induced osteoclastogenesis in vivo. J Immunol. 2004;173:4838–4846. doi: 10.4049/jimmunol.173.8.4838. [DOI] [PubMed] [Google Scholar]
- 32.Gerondakis S, Grossmann M, Nakamura Y, Pohl T, Grumont R. Genetic approaches in mice to understand Rel/NF-κB and IκB function: Transgenics and knockouts. Oncogene. 1999;18:6888–6895. doi: 10.1038/sj.onc.1203236. [DOI] [PubMed] [Google Scholar]
- 33.Wei S, Wang MWH, Teitelbaum SL, Ross FP. RANK ligand activates nuclear factor-κB in osteoclast precursors. Endocrinology. 2001;142:1290–1295. doi: 10.1210/endo.142.3.8031. [DOI] [PubMed] [Google Scholar]
- 34.Abu-Amer Y. IL-4 abrogates osteoclastogenesis through STAT6-dependent inhibition of NF-κB. J Clin Invest. 2001;107:1375–1385. doi: 10.1172/JCI10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin L, et al. Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science. 2001;291:2162–2165. doi: 10.1126/science.1058453. [DOI] [PubMed] [Google Scholar]
- 36.Caamano JH, et al. Nuclear factor (NF)-κB (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med. 1998;187:185–196. doi: 10.1084/jem.187.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirbe AC, et al. Granulocyte colony-stimulating factor enhances bone tumor growth in mice in an osteoclast-dependent manner. Blood. 2007;109:3424–3431. doi: 10.1182/blood-2006-09-048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakewell SJ, et al. Platelet and osteoclast β3 integrins are critical for bone metastasis. Proc Natl Acad Sci USA. 2003;100:14205–14210. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross S, Piwnica-Worms D. Real-time imaging of ligand-induced IKK activation in intact cells and in living mice. Nat Methods. 2005;2:607–614. doi: 10.1038/nmeth779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.