Abstract
Recombination-mediated repair plays a central role in maintaining genomic integrity during DNA replication. The human Mus81–Eme1 endonuclease is involved in recombination repair, but the exact structures it acts on in vivo are not known. Using kinetic and enzymatic analysis of highly purified recombinant enzyme, we find that Mus81–Eme1 catalyzes coordinate bilateral cleavage of model Holliday-junction structures. Using a self-limiting, cruciform-containing substrate, we demonstrate that bilateral cleavage occurs sequentially within the lifetime of the enzyme–substrate complex. Coordinate bilateral cleavage is promoted by the highly cooperative nature of the enzyme and results in symmetrical cleavage of a cruciform structure, thus, Mus81–Eme1 can ensure coordinate, bilateral cleavage of Holliday junction-like structures.
Keywords: nuclease, recombination repair
The maintenance of genomic integrity requires multiple coordinated repair processes during DNA replication. Fork-stalling, recombination repair, and replication restart create a variety of branched structures that are substrates for endonucleases. The Mus81–Eme1 endonuclease was first identified in budding yeast as a mutant that causes sensitivity to replication-associated genotoxic stress (1). Fission yeast strains null for either Mus81 or Eme1 are exquisitely sensitive to replication stress and are inviable during meiosis (2, 3). Based on enzyme activity, damage sensitivity, and the rescue of meiotic segregation defects by the prokaryotic resolvase, RusA, a role in resolving Holliday junctions was proposed for Mus81–Eme1 in fission yeast (3). This function is supported by more recent data showing that the accumulation of X structures in Mus81 delete cells in response to replication pausing and at sites of meiotic recombination (4, 5).
Mus81-null mice are viable, fertile, and have no obvious developmental defects (6, 7). Both mouse and human Mus81- and Eme1-null cell lines are exquisitely sensitive to interstrand crosslink agents including mitomycin C (6–9). Mus81–Eme1 is recruited to sites of UV irradiation specifically during DNA replication (10). To date, no meiotic defects have been reported in null Mus81 mice or Drosophila, however, a role in generating interference-independent crossovers has been reported for budding yeast and Arabidopsis (6, 7, 11–13). Also, Mus81 deficiency is lethal when combined with the disruption of the BLM helicase homologues in budding yeast, fission yeast, Drosophila, and Arabidopsis, suggesting a conserved role for Mus81–Eme1 in recombination repair and possibly Holliday-junction processing (2, 11, 14, 15). Data from several eukaryotic organisms have shown that Mus81–Eme1 has activity on a number of branched DNA structures: Potential in vivo substrates are speculated to include forks, flaps, D-loops, and Holliday junctions (3, 4, 16–22).
In this study, we use highly purified recombinant Mus81–Eme1 to test the enzymatic properties and investigate the mechanism of cleavage of model Holliday junctions. We define the catalytic core of the enzyme complex. By using a plasmid-based substrate, we demonstrate that Mus81–Eme1 uses a highly cooperative, coordinated mechanism that ensures bilateral, symmetrical cleavage of Holliday-junction structures.
Results
Recombinant Human Mus81–Eme1.
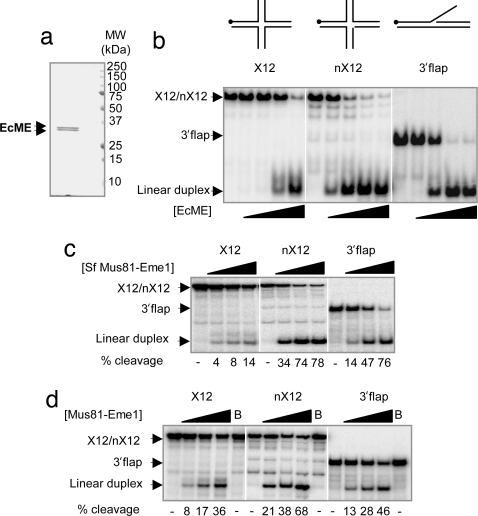
Truncation analysis of both Mus81 and Eme1 was used to define the minimal domains required for endonuclease function [see supplementary information (SI) Fig. 5]. Versions of each protein containing amino acids 260–551 for Mus81 and amino acids 244–571 for Eme1 were expressed in Escherichia coli. The recombinant truncated complex, which we named EcME, was well expressed, largely soluble, and, as detailed below, active. The complex was purified to apparent homogeneity through affinity chromatography, ion exchange, and gel filtration steps (Fig. 1a).
Fig. 1.
Endonuclease activities of recombinant human Mus81–Eme1. (a) Recombinant human Mus81–Eme1 purified from E. coli (EcME). Purified preparations were separated by SDS/PAGE and visualized by Coomassie staining. (b) Endonuclease activity of EcME on multiple substrates. The X12, nX12, and 3′ flap substrates, [200 pM], with 0.02, 0.2, 2, or 20 nM EcME for 20 min. Products were separated by native PAGE. Substrates and DNA products are as indicated. Each substrate is illustrated above the gels, and the 5′ 32P radiolabel is indicated by a circle. (c) Endonuclease activity of Myc.Mus81–Eme1.FLAG purified from Sf9 cells (Sf Mus81–Eme1). The indicated substrates [200 pM] were incubated with purified SfMus81–Eme1 for 20 min, with either 10%, 30%, or 100% of a αFLAG immune precipitate being used. Products were separated by native PAGE. Substrates and products are as indicated. Percentage of cleavage (% cleavage) is indicated below the panel, with a dash representing <1% cut. (d) Endonuclease activity of Mus81–Eme1 immune-precipitated from HeLa cell extracts. The indicated substrates [200 pM] were incubated with purified Mus81–Eme1, or beads (B), for 20 min, with either 10%, 30%, or 100% of a αMus81 immune precipitate.
Endonuclease Properties of a Minimal Mus81–Eme1 Complex.
The activity of the recombinant enzyme was initially tested on three model substrates, X12, nX12, and 3′ flap, and each junction was radiolabeled on the 5′ end of one oligonucleotide (Fig. 1b). The oligo nucleotide sequences are identical to those used previously (4, 16, 23). The X12 substrate has four 25-bp duplex DNA arms, with a 12-bp homologous core that allows branch migration of the junction. The nX12 substrate is identical to X12, except that it contains a nick at the cross-over point. The 3′ flap substrate contains 50 bp of duplex DNA, a central nick, and 25 bases of single-stranded DNA. EcME is able to convert all three substrates to linear duplex product (Fig. 1b). The kinetic parameters of the EcME complex on these substrates were calculated by using nonlinear regression analysis of the reaction velocities obtained from experiments in which substrate concentration was varied (SI Table 1 and SI Fig. 6). EcME has the highest kcat, 0.18 s−1, when acting on an nX12 substrate. The enzyme functioned catalytically in this reaction and converted 95 moles of substrate per mole of enzyme in 10 min (SI Table 2). Consistent with previous observations using full-length Mus81–Eme1 (4, 24), EcME processed an intact X12 junction less efficiently, with a kcat of 0.0047 s−1. Nevertheless, at the highest concentration tested, EcME converted >95% of the intact X12 substrate into linear duplex product (Fig. 1b). The reduced catalytic efficiency of EcME on an intact X12 was driven both by a higher Km and a lower catalytic rate. The observation that Mus81–Eme1 favors cleavage of nX12 over an X12 suggests that the initial cut on an X12 substrate is rate-limiting, and that it is followed by an ≈35-fold faster second cut. A nick-counternick mechanism, in which a rate-limiting initial cut is followed by a kinetically favored second-strand cleavage reaction, has been used to explain the mechanism of action of a number of junction-resolving enzymes (4, 25–28). The kinetic parameters of EcME on a 3′ flap suggests that the complex binds this substrate relatively poorly, but, once bound, the enzyme has a relatively robust rate of catalysis, with a kcat of 0.12 s−1.
To determine whether the endonuclease activity detected in these assays can exclusively be attributed to Mus81–Eme1, versions of EcME enzyme lacking potential catalytic residues were made. We have previously used Mus81D338/339A, a version of Mus81 that has severely compromised, but not completely abolished, endonuclease activity (16, 20). Based on recent structural analysis of related nucleases, PfHEF and ApeXPF (29, 30), we predicted that aspartic acid D307 might be critical for catalysis and that changing this residue to alanine would more effectively compromise catalysis. Both mutant versions of EcME were expressed in E. coli and purified as above.
An X12 substrate labeled on the 5′ end of oligonucleotide 1 was incubated with wild-type or mutant versions of EcME, and the products were examined after denaturing PAGE (SI Fig. 7). Denaturing gels were used for this analysis because nicking of a single strand is not detected on native gels. A trace of endonuclease activity was detected in EcMED338/339A. Significantly, no cleavage was detected when an equivalent amount of EcMED307A was incubated with substrate. The low-to-undetectable nuclease function of these preparations indicates that the endonuclease activity detected in wild-type preparation of EcME is due to the intrinsic properties of the enzyme complex and that the recombinant enzyme preparations are free of contaminating nuclease activities. The residual endonuclease activity seen in EcMED338/339A preparations is explained by structural predications suggesting that these amino acids do not directly coordinate the catalytic cation and thus play a supporting rather than essential role in catalysis (29). The analogous mutation, D599A, in PfHef results in a similar diminished, but not absent, nuclease activity (29).
Substrate Specificity of Endogenous Mus81–Eme1 Endonuclease Activity.
To test whether the truncations of Mus81 and Eme1 affect substrate preference, we compared the activity of EcME to full-length Mus81–Eme1 purified from baculovirus infected insect cells by anti-FLAG immunoprecipitation (Sf Mus81–Eme1). EcME has the same relative substrate preference as the recombinant full-length version of the protein, nX12 > 3′ flap ≫ X12 (Fig. 1c), confirming that the truncations do not profoundly alter the substrate preference of the recombinant EcME enzyme. The activity profile of endogenous Mus81–Eme1 from a human cell line (HeLa) was also compared with that of recombinant full-length Mus81–Eme1 from insect cells (Fig. 1c). As with both recombinant versions of the complex, endogenous Mus81–Eme1 converted all three substrates into linear duplex (Fig. 1d). Notably, endogenous Mus81–Eme1 complex has a higher X12/nX12 activity ratio when compared with the recombinant protein. By comparing the amount of product generated at subsaturating levels of Mus81–Eme1 (i.e., 10% of immune precipitate), we find that the endogenous enzyme is only 3-fold less efficient on X12 than nX12 (Fig. 1d), whereas the recombinant insect cell-expressed enzyme is 10-fold less efficient (Fig. 1c). The endogenous enzyme had the same relative rate of activity when immune-precipitated with either monoclonal mouse or polyclonal rabbit anti-Mus81 antibodies (data not shown).
Gaskell et al. (31) recently concluded that full-length, recombinant, fission yeast Mus81–Eme1 has the same intact X versus nicked X activity ratio as the endogenous protein. However, this comparison was based on assays done in two different laboratories (4, 31). In this study, we compared the activity of recombinant full-length human Mus81–Eme1 to the endogenous complex under identical assay conditions. In these circumstances, increased relative activity on the intact X12 structure is clearly detected. The difference in substrate specificity between the endogenous human and the recombinant enzyme could be due to the presence of associated factors or posttranslational modifications that do not occur in insect cells.
Bilateral Cleavage of a Cruciform-Containing Plasmid.
The cruciform structures formed by extrusion of an inverted repeat sequence in supercoiled plasmids have been extensively used to study the enzymatic properties of Holliday junction-resolving enzymes (25–27, 32). Cruciforms are formed by intrastrand base pairing of inverted repeat sequences and thus form two hairpin loops extruded from closed circular plasmid DNA. The region at the base of the stem loops forms a four-way junction (Fig. 2a). A unique feature of this model substrate is that the cruciform structure is stabilized by the free energy of negative supercoiling in the plasmid. A break in either strand of the DNA leads to relaxation, destabilization, and, thus, reabsorption of both hairpins into duplex DNA (33). An enzyme that cuts a single strand of DNA will thus generate nicked circular DNA. By contrast, an enzyme that simultaneously cleaves both strands of the plasmid generates linear DNA. Likewise, enzymes that make two cleavages sequentially, within the lifetime of the protein DNA complex, generate linear product even though cleavage of the two strands is not simultaneous (25–27).
Fig. 2.
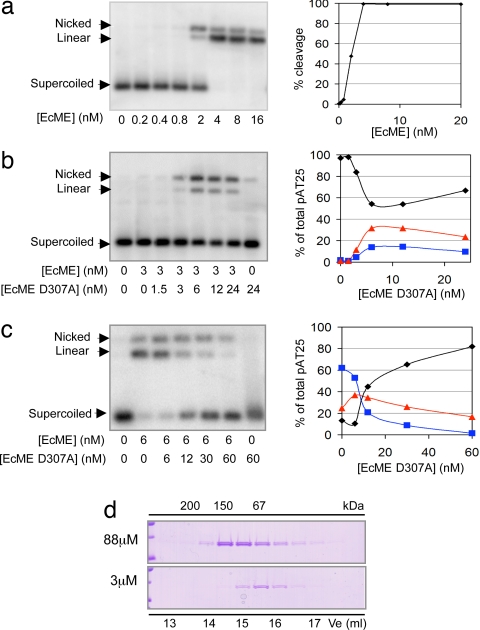
Bilateral cleavage mechanism of the Mus81–Eme1 endonuclease. (a) Bilateral cleavage of the pAT25 cruciform-containing plasmid. The extruded cruciform is depicted in its most abundant isomer, i.e., parallel orientation of the junction arms (44). The consequence of cutting the plasmid either at one or two sites is indicated. (b) Time course of EcME [10 nM] cutting pAT25 [20 pM]. Products were visualized by Southern blot. The percentage of each form of pAT25 is plotted; each point represents the average from three experiments with the standard error of the mean shown. The points represent quantification of supercoiled DNA (black diamonds), linear DNA (blue squares), and nicked DNA (red triangles).
We examined the ability of EcME to process cruciform-containing plasmid DNA. Supercoiled plasmid pAT25 (25), which contains two 25-bp hairpin structures, was incubated with EcME and samples were taken at different time points. The reaction products were analyzed on a native agarose gel. As shown in Fig. 2b, the substrate was converted to linear product, indicating that EcME can make two incisions on this substrate. At early time points, when <30% of substrate had been processed, similar amounts of nicked and linear product were generated. As the reaction proceeded further, the amount of nicked product declined. By the end of the 30-min reaction, when 97% of substrate had been processed, the major product (70%) was linear DNA. The generation of significant quantities of nicked circular DNA early in the reaction suggests that the two incisions made by EcME are sequential. This idea is also supported by the continued appearance of linear product at later time points, when the substrate has been almost exhausted. The relatively slow conversion of nicked to linear product coupled with the observation that 70% of substrate is converted to linear product suggests that EcME restrains the nicked intermediate in a conformation that can be further processed into linear DNA. Data confirming that EcME cuts at the position of the cruciform is presented in SI Figs. 8 and 9.
Cooperative Bilateral Cleavage.
The dose dependence of cruciform processing was investigated (Fig. 3a). Increasing concentrations of EcME were incubated with pAT25. Strikingly, the rate of cleavage catalyzed by EcME was not directly proportional to the concentration of protein. In multiple experiments, a marked threshold concentration of enzyme was required to initiate cruciform cleavage. The sigmoidal relationship between activity and EcME concentration indicates that the EcME-catalyzed reaction is higher than first order. The shape of the curve is most easily explained by cooperative association of two molecules of EcME with DNA. Similar results were obtained when the substrate concentration was 100-fold higher (SI Fig. 8c), indicating that the sigmoidal relationship is not due to low substrate concentration.
Fig. 3.
Cooperative cleavage by Mus81–Eme1. (a) Dose–response of pAT25 cleavage versus EcME concentration. pAT25 substrate [20 pM] was incubated with increasing amounts of Mus81–Eme1 as indicated. Percentage of supercoiled pAT25 substrate cleaved is plotted. Data are from one experiment and are representative of four experiments. (b) Activation of EcME by EcMED307A. pAT25 substrate [20 pM] was incubated with a fixed amount of wild-type EcME [3 nM] and increasing amounts of EcMED307A. Percentage of product and substrate present at each time point is plotted. Data are from one experiment and are representative of three experiments. The points represent supercoiled DNA (black diamonds), linear DNA (blue squares), and nicked DNA (red triangles). (c) Unilateral cleavage by mixed active/inactive EcME. pAT25 substrate [20 pM] was incubated with a fixed amount of wild-type EcME [6 nM] and increasing amounts of EcMED307A. The percentage of product and substrate present at each time point is plotted. Data are from one experiment and are representative of three experiments. (d) Gel filtration analysis of EcME. The indicated concentrations of EcME were fractionated on a Superdex S200 gel-filtration column. Aliquots of each fraction were separated by SDS/PAGE gel, and apparent molecular mass was calculated relative to standards.
If the catalytic activity of EcME depends on the simultaneous presence of two molecules of EcME, then low concentrations of wild-type complex may be stimulated by addition of catalytically inactive EcME. To test this, an amount of wild-type enzyme below the threshold required to initiate cleavage was supplemented with increasing concentrations of endonuclease dead, and the ability of the mix to cleave cruciform DNA was monitored (Fig. 3b). As before, at low concentrations of wild-type enzyme, no processing of the substrate was detected (Fig. 3b, lane 2), significantly, addition of endonuclease-dead EcMED307A stimulated cleavage (Fig. 3b lanes 4–7). Maximal activity was detected when the concentration of endonuclease-dead enzyme was 2-fold greater than the wild-type enzyme (Fig. 3b, lanes 5 and 6). In these reactions, ≈30% of the substrate was converted to nicked circular DNA. Nicked circular DNA can only result from unilateral cleavage of the substrate, thus, the increase in nicked circular product suggests that dimers in which one molecule of Mus81 is active and the other is inactive are formed. The addition of higher concentrations of catalytically inactive EcME also increased the amount of linear DNA that was generated, suggesting that the number or function of active dimers also increases.
The ability of active EcME to function when complexed with inactive EcME was further tested by examining the effects of titrating endonuclease-dead EcMED307A against a fixed concentration of wild-type EcME that is sufficient to cleave ≈90% of substrate. At a 1:1 ratio of wild-type:inactive EcME (Fig. 3c, lane 3), the same amount of substrate was processed, indicating that the presence of the endonuclease-dead enzyme does not significantly inhibit unilateral cleavage catalyzed by the wild-type enzyme. The decrease in linear product was balanced by an increase in nicked product. As the amount of EcMED307A was increased further, the amount of cleaved substrate decreased. At the highest concentrations of EcMED307A, a >90% decrease in linear product and a 30% reduction of nicked product was observed. This result supports earlier evidence suggesting that dimers of active and inactive EcME form under these conditions. The unilateral cleavage seen in these experiments can best be explained if each scission step is catalyzed independently. Taken together, these results suggest that the catalytic function of each unit is independent but cooperative. A coordinate but independent mechanism has been proposed for canonical Holliday junction-resolving enzymes and for restriction endonucleases (25–27, 34, 35).
Ternary Structure of Recombinant Mus81–Eme1.
The presence of two active sites in a single functional complex is thought to be essential for coordinated bilateral cleavage of duplex DNA in the case of restriction endonucleases (36) and Holliday junctions in the case of resolving enzymes (37–39). Several previously characterized resolvases have been shown to exist as highly stable dimeric complexes, whereas others undergo rapid subunit exchange in solution (27, 28). We wished to determine whether EcME can exist as a heterotetramer (containing two molecules of Mus81 and two molecules of Eme1) in solution (Fig. 3d). By using gel filtration, the molecular mass of a 3 μM solution EcME was determined to be 82 kDa [elution volume (Ve) = 15.7 ml]. This apparent molecular mass is consistent with the truncated enzyme forming a functional dimer (i.e., one molecule of EcMus81 and one molecule of EcEme1). Curiously, when more concentrated preparations (88 μM) were analyzed, EcME was found to elute at a position corresponding to a molecular mass of 140 kDa (Ve = 14.5 ml), suggesting that the preparation forms a tetrameric complex.
The observation that EcME can exist in solution as either a heterodimer or a heterotetramer is consistent with the cooperative nature of the enzyme acting on the pAT25 substrate. The concentration of EcME used in the enzyme assays (Fig. 3 a and b) is far below the value needed to form a heterotetramer in solution, however the presence of cruciform-containing DNA could significantly affect this value by acting as a nucleation site for the enzyme.
Symmetrical Cruciform Cleavage by Mus81–Eme1.
Canonical Holliday-junction resolvases cleave junctions such that the linear duplex products contain ligatable nicks that can be sealed directly by ligation (28). Previous studies examining Mus81–Eme1 cleavage of X-shaped oligonucleotide structures found that the enzyme predominantly makes nonsymmetric cuts that result in nonligatable products (3, 16, 18). To determine the structure of the products generated by EcME acting on the cruciform, the reaction products were examined both on native and denaturing gels. Symmetrical bilateral cleavage at the branch point of an extruded cruciform generates linear DNA containing terminal hairpins that can be ligated (Fig. 4a) (39). On a native gel, this structure cannot be distinguished from linear duplex DNA. However, on a denaturing gel, this structure runs as dimer-length single-stranded DNA (Fig. 4b). As expected, EcME generated product runs at the position of linear DNA both on native and denaturing gels. Treatment with T4 ligase did not effect the migration on a native gel (Fig. 4b Upper). Significantly, denaturing gel analysis of T4 ligase-treated products revealed the major species (67%) to be dimer-length single-stranded circular DNA. The identity of the dimer-length ssDNA circle was validated in SI Fig. 10.
Fig. 4.
Symmetrical cleavage of a cruciform structure by Mus81–Eme1. (a) Schematic of ligation assay. The symmetrical cleavage of a cruciform creates nicked hairpin DNA ends that are substrates for DNA ligase. Linear DNA with sealed hairpins runs as a dimer-length ssDNA circle on a denaturing gel. (b) pAT25 substrate [200 pM] was incubated with either EcME [10 nM] or BamHI (10 units) and followed by heat inactivation. Where indicated, T4 DNA ligase was added to the second incubation. Reaction products were analyzed by either native or denaturing gel electrophoresis and detected by Southern blot. (c) pAT25 substrate [2 nM] was incubated with either wild-type or mutant EcME [30 nM]. The reaction products were used as templates for primer extension reactions by using forward (Fwd) or reverse (Rev) primers as indicated. A Maxam–Gilbert sequencing ladder, run on the same gel, is shown at a lighter exposure. The major EcME cleavage sites on the cruciform in pAT25 are illustrated.
The generation of a high proportion of ligatable product suggested that cleavage of pAT25 is predominantly symmetric. The exact sites of cruciform cleavage were mapped by comparing the products of primer-extension reactions to sequencing ladders. As shown in Fig. 4c, the major cleavage sites for EcME mapped to the branch point on the 5′ side of the hairpin. Several minor cleavage sites were also detected within the hairpin arms (Fig. 4c). These results confirm that EcME makes symmetrically related cuts at the site of the junction.
Discussion
The hypothesis that Mus81–Eme1 resolves Holliday junctions in vivo has been challenged on the basis that it preferentially cleaves 3′ flaps and nicked X structures in vitro (8, 17, 22, 40, 41). Our analysis of truncated recombinant human Mus81–Eme1, full-length recombinant human Mus81–Eme1, and endogenous human Mus81–Eme1 is in agreement with previous studies showing that an intact X structure is not the preferred in vitro substrate (18, 40). However, evidence presented here, that Mus81–Eme1 has the specific, intrinsic enzymatic properties needed to catalyze coordinated cleavage of symmetric substrates, containing two independent target bonds, supports the idea that Mus81–Eme1 acts on intact Holliday junctions in vivo.
The use of a cruciform-extruding plasmid as a model Holliday-junction structure has allowed the elucidation of several enzymatic properties of Mus81–Eme1. Because the cruciform structure is unstable in a nicked plasmid (SI Fig. 5), we were able to conclude that two Mus81–Eme1 complexes act sequentially within the lifetime of the enzyme–substrate complex. The sigmoidal relationship between enzyme concentration and activity, coupled with stimulation of wild-type enzyme by the addition of catalytically inactive EcME, demonstrates that the presence of two catalytic units is either required for or greatly stimulates cleavage of the first strand of DNA. These data suggest that allosteric interaction between one Mus81–Eme1 complex and another promotes catalysis. It is also possible that binding of a heterotetrameric enzyme complex to DNA manipulates the junction into a more favorable conformer. These properties are consistent with those expected of an endonuclease that acts on symmetric substrates containing two equivalent cleavage sites. The data also suggest that dimerization is an inherent property of the Mus81–Eme1 complex. Dimerization of endogenous human enzyme has previously been described (20) and was recently confirmed for recombinant yeast proteins (31). We found that high concentrations of EcME are needed to form a heterotetramer in solution. However, in vivo complex formation may be promoted by interaction with DNA, by amino terminal sequences that are not essential for catalysis, and perhaps by other protein factors.
A nick-counternick mechanism has previously been suggested as the mechanism by which resolvases could use independently functioning catalytic units to coordinate bilateral cleavage of Holliday junctions (4, 25–27, 37). Another mechanism used by endonucleases to ensure cleavage of duplex DNA is cooperativity. In the case of type IIS restriction endonucleases, cooperativity and biological function are driven by induced dimerization of the enzyme subunits (35, 36). The highly cooperative kinetic mechanism proposed for Mus81–Eme1 avoids first-strand cleavage unless second-strand cleavage is ensured.
Previous studies found that Mus81–Eme1 cuts oligonucleotide X-shaped structures by cleaving strands of opposite polarity (3, 16, 18). However, the majority of these products were cut nonsymmetrically and contained short flaps or gaps and could not be directly ligated in vitro (3, 16, 18). In contrast, by using a cruciform-containing plasmid as a substrate, we find that cleavage by Mus81–Eme1 is largely symmetric and a significant fraction of the products can be ligated directly. Both oligonucleotide X structures and cruciforms mimic Holliday junctions, however, structural differences may account for differences in the way they are cleaved. For example, the arms of oligonucleotide-based substrates stack in an antiparallel conformation (42, 43), whereas the arms of plasmid-extruded cruciforms predominantly stack in a parallel orientation (44). Equally, it is possible that the cooperativity seen when Mus81–Eme1 acts on the cruciform structure promotes symmetric cleavage. Further studies are required to test the contribution of each of these properties to the symmetrical cleavage of the cruciform junction.
The efficient cleavage of nicked X structures by Mus81–Eme1 has been interpreted as a mechanism, which, if Mus81–Eme1 acts on D-loops before they mature into fully formed X-structures, would favor crossovers in meiosis (4, 19). This is an exciting possibility because it provides a de facto mechanism by which Holliday-junction resolution can ensure crossover. Indeed, meiotic crossovers are greatly reduced in fission yeast mutants of Mus81–Eme1 (3, 5, 19). However, the role of recombination repair in mitotic cells, maintenance of genomic integrity, and facilitating replication restart is somewhat different from its role in meiosis. In mitosis, crossovers occurring between sister chromatids are genetically silent and are therefore assayed by monitoring sister-chromatid exchange. Disruption of Eme1 in mouse embryonic stem cells leads to a slight increase, not a decrease, in sister-chromatid exchange (8). Thus, if Mus81–Eme1 acts on D-loops/nicked Holliday junctions during mitosis in mammalian cells, it is not apparent in assays of sister-chromatid exchange.
Given evidence that Mus81–Eme1 can exist in different multimeric states (Fig. 3d) (20, 31) and the possibility that cofactors may modulate function, it would be interesting to see whether the enzyme functions in different modes in different biological circumstances. The Mus81/XPF/Hef-related endonuclease from Drosophila, Mei9, functions in nucleotide excision repair and interstrand crosslink repair in somatic cells (45, 46). Within meiosis, Mei9 is required to generate crossovers (47). Intriguingly, the crossover function, interstrand crosslink repair function, but not the nucleotide-excision repair function of Mei9 depend on an associated protein, Mus312 (48). Mus312 homologues have not been described in higher eukaryotes, and, indeed, the region that Mus312 binds in Mei9 is not conserved in Mus81 (49). Nevertheless, based on the enhanced X12 activity seen for endogenous Mus81–Eme1, it is tempting to speculate that either changes in the dimerization state, or association with other proteins factors, might direct Mus81–Eme1 activity to different structures.
Methods
Endonuclease Assays on Radiolabeled Substrate.
The radiolabeled junctions were produced and used as previously detailed (4, 16). Reaction products were analyzed by native or denaturing PAGE as outlined in ref. 16.
Endonuclease Assays on pAT25.
Reactions containing pAT25 were initiated by addition of MgCl2 to 2.5 mM, incubated at 37°C for 20 min, and terminated by using stop buffer (10 mM EDTA, 1 mg/ml proteinase K, 0.4% SDS). Products were separated by electrophoresis through 1% agarose at 45 V for 16 h. DNA was transferred by Southern blotting. pAT25 DNA was detected by using a 32P random-labeled probe. Signals were quantified by using a Storm 860 PhosphorImager (Molecular Dynamics).
Ligation Assay on pAT25.
0.2 nM pM pAT25 was incubated in endonuclease buffer [50 mM Tris (pH 8.0), 20 mM NaCl, 2.5 mM MgCl2, 1 mM DTT and 100 μg/ml BSA] with either 10 nM EcME or 10 units of BamHI (NEB) for 2 min at 37°C. The reactions were terminated by heating at 70°C for 20 min. T4 DNA ligase (5U) and 1X T4 ligase buffer (Invitrogen) were added, and the reaction was incubated at room temperature for 30 min. Reaction products were separated by either native or denaturing agarose gel electrophoresis (39) and detected by Southern blotting. SI Methods contains detailed descriptions of enzyme purification, pAT25 purification, and the mapping of pAT25 cut sites.
Supplementary Material
ACKNOWLEDGMENTS.
We thank D. M. J. Lilley (University of Dundee, Dundee, Scotland) for the kind donation of the pAT25 substrate and his encouragement, V. Blais for valuable technical support, and members of the cell cycle group for their input and reading of the manuscript. This work is supported by a Leukemia Lymphomia Society Career Development Award (to E.R.T.) and National Cancer Institute grant support (to C.H.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710291105/DC1.
References
- 1.Interthal H, Heyer WD. MUS81 encodes a novel helix-hairpin-helix protein involved in the response to UV- and methylation-induced DNA damage in Saccharomyces cerevisiae. Mol Gen Genet. 2000;263:812–827. doi: 10.1007/s004380000241. [DOI] [PubMed] [Google Scholar]
- 2.Boddy MN, et al. Damage tolerance protein Mus81 associates with the FHA1 domain of checkpoint kinase Cds1. Mol Cell Biol. 2000;20:8758–8766. doi: 10.1128/mcb.20.23.8758-8766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boddy MN, et al. Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell. 2001;107:537–548. doi: 10.1016/s0092-8674(01)00536-0. [DOI] [PubMed] [Google Scholar]
- 4.Gaillard PH, Noguchi E, Shanahan P, Russell P. The endogenous Mus81-Eme1 complex resolves Holliday junctions by a nick and counternick mechanism. Mol Cell. 2003;12:747–759. doi: 10.1016/s1097-2765(03)00342-3. [DOI] [PubMed] [Google Scholar]
- 5.Cromie GA, et al. Single Holliday junctions are intermediates of meiotic recombination. Cell. 2006;127:1167–1178. doi: 10.1016/j.cell.2006.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPherson JP, et al. Involvement of mammalian Mus81 in genome integrity and tumor suppression. Science. 2004;304:1822–1826. doi: 10.1126/science.1094557. [DOI] [PubMed] [Google Scholar]
- 7.Dendouga N, et al. Disruption of murine Mus81 increases genomic instability and DNA damage sensitivity but does not promote tumorigenesis. Mol Cell Biol. 2005;25:7569–7579. doi: 10.1128/MCB.25.17.7569-7579.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abraham J, et al. Eme1 is involved in DNA damage processing and maintenance of genomic stability in mammalian cells. EMBO J. 2003;22:6137–6147. doi: 10.1093/emboj/cdg580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiyama T, et al. Haploinsufficiency of the Mus81-Eme1 endonuclease activates the intra-S-phase and G2/M checkpoints and promotes rereplication in human cells. Nucleic Acids Res. 2006;34:880–892. doi: 10.1093/nar/gkj495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao H, Chen XB, McGowan CH. Mus81 endonuclease localizes to nucleoli and to regions of DNA damage in human S-phase cells. Mol Biology Cell. 2003;14:4826–4834. doi: 10.1091/mbc.E03-05-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson-Schlitz D, Engels WR. Template disruptions and failure of double Holliday junction dissolution during double-strand break repair in Drosophila BLM mutants. Proc Natl Acad Sci USA. 2006;103:16840–16845. doi: 10.1073/pnas.0607904103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de los Santos T, et al. The Mus81/Mms4 endonuclease acts independently of double-Holliday junction resolution to promote a distinct subset of crossovers during meiosis in budding yeast. Genetics. 2003;164:81–94. doi: 10.1093/genetics/164.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berchowitz LE, Francis KE, Bey AL, Copenhaver GP. The role of AtMUS81 in interference-insensitive crossovers in A. thaliana. PLoS Genet. 2007;3:e132. doi: 10.1371/journal.pgen.0030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullen JR, Kaliraman V, Ibrahim SS, Brill SJ. Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics. 2001;157:103–118. doi: 10.1093/genetics/157.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartung F, Suer S, Bergmann T, Puchta H. The role of AtMUS81 in DNA repair and its genetic interaction with the helicase AtRecQ4A. Nucleic Acids Res. 2006;34:4438–4448. doi: 10.1093/nar/gkl576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen XB, et al. Human Mus81-associated endonuclease cleaves Holliday junctions in vitro. Mol Cell. 2001;8:1117–1127. doi: 10.1016/s1097-2765(01)00375-6. [DOI] [PubMed] [Google Scholar]
- 17.Kaliraman V, Mullen JR, Fricke WM, Bastin-Shanower SA, Brill SJ. Functional overlap between Sgs1-Top3 and the Mms4-Mus81 endonuclease. Genes Dev. 2001;15:2730–2740. doi: 10.1101/gad.932201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Constantinou A, Chen XB, McGowan CH, West SC. Holliday junction resolution in human cells: Two junction endonucleases with distinct substrate specificities. EMBO J. 2002;21:5577–5585. doi: 10.1093/emboj/cdf554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osman F, Dixon J, Doe CL, Whitby MC. Generating crossovers by resolution of nicked Holliday junctions: A role for Mus81-Eme1 in meiosis. Mol Cell. 2003;12:761–774. doi: 10.1016/s1097-2765(03)00343-5. [DOI] [PubMed] [Google Scholar]
- 20.Blais V, et al. RNA interference inhibition of Mus81 reduces mitotic recombination in human cells. Mol Biol Cell. 2004;15:552–562. doi: 10.1091/mbc.E03-08-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doe CL, Ahn JS, Dixon J, Whitby MC. Mus81-Eme1 and Rqh1 involvement in processing stalled and collapsed replication forks. J Biol Chem. 2002;277:32753–32759. doi: 10.1074/jbc.M202120200. [DOI] [PubMed] [Google Scholar]
- 22.Hollingsworth NM, Brill SJ. The Mus81 solution to resolution: Generating meiotic crossovers without Holliday junctions. Genes Dev. 2004;18:117–125. doi: 10.1101/gad.1165904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons CA, Kemper B, West SC. Interaction of a four-way junction in DNA with T4 endonuclease VII. J Biol Chem. 1990;265:9285–9289. [PubMed] [Google Scholar]
- 24.Fricke WM, Bastin-Shanower SA, Brill SJ. Substrate specificity of the Saccharomyces cerevisiae Mus81-Mms4 endonuclease. DNA Rep. 2005;4:243–251. doi: 10.1016/j.dnarep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Giraud-Panis MJ, Lilley DM. Near-simultaneous DNA cleavage by the subunits of the junction-resolving enzyme T4 endonuclease VII. EMBO J. 1997;16:2528–2534. doi: 10.1093/emboj/16.9.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fogg JM, Schofield MJ, Declais AC, Lilley DM. Yeast resolving enzyme CCE1 makes sequential cleavages in DNA junctions within the lifetime of the complex. Biochemistry. 2000;39:4082–4089. doi: 10.1021/bi992785v. [DOI] [PubMed] [Google Scholar]
- 27.Fogg JM, Lilley DM. Ensuring productive resolution by the junction-resolving enzyme RuvC: Large enhancement of the second-strand cleavage rate. Biochemistry. 2000;39:16125–16134. doi: 10.1021/bi001886m. [DOI] [PubMed] [Google Scholar]
- 28.Lilley DM, White MF. The junction-resolving enzymes. Nat Rev. 2001;2:433–443. doi: 10.1038/35073057. [DOI] [PubMed] [Google Scholar]
- 29.Nishino T, Komori K, Ishino Y, Morikawa K. X-ray and biochemical anatomy of an archaeal XPF/Rad1/Mus81 family nuclease: Similarity between its endonuclease domain and restriction enzymes. Structure. 2003;11:445–457. doi: 10.1016/s0969-2126(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 30.Newman M, et al. Structure of an XPF endonuclease with and without DNA suggests a model for substrate recognition. EMBO J. 2005;24:895–905. doi: 10.1038/sj.emboj.7600581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaskell LJ, Osman F, Gilbert RJ, Whitby MC. Mus81 cleavage of Holliday junctions: A failsafe for processing meiotic recombination intermediates? EMBO J. 2007;26:1891–1901. doi: 10.1038/sj.emboj.7601645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lilley DM, Hallam LR. Thermodynamics of the ColE1 cruciform. Comparisons between probing and topological experiments using single topoisomers. J Mol Biol. 1984;180:179–200. doi: 10.1016/0022-2836(84)90436-4. [DOI] [PubMed] [Google Scholar]
- 33.Murchie AI, Lilley DM. Supercoiled DNA, cruciform structures. Methods Enzymol. 1992;211:158–180. doi: 10.1016/0076-6879(92)11010-g. [DOI] [PubMed] [Google Scholar]
- 34.Vanamee ES, Santagata S, Aggarwal AK. FokI requires two specific DNA sites for cleavage. J Mol Biol. 2001;309:69–78. doi: 10.1006/jmbi.2001.4635. [DOI] [PubMed] [Google Scholar]
- 35.Bitinaite J, Wah DA, Aggarwal AK, Schildkraut I. FokI dimerization is required for DNA cleavage. Proc Natl Acad Sci USA. 1998;95:10570–10575. doi: 10.1073/pnas.95.18.10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gemmen GJ, Millin R, Smith DE. Tension-dependent DNA cleavage by restriction endonucleases: Two-site enzymes are “switched off” at low force. Proc Natl Acad Sci USA. 2006;103:11555–11560. doi: 10.1073/pnas.0604463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White MF, Lilley DM. The resolving enzyme CCE1 of yeast opens the structure of the four-way DNA junction. J Mol Biol. 1997;266:122–134. doi: 10.1006/jmbi.1996.0795. [DOI] [PubMed] [Google Scholar]
- 38.Shah R, Cosstick R, West SC. The RuvC protein dimer resolves Holliday junctions by a dual incision mechanism that involves base-specific contacts. EMBO J. 1997;16:1464–1472. doi: 10.1093/emboj/16.6.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwasaki H, Takahagi M, Shiba T, Nakata A, Shinagawa H. Escherichia coli RuvC protein is an endonuclease that resolves the Holliday structure. EMBO J. 1991;10:4381–4389. doi: 10.1002/j.1460-2075.1991.tb05016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciccia A, Constantinou A, West SC. Identification and characterization of the human mus81-eme1 endonuclease. J Biol Chem. 2003;278:25172–25178. doi: 10.1074/jbc.M302882200. [DOI] [PubMed] [Google Scholar]
- 41.Whitby MC. Making crossovers during meiosis. Biochem Soc Trans. 2005;33:1451–1455. doi: 10.1042/BST0331451. [DOI] [PubMed] [Google Scholar]
- 42.Duckett DR, et al. The structure of the Holliday junction, and its resolution. Cell. 1988;55:79–89. doi: 10.1016/0092-8674(88)90011-6. [DOI] [PubMed] [Google Scholar]
- 43.McKinney SA, Declais AC, Lilley DM, Ha T. Structural dynamics of individual Holliday junctions. Nat Struct Biol. 2003;10:93–97. doi: 10.1038/nsb883. [DOI] [PubMed] [Google Scholar]
- 44.Shlyakhtenko LS, Potaman VN, Sinden RR, Lyubchenko YL. Structure and dynamics of supercoil-stabilized DNA cruciforms. J Mol Biol. 1998;280:61–72. doi: 10.1006/jmbi.1998.1855. [DOI] [PubMed] [Google Scholar]
- 45.Boyd JB, Golino MD, Setlow RB. The mei-9 alpha mutant of Drosophila melanogaster increases mutagen sensitivity and decreases excision repair. Genetics. 1976;84:527–544. doi: 10.1093/genetics/84.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sekelsky JJ, McKim KS, Chin GM, Hawley RS. The Drosophila meiotic recombination gene mei-9 encodes a homologue of the yeast excision repair protein Rad1. Genetics. 1995;141:619–627. doi: 10.1093/genetics/141.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker BS, Carpenter AT. Genetic analysis of sex chromosomal meiotic mutants in Drosophilia melanogaster. Genetics. 1972;71:255–286. doi: 10.1093/genetics/71.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yildiz O, Majumder S, Kramer B, Sekelsky JJ. Drosophila MUS312 interacts with the nucleotide excision repair endonuclease MEI-9 to generate meiotic crossovers. Mol Cell. 2002;10:1503–1509. doi: 10.1016/s1097-2765(02)00782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yildiz O, Kearney H, Kramer BC, Sekelsky JJ. Mutational analysis of the Drosophila DNA repair and recombination gene mei-9. Genetics. 2004;167:263–273. doi: 10.1534/genetics.167.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.