Abstract
A Calvin cycle multiprotein complex including phosphoribulokinase (PRK), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and a small protein, CP12, has previously been identified. In this article, we have studied this complex in leaves and have shown that dissociation and reassociation of the PRK/GAPDH/CP12 complex occurs in a time frame of minutes, allowing for rapid regulation of enzyme activity. Furthermore, we have shown that the extent of formation and dissociation of the PRK/GAPDH/CP12 complex correlates with the quantity of light. These data provide evidence linking the status of this complex with the rapid and subtle regulation of GAPDH and PRK activities in response to fluctuations in light availability. We have also demonstrated that dissociation of this complex depends on electron transport chain activity and that the major factor involved in the dissociation of the pea complex was thioredoxin f. We show here that both PRK and GAPDH are present in the reduced form in leaves in the dark, but are inactive, demonstrating the role of the PRK/GAPDH/CP12 complex in deactivating these enzymes in response to reductions in light intensity. Based on our data, we propose a model for thioredoxin f-mediated activation of PRK and GAPDH by two mechanisms: directly through reduction of disulfide bonds within these enzymes and indirectly by mediating the breakdown of the complex in response to changes in light intensity.
Keywords: Calvin cycle, photosynthesis, protein-protein interactions, redox
Nearly all of the organic carbon in the biosphere has been produced by the photosynthetic carbon fixation (Calvin) cycle. The energy and reducing power (ATP and NADPH) required to drive the Calvin cycle are provided by the activity of the electron transport chain in response to illumination. Under natural light regimes, the rate of electron transport is not constant because the amount of light can vary transiently because of a combination of canopy shading and cloud cover, thereby limiting the availability of ATP and NADPH for carbon fixation. Light not only provides the reducing power for the Calvin cycle but also activates the Calvin cycle enzymes, phosphoribulokinase (PRK), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), fructose 1, 6-bisphosphatase (FBPase), and sedoheptulose-1,7-bisphosphatase (SBPase), via the thioredoxin/ferredoxin system (1). Over and above acting as an on/off switch, it is believed that thioredoxin mediated redox regulation allows the activation state of these enzymes to be modulated in response to changes in the activity of the electron transport chain (2, 3); however, this has never been demonstrated in planta. Furthermore, under conditions in which leaves experience a reduction in temperature or are shifted from low to high light, the response of the thioredoxin-modulated enzymes is not uniform (4, 5). Under these two conditions, it has been shown that FBPase and SBPase activity can lead to a temporary limitation of photosynthesis, whereas, in contrast, no limitation is imposed by either PRK or GAPDH. The difference between the responses of the Calvin cycle thioredoxin-modulated enzymes to changes in light and temperature may be attributable to factors known to impact on the regulation of these enzymes such as redox potential, kinetics of the thioredoxin interaction and metabolites (4, 6, 7). However, it is not known which of these factors is involved in the differential activation of individual redox-regulated enzymes under rapidly changing environmental conditions.
In addition to the well described ferredoxin/thioredoxin regulatory system, several lines of evidence have suggested that enzymes of the Calvin cycle associate to form multiprotein complexes, potentially providing another method of regulation (8–10). One stromal complex, involving PRK and GAPDH, was first identified in a green alga and then in spinach (11). When associated in this complex the activity of both PRK and GAPDH was low. Incubation of the isolated, partially purified complex with DTT or NADPH induced dissociation of the complex, which was accompanied by activation of PRK and NADP-GAPDH (12). Evidence has now accumulated indicating that the PRK/GAPDH complex also includes the chloroplast protein, CP12 (13–16). CP12 is a small protein (≈8.5 kDa) containing four highly conserved cysteine residues, which, in the oxidized state, have been shown to form two intramolecular disulfide bridges (15, 17, 18). It has been hypothesized that the function of the PRK/GAPDH/CP12 complex is to provide an additional mode of light/dark regulation of the Calvin cycle mediated by changes in the levels of NADP(H) (16). Much of our more recent knowledge about this complex has been gained from studies on in vitro coexpression of PRK, CP12 and the GapA subunit of GAPDH (13, 17, 19–21), which has led to the proposal that the function of the PRK/GAPDH/CP12 even in higher plants, is to provide redox control of the A4 GAPDH isoform (21). However, in contrast to algae, in higher plants the most abundant and active form of chloroplastic GAPDH is a heterotetramer, containing two distinct subunits, GapA and GapB. Thioredoxin-mediated redox control of this A2B2 form of GAPDH is mediated by the Gap B subunit, which has a C-terminal extension with homology to the CP12 protein (22, 23). Analysis of the PRK/GAPDH/CP12 complex in leaves indicated that both the A and B forms of GAPDH may be present in this complex (16, 24). However, no in vivo analysis of the function of the PRK/GAPDH/CP12 complex in higher plants has been undertaken, and although a cyanobacterial CP12 insertional mutant has been investigated, neither the status of the PRK/GAPDH/CP12 complex nor the activity of PRK or GAPDH was measured in this mutant (25). Therefore, the role of the PRK/GAPDH/CP12 complex in higher plants remains to be addressed.
The aim of the work in this article was to determine the physiological role of the PRK/GAPDH/CP12 complex in leaves and to investigate the importance of this complex in the regulation of PRK and GAPDH activity. To do this, we used a combination of native PAGE together with measurements of PRK and GAPDH activity to investigate the response of the higher plant PRK/GAPDH/CP12 complex to changes in the light environment. A biochemical approach was taken to investigate the links between the changes in status of the PRK/GAPDH/CP12 complex in stromal extracts and the activity of the electron transport chain. This article provides information on the physiological role of the PRK/GAPDH/CP12 complex in leaves in the regulation of photosynthesis in vivo.
Results
PRK and GAPDH Complexes in Pisum sativum.
A combination of Blue Native PAGE (BN/PAGE) and SDS/PAGE analysis were used to investigate factors affecting the status of the PRK/GAPDH/CP12 complex in leaves. Intact chloroplasts were prepared from leaves of P. sativum (pea) plants that had either been dark-adapted (16 h overnight) or illuminated (16 h overnight, followed by 30-min illumination >500 μmol·m−2·s−1). Stromal protein complexes were identified by using PRK or GAPDH A/B specific polyclonal antibodies. In dark-adapted pea leaves PRK was detected only in a band at ≈500 kDa (PRK/GAPDH/CP12), whereas in illuminated leaves the intensity of this band was reduced significantly and a PRK signal was detected at ≈80 kDa; the expected size of the homodimer isoform (Fig. 1A). The aggregation states of GAPDH are more numerous and in samples extracted from both dark-adapted and illuminated leaves complexes of ≈500 kDa (PRK/GAPDH/CP12), 160 kDa (GAPDH A2B2), and 320 kDa [most likely representing the GAPDH 2(A2B2) form] were present (26) (Fig. 1A). High light treatment of leaves reduced the GAPDH signal at 500 kDa (PRK/GAPDH/CP12) and increased the intensity of the band at 160 kDa (A2B2). It should be noted that routinely the signal obtained from the lower molecular mass species of PRK and GAPDH was more intense than that observed for the high molecular mass PRK/GAPDH/CP12 complex, which may be because of differences in transfer efficiency between these different complexes during Western blotting. Although this approach is not quantitative it does allow a comparative analysis of the changes in the amount of each enzyme form between samples. Second dimension SDS/PAGE analysis revealed that the 500-kDa complex contains both GAPDH subunits, GapA (lower band), and GapB (upper band) (Fig. 1B), and also PRK (data not shown). These data suggest that in pea the A2B2 isoform of GAPDH is present in the PRK/GAPDH/CP12 complex. GapA and GapB subunits were also present in the 320-kDa and 160-kDa bands recognized by the GAPDH polyclonal antibodies (16, 24). Mass peptide fingerprinting of protein spots excised from the silver-stained second dimension SDS/PAGE gel confirmed these results (data not shown). We were unable to detect CP12 associated with this complex in pea leaf extracts, using either antibody detection or LC-MS/MS fragmentation, therefore it was not possible to follow CP12 using this approach. However, given that numerous studies have demonstrated the requirement for CP12 in the formation of the complex (13, 15–17, 25) we refer to this complex as the PRK/GAPDH/CP12 throughout the manuscript. The focus of the work presented here is to address the physiological role of the complex in the regulation of the activity of PRK and GAPDH.
Fig. 1.
PRK and GAPDH protein complexes in P. sativum analyzed by using BN/PAGE and SDS/PAGE. Western blots of BN/PAGE analysis of stromal proteins (10 μg) extracted from leaves from dark-adapted plants (■) or after 30-min illumination (□) (A) and 2D SDS/PAGE after separation by BN/PAGE (B). Western blots were probed with PRK or GAPDH A/B polyclonal antibodies.
In Pea, Association and Dissociation of PRK and GAPDH Is Rapid and Reversible and Correlates with Changes in Illumination State and Enzyme Activity.
The response of the PRK/GAPDH/CP12 complex to dark/light transitions was investigated. Dark-adapted pea plants were illuminated for increasing periods of time in high light (>500 μmol·m−2·s−1) before extraction of stromal proteins and BN/PAGE. After 1 min of high light, a decrease in the amount of the 500-kDa PRK/GAPDH/CP12 complex and an increase in the low molecular PRK dimer (80 kDa) and GAPDH heterotetramer (160 kDa) species was evident (Fig. 2A). To investigate the kinetics of complex reassociation, plants were illuminated for 1 h in high light (sufficient for complete dissociation of the complex) and then dark-adapted for 1, 5, and 30 min before sampling. This experiment clearly showed that 1-min dark adaption caused some reassociation of the 500-kDa PRK/GAPDH/CP12 complex and, after 30 min in the dark, the light-induced dissociation was reversed. To explore further the role of the 500-kDa complex in the regulation of PRK and GAPDH, the activity of both enzymes was measured during a dark/light/dark transition (Fig. 2B). On transfer of plants from dark into high light, the initial increase in the activity of PRK and GAPDH correlated with the breakdown of the PRK/GAPDH/CP12 complex. A slow increase in the activity of both enzymes continued after dissociation of the complex. On transfer to dark, a rapid decline in the activity of PRK and GAPDH was evident, which correlated with the formation of the 500-kDa complex.
Fig. 2.
The association state and activity of PRK and GAPDH are rapidly and reversibly altered in response to dark and light. (A) BN/PAGE Western blot of stromal proteins (10 μg) extracted from plants dark-adapted overnight, subjected to increasing periods of light (□) and after transfer back to the dark (■). Blots were probed with either PRK or GAPDH A/B polyclonal antibodies. (B) PRK (▴) and GAPDH (○) activities determined in stromal extracts from leaves of plants after exposure to increasing periods of light (□) or dark (■). Error bars represent SE of mean, n = 3.
The Extent of PRK and GAPDH Association Is Determined by Light Intensity and Correlates Closely with Photosynthetic Carbon Assimilation and Enzyme Activities.
The reversible association and dissociation of the PRK/GAPDH/CP12 complex observed in response to light/dark shifts may suggest that the complex functions as an on/off switch. However, there is also the possibility that this complex responds to changes in light intensity. To explore this, we first measured the photosynthetic light dose–response curve of pea plants grown in the greenhouse (Fig. 3A). These data showed that light was limiting for photosynthesis between 0 and 300 μmol·m−2·s−1, and that maximum rates of photosynthesis were attained at a light level of 500 μmol·m−2·s−1. Based on this analysis, we selected a range of light levels (denoted by arrows, Fig. 3A) over which changes in the status of complex would be expected, if breakdown of the complex had a role in regulating activity of PRK and GAPDH in response to changes in light intensity. Stromal proteins were prepared after transfer of plants from the dark to the different light intensities for 30 min. This analysis showed that under conditions when photosynthesis is limited by light the PRK/GAPDH/CP12 complex was present (Fig. 3B). At the inflection point of the light response curve, between light-limiting and light-saturating conditions, only a small amount of the PRK/GAPDH/CP12 complex remained and under saturating light, PRK and GAPDH are in the active dimeric and heterotetrameric states, respectively. The light response of PRK and GAPDH activities correlated with that of photosynthesis and complex dissociation; with maximal activity of both enzymes occurring when light saturated photosynthesis was attained (Fig. 3C). We have also shown that the reassociation of the PRK/GAPDH/CP12 complex depends on light intensity [supporting information (SI) Figs. 7 and 8 in SI Appendix).
Fig. 3.
Light intensity determines the extent of complex dissociation. (A) Light dose–response of CO2 assimilation rates in pea leaves. Error bars represent SE of mean, n = 3. Arrows indicate light intensity at which the stromal samples were taken for analysis in B and C. (B) Stromal samples (10 μg) were subjected to BN/PAGE followed by Western blotting probed with PRK or GAPDH A/B polyclonal antibodies. (C) PRK (▴) and GAPDH (○) activity in stromal extracts from leaves exposed to different light intensities for 30 min. Error bars represent SE of mean, n = 3.
PRK/GAPDH/CP12 Complex Dissociation Requires Active Photosynthetic Electron Transport.
The response of the PRK/GAPDH/CP12 complex to illumination in intact chloroplasts was similar to that in intact leaves and resulted in the conversion of PRK and GAPDH to the dimeric and tetrameric state, respectively (data not shown). However, light treatment of stromal extracts made from the same darkened leaves did not cause dissociation of the PRK/GAPDH/CP12 complex, suggesting that the thylakoid membranes or products from the electron transport chain are essential for dissociation. To test this hypothesis, the PRK/GAPDH/CP12 complex was studied in stromal extracts from leaves in which electron transport was inhibited (Fig. 4). DCMU was used to inhibit both NADPH and ATP synthesis and methyl viologen (MV) to inhibit NADPH synthesis only. Analysis of stromal extracts from the treated leaves showed that both DCMU and MV inhibited the breakdown of the PRK/GAPDH/CP12 complex (Fig. 4). Similar experiments carried out by using intact chloroplasts again showed that DCMU and MV inhibited complex dissociation, whereas in samples treated with tentoxin, an inhibitor of ATP synthesis, the status of the PRK/GAPDH/CP12 complex was similar to the untreated controls (SI Fig. 9 in SI Appendix).
Fig. 4.
Dissociation of the PRK/GAPDH/CP12 complex requires active electron transport. Leaves were treated with 0.05% Tween (ctrl), 0.05% Tween plus DCMU (0.1 mM), or MV (1 mM) and left in the dark for 10 min, before either a further period in the dark (■) or light (□) for 30 min. After this treatment, chloroplasts were prepared, and stromal proteins were extracted and resolved on BN/PAGE. Western blots were probed with PRK or GAPDH A/B polyclonal antibodies.
Reduced Trx f Mediates Dissociation of the PRK/GAPDH Complex in Pea Stromal Extracts.
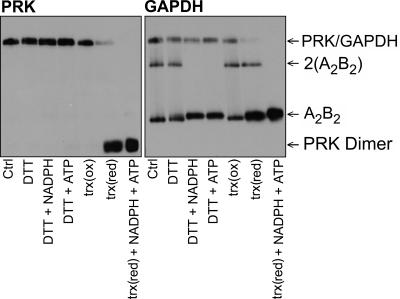
To investigate further the links between electron transport and complex dissociation we tested the effect of NADP, NADPH, ATP, and Trx f on the PRK/GAPDH/CP12 complex. When stromal extracts from dark-adapted leaves were incubated with reduced Trx f almost complete dissociation of the PRK/GAPDH/CP12 complex occurred (Fig. 5). Addition of ATP and NADPH to the reduced Trx f led to complete dissociation of the PRK/GAPDH/CP12 complex (Fig. 5). Interestingly, reduced DTT together with either NADPH or ATP had no effect on the PRK/GAPDH/CP12 complex but resulted in the complete loss of the (320-kDa) form of GAPDH and an increase in the A2B2 heterotetramer (Fig. 5). Although NADPH and ATP alone caused a small increase in the amount of the GAPDH A2B2 heterotetramer, no visible effect on either the 500-kDa (PRK/GAPDH/CP12) or 80-kDa PRK dimer was evident (SI Fig. 10 in SI Appendix).
Fig. 5.
Trx f mediates the dissociation of the PRK/GAPDH/CP12 complex. BN/PAGE Western blot analysis of stromal proteins from darkened leaves incubated with combinations of, NADPH, ATP, DTT (2.5 mM), and/or reduced Trx f (5 μM) for 30 min. Trx f was reduced by using DTT (2.5 mM). Blots were probed with PRK or GAPDH A/B polyclonal antibodies.
PRK Can Be Reduced in the PRK/GAPDH/CP12 Complex but Has Low Activity.
In stromal extracts prepared from dark-adapted pea leaves, all of the PRK was present in the PRK/GAPDH/CP12 complex, and incubation of these extracts with the reducing agent DTT resulted in only minimal breakdown of the complex (Fig. 6A). Parallel analysis of these stromal extracts using nonreducing SDS/PAGE revealed that in these DTT treated samples PRK is completely reduced (Fig. 6B). In contrast, in the untreated samples, two hybridizing bands were seen, representing the reduced and oxidized forms of PRK (Fig. 6B). LC MS/MS analysis of these nonreduced samples after alkylation with iodoacetamide confirmed that in darkened leaves the amino acid residue, C55, a known target for thioredoxin-mediated reductive activation of PRK, was reduced (SI Fig. 11A in SI Appendix). Despite the presence of reduced PRK in the samples from darkened leaves enzyme activity is very low (< 0.1 μmol min−1 mg−1 protein) and was increased by <2-fold after incubation with DTT, despite complete reduction of PRK. In contrast, freeze/thawing of stromal extracts, in the absence of DTT, disrupted the complex and resulted in a 5-fold stimulation in PRK activity (Fig. 6C). Freeze-thawed stromal extracts incubated with DTT led to a further increase in PRK activity (Fig. 6B). Like PRK, the activity of GAPDH was low in darkened extracts, but, in contrast, DTT increased the activity of GAPDH by 5-fold. Freeze thaw dissociation alone increased GAPDH activity by 3-fold, suggesting that some of the GAPDH was in the reduced form in the complex. A further increase in GAPDH activity was observed when the freeze-thawed samples were incubated with DTT (Fig. 6C).
Fig. 6.
Reduced PRK has low activity in the PRK/GAPDH/CP12 complex. Stromal proteins isolated from darkened leaves were either used fresh or freeze-thawed before incubation on ice with or without DTT (0.1 M, 30 min) before analysis. (A) Western blot analysis of BN/PAGE. (B) Nonreducing SDS/PAGE. Western blots were probed with PRK or GAPDH polyclonal antibodies. (C) PRK and GAPDH activity. Error bars represent SE of mean, n = 3.
Discussion
In this article, we provide evidence of a role for the Calvin cycle PRK/GAPDH/CP12 complex in the modulation of carbon fixation in response to changes in the availability of light. We show that in leaves of higher plants this complex is dynamic and mediates the regulation of PRK and GAPDH activity in response to changes in the availability of light. Our data also show that the amount of complex present in pea leaves depends on the quantity of light and that dissociation and reassembly of this complex occurs within minutes when the light level is altered. This rapid response time of complex formation and breakdown is sufficient to allow for the modulation of PRK and GAPDH activity in response to the light/shade transition that plants encounter throughout the day in the field. These data provide strong evidence that the role of this complex in vivo is not simply a light/dark on-off switch as has been proposed (16, 25), but that it could facilitate the coordinate and transient modulation of PRK and GAPDH activity during the day in response to changes in light intensity. We have also shown that in leaves dissociation of the PRK/GAPDH/CP12 complex depended on active electron transport suggesting that a product of these reactions may mediate this process. Previous studies have shown that dissociation of the PRK/GAPDH/CP12 complex could be mediated by NADP and/or NADPH and have demonstrated that reducing conditions caused dissociation of this complex in both algae and higher plants (11, 16, 17, 24, 27). Our work would suggest that, although NADPH and ATP may enhance dissociation of the complex in reducing conditions, the major link between electron transport and complex dissociation is most likely to be reduction via Trx f. This finding is in agreement with the consensus from the literature that dissociation of the PRK/GAPDH/CP12 complex can be mediated by Trx f (17, 21, 24, 27). The target for Trx f-mediated dissociation of the PRK/GAPDH/CP12 complex is not yet known, but our finding that PRK can be fully reduced and the complex remain intact suggests that it is unlikely that a change in the redox state of PRK alone is responsible for complex dissociation. There are two further targets for Trx f within the complex, the redox sensitive C-terminal extension on GapB or the cysteine pairs on the CP12 protein (15, 16, 22, 28). However, it is not yet clear whether all three components of the complex need to be reduced for complete dissociation or, whether reduction of either GAPDH or CP12 reduction is the essential step in this process. Further work will be required to elucidate the details of the redox changes occurring within the complex to bring about dissociation. The lack of response of the pea PRK/GAPDH/CP12 complex to NADPH is not strictly consistent with all of the previous studies on this complex possibly because of differences in the techniques used to visualize the complex (15, 16, 25, 27). Alternatively, this may reflect the differences in metabolic regulation between cyanobacteria, algae, and higher plants (25, 29–32).
A key finding of this study is that the PRK/GAPDH/CP12 complex plays a major role in the light regulation of PRK activity. This is consistent with previous studies that demonstrated a role for this complex in the regulation of PRK activity (13, 17, 20, 30, 33). In the dark, the thioredoxin-activated Calvin cycle enzymes, including PRK and GAPDH, have been shown to be inactive and it has been assumed that this deactivation results from the formation of the oxidized state of the enzyme (3, 27, 34). However, although our results showed that in darkened pea leaves PRK activity is low, unexpectedly, our data provide evidence that in the dark there are significant amounts of the reduced form of PRK. This result is of particular interest as PRK has always been considered as an enzyme displaying “classical” thioredoxin-mediated activation (3). The implication of this finding is that the formation of the PRK/GAPDH/CP12 complex, in response to a reduction in light intensity or on transfer to the dark, is essential for rapid and or complete deactivation of PRK. One role of the PRK/GAPDH/CP12 complex may therefore be to provide a mechanism to maintain a pool of deactivated but reduced PRK, poised for rapid activation when the complex dissociates in response to an increase in light levels. This suggestion is supported by our data showing that the release of PRK and GAPDH from the complex, in the dark and in the absence of any reductive activation, resulted in an increase in activity of both enzymes. Furthermore, we have shown that the activity of both PRK and GAPDH correlates with changes in the status of the complex in response to changes in the light availability. The regulation of PRK and GAPDH by the formation and dissociation of the PRK/GAPDH/CP12 complex is likely to contribute to the rapid activation of these enzymes on transfer from shade to high light (5) and may also explain why PRK and GAPDH do not limit photosynthetic carbon fixation under these conditions (4, 35).
This article provides evidence that in pea leaves the PRK/GAPDH/CP12 complex is the major regulator of PRK activity in response to changes in light availability and that thioredoxin modulates the activity of PRK and GAPDH, not only through direct molecular interaction but also indirectly by mediating changes in the status of the PRK/GAPDH/CP12 complex. Importantly, this study provides the first data suggesting that dynamic changes in the status of the stromal PRK/GAPDH/CP12 complex allows activity of the Calvin cycle to be modulated in response to rapidly changing light levels that occur in the natural environment.
Experimental Procedures
Materials.
Tris base, glycine, and SDS were obtained from Fisher Scientific. Acrylamide/bisacrylamide came from Amresco. All other chemicals were from Sigma–Aldrich.
Plant Growth.
Peas (Onwards) (Nickerson-Zwaan) were grown in vermiculite for 10–12 days in a controlled environment chamber with 16-h light/8-h dark and light levels of 115 μmol·m−2·s−1.
Isolation of Stromal Proteins.
Leaf material was homogenized in ice-cold chloroplast isolation buffer (CIB) containing 30 mM MOPS (pH 7.5), 330 mM mannitol, 2 mM EDTA, and 2 mM MgCl2 using a Polytron (Kinematica) and filtered through two layers of muslin and one layer of miracloth (Calbiochem EMD Biosciences,) (24). Chloroplasts were collected by centrifugation at 2,500 × g for 2 min at 4°C, resuspended, and washed twice in CIB and resuspended in extraction buffer. Stromal extracts were prepared by homogenizing intact chloroplasts in a glass homogenizer (Glass Precision Engineering Ltd.), the resulting suspension centrifuged at 10,000 × g for 5 min at 4°C, and the supernatant filtered through a 0.2-μm filter membrane. Protein concentration was determined by using a Bradford assay.
BN/PAGE and Western Blot Transfer.
BN/PAGE was performed by using the Protean IIxi cell (Bio-Rad) (36). Linear gradient gels (6% acrylamide/bisacrylamide, 25 mM Bis-Tris, pH 7.0, to 12% acrylamide/bisacrylamide, 17.5% glycerol, 25 mM Bis-Tris, pH 7.0) were overlaid with a stacking gel (4% acrylamide/bisacrylamide, 25 mM Bis-Tris, pH 7.0). Electrophoresis was at 4°C, 100 V for 45 min, followed by 500 V (15 mA) for 5 h. Cathode buffer (50 mM Tricine, 15 mM Bis-Tris, pH 7.0, 0.02% Coomassie blue) was exchanged for 0.002% Coomassie blue buffer after 2.5 h. The anode buffer contained 50 mM Bis-Tris, pH 7.0. Before Western transfer or 2D PAGE, gels were denatured for 15 min (25 mM Tris, 190 mM glycine, 0.1% SDS, 3% 2-mercaptoethanol, pH 8.8, 65°C) followed by 15 to 30 min (25 mM Tris, 190 mM glycine SDS 0.1%, pH 7.5). For 2D PAGE, proteins were separated on a Tricine SDS/PAGE gel (4% stacking gel, 10% spacer gel, and 16.5% resolving gel). For Western blots, gels were transferred to an Immobilin-P poly(vinylidene difluoride) (PVDF) membrane (Millipore) and probed by using PRK or GAPDH specific polyclonal antibodies; proteins were detected by using horseradish peroxidase conjugated to a secondary antibody (Promega) with enhanced chemiluminescence (ECL detection reagent; Pierce Biotechnology).
Gas Exchange Analysis.
Gas exchange analysis was carried out by using a portable gas exchange system (LI-6400, LI-COR). Leaf photosynthetic CO2 uptake rate (A) was determined in response to step changes in quantum flux (Q), provided by an integral LED light source. A Peltier cooling system maintained leaf temperature at 25°C and vapor pressure deficit (VPD) was controlled between 0.7 and 1.4.
PRK and GAPDH Activities.
Stromal proteins (20 μg) were assayed in buffer containing 50 mM Hepes, pH 7.8, 40 mM KCl, 8 mM MgCl2, 2 units of pyruvate kinase, 2 units lactate dehydrogenase, 1 mM phosphoenolpyruvate, 1 mM ATP, 4 mM DTT, 0.5 mM ribulose 5-phosphate, and 0.25 mM NADH. Rates of NADH oxidation were followed by measuring the change in absorbance at 340 nm. GAPDH activities were determined as described in ref. 24. Stromal proteins (20 μg) were assayed in reaction buffer containing 50 mM Tris·HCl, pH 7.8, 10 mM MgCl2, 1 mM EDTA, 2.5 mM ATP, 0.15 mM NADPH or NADH, 20 units of 3-phosphoglyceric phosphokinase, 5 mM 3-phosphoglyceric acid, and 4 mM DTT and the rate of NADPH/NADH oxidation at 25°C was followed by measuring the change in absorbance at 340 nm.
Nanoscale LC/MSn Analysis.
Gel slices were excised from nonreducing SDS/PAGE gels, and the Coomassie stain was removed by using NH4HCO3 (25 mM)/acetonitrile (50%) then dehydrated in acetonitrile (100%), air-dried, and rehydrated in iodoacetamide (55 mM)/NH4HCO3 (25 mM) (45 min 56°C) and washed in 25 mM NH4HCO3. The dehydration step was repeated before rehydration in Promega Trypsin Gold (1:40 in 25 mM NH4HCO3). Excess trypsin solution was removed, 25 mM NH4HCO3 was added, and it was incubated overnight at 37°C. Liquid chromatography mass spectrometry of the resulting protein digest was performed by using Ultimate II nano-LC (Dionex) coupled to an Esquire HCT ion trap mass spectrometer (Bruker Daltonics) via online nanoelectrospray ion source. The peptides were separated by a 70-min gradient of acetonitrile (2–40%), flow rate 300 nl·min−1, using a Pepmap 75 reverse-phase C18 nano-column (Dionex). The raw MS/MS data were converted to Mascot generic format files (mgf) using the Bruker Data Analysis program and searched by using the in house Mascot Server (Matrix Science).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Drs. P. Davey and T. Lawson for help with gas exchange analysis and Dr. M. Fryer for advice on the PRK and GAPDH enzyme assays. We also thank Professor M. Salvucci (Arid-Land Agricultural Research Center, Maricopa, AZ) and Dr. N. Wedel (Heinrich-Hecht-Platz, Kiel, Germany) for the kind gift of the PRK and GAPDH antibodies, respectively, and Professor P. Schurmann (University of Neuchâtel, Neuchâtel, Switzerland) for the recombinant Trx f protein. This project was funded by Biotechnology and Biological Sciences Research Council Grant P19403.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710518105/DC1.
References
- 1.Buchanan BB, Balmer Y. Redox regulation: A broadening horizon. Annu Rev Plant Biol. 2005;56:187–220. doi: 10.1146/annurev.arplant.56.032604.144246. [DOI] [PubMed] [Google Scholar]
- 2.Scheibe R. Redox-modulation of chloroplast enzymes: A common principle for individual control. Plant Physiol. 1991;96:1–3. doi: 10.1104/pp.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schurmann P, Jacquot JP. Plant thioredoxin systems revisited. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:371–400. doi: 10.1146/annurev.arplant.51.1.371. [DOI] [PubMed] [Google Scholar]
- 4.Hutchison RS, Groom Q, Ort DR. Differential effects of chilling-induced photooxidation on the redox regulation of photosynthetic enzymes. Biochemistry. 2000;39:6679–6688. doi: 10.1021/bi0001978. [DOI] [PubMed] [Google Scholar]
- 5.Sassenrath-Cole GF, Pearcy RW, Steinmaus S. The role of enzyme activation state in limiting carbon assimilation under variable light conditions. Photosynth Res. 1994;41:295–302. doi: 10.1007/BF00019407. [DOI] [PubMed] [Google Scholar]
- 6.Faske M, et al. Redox equilibria between the regulatory thiols of light dark-modulated chloroplast enzymes and dithiothreitol: Fine-tuning by metabolites. Biochim Biophys Acta. 1995;1247:135–142. doi: 10.1016/0167-4838(94)00203-s. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan BB. Regulation of CO2 assimilation in oxygenic photosynthesis: The ferredoxin thioredoxin system—Perspective on its discovery, present status, and future development. Arch Biochem Biophys. 1991;288:1–9. doi: 10.1016/0003-9861(91)90157-e. [DOI] [PubMed] [Google Scholar]
- 8.Harris GC, Koniger M. The “high” concentrations of enzymes within the chloroplast. Photosynth Res. 1997;54:5–23. [Google Scholar]
- 9.Sainis JK, Harris GC. The association of ribulose-1,5-bisphosphate carboxylase with phosphoriboisomerase and phosphoribulokinase. Biochem Biophys Res Comm. 1986;139:947–954. doi: 10.1016/s0006-291x(86)80269-8. [DOI] [PubMed] [Google Scholar]
- 10.Suss KH, Arkona C, Manteuffel R, Adler K. Calvin cycle multienzyme complexes are bound to chloroplast thylakoid membranes of higher-plants in situ. Proc Natl Acad Sci USA. 1993;90:5514–5518. doi: 10.1073/pnas.90.12.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clasper S, Easterby JS, Powls R. Properties of 2 high-molecular-mass forms of glyceraldehyde-3-phosphate dehydrogenase from spinach leaf, one of which also possesses latent phosphoribulokinase activity. Eur J Biochem. 1991;202:1239–1246. doi: 10.1111/j.1432-1033.1991.tb16496.x. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson S, Easterby JS, Powls R. Properties of a multimeric protein complex from chloroplasts possessing potential activities of NADPH-dependent glyceraldehyde-3-phosphate dehydrogenase and phosphoribulokinase. Eur J Biochem. 1987;162:423–431. doi: 10.1111/j.1432-1033.1987.tb10619.x. [DOI] [PubMed] [Google Scholar]
- 13.Graciet E, et al. The small protein CP12: A protein linker for supramolecular complex assembly. Biochemistry. 2003;42:8163–8170. doi: 10.1021/bi034474x. [DOI] [PubMed] [Google Scholar]
- 14.Pohlmeyer K, Paap BK, Soll J, Wedel N. CP12: A small nuclear-encoded chloroplast protein provides novel insights into higher-plant GAPDH evolution. Plant Mol Biol. 1996;32:969–978. doi: 10.1007/BF00020493. [DOI] [PubMed] [Google Scholar]
- 15.Wedel N, Soll J. Evolutionary conserved light regulation of Calvin cycle activity by NADPH-mediated reversible phosphoribulokinase/CP12/glyceraldehyde-3-phosphate dehydrogenase complex dissociation. Proc Natl Acad Sci USA. 1998;95:9699–9704. doi: 10.1073/pnas.95.16.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wedel N, Soll J, Paap BK. CP12 provides a new mode of light regulation of Calvin cycle activity in higher plants. Proc Natl Acad Sci USA. 1997;94:10479–10484. doi: 10.1073/pnas.94.19.10479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marri L, Trost P, Pupillo P, Sparla F. Reconstitution and properties of the recombinant glyceraldehyde-3-phosphate dehydrogenase/CP12/phosphoribulokinase supramolecular complex of Arabidopsis. Plant Physiol. 2005;139:1433–1443. doi: 10.1104/pp.105.068445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marri L, et al. Spontaneous assembly of photosynthetic supramolecular complexes as mediated by the intrinsically unstructured protein CP12. J Biol Chem. 2007 doi: 10.1074/jbc.M705650200. M705650200. [DOI] [PubMed] [Google Scholar]
- 19.Lebreton S, Andreescu S, Graciet E, Gontero B. Mapping of the interaction site of CP12 with glyceraldehyde-3-phosphate dehydrogenase from Chlamydomonas reinhardtii—Functional consequences for glyceraldehyde-3-phosphate dehydrogenase. FEBS J. 2006;273:3358–3369. doi: 10.1111/j.1742-4658.2006.05342.x. [DOI] [PubMed] [Google Scholar]
- 20.Lebreton S, Graciet E, Gontero B. Modulation, via protein–protein interactions, of glyceraldehyde-3-phosphate dehydrogenase activity through redox phosphoribulokinase regulation. J Biol Chem. 2003;278:12078–12084. doi: 10.1074/jbc.M213096200. [DOI] [PubMed] [Google Scholar]
- 21.Trost P, et al. Thioredoxin-dependent regulation of photosynthetic glyceraldehyde-3-phosphate dehydrogenase: Autonomous vs. CP12-dependent mechanisms. Photosynth Res. 2006;89:263–275. doi: 10.1007/s11120-006-9099-z. [DOI] [PubMed] [Google Scholar]
- 22.Baalmann E, Scheibe R, Cerff R, Martin W. Functional studies of chloroplast glyceraldehyde-3-phosphate dehydrogenase subunits A, B expressed in Escherichia coli: Formation of highly active A (4) and B-4 homotetramers and evidence that aggregation of the B-4 complex is mediated by the B subunit carboxy terminus. Plant Mol Biol. 1996;32:505–513. doi: 10.1007/BF00019102. [DOI] [PubMed] [Google Scholar]
- 23.Fermani S, et al. Molecular mechanism of thioredoxin regulation in photosynthetic A (2)B(2)-glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci USA. 2007;104:11109–11114. doi: 10.1073/pnas.0611636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheibe R, Wedel N, Vetter S, Emmerlich V, Sauermann SM. Co-existence of two regulatory NADP-glyceraldehyde 3-P dehydrogenase complexes in higher plant chloroplasts. Eur J Biochem. 2002;269:5617–5624. doi: 10.1046/j.1432-1033.2002.03269.x. [DOI] [PubMed] [Google Scholar]
- 25.Tamoi M, Miyazaki T, Fukamizo T, Shigeoka S. The Calvin cycle in cyanobacteria is regulated by CP12 via the NAD (H)/NADP(H) ratio under light/dark conditions. Plant J. 2005;42:504–513. doi: 10.1111/j.1365-313X.2005.02391.x. [DOI] [PubMed] [Google Scholar]
- 26.Baalmann E, Backhausen JE, Kitzmann C, Scheibe R. Regulation of NADP-dependent glyceraldehyde-3-phosphate dehydrogenase-activity in spinach-chloroplasts. Botanica Acta. 1994;107:313–320. [Google Scholar]
- 27.Graciet E, Lebreton S, Camadro JM, Gontero B. Thermodynamic analysis of the emergence of new regulatory properties in a phosphoribulokinase-glyceraldehyde 3-phosphate dehydrogenase complex. J Biol Chem. 2002;277:12697–12702. doi: 10.1074/jbc.M111121200. [DOI] [PubMed] [Google Scholar]
- 28.Sparla F, Pupillo P, Trost P. The C-terminal extension of glyceraldehyde-3-phosphate dehydrogenase subunit B acts as an autoinhibitory domain regulated by thioredoxins and nicotinamide adenine dinucleotide. J Biol Chem. 2002;277:44946–44952. doi: 10.1074/jbc.M206873200. [DOI] [PubMed] [Google Scholar]
- 29.Oesterhelt C, et al. Redox regulation of chloroplast enzymes in Galdieria sulphuraria in view of eukaryotic evolution. Plant Cell Physiol. 2007;48:1359–1373. doi: 10.1093/pcp/pcm108. [DOI] [PubMed] [Google Scholar]
- 30.Lebreton S, Gontero B. Memory and imprinting in multienzyme complexes: Evidence for information transfer from glyceraldehyde-3-phosphate dehydrogenase to phosphoribulokinase under reduced state in Chlamydomonas reinhardtii. J Biol Chem. 1999;274:20879–20884. doi: 10.1074/jbc.274.30.20879. [DOI] [PubMed] [Google Scholar]
- 31.Robbens S, Petersen J, Brinkmann H, Rouze P, Van de Peer Y. Unique regulation of the Calvin cycle in the ultrasmall green alga Ostreococcus. J Mol Evol. 2007;64:601–604. doi: 10.1007/s00239-006-0159-y. [DOI] [PubMed] [Google Scholar]
- 32.Michels AK, Wedel N, Kroth PG. Diatom plastids possess a phosphoribulokinase with an altered regulation and no oxidative pentose phosphate pathway. Plant Physiol. 2005;137:911–920. doi: 10.1104/pp.104.055285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avilan L, Lebreton S, Gontero B. Thioredoxin activation of phosphoribulokinase in a bi-enzyme complex from Chlamydomonas reinhardtii chloroplasts. J Biol Chem. 2000;275:9447–9451. doi: 10.1074/jbc.275.13.9447. [DOI] [PubMed] [Google Scholar]
- 34.Porter MA, Stringer CD, Hartman FC. Characterization of the regulatory thioredoxin site of phosphoribulokinase. J Biol Chem. 1988;263:123–129. [PubMed] [Google Scholar]
- 35.Raines CA. The Calvin cycle revisited. Photosynth Res. 2003;75:1–10. doi: 10.1023/A:1022421515027. [DOI] [PubMed] [Google Scholar]
- 36.Schagger H, von Jagow G. Blue native electrophoresis for isolation of membrane-protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.