Abstract
X-ray crystal structures of lactose permease (LacY) reveal pseudosymmetrically arranged N- and C-terminal six-transmembrane helix bundles surrounding a deep internal cavity open on the cytoplasmic side and completely closed on the periplasmic side. The residues essential for sugar recognition and H+ translocation are located at the apex of the cavity and are inaccessible from the outside. On the periplasmic side, helices I/II and VII from the N- and C- six helix bundles, respectively, participate in sealing the cavity from the outside. Three paired double-Cys mutants—Ile-40 → Cys/Asn-245 → Cys, Thr-45 → Cys/Asn-245 → Cys, and Ile-32 → Cys/Asn-245 → Cys—located in the interface between helices I/II and VII on the periplasmic side of LacY were constructed. After cross-linking with homobifunctional reagents less than ≈15 Å in length, all three mutants lose the ability to catalyze lactose transport. Strikingly, however, full or partial activity is observed when cross-linking is mediated by flexible reagents greater than ≈15 Å in length. The results provide direct support for the argument that transport via LacY involves opening and closing of a large periplasmic cavity.
Keywords: cross-linking, membrane proteins, protein dynamics, transport, structure/function
The lactose permease of Escherichia coli (LacY) (1), a member of the major facilitator superfamily (MFS), transduces free energy in an electrochemical H+ gradient (Δμ̄H+) into a concentration gradient of galactopyranosides by coupling the downhill, stoichiometric translocation of H+ to the uphill accumulation of galactopyranosides. LacY can also use free energy released from downhill translocation of sugar in either direction to drive uphill translocation of H+ with generation of Δμ̄H+, the polarity of which depends on the direction of the sugar gradient (1, 2).
X-ray crystal structures of LacY (3–5) combined with a wealth of biochemical and biophysical data (2, 6–10) have led to an alternating access mechanism for sugar translocation across the membrane. By this means, the inward-facing cavity closes with opening of an outward-facing cavity, thereby allowing alternative accessibility of the sugar-binding site to either face of the membrane. A similar model has been proposed for the glycerol phosphate/phosphate antiporter GlpT, a related MFS protein (11), and the ABC transporter Sav 1866 (12). The alternating access model involves a global conformational change, which is consistent with the highly dynamic nature of LacY (2, 13–16).
Although it may seem intuitively obvious that a pathway must open on the periplasmic side of LacY to allow access of sugar to the binding site, insight is cloudy in this respect because all x-ray structures of LacY (3–5) are in the same inward-facing conformation with an open cytoplasmic cavity and a tightly closed periplasmic side. Nonetheless, site-directed alkylation (see refs. 7 and 10), single-molecule fluorescence resonance energy transfer (8) and double electron-electron resonance studies (9) clearly indicate that an outward-facing conformation with a hydrophilic pathway on the periplasmic side of LacY exists during turnover.
In this study, three paired double-Cys replacement mutants distributed in the interface between the N- and C-terminal six-helix bundles on the periplasmic side of LacY are shown to cross-link quantitatively and reversibly with homobifunctional thiol reagents. Strikingly, relatively short (<15 Å) and/or rigid reagents block transport by all three mutants, whereas relatively long (>15 Å), flexible cross-linking agents exhibit partial or even full transport activity. Therefore, the transport mechanism of LacY must involve opening, as well as closing, of a relatively large periplasmic cavity.
Results
Mutant Construction.
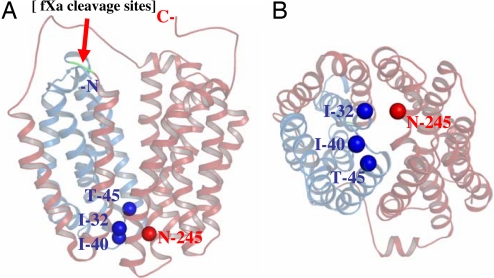
As shown by x-ray crystallography (3–5), helices I, II, and VII are in the interface between the N- and C-terminal six-helix bundles on the periplasmic side of LacY and appear to play a role in sealing the inward-facing cavity from the outside (Fig. 1). A number of single-Cys mutants within the tightly packed periplasmic domain of LacY exhibit sugar-induced increases in reactivity/accessibility with alkylating agents (see refs. 7 and 10) and also react with methanethiosulfonate ethylsulfonate, an impermeant thiol reagent (17–20), suggesting that this region of LacY is not in a fixed state and conditionally accessible to water. To study interactions in this region, three double-Cys mutants with N245C (helix VII) paired with I32C (helix I), I40C (loop I–II), or T45C (helix II), respectively, were constructed, and two tandem factor Xa protease sites were engineered into the loop between helices IV/V (Fig. 1) so that cross-linking could be tested conveniently (21).
Fig. 1.
X-ray crystal structure of LacY (PDB ID 2V8N). Cα of Ile-32 (helix I), Ile-40 (loop I/II), and Thr-45 (helix II) are presented as dark blue spheres, and the Cα of residue N245 (helix VII) is presented as a red sphere. The N-terminal four helices (N4) and the C-terminal helices (C8) are shown in blue and red, respectively, and separated by tandem factor Xa protease sites (Left; green), as indicated by the red arrow. (A) Side view. (B) Periplasmic view.
Cross-Linking.
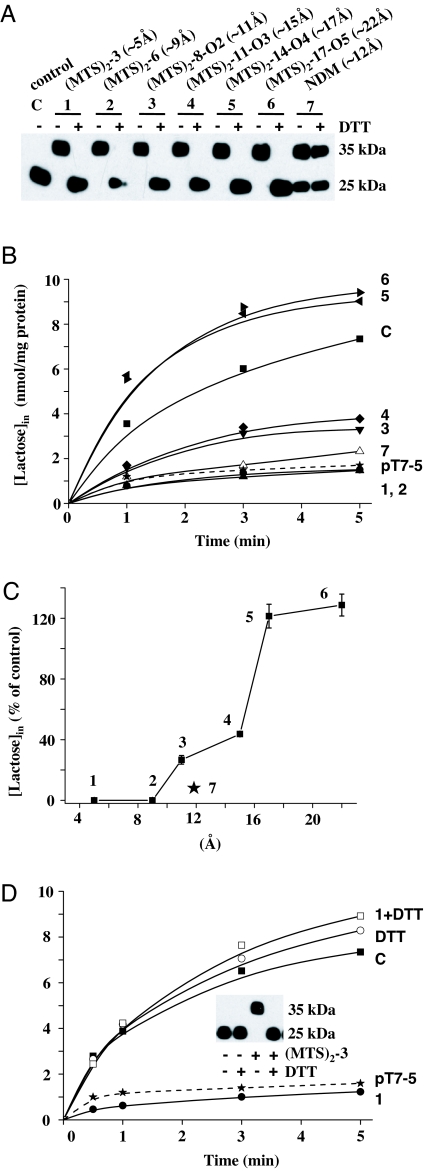
Homobifunctional thiol-reactive reagents (Fig. 2) combined with site-directed factor Xa proteolysis (21, 22) were used to study cross-linking on the periplasmic side of LacY between the N- and C-terminal six-helix bundles (Fig. 1). Because ligand binding increases the accessibility/reactivity of the single-Cys mutants used (7, 10, 18–20), cross-linking was carried out in the presence of β-d-galactopyranosyl 1-thio-β-d-galactopyranoside (TDG). Right-side-out (RSO) membrane vesicles containing given paired-Cys LacY mutants and treated with factor Xa protease in the absence of cross-linker exhibit a single band migrating at an Mr of ≈25 kDa (Fig. 3A; control) corresponding to a LacY fragment containing the eight C-terminal helices plus the loops (C8) (Fig. 1). Therefore, under the conditions described, digestion with factor Xa protease is essentially complete. When cross-linking between the fragments occurs, a band corresponding to intact LacY migrating at ≈35 kDa is observed, demonstrating that the N-terminal four helices (N4) are now covalently bound to the C8 fragment [Fig. 3A; supporting information (SI) Figs. 5A and 6A]. With mutant I40C/N245C, which is located in short loop I–II and helix VII, respectively, cross-linking by each MTS-based reagent (Fig. 2) is complete (Fig. 3A), but with naphthalene-1,5-di-maleimide (NDM; ≈12 Å, rigid; Fig. 2), the reaction is incomplete (Fig. 3A). For mutants I32C/N245C (helix I/VII) (SI Fig. 5A) and T45C/N245C (helix II/VII) (SI Fig. 6A), cross-linking is complete with each reagent tested. Also, with each mutant, no bands are observed >35 kDa, showing that intermolecular cross-linking does not occur (23). Finally, cross-linking with each MTS-based reagent is reversed quantitatively after reduction with DTT (Fig. 3A), but as expected, cross-linking with NDM, a maleimide-based cross-linker, is not reversed (Fig. 3A).
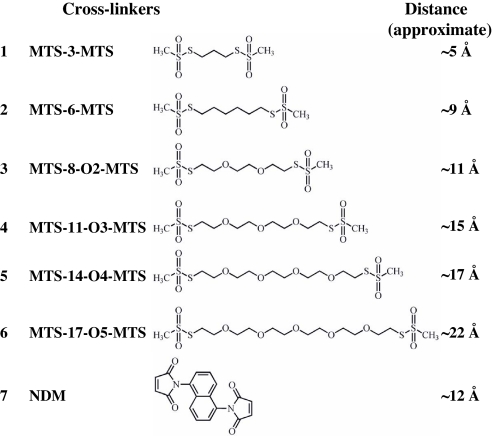
Fig. 2.
Homobifunctional cross-linking reagents. For MTS reagents, the approximate S–S distances between bridging sulfur atoms in the chains are as given by the manufacturer. The distance for NDM is from Green et al. (19).
Fig. 3.
Cross-linking and lactose transport with mutant I40C/N245C. All experiments were performed with RSO vesicles as described in Materials and Methods. C, control sample with no treatment (■); 1, MTS-3-MTS (●); 2, MTS-6-MTS (▴); 3, MTS-8-O2-MTS (▾); 4, MTS-11-O3-MTS (♦); 5, MTS-14-O4-MTS (◀); 6, MTS-17-O5-MTS (▶); 7, NDM (▵); pT7–5, RSO membrane vesicles containing empty vector pT7–5 (★). (A) SDS/PAGE and Western blots with anti-C-terminal antibody. (B) Time courses of transport after cross-linking with various homobifunctional cross-linking agents. Lactose accumulation by RSO membrane vesicles containing given mutants or no LacY (empty vector pT7–5) were assayed as described in Materials and Methods. (C) Correlation between maximum levels of accumulation and cross-linker length. Percentage of lactose accumulation at 5 min from at least three independent measurements with the untreated control with error bars are plotted against the length of the cross-linkers. (D) Reversal of transport and cross-linking by DTT. RSO vesicles containing a given mutant were assayed for transport and cross-linking (Inset) without or with DTT treatment. Effects of DTT on lactose transport were carried out as described in Materials and Methods. ○, DTT only; □, MTS-3-MTS then DTT.
Cross-Linking and Transport Activity.
To study the effect of cross-linking on transport, Δμ̄H+-driven lactose accumulation was tested in the same RSO vesicles used for Western blots. Mutant I40C/N245C (loop I–II/helix VII) completely loses activity after cross-linking with the relatively short reagents 1,3-propanediyl bis-methanethiosulfonate (MTS-3-MTS; ≈5Å and flexible) or 1,6-hexanediyl-bis-methanethiosulfonate (MTS-6-MTS; ≈9 Å and flexible) (Fig. 3 A–C). But strikingly, the mutant exhibits ≈27, 44, 121, and 128% of the control level of accumulation, respectively, after complete cross-linking by flexible MTS reagents ≈11, ≈15, ≈17, and ≈22 Å in length (Fig. 3 A–C). In contrast, little activity is observed after cross-linking with the rigid reagent NDM (≈12 Å) (Fig. 3 B and C), and the low level of activity observed may be because of incomplete cross-linking (Fig. 3A). Gratifyingly, like cross-linking, inactivation of transport by MTS-3-MTS is reversed by reduction with DTT (Fig. 3D). Furthermore, MTS-3-MTS exhibits no effect on the lactose transport with RSO membrane vesicles containing Cys-less LacY indicating that the effect of the cross-linking agents is specific for LacY and does not alter Δμ̄H+.
With mutants I32C/N245C (helix I/VII) (SI Fig. 5 B and C) and T45C/N245C (helix II/VII) (SI Fig. 6 B and C), the effect of cross-linking with each reagent on lactose transport exhibits patterns similar to those observed with mutant I40C/N245C (loop I–II/helix VII). Moreover, inactivation by MTS-3-MTS is fully reversed by reduction by DTT (SI Figs. 5D and 6D). Together, the results reveal that separation of the periplasmic ends of helix I/II including the connecting short loop and helix VII reaches ≈15–17 Å during LacY turnover.
Discussion
All x-ray structures of LacY exhibit a tightly closed periplasmic side with a large hydrophilic cavity facing the cytoplasm. However, findings from site-directed alkylation (see refs. 7 and 10), single molecule fluorescence resonance energy transfer (8), and double electron-electron resonance (9) are consistent with a mechanism that involves opening and closing of hydrophilic cavities on either side of the membrane, resulting in alternating access of the sugar-binding site. In this respect, the experiments presented here, particularly with the double-Cys mutant I40C/N245C, are indeed remarkable in that they provide strong support for the contention that transport of sugar across the membrane by LacY involves opening and closing of a relatively large, water-filled periplasmic cavity that is not apparent in any of the crystal structures obtained thus far (3–5). When Cys replacements at positions 40 and 245 in particular, which presumably lie across the mouth of the putative periplasmic cavity, are cross-linked with bifunctional thiol reagents less than ≈15 Å in length, transport activity is low or nil. However, after quantitative cross-linking with reagents ≈17 Å or ≈22 Å in length, full activity is observed. With two additional double-Cys mutants bridging the presumed cavity, similar but less dramatic effects are observed.
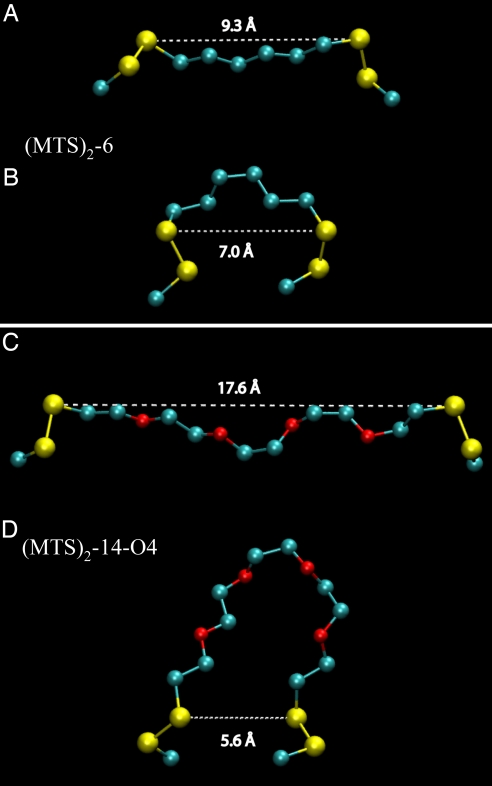
In addition to length, the flexibility of the cross-linking reagents also appears to be an essential factor. Maximum activity is observed when the length of the cross-linker reaches ≈17 Å (MTS-14-O4-MTS) (Fig. 2) with each double mutant (Fig. 3C and SI Figs. 5C and 6C). Further increase in length to ≈22 Å (MTS-17-O5-MTS) exhibits no further effect on lactose transport, whereas significantly lower activity is observed with MTS cross-linkers less than ≈15 Å, and very low or no activity is observed with rigid NDM (≈12.5 Å) (24). In vacuo molecular dynamics simulations were performed to sample the conformational space available to the cross-linking agents. For MTS-3-MTS (data not shown) and MTS-6-MTS (Fig. 4 A and B), as representatives of the cross-linkers that completely inactivate, the whole range of S–S distances in the chains is from ≈3–6 Å and ≈5–10 Å, respectively. In contrast, the whole range of S–S distances with MTS-14-O4-MTS (Fig. 4 C and D) and MTS-17-O5-MTS (data not shown) is from ≈3–19 Å and ≈3–22 Å, respectively. In order for LacY to catalyze transport, the periplasmic cavity must be able to open large enough, as well as close tight enough, and the long MTS cross-linking agents seem to be sufficiently flexible to accommodate both requirements. Although NDM at ≈12.5 Å (24) is sufficiently long for the cavity to open to a distance that might allow partial activity [i.e., mutant I40C/N245C cross-linked with MTS-8-O2-MTS (≈11 Å), exhibits significant activity; Fig. 3 C and D], closure should not be able to occur when cross-linking occurs with this rigid cross-linking agent.
Fig. 4.
Configuration snapshots from MTS-6-MTS and MTS-14-O4-MTS model simulations. For MTS-6-MTS, a fully extended (A) and a disordered (B) configuration exhibit similar distances between indicated sulfur atoms. In contrast, for MTS-14-O-MTS, a fully extended (C) and a bent (D) configuration exhibit markedly different separations between indicated sulfur atoms. The models are shown with the two sulfur atoms and the Cβ carbons of the Cys side chains and without hydrogen atoms for clarity.
Site-directed alkylation (see refs. 7 and 10), single molecule fluorescence resonance energy transfer (8), and double electron-electron resonance (9) provide independent evidence that sugar binding causes opening of a cavity on the periplasmic face of LacY with closing of the large cytoplasmic cavity. Clearly, the results presented here strongly indicate that the maximum size of the opening in the periplasmic hydrophilic cavity formed during turnover of LacY is ≈17 Å, a distance close to that determined by double electron-electron resonance (9).
Materials and Methods
Materials.
Emil Reisler (University of California, Los Angeles) generously provided NDM. Restriction endonucleases, T4 DNA ligase, and Factor Xa protease were purchased from New England Biolabs. The QuikChange II kit was from Stratagene. DNA plasmid purification and DNA fragment gel extraction kits were purchased from Qiagen. MTS-based homobifunctional cross-linking agents were purchased from Toronto Research Chemicals. Site-directed rabbit polyclonal antiserum against a dodecapeptide corresponding to the C terminus of LacY was prepared as described in ref. 25. Micro BCA protein determination and Supersignal West Pico Chemiluminescent substrate kits were from Pierce. All other materials were of reagent grade and obtained from commercial sources.
Construction of Mutants.
Plasmid pT7–5/Cys-less/fXa2 encoding Cys-less LacY with tandem factor Xa sites (Ile-Glu-Gly-Arg)2 between Ser-136 and Asn-137 in cytoplasmic loop IV/V (Fig. 1) was constructed as described in ref. 21. Double-Cys mutants were then generated by two-step replacement of a BamHI/PstI fragment encoding helices I–II and then a BssHII/KpnI fragment encoding helix VII from plasmid pT7–5 containing appropriate single-Cys mutants (18–20) into plasmid pT7–5/Cys-less/fXa2. DNA sequencing of the entire lacY gene confirmed all constructs, and no other mutations were found.
Protein Expression.
E. coli T184 [lacI+O+Z−Y−(A) rpsL,met−,thr−,recA,hsdM,hsdR/F′,lacIqO+ZD118(Y+A+)] transformed with plasmids encoding given double-Cys mutants by electroporation in 0.2-cm cuvettes were grown at 37°C in Luria–Bertani broth with 100 mg/liter ampicillin. Overnight cultures were diluted 10-fold and allowed to grow for 2 h at 37°C before induction with 1 mM isopropyl 1-thio-β-d-galactopyranoside. After additional growth for 2–3 h at 37°C, cells were harvested by centrifugation.
Preparation of RSO Vesicles.
RSO membrane vesicles were prepared by osmotic lysis as described (26, 27), suspended in 100 mM potassium phosphate (KPi; pH 7.5)/10 mM MgSO4 at a protein concentration of ≈10–20 mg/ml, frozen in liquid N2, and stored at −80°C until use.
Cross-Linking.
Unless stated otherwise, cross-linking was carried out at room temperature for 10 min with 0.05 mM MTS reagents or for 30 min with 0.5 mM maleimide reagents at room temperature in presence of 10 mM TDG (final concentrations) (28). After 10-fold dilution with 100 mM cold KPi (pH 7.5), the vesicles were immediately collected by centrifugation at 4°C, washed three times with 100 mM KPi, and resuspended in 100 mM KPi/10 mM MgSO4 at ≈3–5 mg of protein per ml. One portion of the preparation was used to assay lactose transport, the remainder for protein determinations and analysis of cross-linking. Cross-linking was analyzed as follows: aliquots (10 μg of protein) were digested overnight with factor Xa protease as described in ref. 21, and samples (2 μg of protein) were subjected to SDS/12% PAGE and Western blotting with a site-directed polyclonal antibody against the C terminus of LacY followed by an horseradish peroxidase-coupled anti-rabbit antibody (Amersham Biosciences, NA-934) as described in ref. 25.
For reversal of cross-linking, samples cross-linked with MTS-3-MTS were washed twice by centrifugation, resuspended, and incubated for 30 min at room temperature with 40 mM DTT. Two further washes with 100 mM KPi/10 mM MgSO4 were carried out before SDS/PAGE immunoblotting and assaying lactose transport.
Transport Assays.
RSO vesicles containing given LacY mutants were adjusted to an OD600 of 3.0 (3 mg of protein per ml). Transport was assayed by rapid filtration after incubation of samples with 20 mM ascorbate/0.2 mM phenazine methosulfate and 0.4 mM [1-14C]lactose (10 mCi/mmol) under oxygen as described in ref. 29.
Protein Assays.
Protein was assayed with the Micro BCA protein determination kit (Pierce).
Molecular Dynamics Simulations.
All-atom models were built for MTS-3-MTS, MTS-6-MTS, MTS-14-O4-MTS, and MTS-17-O5-MTS, consisting of the spacer moiety from one sulfur to the other, and flanked by two Cys side chains. The initial configuration was submitted to 256 steps of conjugate-gradient energy minimization. In vacuo molecular dynamics simulations at constant temperature were conducted with NAMD 2.6 (30) using the CHARMM force field (31, 32) and a time step of 1 fs. A 100-ns trajectory was run for each model. Configurations were sampled every 10 ps. Molecular graphics and trajectory analyses were performed with VMD 1.8.6 (33).
Supplementary Material
ACKNOWLEDGMENTS.
We are deeply indebted to Emil Reisler for providing NDM. We also thank Yiling Nie for initially providing RSO vesicles with single-Cys mutant I32C and T45C and Gregor Madej for help with Fig. 2. This work was supported by National Institutes of Health Grants DK51131, DK069463, 1 P50 GM073210 and 1 U54 GM074929 and National Science Foundation Grant 0450970 (to H.R.K.). J.A.F. thanks Douglas Tobias and Stephen White for helpful discussions and for support from National Science Foundation Grant CHE-0417158 (to Douglas Tobias) and National Institute of General Medical Sciences Grants GM68002 and GM74637 (to Stephen White.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800825105/DC1.
References
- 1.Kaback HR, Sahin-Toth M, Weinglass AB. The kamikaze approach to membrane transport. Nat Rev Mol Cell Biol. 2001;2:610–620. doi: 10.1038/35085077. [DOI] [PubMed] [Google Scholar]
- 2.Guan L, Kaback HR. Lessons from lactose permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson J, et al. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 4.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural evidence for induced fit and a mechanism for sugar/H(+) symport in LacY. EMBO J. 2006;25:1177–1183. doi: 10.1038/sj.emboj.7601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of wild-type lactose permease. Proc Natl Acad Sci USA. 2007;104:15294–15298. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan L, Kaback HR. Binding affinity of lactose permease is not altered by the H+ electrochemical gradient. Proc Natl Acad Sci USA. 2004;101:12148–12152. doi: 10.1073/pnas.0404936101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaback HR, et al. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci USA. 2007;104:491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majumdar DS, et al. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc Natl Acad Sci USA. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smirnova I, et al. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci USA. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nie Y, Ermolova N, Kaback HR. Site-directed alkylation of LacY: Effect of the proton electrochemical gradient. J Mol Biol. 2007;374:356–364. doi: 10.1016/j.jmb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 12.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 13.le Coutre J, Kaback HR, Patel CK, Heginbotham L, Miller C. Fourier transform infrared spectroscopy reveals a rigid α-helical assembly for the tetrameric Streptomyces lividans K+ channel. Proc Natl Acad Sci USA. 1998;95:6114–6117. doi: 10.1073/pnas.95.11.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patzlaff JS, Moeller JA, Barry BA, Brooker RJ. Fourier transform infrared analysis of purified lactose permease: A monodisperse lactose permease preparation is stably folded, α-helical, and highly accessible to deuterium exchange. Biochemistry. 1998;37:15363–15375. doi: 10.1021/bi981142x. [DOI] [PubMed] [Google Scholar]
- 15.Bogdanov M, Heacock PN, Dowhan W. A polytopic membrane protein displays a reversible topology dependent on membrane lipid composition. EMBO J. 2002;21:2107–2116. doi: 10.1093/emboj/21.9.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nie Y, Smirnova I, Kasho V, Kaback HR. Energetics of ligand-induced conformational flexibility in the lactose permease of Escherichia coli. J Biol Chem. 2006;281:35779–35784. doi: 10.1074/jbc.M607232200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlin A, Akabas MH. Substituted-cysteine accessibility method. Methods Enzymol. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- 18.Ermolova N, Madhvani RV, Kaback HR. Site-directed alkylation of cysteine replacements in the lactose permease of Escherichia coli: Helices I, III, VI, and XI. Biochemistry. 2006;45:4182–4189. doi: 10.1021/bi052631h. [DOI] [PubMed] [Google Scholar]
- 19.Venkatesan P, Liu Z, Hu Y, Kaback HR. Site-directed sulfhydryl labeling of the lactose permease of Escherichia coli: Helix II. Biochemistry. 2000;39:10649–10655. doi: 10.1021/bi0004394. [DOI] [PubMed] [Google Scholar]
- 20.Venkatesan P, Kwaw I, Hu Y, Kaback HR. Site-directed sulfhydryl labeling of the lactose permease of Escherichia coli: Helix VII. Biochemistry. 2000;39:10641–10648. doi: 10.1021/bi000438b. [DOI] [PubMed] [Google Scholar]
- 21.Wolin CD, Kaback HR. Thiol cross-linking of transmembrane domains IV and V in the lactose permease of Escherichia coli. Biochemistry. 2000;39:6130–6135. doi: 10.1021/bi0001269. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Kaback HR. A general method for determining helix packing in membrane proteins in situ: Helices I and II are close to helix VII in the lactose permease of Escherichia coli. Proc Natl Acad Sci USA. 1996;93:14498–14502. doi: 10.1073/pnas.93.25.14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ermolova N, Guan L, Kaback HR. Intermolecular thiol cross-linking via loops in the lactose permease of Escherichia coli. Proc Natl Acad Sci USA. 2003;100:10187–10192. doi: 10.1073/pnas.1434239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green NS, Reisler E, Houk KN. Quantitative evaluation of the lengths of homobifunctional protein cross-linking reagents used as molecular rulers. Protein Sci. 2001;10:1293–1304. doi: 10.1110/ps.51201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrasco N, et al. Intramolecular dislocation of the COOH terminus of the lac carrier protein in reconstituted proteoliposomes. Proc Natl Acad Sci USA. 1984;81:4672–4676. doi: 10.1073/pnas.81.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaback HR. Methods Enzymol, Bacterial membranes. 1971;22:99–120. [Google Scholar]
- 27.Short SA, Kaback HR, Kohn LD. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J Biol Chem. 1975;250:4291–4296. [PubMed] [Google Scholar]
- 28.Guan L, Weinglass AB, Kaback HR. Helix packing in the lactose permease of Escherichia coli: Localization of helix VI. J Mol Biol. 2001;312:69–77. doi: 10.1006/jmbi.2001.4933. [DOI] [PubMed] [Google Scholar]
- 29.Konings WN, Barnes EM, Jr, Kaback HR. Mechanisms of active transport in isolated membrane vesicles. 2. The coupling of reduced phenazine methosulfate to the concentrative uptake of β-galactosides and amino acids. J Biol Chem. 1971;246:5857–5861. [PubMed] [Google Scholar]
- 30.Philips JC, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anisimov VM, Vorobyov IV, Roux B, MacKerell AD., Jr Polarizable empirical force field for the primary and secondary alcohol series based on the classical Drude model. J Chem Theory Comput. 2007;3:1927–1946. doi: 10.1021/ct700100a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacKerell AD, Jr, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 33.Humphrey W, Dalke W, Schulten K. VMD: Visual Molecular Dynamics. J Mol Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.